1. Introduction
Fabry disease is an X-linked inborn error of glycosphingolipid catabolism caused by mutations in
the α-galactosidase A gene encoding the lysosomal hydrolase, alpha-galactosidase A (α-Gal A) [
1,
2]. The marked deficiency or absence of α-Gal A activity results in systemic accumulation of globotriaosylceramide (GB3) and related glycosphingolipids within the lysosomes, particularly in microvascular endothelial cells, vascular smooth muscle cells, renal tubular cells, podocytes, and cardiomyocytes [
3].
Fabry disease cardiomyopathy (FDCM) is a major determinant of patients’ survival and its management actually represents a main therapeutic challenge. Indeed, the impact of enzyme replacement therapy (ERT) on FDCM is still controversial and while there is agreement that early ERT administration, particularly in the pre-hypertrophic phase, prevents progression of the disease, the advanced form is believed to be irreversible [
4,
5].
Mechanisms of resistance to ERT are, however, still unclear.
In Fabry disease, as in other lysosomal storage disorders, substrate deposits in lysosomes fuel multiple pathogenic cascades that ultimately lead to an inflammatory response [
6]. This may be a causative factor in progression of the underlying lysosomal disorder despite initiation of ERT and may be one possible explanation for the clinical failure of therapy. Recently our group demonstrated that an immune-mediated myocarditis can be histologically recognized in 56% of patients with Fabry disease cardiomyopathy and its incidence correlated with disease severity [
7].
Further studies showed that inflammatory and cardiac remodeling biomarkers are elevated in sera of patients with Fabry disease and positively correlated with Mainz Severity Score Index scores, left ventricular hypertrophy, late gadolinium enhancement at cardiac magnetic resonance, and renal dysfunction [
8]. From a pathogenic point of view, GB3 and its metabolites (as for example lysoGB3) bind to toll-like receptor 4, activating nuclear factor kB and T lymphocytes with subsequent production of proinflammatory cytokines, leading to a chronic inflammatory state and associated vasculopathy [
9].
However, both TNF and IL-6 plasma levels are nonspecific markers that resulted to be elevated in several conditions of chronic heart failure, and correlate with a decreasing functional status as well as with all causes mortality [
10]. These observations forward the need of a sensitive and specific circulating biomarker of myocardial inflammation in order to halt, through administration of low dose immunosuppression, its deleterious effect on disease progression and ERT resistance and improve FDCM treatment. This aim appears even more important if we believe that other organs like kidney, brain, nerves and vessels may be involved in the same pathway and theoretically benefit from the same solutions.
2. Materials and Methods
2.1. Methods
Patient Population
We enrolled all the patients with biopsy-proven and genetical diagnosis of FDCM in the study. Circulating levels of anti-GB3 autoantibodies were compared between patients with the histological presence (Group A) or absence (group B) of myocardial inflammation (negative PCR for the cardiotropic viruses and positive anti-heart and anti-myosin abs).
The study complies with the Declaration of Helsinki; the locally appointed ethics committee approved the research protocol and informed consent was obtained from all subjects.
ELISA Test for Circulating anti-Gb3 Antibodies
To test the hypothesis that the presence of circulating anti GB3 autoantibodies could be a marker of myocardial inflammation in Fabry disease, we developed an assay with a classical indirect ELISA format in our laboratory with the support of BioGeM scarl (Ariano Irpino - AV, Italy, Medical Investigational Research, MIR). The assay used Function-Spacer-Lipid (FSL) - GB3 (GB3 conjugated with the FSL group) absorbed on the Meso Scale Discovery (MSD) plate. To this goal a commercially available FSL-GB3 construct, containing the functional component F (the trisaccharide Galα4Galβ4Glcβ), fused through the spacer (S, O(CH2)3NH spacer SA1), to an activated adipate derivative of dioleoyl- phosphatidyl- ethanolamine (L), was used.
The coating of MSD plates with FSL-GB3 was evaluated by using the commercially available rat anti GB3/CD77 monoclonal IgM together with an HRP conjugated anti-Rat IgM secondary.
In order to verify the suitability of the assay for the analysis of human anti-GB3, N=85 human sera from patients with FDCM were used in the assay in combination with a secondary anti-human Pan Ig antibody. A pool of sera collected from healthy individuals was used as “negative controls”; the others were considered instead as “unknown samples”. The stability of coated plates for one month, and the intra- and inter-assay coefficient of variations were determined as well. FSL-Gb3 Coating: Different tests were performed in order to determine the appropriate amount of FSLGB3 to be used in the assay, the coating temperature and timing, the coating buffer, and the plates to be used.
These preliminary experiments were conducted using the commercially available Rat anti-CD77 monoclonal antibody as primary Ab. The conditions were considered as adequate when the curve generated by plotting OD492 values versus Ab dilutions, the absorbance values generated by the two-fold dilution series showed a r2>0,90 (4-PL r2=0,993). Serial dilutions of samples (1:10-1:100) were tested using plates coated with FSL-Gb3 as identified in initial experiments. Secondary anti-human Pan-Ig was tested at different concentration and the one showing the lowest background was used for the assays. A common method to define a cut-off for discriminating positive samples from the negative ones, is calculated by applying the following formula: cut-off = mean + 3 x standard deviation. In order to increase the stringency, we arbitrarily decided to increase the cut-off by adding 5 x standard deviation. This method for cut off calculation may work as a pilot exercise in determining cut-off score but a larger cohort of samples should be employed for determining the upper- and lower limit for either negative (healthy) or positive (diseased) subjects.
ELISA Test for Anti-myosin and Gb3 Antibodies
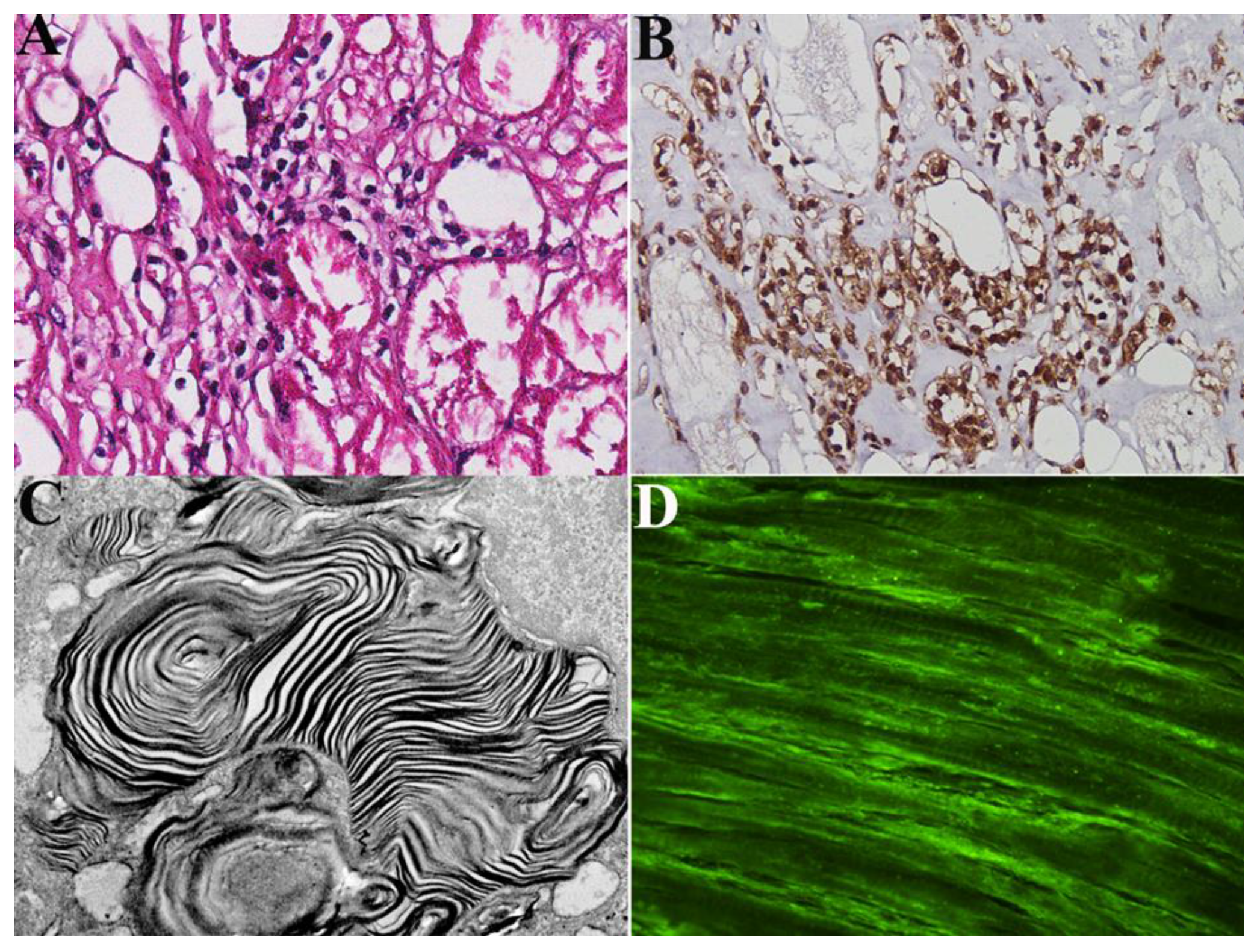
Patient sera were screened for the presence of Gb3 and myosin antibodies, detected by a human myosin and Gb3 ELISA kit. As controls, we used a pool of normal sera from participants matched with patients for age and sex. Standards and undiluted samples were added to the micro-ELISA plate wells and combined with the specific antibody. The optical density proportional to the concentration of human myosin was measured spectrophotometrically at a wavelength of 450 nm2 nm. The detection range of the ELISA kit is 1.56 to 100 ng/mL. The concentration in the samples was calculated comparing the optical density of the samples with the standard curve using the software interface GraphExpert Professional 1.4.0 (Hyams Development) and expressed as ng/mL. Positivity of serum abs for Gb3 was correlated with over-imposition of myocarditis in our patient population of 85 patients with FDCM which diagnosis was obtained with assessment of pathogenic GLA gene mutations and detection of Gb3 accumulation at histology, immunohistochemistry and ultrastructural examination of left ventricular endomyocardial biopsies (
Figure 1).
2.2. Molecular Study
Definition of virus-negative, immune-mediated, overlapping myocarditis followed the presence at histology of ≥ 7 CD3+ T lymphocytes per low microscopic field associated with focal necrosis of the adjacent myocytes (
Figure 1, panel A and B). Negative PCR on two frozen endomyocardial samples for the most common cardiotropic viral genomes including Adenovirus, Epstein Barr Virus, Herpes virus, Parvovirus B19, Cytomegalovirus, Enterovirus, Influenza A/B. Serologic positivity for anti-heart and anti-myosin abs.
2.3. Statistical Analysis
Normal distribution of all continuous variables was checked by visual methods (Q-Q plot and histogram) and by significance test (Kolmogorov-Smirnov normality test and Shapiro-Wilk’s test). For continuous variables, descriptive statistics were provided (number of available observations, mean, standard deviation), while the median (interquartile range) was used for non-normal data. Categorical data were described as a number (percentage). Baseline demographic and clinical characteristics will be presented in table format. Student’s t-test, the χ2 test, and the Fisher exact test were used for comparisons; difference between variables without normal distribution will be tested with the Mann-Whitney U test. For all tests, a P value less than .05 will be considered statistically significant. The receiver-operating characteristics (ROC) curve was used to describe the performance and predictive accuracy of circulating anti-GB3 antibody as biomarker of myocardial inflammation in FDCM. The sensitivity, specificity, and area under the curve (AUC) were calculated, and the cut-off value was determined by the Youden index. All statistical analyses were performed using R statistical analysis software.
3. Results
From January 1996 to December 2021, we enrolled eighty-five patients with histological diagnosis of FDCM; forty-eight (56.5%) of them had an overlapping myocardial inflammation with negative PCR for the common cardiotropic viruses (Group A). Demographic, clinical, and instrumental characteristics of the two Groups are reported in
Table 1. Overall, no significant difference was reported between the two Groups.
Circulating anti-Gb3 Antibodies
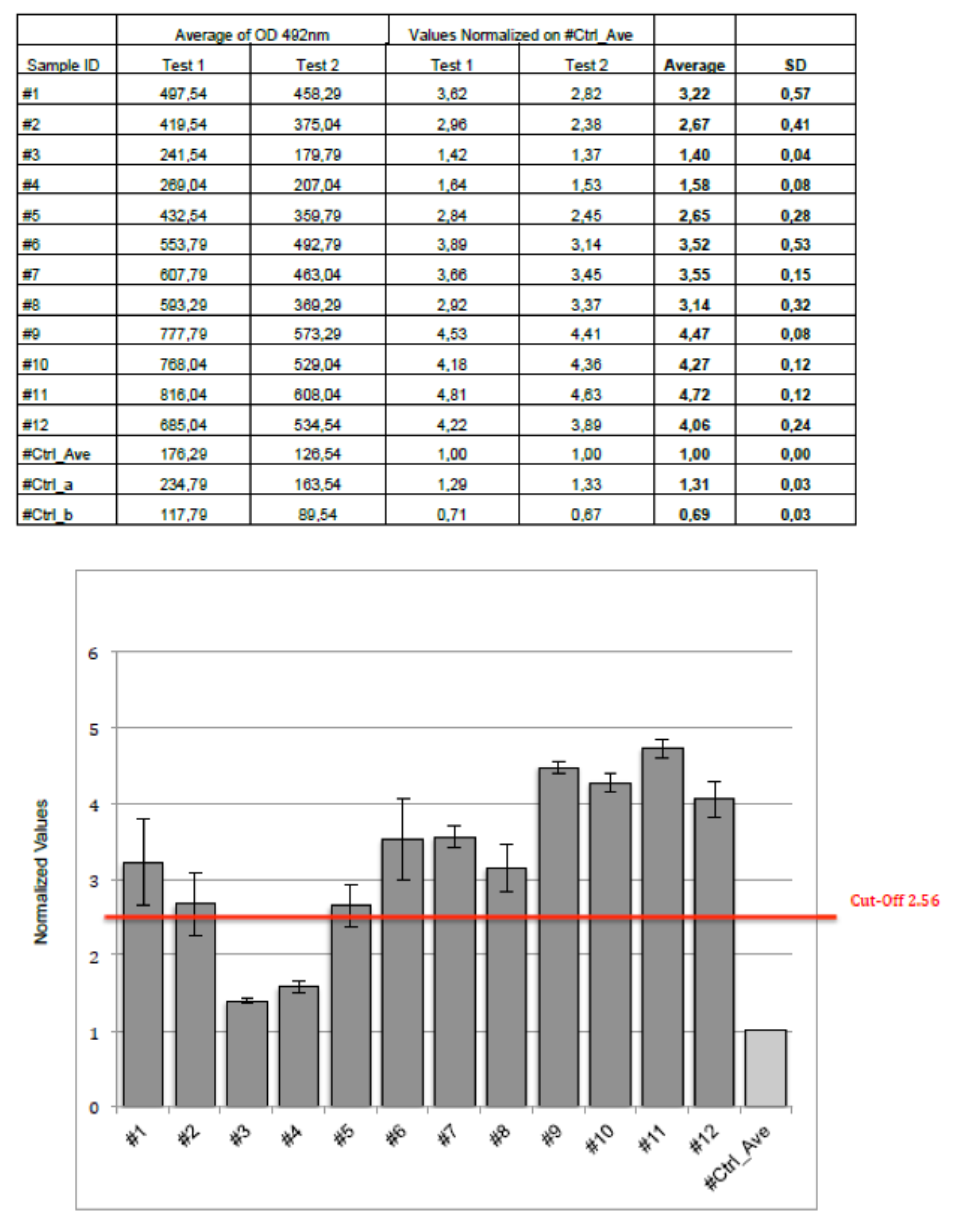
The normalized values of the circulating anti-Gb3 antibodies assays were performed twice on twelve controls; the values were summarized in the
Figure 2. As previously described, the calculated cut-off was 2.56.
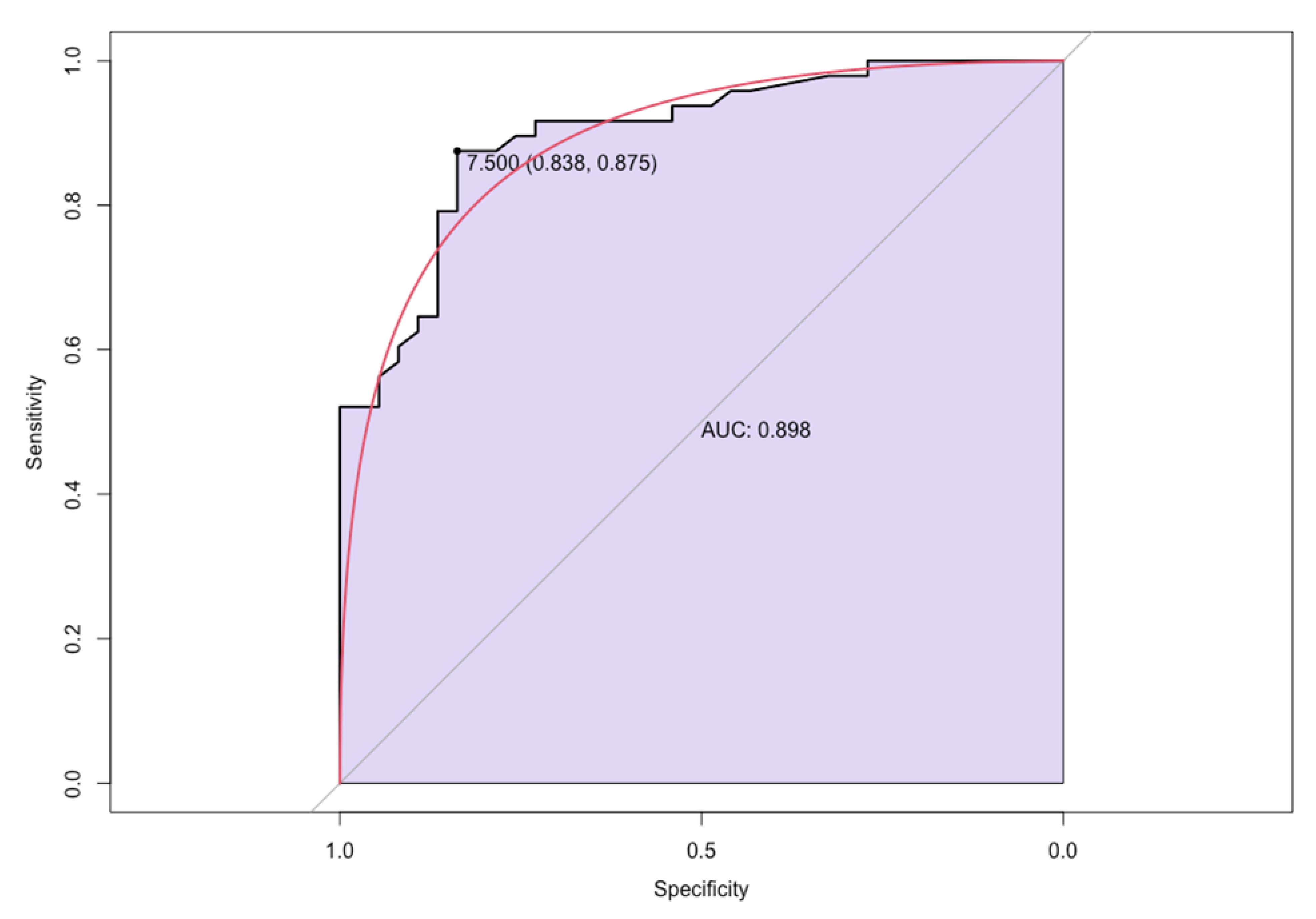
The assay results showed that 42 out of 48 FDCM patients with myocarditis were positive for anti-Gb3 antibodies while 30 FDCM patients on 37 without myocarditis had negative anti GB3 antibodies. The value of the circulating anti-Gb3 antibodies in the Group A was significantly higher than in Group B [15.85 (IQR: 11.4 – 22.8) vs 1.9 (IQR: 1.7 – 2.4); p-value: 0.001]. According to the ROC analysis, the optimal cut-off value of circulating anti- GB3 antibody in FDCM patients for predicting myocardial inflammation was >7.5, with a sensitivity of 83.8% and a specificity of 87.5% (AUC was 0.898, 95% confidence interval 0.854–0.942;
Figure 3).
All patients with overlapping myocarditis at histology were positive for serologic assessment of anti-heart abs (see
Figure 1, panel D).
4. Discussion
There is growing evidence that myocardial inflammation can be a cause of progression of Fabry disease cardiomyopathy as well as an unnoticed mechanism of disease resistance to ERT. Indeed, myocardial inflammation promotes cell dysfunction and death causing expansion of interstitial space through oedema, inflammatory cells infiltration and myocardial fibrosis, that result in both ventricular diastolic and systolic dysfunction.
With regard to the origin of myocardial inflammation associated to Fabry disease cardiomyopathy, the absence of viral genomes in the myocardium and the strong positivity of anti-heart and antimyosin antibodies described, suggest myocardial inflammation to have an autoimmune/dys-reactive origin [
7]. To this regard GB3, the major component of Fabry disease storage material, is constantly released by the engulfed cardiomyocytes along a constitutional secretory pathway or as a result of cell necrosis. GB3 is recognized as a powerful immunogenic molecule that may promote and perpetuate an inflammatory autoimmune disorder. Indeed, a pro-oxidative and pro-inflammatory state that correlates with elevated GB3 levels urinary has been described [
11,
12].
The main objective of the present study was the identification of a reliable biomarker of myocardial inflammation over imposing in human FDCM. Indeed, comparing serologic positivity of anti-Gb3 abs with presence at histology of left ventricular endomyocardial biopsies, presence of inflammation by serology was confirmed with a sensitivity of 83.8 % and a specificity of 87.5%. Identification of a sensitive and specific circulating biomarker of cardiac inflammation in patients with FDCM resistant to ERT administration could allow the introduction of immunosuppressive therapy that may halt the progression of the cardiomyopathy and improve the efficacy of ERT. A better understanding of the autoimmune/inflammatory activation in Fabry disease and its role in the disease morbidity and mortality will change the therapeutic approach to the disease allowing to identify patients with high autoimmune/inflammatory burden who will benefit in addition to ERT of an immunosuppressive treatment. Positive results of a wider HDCM population are, however, necessary for implementation of ERT with immunosuppressive therapy.
5. Conclusions
In conclusion, serologic positivity of anti-Gb3 abs represent a reliable biomarker of myocardial inflammation in FDCM. It may suggest ERT implementation with immune-suppressive therapy to enhance ERT impact and improve patient’s outcome, particularly in those patients with progressive impairment of cardiac function and/or occurrence of complex/life-threatening cardiac arrhythmias. Further perspective double blind clinical studies are, however, necessary for inclusion of immune-suppression in the therapeutic regimen of progressive FDCM with inflammation.
Author Contributions
Author Contributions: AF designed the study, participated in and lead the categorization of the subjects, and wrote the manuscript, RV, RS, and LS categorized and organized the data and experiment. AF, and MAR discussed and verified the cases, MM performed the statistics and collected the data. All authors participated in drafting the manuscript and approved the final manuscript
Funding
The study was supported by Takeda International, grants number: IIR-ITA-00252 and partially by the Italian Health Ministry (IRCCS San Raffaele Roma – Ricerca Corrente #2020/1) and Fondazione Roma (MEBIC # 18/6/2019). Mebic San Raffaele Pisana..
Institutional Review Board Statement
This study was approved by the ethics committee, opinion number 6/2019
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
|
α-Gal A
|
Alpha-galactosidase A. |
| AUC |
Area Under Curve. |
| ERT |
Enzyme Replacement Therapy. |
| FDCM |
Fabry Disease Cardiomyopathy. |
| FSL |
Function-Spacer-Lipid. |
| GB3 |
Globotriaosylceramide. |
| MSD |
Meso Scale Discovery. |
| ROC |
Receiver-Operating Characteristics. |
References
- Desnick, R.J.; Wasserstein, M.P.; Banikazemi, M. Fabry Disease (α-Galactosidase A Deficiency): Renal Involvement and Enzyme Replacement Therapy. In Contributions to Nephrology; Schieppati, A., Daina, E., Sessa, A., Remuzzi, G., Eds.; KARGER: Basel, 2001; Volume 136, pp. 174–192. ISBN 978-3-8055-7278-1. [Google Scholar]
- Zarate, Y.A.; Hopkin, R.J. Fabry’s Disease. The Lancet 2008, 372, 1427–1435. [Google Scholar] [CrossRef] [PubMed]
- Germain, D.P. Fabry Disease. Orphanet J Rare Dis 2010, 5, 30. [Google Scholar] [CrossRef] [PubMed]
- Rombach, S.M.; Smid, B.E.; Bouwman, M.G.; Linthorst, G.E.; Dijkgraaf, M.G.W.; Hollak, C.E.M. Long Term Enzyme Replacement Therapy for Fabry Disease: Effectiveness on Kidney, Heart and Brain. Orphanet J Rare Dis 2013, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Weidemann, F.; Niemann, M.; Störk, S.; Breunig, F.; Beer, M.; Sommer, C.; Herrmann, S.; Ertl, G.; Wanner, C. Long-term Outcome of Enzyme-replacement Therapy in Advanced F Abry Disease: Evidence for Disease Progression towards Serious Complications. J Intern Med 2013, 274, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Mauhin, W.; Lidove, O.; Masat, E.; Mingozzi, F.; Mariampillai, K.; Ziza, J.-M.; Benveniste, O. Innate and Adaptive Immune Response in Fabry Disease. In JIMD Reports, Volume 22; Zschocke, J., Baumgartner, M., Morava, E., Patterson, M., Rahman, S., Peters, V., Eds.; JIMD Reports; Springer Berlin Heidelberg: Berlin, Heidelberg, 2015; Volume 22, ISBN 978-3-662-47452-5. [Google Scholar]
- Frustaci, A.; Verardo, R.; Grande, C.; Galea, N.; Piselli, P.; Carbone, I.; Alfarano, M.; Russo, M.A.; Chimenti, C. Immune-Mediated Myocarditis in Fabry Disease Cardiomyopathy. JAHA 2018, 7, e009052. [Google Scholar] [CrossRef] [PubMed]
- Yogasundaram, H.; Nikhanj, A.; Putko, B.N.; Boutin, M.; Jain-Ghai, S.; Khan, A.; Auray-Blais, C.; West, M.L.; Oudit, G.Y. Elevated Inflammatory Plasma Biomarkers in Patients With Fabry Disease: A Critical Link to Heart Failure With Preserved Ejection Fraction. JAHA 2018, 7, e009098. [Google Scholar] [CrossRef] [PubMed]
- Rozenfeld, P.; Feriozzi, S. Contribution of Inflammatory Pathways to Fabry Disease Pathogenesis. Molecular Genetics and Metabolism 2017, 122, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Rauchhaus, M.; Doehner, W.; Francis, D.P.; Davos, C.; Kemp, M.; Liebenthal, C.; Niebauer, J.; Hooper, J.; Volk, H.-D.; Coats, A.J.S.; et al. Plasma Cytokine Parameters and Mortality in Patients With Chronic Heart Failure. Circulation 2000, 102, 3060–3067. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, P.N.; Mucci, J.M.; Ceci, R.; Fossati, C.A.; Rozenfeld, P.A. Higher Apoptotic State in Fabry Disease Peripheral Blood Mononuclear Cells. Molecular Genetics and Metabolism 2011, 104, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Biancini, G.B.; Vanzin, C.S.; Rodrigues, D.B.; Deon, M.; Ribas, G.S.; Barschak, A.G.; Manfredini, V.; Netto, C.B.O.; Jardim, L.B.; Giugliani, R.; et al. Globotriaosylceramide Is Correlated with Oxidative Stress and Inflammation in Fabry Patients Treated with Enzyme Replacement Therapy. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 2012, 1822, 226–232. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).