Submitted:
14 April 2023
Posted:
17 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Material and Methods
2.1. Plant material and growth conditions
2.2. Growth conditions, treatments and experimental designs
2.3. Drought treatment
2.4. GA3 and TRinexapac application
2.5. Light/Dark Cycle Treatment
2.6. Phenology and phenotypic evaluation
2.7. Anther morphology evaluation
2.8. Pollen viability and morphology evaluation
2.9. Chlorophyll fluorescence measurements
2.10. Relative Water Content
2.11. Microscopic observations
2.12. Light microscopy observation
2.13. Electron microscopy observation
2.14. RNA Extraction, Reverse Transcription, and Real-Time PCR
2.15. Statistical analysis
3. Results
3.1. Differences in plant phenology, chlorophyll fluorescence kinetics and yield reduction in drought
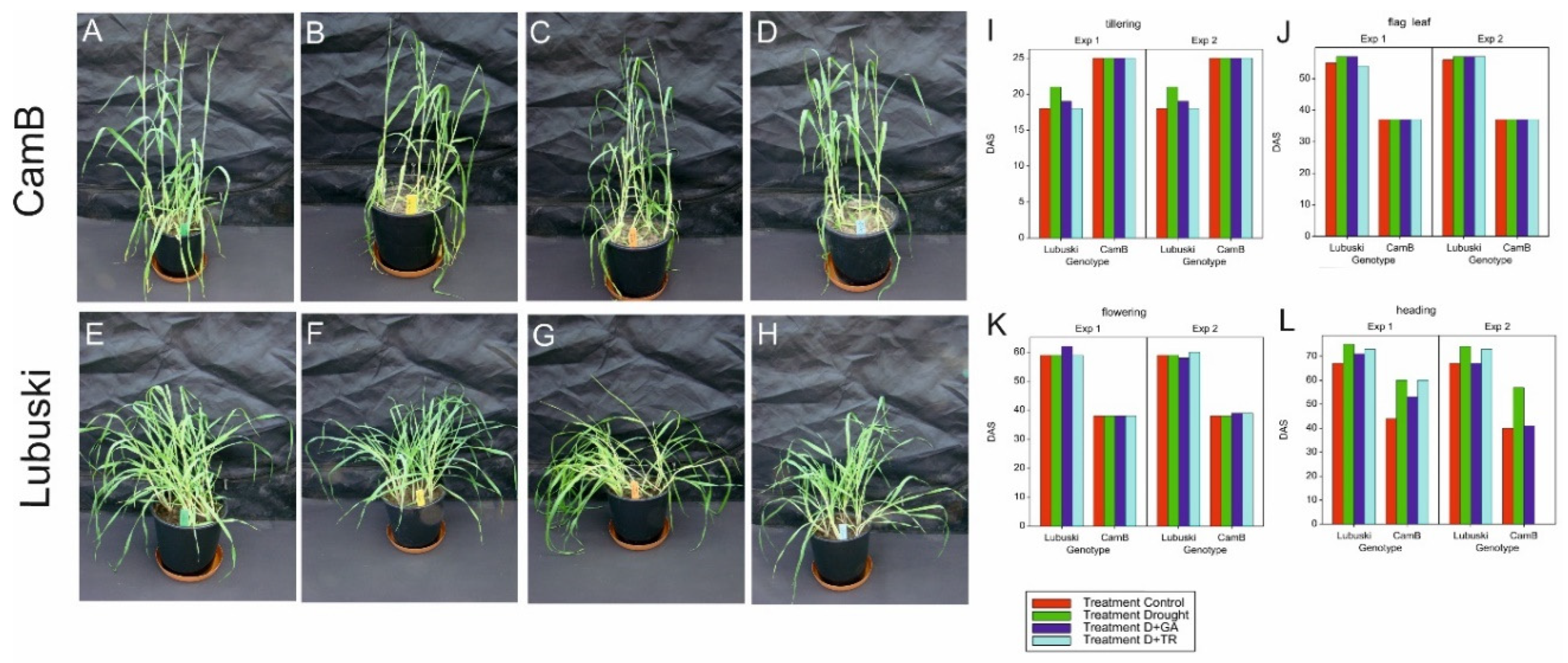
3.1.1. Phenology
3.1.2. Yield performance
3.1.3. Chlorophyll fluorescence kinetics and RWC
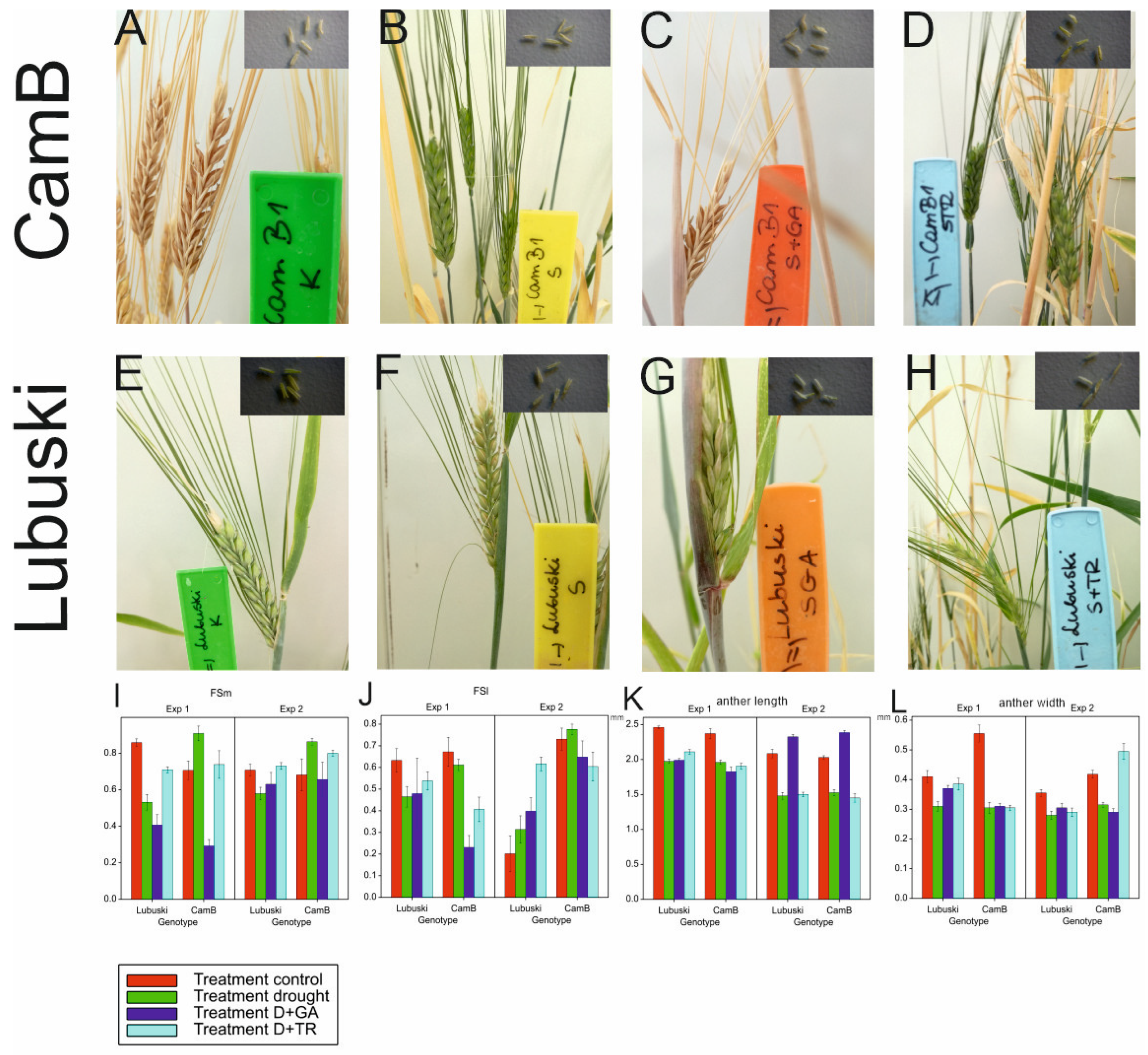
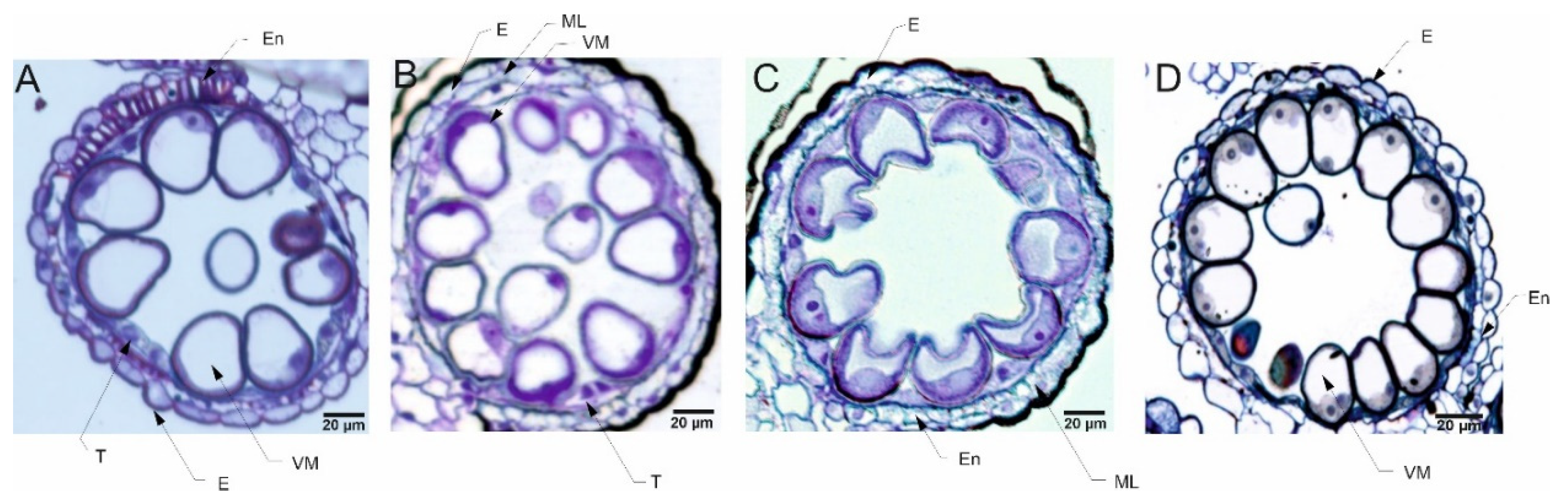
3.2. Spike fertility and anther morphology
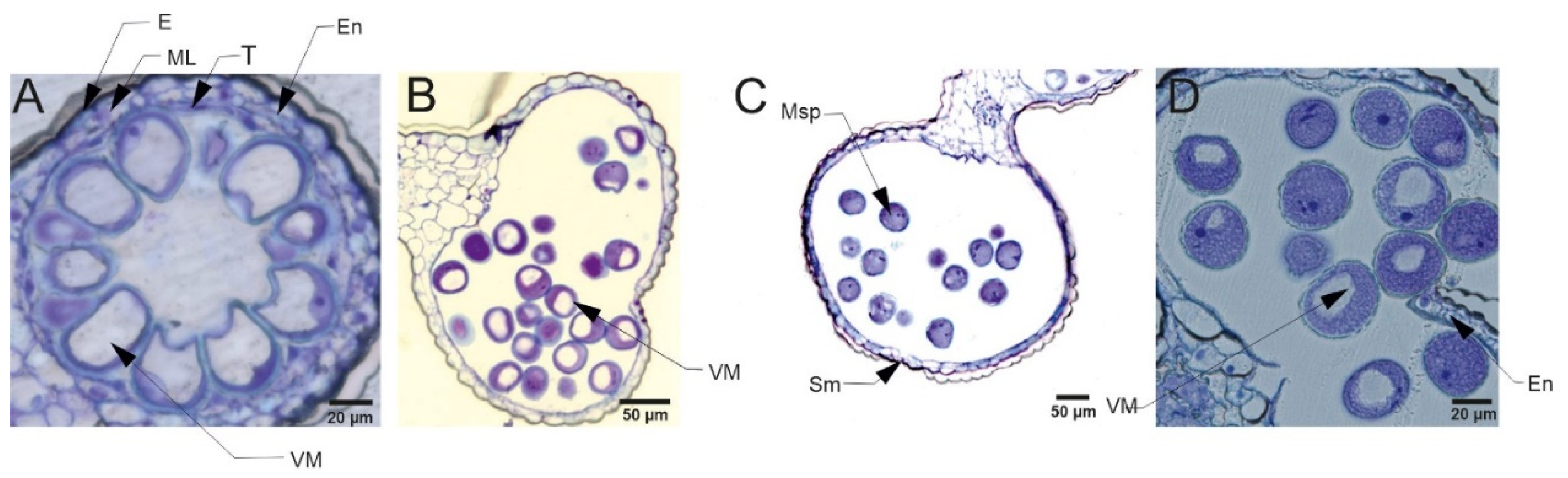
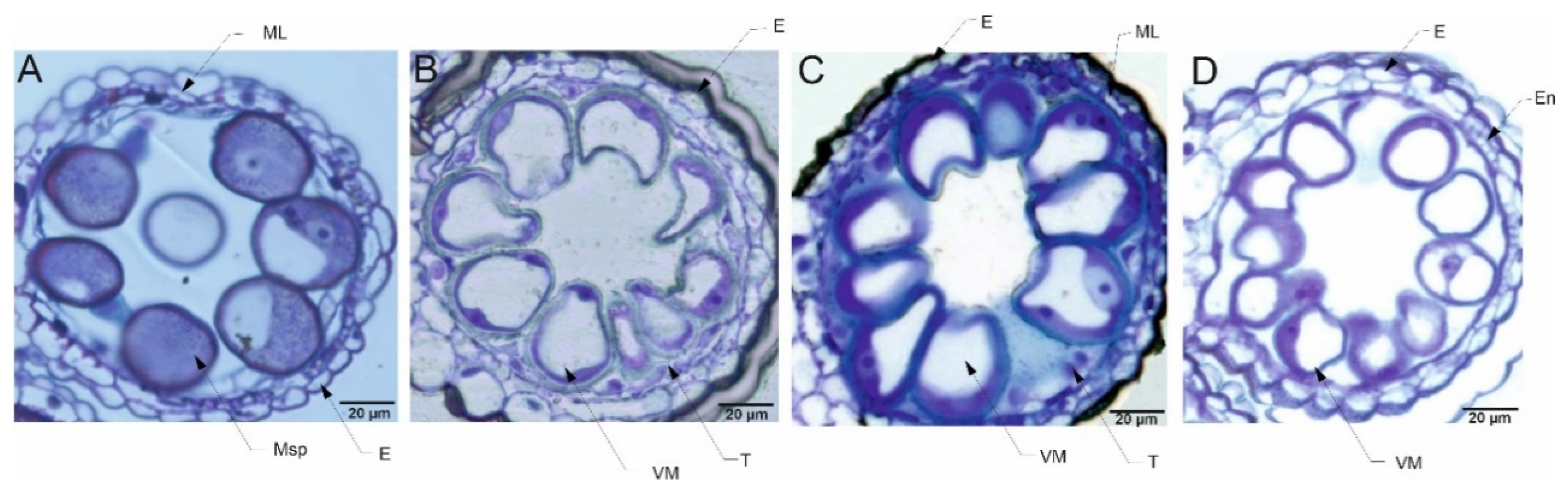
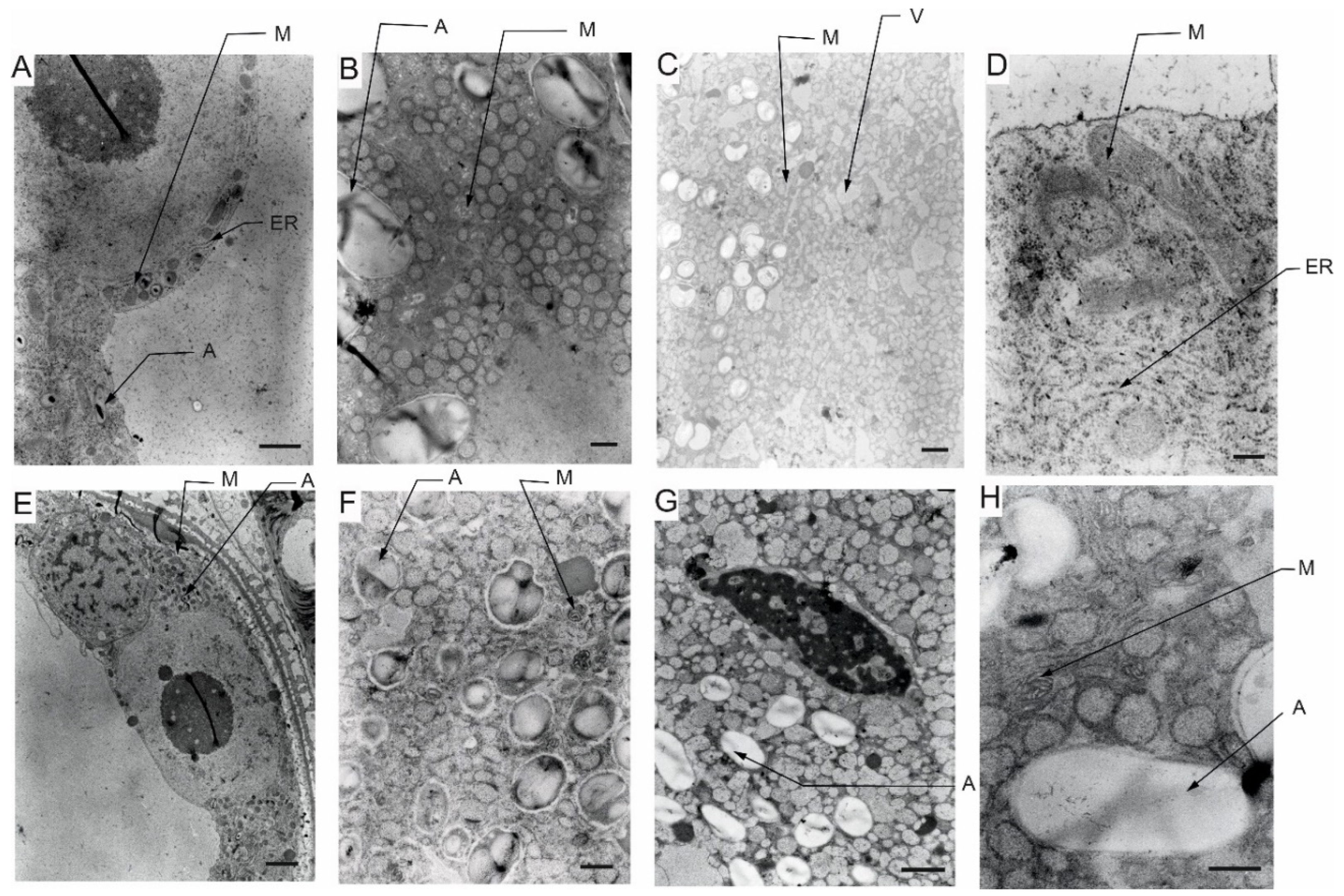
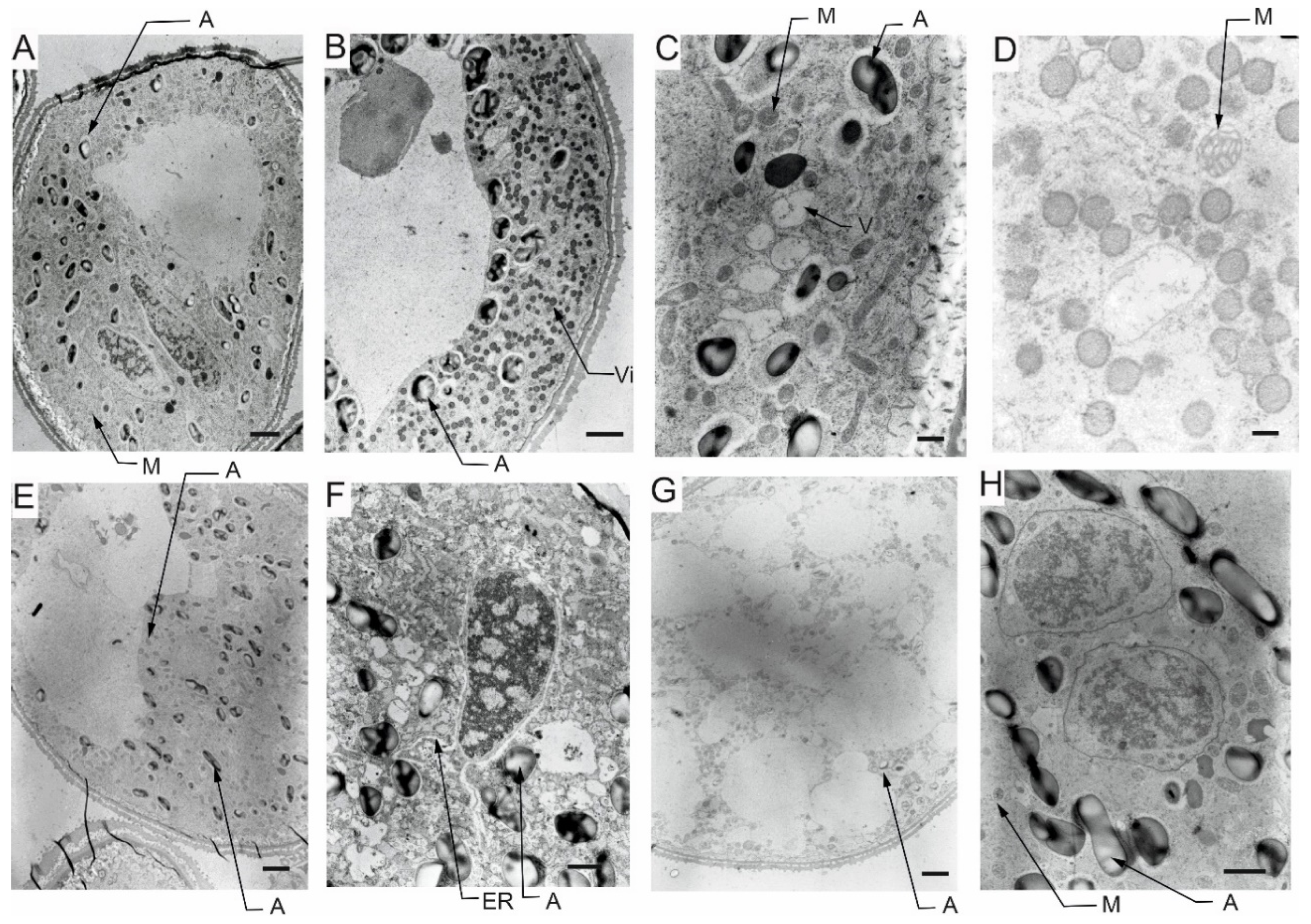
3.3. Pollen micromorphology and ultrastructure
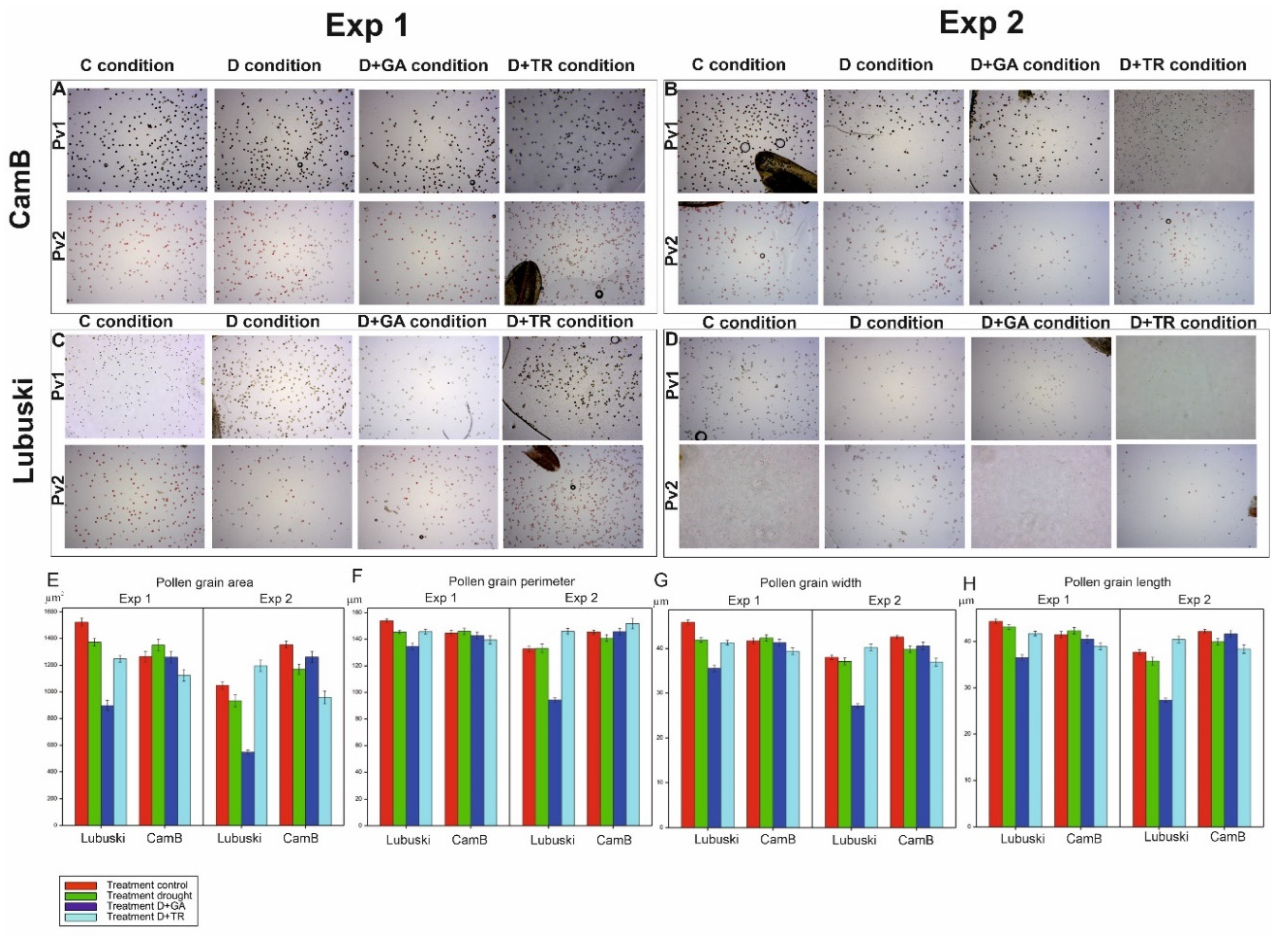
3.3.1. Pollen grain morphology and viability
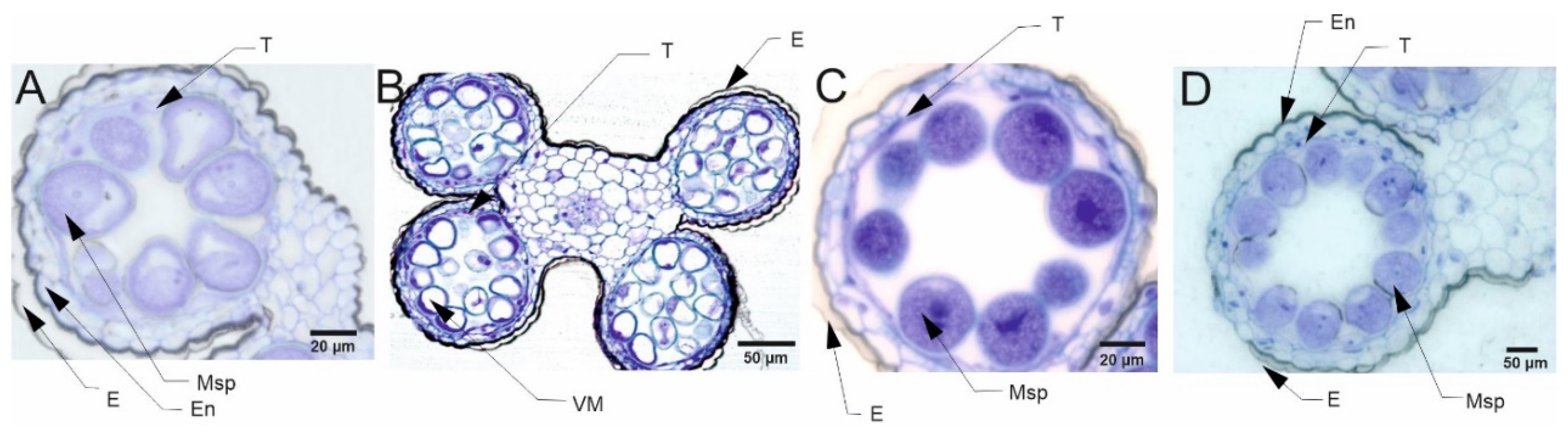
3.3.2. Pollen cytological observations
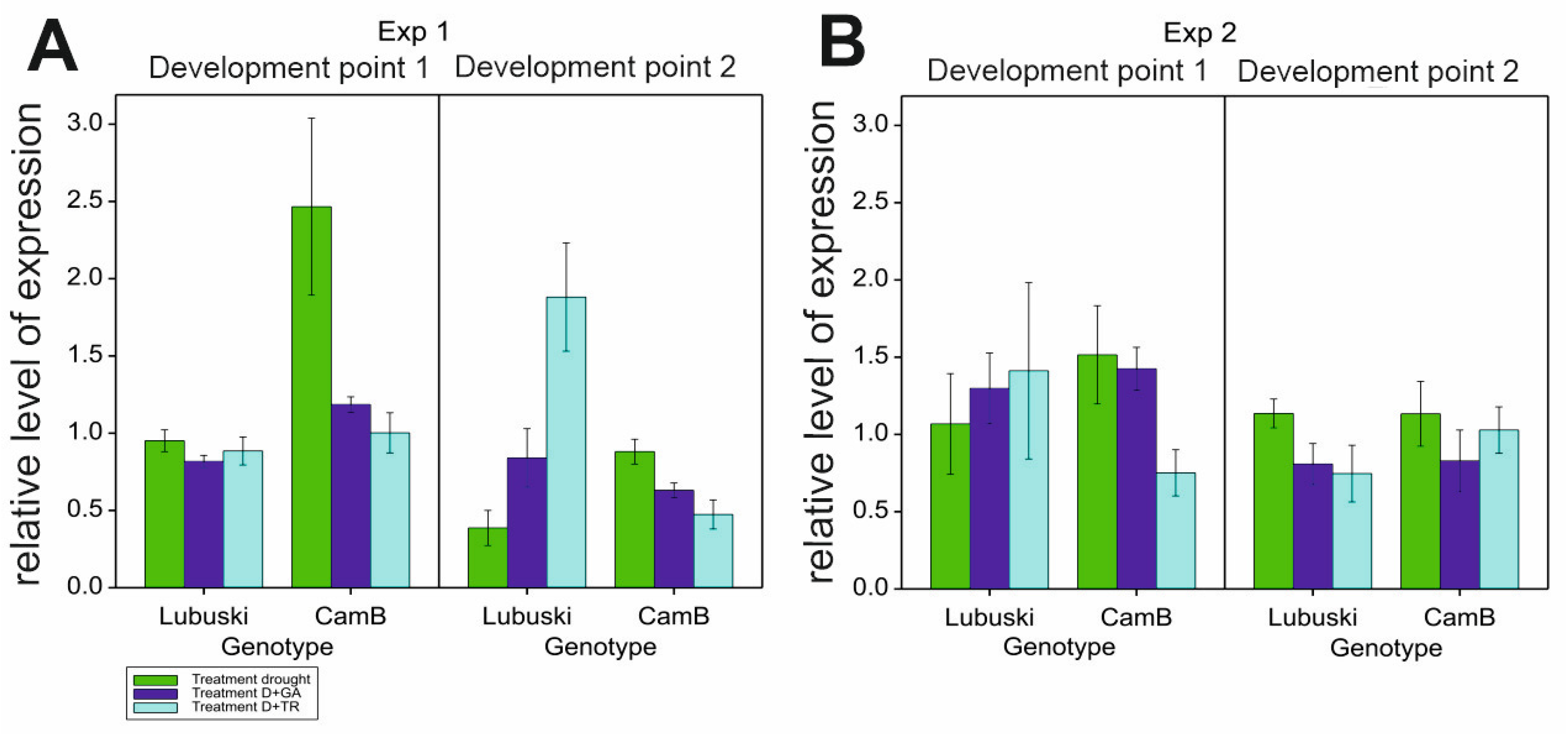
4. HvGAMYB transcript level
5. Discussion
5.1. Differences between studied genotypes
5.2. Spike fertility and anther morphology
5.3. Pollen viability and micromorphology
5.4. Pollen cytological observations - LM
5.5. Pollen ultrastructure observations - TEM
5.6. HvGAMYB expression fluctuation
6. Conclusions
Supplementary Materials
Funding
Author Contribution
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflict of Interest
References
- Dong, B.; Zheng, X.; Liu, H.; Able, J.A.; Yang, H.; Zhao, H.; Zhang, M.; Qiao, Y.; Wang, Y.; Liu, M. Effects of Drought Stress on Pollen Sterility, Grain Yield, Abscisic Acid and Protective Enzymes in Two Winter Wheat Cultivars. Front. Plant Sci. 2017, 8, 1008. [Google Scholar] [CrossRef] [PubMed]
- Calleja-Cabrera, J.; Boter, M.; Oñate-Sánchez, L.; Pernas, M. Root Growth Adaptation to Climate Change in Crops. Front. Plant Sci. 2020, 11, 544. [Google Scholar] [CrossRef] [PubMed]
- Jaleel, C.A.; Gopi, R.; Sankar, B.; Gomathinayagam, M.; Panneerselvam, R. Differential Responses in Water Use Efficiency in Two Varieties of Catharanthus Roseus under Drought Stress. Comptes. Rendus. Biol. 2008, 331, 42–47. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Santiago, J.P.; Sharkey, T.D. Pollen development at high temperature and role of carbon and nitrogen metabolites. Plant Cell Environ. 2019, 42, 2759–2775. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Jiang, M.; Guo, C. Crop Pollen Development under Drought: From the Phenotype to the Mechanism. Int. J. Mol. Sci. 2019, 20, 1550. [Google Scholar] [CrossRef] [PubMed]
- Alqudah, A.M.; Samarah, N.H.; Mullen, R.E. Drought stress effect on crop pollination, seed set, yield and quality. 2011, pp. 193-213. In: Lichtfouse E. (ed), Alternative farming systems, biotechnology, drought stress and ecological fertilisation, Sustainable Agriculture Reviews 6. Springer Science+Business Media B.V. ISBN 978-94-007-0186-1. [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop Production under Drought and Heat Stress: Plant Responses and Management Options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought Stress Impacts on Plants and Different Approaches to Alleviate Its Adverse Effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, L. Specification of tapetum and microsporocyte cells within the anther. Curr. Opin. Plant Biol. 2014, 17, 49–55. [Google Scholar] [CrossRef]
- Gómez, J.F.; Talle, B.; Wilson, Z.A. Anther and pollen development: A conserved developmental pathway. J. Integr. Plant Biol. 2015, 57, 876–91. [Google Scholar] [CrossRef]
- Kwon, C.T.; Kim, S.H.; Kim, D.; Paek, N.C. The rice floral repressor Early flowering1 affects spikelet fertility by modulating gibberellin signaling. Rice, 2015, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Plackett. A.R.; Thomas, S.G.; Wilson, Z.A.; Hedden, P. Gibberellin control of stamen development: a fertile field. Trends Plant Sci. 2011, 16, 568–78. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, H.; Aya, K.; Ueguchi-Tanaka, M.; Shimada, Y.; Nakazono, M.; Watanabe, R.; Nishizawa, N.; Gomi, K.; Shimada, A.; Kitano, H.; et al. GAMYB controls different sets of genes and is differentially regulated by microRNA in aleurone cells and anthers. Plant J, 2006, 47, 427–444. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, Y.; Zhu, J.K. Thriving under stress: how plants balance growth and the stress response. Dev. Cell 2020, 55, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Aya, K.; Ueguchi-Tanaka, M.; Kondo, M.; Hamada, K.; Yano, K.; Nishimura, M.; Matsuoka, M. Gibberellin modulates anther development in rice via the transcriptional regulation of GAMYB. Plant Cell. 2009, 21, 1453–72. [Google Scholar] [CrossRef] [PubMed]
- Osnato, M.; Castillejo, C.; Matías-Hernández, L.; Pelaz, S. TEMPRANILLO genes link photoperiod and gibberellin pathways to control flowering in Arabidopsis. Nat Commun. 2012, 3, 808. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Pan, J.; Li, Y.; Lou, D.; Hu, Y.; Yu, D. The DELLA-CONSTANS Transcription Factor Cascade Integrates Gibberellic Acid and Photoperiod Signaling to Regulate Flowering. Plant Physiol. 2016, 172, 479–88. [Google Scholar] [CrossRef]
- Quiroz, S.; Yustis, J.C.; Chávez-Hernández, E.C.; Martínez, T.; Sanchez, M.d.l.P.; Garay-Arroyo, A.; Álvarez-Buylla, E.R.; García-Ponce, B. Beyond the Genetic Pathways, Flowering Regulation Complexity in Arabidopsis thaliana. Int. J. Mol. Sci. 2021, 22, 5716. [Google Scholar] [CrossRef]
- Roy, S. Function of MYB domain transcription factors in abiotic stress and epigenetic control of stress response in plant genome. Plant Signal Behav. 2016, 11, e1117723. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, Y.; Wang, Y.; Liu, Z.; Gao, C. Comprehensive Analysis of MYB Gene Family and Their Expressions Under Abiotic Stresses and Hormone Treatments in Tamarix hispida. Front. Plant Sci. 2018, 9, 1303. [Google Scholar] [CrossRef]
- Jackson, R.B.; Sperry, J.S.; Dawson, T.E. Root water uptake and transport: using physiological predictions in global predictions. Trends in Plant Sci. 2000, 5, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.M. Osmoregulation and water stress in higher plants. Annu. Rev. Plant Physiol. 1984, 35, 299–319. [Google Scholar] [CrossRef]
- Bartoli, C.G.; Simontacchi, M.; Tambussi, E.; Beltrano, J.; Montaldi, E.; Puntarulo, S. Drought and watering-dependent oxidative stress: effect on antioxidant content in Triticum aestivum L. leaves. J. Exp. Bot. 1999, 50, 375–383. [Google Scholar] [CrossRef]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding plant responses to drought - from genes to the whole plant. Funct Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef] [PubMed]
- Maherali, H.; Caruso, C.M.; Sherrard, M.E.; Latta, R.G. Adaptive value and costs of physiological plasticity to soil moisture limitation in recombinant inbred lines of Avena barbata. Am. Nat. 2010, 175, 211–224. [Google Scholar] [CrossRef]
- Dolferus, R. To grow or not to grow: a stressful decision for plants. Plant Sci. 2014, 229, 247–261. [Google Scholar] [CrossRef]
- Willige, B.C.; Ogiso-tanaka, E.; Zourelidou, M.; Schwechheimer, C. (2012). WAG2 represses apical hook opening downstream from gibberellin and PHYTOCHROME INTERACTING FACTOR 5. Development 2012, 139, 4020–4028. [Google Scholar] [CrossRef]
- de Lucas, M.; Davière, J.M.; Rodríguez-Falcón, M.; Pontin, M.; Iglesias-Pedraz, J.M.; Lorrain, S.; Fankhauser, C.; Blázquez, M.A.; Titarenko, E.; Prat, S. A molecular framework for light and gibberellin control of cell elongation. Nature 2008, 451, 480–4. [Google Scholar] [CrossRef]
- van der Graaff, E.; Schwacke, R.; Schneider, A.; Desimone, M.; Flugge, U.I.; Kunze, R. Transcription analysis of Arabidopsis membrane transporters and hormone pathways during developmental and induced leaf senescence. Plant Physiol. 2006, 141, 776–792. [Google Scholar] [CrossRef]
- Guo, Y.; Gan, S.S. Convergence and divergence in gene expression profiles induced by leaf senescence and 27 senescence-promoting hormonal, pathological and environmental stress treatments. Plant Cell Environ. 2012, 35, 644–655. [Google Scholar] [CrossRef]
- Roberts, I.N.; Veliz, C.G.; Criado, M.V.; Signorini, A.; Simonetti, E.; Caputo, C. Identification and expression analysis of 11 subtilase genes during natural and induced senescence of barley plants. J. Plant Physiol. 2017, 211, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Rankenberg, T.; Geldhof, B.; van Veen, H.; Holsteens, K.; Van de Poel, B.; Sasidharan, R. Age-dependent abiotic stress resilience in plants. Trends Plant Sci. 2021, 26, 692–705. [Google Scholar] [CrossRef] [PubMed]
- Gürel, F.; Öztürk, Z.N.; Uçarlı, C.; Rosellini, D. (2016) Barley Genes as Tools to Confer Abiotic Stress Tolerance in Crops. Front. Plant Sci. 2016, 7, 1137. [Google Scholar] [CrossRef] [PubMed]
- Gómez, J.F.; Wilson, Z.A. Non-destructive staging of barley reproductive development for molecular analysis based upon external morphology. J. Exp. Bot. 2012, 63, 4085–94. [Google Scholar] [CrossRef] [PubMed]
- Ogrodowicz, P.; Adamski, T.; Mikołajczak, K.; Kuczyńska, A.; Surma, M.; Krajewski, P.; Sawikowska, A.; Górny, A.G.; Gudyś, K.; Szarejko, I.; et al. QTLs for earliness and yield-forming traits in the Lubuski × CamB barley RIL population under various water regimes. J. Appl. Genet. 2017, 58, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Ogrodowicz, P.; Kuczyńska, A.; Krajewski, P.; Kempa, M. The effects of heading time on yield performance and HvGAMYB expression in spring barley subjected to drought. J. Appl. Genet. 2023, 64, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Ogrodowicz, P.; Mikołajczak, K.; Kempa, M.; Mokrzycka, M.; Krajewski, P.; Kuczyńska, A. Genome-wide association study of agronomical and root-related traits in spring barley collection grown under field conditions. Front. Plant Sci. 2023, 14, 1077631. [Google Scholar] [CrossRef] [PubMed]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Kuczyńska, A.; Cardenia, V.; Ogrodowicz, P.; Kempa, M.; Rodriguez-Estrada, M.T.; Mikołajczak, K. Effects of multiple abiotic stresses on lipids and sterols profile in barley leaves (Hordeum vulgare L.). Plant Physiol. Biochem. 2019, 141, 215–224. [Google Scholar] [CrossRef]
- Mikołajczak, K.; Kuczyńska, A.; Krajewski, P.; Sawikowska, A.; Surma, M.; Ogrodowicz, P.; Adamski, T.; Krystkowiak, K.; Górny, A.G.; Kempa, M.; et al. Quantitative trait loci for plant height in Maresi × CamB barley population and their associations with yield-related traits under different water regimes. J. Appl. Genet. 2017, 58, 23–35. [Google Scholar] [CrossRef]
- Boden, S.A.; Weiss, D.; Ross, J.J.; Davies, N.W.; Trevaskis, B.; Chandler, P.M.; Swain, S.M. EARLY FLOWERING3 regulates flowering in spring barley by mediating gibberellin production and FLOWERING LOCUS T expression. Plant Cell 2014, 26, 1557–1569. [Google Scholar] [CrossRef]
- Grijalva-Contreras, R.L.; Macías-Duarte, R.; Martínez-Díaz, G.; Robles-Contreras, F.; Nuñez-Ramírez, F. Effects of trinexapac-ethyl on different wheat varieties under desert conditions of Mexico. Agric. Sci. 2012, 3, 658–662. [Google Scholar] [CrossRef]
- Browne, R.G.; Iacuone, S.; Li, S.F.; Dolferus, R.; Parish, R.W. Anther Morphological Development and Stage Determination in Triticum aestivum. Front. Plant Sci. 2018, 9, 228. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Huang, H.J.; Ren, S.T.; Li, J.J.; Sun, Y.; Sun, D.Y. Zhang, S.Q. The rice wall-associated receptor-like kinase gene OsDEES1 plays a role in female gametophyte development. Plant Physiol. 2012, 160, 696–707. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, J.; Dong, J.; Yu, J.; Huang, S.; Lin, H.; Hu, S.; Wang, J. Optimized qualitative and quantitative methods for barley viability testing using triphenyl tetrazolium chloride staining. Cereal Chem. 2019, 96, 421–428. [Google Scholar] [CrossRef]
- Impe, D.; Reitz, J.; Köpnick, C.; Rolletschek, H.; Börner, A.; Senula, A.; Nagel, M. Assessment of pollen viability for wheat. Front. Plant Sci. 2020, 10, 1588. [Google Scholar] [CrossRef]
- Barrs, H.D.; Weatherley, P.E. A Re-Examination of the Relative Turgidity Techniques for Estimating Water Deficits in Leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–22. [Google Scholar] [CrossRef]
- Dawidziuk, A.; Koczyk, G.; Popiel, D.; Kaczmarek, J.; Buśko, M. Molecular diagnostics on the toxigenic potential of Fusarium spp. Plan pathogens. J. Appl. Microbio. 2014, 16, 1607–1620. [Google Scholar] [CrossRef] [PubMed]
- Box, G.E.P.; Cox, D.R. An analysis of transformations. J. R. Stat. Soc. Series B. 1964, 26, 211–252. [Google Scholar] [CrossRef]
- VSN International. Genstat for Windows 22nd Edition. VSN International 2022, Hemel Hempstead, UK. Web page: Genstat.co.uk.
- Guo, C.; Yao, L.; You, C.; Wang, S.; Cui, J.; Ge, X.; Ma, H. MID1 plays an important role in response to drought stress during reproductive development. Plant J. 2016, 88, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Saini, H.S. Effects of water stress on male gametophyte development in plants. Sex. Plant Reprod. 1997, 10, 67–73. [Google Scholar] [CrossRef]
- Bakhtiari, M.; Formenti, L.; Caggia, V.; Glauser, G.; Rasmann, S. Variable effects on growth and defense traits for plant ecotypic differentiation and phenotypic plasticity along elevation gradients. Ecol. Evol. 2019, 9, 3740–3755. [Google Scholar] [CrossRef] [PubMed]
- Zare, M.; Azizi, M.H.; Bazrafshan, F. Effect of drought stress on some agronomic traits in ten barley (Hordeum vulgar) cultivars. TJEAS 2011, 1, 57–62. [Google Scholar]
- Ellis, R.P.; Forster, B.P.; Robinson, D.; Handley, L.L.; Gordon, D.C.; Russell, J.R.; Powell, W. Wild barley: A source of genes for crop improvement in the 21st century? J. Exp. Bot. 2000, 51, 9–17. [Google Scholar] [CrossRef]
- Mikołajczak, K.; Ogrodowicz, P.; Gudyś, K.; Krystkowiak, K.; Sawikowska, A.; Frohmberg, W.; Górny, A.; Kędziora, A.; Jankowiak, J.; Józefczyk, D.; et al. Quantitative Trait Loci for Yield and Yield-Related Traits in Spring Barley Populations Derived from Crosses between European and Syrian Cultivars. PLoS ONE 2016, 11(5), e0155938. [Google Scholar] [CrossRef]
- Ogrodowicz, P.; Kuczyńska, A.; Mikołajczak, K.; Adamski, T.; Surma, M.; Krajewski, P.; Ćwiek-Kupczyńska, H.; Kempa, Michał.; Rokicki, Michał.; Jasińska, D. Mapping of quantitative trait loci for traits linked to fusarium head blight in barley. PLoS ONE 2020, 15(2), e0222375. [Google Scholar] [CrossRef]
- Zhang, Z.; Shi, Y.; Ma, Y.; Yang, X.; Yin, X.; Zhang, Y.; Xiao, Y.; Liu, W.; Li, Y.; Li, S.; et al. The Strawberry Transcription Factor Farav1 Positively Regulates Anthocyanin Accumulation by Activation of Famyb10 and Anthocyanin Pathway Genes. Plant Biotechnol. J. 2020, 18, 2267–2279. [Google Scholar] [CrossRef]
- Gol, L.; Haraldsson, E.B.; von Korff, M. Ppd-H1 integrates drought stress signals to control spike development and flowering time in barley. J. Exp. Bot. 2021, 72, 122–136. [Google Scholar] [CrossRef]
- Oguz, M.C.; Aycan, M.; Oguz, E.; Poyraz, I.; Yildiz, M. Drought Stress Tolerance in Plants: Interplay of Molecular, Biochemical and Physiological Responses in Important Development Stages. Physiologia 2022, 2, 180–197. [Google Scholar] [CrossRef]
- Goltsev, V.; Chernev, P.; Zaharieva, I.; Lambrev, P.; Strasser, R.J. Kinetics of delayed chlorophyll a fluorescence registered in milliseconds time range. Photosynth. Res. 2005, 84, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant 2016, 38, 1–11. [Google Scholar] [CrossRef]
- Dąbrowski, P.; Baczewska-Dąbrowska, A.H.; Kalaji, H.M.; Goltsev, V.; Paunov, M.; Rapacz, M.; Wójcik-Jagła, M.; Pawluśkiewicz, B.; Bąba, W.; Brestic, M. Exploration of Chlorophyll a Fluorescence and Plant Gas Exchange Parameters as Indicators of Drought Tolerance in Perennial Ryegrass. Sensors 2019, 19, 2736. [Google Scholar] [CrossRef] [PubMed]
- Daszkowska-Golec, A.; Collin, A.; Sitko, K.; Janiak, A.; Kalaji, H.M.; Szarejko, I. Genetic and Physiological Dissection of Photosynthesis in Barley Exposed to Drought Stress. Int. J. Mol. Sci. 2019, 20, 6341. [Google Scholar] [CrossRef] [PubMed]
- Rapacz, M.; Wójcik-Jagła, M.; Fiust, A.; Kalaji, H.M. Kościelniak, J. Genome-Wide Associations of Chlorophyll Fluorescence OJIP Transient Parameters Connected With Soil Drought Response in Barley. Front. Plant Sci. 2019, 10, 78. [Google Scholar] [CrossRef] [PubMed]
- Soltys-Kalina, D.; Plich, J.; Strzelczyk-Żyta, D.; Śliwka, J.; Marczewski, W. The effect of drought stress on the leaf relative water content and tuber yield of a half-sib family of 'Katahdin'-derived potato cultivars. Breed. Sci. 2016, 66, 328–331. [Google Scholar] [CrossRef]
- Longstaff, B.J.; Loneragan, N.R.; O’Donohue, M.J.; Dennison, W.J. Effects of light deprivation on the survival and recovery of the seagrass Halophila ovalis (R.Br.) Hook. J. Exp. Mar. Biol. Ecol. 1999, 234, 1–27. [Google Scholar] [CrossRef]
- Lu, D.; Liu, B.; Ren, M.; Wu, C.; Ma, J.; Shen, Y. Light Deficiency Inhibits Growth by Affecting Photosynthesis Efficiency as well as JA and Ethylene Signaling in Endangered Plant Magnolia sinostellata. Plants 2021, 10, 2261. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Ren, K.; Li, K.; Wei, M.; Wang, W.; Sheng, X. Light Deprivation-Induced Inhibition of Chloroplast Biogenesis Does Not Arrest Embryo Morphogenesis But Strongly Reduces the Accumulation of Storage Reserves during Embryo Maturation in Arabidopsis. Front. Plant Sci. 2017, 8, 1287. [Google Scholar] [CrossRef]
- Paluch-Lubawa, E.; Stolarska, E.; Sobieszczuk-Nowicka, E. Dark-Induced Barley Leaf Senescence – A Crop System for Studying Senescence and Autophagy Mechanisms. Front. Plant Sci. 2021, 12, 635619. [Google Scholar] [CrossRef]
- Kebrom, T.H.; Chandler, P.M.; Swain, S.M.; King, R.W.; Richards, R.A.; Spielmeyer, W. Inhibition of tiller bud outgrowth in the tin mutant of wheat is associated with precocious internode development. Plant Physiol. 2012, 160, 308–18. [Google Scholar] [CrossRef] [PubMed]
- Jagadish, S.V.K. Heat stress during flowering in cereals – effects and adaptation strategies. New Phytol. 2020, 226, 1567–1572. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, A.; Sita, K.; Siddique, K.H.M.; Kumar, R.; Bhogireddy, S.; Varshney, R.K.; HanumanthaRao, B.; Nair, R.M.; Prasad, P.V.V.; Nayyar, H. Drought or/and Heat-Stress Effects on Seed Filling in Food Crops: Impacts on Functional Biochemistry, Seed Yields, and Nutritional Quality. Front. Plant Sci. 2018, 9, 1705. [Google Scholar] [CrossRef] [PubMed]
- Firon, N.; Nepi, M.; Pacini, E. Water status and associated processes mark critical stages in pollen development and functioning. Ann. Bot. 2012, 109, 1201–1214. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Riano, T.; Dafni, A. A new procedure to asses pollen viability. Sex. Plant Reprod. 2000, 12, 241–244. [Google Scholar] [CrossRef]
- Pacini, E.; Jacquard, C.; Clément, C. Pollen vacuoles and their significance. Planta 2011, 234, 217–227. [Google Scholar] [CrossRef]
- Hoekstra, F.A.; Golovina, E.A.; Buitink, J. Mechanisms of plant desiccation tolerance. Trends in Plant Sci. 2001, 6, 431–438. [Google Scholar] [CrossRef]
- Williams, J.H.; Taylor, M.L.; O'Meara, B.C. Repeated evolution of tricellular (and bicellular) pollen. Am. J. Bot. 2014, 101, 559–71. [Google Scholar] [CrossRef]
- Martinoia, E.; Maeshima, M.; Neuhaus, H.E. Vacuolar transporters and their essential role in plant metabolism. J. Exp. Bot. 2007, 58, 83–102. [Google Scholar] [CrossRef]
- Signorelli, S.; Tarkowski, Ł.P.; Van den Ende, W.; Bassham, D.C. Linking Autophagy to Abiotic and Biotic Stress Responses. Trends Plant Sci. 2019, 24, 413–430. [Google Scholar] [CrossRef]
- Han, S.K.; Wagner, D. Role of chromatin in water stress responses in plants. J. Exp. Bot. 2014, 65, 2785–2799. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-M.; Sasaki, T.; Ueda, M.; Sako, K.; Seki, M. Chromatin changes in response to drought, salinity, heat, and cold stresses in plants. Front. Plant Sci. 2015, 6, 114. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lu, M.; Wang, Y.; Wang, Y.; Liu, Z.; Chen, S. Response Mechanism of Plants to Drought Stress. Horticulturae 2021, 7, 50. [Google Scholar] [CrossRef]
- Boutté, Y.; Vernhettes, S.; Satiat-Jeunemaitre, B. Involvement of the cytoskeleton in the secretory pathway and plasma membrane organisation of higher plant cells. Cell Biol. Int. 2007, 31, 649–54. [Google Scholar] [CrossRef] [PubMed]
- Grigorova, B.; Vassileva, V.; Klimchuk, D.; Vaseva, I.; Demirevska, K.; Feller, U. (2012) Drought, high temperature, and their combination affect ultrastructure of chloroplasts and mitochondria in wheat (Triticum aestivum L.) leaves. J. Plant Interact. 2012, 7, 204–213. [Google Scholar] [CrossRef]
- Liu, J.X.; Bennett, J. Reversible and irreversible drought-induced changes in the anther proteome of rice (Oryza sativa L.) genotypes IR64 and Moroberekan. Mol. Plant. 2011, 4, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Kong, F.; Zhang, S.; Meng, X.; Wang, Y.; Meng, Q. A tomato chloroplast-targeted DnaJ protein protects Rubisco activity under heat stress. J. Exp. Bot. 2015, 66, 3027–3040. [Google Scholar] [CrossRef]
- Tang, H.; Zhu, H. Specific Changes in Morphology and Dynamics of Plant Mitochondria under Abiotic Stress. Horticulturae 2023, 9, 11. [Google Scholar] [CrossRef]
- Vassileva, V.; Simova-Stoilova, L.; Demirevska, K.; Feller, U. Variety-specific response of wheat (Triticum aestivum L.) leaf mitochondria to drought stress. J. Plant Res. 2009, 122, 445–454. [Google Scholar] [CrossRef]
- Wan, S.; Jiang, L. Endoplasmic reticulum (ER) stress and the unfolded protein response (UPR) in plants. Protoplasma 2016, 253, 753–764. [Google Scholar] [CrossRef]
- Wang, X.; Xu, M.; Gao, C.; Zeng, Y.; Cui, Y.; Shen, W.; Jiang, L. The roles of endomembrane trafficking in plant abiotic stress responses. J. Integr. Plant Biol. 2020, 62, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Murray, F.; Kalla, R.; Jacobsen, J.; Gubler, F. A role of HvGAMYB in anther development. Plant J. 2003, 33, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Duca, M.; Port, A.; Orozco-Cardenas, M.; Lovatt, C. Gibberellin-Induced Gene Expression Associated with Cytoplasmic Male Sterility in Sunflower. Biotechnol. Biotechnol. Equip. 2008, 22, 691–698. [Google Scholar] [CrossRef]
- Begcy, K.; Dresselhaus, T. Epigenetic responses to abiotic stresses during reproductive development in cereals. Plant Reprod. 2018, 31, 343–355. [Google Scholar] [CrossRef]
- Pacini, E.; Dolferus, R. Pollen Developmental Arrest: Maintaining Pollen Fertility in a World With a Changing Climate. Front. Plant Sci. 2019, 10, 679. [Google Scholar] [CrossRef]
| Trait (unit), (abbrev.) | Trait description |
| Total number of tillers, (Tn) | Number of tillers with fertile and non-fertile (without grains) spikes |
| Number of productive tillers, (PTn) | Number of tillers with fertile spikes |
| Length of main spike (cm), (LSm) | Length of main spike from 10 randomly selected spikes in a pot (without awns) |
| Number of spikelets per main spike, (NSSm) | Number of spikelets in spike of main stem-average e for 10 main spikes in a pot |
| Number of grains per main spike, (NGSm) | Number of grains collected from one spike of main stem - average for 10 main spikes in a pot |
| Weight of grains per main spike, (WGSm) | Weight of grain collected from one spike of the main stem - average for 10 main spikes in a pot |
| Length of lateral spike (cm), (LSl) | Length of spike from lateral stem - average for 10 lateral spikes in a pot (without awns) |
| Number of spikelets per lateral spike, (NSSl) | Number of spikelets per spike of lateral stem - average for 10 lateral spikes in a pot |
| Number of grains per lateral spike, (NGSl) | Number of grains collected from spike of lateral stem - average for 10 lateral spikes in a pot |
| Weight of grains per main spike, (WGSl) | Weight of grain collected from one spike of the lateral stem - average for 10 lateral spikes in a pot |
| Grain yield (g), (GY) | Average weight of grains collected from one plant, calculated as average of measurements of grain weight for 10 plants. |
| Thousand grain weight (g), (TGW) | Average weight of 1000 grains, calculated as average of 1000 * average weight of one grain for 20 spikes in a pot |
| Fertility of the main spike, (FSm) | NGSm/NSSm ratio |
| Fertility of the lateral spike, (FSl) | NGSl/NSSl ratio |
| Trait | Abbrev. |
| Quantify the PSII behavior were the absorbed energy flux | ABS_RC |
| Trapped energy flux | TRo_RC |
| Electron transport flux | Eto_RC |
| Dissipated energy flux | DIo_RC |
| Maximum quantum yield of primary photochemistry | Fv_Fm |
| Probability/efficiency that a trapped exciton moves an electron into the electron transport chain beyond QA | Ψ_o |
| Quantum yield of electron transport | Φ_Eo |
| Probability that the energy of an absorbed photon is dissipated as heat | Φ_Do |
| Performance index | Pi _Abs |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





