Submitted:
14 April 2023
Posted:
17 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Preparation of Extract
2.3. Animal Housing and Care
2.4. Experimental Procedure
2.4.1. The 14-Day Acute Toxicity Study
2.4.2. The 28-Day Subacute Toxicity Study
2.5. Clinical Observations
2.6. Sample Collection
2.7. Hematological Indices
2.8. Biochemical Analysis
2.9. Histopathology
2.10. Statistical Analyses
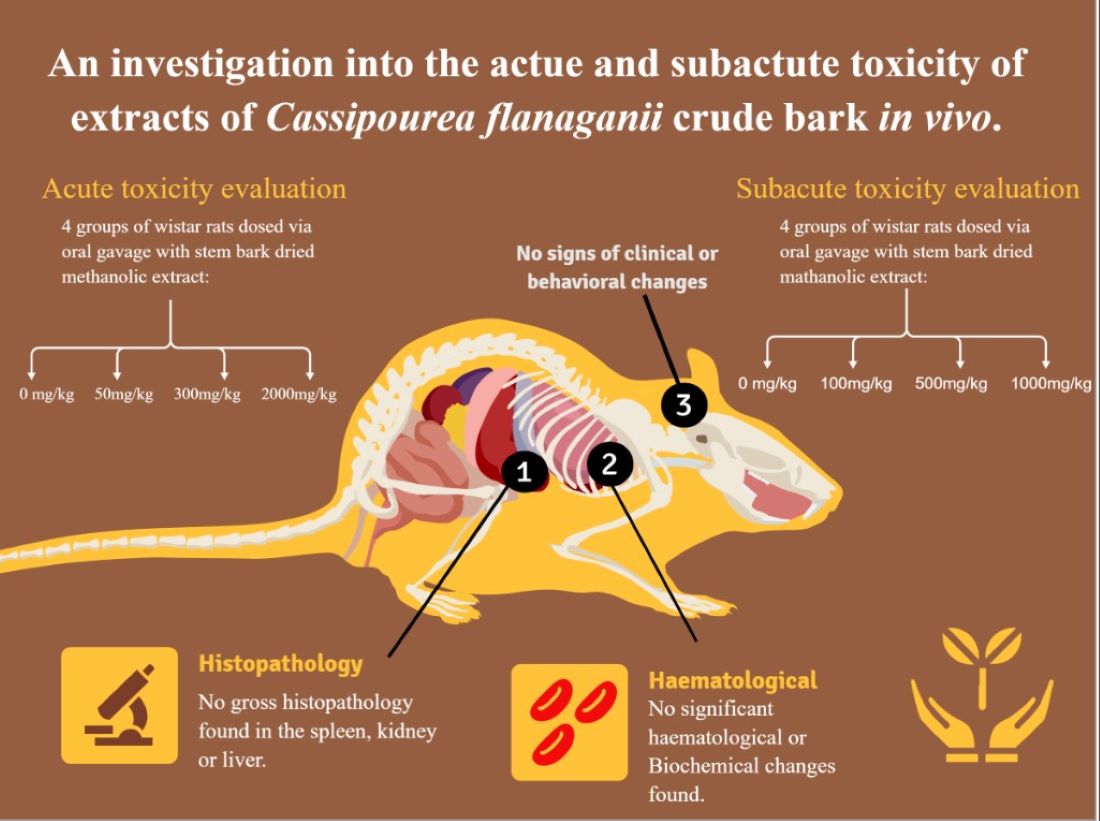
3. RESULTS
3.1. Morphological Alteration
3.2. 14-Day Acute Toxicity
3.3. 14-Day Acute Repeat Toxicity Results
3.3.1. Subacute Biochemical Analyses
3.4. Haematological Analyses
- White cell count (500 mg/kg) with Baseline (4.50±2.06) and End (6.77±2.28), the difference being statistically significant (SIG);
- Monocytes ABS (500 mg/kg) with Baseline (0.07±0.04) and End (0.16±0.60), the difference being statistically significant (SIG); and
- Monocytes ABS (Control) with Baseline (0.08±0.03) and End (0.16±0.05), the difference being statistically significant (SIG).
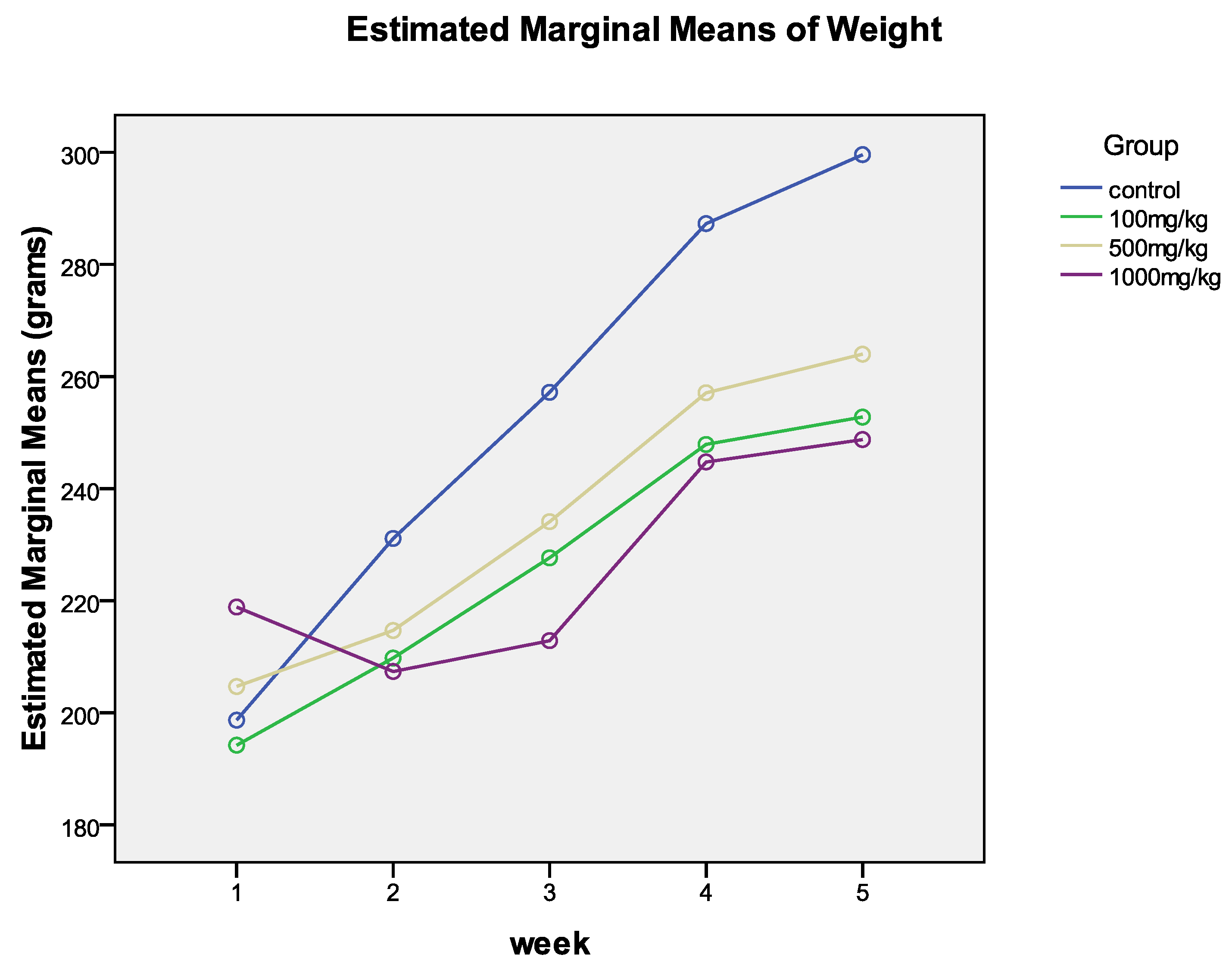
3.5. Relative Organ Weight and Weekly Weight
3.6. Changes in Weight Over Time
4. DISCUSSION
5. CONCLUSIONS
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Licata, A., F.S. Macaluso, and A. Craxì, Herbal hepatotoxicity: a hidden epidemic. Internal and emergency medicine, 2013. 8(1): p. 13-22. [CrossRef]
- Gomes, C., et al., Evaluation of subchronic toxicity of the hydroethanolic extract of Tropaeolum majus in Wistar rats. Journal of Ethnopharmacology, 2012. 142(2): p. 481-487. [CrossRef]
- Organization, W.H., WHO guidelines on safety monitoring of herbal medicines in pharmacovigilance systems. 2004: World Health Organization.
- Buwa-Komoren, L.V., et al., An ethnobotanical and ethnomedicinal survey of traditionally used medicinal plants in Seymour, South Africa: An attempt toward digitization and preservation of ethnic knowledge. Pharmacognosy Magazine, 2019. 15(60): p. 115. [CrossRef]
- Organization, W.H., Fact sheet no. 134: Traditional medicine. Geneva: World Health Organization, 2008.
- Gupta, D., B. Bleakley, and R.K. Gupta, Dragon's blood: botany, chemistry and therapeutic uses. Journal of ethnopharmacology, 2008. 115(3): p. 361-380. [CrossRef]
- Jordan, S.A., D.G. Cunningham, and R.J. Marles, Assessment of herbal medicinal products: challenges, and opportunities to increase the knowledge base for safety assessment. Toxicology and applied pharmacology, 2010. 243(2): p. 198-216. [CrossRef]
- Oyedemi, S., M. Yakubu, and A. Afolayan, Effect of aqueous extract of Leonotis leonurus (L.) R. Br. leaves in male Wistar rats. Human & experimental toxicology, 2010. 29(5): p. 377-384. [CrossRef]
- Ashafa, A.O.T., L.O. Orekoya, and M.T. Yakubu, Toxicity profile of ethanolic extract of Azadirachta indica stem bark in male Wistar rats. Asian Pacific journal of tropical biomedicine, 2012. 2(10): p. 811-817. [CrossRef]
- Seeff, L.B., Herbal hepatotoxicity. Clinics in liver disease, 2007. 11(3): p. 577-596. [CrossRef]
- Lall, N. and N. Kishore, Are plants used for skin care in South Africa fully explored? Journal of ethnopharmacology, 2014. 153(1): p. 61-84. [CrossRef]
- Makunga, N., L. Philander, and M. Smith, Current perspectives on an emerging formal natural products sector in South Africa. Journal of Ethnopharmacology, 2008. 119(3): p. 365-375. [CrossRef]
- Langat, M.K., et al., The effect of isolates from Cassipourea flanaganii (Schinz) alston, a plant used as a skin lightning agent, on melanin production and tyrosinase inhibition. Journal of Ethnopharmacology, 2021. 264: p. 113272. [CrossRef]
- Dold, T. and M. Cocks, Imbhola yesiXhosa: traditional Xhosa cosmetics. Veld & Flora, 2005. 91(3): p. 123-125.
- Thibane, V., et al., Phytochemistry and cosmetic importance of medicinal plants used for skin beauty and healthcare from the Eastern Cape Province, South Africa. South African Journal of Botany, 2017. 100(109): p. 371. [CrossRef]
- Thibane, V., et al., Modulation of the enzyme activity of secretory phospholipase A2, lipoxygenase and cyclooxygenase involved in inflammation and disease by extracts from some medicinal plants used for skincare and beauty. South African Journal of Botany, 2019. 120: p. 198-203. [CrossRef]
- Mpofana, N. and H. Abrahamse, The management of melasma on skin types V and VI using light emitting diode treatment. Photomedicine and Laser Surgery, 2018. 36(10): p. 522-529. [CrossRef]
- Kwon, S.H., et al., Melasma: Updates and perspectives. Experimental dermatology, 2019. 28(6): p. 704-708. [CrossRef]
- Masub, N., et al., The vascular component of melasma: a systematic review of laboratory, diagnostic, and therapeutic evidence. Dermatologic Surgery, 2020. 46(12): p. 1642-1650. [CrossRef]
- Pichardo, R., et al., The prevalence of melasma and its association with quality of life in adult male Latino migrant workers. International journal of dermatology, 2009. 48(1): p. 22-26. [CrossRef]
- Uyanikoglu, H. and M. Aksoy, Quality of life in patients with melasma in Turkish women. Dermatology reports, 2017. 9(2). [CrossRef]
- Gupta, S., et al., A study of the clinical profile and assessment of the quality of life in patients of Melasma. The Pharma Innovation, 2017. 6(7, Part C): p. 190.
- Lueangarun, S., C. Namboonlue, and T. Tempark, Postinflammatory and rebound hyperpigmentation as a complication after treatment efficacy of telangiectatic melasma with 585 nanometers Q-switched Nd: YAG laser and 4% hydroquinone cream in skin phototypes III-V. Journal of Cosmetic Dermatology, 2021. 20(6): p. 1700-1708. [CrossRef]
- Perper, M., et al., Tranexamic acid in the treatment of melasma: a review of the literature. American journal of clinical dermatology, 2017. 18(3): p. 373-381. [CrossRef]
- Sarkar, R., et al., The combination of glycolic acid peels with a topical regimen in the treatment of melasma in dark-skinned patients: a comparative study. Dermatologic surgery, 2002. 28(9): p. 828-832. [CrossRef]
- Kooyers, T. and W. Westerhof, Toxicology and health risks of hydroquinone in skin lightening formulations. Journal of the European academy of Dermatology and Venereology, 2006. 20(7): p. 777-780. [CrossRef]
- Mukherjee, P.K., et al., Validation of medicinal herbs for anti-tyrosinase potential. Journal of herbal medicine, 2018. 14: p. 1-16. [CrossRef]
- Ekor, M., The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Frontiers in pharmacology, 2014. 4: p. 177. [CrossRef]
- Bello, I., et al., Acute and sub-acute toxicity evaluation of the methanolic extract of Alstonia scholaris stem bark. Medical Sciences, 2016. 4(1): p. 4. [CrossRef]
- Maregesi, S.M., A.R. Mwakigonja, and P. Urio, Toxicity evaluation of Abrus precatorius seeds collected from Bunda District, Tanzania. Sch Acad J Pharm, 2016. 5: p. 399-405. [CrossRef]
- Mlozi, S.H., J.A. Mmongoyo, and M. Chacha, The in vivo toxicity evaluation of leaf and root methanolic extracts of Tephrosia vogelii Hook. f using animal model. Clinical Phytoscience, 2020. 6(1): p. 1-9. [CrossRef]
- Olson, H., et al., Concordance of the toxicity of pharmaceuticals in humans and in animals. Regulatory Toxicology and Pharmacology, 2000. 32(1): p. 56-67. [CrossRef]
- Kowalczyk, E., et al., Effect of anthocyanins on selected biochemical parameters in rats exposed to cadmium. Acta Biochimica Polonica, 2003. 50(2): p. 543-548. [CrossRef]
- Adedapo, A.A., M.O. Abatan, and O.O. Olorunsogo, Effects of some plants of the spurge family on haematological and biochemical parameters in rats. Veterinarski Arhiv, 2007. 77(1): p. 29-38.
- Lezoul, N.E.H., et al., Extraction processes with several solvents on total bioactive compounds in different organs of three medicinal plants. Molecules, 2020. 25(20): p. 4672. [CrossRef]
- Kilkenny, C., et al., Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. Journal of Pharmacology and Pharmacotherapeutics, 2010. 1(2): p. 94-99. [CrossRef]
- Percie du Sert, N., et al., The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. Journal of Cerebral Blood Flow & Metabolism, 2020. 40(9): p. 1769-1777. [CrossRef]
- Shapiro, S.S. and M.B. Wilk, An analysis of variance test for normality (complete samples). Biometrika, 1965. 52(3/4): p. 591-611. [CrossRef]
- Jatsa, H.B., et al., Efficacy of Sida pilosa Retz aqueous extract against Schistosoma mansoni–induced granulomatous inflammation in the liver and the intestine of mice: histomorphometry and gastrointestinal motility evaluation. BMC complementary and alternative medicine, 2018. 18(1): p. 1-15. [CrossRef]
- Würbel, H. and J.P. Garner, Refinement of rodent research through environmental enrichment and systematic randomization. NC3Rs, 2007. 9: p. 1-9.
- Asare, G., et al., Acute toxicity studies of aqueous leaf extract of. Interdisciplinary toxicology, 2011. 4(4): p. 206-210. [CrossRef]
- Giknis, M. and C. Clifford, Clinical laboratory parameters for Crl: WI (Han) rats. Accel Drug Dev, 2008: p. 1-14.
- Reece, W.O., et al., Dukes' physiology of domestic animals. 2015: John Wiley & Sons.
| Toxicity Test | Control Baseline | End Value | p-Value | Decision | 50mg/kg Baseline | End Value | p-Value | Decision | ||||||||
| Urea | 7.90 | ± | 1.79 | 6.67 | ± | 0.57 | 0.118 | NS | 8.10 | ± | 0.43 | 6.33 | ± | 0.57 | 0.005 | SIG |
| LDL Cholesterol | N/A | N/A | N/A | N/A | N/A | N/A | ||||||||||
| Triglyceride | 0.66 | ± | 0.42 | 0.65 | ± | 0.54 | 0.716 | NS | 0.73 | ± | 0.74 | 1.16 | ± | 0.73 | 0.290 | NS |
| Total protein (g/L) | 68.00 | ± | 8.96 | 63.00 | ± | 6.57 | 0.013 | SIG | 70.67 | ± | 11.20 | 64.67 | ± | 5.17 | 0.188 | NS |
| Albumin (g/L) | 35.00 | ± | 4.97 | 32.00 | ± | 2.48 | 0.035 | SIG | 36.00 | ± | 4.97 | 32.67 | ± | 2.87 | 0.130 | NS |
| ± | 33.00 | 4.30 | 31.00 | ± | 4.30 | N/A | N/A | 34.67 | ± | 6.25 | 32.00 | ± | 2.48 | 0.270 | NS | |
| Alb/Glob ratio | 1.07 | ± | 0.14 | 1.03 | ± | 0.14 | 0.423 | NS | 103 | ± | 0.14 | 1.00 | ± | N/A | 0.423 | NS |
| Total Bilirubin | 20.0 | ± | N/A | 2.00 | ± | N/A | N/A | 2.33 | ± | 1.43 | 2.00 | ± | N/A | 0.423 | NS | |
| ALK (IU/L) | 107.00 | ± | 36.08 | 106.67 | ± | 36.62 | 0.979 | NS | 103.33 | ± | 72.10 | 84.67 | ± | 33.08 | 0.333 | NS |
| ALT (IU/L) | 42.67 | ± | 11.20 | 37.33 | ± | 11.20 | 0.004 | SIG | 42.00 | ± | 22.77 | 35.00 | ± | 15.51 | 0.379 | NS |
| AST (IU/L) | 102.67 | ± | 11.20 | 88.00 | ± | 10.83 | 0.065 | NS | 107.33 | ± | 42.33 | 68.67 | ± | 10.04 | 0.054 | NS |
| Toxicity Test | 300mg/kg Baseline | End | p-Value | Decision | 2000mg/kg Baseline | End | p-Value | Decision | ||||||||
| Urea | 8.37 | ± | 1.88 | 7.20 | ± | 1.08 | 0.194 | NS | 7.17 | ± | 1.37 | 7.15 | ± | 0.87 | 0.939 | NS |
| LDL Cholesterol | N/A | N/A | N/A | N/A | N/A | N/A | ||||||||||
| Triglyceride | 0.77 | ± | 0.68 | 0.55 | ± | 0.53 | 0.105 | NS | 0.77 | ± | 0.70 | 0.74 | ± | 0.04 | 0.843 | NS |
| Total protein (g/L) | 71.67 | ± | 5.17 | 63.00 | ± | 8.96 | 0.069 | NS | 71.33 | ± | 10.04 | 53.50 | ± | 1.24 | 0.013 | SIG |
| Albumin (g/L) | 35.33 | ± | 2.87 | 32.67 | ± | 3.79 | 0.015 | SIG | 35.00 | ± | 6.57 | 27.50 | ± | 1.24 | 0.027 | SIG |
| Globulin (g/L) | 36.33 | ± | 3.79 | 30.33 | ± | 5.74 | 0.102 | NS | 36.33 | ± | 3.79 | 26.00 | ± | N/A | 0.007 | SIG |
| Alb/Glob ratio | 0.93 | ± | 0.14 | 1.07 | ± | 0.14 | 0.184 | NS | 0.97 | ± | 0.14 | 1.05 | ± | 0.12 | 0.038 | SIG |
| Total Bilirubin | 2.33 | ± | 1.43 | 2.00 | ± | N/A | 0.423 | NS | 5.33 | ± | 10.04 | 2.00 | ± | N/A | 0.289 | NS |
| ALK (IU/L) | 102.00 | ± | 31.13 | 102.67 | ± | 21.70 | 0.958 | NS | 121.67 | ± | 47.02 | 109.50 | ± | 16.15 | 0.387 | NS |
| ALT (IU/L) | 50.00 | ± | 2.48 | 39.00 | ± | 14.90 | 0.062 | NS | 45.00 | ± | 21.22 | 135.50 | ± | 232.27 | 0.249 | NS |
| AST (IU/L) | 101.00 | ± | 33.42 | 88.33 | ± | 33.73 | 0.201 | NS | 88.67 | ± | 19.92 | 312.00 | ± | 474.47 | 0.191 | NS |
| Toxicity Test | Control Baseline | Control End | p-Value | Decision | 50mg/kgBaseline | 50mg/kgEnd | p-Value | Decision | ||||||||
| Urea | 6.90 | ± | 1.79 | 6.23 | ± | 0.72 | 0.171 | NS | 17.07 | ± | 31.43 | 7.37 | ± | 1.74 | 0.334 | NS |
| LDL Cholesterol | N/A | N/A | N/A | N/A | N/A | N/A | ||||||||||
| Triglyceride | 0.98 | ± | 0.39 | 1.09 | ± | 0.60 | 0.594 | NS | 0.91 | ± | 0.64 | 0.88 | ± | 0.93 | 0.939 | NS |
| Total protein (g/L) | 65.67 | ± | 6.25 | 61.00 | ± | 4.30 | 0.020 | SIG | 68.33 | ± | 1.43 | 59.33 | ± | 3.79 | 0.016 | SIG |
| Albumin (g/L) | 34.33 | ± | 3.79 | 32.33 | ± | 2.87 | 0.074 | NS | 34.33 | ± | 3.79 | 31.33 | ± | 1.43 | 0.122 | NS |
| Globulin (g/L) | 31.33 | ± | 2.87 | 28.67 | ± | 1.43 | 0.015 | SIG | 34.00 | ± | 2.48 | 28.00 | ± | 2.48 | N/A | |
| Alb/Glob ratio | 1.10 | ± | N/A | 1.10 | ± | N/A | N/A | 1.00 | ± | 0.25 | 1.10 | ± | N/A | 0.225 | NS | |
| Total Bilirubin | 7.00 | ± | N/A | 9.33 | ± | 3.79 | 0.118 | NS | 4.00 | ± | 25.41 | 3.67 | ± | 7.17 | 0.500 | NS |
| ALK (IU/L) | 189.33 | ± | 106.63 | 144.67 | ± | 33.08 | 0.121 | NS | 170.33 | ± | 55.40 | 121.67 | ± | 39.85 | 0.084 | NS |
| ALT (IU/L) | 39.33 | ± | 1.43 | 37.00 | ± | 4.30 | 0.118 | NS | 42.00 | ± | 21.66 | 41.33 | ± | 11.74 | 0.866 | NS |
| AST (IU/L) | 96.67 | ± | 21.13 | 84.33 | ± | 19.92 | 0.011 | SIG | 122.67 | ± | 22.27 | 139.67 | ± | 231.24 | 0.792 | NS |
| Toxicity Test | 300mg/kg Baseline | 300mg/kg End | p-Value | Decision | 2000mg/kg Baseline | 2000mg/kg End | p-Value | Decision | ||||||||
| Urea | 11.23 | ± | 12.82 | 7.83 | ± | 1.60 | 0.325 | NS | 6.13 | ± | 5.09 | 7.47 | ± | 1.12 | 0.307 | NS |
| LDL Cholesterol | N/A | N/A | N/A | N/A | N/A | N/A | ||||||||||
| Triglyceride | 0.87 | ± | 0.37 | 1.19 | ± | 1.09 | 0.317 | NS | 0.88 | ± | 0.25 | 0.83 | ± | 0.64 | 0.768 | NS |
| Total protein (g/L) | 67.67 | ± | 5.17 | 61.00 | ± | 8.96 | 0.179 | NS | 67.33 | ± | 3.79 | 59.67 | ± | 3.79 | 0.013 | SIG |
| Albumin (g/L) | 35.33 | ± | 1.43 | 32.67 | ± | 1.43 | 0.057 | NS | 35.00 | ± | 2.48 | 31.00 | ± | 2.48 | 0.020 | SIG |
| Globulin (g/L) | 32.33 | ± | 3.79 | 28.33 | ± | 7.59 | 0.270 | NS | 32.33 | ± | 1.43 | 28.67 | ± | 1.43 | 0.008 | SIG |
| Alb/Glob ratio | 1.10 | ± | N/A | 1.17 | ± | 0.29 | 0.423 | NS | 1.10 | ± | N/A | 1.10 | ± | N/A | N/A | |
| Total Bilirubin | 6.33 | ± | 9.40 | 8.50 | ± | 6.35 | N/A | 5.67 | ± | 7.99 | 5.67 | ± | 7.99 | N/A | ||
| ALK (IU/L) | 167.00 | ± | 52.58 | 124.67 | ± | 77.22 | 0.061 | NS | 15.67 | ± | 11.47 | 132.67 | ± | 19.30 | 0.027 | SIG |
| ALT (IU/L) | 38.67 | ± | 2.87 | 34.00 | ± | 8.61 | 0.118 | NS | 38.33 | ± | 7.99 | 41.33 | ± | 13.68 | 0.483 | NS |
| AST (IU/L) | 99.33 | ± | 29.43 | 105.00 | ± | 43.03 | 0.598 | NS | 86.67 | ± | 29.64 | 80.00 | ± | 8.61 | 0.511 | NS |
| Variable | 100mg/kg | 500mg/kg | 1000mg/kg | Control | ||||||||
| Baseline | End | p-Value | Baseline | End | p-Value | Baseline | End | p-Value | Baseline | End | p-Value | |
| Urea | 6.08±0.62 | 7.74±2.21 | NS | 6.47±0.80 | 9.25±0.56 | SIG | 6.14±0.61 | 8.15±2.37 | NS | 6.26±0.44 | 9.14±0.73 | SIG |
| LDL Cholesterol | 0.15±0.00 | 0.14±0.03 | NS | 0.15±0.00 | 0.15±0.00 | N/A | 0.15±0.00 | 0.17±0.03 | NS | 0.15±0.00 | 0.15±0.00 | N/A |
| Triglyceride | 1.00±0.16 | 1.06±0.37 | NS | 0.96±0.17 | 1.27±0.26 | NS | 1.09±0.20 | 0.99±0.34 | NS | 1.17±0.22 | 1.31±0.43 | NS |
| Total Protein | 59.80±2.65 | 53.10±13.44 | NS | 59.90±4.00 | 60.70±2.31 | NS | 59.70±4.33 | 54.60±13.89 | NS | 59.40±3.54 | 59.10±1.67 | NS |
| Albumin | 32.20±1.71 | 28.50±7.30 | NS | 32.40±2.27 | 32.60±2.08 | NS | 32.20±2.82 | 32.33±1.63 | NS | 31.70±2.16 | 30.90±1.37 | NS |
| Globulin | 21.60±1.02 | 24.60±6.22 | NS | 27.50±1.92 | 28.10±2.17 | NS | 27.50±1.69 | 25.50±6.54 | NS | 27.70±1.47 | 28.20±1.21 | NS |
| Alb/Glob ratio | 1.18±0.03 | 1.04±0.27 | NS | 1.19±0.05 | 1.17±0.14 | NS | 1.18±0.07 | 1.04±0.27 | NS | 1.15±0.04 | 1.10±0.08 | NS |
| Total Bilirubin | 4.30±2.50 | 4.50±2.72 | NS | 3.80±1.87 | 4.70±2.82 | NS | 2.50±1.92 | 2.70±2.41 | NS | 2.50±1.73 | 2.70±1.55 | NS |
| ALK | 255.20±72.65 | 136.70±51.53 | SIG | 252.40±86.03 | 143.40±46.25 | SIG | 239.10±61.87 | 136.80±49.24 | SIG | 288.50±58.88 | 193.40±45.39 | SIG |
| AST | 100.50±7.81 | 111.90±37.80 | NS | 96.30±11.06 | 81.40±9.82 | NS | 92.00±7.80 | 78.70±24.08 | NS | 83.20±6.89 | 86.60±14.42 | NS |
| Variable | 100mg/g | 500mg/kg | 1000mg/kg | Control | P-value | Decision |
| Urea | 7.74±2.24 | 9.25±0.56 | 8.15±2.37 | 9.14±0.73 | 0.412 | NS |
| LDL Cholesterol | 0.14±0.03 | 0.15±0.00 | 0.17±0.03 | 0.15±0.00 | 0.279 | NS |
| Triglyceride | 1.06±0.37 | 1.27±0.26 | 0.99±0.34 | 1.31±0.43 | 0.427 | NS |
| Total Protein | 53.10±13.44 | 60.70±2.31 | 54.60±13.89 | 59.10±1.67 | 0.560 | NS |
| Albumin | 28.50±7.30 | 32.50±2.08 | 32.33±1.63 | 30.90±1.37 | 0.611 | NS |
| Globulin | 24.60±6.22 | 28.10±2.17 | 25.50±6.54 | 28.20±1.21 | 0.513 | NS |
| Alb/Glob ratio | 1.04±0.27 | 1.17±0.14 | 1.04±0.27 | 1.10±0.08 | 0.709 | NS |
| Total Bilirubin | 4.50±2.72 | 4.70±2.82 | 2.70±2.41 | 2.70±1.55 | 0.382 | NS |
| ALK | 136.70±51.53 | 143.40±46.25 | 136.80±49.24 | 193.40±45.39 | 0.194 | NS |
| AST | 111.90±37.80 | 81.40±9.82 | 78.70±24.08 | 86.60±14.42 | 0.125 | NS |
| Variable | 100mg/kg | 500mg/kg | 1000mg/kg | Control | ||||||||
| Baseline | End | p-Value | Baseline | End | p-Value | Baseline | End | p-Value | Baseline | End | p-Value | |
| Red Cell Count | 4.23±2.65 | 5.92±2.24 | NS | 4.89±2.48 | 6.53±1.67 | NS | 6.12±1.59 | 7.30±0.17 | NS | 5.69±2.16 | 7.42±0.16 | NS |
| Haemoglobin | 8.69±5.41 | 11.47±4.35 | NS | 12.53±4.51 | 13.94±0.56 | NS | 14.02±0.66 | 12.85±3.27 | NS | 11.72±4.43 | 14.39±0.27 | NS |
| Haematocrit | 0.30±0.19 | 0.39±0.15 | NS | 0.34±0.17 | 0.43±0.11 | NS | 0.43±0.11 | 0.44±0.11 | NS | 0.40±0.15 | 0.95±1.02 | NS |
| MCV | 42.50±26.22 | 52.70±19.87 | NS | 54.22±23.67 | 60.10±15.14 | NS | 63.90±16.19 | 60.20±15.22 | NS | 56.50±21.38 | 66.80±1.30 | NS |
| MCH | 12.40±7.65 | 15.50±5.85 | NS | 14.30±7.07 | 17.40±4.40 | NS | 20.67±0.54 | 17.70±4.47 | NS | 16.50±6.25 | 19.50±0.51 | NS |
| MCHC | 14.70±9.38 | 18.50±7.77 | NS | 17.80±9.22 | 22.20±6.67 | NS | 25.11±3.55 | 22.10±6.45 | NS | 19.80±8.08 | 24.30±3.89 | NS |
| RDW | 8.06±5.06 | 9.70±3.70 | NS | 9.44±4.74 | 11.18±2.85 | NS | 11.05±3.82 | 11.51±2.95 | NS | 9.91±4.65 | 11.80±0.46 | NS |
| White cell count | 3.51±2.28 | 5.26±2.23 | NS | 4.50±2.06 | 6.77±2.28 | SIG | 4.70±1.31 | 3.59±1.78 | NS | 4.30±1.69 | 5.92±1.07 | NS |
| Neutrophils | 5.15±5.47 | 7.14±4.99 | NS | 5.00±5.61 | 6.25±4.67 | NS | 9.80±8.94 | 11.37±10.78 | NS | 8.17±6.77 | 9.24±7.73 | NS |
| Lymphocytes ABS | 25.65±27.15 | 43.03±30.00 | NS | 26.56±27.76 | 36.37±28.96 | NS | 31.92±26.32 | 29.96±25.56 | NS | 33.52±28.15 | 41.72±28.33 | NS |
| Monocytes ABS | 0.07±0.04 | 0.11±0.06 | NS | 0.07±0.04 | 0.16±0.60 | SIG | 0.08±0.03 | 0.11±0.06 | NS | 0.08±0.03 | 0.16±0.05 | SIG |
| Eosinophils | 0.03±0.02 | 0.06±0.03 | NS | 0.04±0.03 | 0.10±0.07 | NS | 0.05±0.02 | 0.03±0.02 | NS | 0.05±0.03 | 0.06±0.03 | NS |
| Basophils ABS | 0.01±0.01 | 0.01±0.01 | NS | 0.01±0.00 | 0.01±0.01 | NS | 0.01±0.00 | 0.03±0.03 | NS | 0.01±0.00 | 0.01±0.01 | NS |
| Platelet count | 385.80±257.90 | 583.10±231.32 | NS | 600.30±314.00 | 745.80±203.92 | NS | 670.20±185.37 | 780.00±227.54 | NS | 674.40±285.65 | 848.30±90.65 | NS |
| Variable | 100mg/g | 500mg/kg | 1000mg/kg | Control | P-value | Decision |
| Red Cell Count | 5.92±2.24 | 6.53±1.67 | 7.30±.17 | 7.42±0.16 | 0.535 | NS |
| Haemoglobin | 11.47±4.35 | 13.94±0.56 | 12.85±3.27 | 14.39±0.27 | 0.531 | NS |
| Haematocrit | 0.39±0.15 | 0.43±0.11 | 0.44±0.11 | 0.95±1.02 | 0.287 | NS |
| MCV | 52.70±19.87 | 60.10±15.14 | 60.20±15.22 | 66.80±1.30 | 0.506 | NS |
| MCH | 15.50±5.85 | 17.40±4.40 | 17.70±4.47 | 19.50±0.51 | 0.533 | NS |
| MCHC | 18.50±7.77 | 22.20±6.67 | 22.10±6.45 | 24.30±3.89 | 0.538 | NS |
| RDW | 9.70±3.70 | 11.18±2.85 | 11.51±2.95 | 11.80±0.46 | 0.631 | NS |
| White cell count | 5.26±2.23 | 6.77±2.28 | 3.59±1.78 | 5.92±1.07 | 0.070 | NS |
| Neutrophils | 7.14±4.99 | 6.25±4.67 | 11.37±10.78 | 9.24±7.73 | 0.796 | NS |
| Lymphocytes ABS | 43.03±30.00 | 36.37±28.96 | 29.96±25.56 | 41.72±28.33 | 0.8777 | NS |
| Monocytes ABS | 0.11±0.06 | 0.16±0.06 | 0.11±0.06 | 0.16±0.5 | 0.375 | NS |
| Eosinophils | 0.06±0.03 | 0.10±0.07 | 0.03±0.02 | 0.06±0.03 | 0.088 | NS |
| Basophils ABS | 0.01±0.01 | 0.01±0.01 | 0.03±0.03 | 0.01±0.01 | 0.539 | NS |
| Platelet count | 583.10±231.32 | 745.80±203.92 | 780.00±227.54 | 848.30±90.65 | 0.190 | NS |
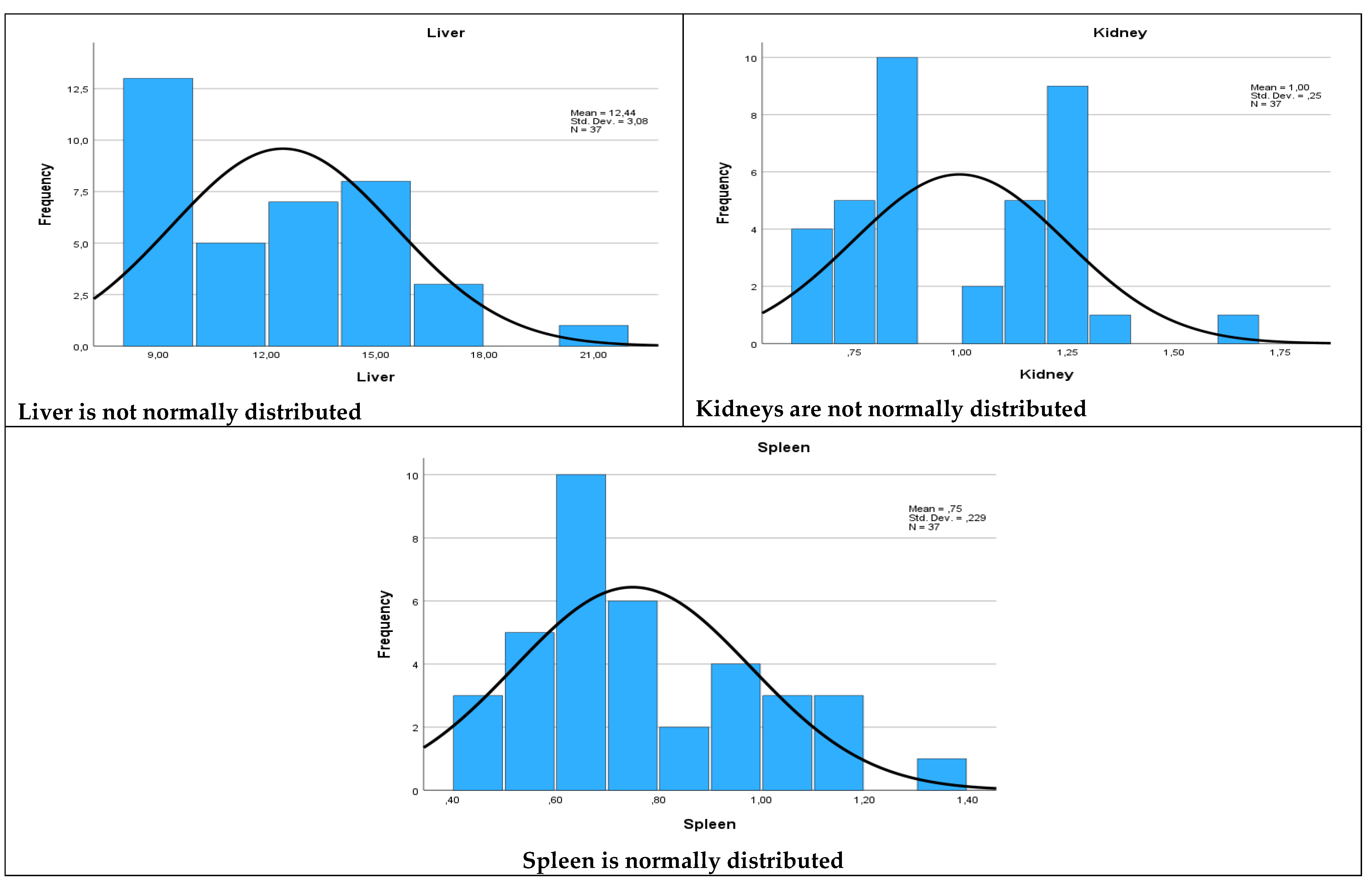
| Organ | W Statistic |
Degrees of Freedom (df) |
p- Value |
| Liver | 0.931 | 37 | 0.023 |
| Spleen | 0.948 | 37 | 0.081 |
| Kidney | 0.913 | 37 | 0.007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





