Submitted:
13 April 2023
Posted:
14 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
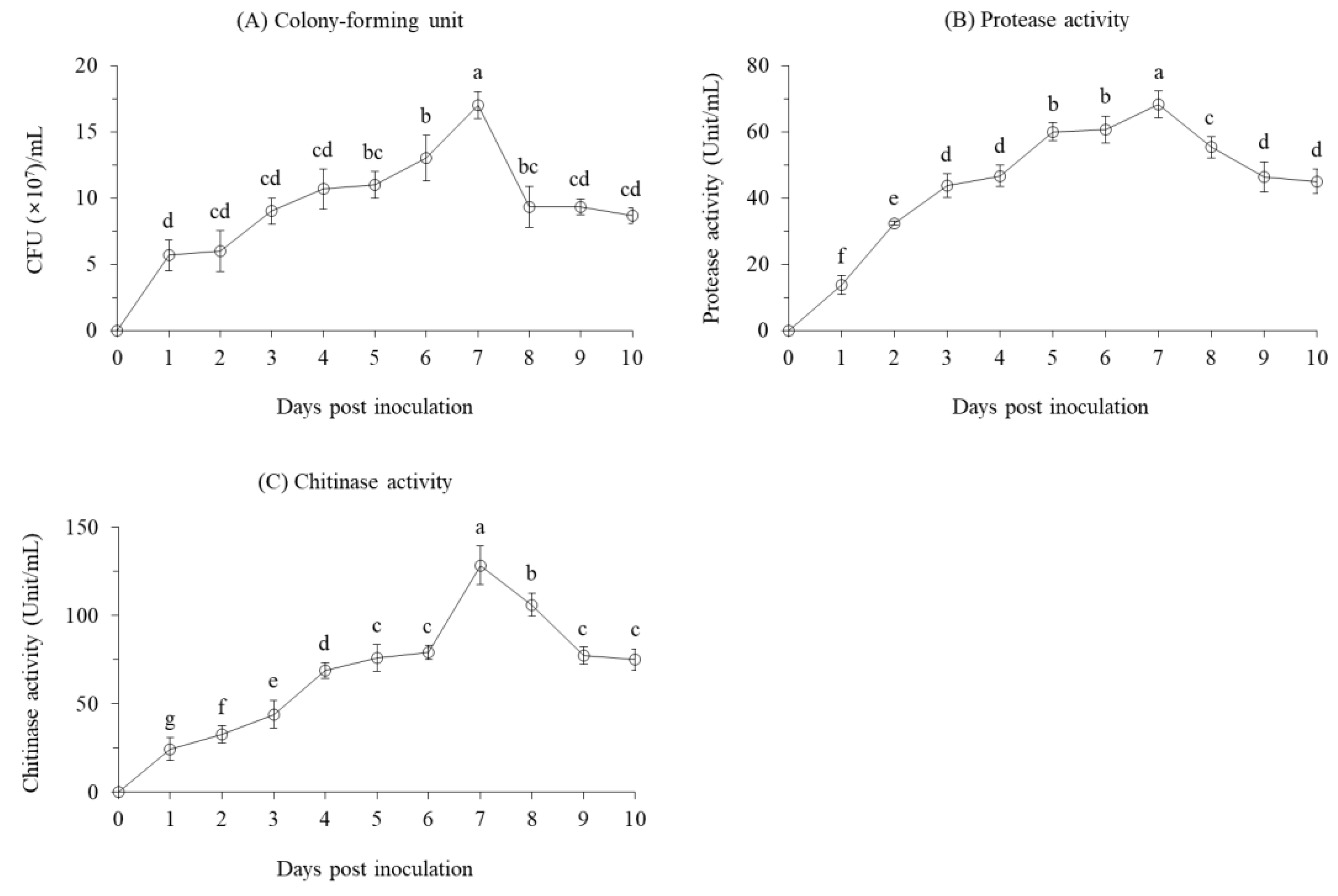
2.1. Cell Growth, Protease and Chitinase Activity of B. velezensis CE 100
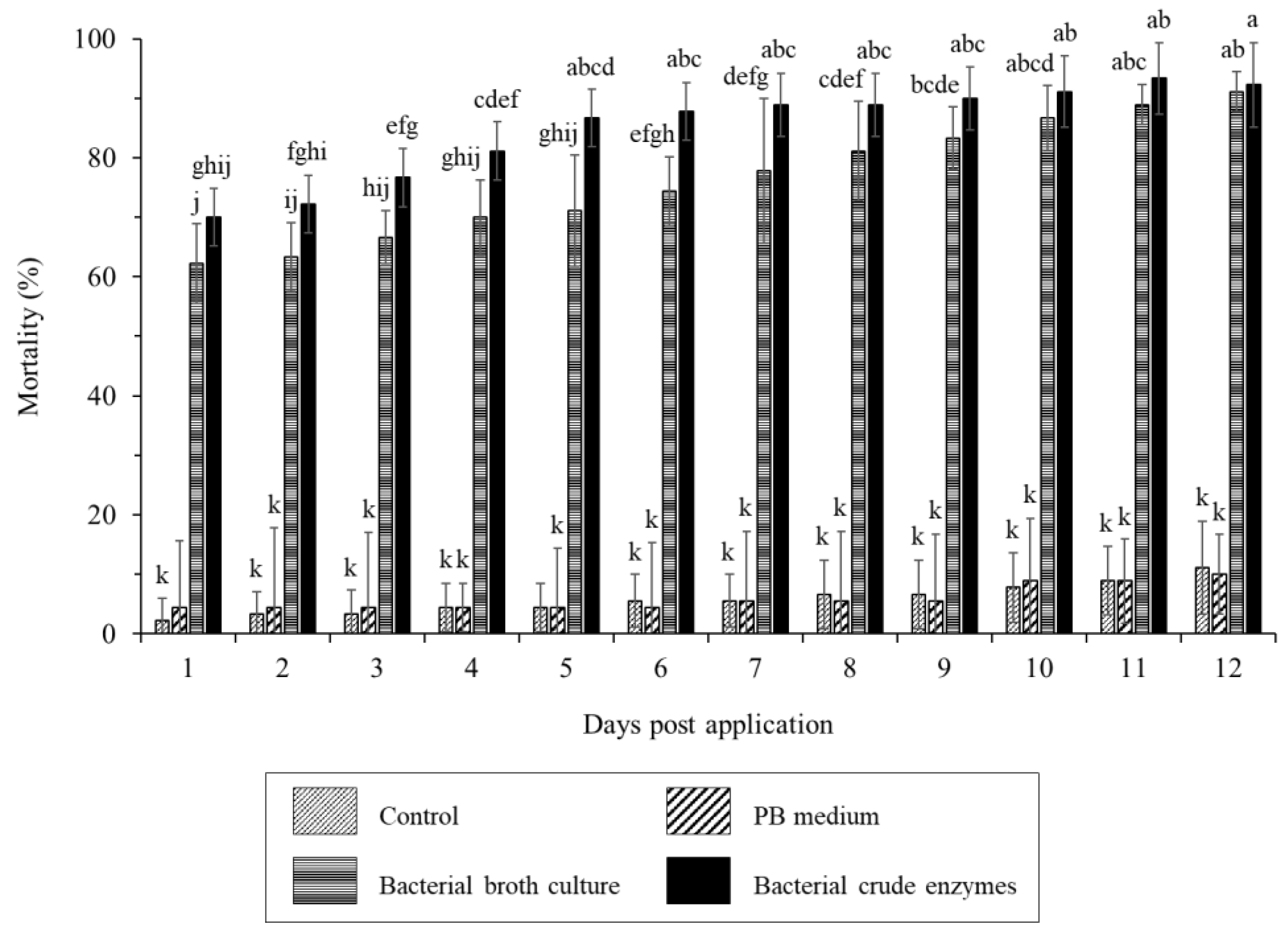
2.2. Termiticidal Activity of B. velezensis CE 100 against R. speratus Worker Termites
2.3. Morphological Deformation of Termite Cuticles after Treatment
3. Discussion
4. Materials and Methods
4.1. Preparation of Bacterial Broth Culture and Termites Samples
4.2. Cell Growth, Proteolytic Activity and Chitinolytic Activity of B. velezensis CE 100
4.3. Preparing the Crude Enzyme Fraction from B. velezensis CE 100 Bacterial Broth Culture
4.4. Termiticidal Activity of B. licheniformis PR2 Broth Culture and Crude Enzymes on R. speratus Worker Termites
4.5. Analysis of the Morphological Changes in Termite cuticles after Treatment
4.6. Statistical Analysis
5. Conclusion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eggleton, P.; Tayasu, I. Feeding groups, lifetypes and the global ecology of termites. Ecol. Res. 2001, 16, 941–960. [Google Scholar] [CrossRef]
- Kim, S.-H.; Chung, Y.-J. Analysis of factors affecting termite damage to wooden architectural heritage buildings in Korea. Forests 2022, 13, 465. [Google Scholar] [CrossRef]
- Novita, N.; Amiruddin, H.; Ibrahim, H.; Jamil, T. M.; Syaukani, S.; Oguri, E.; Eguchi, K. Investigation of termite attack on cultural heritage buildings: A case study in Aceh province, Indonesia. Insects 2020, 11, 385. [Google Scholar] [CrossRef] [PubMed]
- Traniello, J.F.A.; Leuthold, R.H. Behavior and Ecology of Foraging in Termites. In Termites: evolution, sociality, symbioses, ecology.; Abe, T., Bignell, D.E., Higashi, M., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; pp. 141–168. [Google Scholar] [CrossRef]
- Im, I.-G.; Cha, H.-S.; Kang, W.-C.; Lee, S.-B.; Han, G.-S. The status of damage and monitoring of subterranean termite (Reticulitermes spp.)(Blattodea: Rhinotermitidae) for wooden cultural heritage in Korea. J. Conserv. Sci. 2021, 37, 191–208. [Google Scholar] [CrossRef]
- Su, N.-Y.; Scheffrahn, R.H. Termites as pests of buildings. In Termites: evolution, sociality, symbioses, ecology.; Abe, T., Bignell, D.E., Higashi, M., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000. [Google Scholar] [CrossRef]
- Jung-Kwon, O.; Chul-Ki, K.; Jung-Pyo, H.; Jun-Jae, L. Improvement of robustness in ultrasonic attenuation spectroscopy for detecting internal insect damage in wood member of cultural heritage. J. Wood Sci. 2015, 61, 136–142. [Google Scholar] [CrossRef]
- Han, S.-H.; Lee, K.-S.; Chung, Y.-J. Characteristic of termite inhabits in South Korea and the control. Conserv. Sutides 1998, 19, 133–158. [Google Scholar]
- Buczkowski, G.; Bertelsmeier, C. Invasive termites in a changing climate: A global perspective. Ecol. Evol. 2017, 7, 974–985. [Google Scholar] [CrossRef]
- Ewart, D.; Nunes, L.; de Troya, T.; Kutnik, M.; Dhang, P. Termites and a changing climate. In Climat change impacts on urban pests.; Dhang, P., Ed.; CAB International: Preston, UK, 2016. [Google Scholar]
- Shim, J.; Park, H.; Ju, H.-J.; Song, J.-H. First record of the termite family Kalotermitidae (Blattodea: Termitoidae) in Korea. J. Asia Pac. Entomol. 2021, 24, 1266–1269. [Google Scholar] [CrossRef]
- Pareek, A.; Meena, B.M.; Sharma, S.; Tetarwal, M.L.; Kalyan, R.K.; Meena, B.L. Impact of climate change on insect pests and their management strategies. In Climate change and sustainable agriculture.; Kumar, P.S., Kanwat, M., Meena, P.D., Kumar, V., Alone, R.A., Eds.; New India Publishing Agency: New Delhi, India, 2017; pp. 253–286. ISBN 9789385516726. [Google Scholar]
- Lee, S.-B.; Tong, R. L.; Kim, S.-H.; Im, I. G.; Su, N.-Y. Potential pest status of the Formosan subterranean termite, Coptotermes formosanus Shiraki (Blattodea: Isoptera: Rhinotermitidae), in response to climate change in the Korean peninsula. Fla. Entomol. 2021, 103, 431–437. [Google Scholar] [CrossRef]
- Verma, M.; Sharma, S.; Prasad, R. Biological alternatives for termite control: A review. Int. Biodeterior. Biodegrad. 2009, 63, 959–972. [Google Scholar] [CrossRef]
- Lee, S.; Im, I.; Kim, S. A history of termite control and improvements to prevent termites in wooden architectural heritage. J. Cult. Herit. Stud. (Munhwaje) 2021, 54, 194–215. [Google Scholar] [CrossRef]
- Gordon, J. M.; Velenovsky IV, J. F.; Chouvenc, T. Subterranean termite colony elimination can be achieved even when only a small proportion of foragers feed upon a CSI bait. J. Pest Sci. 2022, 95, 1207–1216. [Google Scholar] [CrossRef]
- Lemus, R.; Abdelghani, A. Chlorpyrifos: an unwelcome pesticide in our homes. Rev. Environ. Health 2000, 15, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Hamm, R. L.; DeMark, J. J.; Chin-Heady, E.; Tolley, M. P. Consumption of a durable termite bait matrix by subterranean termites (Isoptera: Rhinotermitidae) and resulting insecticidal activity. Pest Manag. Sci. 2013, 69, 507–511. [Google Scholar] [CrossRef]
- Sajap, A. S.; Lee, L.; Shah, Z. M. Elimination of subterranean termite colonies with hexaflumuron in an improved bait matrix, preferred textured cellulose (PTC). Sociobiology 2009, 53, 891–902. [Google Scholar]
- Chouvenc, T.; Su, N.-Y. Subterranean termites feeding on CSI baits for a short duration still results in colony elimination. J. Econ. Entomol. 2017, 110, 2534–2538. [Google Scholar] [CrossRef] [PubMed]
- Ghaly, A.; Edwards, S. Termite damage to buildings: Nature of attacks and preventive construction methods. Am. J. Eng. Appl. Sci. 2011, 4, 187–200. [Google Scholar] [CrossRef]
- Hong, S.-H.; An, M.-Y.; Kang, R.-Y.; Choi, S.-H. Effectiveness of controlling micro climate by the pine (Pinus Densiflora) forests of the temple in southeast area of Korea. Korean J. Environ.Ecol. 2020, 34, 294–303. [Google Scholar] [CrossRef]
- Matthews, J. Sonamu–The cultural significance of the Korean red pine. Int. Dendrol. Soc. Yearbook 2019, 72–79. [Google Scholar] [CrossRef]
- He, B.; Ni, Y.; Jin, Y.; Fu, Z. Pesticides-induced energy metabolic disorders. Sci. Total Environ. 2020, 729, 139033. [Google Scholar] [CrossRef]
- Meftaul, I. M.; Venkateswarlu, K.; Dharmarajan, R.; Annamalai, P.; Megharaj, M. Pesticides in the urban environment: A potential threat that knocks at the door. Sci. Total Environ. 2020, 711, 134612. [Google Scholar] [CrossRef] [PubMed]
- Rezende-Teixeira, P.; Dusi, R. G.; Jimenez, P. C.; Espindola, L. S.; Costa-Lotufo, L. V. What can we learn from commercial insecticides? Efficacy, toxicity, environmental impacts, and future developments. Environ. Pollut. 2022, 300, 118983. [Google Scholar] [CrossRef]
- Osbrink, W. L.; Lax, A. R.; Brenner, R. J. Insecticide susceptibility in Coptotermes formosanus and Reticulitermes virginicus (Isoptera: Rhinotermitidae). J. Econ. Entomol. 2001, 94, 1217–1228. [Google Scholar] [CrossRef] [PubMed]
- Rakshiya, Y.; Verma, M.; Sindhu, S. Efficacy of antagonistic soil bacteria in management of subterranean termites (Isoptera). Res. Environ. Life Sci. 2016, 9, 949–955. [Google Scholar]
- Sindhu, S.S.; Rakshiya, Y.S.; Verma, M.K. Biological control of termites by antagonistic soil microorganisms. In Bioaugmentation, Biostimulation and Biocontrol, Singh, A., Parmar, N., Kuhad R.C., Eds.; Springer: Berlin, Germany, 2011; pp. 261–309. [Google Scholar] [CrossRef]
- Hussin, N. A.; Ab Majid, A. H. Termiticidal activity of chitinase enzyme of Bacillus licheniformis, a symbiont isolated from the gut of Globitermes sulphureus worker. Biocatal. Agric. Biotechnol. 2020, 24, 101548. [Google Scholar] [CrossRef]
- Choi, S.-I.; Ajuna, H. B.; Won, S.-J.; Choub, V.; Kim, C.-W.; Moon, J.-H.; Ahn, Y. S. The insecticidal potential of Bacillus velezensis CE 100 against Dasineura jujubifolia Jiao & Bu (Diptera: Cecidomyiidae) larvae infestation and its role in the enhancement of yield and fruit quality of jujube (Zizyphus jujuba Miller var. inermis Rehder). Crop Prot. 2022, 106098. [Google Scholar] [CrossRef]
- Moon, J.-H.; Won, S.-J.; Choub, V.; Choi, S.-I.; Ajuna, H. B.; Ahn, Y. S. Biological control of fall webworm larva (Hyphantria cunea Drury) and growth promotion of poplar seedlings (Populus× canadensis Moench) with Bacillus licheniformis PR2. For. Ecol. Manag. 2022, 525, 120574. [Google Scholar] [CrossRef]
- Leger, R. S.; Cooper, R. M.; Charnley, A. K. Cuticle-degrading enzymes of entomopathogenic fungi: cuticle degradation in vitro by enzymes from entomopathogens. J. Invertebr. Pathol. 1986, 47, 167–177. [Google Scholar] [CrossRef]
- Moon, J.-H.; Ajuna, H. B.; Won, S.-J.; Choub, V.; Choi, S.-I.; Yun, J.-Y.; Park, S. W.; Ahn, Y. S. Anti-termitic activity of Bacillus licheniformis PR2 against subterranean termites (Rhinotermitidae: Reticulitermes speratus kyushuensis Morimoto). Forests Submitted (under review). 2023. [Google Scholar] [CrossRef]
- Zukowski, J.; Su, N.-Y. Survival of termites (Isoptera) exposed to various levels of relative humidity (RH) and water availability, and their RH preferences. Fla. Entomol. 2017, 100, 532–538. [Google Scholar] [CrossRef]
- Zukowski, J.; Su, N.-Y. A comparison of morphology among four termite species with different moisture requirements. Insects 2020, 11, 262. [Google Scholar] [CrossRef]
- Moon, J.-H.; Won, S.-J.; Maung, C. E. H.; Choi, J.-H.; Choi, S.-I.; Ajuna, H. B.; Ahn, Y. S.; Jo, Y. H. The role of Lysobacter antibioticus HS124 on the control of fall webworm (Hyphantria cunea Drury) and growth promotion of Canadian poplar (Populus canadensis Moench) at Saemangeum reclaimed land in Korea. Microorganisms 2021, 9, 1580. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, G.; Chouvenc, T.; Su, N.-Y. Postecdysis sclerotization of mouthparts of the Formosan subterranean termites (Isoptera: Rhinotermitidae). J. Econ. Entomol. 2016, 109, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J. F. Arthropod cuticle: a natural composite shell system. Compos. - A: Appl. Sci. Manuf. 2002, 33, 1311–1315. [Google Scholar] [CrossRef]
- Vincent, J. F.; Wegst, U. G. Design and mechanical properties of insect cuticle. Arthropod Struct. Dev. 2004, 33, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S. Insect Cuticle as a Covalently Crosslinked Protein Network. ACS Synth. Biol. 1988, 141–150. [Google Scholar] [CrossRef]
- Moussian, B. The arthropod cuticle. In Arthropod biology and evolution: molecules, development, morphology, 1 Ed.; Minelli, A., Boxshall, G., Fusco, G., Eds.; Springer: Berlin, Germany, 2013. [Google Scholar] [CrossRef]
- Choi, T. G.; Maung, C. E. H.; Lee, D. R.; Henry, A. B.; Lee, Y. S.; Kim, K. Y. Role of bacterial antagonists of fungal pathogens, Bacillus thuringiensis KYC and Bacillus velezensis CE 100 in control of root-knot nematode, Meloidogyne incognita and subsequent growth promotion of tomato. Biocontrol Sci. Technol. 2020, 30, 685–700. [Google Scholar] [CrossRef]
- Choub, V.; Ajuna, H. B.; Won, S.-J.; Moon, J.-H.; Choi, S.-I.; Maung, C. E. H.; Kim, C.-W.; Ahn, Y. S. Antifungal activity of Bacillus velezensis CE 100 against anthracnose disease (Colletotrichum gloeosporioides) and growth promotion of walnut (Juglans regia L.) trees. Int. J. Mol. Sci. 2021, 22, 10438. [Google Scholar] [CrossRef]
- Moon, J.-H.; Won, S.-J.; Maung, C. E. H.; Choi, J.-H.; Choi, S.-I.; Ajuna, H. B.; Ahn, Y. S. Bacillus velezensis CE 100 inhibits root rot diseases (Phytophthora spp.) and promotes growth of Japanese cypress (Chamaecyparis obtusa Endlicher) seedlings. Microorganisms 2021, 9, 821. [Google Scholar] [CrossRef]
- Hong, S.; Kim, T. Y.; Won, S.-J.; Moon, J.-H.; Ajuna, H. B.; Kim, K. Y.; Ahn, Y. S. Control of fungal diseases and fruit yield improvement of strawberry using Bacillus velezensis CE 100. Microorganisms 2022, 10, 365. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




