Submitted:
17 April 2023
Posted:
18 April 2023
You are already at the latest version
Abstract
Keywords:
Introduction
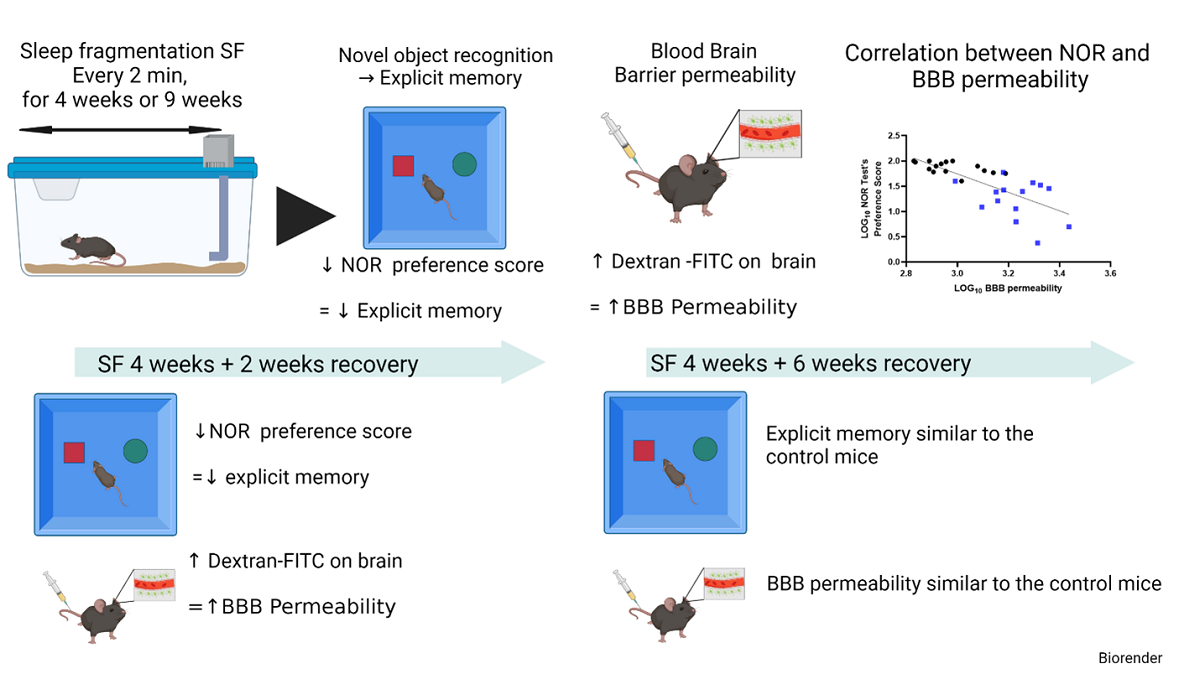
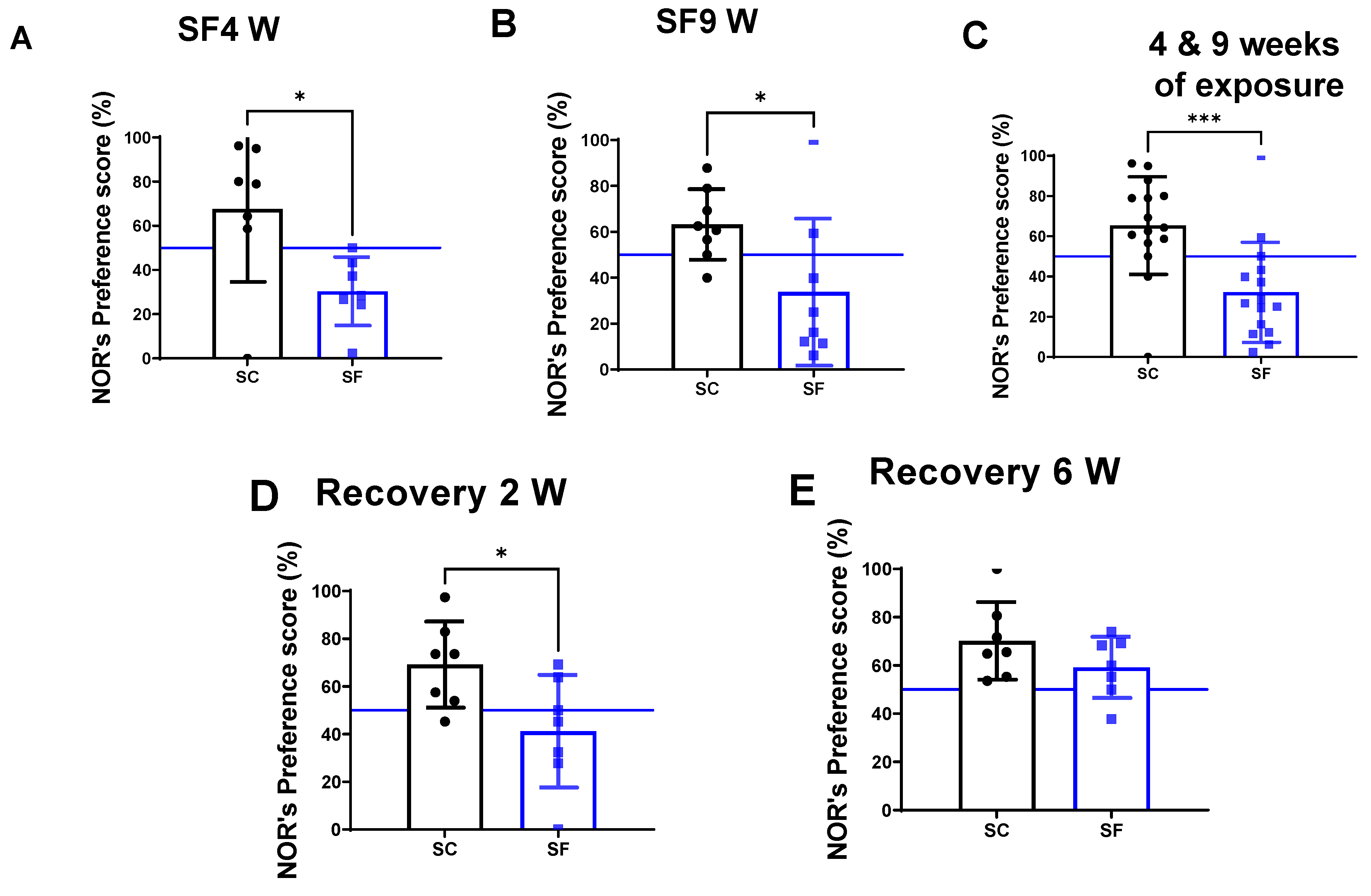
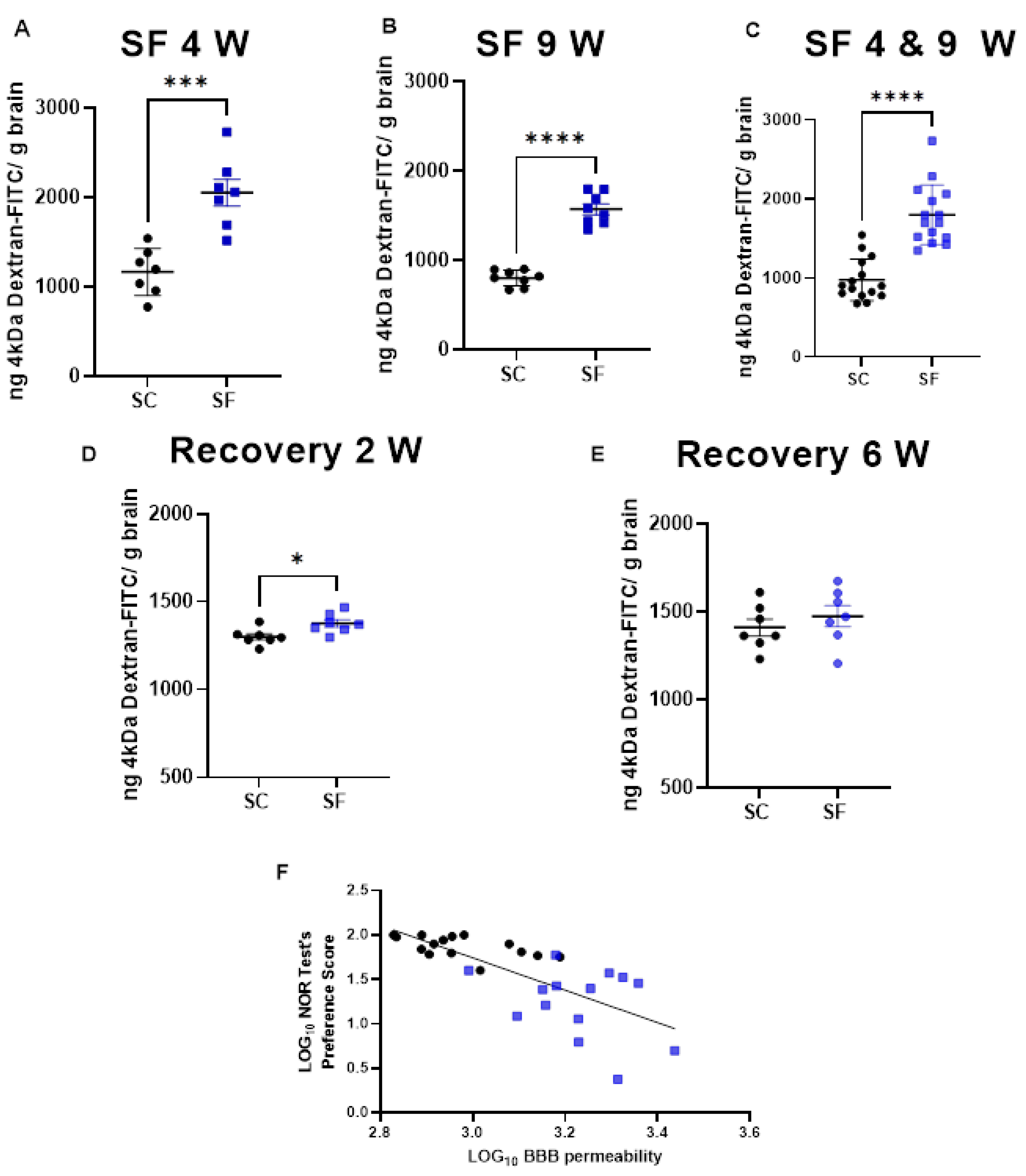
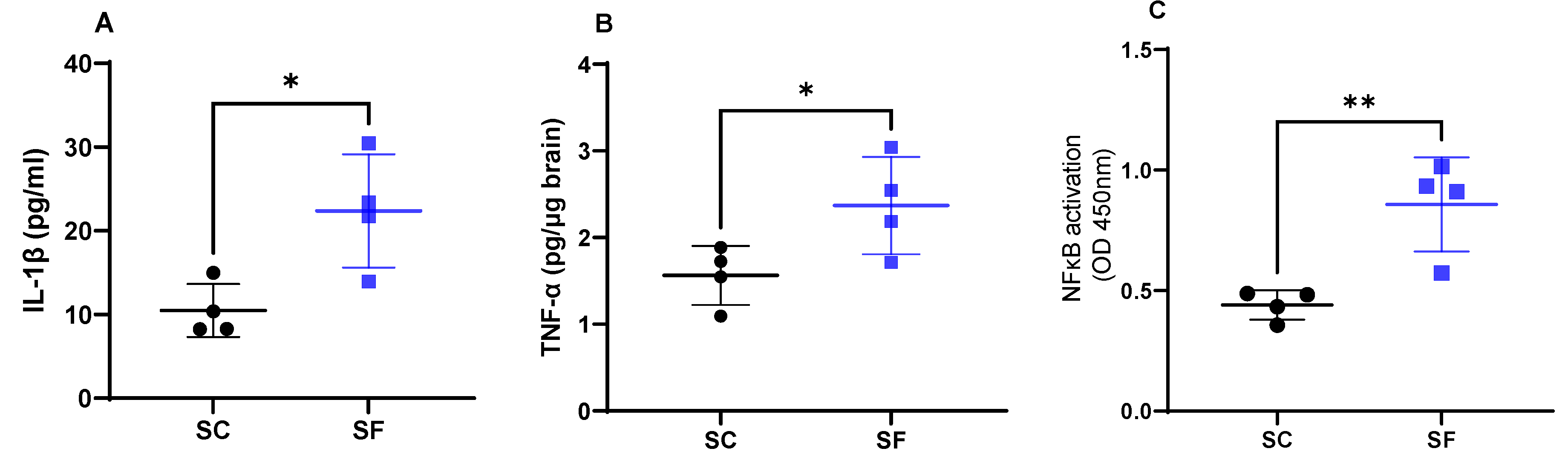
Results
Discussion
Materials and Methods
Sleep Fragmentation (SF)
Novel Object Recognition (NOR) Test
Inflammatory Markers
Blood Brain Permeability
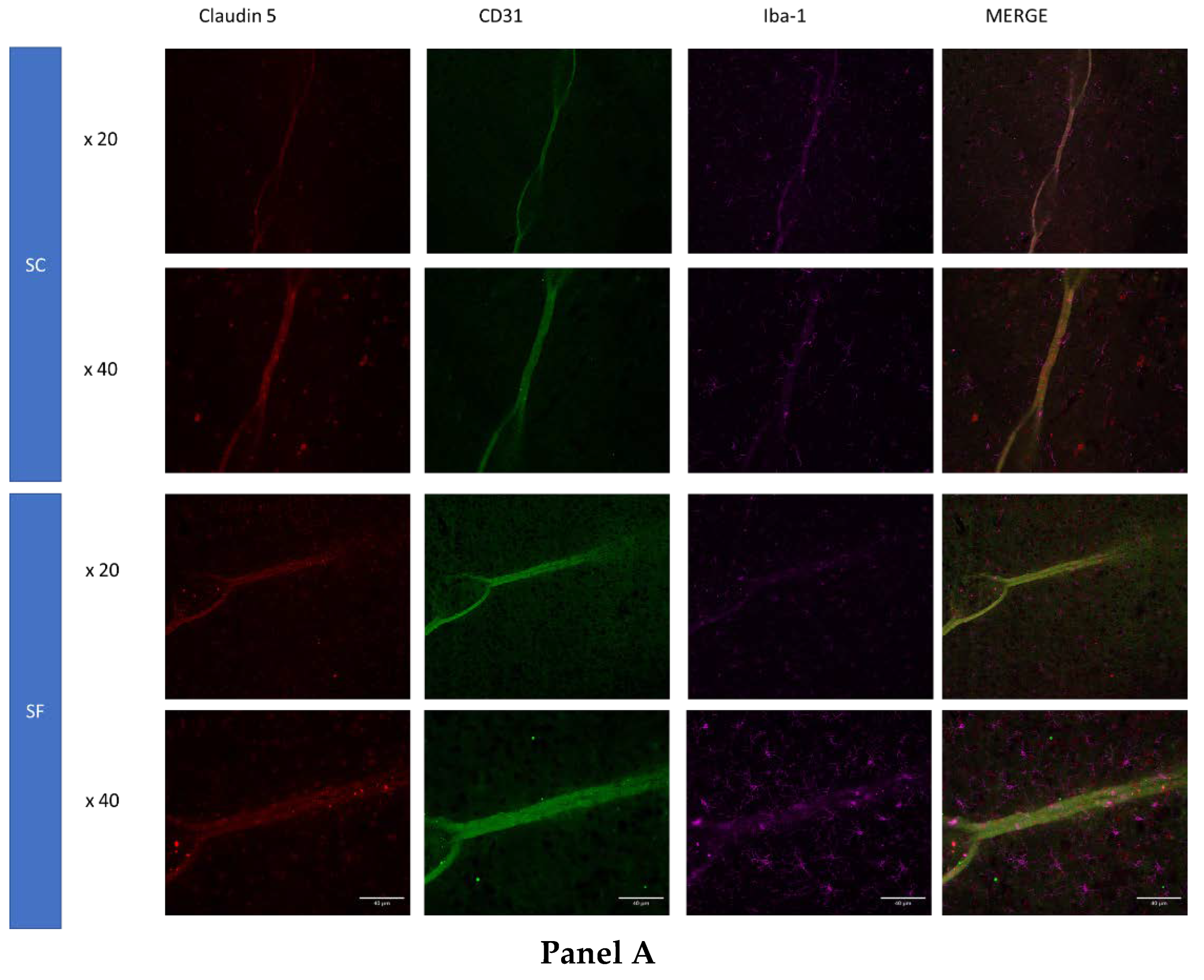
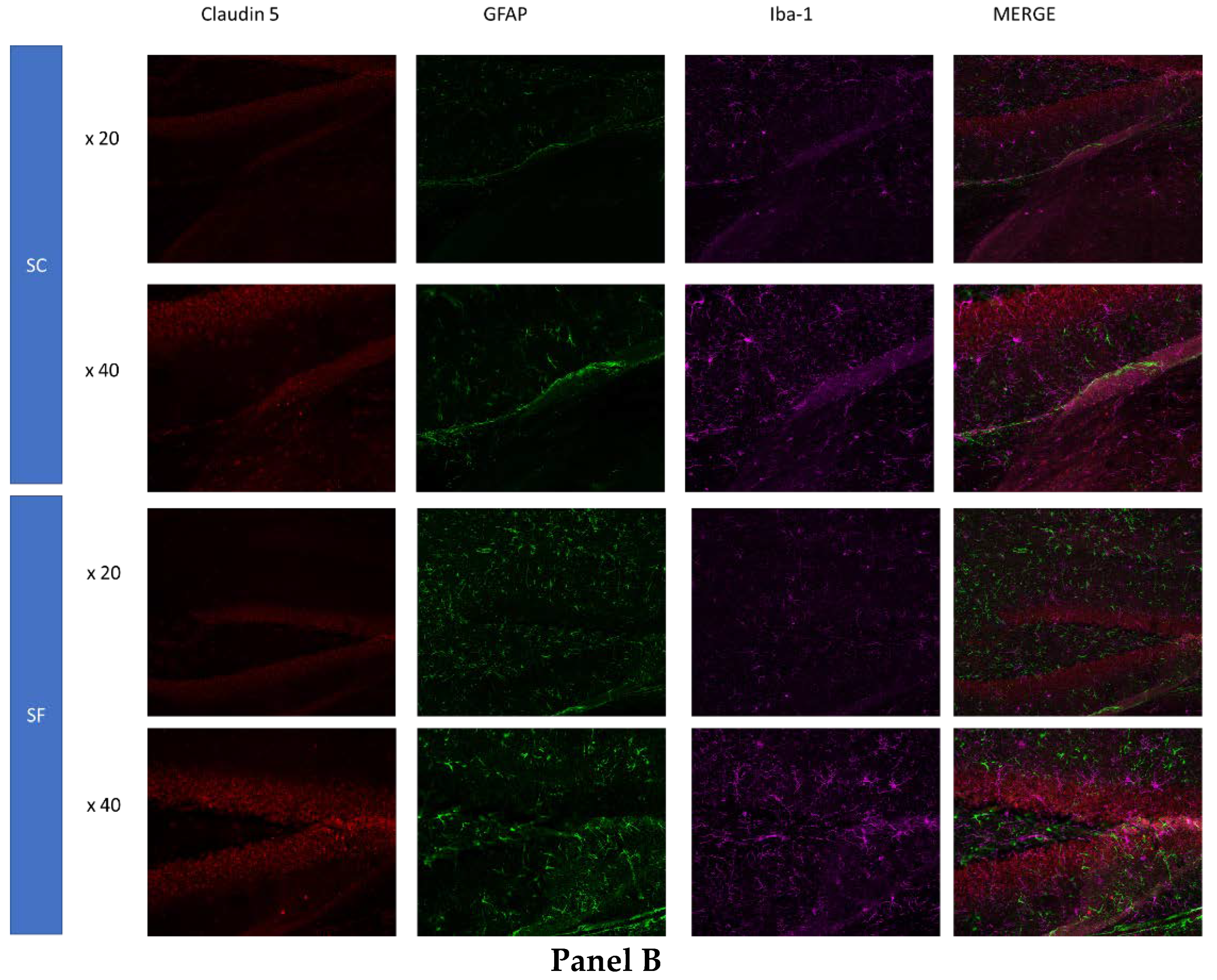
Immunohistochemistry
Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Almendros, I.; Martinez-Garcia, M.A.; Farré, R.; Gozal, D. Obesity, Sleep Apnea, and Cancer. Int. J. Obes. 2020, 44, 1653–1667. [Google Scholar] [CrossRef] [PubMed]
- Badran, M.; Khalyfa, A.; Ericsson, A.; Gozal, D. Fecal Microbiota Transplantation from Mice Exposed to Chronic Intermittent Hypoxia Elicits Sleep Disturbances in Naïve Mice. Exp. Neurol. 2020, 334, 113439. [Google Scholar] [CrossRef] [PubMed]
- Stranks, E.K.; Crowe, S.F. The Cognitive Effects of Obstructive Sleep Apnea: An Updated Meta-Analysis. Arch. Clin. Neuropsychol. Off. J. Natl. Acad. Neuropsychol. 2016, 31, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Krysta, K.; Bratek, A.; Zawada, K.; Stepańczak, R. Cognitive Deficits in Adults with Obstructive Sleep Apnea Compared to Children and Adolescents. J. Neural Transm. 2017, 124, 187–201. [Google Scholar] [CrossRef]
- Lajoie, A.C.; Lafontaine, A.-L.; Kimoff, R.J.; Kaminska, M. Obstructive Sleep Apnea in Neurodegenerative Disorders: Current Evidence in Support of Benefit from Sleep Apnea Treatment. J. Clin. Med. 2020, 9, E297. [Google Scholar] [CrossRef]
- Senaratna, C.V.; Perret, J.L.; Lodge, C.J.; Lowe, A.J.; Campbell, B.E.; Matheson, M.C.; Hamilton, G.S.; Dharmage, S.C. Prevalence of Obstructive Sleep Apnea in the General Population: A Systematic Review. Sleep Med. Rev. 2017, 34, 70–81. [Google Scholar] [CrossRef]
- Gileles-Hillel, A.; Kheirandish-Gozal, L.; Gozal, D. Biological Plausibility Linking Sleep Apnoea and Metabolic Dysfunction. Nat. Rev. Endocrinol. 2016, 12, 290–298. [Google Scholar] [CrossRef]
- Yeghiazarians, Y.; Jneid, H.; Tietjens, J.R.; Redline, S.; Brown, D.L.; El-Sherif, N.; Mehra, R.; Bozkurt, B.; Ndumele, C.E.; Somers, V.K. Obstructive Sleep Apnea and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 144, e56–e67. [Google Scholar] [CrossRef]
- Abad, V.C. Profile of Solriamfetol in the Management of Excessive Daytime Sleepiness Associated with Narcolepsy or Obstructive Sleep Apnea: Focus on Patient Selection and Perspectives. Nat. Sci. Sleep 2021, 13, 75–91. [Google Scholar] [CrossRef]
- Bucks, R.S.; Olaithe, M.; Rosenzweig, I.; Morrell, M.J. Reviewing the Relationship between OSA and Cognition: Where Do We Go from Here? Respirol. Carlton Vic 2017, 22, 1253–1261. [Google Scholar] [CrossRef]
- Hirsch Allen, A.J.M.; Bansback, N.; Ayas, N.T. The Effect of OSA on Work Disability and Work-Related Injuries. Chest 2015, 147, 1422–1428. [Google Scholar] [CrossRef] [PubMed]
- Vanek, J.; Prasko, J.; Genzor, S.; Ociskova, M.; Kantor, K.; Holubova, M.; Slepecky, M.; Nesnidal, V.; Kolek, A.; Sova, M. Obstructive Sleep Apnea, Depression and Cognitive Impairment. Sleep Med. 2020, 72, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Lal, C.; Ayappa, I.; Ayas, N.; Beaudin, A.E.; Hoyos, C.; Kushida, C.A.; Kaminska, M.; Mullins, A.; Naismith, S.L.; Osorio, R.S.; et al. The Link between Obstructive Sleep Apnea and Neurocognitive Impairment: An Official American Thoracic Society Workshop Report. Ann. Am. Thorac. Soc. 2022, 19, 1245–1256. [Google Scholar] [CrossRef] [PubMed]
- Gozal, D.; Kheirandish-Gozal, L.; Bhattacharjee, R.; Spruyt, K. Neurocognitive and Endothelial Dysfunction in Children With Obstructive Sleep Apnea. Pediatrics 2010, 126, e1161–e1167. [Google Scholar] [CrossRef] [PubMed]
- Cistulli, P.A.; Celermajer, D.S. Endothelial Dysfunction and Obstructive Sleep Apnea: The Jury Is Still Out! Am. J. Respir. Crit. Care Med. 2017, 195, 1135–1137. [Google Scholar] [CrossRef]
- Budhiraja, R.; Parthasarathy, S.; Quan, S.F. Endothelial Dysfunction in Obstructive Sleep Apnea. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2007, 3, 409–415. [Google Scholar] [CrossRef]
- Atkeson, A.; Yeh, S.Y.; Malhotra, A.; Jelic, S. Endothelial Function in Obstructive Sleep Apnea. Prog. Cardiovasc. Dis. 2009, 51, 351–362. [Google Scholar] [CrossRef]
- Lal, C.; Strange, C.; Bachman, D. Neurocognitive Impairment in Obstructive Sleep Apnea. Chest 2012, 141, 1601–1610. [Google Scholar] [CrossRef]
- Hoyos, C.M.; Melehan, K.L.; Liu, P.Y.; Grunstein, R.R.; Phillips, C.L. Does Obstructive Sleep Apnea Cause Endothelial Dysfunction? A Critical Review of the Literature. Sleep Med. Rev. 2015, 20, 15–26. [Google Scholar] [CrossRef]
- Schwarz, E.I.; Puhan, M.A.; Schlatzer, C.; Stradling, J.R.; Kohler, M. Effect of CPAP Therapy on Endothelial Function in Obstructive Sleep Apnoea: A Systematic Review and Meta-Analysis. Respirology 2015, 20, 889–895. [Google Scholar] [CrossRef]
- Kerner, N.A.; Roose, S.P. Obstructive Sleep Apnea Is Linked to Depression and Cognitive Impairment: Evidence and Potential Mechanisms. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry 2016, 24, 496–508. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.C.; Pack, A.I. Obstructive Sleep Apnea and Cognitive Impairment: Addressing the Blood-Brain Barrier. Sleep Med. Rev. 2014, 18, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Kim, L.J.; Martinez, D.; Fiori, C.Z.; Baronio, D.; Kretzmann, N.A.; Barros, H.M.T. Hypomyelination, Memory Impairment, and Blood–Brain Barrier Permeability in a Model of Sleep Apnea. Brain Res. 2015, 1597, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Zolotoff, C.; Voirin, A.-C.; Puech, C.; Roche, F.; Perek, N. Intermittent Hypoxia and Its Impact on Nrf2/HIF-1α Expression and ABC Transporters: An in Vitro Human Blood-Brain Barrier Model Study. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2020, 54, 1231–1248. [Google Scholar] [CrossRef]
- Kilicarslan, R.; Alkan, A.; Sharifov, R.; Akkoyunlu, M.E.; Aralasmak, A.; Kocer, A.; Kart, L. The Effect of Obesity on Brain Diffusion Alteration in Patients with Obstructive Sleep Apnea. ScientificWorldJournal 2014, 2014, 768415. [Google Scholar] [CrossRef]
- Kheirandish-Gozal, L.; Khalyfa, A.; Gozal, D. Exosomes, Blood–Brain Barrier, and Cognitive Dysfunction in Pediatric Sleep Apnea. Sleep Biol. Rhythms 2017, 15, 261–267. [Google Scholar] [CrossRef]
- Voirin, A.-C.; Celle, S.; Perek, N.; Roche, F. Sera of Elderly Obstructive Sleep Apnea Patients Alter Blood-Brain Barrier Integrity in Vitro: A Pilot Study. Sci. Rep. 2020, 10, 11309. [Google Scholar] [CrossRef]
- Khalyfa, A.; Gozal, D.; Kheirandish-Gozal, L. Plasma Extracellular Vesicles in Children with OSA Disrupt Blood–Brain Barrier Integrity and Endothelial Cell Wound Healing In Vitro. Int. J. Mol. Sci. 2019, 20, 6233. [Google Scholar] [CrossRef]
- Khalyfa, A.; Gozal, D.; Kheirandish-Gozal, L. Plasma Exosomes Disrupt the Blood–Brain Barrier in Children with Obstructive Sleep Apnea and Neurocognitive Deficits. Am. J. Respir. Crit. Care Med. 2018, 197, 1073–1076. [Google Scholar] [CrossRef]
- Daneman, R.; Prat, A. The Blood–Brain Barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef]
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and Function of the Blood-Brain Barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zheng, T.; Yang, X.; Fan, M.; Zhu, L.; Liu, S.; Wu, L.; Sun, C. Cryptotanshinone Attenuates Oxygen-Glucose Deprivation/ Recovery-Induced Injury in an in Vitro Model of Neurovascular Unit. Front. Neurol. 2019, 10, 381. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.S.; Foster, C.G.; Courtney, J.-M.; King, N.E.; Howells, D.W.; Sutherland, B.A. Pericytes and Neurovascular Function in the Healthy and Diseased Brain. Front. Cell. Neurosci. 2019, 13, 282. [Google Scholar] [CrossRef] [PubMed]
- Cuddapah, V.A.; Zhang, S.L.; Sehgal, A. Regulation of the Blood-Brain Barrier by Circadian Rhythms and Sleep. Trends Neurosci. 2019, 42, 500–510. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, S.; Sonobe, Y.; Cheng, Y.; Horiuchi, H.; Parajuli, B.; Kawanokuchi, J.; Mizuno, T.; Takeuchi, H.; Suzumura, A. Interleukin-1β Induces Blood-Brain Barrier Disruption by Downregulating Sonic Hedgehog in Astrocytes. PLoS ONE 2014, 9, e110024. [Google Scholar] [CrossRef] [PubMed]
- Venancio, D.P.; Suchecki, D. Prolonged REM Sleep Restriction Induces Metabolic Syndrome-Related Changes: Mediation by pro-Inflammatory Cytokines. Brain. Behav. Immun. 2015, 47, 109–117. [Google Scholar] [CrossRef]
- Yehuda, S.; Sredni, B.; Carasso, R.L.; Kenigsbuch-Sredni, D. REM Sleep Deprivation in Rats Results in Inflammation and Interleukin-17 Elevation. J. Interferon Cytokine Res. Off. J. Int. Soc. Interferon Cytokine Res. 2009, 29, 393–398. [Google Scholar] [CrossRef]
- Hurtado-Alvarado, G.; Domínguez-Salazar, E.; Pavon, L.; Velázquez-Moctezuma, J.; Gómez-González, B. Blood-Brain Barrier Disruption Induced by Chronic Sleep Loss: Low-Grade Inflammation May Be the Link. J. Immunol. Res. 2016, 2016, 4576012. [Google Scholar] [CrossRef]
- Hurtado-Alvarado, G.; Becerril-Villanueva, E.; Contis-Montes de Oca, A.; Domínguez-Salazar, E.; Salinas-Jazmín, N.; Pérez-Tapia, S.M.; Pavon, L.; Velázquez-Moctezuma, J.; Gómez-González, B. The Yin/Yang of Inflammatory Status: Blood-Brain Barrier Regulation during Sleep. Brain. Behav. Immun. 2018, 69, 154–166. [Google Scholar] [CrossRef]
- Opp, M.R.; George, A.; Ringgold, K.M.; Hansen, K.M.; Bullock, K.M.; Banks, W.A. Sleep Fragmentation and Sepsis Differentially Impact Blood–Brain Barrier Integrity and Transport of Tumor Necrosis Factor-α in Aging. Brain. Behav. Immun. 2015, 50, 259–265. [Google Scholar] [CrossRef]
- Ramesh, V.; Nair, D.; Zhang, S.X.L.; Hakim, F.; Kaushal, N.; Kayali, F.; Wang, Y.; Li, R.C.; Carreras, A.; Gozal, D. Disrupted Sleep without Sleep Curtailment Induces Sleepiness and Cognitive Dysfunction via the Tumor Necrosis Factor-α Pathway. J. Neuroinflammation 2012, 9, 91. [Google Scholar] [CrossRef]
- Chennaoui, M.; Sauvet, F.; Drogou, C.; Van Beers, P.; Langrume, C.; Guillard, M.; Gourby, B.; Bourrilhon, C.; Florence, G.; Gomez-Merino, D. Effect of One Night of Sleep Loss on Changes in Tumor Necrosis Factor Alpha (TNF-α) Levels in Healthy Men. Cytokine 2011, 56, 318–324. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Hsuchou, H.; He, Y.; Kastin, A.J.; Wang, Y.; Pan, W. Sleep Restriction Impairs Blood–Brain Barrier Function. J. Neurosci. 2014, 34, 14697–14706. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Gonzalez, B.; Hurtado-Alvarado, G.; Esqueda-Leon, E.; Santana- Miranda, R.; Rojas-Zamorano, J.A.; Velazquez-Moctezuma, J. REM Sleep Loss and Recovery Regulates Blood-Brain Barrier Function. Curr. Neurovasc. Res. 2013, 10, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood–Brain Barrier Breakdown in Alzheimer’s Disease and Other Neurodegenerative Disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Hussain, B.; Fang, C.; Chang, J. Blood–Brain Barrier Breakdown: An Emerging Biomarker of Cognitive Impairment in Normal Aging and Dementia. Front. Neurosci. 2021, 15. [Google Scholar] [CrossRef]
- Knox, E.G.; Aburto, M.R.; Clarke, G.; Cryan, J.F.; O’Driscoll, C.M. The Blood-Brain Barrier in Aging and Neurodegeneration. Mol. Psychiatry 2022, 27, 2659–2673. [Google Scholar] [CrossRef]
- Bilyukov, R.G.; Nikolov, M.S.; Pencheva, V.P.; Petrova, D.S.; Georgiev, O.B.; Mondeshki, T.L.; Milanova, V.K. Cognitive Impairment and Affective Disorders in Patients With Obstructive Sleep Apnea Syndrome. Front. Psychiatry 2018, 9, 357. [Google Scholar] [CrossRef]
- Ferini-Strambi, L.; Baietto, C.; Di Gioia, M.R.; Castaldi, P.; Castronovo, C.; Zucconi, M.; Cappa, S.F. Cognitive Dysfunction in Patients with Obstructive Sleep Apnea (OSA): Partial Reversibility after Continuous Positive Airway Pressure (CPAP). Brain Res. Bull. 2003, 61, 87–92. [Google Scholar] [CrossRef]
- Puech, C.; Badran, M.; Runion, A.R.; Barrow, M.B.; Qiao, Z.; Khalyfa, A.; Gozal, D. Explicit Memory, Anxiety and Depressive like Behavior in Mice Exposed to Chronic Intermittent Hypoxia, Sleep Fragmentation, or Both during the Daylight Period. Neurobiol. Sleep Circadian Rhythms 2022, 100084. [Google Scholar] [CrossRef]
- Xu, L.-H.; Xie, H.; Shi, Z.-H.; Du, L.-D.; Wing, Y.-K.; Li, A.M.; Ke, Y.; Yung, W.-H. Critical Role of Endoplasmic Reticulum Stress in Chronic Intermittent Hypoxia-Induced Deficits in Synaptic Plasticity and Long-Term Memory. Antioxid. Redox Signal. 2015, 23, 695–710. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, N.; Ramesh, V.; Gozal, D. Human Apolipoprotein E4 Targeted Replacement in Mice Reveals Increased Susceptibility to Sleep Disruption and Intermittent Hypoxia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 303, R19–R29. [Google Scholar] [CrossRef] [PubMed]
- Ward, C.P.; McCoy, J.G.; McKenna, J.T.; Connolly, N.P.; McCarley, R.W.; Strecker, R.E. Spatial Learning and Memory Deficits Following Exposure to 24 h of Sleep Fragmentation or Intermittent Hypoxia in a Rat Model of Obstructive Sleep Apnea. Brain Res. 2009, 1294, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Row, B.W. Intermittent Hypoxia and Cognitive Function: Implications from Chronic Animal Models. Adv. Exp. Med. Biol. 2007, 618, 51–67. [Google Scholar] [CrossRef] [PubMed]
- Nair, D.; Zhang, S.X.L.; Ramesh, V.; Hakim, F.; Kaushal, N.; Wang, Y.; Gozal, D. Sleep Fragmentation Induces Cognitive Deficits via Nicotinamide Adenine Dinucleotide Phosphate Oxidase-Dependent Pathways in Mouse. Am. J. Respir. Crit. Care Med. 2011, 184, 1305–1312. [Google Scholar] [CrossRef]
- Nair, D.; Ramesh, V.; Gozal, D. Cognitive Deficits Are Attenuated in Neuroglobin Overexpressing Mice Exposed to a Model of Obstructive Sleep Apnea. Front. Neurol. 2018, 9. [Google Scholar] [CrossRef]
- Kaushal, N.; Nair, D.; Gozal, D.; Ramesh, V. Socially Isolated Mice Exhibit a Blunted Homeostatic Sleep Response to Acute Sleep Deprivation Compared to Socially Paired Mice. Brain Res. 2012, 1454, 65–79. [Google Scholar] [CrossRef]
- Puech, C.; Badran, M.; Barrow, M.B.; Runion, A.R.; Gozal, D. Solriamfetol Improves Chronic Sleep Fragmentation-Induced Increases in Sleep Propensity and Ameliorates Explicit Memory in Male Mice. Sleep 2023, zsad057. [Google Scholar] [CrossRef]
- Antunes, M.; Biala, G. The Novel Object Recognition Memory: Neurobiology, Test Procedure, and Its Modifications. Cogn. Process. 2012, 13, 93–110. [Google Scholar] [CrossRef]
- Hammond, R.S.; Tull, L.E.; Stackman, R.W. On the Delay-Dependent Involvement of the Hippocampus in Object Recognition Memory. Neurobiol. Learn. Mem. 2004, 82, 26–34. [Google Scholar] [CrossRef]
- Mazzucco, M.R.; Vartanain, T.; Linden, J.R. In Vivo Blood-Brain Barrier Permeability Assays Using Clostridium Perfringens Epsilon Toxin. Bio-Protoc. 2020, 10, e3709. [Google Scholar] [CrossRef] [PubMed]
- Baganha, F.; Ritsma, L.; Quax, P.H.A.; de Vries, M.R. Assessment of Microvessel Permeability in Murine Atherosclerotic Vein Grafts Using Two-Photon Intravital Microscopy. Int. J. Mol. Sci. 2020, 21, 9244. [Google Scholar] [CrossRef] [PubMed]
- Egawa, G.; Nakamizo, S.; Natsuaki, Y.; Doi, H.; Miyachi, Y.; Kabashima, K. Intravital Analysis of Vascular Permeability in Mice Using Two-Photon Microscopy. Sci. Rep. 2013, 3, 1932. [Google Scholar] [CrossRef] [PubMed]
- Devraj, K.; Guérit, S.; Macas, J.; Reiss, Y. An In Vivo Blood-Brain Barrier Permeability Assay in Mice Using Fluorescently Labeled Tracers. J. Vis. Exp. JoVE 2018, 57038. [Google Scholar] [CrossRef]
- Verstraeten, E. Neurocognitive Effects of Obstructive Sleep Apnea Syndrome. Curr. Neurol. Neurosci. Rep. 2007, 7, 161–166. [Google Scholar] [CrossRef]
- Ferrara, M.; De Gennaro, L.; Casagrande, M.; Bertini, M. Selective Slow-Wave Sleep Deprivation and Time-of-Night Effects on Cognitive Performance upon Awakening. Psychophysiology 2000, 37, 440–446. [Google Scholar] [CrossRef]
- Stewart, C.A.; Auger, R.R.; Enders, F.T.B.; Felmlee-Devine, D.; Smith, G.E. The Effects of Poor Sleep Quality on Cognitive Function of Patients with Cirrhosis. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2014, 10, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wu, J.; Hua, F.; Chen, Y.; Zhan, F.; Xu, G. Sleep Deprivation Induces Cognitive Impairment by Increasing Blood-Brain Barrier Permeability via CD44. Front. Neurol. 2020, 11. [Google Scholar] [CrossRef]
- Zielinski, M.R.; Kim, Y.; Karpova, S.A.; McCarley, R.W.; Strecker, R.E.; Gerashchenko, D. Chronic Sleep Restriction Elevates Brain Interleukin-1 Beta and Tumor Necrosis Factor-Alpha and Attenuates Brain-Derived Neurotrophic Factor Expression. Neurosci. Lett. 2014, 580, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Voirin, A.-C.; Perek, N.; Roche, F. Inflammatory Stress Induced by a Combination of Cytokines (IL-6, IL-17, TNF-α) Leads to a Loss of Integrity on BEnd.3 Endothelial Cells in Vitro BBB Model. Brain Res. 2020, 1730, 146647. [Google Scholar] [CrossRef]
- Jewett, K.A.; Krueger, J.M. Humoral Sleep Regulation; Interleukin-1 and Tumor Necrosis Factor. Vitam. Horm. 2012, 89, 241–257. [Google Scholar] [CrossRef] [PubMed]
- Woodburn, S.C.; Bollinger, J.L.; Wohleb, E.S. The Semantics of Microglia Activation: Neuroinflammation, Homeostasis, and Stress. J. Neuroinflammation 2021, 18, 258. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Gamdzyk, M.; Lenahan, C.; Tang, J.; Tan, S.; Zhang, J.H. The Dual Role of Microglia in Blood-Brain Barrier Dysfunction after Stroke. Curr. Neuropharmacol. 2020, 18, 1237–1249. [Google Scholar] [CrossRef]
- Ronaldson, P.T.; Davis, T.P. Regulation of Blood–Brain Barrier Integrity by Microglia in Health and Disease: A Therapeutic Opportunity. J. Cereb. Blood Flow Metab. 2020, 40, S6–S24. [Google Scholar] [CrossRef] [PubMed]
- Al-Bachari, S.; Naish, J.H.; Parker, G.J.M.; Emsley, H.C.A.; Parkes, L.M. Blood–Brain Barrier Leakage Is Increased in Parkinson’s Disease. Front. Physiol. 2020, 11, 593026. [Google Scholar] [CrossRef]
- Lee, R.-L.; Funk, K.E. Imaging Blood–Brain Barrier Disruption in Neuroinflammation and Alzheimer’s Disease. Front. Aging Neurosci. 2023, 15. [Google Scholar] [CrossRef]
- Lee, H.; Pienaar, I.S. Disruption of the Blood-Brain Barrier in Parkinson’s Disease: Curse or Route to a Cure? Front. Biosci. Landmark Ed. 2014, 19, 272–280. [Google Scholar] [CrossRef]
- Grubac, Z.; Sutulovic, N.; Ademovic, A.; Velimirovic, M.; Rasic-Markovic, A.; Macut, D.; Petronijevic, N.; Stanojlovic, O.; Hrncic, D. Short-Term Sleep Fragmentation Enhances Anxiety-Related Behavior: The Role of Hormonal Alterations. PLoS ONE 2019, 14, e0218920. [Google Scholar] [CrossRef]
- Manchanda, S.; Singh, H.; Kaur, T.; Kaur, G. Low-Grade Neuroinflammation Due to Chronic Sleep Deprivation Results in Anxiety and Learning and Memory Impairments. Mol. Cell. Biochem. 2018, 449, 63–72. [Google Scholar] [CrossRef]
- Irwin, M.R.; Wang, M.; Ribeiro, D.; Cho, H.J.; Olmstead, R.; Breen, E.C.; Martinez-Maza, O.; Cole, S. Sleep Loss Activates Cellular Inflammatory Signaling. Biol. Psychiatry 2008, 64, 538–540. [Google Scholar] [CrossRef]
- Chen, Z.; Gardi, J.; Kushikata, T.; Fang, J.; Krueger, J.M. Nuclear Factor-KappaB-like Activity Increases in Murine Cerebral Cortex after Sleep Deprivation. Am. J. Physiol. 1999, 276, R1812–1818. [Google Scholar] [CrossRef] [PubMed]
- Hurtado-Alvarado, G.; Velázquez-Moctezuma, J.; Gómez-González, B. Chronic Sleep Restriction Disrupts Interendothelial Junctions in the Hippocampus and Increases Blood-Brain Barrier Permeability. J. Microsc. 2017, 268, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Serna-Rodríguez, M.F.; Bernal-Vega, S.; de la Barquera, J.A.O.-S.; Camacho-Morales, A.; Pérez-Maya, A.A. The Role of Damage Associated Molecular Pattern Molecules (DAMPs) and Permeability of the Blood-Brain Barrier in Depression and Neuroinflammation. J. Neuroimmunol. 2022, 371, 577951. [Google Scholar] [CrossRef]
- Gozal, D.; Kheirandish-Gozal, L.; Bhattacharjee, R.; Spruyt, K. Neurocognitive and Endothelial Dysfunction in Children with Obstructive Sleep Apnea. Pediatrics 2010, 126, e1161–e1167. [Google Scholar] [CrossRef] [PubMed]
- Palomares, J.A.; Tummala, S.; Wang, D.J.J.; Park, B.; Woo, M.A.; Kang, D.W.; St Lawrence, K.S.; Harper, R.M.; Kumar, R. Water Exchange across the Blood-Brain Barrier in Obstructive Sleep Apnea: An MRI Diffusion-Weighted Pseudo-Continuous Arterial Spin Labeling Study. J. Neuroimaging Off. J. Am. Soc. Neuroimaging 2015, 25, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Dion-Albert, L.; Dudek, K.A.; Russo, S.J.; Campbell, M.; Menard, C. Neurovascular Adaptations Modulating Cognition, Mood, and Stress Responses. Trends Neurosci. 2023, 46, 276–292. [Google Scholar] [CrossRef]
- Hüls, A.; Robins, C.; Conneely, K.N.; Edgar, R.; De Jager, P.L.; Bennett, D.A.; Wingo, A.P.; Epstein, M.P.; Wingo, T.S. Brain DNA Methylation Patterns in CLDN5 Associated With Cognitive Decline. Biol. Psychiatry 2022, 91, 389–398. [Google Scholar] [CrossRef]
- Debette, S.; Ibrahim Verbaas, C.A.; Bressler, J.; Schuur, M.; Smith, A.; Bis, J.C.; Davies, G.; Wolf, C.; Gudnason, V.; Chibnik, L.B.; et al. Genome-Wide Studies of Verbal Declarative Memory in Nondemented Older People: The Cohorts for Heart and Aging Research in Genomic Epidemiology Consortium. Biol. Psychiatry 2015, 77, 749–763. [Google Scholar] [CrossRef]
- Menard, C.; Pfau, M.L.; Hodes, G.E.; Kana, V.; Wang, V.X.; Bouchard, S.; Takahashi, A.; Flanigan, M.E.; Aleyasin, H.; LeClair, K.B.; et al. Social Stress Induces Neurovascular Pathology Promoting Depression. Nat. Neurosci. 2017, 20, 1752–1760. [Google Scholar] [CrossRef]
- Johnson, A.C.; Uhlig, F.; Einwag, Z.; Cataldo, N.; Erdos, B. The Neuroendocrine Stress Response Impairs Hippocampal Vascular Function and Memory in Male and Female Rats. Neurobiol. Dis. 2022, 168, 105717. [Google Scholar] [CrossRef]
- Minett, T.; Classey, J.; Matthews, F.E.; Fahrenhold, M.; Taga, M.; Brayne, C.; Ince, P.G.; Nicoll, J.A.R.; Boche, D. ; MRC CFAS Microglial Immunophenotype in Dementia with Alzheimer’s Pathology. J. Neuroinflammation 2016, 13, 135. [Google Scholar] [CrossRef] [PubMed]
- Ito, D.; Imai, Y.; Ohsawa, K.; Nakajima, K.; Fukuuchi, Y.; Kohsaka, S. Microglia-Specific Localisation of a Novel Calcium Binding Protein, Iba1. Brain Res. Mol. Brain Res. 1998, 57, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lituma, P.J.; Woo, E.; O’Hara, B.F.; Castillo, P.E.; Sibinga, N.E.S.; Nandi, S. Altered Synaptic Connectivity and Brain Function in Mice Lacking Microglial Adapter Protein Iba1. Proc. Natl. Acad. Sci. USA 2021, 118, e2115539118. [Google Scholar] [CrossRef] [PubMed]
- Umpierre, A.D.; Wu, L.-J. How Microglia Sense and Regulate Neuronal Activity. Glia 2021, 69, 1637–1653. [Google Scholar] [CrossRef]
- Kopp, C.; Longordo, F.; Nicholson, J.R.; Lüthi, A. Insufficient Sleep Reversibly Alters Bidirectional Synaptic Plasticity and NMDA Receptor Function. J. Neurosci. Off. J. Soc. Neurosci. 2006, 26, 12456–12465. [Google Scholar] [CrossRef]
- Huber, R.; Deboer, T.; Tobler, I. Effects of Sleep Deprivation on Sleep and Sleep EEG in Three Mouse Strains: Empirical Data and Simulations. Brain Res. 2000, 857, 8–19. [Google Scholar] [CrossRef]
- Rabat, A.; Gomez-Merino, D.; Roca-Paixao, L.; Bougard, C.; Van Beers, P.; Dispersyn, G.; Guillard, M.; Bourrilhon, C.; Drogou, C.; Arnal, P.J.; et al. Differential Kinetics in Alteration and Recovery of Cognitive Processes from a Chronic Sleep Restriction in Young Healthy Men. Front. Behav. Neurosci. 2016, 10, 95. [Google Scholar] [CrossRef]
- Seda, G.; Matwiyoff, G.; Parrish, J.S. Effects of Obstructive Sleep Apnea and CPAP on Cognitive Function. Curr. Neurol. Neurosci. Rep. 2021, 21, 32. [Google Scholar] [CrossRef]
- Chang, E.-T.; Wang, H.-M. Cognitive Function Was Improved after Continuous Positive Airway Pressure Treatment in Obstructive Sleep Apnea Syndrome. Eur. Respir. J. 2016, 48. [Google Scholar] [CrossRef]
- D’Rozario, A.L.; Hoyos, C.M.; Wong, K.K.H.; Unger, G.; Kim, J.W.; Vakulin, A.; Kao, C.-H.; Naismith, S.L.; Bartlett, D.J.; Grunstein, R.R. Improvements in Cognitive Function and Quantitative Sleep Electroencephalogram in Obstructive Sleep Apnea after Six Months of Continuous Positive Airway Pressure Treatment. Sleep 2022, 45, zsac013. [Google Scholar] [CrossRef]
- Kushida, C.A.; Nichols, D.A.; Holmes, T.H.; Quan, S.F.; Walsh, J.K.; Gottlieb, D.J.; Simon, R.D.; Guilleminault, C.; White, D.P.; Goodwin, J.L.; et al. Effects of Continuous Positive Airway Pressure on Neurocognitive Function in Obstructive Sleep Apnea Patients: The Apnea Positive Pressure Long-Term Efficacy Study (APPLES). Sleep 2012, 35, 1593–1602. [Google Scholar] [CrossRef] [PubMed]
- Crawford-Achour, E.; Dauphinot, V.; Martin, M.S.; Tardy, M.; Gonthier, R.; Barthelemy, J.C.; Roche, F. Protective Effect of Long-Term CPAP Therapy on Cognitive Performance in Elderly Patients with Severe OSA: The PROOF Study. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2015, 11, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, M.E.; Arnedt, J.T.; Stanchina, M.; Millman, R.P.; Aloia, M.S. Normalization of Memory Performance and Positive Airway Pressure Adherence in Memory-Impaired Patients with Obstructive Sleep Apnea. Chest 2006, 130, 1772–1778. [Google Scholar] [CrossRef] [PubMed]
- Franks, K.H.; Rowsthorn, E.; Nicolazzo, J.; Boland, A.; Lavale, A.; Baker, J.; Rajaratnam, S.M.W.; Cavuoto, M.G.; Yiallourou, S.R.; Naughton, M.T.; et al. The Treatment of Sleep Dysfunction to Improve Cognitive Function: A Meta-Analysis of Randomized Controlled Trials. Sleep Med. 2023, 101, 118–126. [Google Scholar] [CrossRef]
- Marcus, C.L.; Moore, R.H.; Rosen, C.L.; Giordani, B.; Garetz, S.L.; Taylor, H.G.; Mitchell, R.B.; Amin, R.; Katz, E.S.; Arens, R.; et al. A Randomized Trial of Adenotonsillectomy for Childhood Sleep Apnea. N. Engl. J. Med. 2013, 368, 2366–2376. [Google Scholar] [CrossRef]
- Felver, -Gant Joshua C. ; Bruce, A.S.; Zimmerman, M.; Sweet, L.H.; Millman, R.P.; Aloia, M.S. Working Memory in Obstructive Sleep Apnea. J. Clin. Sleep Med. 2007, 03, 589–594. [Google Scholar] [CrossRef]
- Gozal, D.; Khalyfa, A.; Qiao, Z.; Almendros, I.; Farré, R. Temporal Trajectories of Novel Object Recognition Performance in Mice Exposed to Intermittent Hypoxia. Eur. Respir. J. 2017, 50. [Google Scholar] [CrossRef]
| Primary Antibodies | Dilution | Manufacturer | Order |
|---|---|---|---|
| Rabbit monoclonal anti Claudin 5 | 1/1000 | Abcam (Abcam (Cambridge, UK)Ab131259 | 1 |
| Rat monoclonal anti Iba1 | 1/500 | Invitrogen (Waltham, MA, USA)MA5-38266 | 2 |
| Goat Polyclonal anti-CD31/PECAM | 10ⴞg/ml | Novus Biological (Englewood, CO, USA)AF3628 | 3 (panel 1) |
| Chicken polyclonal anti GFAP | 1/5000 | Invitrogen (Waltham, MA, USA)PA1-10004 | 3(panel 2) |
| Secondary Antibodies | Dilution | Manufacturer | Order |
| Donkey anti-RabbitAlexa fluor 555 | 1/1000 | Thermofischer (Waltham, MA, USA) A32794 | 1 |
| Donkey anti-RatAlexa fluor 647 | 1/1000 | Thermofischer (Waltham, MA, USA) A48272 | 2 |
| Donkey anti-GoatAlexa fluor 488 | 1/1000 | Thermofischer (Waltham, MA, USA) A32814 | 3 (panel 1) |
| Donkey anti ChickenAlexa fluor 488 | 1/5000 | Jackson ImmunoResearch (West Grove, PA, USA) 703-545-155 | 3 (panel 2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





