Submitted:
17 April 2023
Posted:
18 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
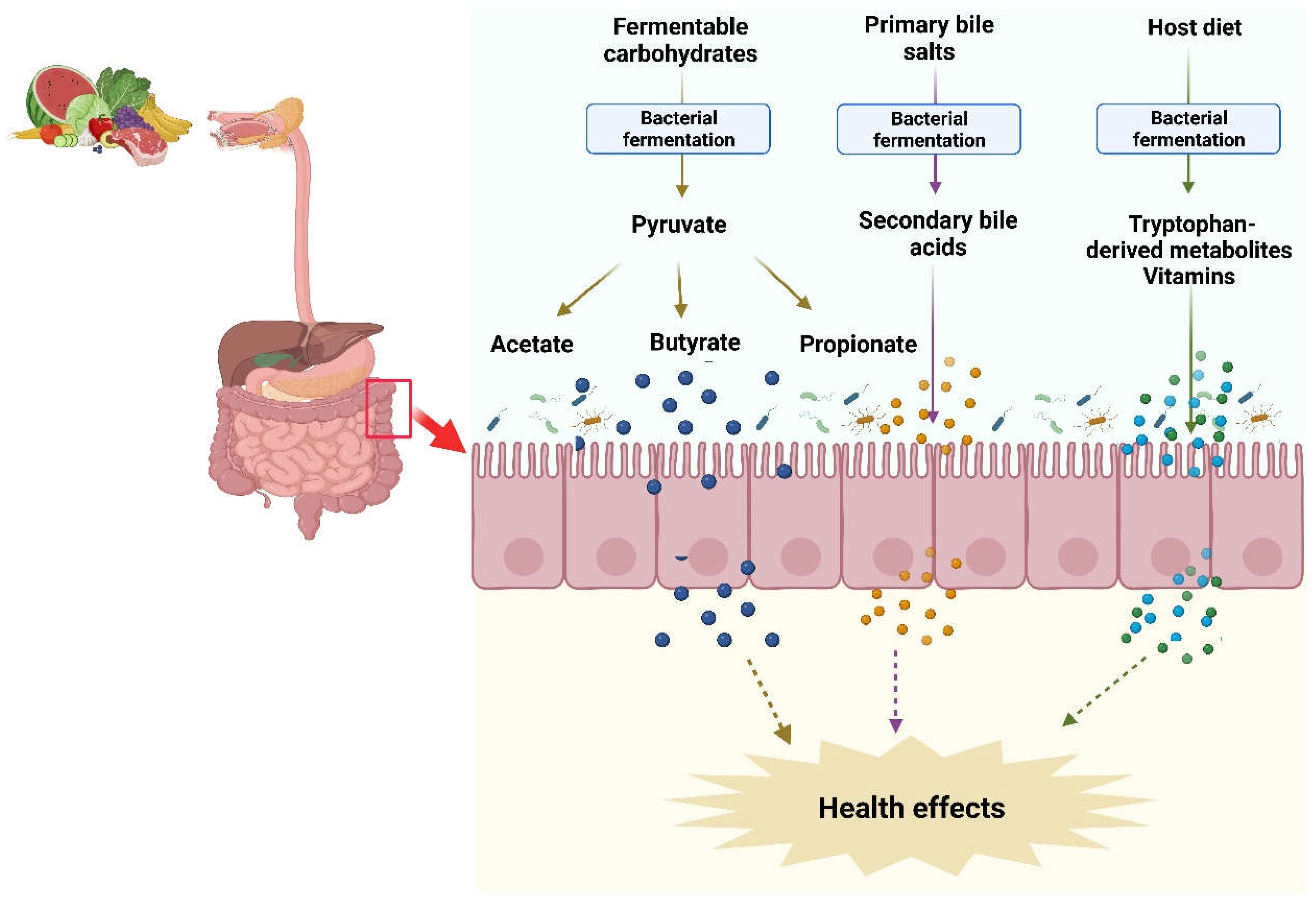
2. Gut microbiota and metabolites
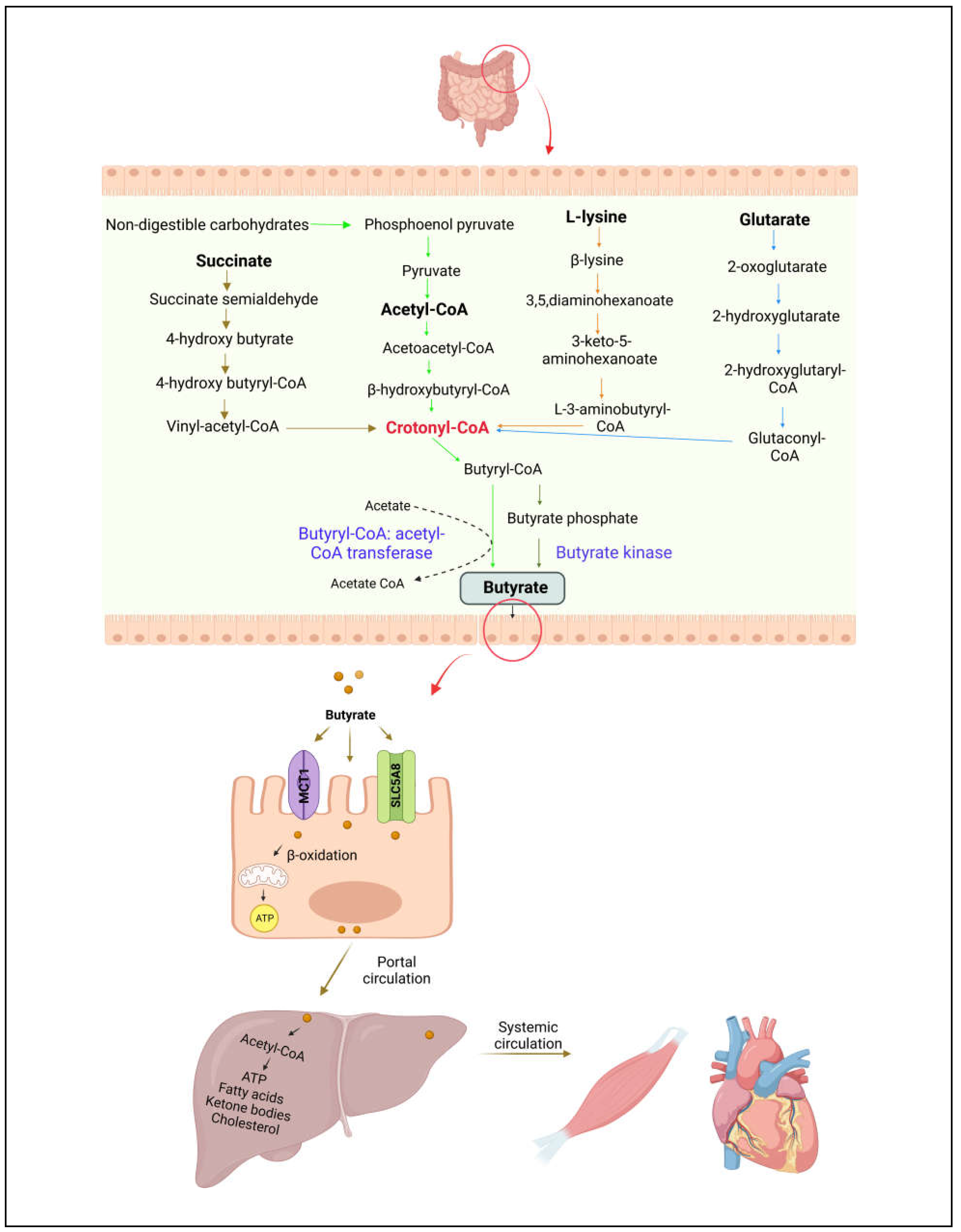
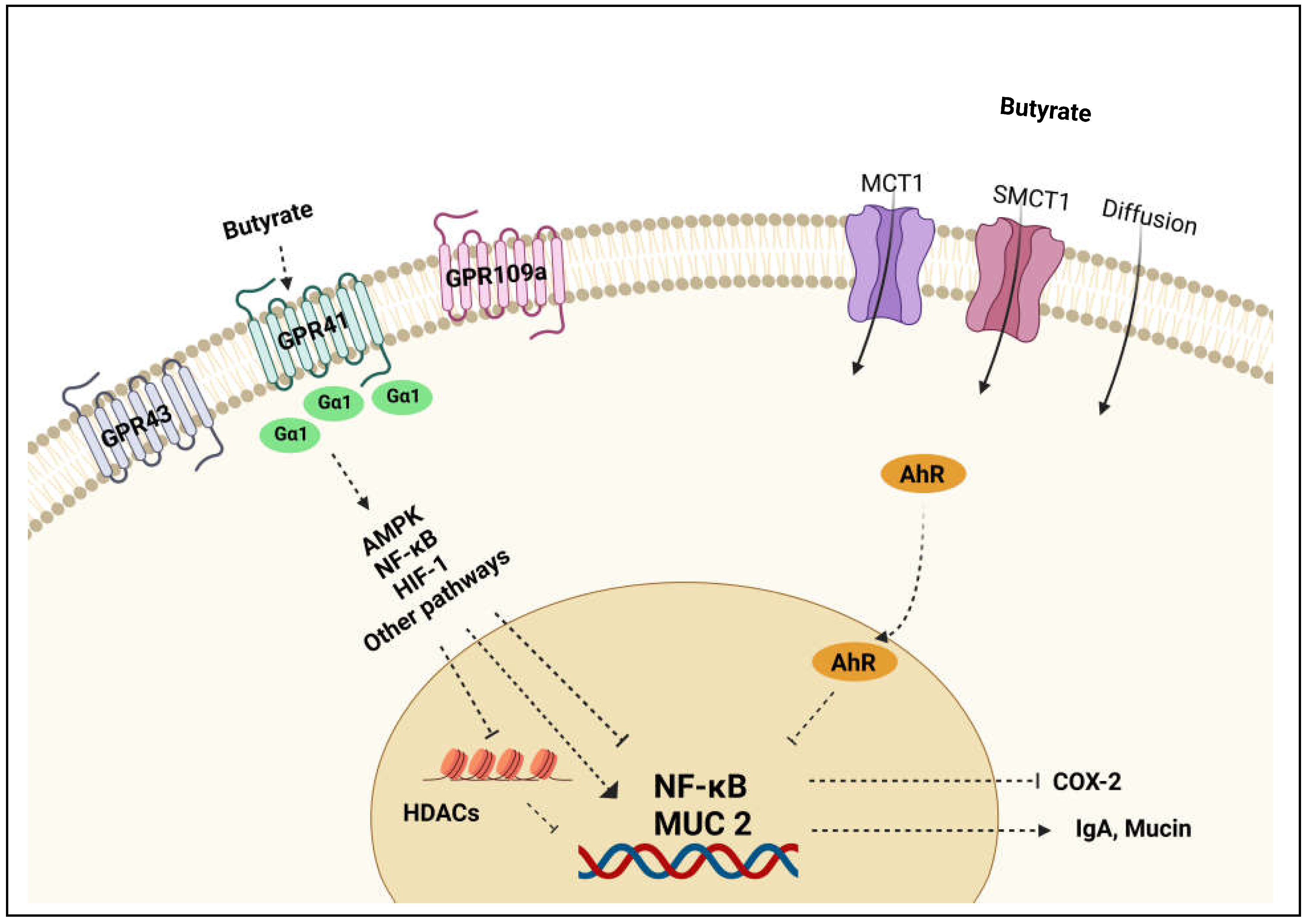
3. Butyrate production, absorption, and metabolism
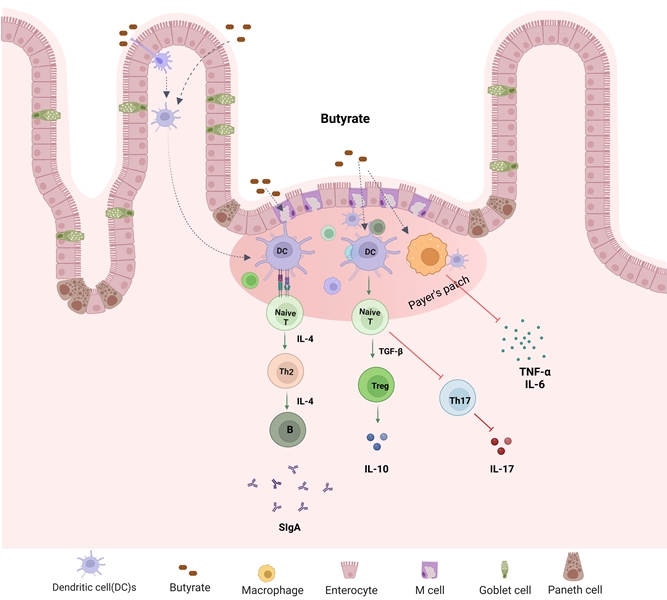
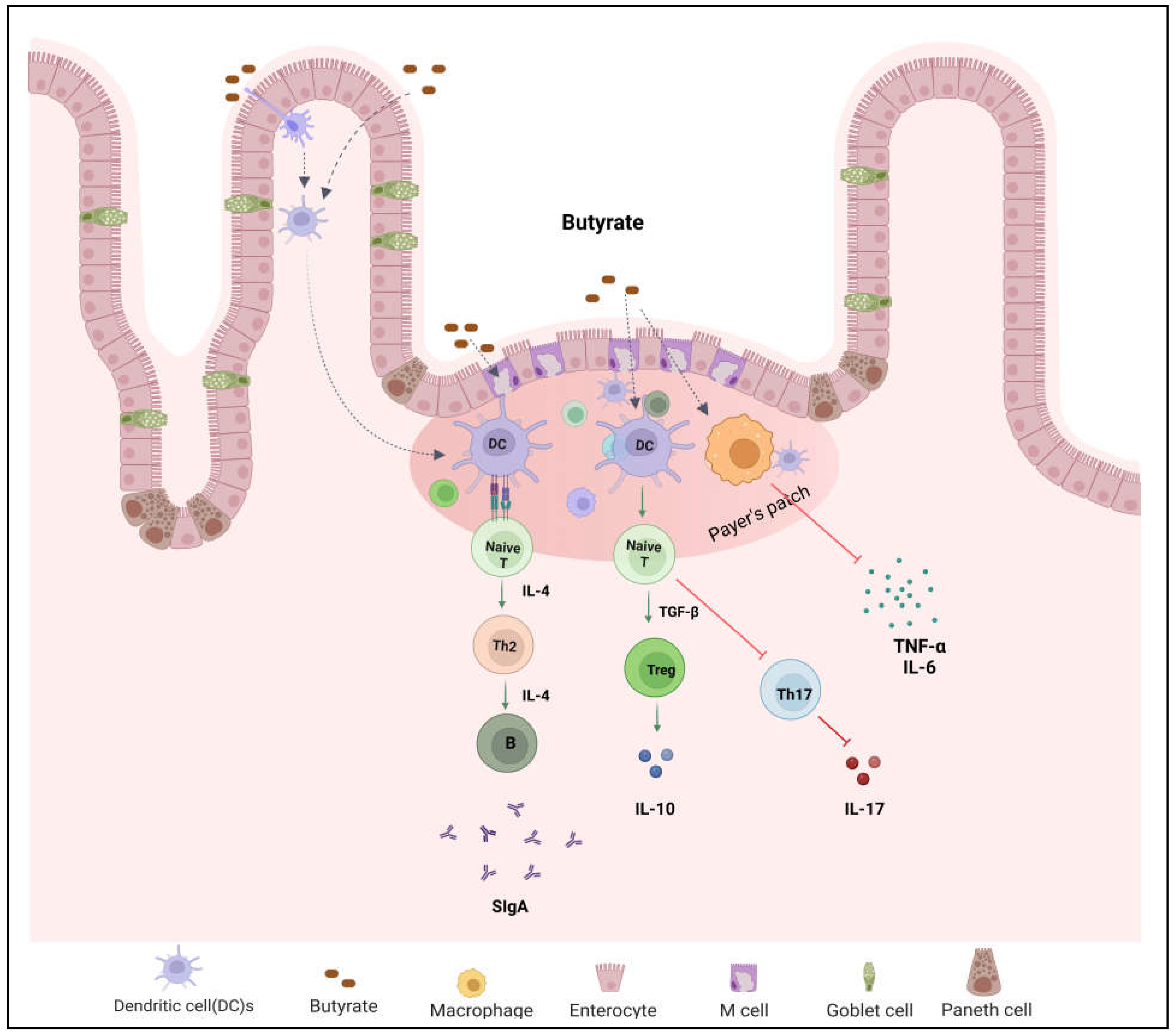
4. Role and mechanisms of butyrate in the regulation of barrier function and immune response
5. Therapeutic implications of butyrate for IBD
5.1. Butyrate supplements
5.1. a. Oral administration
| Treatment name | Concentration | Colitis model | Effects | Authors | |
|---|---|---|---|---|---|
| Mice | Sodium butyrate | 0.5% of sodium butyrate | DSS-induced colitis | Decreased mucosal inflammation | Vieira et al. [74] |
| Mice | Butyrate-releasing polysaccharide derivative | 200 mg/kg | DSS-induced colitis | Reduced disease activity index, rebalanced gut microbiota and reversed the imbalance between pro- and anti-inflammatory cytokines | Zha et al. [65] |
| Mice | Balatable butyrate-releasing derivative, N-(1-carbamoyl-2-phenylethyl) butyramide (FBA) | 42.5 mg/kg | DSS-induced colitis | Reduced disease activity index | Simeoli et al. [75] |
| Mice | Sodium butyrate | 200 mM | Citrobacter rodentium infection model | Prevented mice from weight loss and suppressed intestinal inflammation | Zhou et al. [76] |
| Mice | Sodium butyrate | 200 mM | DSS-induced colitis | Suppressed intestinal inflammation and lowered pathology scores | Zhou et al. [76] |
| Mice | Sodium butyrate | 5 g/L | TNBS induced colitis | Decreased disease activity index and suppressed inflammation | Chen et al. [77] |
| Mice | Sodium butyrate | 100 mg/kg/day | DSS-induced acute colitis Piroxicam-induced chronic colitis |
Decreased colitis scores and prevented weight loss | Lee et al. [67] |
| Mice | Sodium butyrate | DSS-induced colitis | Decreased disease activity index, and restored the balance of gut microbial communities | Dou et al. [70] | |
| Mice | Sodium butyrate150 mM | 150 mM sodium butyrate | DSS-induced colitis | No significant difference in histologic scores | Lee et al. [78] |
| Human | Enteric-coated tablets | 4 g/day | Crohn's disease | Induced clinical improvement and reduced disease activity index | Sabatino et al. [71] |
| Human | Sodium butyrate tablets | 4 g/day | Crohn's disease | Induced clinical improvement or remission | Di Sabatino et al. [79] |
| Human | Microencapsulated-sodium-butyrate | 1800 mg/day | IBD-both CD and UC | Increases the growth of bacteria able to produce SCFA with potential anti-inflammatory action | Faccin et al. [66] |
| Human | Microencapsulated sodium butyrate | 1000 mg/day | UC in clinical remission | Helped to maintain clinical remission | Vernero et al. [72] |
| Human | Sodium butyrate | 150 mg/twice a day | IBD-both CD and UC | No significant effects in newly diagnosed children and adolescents | Pietrzak et al. [80] |
5.1. b. Butyrate enemas
5.2. Butyrogenic diets
5.3. Combination therapies
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest statement
References
- Xavier, R.J.; Podolsky, D.K. Unravelling the Pathogenesis of Inflammatory Bowel Disease. Nature 2007, 448, 427–434. [CrossRef]
- Dassopoulos, T.; Sultan, S.; Falck–Ytter, Y.T.; Inadomi, J.M.; Hanauer, S.B. American Gastroenterological Association Institute Technical Review on the Use of Thiopurines, Methotrexate, and Anti–TNF-α Biologic Drugs for the Induction and Maintenance of Remission in Inflammatory Crohn’s Disease. Gastroenterology 2013, 145, 1464–1478. [CrossRef]
- Ghishan, F.K.; Kiela, P.R. Epithelial Transport in Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2014, 20, 1099–1109. [CrossRef]
- Sokol, H.; Seksik, P.; Furet, J.P.; Firmesse, O.; Nion-Larmurier, I.; Beaugerie, L.; Cosnes, J.; Corthier, G.; Marteau, P.; Doré, J. Low Counts of Faecalibacterium Prausnitzii in Colitis Microbiota. Inflamm. Bowel Dis. 2009, 15, 1183–1189. [CrossRef]
- Machiels, K.; Joossens, M.; Sabino, J.; De Preter, V.; Arijs, I.; Eeckhaut, V.; Ballet, V.; Claes, K.; Van Immerseel, F.; Verbeke, K. A Decrease of the Butyrate-Producing Species Roseburia Hominis and Faecalibacterium Prausnitzii Defines Dysbiosis in Patients with Ulcerative Colitis. Gut 2014, 63, 1275–1283. [CrossRef]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, Stability and Resilience of the Human Gut Microbiota. Nature 2012, 489, 220–230. [CrossRef]
- Rooks, M.G.; Garrett, W.S. Gut Microbiota, Metabolites and Host Immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [CrossRef]
- Feng, W.; Ao, H.; Peng, C. Gut Microbiota, Short-Chain Fatty Acids, and Herbal Medicines. Front. Pharmacol. 2018, 9, 1354. [CrossRef]
- Ridlon, J.M.; Kang, D.-J.; Hylemon, P.B. Bile Salt Biotransformations by Human Intestinal Bacteria. J. Lipid Res. 2006, 47, 241–259. [CrossRef]
- Jia, W.; Xie, G.; Jia, W. Bile Acid–Microbiota Crosstalk in Gastrointestinal Inflammation and Carcinogenesis. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 111–128. [CrossRef]
- Ridlon, J.M.; Hylemon, P.B. Identification and Characterization of Two Bile Acid Coenzyme A Transferases from Clostridium Scindens, a Bile Acid 7α-Dehydroxylating Intestinal Bacterium. J. Lipid Res. 2012, 53, 66–76. [CrossRef]
- Pavlović, N.; Goločorbin-Kon, S.; Ðanić, M.; Stanimirov, B.; Al-Salami, H.; Stankov, K.; Mikov, M. Bile Acids and Their Derivatives as Potential Modifiers of Drug Release and Pharmacokinetic Profiles. Front. Pharmacol. 2018, 9, 1283. [CrossRef]
- Su, X.; Gao, Y.; Yang, R. Gut Microbiota-Derived Tryptophan Metabolites Maintain Gut and Systemic Homeostasis. Cells 2022, 11, 2296. [CrossRef]
- Zhang, J.; Zhu, S.; Ma, N.; Johnston, L.J.; Wu, C.; Ma, X. Metabolites of Microbiota Response to Tryptophan and Intestinal Mucosal Immunity: A Therapeutic Target to Control Intestinal Inflammation. Med. Res. Rev. 2021, 41, 1061–1088. [CrossRef]
- Hugenholtz, F.; Mullaney, J.A.; Kleerebezem, M.; Smidt, H.; Rosendale, D.I. Modulation of the Microbial Fermentation in the Gut by Fermentable Carbohydrates. Bioact. Carbohydrates Diet. Fibre 2013, 2, 133–142. [CrossRef]
- Wang, G.; Huang, S.; Wang, Y.; Cai, S.; Yu, H.; Liu, H.; Zeng, X.; Zhang, G.; Qiao, S. Bridging Intestinal Immunity and Gut Microbiota by Metabolites. Cell. Mol. Life Sci. 2019, 76, 3917–3937.
- Louis, P.; Hold, G.L.; Flint, H.J. The Gut Microbiota, Bacterial Metabolites and Colorectal Cancer. Nat. Rev. Microbiol. 2014, 12, 661–672. [CrossRef]
- Høverstad, T. Studies of Short-Chain Fatty Acid Absorption in Man. Scand. J. Gastroenterol. 1986, 21, 257–260. [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The Role of Short-Chain Fatty Acids in Microbiota–Gut–Brain Communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [CrossRef]
- Levine, M.; Lohinai, Z.M. Resolving the Contradictory Functions of Lysine Decarboxylase and Butyrate in Periodontal and Intestinal Diseases. J. Clin. Med. 2021, 10, 2360. [CrossRef]
- Liu, H.; Wang, J.; He, T.; Becker, S.; Zhang, G.; Li, D.; Ma, X. Butyrate: A Double-Edged Sword for Health? Adv. Nutr. 2018, 9, 21–29. [CrossRef]
- Fu, X.; Liu, Z.; Zhu, C.; Mou, H.; Kong, Q. Nondigestible Carbohydrates, Butyrate, and Butyrate-Producing Bacteria. Crit. Rev. Food Sci. Nutr. 2019, 59, S130–S152. [CrossRef]
- Louis, P.; Flint, H.J. Diversity, Metabolism and Microbial Ecology of Butyrate-Producing Bacteria from the Human Large Intestine. FEMS Microbiol. Lett. 2009, 294, 1–8. [CrossRef]
- Vital, M.; Howe, A.C.; Tiedje, J.M. Revealing the Bacterial Butyrate Synthesis Pathways by Analyzing (Meta) Genomic Data. MBio 2014, 5, e00889-14. [CrossRef]
- Bui, T.P.N.; Ritari, J.; Boeren, S.; De Waard, P.; Plugge, C.M.; De Vos, W.M. Production of Butyrate from Lysine and the Amadori Product Fructoselysine by a Human Gut Commensal. Nat. Commun. 2015, 6, 10062. [CrossRef]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; Van Harsselaar, J. Short Chain Fatty Acids in Human Gut and Metabolic Health. Benef. Microbes 2020, 11, 411–455. [CrossRef]
- Nedjadi, T.; Moran, A.W.; Al-Rammahi, M.A.; Shirazi-Beechey, S.P. Characterization of Butyrate Transport across the Luminal Membranes of Equine Large Intestine. Exp. Physiol. 2014, 99, 1335–1347. [CrossRef]
- Takebe, K.; Nio, J.; Morimatsu, M.; Karaki, S.-I.; Kuwahara, A.; Kato, I.; Iwanaga, T. Histochemical Demonstration of a Na+-Coupled Transporter for Short-Chain Fatty Acids (Slc5a8) in the Intestine and Kidney of the Mouse. Biomed. Res. 2005, 26, 213–221. [CrossRef]
- Guilloteau, P.; Martin, L.; Eeckhaut, V.; Ducatelle, R.; Zabielski, R.; Van Immerseel, F. From the Gut to the Peripheral Tissues: The Multiple Effects of Butyrate. Nutr. Res. Rev. 2010, 23, 366–384. [CrossRef]
- Wong, J.M.W.; De Souza, R.; Kendall, C.W.C.; Emam, A.; Jenkins, D.J.A. Colonic Health: Fermentation and Short Chain Fatty Acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [CrossRef]
- Boets, E.; Gomand, S. V; Deroover, L.; Preston, T.; Vermeulen, K.; De Preter, V.; Hamer, H.M.; Van den Mooter, G.; De Vuyst, L.; Courtin, C.M. Systemic Availability and Metabolism of Colonic-derived Short-chain Fatty Acids in Healthy Subjects: A Stable Isotope Study. J. Physiol. 2017, 595, 541–555. [CrossRef]
- Laukoetter, M.G.; Nava, P.; Nusrat, A. Role of the Intestinal Barrier in Inflammatory Bowel Disease. World J. Gastroenterol. WJG 2008, 14, 401. [CrossRef]
- Vereecke, L.; Beyaert, R.; van Loo, G. Enterocyte Death and Intestinal Barrier Maintenance in Homeostasis and Disease. Trends Mol. Med. 2011, 17, 584–593. [CrossRef]
- Hering, N.A.; Fromm, M.; Schulzke, J. Determinants of Colonic Barrier Function in Inflammatory Bowel Disease and Potential Therapeutics. J. Physiol. 2012, 590, 1035–1044. [CrossRef]
- Sánchez de Medina, F.; Romero-Calvo, I.; Mascaraque, C.; Martínez-Augustin, O. Intestinal Inflammation and Mucosal Barrier Function. Inflamm. Bowel Dis. 2014, 20, 2394–2404. [CrossRef]
- Takiishi, T.; Fenero, C.I.M.; Câmara, N.O.S. Intestinal Barrier and Gut Microbiota: Shaping Our Immune Responses throughout Life. Tissue barriers 2017, 5, e1373208. [CrossRef]
- Foti, M.; Ricciardi-Castagnoli, P. Antigen Sampling by Mucosal Dendritic Cells. Trends Mol. Med. 2005, 11, 394–396. [CrossRef]
- Peterson, L.W.; Artis, D. Intestinal Epithelial Cells: Regulators of Barrier Function and Immune Homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [CrossRef]
- Chehade, M.; Mayer, L. Oral Tolerance and Its Relation to Food Hypersensitivities. J. Allergy Clin. Immunol. 2005, 115, 3–12. [CrossRef]
- Peng, L.; He, Z.; Chen, W.; Holzman, I.R.; Lin, J. Effects of Butyrate on Intestinal Barrier Function in a Caco-2 Cell Monolayer Model of Intestinal Barrier. Pediatr. Res. 2007, 61, 37–41. [CrossRef]
- Peng, L.; Li, Z.-R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate Enhances the Intestinal Barrier by Facilitating Tight Junction Assembly via Activation of AMP-Activated Protein Kinase in Caco-2 Cell Monolayers. J. Nutr. 2009, 139, 1619–1625. [CrossRef]
- Yan, H.; Ajuwon, K.M. Butyrate Modifies Intestinal Barrier Function in IPEC-J2 Cells through a Selective Upregulation of Tight Junction Proteins and Activation of the Akt Signaling Pathway. PLoS One 2017, 12, e0179586. [CrossRef]
- Marinelli, L.; Martin-Gallausiaux, C.; Bourhis, J.-M.; Béguet-Crespel, F.; Blottière, H.M.; Lapaque, N. Identification of the Novel Role of Butyrate as AhR Ligand in Human Intestinal Epithelial Cells. Sci. Rep. 2019, 9, 643. [CrossRef]
- Isobe, J.; Maeda, S.; Obata, Y.; Iizuka, K.; Nakamura, Y.; Fujimura, Y.; Kimizuka, T.; Hattori, K.; Kim, Y.-G.; Morita, T. Commensal-Bacteria-Derived Butyrate Promotes the T-Cell-Independent IgA Response in the Colon. Int. Immunol. 2020, 32, 243–258. [CrossRef]
- Zheng, L.; Kelly, C.J.; Battista, K.D.; Schaefer, R.; Lanis, J.M.; Alexeev, E.E.; Wang, R.X.; Onyiah, J.C.; Kominsky, D.J.; Colgan, S.P. Microbial-Derived Butyrate Promotes Epithelial Barrier Function through IL-10 Receptor–Dependent Repression of Claudin-2. J. Immunol. 2017, 199, 2976–2984. [CrossRef]
- Wang, H.-B.; Wang, P.-Y.; Wang, X.; Wan, Y.-L.; Liu, Y.-C. Butyrate Enhances Intestinal Epithelial Barrier Function via Up-Regulation of Tight Junction Protein Claudin-1 Transcription. Dig. Dis. Sci. 2012, 57, 3126–3135. [CrossRef]
- Burger-van Paassen, N.; Vincent, A.; Puiman, P.J.; van Der Sluis, M.; Bouma, J.; Boehm, G.; Van Goudoever, J.B.; Van Seuningen, I.; Renes, I.B. The Regulation of Intestinal Mucin MUC2 Expression by Short-Chain Fatty Acids: Implications for Epithelial Protection. Biochem. J. 2009, 420, 211–219. [CrossRef]
- Fachi, J.L.; de Souza Felipe, J.; Pral, L.P.; da Silva, B.K.; Corrêa, R.O.; de Andrade, M.C.P.; da Fonseca, D.M.; Basso, P.J.; Câmara, N.O.S.; e Souza, É.L. de S. Butyrate Protects Mice from Clostridium Difficile-Induced Colitis through an HIF-1-Dependent Mechanism. Cell Rep. 2019, 27, 750–761. [CrossRef]
- Brown, S.J.; Mayer, L. The Immune Response in Inflammatory Bowel Disease. Off. J. Am. Coll. Gastroenterol. ACG 2007, 102, 2058–2069. [CrossRef]
- Guan, Q.; Zhang, J. Recent Advances: The Imbalance of Cytokines in the Pathogenesis of Inflammatory Bowel Disease. Mediators Inflamm. 2017, 2017. [CrossRef]
- Maloy, K.J.; Powrie, F. Intestinal Homeostasis and Its Breakdown in Inflammatory Bowel Disease. Nature 2011, 474, 298–306. [CrossRef]
- Luckheeram, R.V.; Zhou, R.; Verma, A.D.; Xia, B. CD4+ T Cells: Differentiation and Functions. Clin. Dev. Immunol. 2012, 2012. [CrossRef]
- Zhang, M.; Zhou, Q.; Dorfman, R.G.; Huang, X.; Fan, T.; Zhang, H.; Zhang, J.; Yu, C. Butyrate Inhibits Interleukin-17 and Generates Tregs to Ameliorate Colorectal Colitis in Rats. BMC Gastroenterol. 2016, 16, 1–9. [CrossRef]
- Bouma, G.; Strober, W. The Immunological and Genetic Basis of Inflammatory Bowel Disease. Nat. Rev. Immunol. 2003, 3, 521–533. [CrossRef]
- Zimmerman, M.A.; Singh, N.; Martin, P.M.; Thangaraju, M.; Ganapathy, V.; Waller, J.L.; Shi, H.; Robertson, K.D.; Munn, D.H.; Liu, K. Butyrate Suppresses Colonic Inflammation through HDAC1-Dependent Fas Upregulation and Fas-Mediated Apoptosis of T Cells. Am. J. Physiol. Liver Physiol. 2012, 302, G1405–G1415. [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T. Commensal Microbe-Derived Butyrate Induces the Differentiation of Colonic Regulatory T Cells. Nature 2013, 504, 446–450. [CrossRef]
- Chen, L.; Sun, M.; Wu, W.; Yang, W.; Huang, X.; Xiao, Y.; Ma, C.; Xu, L.; Yao, S.; Liu, Z. Microbiota Metabolite Butyrate Differentially Regulates Th1 and Th17 Cells’ Differentiation and Function in Induction of Colitis. Inflamm. Bowel Dis. 2019, 25, 1450–1461. [CrossRef]
- Venkatraman, A.; Ramakrishna, B.S.; Shaji, R. V; Kumar, N.S.N.; Pulimood, A.; Patra, S. Amelioration of Dextran Sulfate Colitis by Butyrate: Role of Heat Shock Protein 70 and NF-ΚB. Am. J. Physiol. Liver Physiol. 2003, 285, G177–G184. [CrossRef]
- Segain, J.P.; De La Blétiere, D.R.; Bourreille, A.; Leray, V.; Gervois, N.; Rosales, C.; Ferrier, L.; Bonnet, C.; Blottiere, H.M.; Galmiche, J.P. Butyrate Inhibits Inflammatory Responses through NFκB Inhibition: Implications for Crohn’s Disease. Gut 2000, 47, 397–403. [CrossRef]
- Inan, M.S.; Rasoulpour, R.J.; Yin, L.; Hubbard, A.K.; Rosenberg, D.W.; Giardina, C. The Luminal Short-Chain Fatty Acid Butyrate Modulates NF-ΚB Activity in a Human Colonic Epithelial Cell Line. Gastroenterology 2000, 118, 724–734. [CrossRef]
- Yin, L.; Laevsky, G.; Giardina, C. Butyrate Suppression of Colonocyte NF-ΚB Activation and Cellular Proteasome Activity. J. Biol. Chem. 2001, 276, 44641–44646. [CrossRef]
- Lee, H.U.; McPherson, Z.E.; Tan, B.; Korecka, A.; Pettersson, S. Host-Microbiome Interactions: The Aryl Hydrocarbon Receptor and the Central Nervous System. J. Mol. Med. 2017, 95, 29–39. [CrossRef]
- Yu, M.; Wang, Q.; Ma, Y.; Li, L.; Yu, K.; Zhang, Z.; Chen, G.; Li, X.; Xiao, W.; Xu, P. Aryl Hydrocarbon Receptor Activation Modulates Intestinal Epithelial Barrier Function by Maintaining Tight Junction Integrity. Int. J. Biol. Sci. 2018, 14, 69. [CrossRef]
- Jin, U.-H.; Cheng, Y.; Park, H.; Davidson, L.A.; Callaway, E.S.; Chapkin, R.S.; Jayaraman, A.; Asante, A.; Allred, C.; Weaver, E.A. Short Chain Fatty Acids Enhance Aryl Hydrocarbon (Ah) Responsiveness in Mouse Colonocytes and Caco-2 Human Colon Cancer Cells. Sci. Rep. 2017, 7, 10163. [CrossRef]
- Zha, Z.; Lv, Y.; Tang, H.; Li, T.; Miao, Y.; Cheng, J.; Wang, G.; Tan, Y.; Zhu, Y.; Xing, X. An Orally Administered Butyrate-Releasing Xylan Derivative Reduces Inflammation in Dextran Sulphate Sodium-Induced Murine Colitis. Int. J. Biol. Macromol. 2020, 156, 1217–1233. [CrossRef]
- Facchin, S.; Vitulo, N.; Calgaro, M.; Buda, A.; Romualdi, C.; Pohl, D.; Perini, B.; Lorenzon, G.; Marinelli, C.; D’Incà, R. Microbiota Changes Induced by Microencapsulated Sodium Butyrate in Patients with Inflammatory Bowel Disease. Neurogastroenterol. Motil. 2020, 32, e13914. [CrossRef]
- Lee, C.; Kim, B.G.; Kim, J.H.; Chun, J.; Im, J.P.; Kim, J.S. Sodium Butyrate Inhibits the NF-Kappa B Signaling Pathway and Histone Deacetylation, and Attenuates Experimental Colitis in an IL-10 Independent Manner. Int. Immunopharmacol. 2017, 51, 47–56. [CrossRef]
- Huda-Faujan, N.; Abdulamir, A.S.; Fatimah, A.B.; Anas, O.M.; Shuhaimi, M.; Yazid, A.M.; Loong, Y.Y. The Impact of the Level of the Intestinal Short Chain Fatty Acids in Inflammatory Bowel Disease Patients versus Healthy Subjects. Open Biochem. J. 2010, 4, 53. [CrossRef]
- Chang, P. V; Hao, L.; Offermanns, S.; Medzhitov, R. The Microbial Metabolite Butyrate Regulates Intestinal Macrophage Function via Histone Deacetylase Inhibition. Proc. Natl. Acad. Sci. 2014, 111, 2247–2252. [CrossRef]
- Dou, X.; Gao, N.; Yan, D.; Shan, A. Sodium Butyrate Alleviates Mouse Colitis by Regulating Gut Microbiota Dysbiosis. Animals 2020, 10, 1154. [CrossRef]
- Sabatino, A. Di; Morera, R.; Ciccocioppo, R.; Cazzola, P.; Gotti, S.; Tinozzi, F.P.; Tinozzi, S.; Corazza, G.R. Oral Butyrate for Mildly to Moderately Active Crohn’s Disease. Aliment. Pharmacol. Ther. 2005, 22, 789–794. [CrossRef]
- Vernero, M.; De Blasio, F.; Ribaldone, D.G.; Bugianesi, E.; Pellicano, R.; Saracco, G.M.; Astegiano, M.; Caviglia, G.P. The Usefulness of Microencapsulated Sodium Butyrate Add-on Therapy in Maintaining Remission in Patients with Ulcerative Colitis: A Prospective Observational Study. J. Clin. Med. 2020, 9, 3941. [CrossRef]
- Wang, R.; Cao, S.; Bashir, M.E.H.; Hesser, L.A.; Su, Y.; Hong, S.M.C.; Thompson, A.; Culleen, E.; Sabados, M.; Dylla, N.P. Treatment of Peanut Allergy and Colitis in Mice via the Intestinal Release of Butyrate from Polymeric Micelles. Nat. Biomed. Eng. 2022, 1–18. [CrossRef]
- Vieira, E.L.M.; Leonel, A.J.; Sad, A.P.; Beltrão, N.R.M.; Costa, T.F.; Ferreira, T.M.R.; Gomes-Santos, A.C.; Faria, A.M.C.; Peluzio, M.C.G.; Cara, D.C. Oral Administration of Sodium Butyrate Attenuates Inflammation and Mucosal Lesion in Experimental Acute Ulcerative Colitis. J. Nutr. Biochem. 2012, 23, 430–436. [CrossRef]
- Simeoli, R.; Mattace Raso, G.; Pirozzi, C.; Lama, A.; Santoro, A.; Russo, R.; Montero-Melendez, T.; Berni Canani, R.; Calignano, A.; Perretti, M. An Orally Administered Butyrate-releasing Derivative Reduces Neutrophil Recruitment and Inflammation in Dextran Sulphate Sodium-induced Murine Colitis. Br. J. Pharmacol. 2017, 174, 1484–1496. [CrossRef]
- Zhou, Z.; Cao, J.; Liu, X.; Li, M. Evidence for the Butyrate Metabolism as Key Pathway Improving Ulcerative Colitis in Both Pediatric and Adult Patients. Bioengineered 2021, 12, 8309–8324. [CrossRef]
- Chen, G.; Ran, X.; Li, B.; Li, Y.; He, D.; Huang, B.; Fu, S.; Liu, J.; Wang, W. Sodium Butyrate Inhibits Inflammation and Maintains Epithelium Barrier Integrity in a TNBS-Induced Inflammatory Bowel Disease Mice Model. EBioMedicine 2018, 30, 317–325. [CrossRef]
- Lee, J.G.; Lee, J.; Lee, A.; Jo, S.V.; Park, C.H.; Han, D.S.; Eun, C.S. Impact of Short-Chain Fatty Acid Supplementation on Gut Inflammation and Microbiota Composition in a Murine Colitis Model. J. Nutr. Biochem. 2022, 101, 108926. [CrossRef]
- Di Sabatino, A.; Cazzola, P.; Ciccocioppo, R.; Morera, R.; Biancheri, P.; Rovedatti, L.; Cantoro, L.; Vanoli, A.; Tinozzi, F.P.; Tinozzi, S. Efficacy of Butyrate in the Treatment of Mild to Moderate Crohn’s Disease. Dig. Liver Dis. Suppl. 2007, 1, 31–35. [CrossRef]
- Pietrzak, A.; Banasiuk, M.; Szczepanik, M.; Borys-Iwanicka, A.; Pytrus, T.; Walkowiak, J.; Banaszkiewicz, A. Sodium Butyrate Effectiveness in Children and Adolescents with Newly Diagnosed Inflammatory Bowel Diseases—Randomized Placebo-Controlled Multicenter Trial. Nutrients 2022, 14, 3283. [CrossRef]
- Lührs, H.; Gerke, T.; Müller, J.G.; Melcher, R.; Schauber, J.; Boxberger, F.; Scheppach, W.; Menzel, T. Butyrate Inhibits NF-ΚB Activation in Lamina Propria Macrophages of Patients with Ulcerative Colitis. Scand. J. Gastroenterol. 2002, 37, 458–466. [CrossRef]
- Hamer, H.M.; Jonkers, D.M.A.E.; Vanhoutvin, S.A.L.W.; Troost, F.J.; Rijkers, G.; de Bruïne, A.; Bast, A.; Venema, K.; Brummer, R.-J.M. Effect of Butyrate Enemas on Inflammation and Antioxidant Status in the Colonic Mucosa of Patients with Ulcerative Colitis in Remission. Clin. Nutr. 2010, 29, 738–744. [CrossRef]
- Steinhart, A.H.; Hiruki, T.; Brzezinski, A.; Baker, J.P. Treatment of Left-sided Ulcerative Colitis with Butyrate Enemas: A Controlled Trial. Aliment. Pharmacol. Ther. 1996, 10, 729–736. [CrossRef]
- Okamoto, T.; Sasaki, M.; Tsujikawa, T.; Fujiyama, Y.; Bamba, T.; Kusunoki, M. Preventive Efficacy of Butyrate Enemas and Oral Administration of Clostridium Butyricum M588 in Dextran Sodium Sulfate-Induced Colitis in Rats. J. Gastroenterol. 2000, 35, 341–346. [CrossRef]
- Butzner, J.D.; Parmar, R.; Bell, C.J.; Dalal, V. Butyrate Enema Therapy Stimulates Mucosal Repair in Experimental Colitis in the Rat. Gut 1996, 38, 568–573. [CrossRef]
- Kanauchi, O.; Iwanaga, T.; Mitsuyama, K.; Saiki, T.; Tsuruta, O.; Noguchi, K.; Toyonaga, A. Butyrate from Bacterial Fermentation of Germinated Barley Foodstuff Preserves Intestinal Barrier Function in Experimental Colitis in the Rat Model. J. Gastroenterol. Hepatol. 1999, 14, 880–888. [CrossRef]
- Scheppach, W.; Sommer, H.; Kirchner, T.; Paganelli, G.-M.; Bartram, P.; Christl, S.; Richter, F.; Dusel, G.; Kasper, H. Effect of Butyrate Enemas on the Colonic Mucosa in Distal Ulcerative Colitis. Gastroenterology 1992, 103, 51–56. [CrossRef]
- Fernandez-Banares, F.; Hinojosa, J.; Sanchez-Lombrana, J.L.; Navarro, E.; Martınez-Salmerón, J.F.; Garcıa-Pugés, A.; González-Huix, F.; Riera, J.; González-Lara, V.; Domınguez-Abascal, F. Randomized Clinical Trial of Plantago Ovata Seeds (Dietary Fiber) as Compared with Mesalamine in Maintaining Remission in Ulcerative Colitis. Am. J. Gastroenterol. 1999, 94, 427–433. [CrossRef]
- Hallert, C.; Björck, I.; Nyman, M.; Pousette, A.; Grännö, C.; Svensson, H. Increasing Fecal Butyrate in Ulcerative Colitis Patients by Diet: Controlled Pilot Study. Inflamm. Bowel Dis. 2003, 9, 116–121. [CrossRef]
- Hanai, H.; Kanauchi, O.; Mitsuyama, K.; Andoh, A.; Takeuchi, K.; Takayuki, I.; Araki, Y.; Fujiyama, Y.; Toyonaga, A.; Sata, M. Germinated Barley Foodstuff Prolongs Remission in Patients with Ulcerative Colitis. Int. J. Mol. Med. 2004, 13, 643–647. [CrossRef]
- Chen, M.; Tian, S.; Li, S.; Pang, X.; Sun, J.; Zhu, X.; Lv, F.; Lu, Z.; Li, X. β-Glucan Extracted from Highland Barley Alleviates Dextran Sulfate Sodium-Induced Ulcerative Colitis in C57BL/6J Mice. Molecules 2021, 26, 5812. [CrossRef]
- Bai, J.; Zhao, J.; Waleed, A.-A.; Wang, J.; Xue, L.; Liu, J.; Wang, Y.; Fan, M.; Qian, H.; Li, Y. Oat β-Glucan Alleviates DSS-Induced Colitis via Regulating Gut Microbiota Metabolism in Mice. Food Funct. 2021, 12, 8976–8993. [CrossRef]
- Nyman, M.; Nguyen, T.D.; Wikman, O.; Hjortswang, H.; Hallert, C. Oat Bran Increased Fecal Butyrate and Prevented Gastrointestinal Symptoms in Patients with Quiescent Ulcerative Colitis—Randomized Controlled Trial. Crohn’s Colitis 360 2020, 2, otaa005. [CrossRef]
- Wedlake, L.; Slack, N.; Andreyev, H.J.N.; Whelan, K. Fiber in the Treatment and Maintenance of Inflammatory Bowel Disease: A Systematic Review of Randomized Controlled Trials. Inflamm. Bowel Dis. 2014, 20, 576–586. [CrossRef]
- Limketkai, B.N.; Iheozor-Ejiofor, Z.; Gjuladin-Hellon, T.; Parian, A.; Matarese, L.E.; Bracewell, K.; MacDonald, J.K.; Gordon, M.; Mullin, G.E. Dietary Interventions for Induction and Maintenance of Remission in Inflammatory Bowel Disease. Cochrane Database Syst. Rev. 2019. [CrossRef]
- Xu, Z.; Chen, W.; Deng, Q.; Huang, Q.; Wang, X.; Yang, C.; Huang, F. Flaxseed Oligosaccharides Alleviate DSS-Induced Colitis through Modulation of Gut Microbiota and Repair of the Intestinal Barrier in Mice. Food Funct. 2020, 11, 8077–8088. [CrossRef]
- Kang, S.; You, H.J.; Ju, Y.; Kim, H.J.; Jeong, Y.J.; Johnston, T. V; Ji, G.E.; Ku, S.; Park, M.S. Butyl-Fructooligosaccharides Modulate Gut Microbiota in Healthy Mice and Ameliorate Ulcerative Colitis in a DSS-Induced Model. Food Funct. 2022, 13, 1834–1845. [CrossRef]
- Liu, J.; Wang, Z.; Mai, P.; Hao, Y.; Wang, Z.; Wang, J. Quinoa Bran Soluble Dietary Fiber Ameliorates Dextran Sodium Sulfate Induced Ulcerative Colitis in BALB/c Mice by Maintaining Intestinal Barrier Function and Modulating Gut Microbiota. Int. J. Biol. Macromol. 2022, 216, 75–85. [CrossRef]
- Wang, N.; Chen, W.; Cui, C.; Zheng, Y.; Yu, Q.; Ren, H.; Liu, Z.; Xu, C.; Zhang, G. The Peanut Skin Procyanidins Attenuate DSS-Induced Ulcerative Colitis in C57BL/6 Mice. Antioxidants 2022, 11, 2098. [CrossRef]
- De Preter, V.; Falony, G.; Windey, K.; Hamer, H.M.; De Vuyst, L.; Verbeke, K. The Prebiotic, Oligofructose-enriched Inulin Modulates the Faecal Metabolite Profile: An in Vitro Analysis. Mol. Nutr. Food Res. 2010, 54, 1791–1801. [CrossRef]
- Gholami, M.; Ghasemi-Niri, S.F.; Maqbool, F.; Baeeri, M.; Memariani, Z.; Pousti, I.; Abdollahi, M. Experimental and Pathalogical Study of Pistacia Atlantica, Butyrate, Lactobacillus Casei and Their Combination on Rat Ulcerative Colitis Model. Pathol. Pract. 2016, 212, 500–508. [CrossRef]
- Moeinian, M.; Ghasemi-Niri, S.F.; Mozaffari, S.; Abdolghaffari, A.H.; Baeeri, M.; Navaea-Nigjeh, M.; Abdollahi, M. Beneficial Effect of Butyrate, Lactobacillus Casei and L-Carnitine Combination in Preference to Each in Experimental Colitis. World J. Gastroenterol. WJG 2014, 20, 10876. [CrossRef]
- Breuer, R.I.; Soergel, K.H.; Lashner, B.A.; Christ, M.L.; Hanauer, S.B.; Vanagunas, A.; Harig, J.M.; Keshavarzian, A.; Robinson, M.; Sellin, J.H. Short Chain Fatty Acid Rectal Irrigation for Left-Sided Ulcerative Colitis: A Randomised, Placebo Controlled Trial. Gut 1997, 40, 485–491. [CrossRef]
- Vernia, P.; Monteleone, G.; Grandinetti, G.; Villotti, G.; Di Giulio, E.; Frieri, G.; Marcheggiano, A.; Pallone, F.; Caprilli, R.; Torsoli, A. Combined Oral Sodium Butyrate and Mesalazine Treatment Compared to Oral Mesalazine Alone in Ulcerative Colitis. Dig. Dis. Sci. 2000, 45, 976–981. [CrossRef]
- Gibbs, B.; Brown, B.I. Butyrate Therapy for Treatment-Resistant Ulcerative Colitis: A Case Study. 2022.
| Features | Crohn’s disease | Ulcerative colitis |
|---|---|---|
| Location | Any part of the GI tract | Large intestine |
| Inflammation | Transmural | Superficial |
| Complications | Fistula development, obstruction | No fistula, Hemorrhage |
| Distribution | Continuous | Discontinuous and patchy |
| Rectal involvement | Common | Occasional |
| Concentration | Colitis model | Duration | Effects | Authors | |
|---|---|---|---|---|---|
| Rat | 3 ml of 100 mM | DSS-induced colitis | 17 days | Decreased ulcer index and myeloperoxidase activity | Okamoto et al. [84] |
| Rat | 100 mM sodium butyrate | TNBS induced colitis | Day 5 to 23 | Decreased inflammation and improved clinical recovery | Butzner et al. [85] |
| Rat | 3% of sodium butyrate | DSS-induced colitis | Decreased mucosal damage, no difference in the incidence of diarrhea | Kanauchi et al. [86] | |
| Rat | 100 mM sodium butyrate | TNBS induced colitis | 2 wks | Decreased inflammation and stimulated mucosal repair | Segain et al. [59] |
| Human | 100 mM sodium butyrate | Ulcerative colitis | 2 wks | Decreased disease activity index and inflammation | Scheppach et al. [87] |
| Human | 60 mL of 80 mM sodium butyrate | Ulcerative colitis | 3 and 6 wks | Nightly butyrate enema was not efficacious for distal ulcerative colitis | Steinhart et al. [83] |
| Human | 60 mL of 100 mM sodium butyrate | Ulcerative colitis | 4 and 8 wks | Decreased disease activity index and mucosal inflammation after 8 wks | Luhrs et al. [81] |
| Human | 60 mL of 100 mM sodium butyrate | Ulcerative colitis | 20 days | No significant effects of butyrate administration on parameters of oxidative stress were found | Hamer et al. [82] |
| Treatment | Disease or model | Effects | Authors | |
|---|---|---|---|---|
| Rat | Germinated barley foodstuff | DSS-induced colitis | Bloody diarrhea and mucosal damage were dose dependently decreased | Kanauchi et al. [86] |
| Mice | Flaxseed oligosaccharides | DSS-induced colitis | Decreased disease activity index, improved colon histology, and increased cecal SCFAs levels | Xu et al. [96] |
| Mice | Oat β-glucan | DSS-induced colitis | Suppressed colonic inflammatory infiltration and increased SCFAs concentrations | Bai et al. [92] |
| Mice | Butyl-fructooligosaccharides | DSS-induced colitis | Increased cecal butyrate concentration, increased occludin mRNA expression | Kang et al. [97] |
| Mice | Soluble dietary fiber from quinoa bran | DSS-induced colitis | Decreased disease activity index, increased microbial diversity and SCFAs | Liu et al. [98] |
| Mice | Peanut skin procyanidins extract | DSS-induced colitis | Suppressed inflammatory responses, increased butyrate producing bacterial abundance and colon SCFAs | Wang et al. [99] |
| Human | Plantago ovata seeds | Ulcerative colitis in remission | Increased fecal butyrate levels | Fernandez-Banares et al. [88] |
| Human | Oat bran | Ulcerative colitis | Increased fecal butyrate and maintained the remission phase | Hallert et al. [89] |
| Human | Germinated barley food stuff | Ulcerative colitis in remission | Effective in maintenance of prolonged remission | Hanai et al. [90] |
| Human | Prebiotic oligofructose-enriched inulin | Crohn's disease | The relative levels of butyrate and acetaldehyde increased compared to baseline | De Preter et al. [100] |
| Human | Oat bran | Ulcerative colitis in remission | Increased fecal SCFAs including butyric acid and reduced the risk of relapse | Nyman et al. [93] |
| Treament name | Concentration | Colitis model | Duration | Effects | Authors | |
|---|---|---|---|---|---|---|
| Mice | SCFAs | 67.5 mM acetate, 40 mM butyrate, 25.9 mM propionate | DSS-induced colitis | No significant difference in histologic scores but IL-17A producing T cells increased | Lee et al. [78] | |
| Rat | Pistacia atlantica, butyrate, Lactobacillus casei | 25 mg/kg atlantica, 0.5% butyrate, and 108 CFU of Lactbacillus | TNBS-induced colitis | 10 days | Reduced the severity of inflammation | Gholami et al. [101] |
| Human | Plantago ovata seeds and mesalamine | 20 g seeds and 1.5 g mesalamine/day | Ulcerative colitis remission | 12 months | Effective in remission maintenance | Fernandez-Banares et al. [88] |
| Human | Sodium butyrate and mesalazine | 4 g/day butyrate and 2.4 g/day mesalamine | Ulcerative colitis | 6 wks | Improved the efficacy of mesalazine | Vernia et al. [104] |
| Human | Calcium magnesium butyrate along with Mezavant treatment | 1.2 g/day magnesium butyrate and 4.8 g/day mezavant |
Ulcerative colitis | Relief of symptoms | Gibbs and Brown. [105] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




