Submitted:
14 April 2023
Posted:
17 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Experimental design and growth conditions
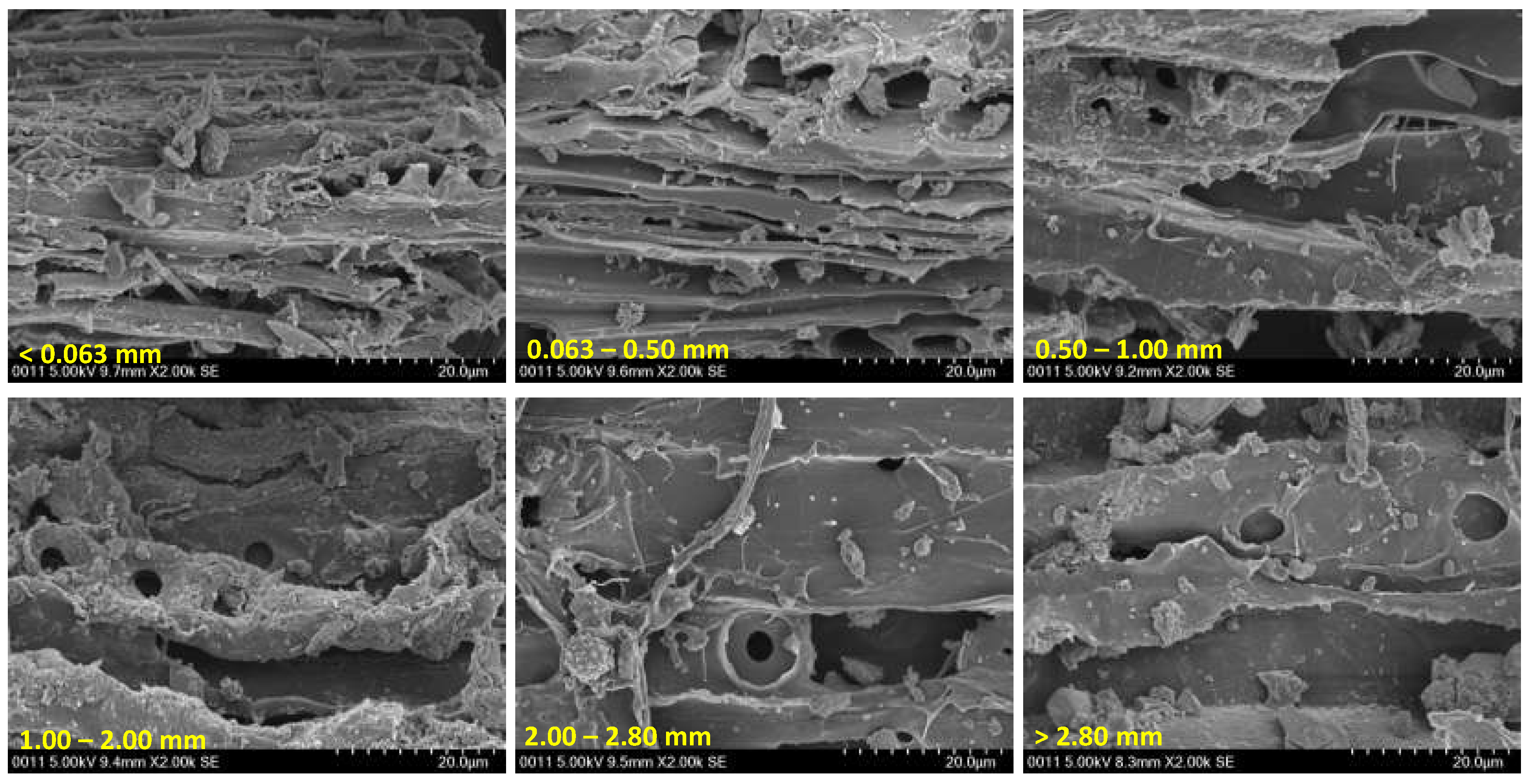
2.2. Soil and biochar characterization
2.3. Plant performance measurements
2.4. Statistical Analysis
3. Results
3.1. Biochar properties
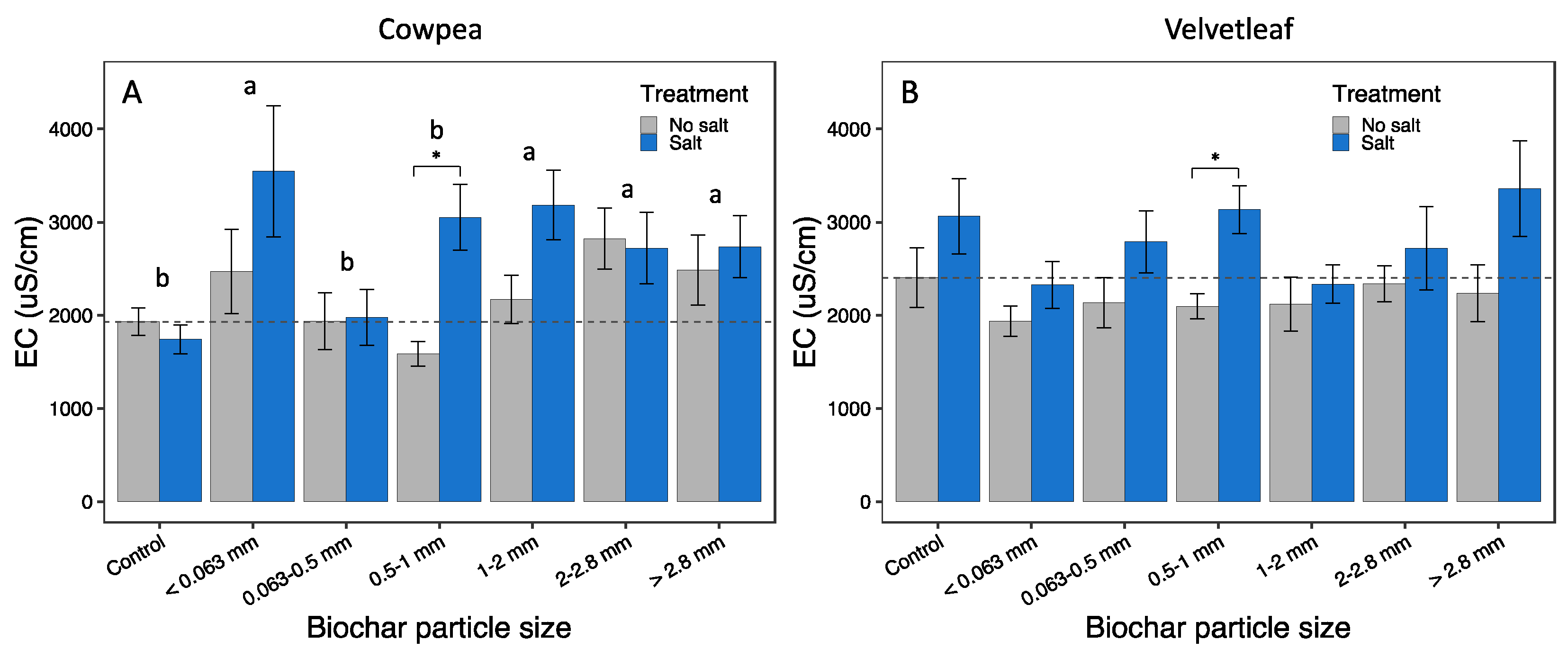
3.2. Soil properties
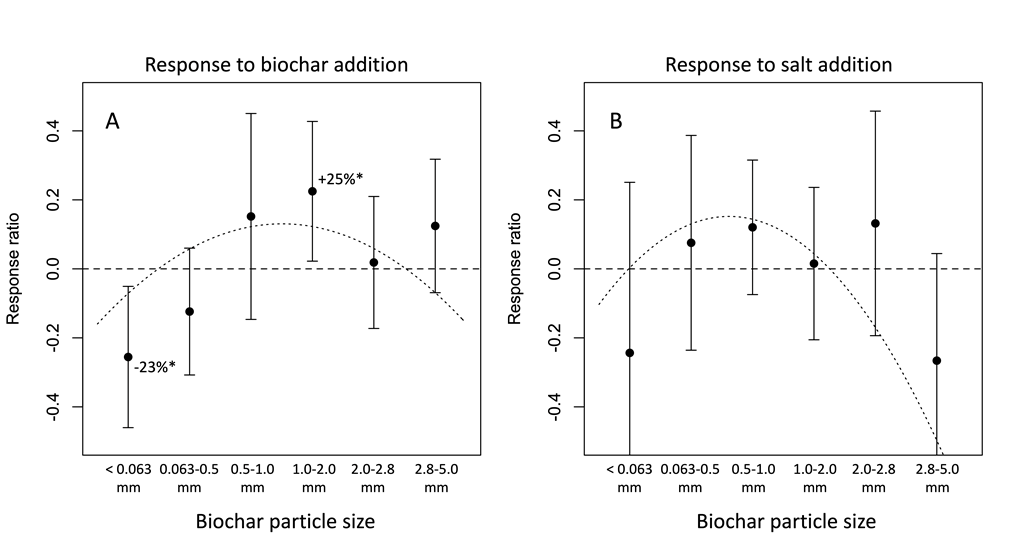
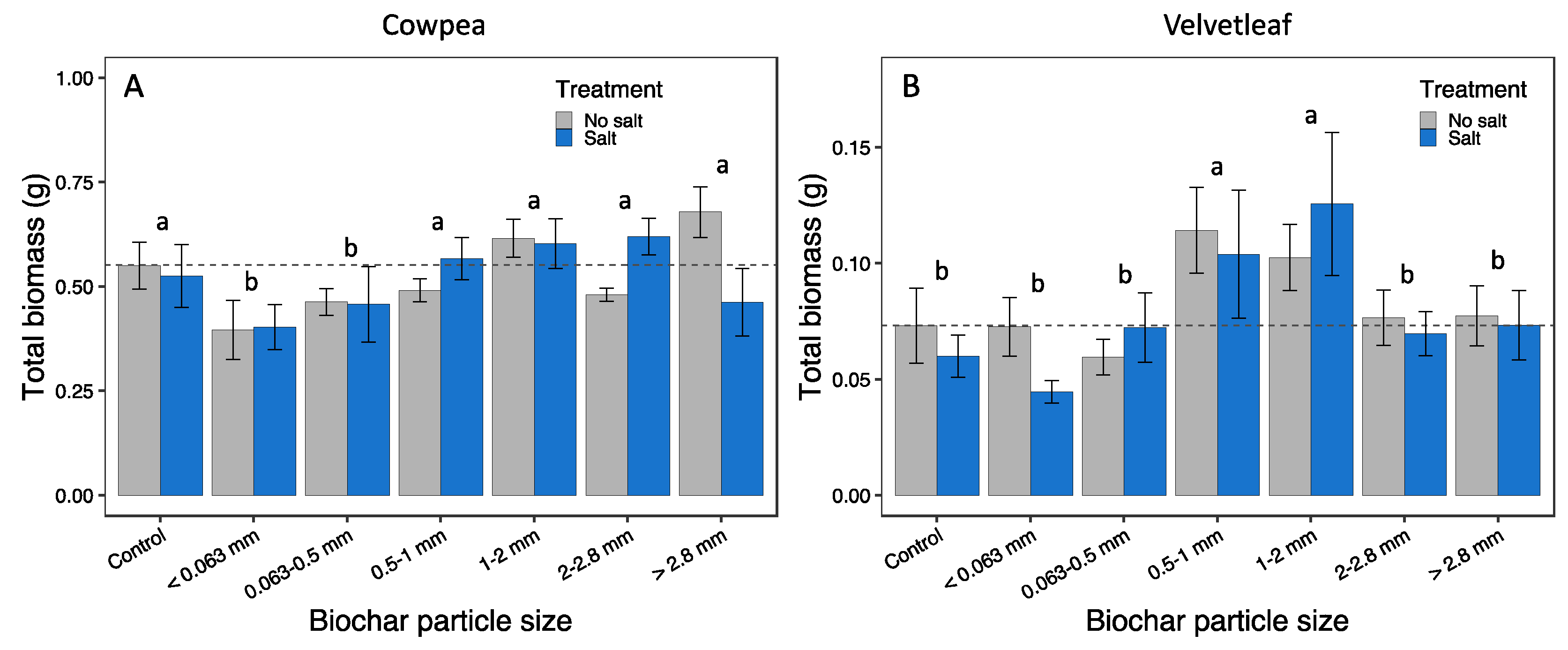
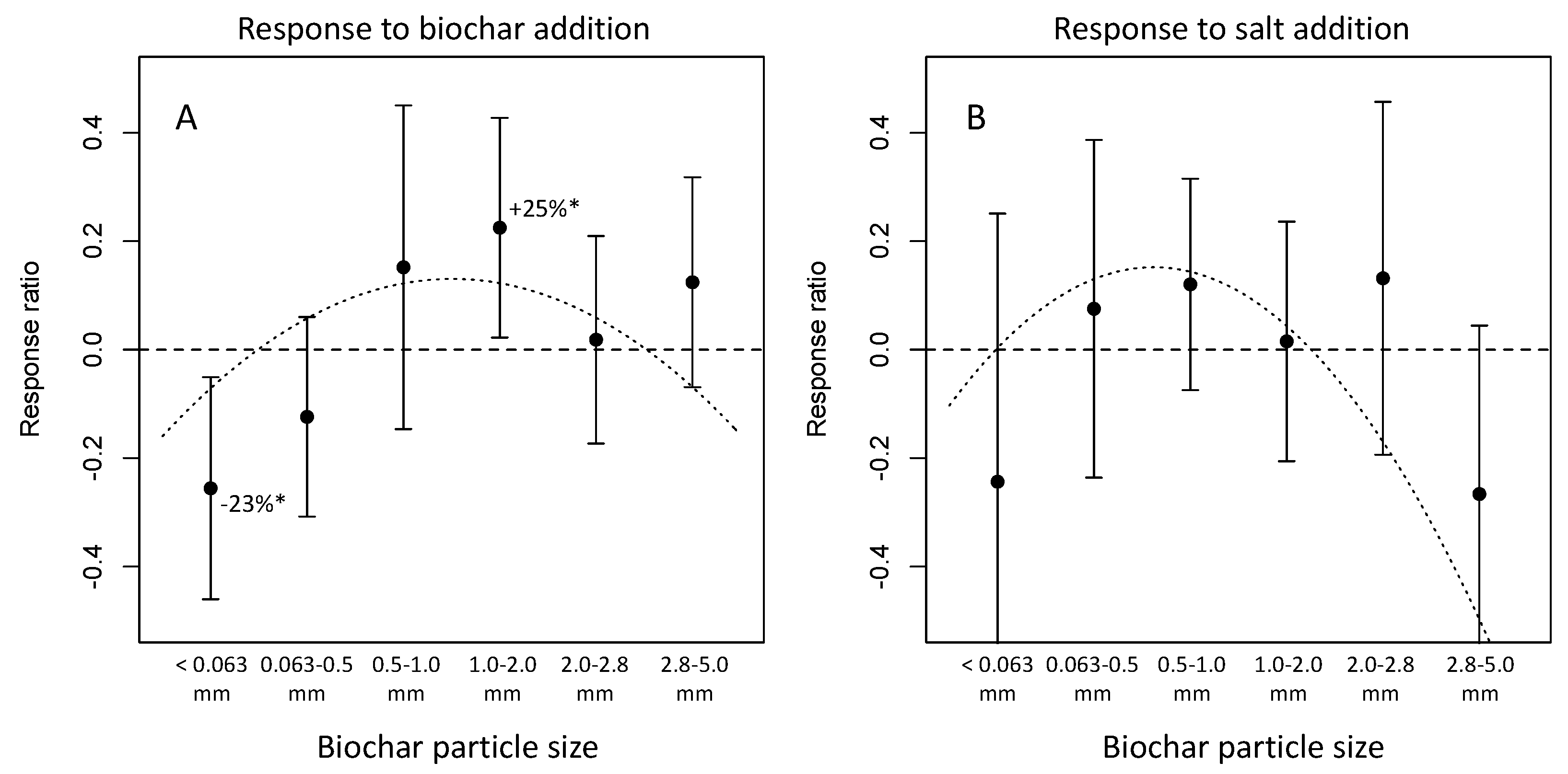
3.3. Plant growth responses
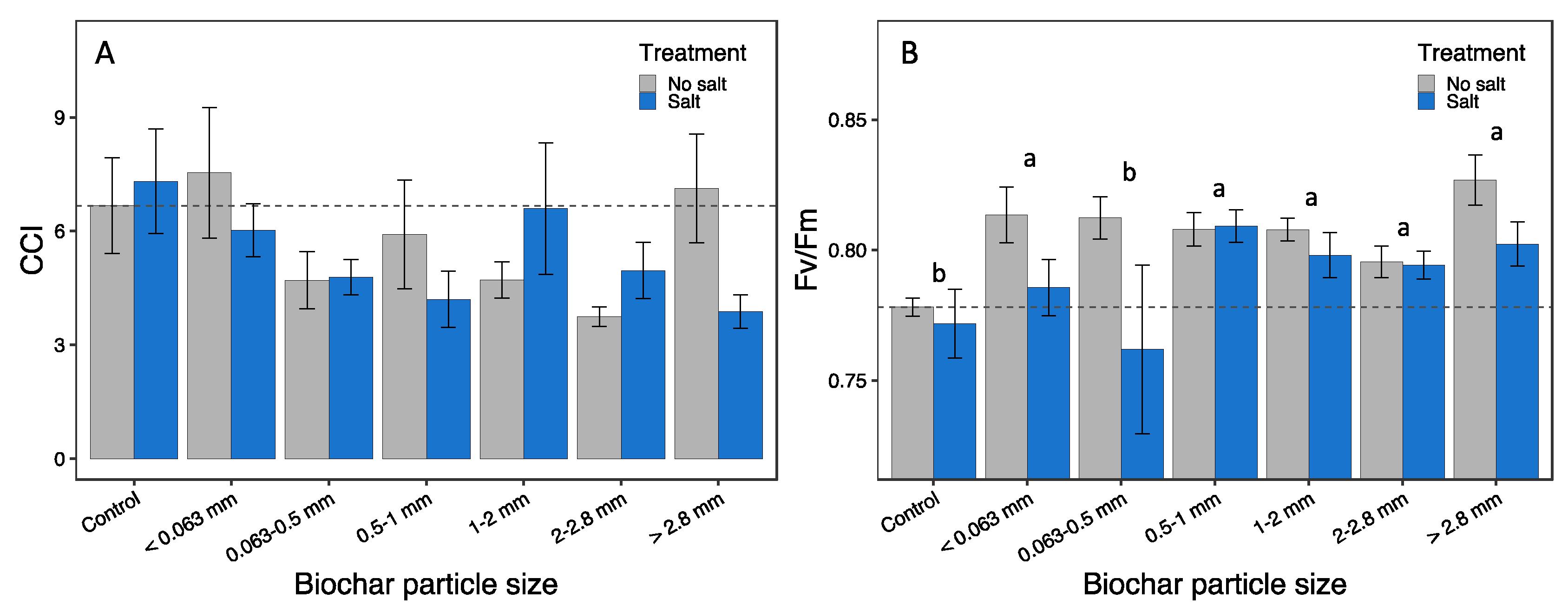
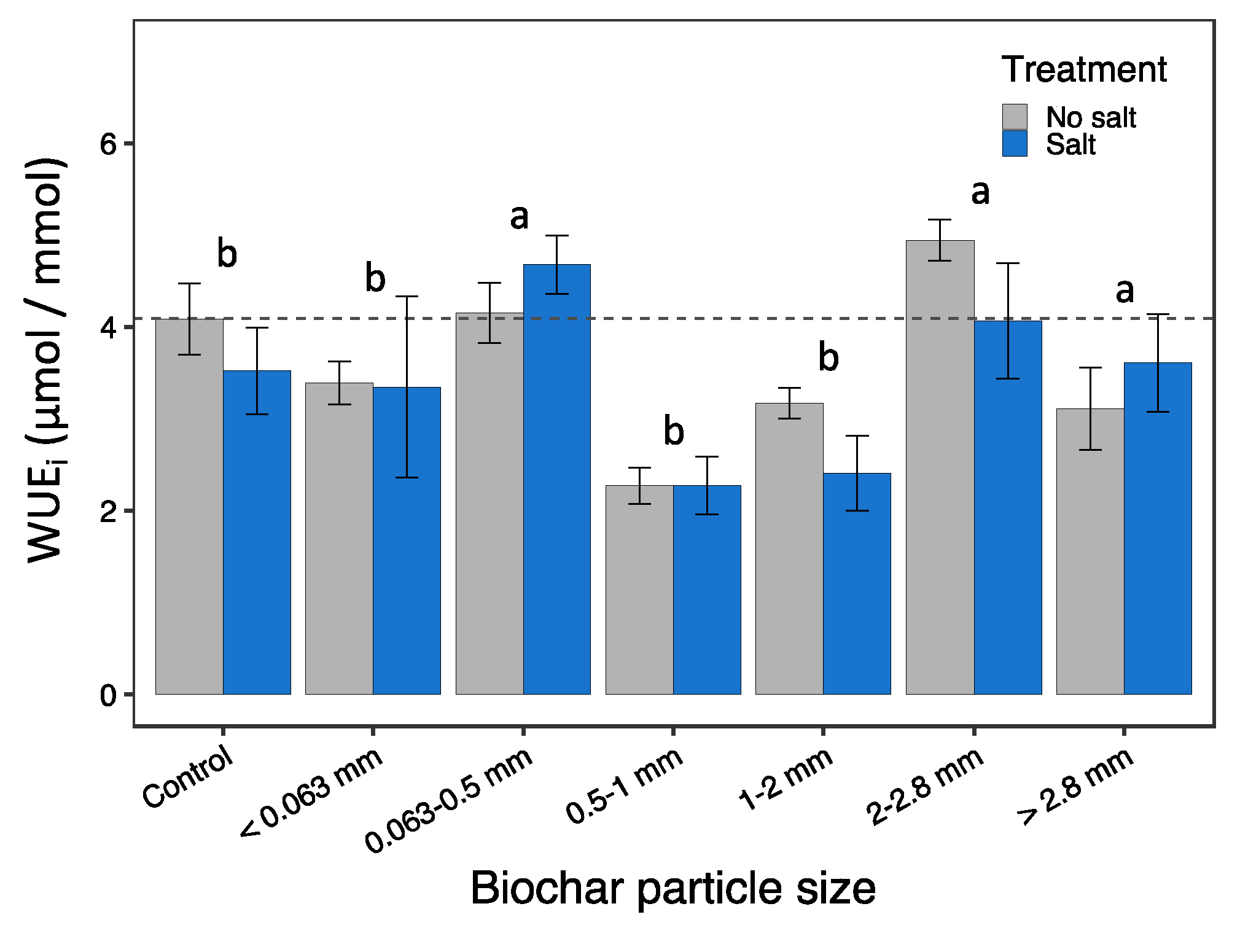
3.4. Physiological responses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, J.; Pu, L.; Han, M.; Zhu, M.; Zhang, R.; Xiang, Y. Soil salinization research in China: advances and prospects. J Geogr Sci 2014, 24, 943–960. [Google Scholar] [CrossRef]
- Shahid, S.A.; Zaman, M.; Heng, L. Soil salinity: historical perspectives and a world overview of the problem. In Guideline for salinity assessment, mitigation and adaptation using nuclear and related techniques; Cham: Springer International Publishing, 2018; pp. 43–53. [Google Scholar] [CrossRef]
- FAO Global map of salt-affected soils; Rome, Italy. 2021. Available from: https://www.fao.org/3/cb7247en/cb7247en.pdf.
- Hillel, D. Salinity management for sustainable irrigation: integrating science, environment, and economics; World Bank Publications, 2000; p. 102. [Google Scholar] [CrossRef]
- Bohn, H.; McNeal, B.; O’Conner, G. Soil chemistry; Wiley: New York, USA, 1985; pp. 217–246. [Google Scholar]
- Li, Z.; Liang, Y.; Zhou, J.; Sun, X. Impacts of de-icing salt pollution on urban road greenspace: a case study of Beijing. Front Environ Sci Eng 2014, 8, 747–756. [Google Scholar] [CrossRef]
- Kaushal, S.S. Increased salinization decreases safe drinking water. Environ Sci Technol 2016, 50, 2765–2766. [Google Scholar] [CrossRef]
- Corsi, S.R.; Graczyk, D.J.; Geis, S.W.; Booth, N.L.; Richards, K.D. A fresh look at road salt: aquatic toxicity and water-quality impacts on local, regional, and national scales. Environ Sci Technol 2010, 44, 7376–7382. [Google Scholar] [CrossRef]
- Shannon, T.P.; Ahler, S.J.; Mathers, A.; Ziter, C.D.; Dugan, H.A. Road salt impact on soil electrical conductivity across an urban landscape. J Urban Ecol 2020, 6, juaa006. [Google Scholar] [CrossRef]
- Williams, D.D.; Williams, N.E.; Cao, Y. Road salt contamination of groundwater in a major metropolitan area and development of a biological index to monitor its impact. Water Res 2000, 34, 127–138. [Google Scholar] [CrossRef]
- Jamshidi, A.; Goodarzi, A.R.; Razmara, P. Long-term impacts of road salt application on the groundwater contamination in urban environments. Environ Sci Pollut Res 2020, 27, 30162–30177. [Google Scholar] [CrossRef] [PubMed]
- Green, S.M.; Machin, R.; Cresser, M.S. Effect of long-term changes in soil chemistry induced by road salt applications on N-transformations in roadside soils. Environ Pollut 2008, 152, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Van Meter, R.J.; Swan, C.M.; Leips, J.; Snodgrass, J.W. Road salt stress induces novel food web structure and interactions. Wetlands 2011, 31, 843–851. [Google Scholar] [CrossRef]
- Tiwari, A.; Rachlin, J.W. A review of road salt ecological impacts. Northeast Nat 2018, 25, 123–142. [Google Scholar] [CrossRef]
- Miklovic, S.; Galatowitsch, S.M. Effect of NaCl and Typha angustifolia L. on marsh community establishment: a greenhouse study. Wetlands 2005, 25, 420–429. [Google Scholar] [CrossRef]
- Mineau, P.; Brownlee, L.J. Road salts and birds: an assessment of the risk with particular emphasis on winter finch mortality. Wildl Soc Bull 2005, 33, 835–841. [Google Scholar] [CrossRef]
- Dirr, M. Selection of trees for tolerance to salt injury. Arboric Urban For 1976, 2, 209–216. https://www.nswooa.ca/uploads/5/9/6/9/59690537/salt-tolerant-trees.pdf. [CrossRef]
- Geilfus, C. Chloride: from nutrient to toxicant. Plant Cell Physiol 2018, 59, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Dmuchowski, W.; Baczewska-Dąbrowska, A.; Gozdowski, D.; Brągoszewska, P.; Gworek, B.; Suwara, I.; Chojnaki, T.; Jóźwiak, A.; Swiezewska, E. Effect of salt stress in urban conditions on two Acer species with different sensitivity. PeerJ 2021, 9, e10577. [Google Scholar] [CrossRef] [PubMed]
- Tavakkoli, E.; Rengasamy, P.; McDonald, G.K. High concentrations of Na+ and Cl– ions in soil solution have simultaneous detrimental effects on growth of faba bean under salinity stress. J Exp Bot 2010, 61, 4449–4459. [Google Scholar] [CrossRef]
- Terry, L.G.; Conaway, K.; Rebar, J.; Graettinger, A.J. Alternative deicers for winter road maintenance—a review. Water Air Soil Pollut 2020, 231, 394. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science, Technology and Implementation; Routledge: Oxon, USA, 2015; https://www.taylorfrancis.com/books/9781134489534.
- Atkinson, C.J.; Fitzgerald, J.D.; Hipps, N.A. Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: a review. Plant Soil 2010, 337, 1–18. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, A.; Ji, C.; Joseph, S.; Bian, R.; Li, L.; Pan, G.; Paz-Ferreiro, J. Biochar’s effect on crop productivity and the dependence on experimental conditions—a meta-analysis of literature data. Plant Soil 2013, 373, 583–594. [Google Scholar] [CrossRef]
- Woolf, D.; Amonette, J.E.; Street-Perrott, F.A.; Lehmann, J.; Joseph, S. Sustainable biochar to mitigate global climate change. Nat Commun 2010, 1, 56. [Google Scholar] [CrossRef]
- Smith, P. Soil carbon sequestration and biochar as negative emission technologies. Glob Change Biol 2016, 22, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.C.; Gale, N. Biochar and forest restoration: a review and meta-analysis of tree growth responses. New For 2015, 46, 931–946. [Google Scholar] [CrossRef]
- Inyang, M.; Dickenson, E. The potential role of biochar in the removal of organic and microbial contaminants from potable and reuse water: A review. Chemosphere 2015, 134, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, H.; He, L.; Lu, K.; Sarmah, A.; Li, J.; Bolan, N.S.; Pei, J.; Huang, H. Using biochar for remediation of soils contaminated with heavy metals and organic pollutants. Environ Sci Pollut Res 2013, 20, 8472–8483. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, D.; Peng, T.; Zhang, J.; Tsang, D.C.W.; Alessi, D.S.; Shen, Z.; Bolan, N.S.; Hou, D. Biochar application for the remediation of heavy metal polluted land: a review of in situ field trials. Sci Total Environ 2018, 619–620, 815–826. [Google Scholar] [CrossRef]
- Thomas, S.C.; Frye, S.; Gale, N.; Garmon, M.; Launchbury, R.; Machado, N.; Melamed, S.; Murray, J.; Petroff, A. Biochar mitigates negative effects of salt additions on two herbaceous plant species. J Environ Manage 2013, 129, 62–68. [Google Scholar] [CrossRef]
- Gunarathne, V.; Senadeera, A.; Gunarathne, U.; Biswas, J.K.; Almaroai, Y.A.; Vithanage, M. Potential of biochar and organic amendments for reclamation of coastal acidic-salt affected soil. Biochar 2020, 2, 107–120. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Andersen, M.N.; Liu, F. Biochar mitigates salinity stress in potato. J Agron Crop Sci 2015, 201, 368–378. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Andersen, M.N.; Liu, F. Residual effects of biochar on improving growth, physiology and yield of wheat under salt stress. Agric Water Manag 2015, 158, 61–68. [Google Scholar] [CrossRef]
- Liang, B.; Lehmann, J.; Solomon, D.; Kinyangi, J.; Grossman, J.; O’Neill, B.; Skjemstad, J.O.; Luizão, F.J.; Petersen, J.; Naves, F.G. Black carbon increases cation exchange capacity in soils. Soil Sci Soc Am J 2006, 70, 1719–1730. [Google Scholar] [CrossRef]
- Karhu, K.; Mattila, T.; Bergström, I.; Regina, K. Biochar addition to agricultural soil increased CH4 uptake and water holding capacity – results from a short-term pilot field study. Agric Ecosyst Environ. 2011, 140, 309–313. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Cui, L.; Kammann, C.; Wrage-Mönnig, N.; Estavillo, J.M.; Fuertes-Mendizabal, T.; Cayuela, M.L.; Sigua, G.; Novak, J.; Spokas, K. Feedstock choice, pyrolysis temperature and type influence biochar characteristics: a comprehensive meta-data analysis review. Biochar 2020, 2, 421–438. [Google Scholar] [CrossRef]
- Thomas, S.C. Post-processing of biochars to enhance plant growth responses: a review and meta-analysis. Biochar 2021, 3, 437–455. [Google Scholar] [CrossRef]
- Sangani, M.; Abrishamkesh, S.; Owens, G. Physicochemical characteristics of biochars can be beneficially manipulated using post-pyrolyzed particle size modification. Bioresour Technol 2020, 306, 123157. [Google Scholar] [CrossRef]
- Liao, W.; Thomas, S.C. Biochar particle size and post-pyrolysis mechanical processing affect soil pH, water retention capacity, and plant performance. Soil Syst 2019, 3, 14. [Google Scholar] [CrossRef]
- Gale, N.V.; Sackett, T.E.; Thomas, S.C. Thermal treatment and leaching of biochar alleviates plant growth inhibition from mobile organic compounds. PeerJ 2016, 4, e2385. [Google Scholar] [CrossRef] [PubMed]
- Blatt-Janmaat, K.L.; MacQuarrie, S.L.; Sit, C.S. Does size matter? An investigation into the impact of coarse and fine ground inoculated biochar on Hordeum vulgare (barley) growth and yield. Rhizosphere 2020, 13, 100184. [Google Scholar] [CrossRef]
- Lim, T.J.; Spokas, K.A.; Feyereisen, G.W.; Weis, R.; Koskinen, W.C. Influence of biochar particle size and shape on soil hydraulic properties. J Environ Sci Eng Technol 2017, 5, 8–15. https://www.ars.usda.gov/ARSUserFiles/39868/Updates/2017_Lim_InfluenceofBiochar.pdf. [CrossRef]
- Liu, Z.; Dugan, B.; Masiello, C.A.; Gonnermann, H.M. Biochar particle size, shape, and porosity act together to influence soil water properties. PLoSone 2017, 12, e0179079. [Google Scholar] [CrossRef]
- Ehlers, J.D.; Hall, A.E. Cowpea (Vigna unguiculata L. Walp.). Field Crops Res 1997, 53, 187–204. [Google Scholar] [CrossRef]
- Spencer, N.R. Velvetleaf, Abutilon theophrasti (malvaceae), history and economic impact in the United States. Econ Bot 1984, 38, 407–16. [Google Scholar] [CrossRef]
- Denyes, M.; Matovic, D.; Zeeb, B.; Rutter, A. Report on the production and characterization of biochar produced at Burt’s Greenhouses (Odessa, ON, Canada). 2013. http://burtsgh.com/wpr/wp-content/uploads/2013/12/Report_on_the_Production_and_Characterization_Biochar_Produced_at_BurtsGreenhouses_Final_O1.pdf.
- Gale, N.V.; Thomas, S.C. Dose-dependence of growth and ecophysiological responses of plants to biochar. Sci Total Environ 2019, 658, 1344–1354. [Google Scholar] [CrossRef] [PubMed]
- Kuttner, B.G.; Thomas, S.C. Interactive effects of biochar and an organic dust suppressant for revegetation and erosion control with herbaceous seed mixtures and willow cuttings: biochar, erosion control, and revegetation. Restor Ecol 2017, 25, 367–375. [Google Scholar] [CrossRef]
- Williams, J.M.; Thomas, S.C. Effects of high-carbon wood ash biochar on volunteer vegetation establishment and community composition on metal mine tailings. Restor Ecol 2023, e13861. [Google Scholar] [CrossRef]
- ASTM D1762-84; ASTM Standard test method for chemical analysis of wood charcoal. ASTM International: West Conshohocken, PA, 2007.
- Scott, A.J.; Knott, M. A cluster analysis method for grouping means in the analysis of variance. Biometrics 1974, 30, 507. [Google Scholar] [CrossRef]
- Jelihovschi, E.; Faria, J.C.; Allaman, I.B. ScottKnott: A package for performing the Scott-Knott clustering algorithm in R. TEMA São Carlos 2014, 15, 003. [Google Scholar] [CrossRef]
- Viechtbauer, W. Conducting meta-analyses in R with the metafor Package. J Stat Softw 2010, 36, 3. [Google Scholar] [CrossRef]
- Prodana M, Silva C, Gravato C, Verheijen FGA, Keizer JJ, Soares AMVM, Loureriro S, Bastos AC. Influence of biochar particle size on biota responses. Ecotoxicol Environ Saf 2019, 174, 120–128. [Google Scholar] [CrossRef]
- He, M.; Xiong, X.; Wang, L.; Hou, D.; Bolan, N.S.; Ok, Y.S.; Rinklebe, J.; Tsang, D.C.W. A critical review on performance indicators for evaluating soil biota and soil health of biochar-amended soils. J Hazard Mater 2021, 414, 125378. [Google Scholar] [CrossRef]
- Zheng, R.; Li, C.; Sun, G.; Xie, Z.; Chen, J.; Wu, J.; Wang, Q. The influence of particle size and feedstock of biochar on the accumulation of Cd, Zn, Pb, and As by Brassica chinensis L. Environ Sci Pollut Res 2017, 24, 22340–22352. [Google Scholar] [CrossRef]
- Albert, H.A.; Li, X.; Jeyakumar, P.; Wei, L.; Huang, L.; Huang, Q.; Kamran, M.; Shaheen, S.M.; Hou, D.; Rinklebe, J.; Lin, Z.; Wang, H. Influence of biochar and soil properties on soil and plant tissue concentrations of Cd and Pb: a meta-analysis. Sci Total Environ 2021, 755, 142582. [Google Scholar] [CrossRef] [PubMed]
- de Jesus Duarte, S.; Glaser, B.; Pellegrino Cerri, C. Effect of biochar particle size on physical, hydrological and chemical properties of loamy and sandy tropical soils. Agronomy 2019, 9, 165. [Google Scholar] [CrossRef]
- Werdin, J.; Conn, R.; Fletcher, T.D.; Rayner, J.P.; Williams, N.S.G.; Farrell, C. Biochar particle size and amendment rate are more important for water retention and weight of green roof substrates than differences in feedstock type. Ecol Eng 2021, 171, 106391. [Google Scholar] [CrossRef]
- Lebrun, M.; Miard, F.; Nandillon, R.; Hattab-Hambli, N.; Scippa, G.S.; Bourgerie, S.; Morabito, D. Eco-restoration of a mine technosol according to biochar particle size and dose application: study of soil physico-chemical properties and phytostabilization capacities of Salix viminalis. J Soils Sediments 2018, 18, 2188–2202. [Google Scholar] [CrossRef]
- Billah, M.M.; Ahmad, W.; Ali, M. Biochar particle size and Rhizobia strains effect on the uptake and efficiency of nitrogen in lentils. J Plant Nutr 2019, 42, 1709–1725. [Google Scholar] [CrossRef]
- Edeh, I.G.; Mašek, O.; Buss, W. A meta-analysis on biochar’s effects on soil water properties – new insights and future research challenges. Sci Total Environ 2020, 714, 136857. [Google Scholar] [CrossRef]
- He, P.; Liu, Y.; Shao, L.; Zhang, H.; Lü, F. Particle size dependence of the physicochemical properties of biochar. Chemosphere 2018, 212, 385–392. [Google Scholar] [CrossRef]
- Zhang, J.; Amonette, J.E.; Flury, M. Effect of biochar and biochar particle size on plant-available water of sand, silt loam, and clay soil. Soil Tillage Res 2021, 212, 104992. [Google Scholar] [CrossRef]
- Edeh, I.G.; Mašek, O. The role of biochar particle size and hydrophobicity in improving soil hydraulic properties. Eur J Soil Sci 2022, 73, e13138. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, G.; Shao, H.B. Furfural and its biochar improve the general properties of a saline soil. Solid Earth 2014, 5, 665–671. [Google Scholar] [CrossRef]
- Abbas, T.; Rizwan, M.; Ali, S.; Adrees, M.; Zia-ur-Rehman, M.; Qayyum, M.F.; Ok, Y.S.; Murtaza, G. Effect of biochar on alleviation of cadmium toxicity in wheat (Triticum aestivum L.) grown on Cd-contaminated saline soil. Environ Sci Pollut Res 2018, 25, 25668–25680. [Google Scholar] [CrossRef] [PubMed]
- She, D.; Sun, X.; Gamareldawla, A.H.D.; Nazar, E.A.; Hu, W.; Edith, K.; Yu, S. Benefits of soil biochar amendments to tomato growth under saline water irrigation. Sci Rep 2018, 8, 14743. [Google Scholar] [CrossRef] [PubMed]
- Kargar, M.; Clark, O.G.; Hendershot, W.H.; Jutras, P.; Prasher, S.O. Immobilization of trace metals in contaminated urban soil amended with compost and biochar. Water Air Soil Pollut 2015, 226, 191. [Google Scholar] [CrossRef]
- Shen, Y.; Song, S.; Thian, B.W.Y.; Fong, S.L.; Ee, A.W.L.; Arora, S.; Ghosh, S.; Li, S.F.Y.; Tan, H.T.W.; Dai, Y.; Wang, C. Impacts of biochar concentration on the growth performance of a leafy vegetable in a tropical city and its global warming potential. J Clean Prod 2020, 264, 121678. [Google Scholar] [CrossRef]
- Sifton, M.A.; Lim, P.; Smith, S.M.; Thomas, S.C. Interactive effects of biochar and N-fixing companion plants on growth and physiology of Acer saccharinum. Urban For Urban Green 2022, 74, 127652. [Google Scholar] [CrossRef]
- Scharenbroch, B.C.; Meza, E.N.; Catania, M.; Fite, K. Biochar and biosolids increase tree growth and improve soil quality for urban landscapes. J Environ Qual 2013, 42, 1372–1385. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.; Cowie, A.L.; Van Zwieten, L.; Bolan, N.; Budai, A.; Buss, W.; Cayuela, M.L.; Grber, E.R.; Ippolito, J.A.; Kuzyakov, Y.; Luo, Y.; Ok, Y.S.; Palansooriya, K.N.; Shephard, J.; Stephens, S.; Weng, Z.; Lehmann, J. How biochar works, and when it doesn’t: a review of mechanisms controlling soil and plant responses to biochar. GCB Bioenergy 2021, 13, 1731–1764. [Google Scholar] [CrossRef]
- Rumpel, C.; Leifeld, F.; Santin, C.; Doerr, S. Movement of biochar in the environment. In Biochar for environmental management science, technology and implementation; Routledge: Oxon, USA, 2015; pp. 283–299. [Google Scholar]
- Gelardi, D.L.; Li, C.; Parikh, S.J. An emerging environmental concern: biochar-induced dust emissions and their potentially toxic properties. Sci Total Environ 2019, 678, 813–820. [Google Scholar] [CrossRef]
- Ravi, S.; Sharratt, B.S.; Li, J.; Olshevski, S.; Meng, Z.; Zhang, J. Particulate matter emissions from biochar-amended soils as a potential tradeoff to the negative emission potential. Sci Rep 2016, 6, 35984. [Google Scholar] [CrossRef]
| Biochar size (mm) | |||||||
|---|---|---|---|---|---|---|---|
| Size category: | 0 | 1 | 2 | 3 | 4 | 5 | |
| Attribute | < 0.063 | 0.063-0.50 | 0.50-1.00 | 1.00-2.00 | 2.00-2.80 | > 2.8 | Soil |
| pH | 9.1 (0.04) | 9.0 (0.03) | 9.0 (0.01) | 9.0 (0.02) | 9.2 (0.02) | 9.5 (0.05) | 7.5 |
| EC (mS/cm) | 1.70 (0.01) | 1.47 (0.03) | 1.21 (0.02) | 0.83 (0.00) | 0.96 (0.06) | 0.89 (0.04) | 1.00 (0.04) |
| Bulk density (g/cm3) | 0.31 (0.01) | 0.25 (0.01) | 0.17 (0.01) | 0.14 (0.00) | 0.11 (0.00) | 0.12 (0.00) | 0.44 (0.01) |
| Tap density (g/cm3) | 0.38 (0.01) | 0.33 (0.01) | 0.20 (0.00) | 0.15 (0.01) | 0.13 (0.00) | 0.14 (0.01) | 0.49 (0.03) |
| Compression ratio | 1.23 (0.08) | 1.33 (0.04) | 1.17 (0.03) | 1.07 (0.01) | 1.23 (0.04) | 1.23 (0.01) | 1.13 (0.04) |
| Biochar size | Salt | Size x Salt | Scott-Knott clusters* | ||||
|---|---|---|---|---|---|---|---|
| Attribute | F | p | F | p | F | p | (for biochar size) |
| Cowpea | |||||||
| Soil pH | 5.63 | <0.001*** | 0.09 | 0.760 | 1.09 | 0.370 | (c,1,3) (0,2,4,5) |
| Soil EC (µS/cm) | 2.84 | 0.015 | 7.06 | 0.009 | 1.69 | 0.134 | (c,1,2) (0,3,4,5) |
| Early leaf area (cm2) | 3.11 | 0.004** | 0.22 | 0.612 | 2.83 | 0.015 | (c,0,1,2) (3,4,5) |
| Total biomass (g) | 3.03 | 0.010** | 0.02 | 0.871 | 1.84 | 0.101 | (0,1) (c,2-5) |
| Aboveground biomass (g) | 3.07 | 0.009** | 0.01 | 0.918 | 1.49 | 0.191 | (0,1) (c,2-5) |
| Belowground biomass (g) | 2.62 | 0.022* | 0.52 | 0.471 | 2.56 | 0.025 | (0,1,5) (c,2-4) |
| Root fraction | 2.47 | 0.030* | 0.93 | 0.337 | 1.22 | 0.306 | (5) (c,0-4) |
| Final leaf area (cm2) | 1.46 | 0.204 | 0.00 | 0.978 | 0.87 | 0.522 | - |
| LMA (g/cm2) | 1.32 | 0.255 | 1.03 | 0.313 | 1.14 | 0.348 | - |
| CCI | 1.67 | 0.140 | 0.46 | 0.499 | 1.46 | 0.201 | - |
| Fv/Fm | 2.52 | 0.027 | 7.49 | 0.008 | 1.25 | 0.289 | (c,1) (0,2-5) |
| Amax (µmol m-2 s-1) | 0.29 | 0.939 | 0.59 | 0.445 | 0.235 | 0.964 | - |
| gs (mmol m-2 s-1) | 0.55 | 0.772 | 0.19 | 0.667 | 0.56 | 0.758 | - |
| WUEi | 2.87 | 0.015 | 1.72 | 0.194 | 0.47 | 0.827 | (c,0,2,3) (1,4,5) |
| Velvetleaf | |||||||
| Soil pH | 2.61 | 0.027* | 1.64 | 0.203 | 1.11 | 0.364 | (c,1,4,5) (0,2,3) |
| Soil EC (µS/cm) | 1.28 | 0.275 | 14.95 | <0.001 | 0.59 | 0.740 | - |
| Early leaf area (cm2) | 1.04 | 0.408 | 0.29 | 0.593 | 0.67 | 0.672 | - |
| Total biomass (g) | 3.89 | 0.002** | 0.29 | 0.591 | 0.60 | 0.727 | (c,0,1,4,5) (2,3) |
| Aboveground biomass (g) | 3.97 | 0.002** | 0.26 | 0.610 | 0.60 | 0.730 | (c,0,1,4,5) (2,3) |
| Belowground biomass (g) | 3.44 | 0.004** | 0.36 | 0.548 | 0.62 | 0.714 | (c,0,1,4,5) (2,3) |
| Root fraction | 3.81 | 0.002** | 0.745 | 0.391 | 0.67 | 0.676 | (1-5) (c,0) |
| Final leaf area (cm2) | 3.15 | 0.008** | 2.19 | 0.143 | 0.73 | 0.627 | (c,0,1,4,5) (2,3) |
| LMA (g/cm2) | 1.83 | 0.103 | 1.57 | 0.214 | 1.59 | 0.161 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





