1. Introduction
Taking part in physical activity and/or exercise generates a varied response across different physiological parameters. Among them, there are positive effects on Heart Rate (HR) - the resting heart rate, the maximal heart rate, and the submaximal heart rate in patients with cardiac issues [
1]. Blood Pressure (BP) is also positively impacted [
2], with studies showing that physical activity causes it to fall both at rest and during daily activities [
3,
4,
5]. It has also been shown that taking part in aerobic exercise for at least two weeks decreases systolic and diastolic pressure [
6], which are changes brought about not only after moderate activity such as walking [
7] but also six weeks of strength work [
8]. At the same time, physical exercise, as a physiological and psychological stressor, activates the hypothalamic-pituitary-adrenal (HPA) axis, and the secretion of cortisol increases in response to this situation of physical stress [
9].
Cortisol is a hormone generated by the adrenal glands. Under normal conditions, cortisol secretion varies according to the time of day, as it is linked to circadian rhythms. Its highest levels occur 30 minutes after waking and progressively decrease during the day until sleep, at which time they begin to climb again [
10,
11,
12,
13]. However, when a physical or mental challenge presents itself, or a threat is perceived, the HPA axis is activated and an increase in cortisol occurs. Once the challenge has been resolved, cortisol levels return to their basal state [
14]. These states and fluctuations are important, as abnormal cortisol levels provoke changes in the immune system and an inflammatory response [
10].
Cortisol levels, as detected both in serum and saliva, also increase according to the type and intensity of exercise performed [
15,
16,
17,
18,
19,
20], and the study of these levels is therefore used to assess the acute and chronic effects of training [
21]. Therefore, cortisol levels are used as a biomarker of psychological and physical stress [
20,
22,
23]. To analyze the concentration of cortisol, both blood and saliva tests are commonly used [
24]. As a non-invasive method which has a high correlation with serum levels, salivary cortisol analysis is widely used [
23,
25,
26,
27], although it can be affected by the consumption of food or coffee, or by smoking or chewing gum [
22].
Meanwhile, the use of active video games or exergames has been booming recently, as they are motivating and promote exercise and healthy lifestyle habits for their users [
28]. Some studies have recently explored whether these immersive virtual reality (IVR) exercise programs could be compared to traditional physical exercise programs [
29,
30,
31,
32,
33].
So far these studies have focused on whether these programs are feasible for different populations and whether the improvements in physical, cognitive and functional abilities generally attributed to traditional exercise, can be replicated or even improved by the use of fully immersive virtual environments [
34,
35,
36,
37,
38,
39,
40,
41]. To the best of our knowledge, however, physiological responses, such as raised levels of cortisol in the saliva after exposure to IVR exergames have not yet been studied.
Therefore, the main objective of our research was to evaluate the response across certain physiological parameters (heart rate, blood pressure and stress) after exposure to an IVR exergame in a sample of healthy adults, and to explore its usefulness as a tool for facilitating physical exercise. A secondary objective was to report on the safety and usability of the IVR tool, as well as the participant satisfaction with the experience.
3. Results
All participants completed the IVR session successfully without significant adverse effects. The main findings were that after the IVR intervention, the physiological parameters analyzed (heart rate, blood pressure and cortisol concentration in saliva) increased in relation to their pre-intervention level (see
Table 2). Additionally, the level of perceived exertion corresponded to the values for moderate to intense exercise (6.30±0.50/10 on the modified Borg scale). Furthermore, as can be seen in
Table 3, the participants gave good usability values for the IVR device (>76%), as well as low values in relation to any negative experiences, as measured with the post-game GEQ (0.05±0.12/4). This perception is supported by the fact that 100% of the participants considered the experience as good or very good and would recommend it (see
Table 4).
Finally, responses to the SSQ questionnaire showed no serious adverse symptoms linked to cybersickness in any of the participants. Only 1/37 reported feeling a moderate symptom (stomach awareness), 7/37 reported mild symptoms related (difficulty focusing) on the end of the session, but most participants reported a total absence of symptoms related to virtual exposure.
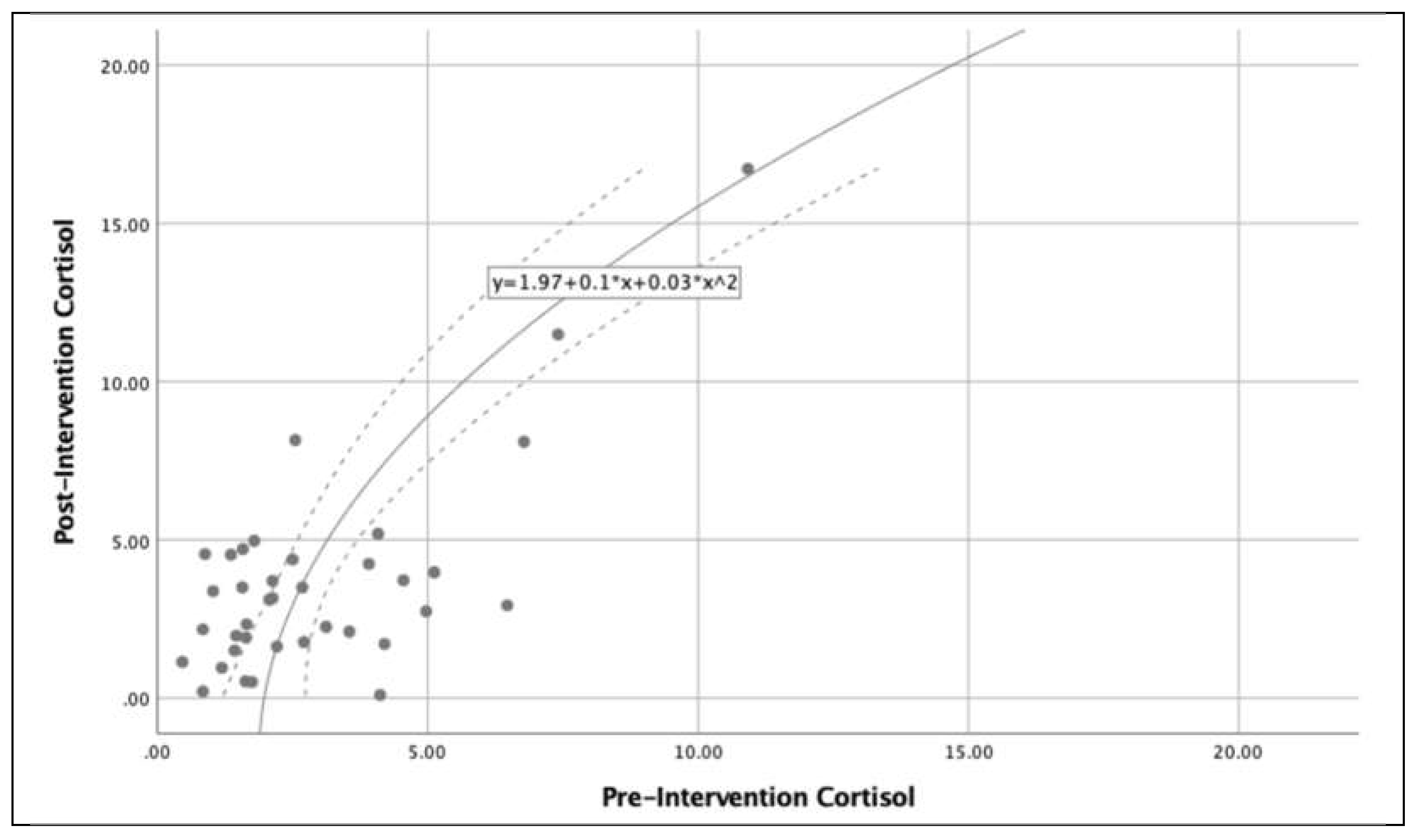
Figure 3 shows the relationship between cortisol levels (pre and post intervention) in the subjects analyzed, there is an exponential growth of cortisol levels after completion of the IVR exercise. Additionally,
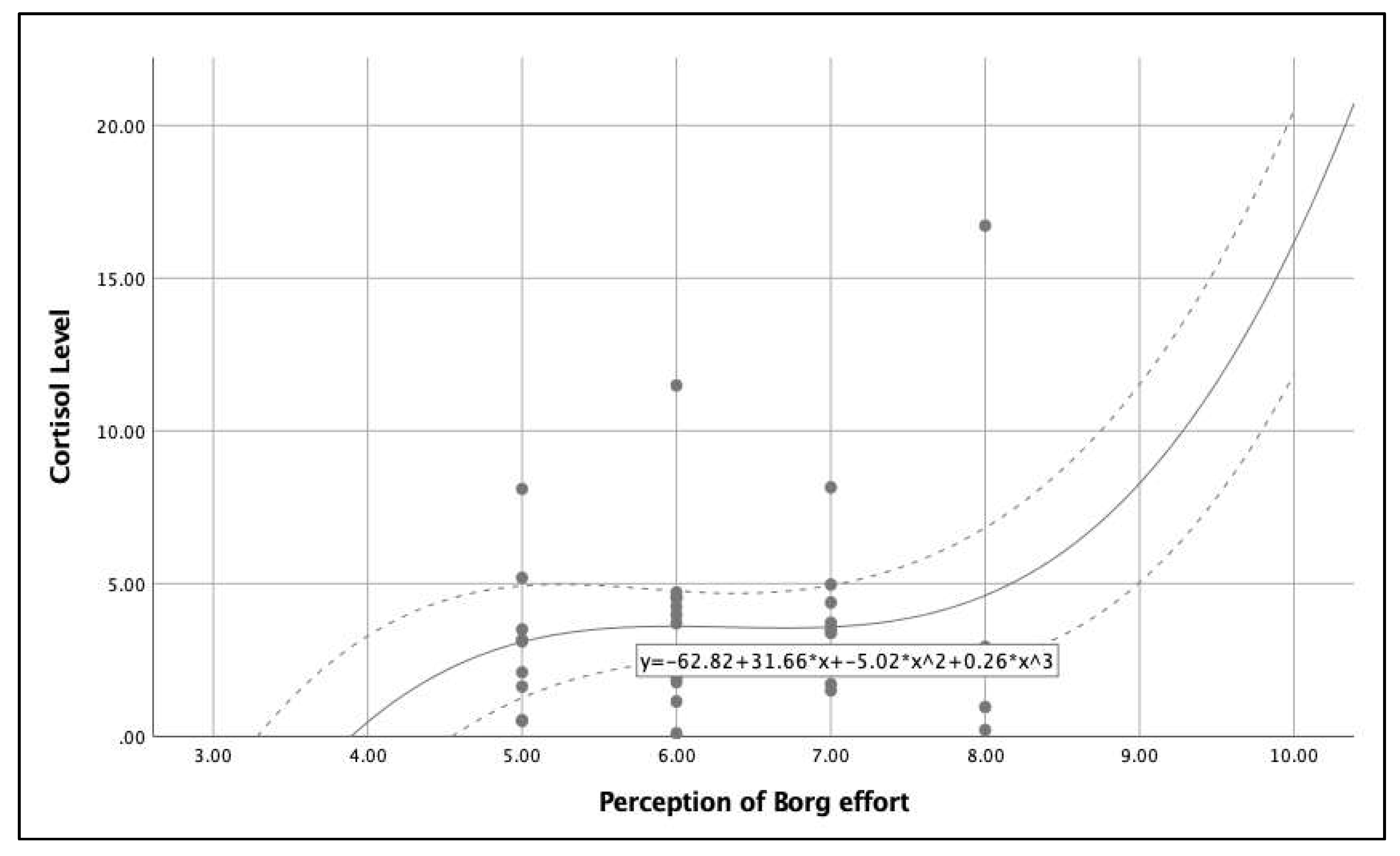
Figure 4 shows the relationship between the post-intervention cortisol levels and perceived exertion and indicates that low levels of perceived exertion is related to low cortisol levels and high levels of perceived exertion is related to high cortisol levels. It is important to note that in the perceived exertion range 5-8, the cortisol level is stable (4.6-5.0). Finally,
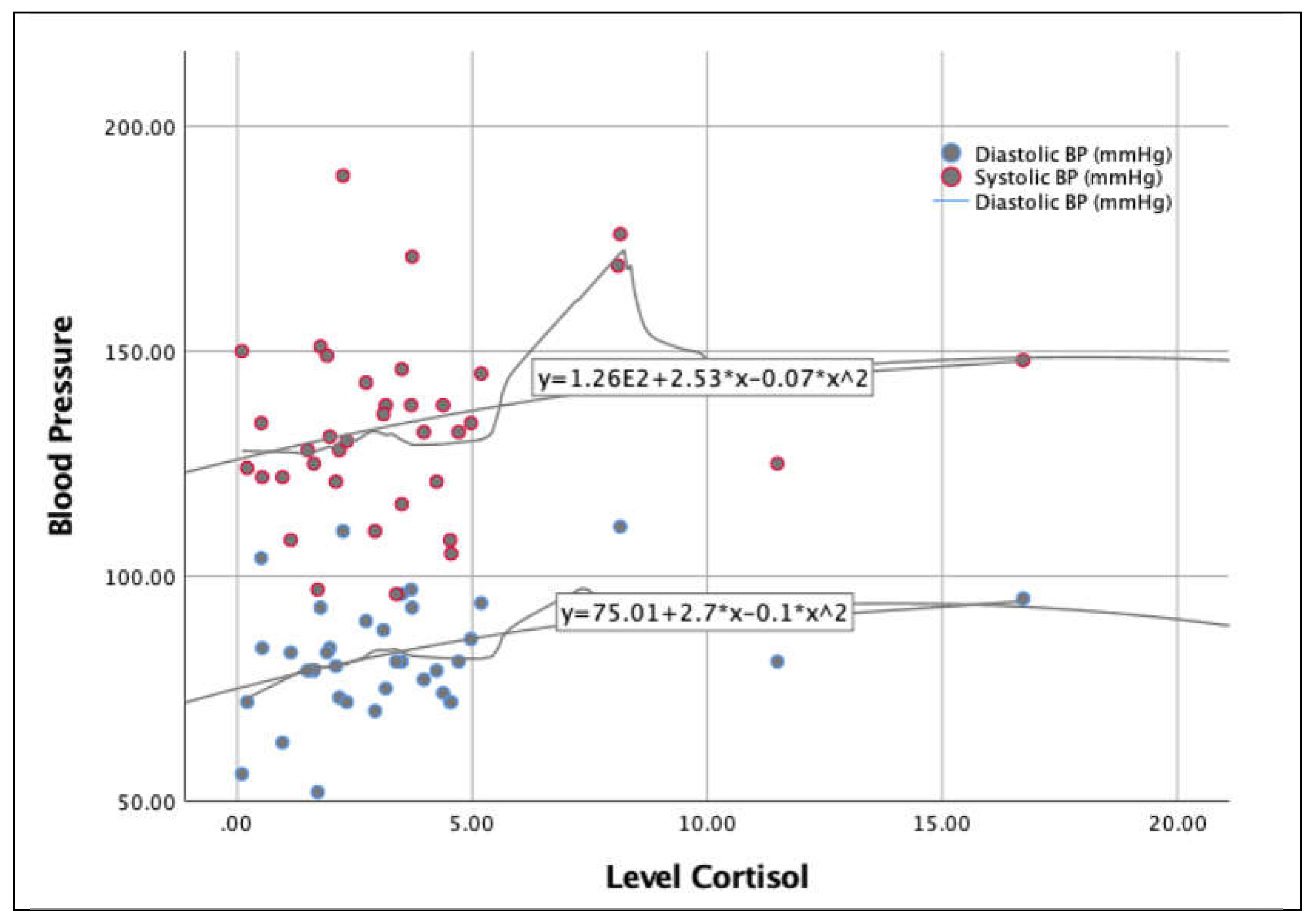
Figure 5 shows the influence of cortisol level on blood pressure. The distribution shows very similar behavior, with the exception of the 5 – 10 cortisol range, in which the systolic pressure rises much more than the diastolic pressure.
4. Discussion
Our study evaluated the response of certain physiological parameters in a sample of healthy adults to participation in an immersive exergame. The findings indicate that participating in an IVR session involved a perception of effort similar to that of a moderate to intense activity, generating significant increases in heart rate, blood pressure and cortisol concentration in saliva.
The increase in heart rate and blood pressure following the performance of a one-off activity with these levels of perceived effort is in line with other studies [
31,
32,
50,
51,
52]. González-Camarena et al. [
50] found similar results after an intervention of a similar length - 6 min - although rather than being an IVR intervention, they investigated quadriceps work at 30% of the maximum voluntary contraction, or cycling efforts at 30 or 60% of VO2max. Meanwhile Silva et al. [
31] pointed out that although an exergame training session and a conventional training session both resulted in similar acute effects on Heart Rate and Blood Pressure, their results differed from those of this study in that systolic blood pressure decreased significantly immediately after the session. This result was also arrived at by Kim et al. [
53], in the case of people who had suffered cybersickness after using IVR. In our case, where we used a boxing application, the increase in post-intervention blood pressure may have been due to the overall movement demands, which could have been more physically demanding for our sample. In addition, the fact that it was a single session - a one-off activity - may explain this increase in blood pressure, an aspect that leads us to suggest that longer interventions are needed clarify if certain types of IVR training lead to lower blood pressure.
McDonough et al. [
32] report that while systolic blood pressure was significantly higher in a 20 min session of IVR cycling than in a session of exergaming cycling (non-immersive), this was not the case when it was compared to a session of traditional exercise cycling. Similar results to McDonough et al. [
32] were published by Saiz-Gonzalez et al. [
51], who found that IVR cycling and traditional cycling interventions significantly increased systolic blood pressure when compared to non-immersive VR cycling. They found no significant differences between IVR cycling and traditional cycling (both studies were also one-off sessions and not part of a long-term programme). The results they found led Saiz-Gonzalez et al. [
51] to claim that IVR may be as effective as traditional cycling in relation to cardiovascular health. Our data could also be interpreted this way, suggesting that IVR boxing may be a form of exercise with potential for certain clinical situations that, for whatever reason, do not favour traditional exercise - for example, in the case of patients suffering from functional impairment or having mobility issues.
The levels of cortisol in saliva also respond in a similar way to HR and blood pressure. Our data shows significant increase immediately after the IVR intervention. As reported in other studies [
19,
20,
54,
55,
56,
57,
58], it appears that high-intensity exercise increases salivary cortisol values, while low to moderate intensity exercise either causes them to decrease, causes no change, or the variation recorded is statistically insignificant. The IVR intervention detailed here was moderate to high intensity exercise, according to our sample's rating of perceived exertion (6.3 on a scale of 0-10). This is data similar to that recorded in a study carried out by Budde et al. [
56], with a sample of adolescents, or Hill et al. [
55], who analysed what level of exercise intensity leads to an increase in circulating cortisol level. To do this, a 30-min intervention was designed, using a cycloergometer at 40, 60 and 80% of VO2max, and a sample of moderately trained active men with a mean age of 26 years. The results indicated that moderate to high intensity exercise leads to an increase in cortisol levels, which is consistent with the data produced by the present study, although the intervention carried out by Hill et al. is clearly different to this one. Jacks et al. [
20] add some qualifiers to these results, as in their study it was only participants who exercised at a high intensity who significantly increased post-exercise salivary cortisol levels, and not those who exercised at a low or moderate intensity. Similar results were found by Begdache et al. [
59], who pointed out that cortisol level can be a gauge of workout intensity, since in their study, with a sample of 48 healthy college students made up of 23 athletes and 25 non-athletes who performed a cycling session at a minimum of 65% of age-adjusted maximal heart rate, the cortisol level increased only in the athletes. As the authors themselves suggest, this could be because the athletes performed the activity at a higher intensity than the non-athletes. All of the above suggests that there are several factors at play, which means that further research is needed.
It can also be interpreted that the variation in cortisol levels depends on the training load in the case of athletes, or as pointed out by Kudielka et al. [
54], that it depends on the level of habituation to the stressor, since habituation to effort or to a given stressor may lead to a decrease in cortisol levels as the sessions go on. This could explain our results, since for most of the sample (32/37), it was their first experience with IVR and there was, therefore, a lack of habituation to this type of stimuli and situation. This is in agreement with the findings of Popovic et al. [
60], who indicate that untrained participants show a greater increase in cortisol levels than athletes after performing a progressive continuous cardiopulmonary exercise test on a treadmill, and also with Roberts [
22], who found that in a group of healthy college students, those who exercised regularly had a lower cortisol level post-exercise than those who did not. A similar situation can also be observed in the study by Parastesh et al. [
61], in which, after a session of exercise to exhaustion on an ergometer, fifteen female athletes found that there were no significant differences in cortisol levels pre- and post-intervention.
Regarding this habituation to the stressor, there is also the possible influence of a cognitive-emotional load [
26] on the cortisol level, which can occur when facing a new situation - as was the case in our research - and which could be similar to what athletes experience when competing. Thus, despite habituation to high-intensity exercise, Viru et al. [
62] found that competition-like situations led to an increase in post-exercise cortisol level.
The physical exercise performed during this IVR intervention seems to be similar to and comparable with traditional physical exercise in terms of the variation shown in the physiological parameters analyzed [
29,
30,
31,
32,
33]. However, the absence of study variables relating to physical condition and functionality only allows us to suggest this, and future studies are needed to analyze this aspect. There is also another parameter to consider, which may relativize the value of our findings and which also requires further research, and that is the duration of intervention. Our IVR intervention, which lasted approximately 6 min, resulted in an increase in heart rate, blood pressure and cortisol level in saliva. As noted previously, similar results in times like ours have also been seen in the work of Gonzalez-Camarena et al. [
50], although with quadriceps or cycling work, not IVR. However, with their study using a cycloergometer intervention, Jacks et al. [
20] found that with a workout of less than 40 min there were no modifications in post-exercise cortisol levels. Other previously noted work carried out interventions that included VR+cycling of 12[
51], 20[
32], 50[
31] or 60[
59] min. This disparity in intervention durations and outcomes calls for protocols to be put in place, and for further research, not only to see to what degree duration affects physiological parameters, but also to analyze what type of exergame could be more effective in inducing changes to physiological markers.
In addition, this intervention scarcely had any adverse effects, these being slight and residual, confirming good usability of the commercial HMD device and a very positive evaluation of the experience by the participants. This positive and motivating evaluation of IVR use is in line with previous studies [
32,
33,
34,
35,
37,
41,
51,
63], although there are also studies which state the opposite [
52]. In any case, our results did not allow us to verify whether the presence of severe cybersickness symptoms would produce variations in the physiological parameters studied, nor whether a certain stability in these guarantees the absence of cybersickness, aspects to be evaluated in future studies. It is possible that the non-generation of cybersickness symptoms in our case is due to the fact that the exergame used does not lead to a conflict in the sample between its vestibular system and its visual perception of body movement [
53].
One benefit of this study – though it was not a study objective – was that it seems IVR training may attract people to exercise, especially those with physical difficulties or a previous lack of interest, and that it may also promote adherence to such physical training programs [
34,
35,
64], and also to programs promoting healthy living, and disease prevention and treatment – as other research has previously illustrated [
33,
34,
35,
37,
65,
66,
67,
68,
69]. Initially, at least, this is suggested by what our sample reported when answering the SUS, SSQ, post-game GEQ and ad hoc satisfaction questionnaire.
Limitations
Although our study suggests that the proposed IVR based on a boxing exergame can lead to moderate to high intensity exercise for the physiological parameters analyzed, as well as being motivating for the subjects, it is not without several limitations. Firstly, we only assessed the acute effects of the IVR on heart rate, blood pressure and cortisol in saliva immediately post-intervention but not at different times after the intervention (20, 30 or 60 minutes), so we do not know how long they remained elevated, or how long it took them to return to their pre-intervention baseline values. Neither was it posible to determine whether this variation occurs at any time during a person's daily circadian rhythm. Secondly, the data obtained cannot be generalized to other ages (preadolescents, adolescents or older), or to populations with health problems, or to other times of the day (afternoon or evening). Furthermore, the lack of a sample size calculation also precludes these generalisations. Regarding the measurement of the level of cortisol, the sensitivity of cortisol measurement is greater in blood than in saliva, which forces us to be cautious with the data obtained here, although it is a fact that saliva has the advantage of being easy to collect and of being an uninvasive method. The lack of control over the participants´ possible ingestion of coffee or food, of smoking or chewing gum, as well as the possible differences that may have occurred between the time of waking up and the taking of the initial sample of cortisol in saliva, or the time elapsed between waking up and taking part in the intervention – all of which may have altered the levels of cortisol in saliva or, at least, may not be the same as in a situation of total control over these variables - oblige us to be cautious.