1. Introduction
Ultrafine bubbles (UFBs), which are in some cases called nanobubbles, are defined as gas-filled bubbles with a volume equivalent diameter of less than 1 μm in ISO 20480-1:2017[
1]. The existence of UFBs and their peculiar effect is increasingly attracting attention in pure and applied science and technology, covering many areas, such as the physicochemical, medical, and biological fields, among others [
2,
3,
4,
5,
6,
7]. In the agricultural field, there is now accumulating evidence that UFBs and microbubbles enhance the growth process. Park and Kurata reported that microbubbles in hydroponic nutrient solution resulted in about twice as much lettuce growth compared to that achieved with the control solution [
8]. It was also reported that microbubble generation in nutrient solutions promoted lettuce growth [
9]. Additionally, it was found that the fresh and dry weights of shoots were higher in tap water with UFBs than in tap water without UFBs in another application of UFBs to lettuce production in hydroponic systems [
10]. A similar effect was shown by Ebina et al., where the growth of
Brassica campestris cultured hydroponically for 4 weeks within air nanobubble water was significantly promoted compared to normal water [
11]. The effect of UFB on rice production was also examined not only in a laboratory experiment, but also in a field experiment by Wang et al. [
12]. They found that UFB stimulated gibberellin growth hormone synthesis and upregulated the plant nutrient absorption genes in rice seedlings and that UFB treatment significantly increased rice yield by almost 8% more than the control, also resulting in approximately 25% less fertilizer compared to the control in a field experiment.
As seed germination is very important at the beginning stages of plant growth, the effect of UFBs on seed germination has also been investigated. Liu et al. reported that UFB water exhibited a longer NMR
T2 value than that of control water, which resulted in an increase in the mobility of water molecules and a higher germination ratio in barley seeds [
13]. The promotion of seed germination due to UFB has also been examined in several aspects: ROS (reactive oxygen species) production in UFB water [
14,
15]; a change in gene expression within the seed [
16]; the number concentration of UFBs [
17]; different kinds of gases composing UFBs [
18]; and via a comparison between various priming treatments [
19].
These studies proved that the growth of plants, starting from seed germination, is promoted by UFB. This fact induces the following question: what is the role of UFB number concentration in growth promotion? However, the answer is yet to be elucidated. As for the UFB promotion effect mechanism, it was suggested that OH radicals—a type of reactive oxygen species (ROS)—detected using a sensitive fluorescence probe, APF, in UFB water are one of the factors that stimulate the promotion of growth [
15,
18]. On the other hand, the opposite finding was reported using numerical simulation, suggesting that no OH radicals are produced from dissolving UFBs, and that hydrogen peroxide (H
2O
2) is produced inside an ozone or oxygen microbubble in water during hydrodynamic or acoustic cavitation due to violent collapse [
20,
21,
22,
23].
In light of this situation, in this study, the promotion effect of UFB on seed germination was examined in order to ensure the UFB number concentration role. As a target sample, barley seeds were selected because barley is very well known as a model crop in plant breeding methodology, genetics, cytogenetics, pathology, virology, and biotechnology studies [
24]. In parallel to germination examination, the detection of OH radicals in oxygen (O
2) UFB water was reattempted using ESR to confirm OH radical generation in UFB water with the presence of no external stimuli.
2. Materials and Methods
2.1. Seed Germination
2.1.1. Air UFBs for the use of seed germination and its measuring device
An ultrafine bubble generator (GALF FZ1N-10, IDEC Corporation, Japan), a kind of venturi-type generator [
7] with a pressure dissolution system, the pressure of which (just after the pressurizing pump) is around 700 kPa, and a saturator around 300 kPa were used for generating air UFBs in water. The UFB generator was equipped with a 15 L water tank and the circulation flow rate of distilled water (Autostill WA-53, Yamato Scientific Co., Lid.) was 0.83 L min
-1. An air filter (KIC-T6, AS ONE Corporation, Japan) with 0.01 μm filtration accuracy was mounted on the front of the gas inlet. The UFB generator was operated for 10 to 120 min to generate the roughly desired number concentrations of UFBs. After the generation of air UFBs, UFB waters were stored at 25 ℃ overnight in order to stabilize UFBs. They were then used for a seed germination test.
The number concentration and bubble size distribution of the UFBs were measured using a commercial device via the particle tracking analysis method (NanoSight-LM10, Quantum Design Inc., Japan), the measuring range of which was between 50 and 1000 nm, with a laser light source wavelength of 635 nm and 40 mW of power, a black and white CCD mounted camera and NTA 3.1 Build 3.1.46 analysis software. Measurements were made at a room temperature of around 22 degrees Celsius.
2.1.2. Seed material
To examine the fundamental UFB number concentration effect, barley seeds (
Hordeum vulagre L., cv. Kobinkatagi) were used as the material. A germination ratio of about 100% is normally expected due to the good quality of this type of barley seed. For this reason, the fundamental aspect of UFBs’ effect on germination can be examined by simply evaluating the germination process using only T
50, as outlined in
Section 2.1.4.
To examine the effect of excess UFB number concentration, barley seeds (
Hordeum vulagre L., cv. Yumesakiboshi) were used as the material. This barley seed is known to have a low germination ratio by nature even though the seed’s quality is good, and UFBs’ effect on germination is expected to be observed more easily according to two parameters, T
50 and G
max, as outlined in
Section 2.1.4. There is only one report indicating the possibility of carrot seed germination suppression at higher UFB number concentrations [
15]. The experimental setup in this study aimed to indicate that there is a certain upper limit of UFB number concentration beyond which UFBs negatively affect seed germination.
2.1.3. Germination test
Germination tests were performed with three seed groups for UFB water and control water sections. Each group was composed of 50 seeds in a net-like plastic bag. Each group of barley seeds was submerged in glass beakers with a volume of 2 L of distilled water (control) and UFB waters containing different UFB number concentrations in a ratio of 10 mL of water per seed. UFB waters containing 4 different number concentrations were used to examine the fundamental effect and those containing 3 different number concentrations were used to examine the excess number concentration effect. During the germination tests, control water and UFB waters were changed twice daily to avoid a lack of oxygen and to maintain a certain amount of UFBs in water. Germination tests were performed in the dark at 25℃. The germination ratio obtained from three independent replicates of each group was shown as the mean value.
2.1.4. Analysis of germination process
The germination process was analyzed using a dose–response model, as follows [
25]:
where G
max is the maximum germination ratio, t
i is the time for each inspection, T
50 is the time at which the inferred germination ratio is 50% of G
max, G(t
i) is the observed germination ratio for each inspection and B is the slope at the time T
50. We set t
0.1 as the starting time of calculation instead of t
0 in order to avoid the calculation of log 0 for smooth data analysis, which was conducted using VBA software developed with Microsoft Excel.
2.2. Evaluation of ROS in UFB water
2.2.1. Oxygen UFBs for ROS detection
The same UFB generator described above was used to generate O
2 UFBs with the aid of the IDEC Corporation. Distilled water (FUJIFILM Wako chemicals, Japan) was poured into the water tank and pure O
2 was supplied from a gas inlet during the operation of the UFB generator for about 1 h to generate O
2 UFBs. After O
2 UFB water was poured into glass bottles and sealed without headspace, it was stored for 20 d at 4 ℃. Then, it was concentrated using vaporization under reduced pressure to reach about 1/200 of the initial volume, according to the procedure described in the patent [
26]. Foreign matter was then filtered out from the concentrated O
2 UFB water using a polycarbonate membrane with a filtration accuracy of 0.20 μm (K020A025A, ADVANTEC TOYO KAISHA, LTD., Japan). After filtration, a high concentration of O
2 UFB water (“O
2 UFB water” is used hereinafter) was poured into 4 vials with a volume of 3 mL and sealed without headspace. They were then stored for another 10 d at 4 ℃ before ESR measurements were conducted.
2.2.2. Electron spin resonance (ESR) measurement
An X-band (9.8 GHz) ESR spectrometer (EMX-plus, Bruker Japan) equipped with 100 kHz field modulation was used for the ESR measurements for the detection of ROS. The spectrometer settings were as follows: resonance field of ∼3372–3672 G; 1 G field modulation width; 6 mW microwave power; 0.1 s time constant. ESR spectra were accumulated at room temperature. As a spin trapping reagent, 80 mM of 5-(2,2-dimethyl-1, 3-propoxy cyclophoryl)-55-methil-1-pyrroline N-oxide (CYPMPO, MW=247.23) was used to detect OH radicals.
O2 UFB water with 80 mM of CYPMPO was added to a disposable flat cell with a RDC-60-S syringe (FlashPoint Co., Ltd., Japan). ESR measurements were then conducted to examine the formation of adducts between ROS and CYPMPO under the condition that no dynamic stimuli were applied to the O2 UFB water. Another ESR measurement was also conducted to confirm the generation of OH radicals by detecting the hydroxyl adduct of CYPMPO (CYPMPO-OH adduct) induced by the application of ultrasonic sound (43 kHz, 80W) for 30 s to the O2 UFB water with 80 mM of CYPMPO in a disposable flat cell. All measurements were carried out at room temperature.
3. Results and Discussion
3.1. Fundamental aspect of the effect of UFBs on seed germination
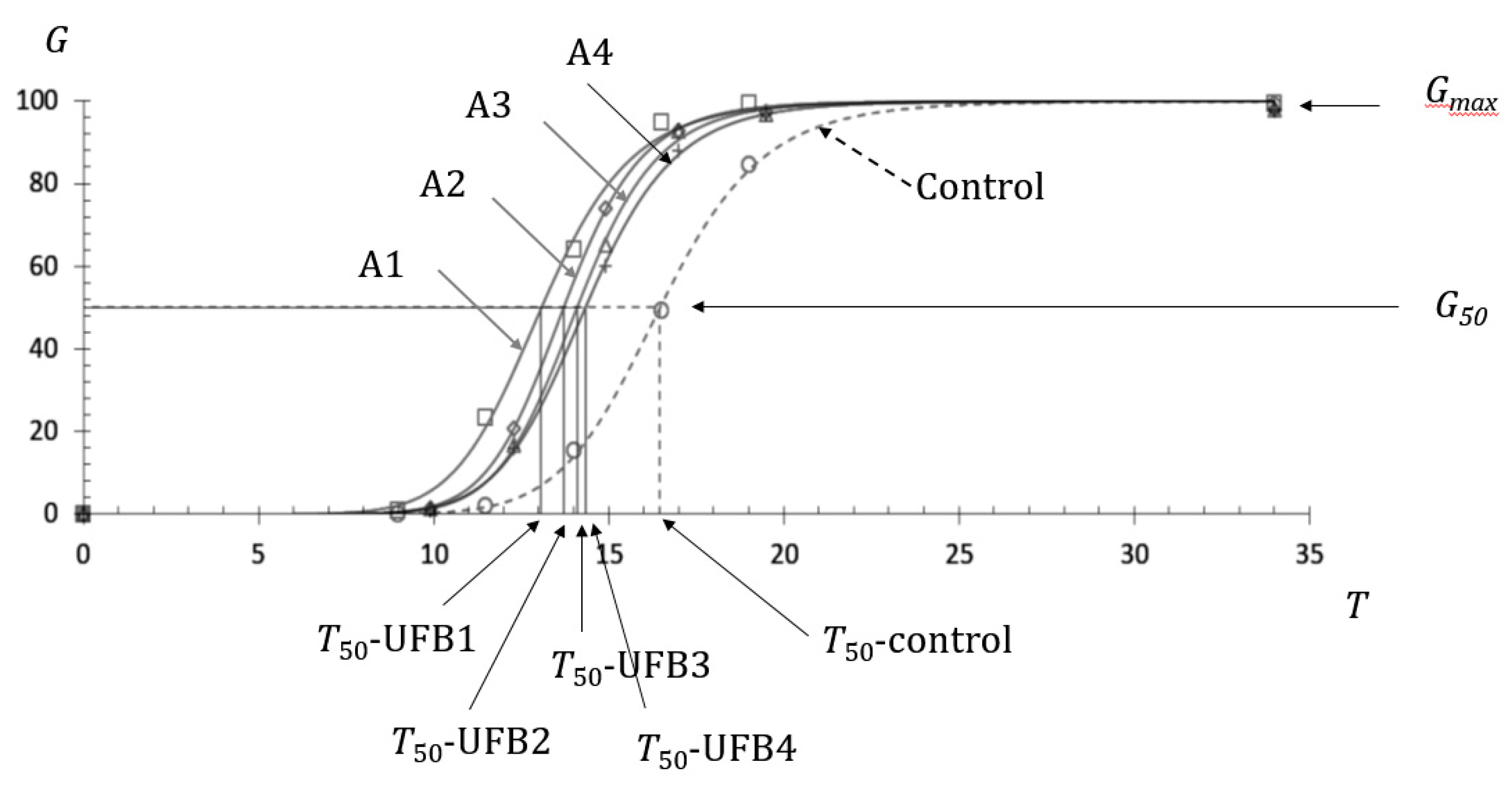
Figure 1 shows the observed germination ratios of seeds submerged in UFB1 (□), UFB2 (◇), UFB3 (△), and UFB4 (+) water at each inspection time, together with those of seeds in control water (○). Each regression curve indicating the seed germination process in UFB1, UFB2, UFB3, UFB4, and control water was obtained using Equation (1) and denoted as A1, A2, A3, A4, and control, respectively. The number concentration of UFB1 to UFB4 showed the relationship (UFB1>UFB2>UFB3>UFB4), as shown in
Table 1, together with each mean diameter.
The T
50 of seeds in UFB1 to UFB4 are indicated in
Figure 1 and the actual values of T
50 are provided in
Table 1. Their relationship is shown as (T
50-UFB1< T
50-UFB2< T
50-UFB3< T
50-UFB4). A significant difference between T
50-UFB1 and T
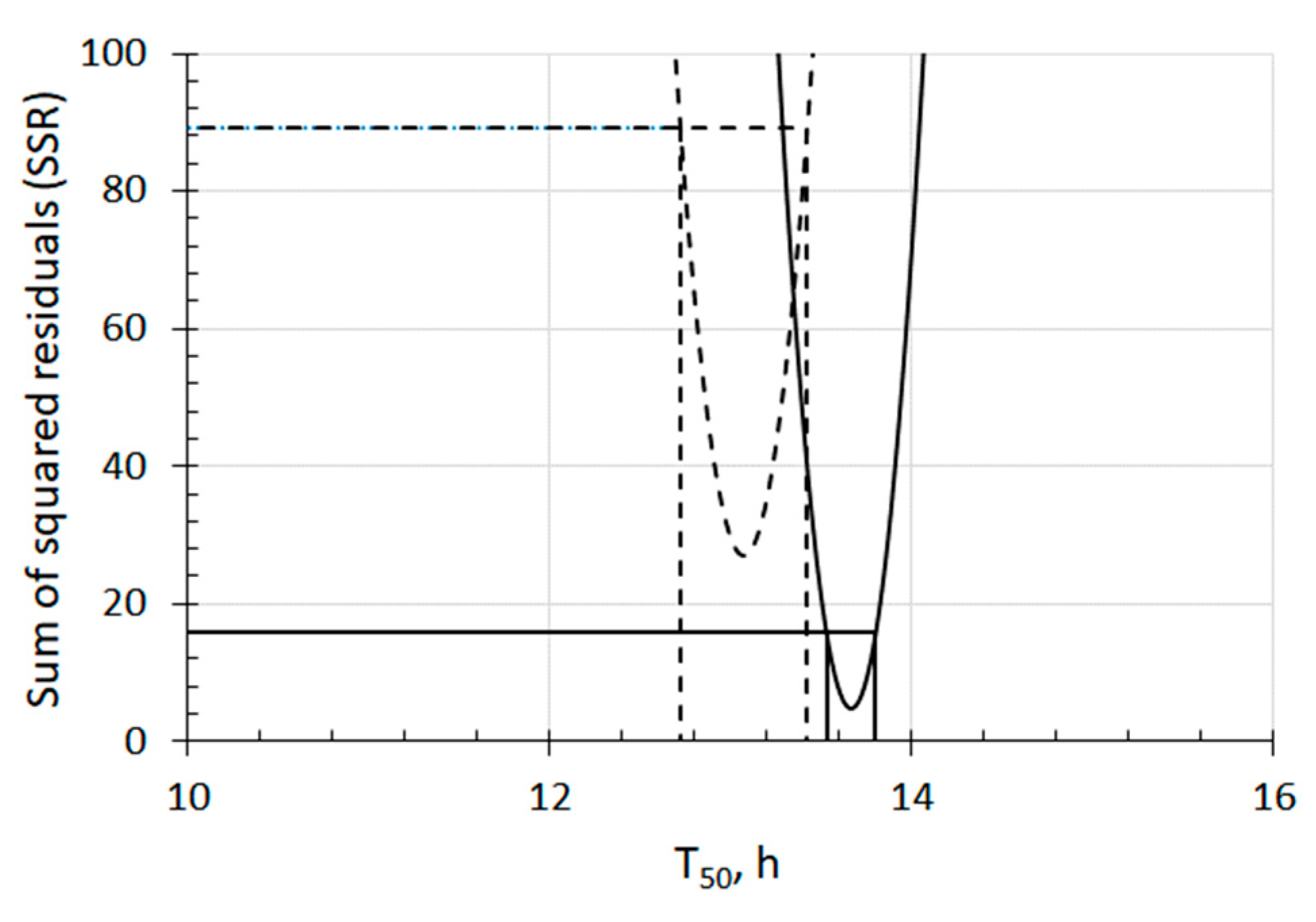
50-UFB2 was observed, as supported by
Figure 2. In the same way, a significant difference was also observed among T
50-UFB2, T
50-UFB3 and T
50-UFB4 (data not shown). As the T
50-control was 16.5 h, as seen in
Figure 1, with a shorter T
50 indicating earlier seed germination, all UFB waters from UFB1 to UFB4 induced the promotion of seed germination compared with the seeds in the control water.
Dissolved oxygen concentrations (DO) of UFB waters measured just after they were added glass beakers were 9.4 mgL-1, 9.2 mgL-1, 8.8 mgL-1, 8.8 mgL-1, and 7.1 mgL-1 for UFB1, UFB2, UFB3, UFB4, and control water, respectively. It can therefore be said that germination promotion did not only occur due to the high DO of UFB waters. This is supported by our preliminary experiment using another group of barley seeds, where the germination ratio of seeds in UFB water for which DO was adjusted to 8.2 mgL-1 was 61% and that in control water with the same DO was 43% at an inspection time of 12 h after submerging. In other words, UFB affects seed germination promotion even under the same DO.
Although UFB growth promotion is acknowledged as described in the Introduction, the number concentration role remains unaccounted for. About this issue, the results shown here presented the fundamental aspect of the effect of UFBs on seed germination. That is to say, a higher UFB number concentration leads to a greater seed germination promotion effect within a proper number concentration range, such as the range set in this experiment.
Figure 1.
Promotion of UFBs on barley seed germination of cv. Kobinkatagi in four different UFB number concentrations in water, indicating the highest number concentration (UFB1), which resulted in the highest promotion effect. G indicates the germination ratio in %, T indicates time in h and A1, A2, A3, and A4 indicate the germination process of seeds submerged in water containing number concentrations of UFB1, UFB2, UFB3, and UFB4, respectively.
Figure 1.
Promotion of UFBs on barley seed germination of cv. Kobinkatagi in four different UFB number concentrations in water, indicating the highest number concentration (UFB1), which resulted in the highest promotion effect. G indicates the germination ratio in %, T indicates time in h and A1, A2, A3, and A4 indicate the germination process of seeds submerged in water containing number concentrations of UFB1, UFB2, UFB3, and UFB4, respectively.
Figure 2.
Sum of squared residuals (SSR) and 95% confidence intervals of T50 of both UFB1 and UFB2 barley seed sections. Dashed and solid curves show an SSR of T50 for seeds in UFB1 and UFB2 water, respectively. The segments of horizontal lines bounded by curves of SSR indicate a 95% confidence interval. A significant difference is observed, as they do not overlap with each other.
Figure 2.
Sum of squared residuals (SSR) and 95% confidence intervals of T50 of both UFB1 and UFB2 barley seed sections. Dashed and solid curves show an SSR of T50 for seeds in UFB1 and UFB2 water, respectively. The segments of horizontal lines bounded by curves of SSR indicate a 95% confidence interval. A significant difference is observed, as they do not overlap with each other.
Table 1.
Characteristics of UFB water from UFB1 to UFB4 and T50 of seeds germinated in each different UFB water.
Table 1.
Characteristics of UFB water from UFB1 to UFB4 and T50 of seeds germinated in each different UFB water.
| |
UFB1 |
UFB2 |
UFB3 |
UFB4 |
| Number concentration ± SD, mL-1
|
8.5 x 108±3.6 x 107
|
5.0 x 108±3.7x107
|
3.8 x 108±4.8x107
|
1.4 x 108±1.7x107
|
| Mean diameter ±SD, nm |
127.3±4.7 |
123.5±2.8 |
135.9±4.1 |
132.5±7.5 |
| T50, h |
13.1 |
13.7 |
14.1 |
14.3 |
3.2. Negative effect on seed germination caused by excess number concentration
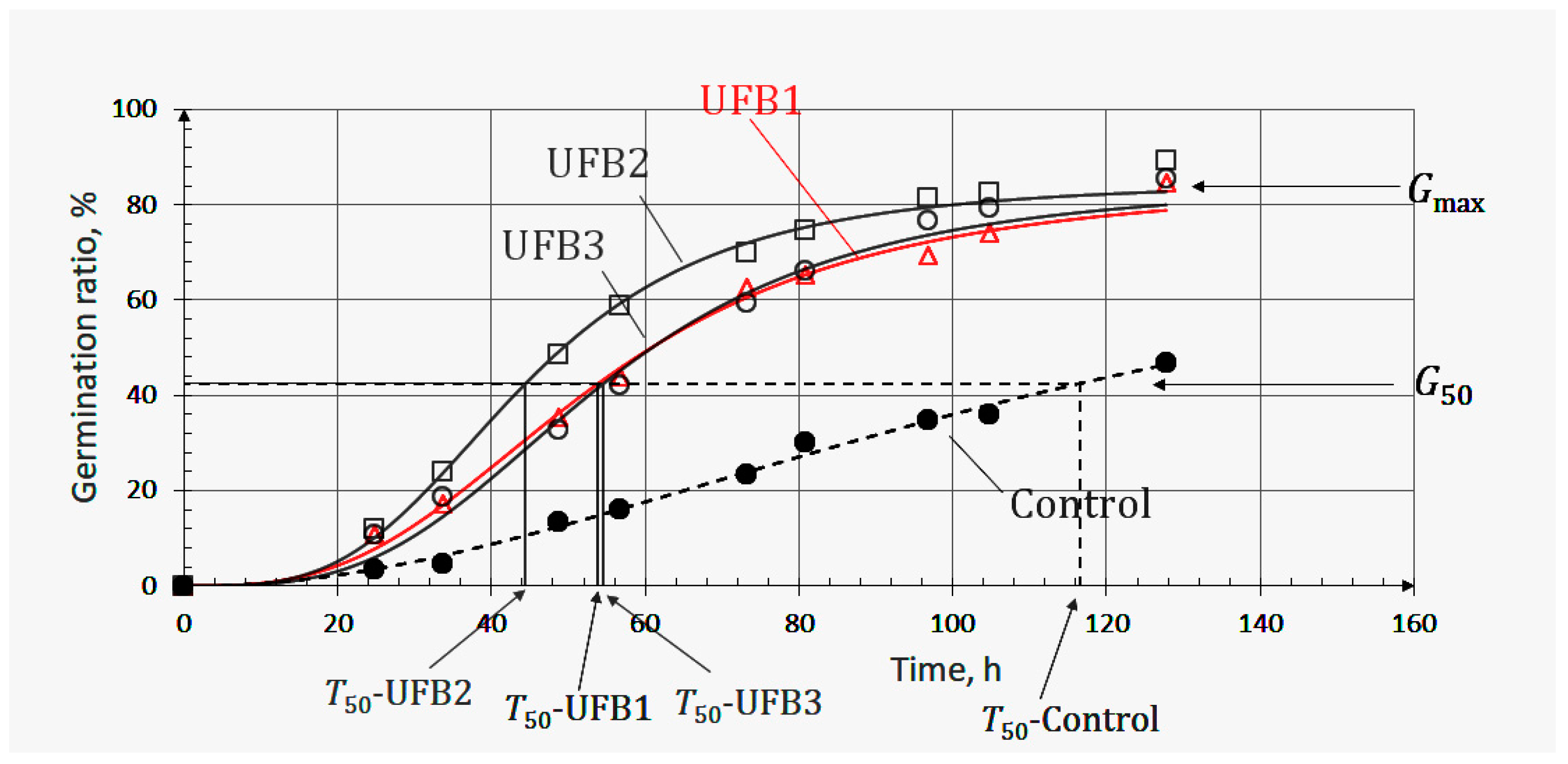
Figure 3 shows the observed germination ratios of seeds submerged in UFB1 (△), UFB2 (□), and UFB3 (○) water at each inspection time together with those of seeds in control water (●). Each regression curve indicating the germination process of seeds in UFB1, UFB2, UFB3, and control water was obtained using Equation (1) and denoted by UFB1, UFB2, UFB3, and control, respectively. The number concentration of UFB1 to UF3 showed the relationship (UFB1>UFB2>UFB3), as shown in
Table 2, together with each mean diameter.
In the context of the knowledge clarified in the previous section, a higher UFB number concentration is expected to lead to greater seed germination promotion. However, the seed regression curve in UFB1 water containing the highest number concentration appeared under that of seeds in UFB2 water, of which the number concentration was lower than that of UFB1. This is also supported quantitatively by the T50 and Gmax parameters, that is, (T50-UFB1>T50-UFB2) and (Gmax-UFB1<Gmax-UFB2). In other words, UFB1 water had a negative effect on seed germination.
On the other hand, seed germination in UFB2 and UFB3 water followed the fundamental aspect of the effect of UFB observed in the previous section. The higher number concentration led to a higher germination ratio, that is, the relationship (UFB2>UFB3) achieved the results as (T50-UFB2<T50-UFB3) and (Gmax-UFB2>Gmax-UFB3).
The results shown here mean that there is an upper limit of UFB number concentration beyond which seed germination is suppressed, and UFB1 is thought to have exceeded this upper limit. This understanding is supported by previous research in plant physiology field. Bailly et al. suggested a model named the “oxidative window” to account for the dual role, toxic or signaling effects of ROS generated within seeds, indicating that seed germination is only possible when the ROS content of the seed is within the range of the oxidative window [
27]. Our previous paper indicated that the submerging of seeds in UFB water contributes to producing higher levels of endogenous ROS (superoxide radicals, O
2●-) than that in distilled (control) water [
14]. Considering these factors, only the number concentration of UFB1 water induced ROS in seeds, of which the content was beyond the upper limit of the oxidative window, and the number concentrations of other UFB waters stimulated to produce ROS in seeds, the levels of which were maintained at an amount that triggered regular cellular events associated with germination, such as hormone signaling. In other words, UFB was proven to stimulate seeds to produce endogenous ROS, upregulating seed germination, which had a positive correlation with the UFB number concentration as long as the content of endogenous ROS were within the oxidative window.
Figure 3.
Positive and negative effect of UFB on germination of barley seeds (cv. Yumesakiboshi) in three different UFB number concentrations in water, with the highest number concentration (UFB1) exerting a negative effect on seed germination. UFB1, UFB2, and UFB3 indicate the germination process of seeds submerged in water containing the number concentrations of UFB1, UFB2, and UFB3, respectively.
Figure 3.
Positive and negative effect of UFB on germination of barley seeds (cv. Yumesakiboshi) in three different UFB number concentrations in water, with the highest number concentration (UFB1) exerting a negative effect on seed germination. UFB1, UFB2, and UFB3 indicate the germination process of seeds submerged in water containing the number concentrations of UFB1, UFB2, and UFB3, respectively.
Table 2.
Characteristics of UFB water from UFB1 to UFB3 and T50 of seeds germinated in each UFB water sample.
Table 2.
Characteristics of UFB water from UFB1 to UFB3 and T50 of seeds germinated in each UFB water sample.
| |
UFB1 |
UFB2 |
UFB3 |
| Number concentration ± SD, mL-1
|
1.0 x 109±4.4 x 107
|
7.4 x 108±9.0x107
|
2.4 x 108±3.3x107
|
| Mean diameter ±SD, nm |
136.8±3.4 |
147.1±5.4 |
143.6±5.7 |
| T50, h |
53.9 |
44.4 |
54.6 |
3.3. A possible factor promoting seed germination
In the previous two sections, two aspects of UFB are shown. One is that a higher UFB number concentration leads to a greater seed germination promotion effect within a proper number concentration range, corresponding with the oxidative window. The other is that there is an UFB number concentration upper limit, beyond which seed germination is suppressed. In light of this, we pose the following question: what is the factor attributed to UFB water that affects the promotion or suppression of seed germination? A powerful clue is provided in many studies explaining that exogenous H
2O
2, one of ROS, plays a role in promoting seed germination [
28,
29,
30,
31]. We paid particular attention to this and found in the previous paper that OH radicals, but not H
2O
2, were generated in UFB water, which stimulated the generation of endogenous ROS to promote/suppress vegetable seed germination [
15]. However, negative opinions about the generation of OH radicals in UFB water free from the influence of cavitation have also been published [
20,
21,
22,
23]. Thus, we conducted systematic germination tests on barley seeds and found that UFB number concentration has either a positive or negative correlation with germination depending on its concentration. This suggested that the OH radicals are produced in UFB water, as described in the next section.
3.4. Detection of ROS in O2 UFB water
The O
2 UFB water number concentrations in the four vials were 1.1 x 10
11 mL
-1, 1.0 x 10
11 mL
-1, 1.3 x 10
11 mL
-1, and 1.2 x 10
11 mL
-1.
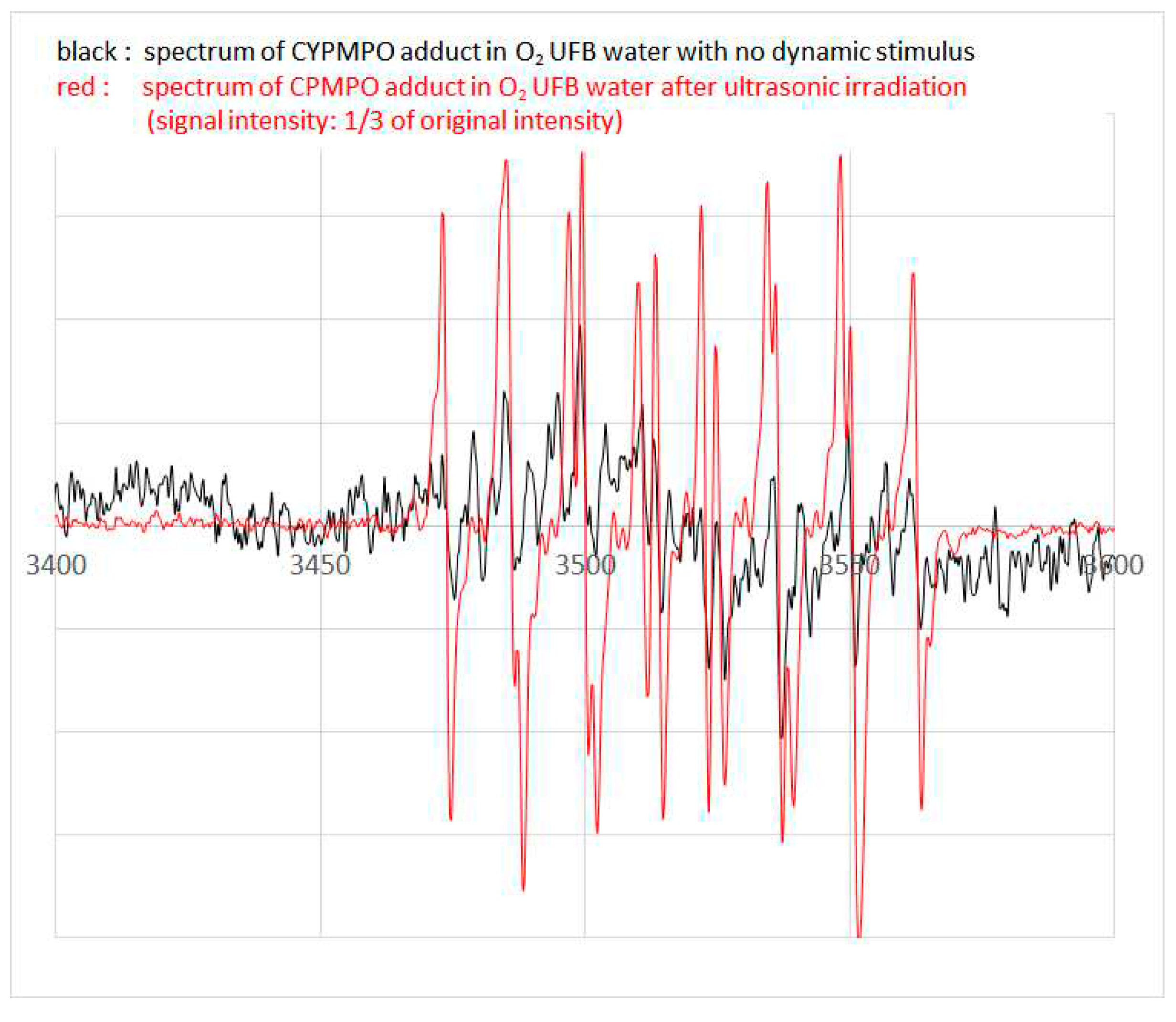
Figure 4 shows the representative spectra observed from O
2 UFB water without exposure to any dynamic stimuli (black line) and that after ultrasonic irradiation (red line). The signal intensity of the latter was reduced three times from its original signal intensity, producing a visible improvement. Both spectra clearly demonstrate the typical spectra of the CYPMPO-OH adduct [
32,
33]. Similar spectra were also observed in other O
2 UFB waters with and without ultrasonic irradiation taken from three different vials (data not shown).
The detection of the CYPMPO-OH adduct in O
2 UFB water after ultrasonic irradiation is a natural consequence, as the violent collapse of UFBs caused by ultrasonic irradiation results in the formation of OH radicals [
22,
23,
34,
35]. Interestingly, we observed the CYPMPO-OH adduct in the O
2 UFB water without the presence of any dynamic stimuli.
In our previous paper, the OH radicals was observed in O
2 UFB water using an APF fluorescence probe [
14,
15]. However, numerical simulations indicate that the ROS signals observed after the generation of UFBs did not originate in OH radicals, but instead originated in H
2O
2 [
21,
22,
23]. This opinion is based on the phenomenon that an appreciable amount of H
2O
2 is produced from violent collapses of cavitation bubbles during UFB generation. The lifetime of OH radicals is as short as 1 ns [
36] or 20 ns [
22] and that of H
2O
2 is generally between hours and days [
37]. Therefore, the O
2 UFB water used in this study was stored for a total of 30 d before ESR measurements were conducted to assure the disappearance of not only OH radicals, but also the H
2O
2 produced during UFB generation. With this storage treatment, both OH radicals and H
2O
2 produced during cavitation [
23] can be excluded from O
2 UFB water used for ESR measurement. The possibility of OH radicals production by causing a chemical reaction between H
2O
2 and O
3 [
23] can also be excluded because the O
2 UFB water used at the time of ESR measurement contains neither H
2O
2 nor O
3. Furthermore, it is also suggested that the OH radicals detected in the experiments could not have originated from dissolving bubbles [
23]. The numerical simulations quoted here seem to be convincing; however, we must assume that the detected signals observed in this study were from OH radicals that existed in O
2 UFB water stored for a long period without being exposed to any dynamic stimuli. For this reason, the following question still remains: how can OH radicals be generated in O
2 UFB water without being exposed to dynamic stimuli?
From another perspective, the observation of OH radicals in O2 UFB water without any dynamic stimuli during a long storage period provides evidence of the long-term existence of UFBs as distinct from the foreign matter that is inevitably found in water, although this is a qualitative estimation.
Figure 4.
ESR spectra of CYPMPO-OH adducts observed from O2 UFB waters. The black line shows the signal intensity of CYPMPO-OH adduct observed from O2 UFB water without any dynamic stimuli and the red line shows that observed from O2 UFB water after ultrasonic irradiation at 1/3 of its original signal intensity.
Figure 4.
ESR spectra of CYPMPO-OH adducts observed from O2 UFB waters. The black line shows the signal intensity of CYPMPO-OH adduct observed from O2 UFB water without any dynamic stimuli and the red line shows that observed from O2 UFB water after ultrasonic irradiation at 1/3 of its original signal intensity.
4. Conclusions
In this study, barley seed germination tests were systematically conducted to examine the role of UFB number concentration. It was found that UFB number concentration has either a positive or negative correlation with germination depending on concentration. This implies that the production of ROS in UFB water may play an exogenous role in ROS stimulation, generating endogenous ROS in seeds to promote/suppress seed germination. Thus, we conducted ESR measurements and detected OH radicals in O2 UFB water without exposure to dynamic stimuli. Based on these results, it is suggested that UFB number concentration has a correlation with OH radicals production, which induces the promotion or suppression of germination depending on its content. Our finding on the role of number concentration of UFBs will provide scientific information when UFB water is applied to seeds aiming to germination promotion as one of applications of UFBs to agricultural production. Finally, it is necessary to consider the possibility of a phenomenon in which OH radicals can be produced in UFB water other than during the process of UFB dissolution.
Author Contributions
Conceptualization and methodology, S.O., S.B., H.K. and M.Y.; investigation, S.O., S.B., H.K., M.Y. and I.S.; writing—original draft preparation, S.O.; writing—review and editing, S.B., H.K., M.Y. and I.S.; funding acquisition, S.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partly funded by the Ministry of Economy, Trade and Industry (METI), Japan, as a part of the international standardization project for fine bubbles.
Acknowledgments
We acknowledge the Fine Bubble Department, IDEC CORPORATION, Japan for their kind cooperation in the production of highly concentrated O2 UFB water. We also acknowledge Prof. Hideo Nakamura at Hokkaido University of Education for his fruitful discussion on the characteristic features of CYPMPO.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- ISO20480-1:2017. Available online: https://www.iso.org/obp/ui/#iso:std:iso:20480:-1:ed-1:v1:en (accessed on 22 February, 2023).
- Ushikubo, F.Y.; Furukawa, T.; Nakagawa, R.; Enari, M.; Makino, Y.; Kawagoe, Y.; Shiina, T.; Oshita, S. Evidence of the existence and the stability of nano-bubbles in water. Colloids Surfaces A: Physicochem. Eng. Asp. 2010, 361, 31–37, . [CrossRef]
- Uchida, T.; Oshita, S.; Ohmori, M.; Tsuno, T.; Soejima, K.; Shinozaki, S.; Take, Y.; Mitsuda, K. Transmission electron microscopic observations of nanobubbles and their capture of impurities in wastewater. Nanoscale Res. Lett. 2011, 6, 295–295, . [CrossRef]
- Alheshibri, M.; Qian, J.; Jehannin, M.; Craig, V.S.J. A History of Nanobubbles. Langmuir 2016, 32, 11086–11100, . [CrossRef]
- Wang, X.; Lei, Z.; Shimizu, K.; Zhang, Z.; Lee, D.-J. Recent advancements in nanobubble water technology and its application in energy recovery from organic solid wastes towards a greater environmental friendliness of anaerobic digestion system. Renew. Sustain. Energy Rev. 2021, 145, . [CrossRef]
- Patel, A.K.; Singhania, R.R.; Chen, C.-W.; Tseng, Y.-S.; Kuo, C.-H.; Wu, C.-H.; Di Dong, C. Advances in micro- and nano bubbles technology for application in biochemical processes. Environ. Technol. Innov. 2021, 23, 101729, . [CrossRef]
- Terasaka, K.; Yasui, K.; Kanematsu, W.; Aya, N. Eds. Ultrafine Bubbles. Jenny Stanford: Singapore 2022.
- Park, J. S.; Kurata, K. Application of microbubbles to hydroponics solution promotes lettuce growth. HortTechnology 2009, 19, 212-215.
- Park, J. S.; Ohashi, K.; Kurata, K.; Lee, J. W. Promotion of lettuce growth by application of microbubbles in nutrient solution using different rates of electrical conductivity and under periodic intermittent generation in a deep flow technique culture system. Europ. J. Hort. Sci. 2010, 75(5), 198-203.
- Kobayashi, N.; Yamaji, K. Leaf lettuce (Lactuca sativa L. ‘L-121’) growth in hydroponics with different nutrient solutions used to generate ultrafine bubbles. J. Plant Nutr. 2021, 45, 816–827, . [CrossRef]
- Ebina, K.; Shi, K.; Hirao, M.; Hashimoto, J.; Kawato, Y.; Kaneshiro, S.; Morimoto, T.; Koizumi, K.; Yoshikawa, H. Oxygen and Air Nanobubble Water Solution Promote the Growth of Plants, Fishes, and Mice. PLOS ONE 2013, 8, e65339, . [CrossRef]
- Wang, Y.; Wang, S.; Sun, J.; Dai, H.; Zhang, B.; Xiang, W.; Hu, Z.; Li, P.; Yang, J.; Zhang, W. Nanobubbles promote nutrient utilization and plant growth in rice by upregulating nutrient uptake genes and stimulating growth hormone production. Sci. Total. Environ. 2021, 800, 149627, . [CrossRef]
- Liu, S.; Kawagoe, Y.; Makino, Y.; Oshita, S. Effects of nanobubbles on the physicochemical properties of water: The basis for peculiar properties of water containing nanobubbles. Chem. Eng. Sci. 2013, 93, 250–256, . [CrossRef]
- Liu, S.; Oshita, S.; Makino, Y.; Wang, Q.; Kawagoe, Y.; Uchida, T. Oxidative Capacity of Nanobubbles and Its Effect on Seed Germination. ACS Sustain. Chem. Eng. 2015, 4, 1347–1353, . [CrossRef]
- Liu, S.; Oshita, S.; Kawabata, S.; Makino, Y.; Yoshimoto, T. Identification of ROS Produced by Nanobubbles and Their Positive and Negative Effects on Vegetable Seed Germination. Langmuir 2016, 32, 11295–11302, . [CrossRef]
- Liu, S.; Oshita, S.; Kawabata, S.; Thuyet, D. Q. Nanobubble water’s promotion effect of barley (Hordeum vulgare L.) sprouts supported by RNA-Seq analysis. Langmuir. 2017, 33, 12478-12486. [CrossRef]
- Oshita, S.; Kamijo, Y.; Pham, T. Q. A.; Yoshimura, M.; Sotome, I.; Kameya, H.; Fujita, T.; Liu, S. Number concentration of ultrafince bubble being effective in promoting barley seed. Japanese J. Multiphase Flow. 2020, 34 (1), 194-204.
- Ahmed, A.K.A.; Shi, X.; Hua, L.; Manzueta, L.; Qing, W.; Marhaba, T.; Zhang, W. Influences of Air, Oxygen, Nitrogen, and Carbon Dioxide Nanobubbles on Seed Germination and Plant Growth. J. Agric. Food Chem. 2018, 66, 5117–5124, . [CrossRef]
- Siregar, I.Z.; Muharam, K.F.; Purwanto, Y.A.; Sudrajat, D.J. Seed germination characteristics in different storage time of Gmelina arborea treated with ultrafine bubbles priming. Biodiversitas J. Biol. Divers. 2020, 21, . [CrossRef]
- Yasui, K.; Tuziuti, T.; Kanematsu, W. Extreme conditions in a dissolving air nanobubble. Phys. Rev. E 2016, 94, 013106–013106, . [CrossRef]
- Yasui, K.; Tuziuti, T.; Kanematsu, W. Mysteries of bulk nanobubbles (ultrafine bubbles); stability and radical formation. Ultrason. Sonochemistry 2018, 48, 259–266, . [CrossRef]
- Yasui, K.; Tuziuti, T.; Kanematsu, W. Mechanism of OH radical production from ozone bubbles in water after stopping cavitation. Ultrason. Sonochemistry 2019, 58, 104707, . [CrossRef]
- Yasui, K. On Some Aspects of Nanobubble-Containing Systems. Nanomaterials 2022, 12, 2175, . [CrossRef]
- Akar, T.; Avci, M.; Dusunceli, F. Barley: Post-Harvest Operations 2004. FAO, Post_Harvest_Compendium_-_BARLEY.pdf (fao.org) (accessed on 22 February, 2023).
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-Response Analysis Using R. PLOS ONE 2015, 10, e0146021, . [CrossRef]
- IDEC Corporation, High density fine bubble liquid generation method and high density fine bubble liquid generation apparatus, JP Patent JP5715272B2, 20 March 2015.
- Bailly, C.; El-Maarouf-Bouteau, H.; Corbineau, F. From intracellular signaling networks to cell death: the dual role of reactive oxygen species in seed physiology. Comptes Rendus Biol. 2008, 331, 806–814, . [CrossRef]
- Sarath, G.; Hou, G.; Baird, L.M.; Mitchell, R.B. Reactive oxygen species, ABA and nitric oxide interactions on the germination of warm-season C4-grasses. Planta 2007, 226, 697–708, . [CrossRef]
- Ishibashi, Y.; Tawaratsumida, T.; Kondo, K.; Kasa, S.; Sakamoto, M.; Aoki, N.; Zheng, S.-H.; Yuasa, T.; Iwaya-Inoue, M. Reactive Oxygen Species Are Involved in Gibberellin/Abscisic Acid Signaling in Barley Aleurone Cells. Plant Physiol. 2012, 158, 1705–1714, . [CrossRef]
- Liu, Y.; Ye, N.; Liu, R.; Chen, M.; Zhang, J. H2O2 mediates the regulation of ABA catabolism and GA biosynthesis in Arabidopsis seed dormancy and germination. J. Exp. Bot. 2010, 61, 2979–2990, . [CrossRef]
- Ishibashi, Y.; Koda, Y.; Zheng, S.-H.; Yuasa, T.; Iwaya-Inoue, M. Regulation of soybean seed germination through ethylene production in response to reactive oxygen species. Ann. Bot. 2012, 111, 95–102, . [CrossRef]
- Kameya, H. Evaluation of Hydroxyl Radical and Alkyl-oxy Radical Scavenging Activity of Coffee by ESR Spin Trapping Method. J. Food Sci. Eng. 2017, 7, . [CrossRef]
- Oowada, S.; Endo, N.; Kameya, H.; Shimmei, M.; Kotake, Y. Multiple free-radical scavenging capacity in serum. J. Clin. Biochem. Nutr. 2012, 51, 117–121, . [CrossRef]
- Fang, X.; Mark, G.; von Sonntag, C. OH radical formation by ultrasound in aqueous solutions Part I: the chemistry underlying the terephthalate dosimeter. Ultrason. Sonochemistry 1996, 3, 57–63, . [CrossRef]
- Gogate, P.R.; Shirgaonkar, I.Z.; Sivakumar, M.; Senthilkumar, P.; Vichare, N.P.; Pandit, A.B. Cavitation reactors: Efficiency assessment using a model reaction. AIChE J. 2001, 47, 2526–2538, . [CrossRef]
- Donoghue, M.A.; Xu, X.; Bernlohr, D.A.; Arriaga, E.A. Capillary electrophoretic analysis of hydroxyl radicals produced by respiring mitochondria.. Anal. Bioanal. Chem. 2013, 405, 6053–60, . [CrossRef]
- Petri, B. G.; Watts, R. J.; Teel, A. L.; Huling, S. G.; Brown, R. A. Fundamentals of ISCO using hydrogen peroxide. In In Situ Cemical Oxidation for Groundwater Remediation; Siegrist, R. L.; Crimi, M.; Simpkin, T. J., Eds.; Springer Science+Business Media: New York, 2011, 33-88.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).