Submitted:
14 April 2023
Posted:
14 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
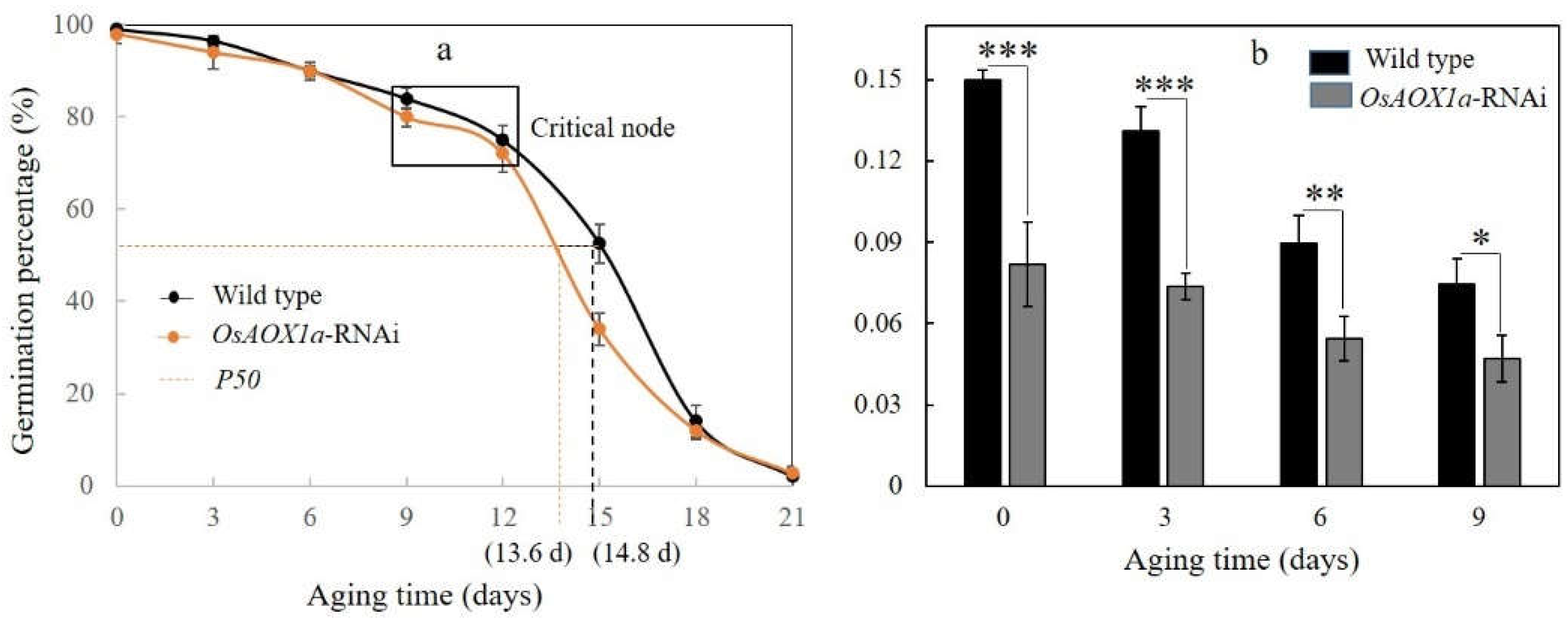
2.1. Pattern of survival curves between WT and OsAOX1a-RNAi seeds after AA treatment
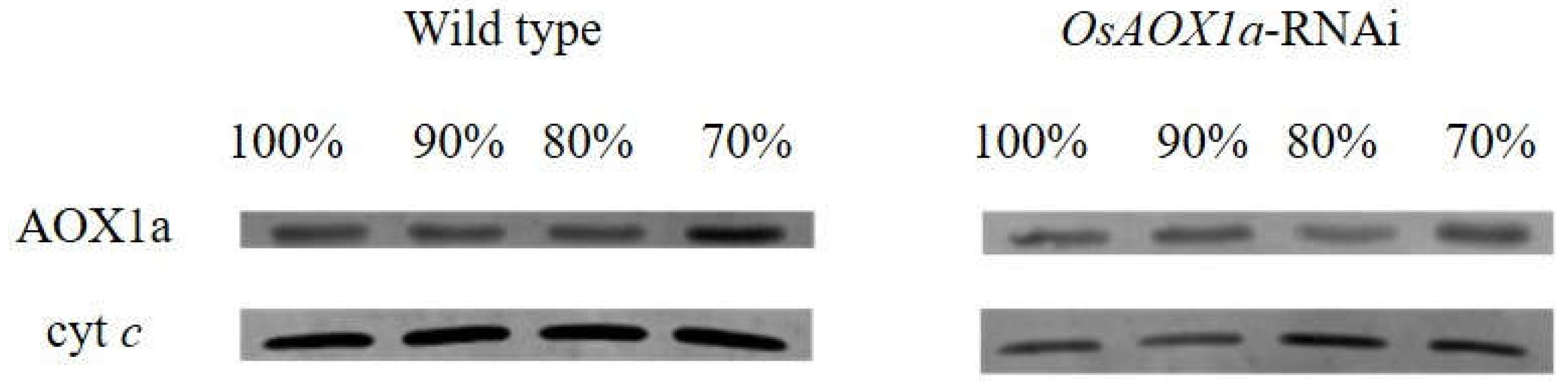
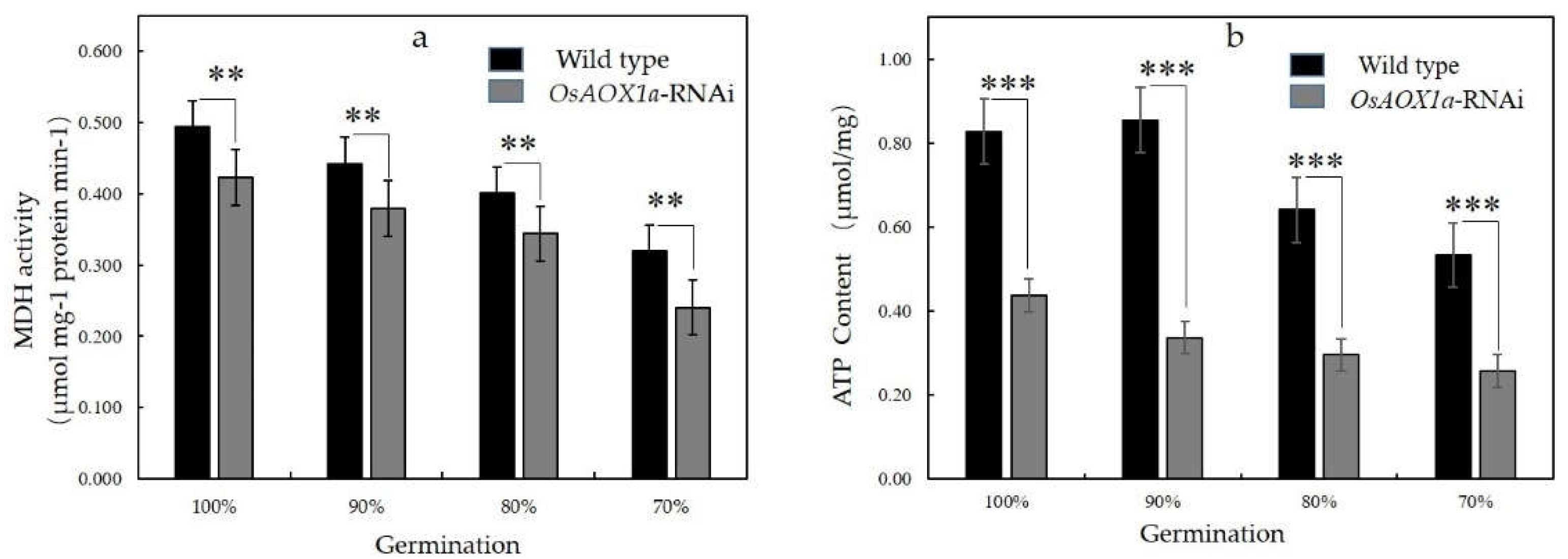
2.2. Assessment of mitochondria status during AA treatments
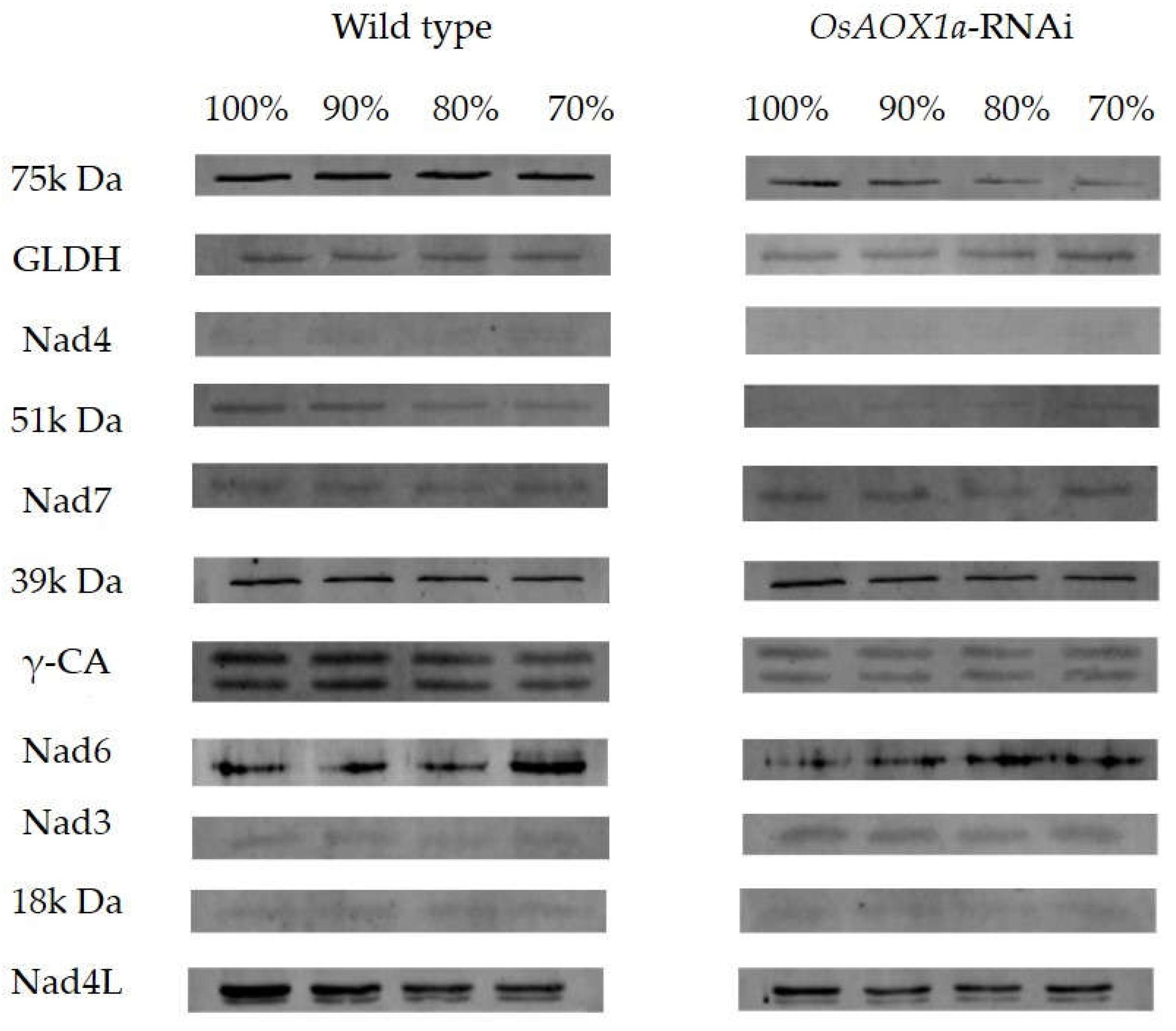
2.3. The abundance of complex I subunits during AA treatments
3. Discussion
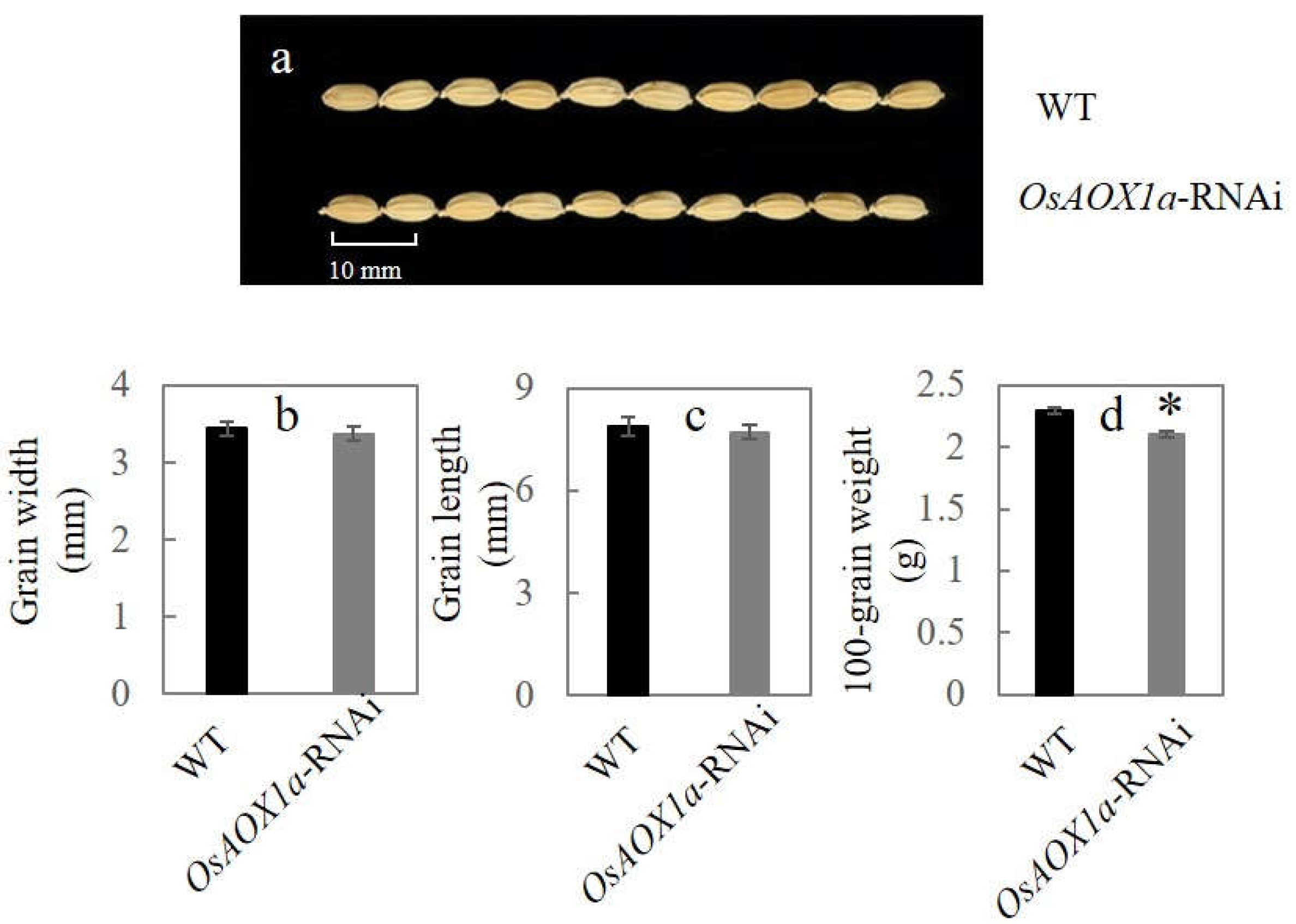
3.1. Low AOX1a impaired seed development and storability
3.2. Low AOX1a impaired mitochondrial activity during seed imbibition
3.3. Low AOX1a altered mitochondrial electron transfer chain complex I during seed imbibition
4. Materials and Methods
4.1. Materials and treatments
4.2. Crude mitochondria purification
4.3. Mitochondrial respiration rate assay
4.4. ATP content determination
4.5. Mitochondrial malate dehydrogenase activity
4.6. Western blot t analysis
4.7. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Carrie, C.; Murcha, M.W.; Giraud, E.; Ng, S.; Zhang, M.F.; Narsai, R.; Whelan, J. How do plants make mitochondria? Planta 2013, 237, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Law, S.R.; Narsai, R.; Taylor, N.L.; Delannoy, E.; Carrie, C.; Giraud, E.; Millar, A.H.; Small, I.; Whelan, J. Nucleotide and RNA metabolism prime translational initiation in the earliest events of mitochondrial biogenesis during Arabidopsis germination. Plant Physiol. 2012, 158, 1610–1627. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Tian, Q.; Yin, G.; Chen, X.; Zhang, J.; Ng, S.; Lu, X. Reduced mitochondrial and ascorbate-glutathione activity after artificial ageing in soybean seed. J Plant Physiol. 2014, 171, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Xue, H.; Pritchard, H.W.; Wang, X. Reactive oxygen species-provoked mitochondria-dependent cell death during ageing of elm (Ulmus pumila L.) seeds. Plant J. 2015, 81, 438–452. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.; Whelan, J.; Wu, S.; Zhou, J.; Chen, B.; Chen, X.; Zhang, J.; He, J.; Xin, X.; Lu, X. Comprehensive mitochondrial metabolic shift during the critical node of Seed Ageing in Rice. PLoS One 2016, 11, e0148013. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Xue, H.; Pritchard, H.W.; Wang, X. Changes in the mitochondrial protein profile due to ROS eruption during ageing of elm (Ulmus pumila L.) seeds. Plant Physiol Biochem. 2017, 114, 72–87. [Google Scholar] [CrossRef]
- Sun, H.; Li, L.; Wang, X.; Wu, S.; Wang, X. Ascorbate-glutathione cycle of mitochondria in osmoprimed soybean cotyledons in response to imbibitional chilling injury. J Plant Physiol. 2011, 168, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Stadtman, E.R.; Levine, R.L. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids 2003, 25, 207–218. [Google Scholar] [CrossRef]
- Ramallo Guevara, C.; Philipp, O.; Hamann, A.; Werner, A.; Osiewacz, H.D.; Rexroth, S.; Rögner, M.; Poetsch, A. Global protein oxidation profiling suggests efficient mitochondrial proteome homeostasis during aging. Mol Cell Proteomics. 2016, 15, 1692–1709. [Google Scholar] [CrossRef]
- Yin, G.; Xin, X.; Fu, S.; An, M.; Wu, S.; Chen, X.; Zhang, J.; He, J.; Whelan, J.; Lu, X. Proteomic and carbonylation profile analysis at the critical node of Seed Ageing in Oryza sativa. Sci Rep. 2017, 7, 40611. [Google Scholar] [CrossRef]
- Fu, S.; Yin, G.; Xin, X.; Wu, S.; Wei, X.; Lu, X. Levels of crotonaldehyde and 4-hydroxy-(E)-2-nonenal and expression of genes encoding carbonyl-Scavenging enzyme at critical node during rice seed aging. Rice Sci. 2018, 25, 152–160. [Google Scholar]
- Millar, A.H.; Whelan, J.; Soole, K.L.; Day, D.A. Organization and regulation of mitochondrial respiration in plants. Annu Rev Plant Biol. 2011, 62, 79–104. [Google Scholar] [CrossRef] [PubMed]
- Moller, I.M. Plant mitochondria and oxidative stress: electron transport, NADPH Turnover, and metabolism of reactive oxygen species. Annu Rev Plant Physiol Plant Mol Biol. 2001, 52, 561–591. [Google Scholar] [CrossRef] [PubMed]
- Fromm, S.; Senkler, J.; Zabaleta, E.; Peterhänsel, C.; Braun, H.P. The carbonic anhydrase domain of plant mitochondrial complex I. Physiol Plant. 2016, 157, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Senkler, J.; Senkler, M.; Braun, H.P. Structure and function of complex I in animals and plants—A comparative view. Physiol Plant 2017, 161, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Selinski, J.; Scheibe, R.; Day, D.A.; Whelan, J. Alternative oxidase is positive for plant performance. Trends Plant Sci. 2018, 23, 588–597. [Google Scholar] [CrossRef]
- Kühn, K.; Yin, G.; Duncan, O.; Law, S.R.; Kubiszewski-Jakubiak, S.; Kaur, P.; Meyer, E.; Wang, Y.; Small, C.C.; Giraud, E.; Narsai, R.; Whelan, J. Decreasing electron flux through the cytochrome and/or alternative respiratory pathways triggers common and distinct cellular responses dependent on growth conditions. Plant Physiol. 2015, 167, 228–250. [Google Scholar] [CrossRef]
- Costa, J.H.; McDonald, A.E.; Arnholdt-Schmitt, B.; Fernandes de Melo, D. A classification scheme for alternative oxidases reveals the taxonomic distribution and evolutionary history of the enzyme in angiosperms. Mitochondrion 2014, 19 Pt B, 172–183. [Google Scholar] [CrossRef]
- Chen, B.; Yin, G.; Whelan, J.; Zhang, Z.; Xin, X.; He, J.; Chen, X.; Zhang, J.; Zhou, Y.; Lu, X. Composition of mitochondrial Complex I during the critical node of seed aging in Oryza sativa. J Plant Physiol. 2019, 236, 7–14. [Google Scholar] [CrossRef]
- Garmash, E.V.; Velegzhaninov, I.O.; Ermolina, K.V.; Rybak, A.V.; Malyshev, R.V. Altered levels of AOX1a expression result in changes in metabolic pathways in Arabidopsis thaliana plants acclimated to low dose rates of ultraviolet B radiation. Plant Sci. 2020, 291, 110332. [Google Scholar] [CrossRef]
- Clifton, R.; Millar, A.H.; Whelan, J. Alternative oxidases in Arabidopsis: a comparative analysis of differential expression in the gene family provides new insights into function of non-phosphorylating bypasses. Biochimica et biophysica Acta 2006, 1757, 730–741. [Google Scholar] [CrossRef]
- Fiorani, F.; Umbach, A.L.; Siedow, J.N. The alternative oxidase of plant mitochondria is involved in the acclimation of shoot growth at low temperature. A study of Arabidopsis AOX1a transgenic plants. Plant Physiol. 2005, 139, 1795–1805. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Yuan, S.; Zhang, D.W.; Lv, X.; Lin, H.H. The role of alternative oxidase in tomato fruit ripening and its regulatory interaction with ethylene. J Exp Bot. 2012, 63, 5705–5716. [Google Scholar] [CrossRef]
- Selinski, J.; Scheibe, R.; Day, D.A.; Whelan, J. Alternative oxidase is ppositive for plant performance. Trends in Plant Sci. 2018, 23, 588–597. [Google Scholar] [CrossRef]
- Giraud, E.; Ho, L.H.; Clifton, R.; Carroll, A.; Estavillo, G.; Tan, Y.F.; Howell, K.A.; Ivanova, A.; Pogson, B.J.; Millar, A.H.; Whelan, J. The absence of alternative oxidase1a in Arabidopsis results in acute sensitivity to combined light and drought stress. Plant Physiol. 2008, 147, 595–610. [Google Scholar] [CrossRef] [PubMed]
- Gandin, A.; Duffes, C.; Day, D.A.; Cousins, A.B. The absence of alternative oxidase AOX1A results in altered response of photosynthetic carbon assimilation to increasing CO2 in Arabidopsis thaliana. Plant Cell Physiol. 2012, 53, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- Imsande, J.; Pittig, J.; Palmer, R.G.; Wimmer, C.; Gietl, C. Independent spontaneous mitochondrial malate dehydrogenase null mutants in soybean are the result of deletions. The Journal of heredity 2001, 92, 333–338. [Google Scholar] [CrossRef]
- Day, D.A.; Millar, A.H.; Whelan, J. Plant mitochondria: from genome to function. In Advances in Photosynthesis and Respiration; Govindjee Kluwer, Ed.; Academic Press: Dordrecht, 2004; pp. 2–7. [Google Scholar]
- Hunte, C.; Zickermann, V.; Brandt, U. Functional modules and structural basis of conformational coupling in mitochondrial complex I. Science 2010, 329, 448–451. [Google Scholar] [CrossRef]
- Braun, H.P.; Binder, S.; Brennicke, A.; Eubel, H.; Fernie, A.R.; Finkemeier, I.; Klodmann, J.; König, A.C.; Kühn, K.; Meyer, E.; Obata, T.; Schwarzländer, M.; Takenaka, M.; Zehrmann, A. The life of plant mitochondrial complex I. Mitochondrion 2014, 19 Pt B, 295–313. [Google Scholar] [CrossRef]
- Sweetman, C.; Waterman, C.D.; Rainbird, B.M.; Smith, P.M.C.; Jenkins, C.D.; Day, D.A.; Soole, K.L. AtNDB2 is the main external NADH dehydrogenase in mitochondria and is important for tolerance to environmental stress. Plant Physiol. 2019, 181, 774–788. [Google Scholar] [CrossRef]
- Sweetman, C.; Miller, T.K.; Booth, N.J.; Shavrukov, Y.; Jenkins, C.L.D.; Soole, K.L.; Day, D.A. Identification of alternative mitochondrial electron transport pathway components in chickpea indicates a differential response to salinity stress between cultivars. Int J Mol Sci. 2020, 21, 3844. [Google Scholar] [CrossRef] [PubMed]
- Walters, C.; Wheeler, L.; Grotenhuis, J. Longevity of seeds stored in a genebank: Species characteristics. Seed Sci Res. 2005, 15, 1–20. [Google Scholar] [CrossRef]
- Logan, D.C.; Millar, A.H.; Sweetlove, L.J.; Hill, S.A.; Leaver, C.J. Mitochondrial biogenesis during germination in maize embryos. Plant physiol. 2001, 125, 662–672. [Google Scholar] [CrossRef] [PubMed]
| Materials | Substrate | O2 consumption rate (nmolO2·min−1mg−1 protein) | |||
|---|---|---|---|---|---|
| 100% | 90% | 80% | 70% | ||
| Wild type | NADH | 44.4 ± 3.2 | 38.1 ± 2.2 | 18.9 ± 0.6 | 11.4 ± 2.6 |
| NADH + ADP | 97.8 ± 3.2 | 65.5 ± 2.9 | 43.3 ± 3.0 | 22.7 ± 2.6 | |
| Succinate | 24.5 ± 2.0 | 18.8 ± 0.8 | 11.5 ± 1.0 | 7.9 ± 0.7 | |
| Succinate + ADP | 40.5 ± 1.9 | 25.8 ± 1.8 | 14.2 ± 1.7 | 9.8 ± 2.6 | |
| OsAOX1a-RNAi | NADH | 17.3 ± 1.9 | 11.8 ± 1.2 | 8.3 ± 0.7 | 6.6 ± 0.4 |
| NADH + ADP | 36.7 ± 0.9 | 25.2 ± 1.1 | 12.9 ± 0.7 | 10.5 ± 1.0 | |
| Succinate | 7.9 ± 0.2 | 6.0 ± 0.3 | 4.5 ± 0.5 | 4.1 ± 0.2 | |
| Succinate + ADP | 32.0 ± 1.2 | 18.5 ± 0.7 | 8.8 ± 0.6 | 7.9 ± 0.5 | |
| name | Primer (5'→3') | restriction site |
|---|---|---|
| OsAOX1a-F | CGGGTACCACTAGTGTGCGGCGTACGAAAAACAG | Kpn I + Spe I |
| OsAOX1a-R | GGGGATCCGAGCTCTGCCGAGGATTTGCATCACT | BamH I + Sac I |
| HygF | CTATTTCTTTGCCCTCGGAC | |
| HygR | AAGCCTGAACTCACCGCGAC | |
| OsUBQ5-F | ACCACTTCGACCGCCACTACT | |
| OsUBQ5-R | ACGCCTAAGCCTGCTGGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




