Submitted:
19 April 2023
Posted:
20 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
Working memory deficits in PD
Episodic memory deficits in PD
2. Materials and Methods
2.1. Participants
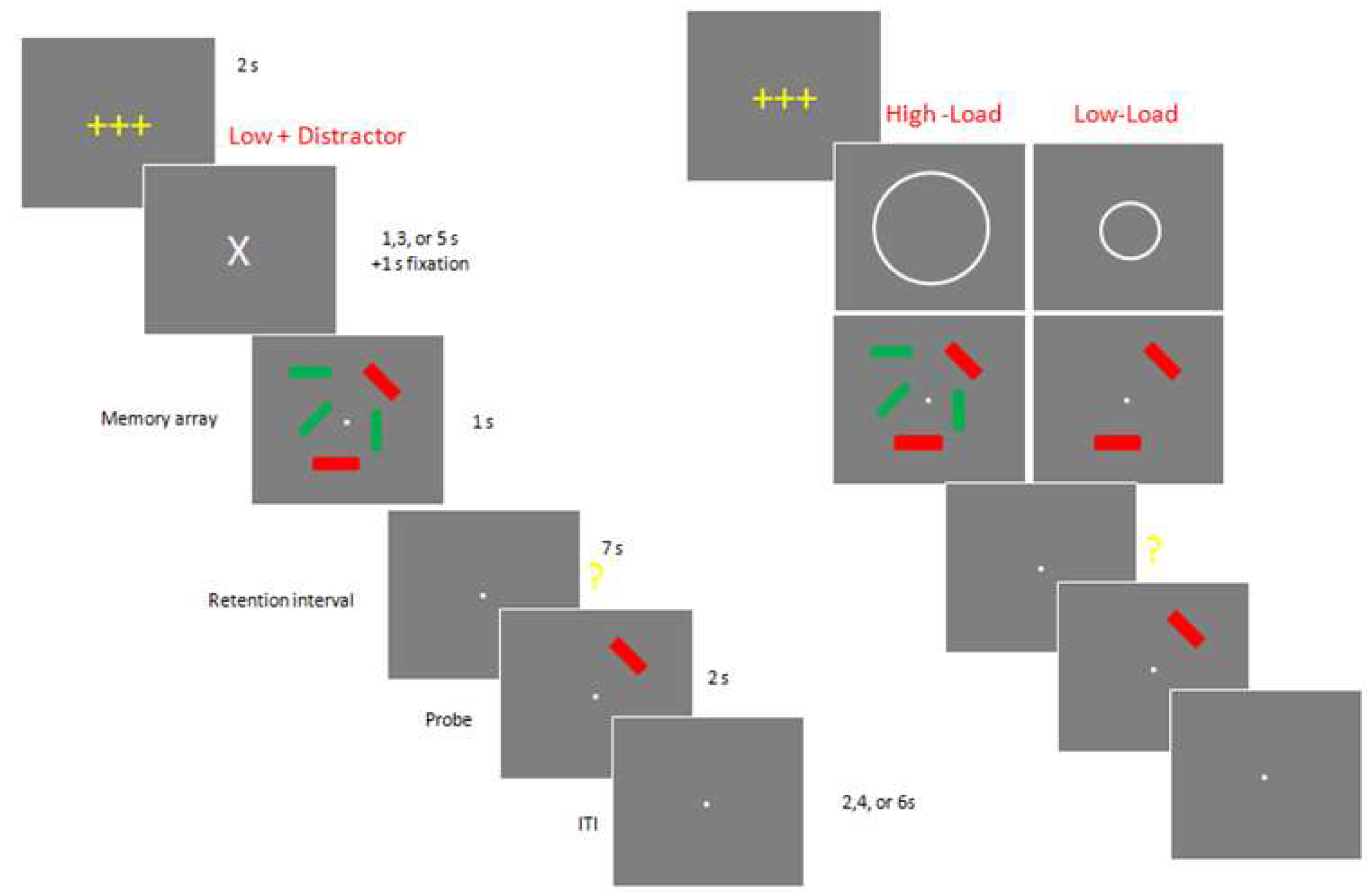
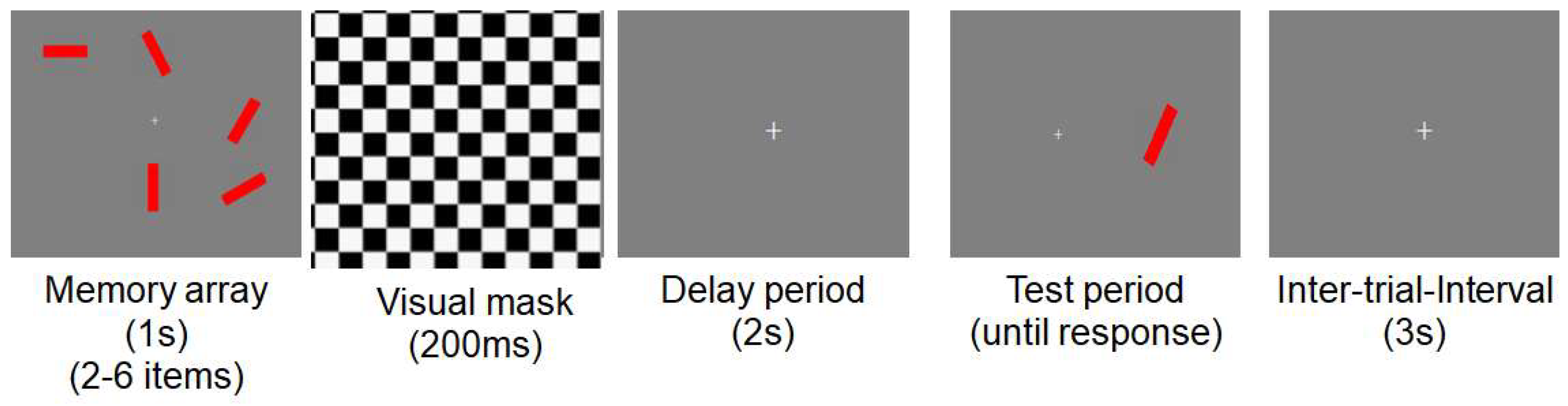
2.2. Stimuli and Procedures for Neuropsychological Experiments
2.3. MRI image acquisition and image processing
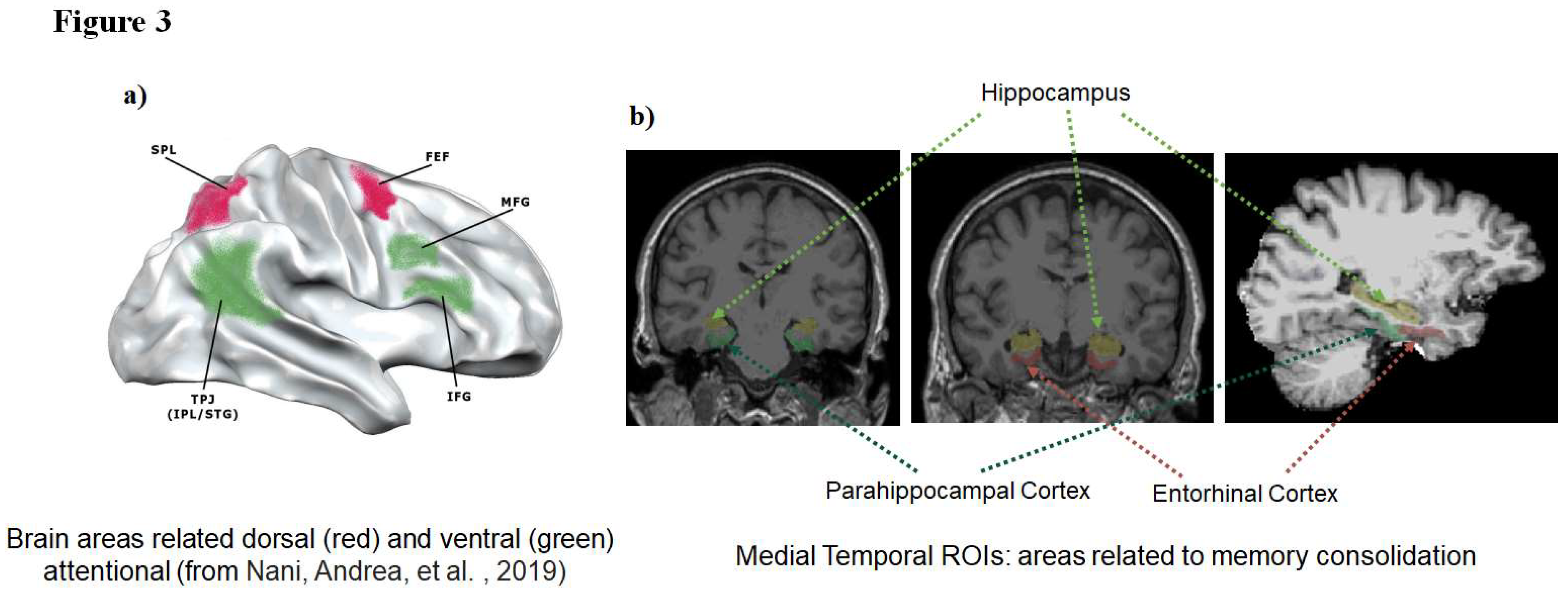
2.3.1. Brain regions of interest
2.3.2. Hippocampal volumes and cortical thickness
2.4. Statistical Analysis
3. Results
3.1. Demographics
| Controls (N=22) |
PD Patients (N=19) |
p-values | ||
|---|---|---|---|---|
| a. Demographics | ||||
| Age (y) | 69.05 ± 5.58 | 66.16 ± 8.81 | 0.211 | |
| Gender (m/f) | 12/10 | 14/5 | 0.205 | |
| Education (years) | 14.77 ± 3.19 | 16.63 ± 3.25 | 0.073 | |
| MMSE | 29.40 ± 0.99 | 29.00 ± 1.29 | 0.284 | |
| Hoehn & Yahr Scale | 0 | 2.03 ± 0.77 | ||
| Disease duration (years) | 0 | 6.65 ± 4.76 | ||
| GDS | 2.78 | 4.11 ± 3.11 | 0.147 | |
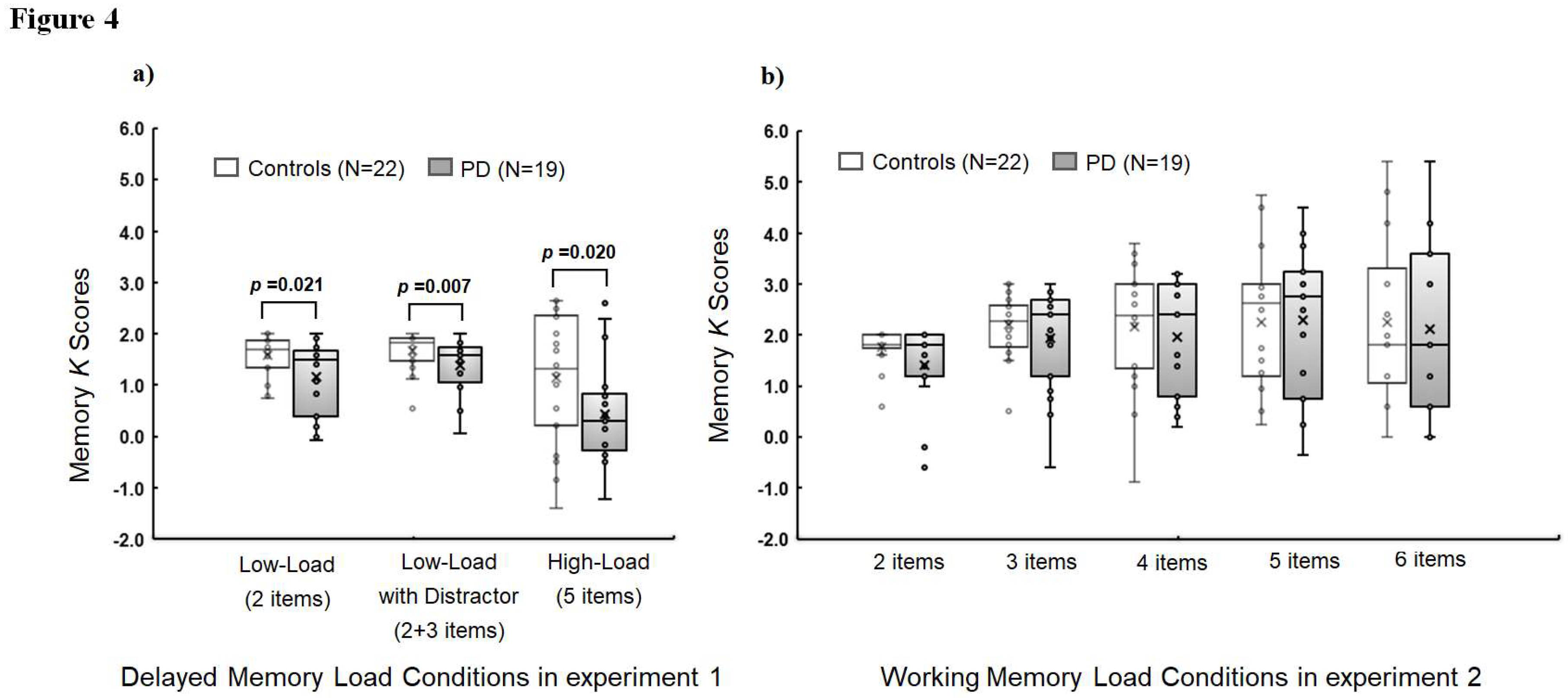
3.2. Group comparison of memory metrics
3.3. Group comparison of MRI structural metrics
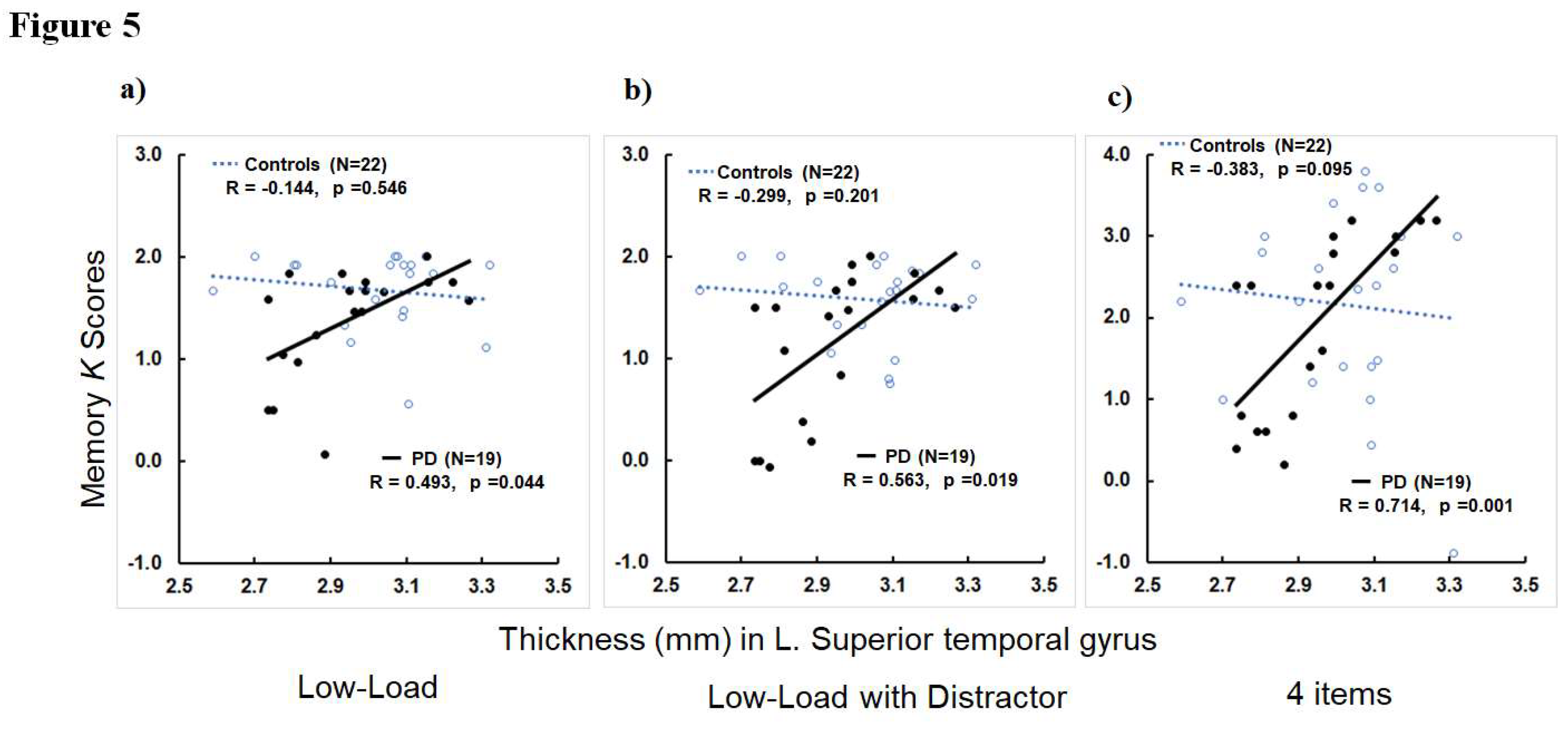
3.4. Associations of MRI structural metrics with memory metrics
3.5. Stepwise regression analysis to determine factors predicting memory metrics
4. Discussion
4.1. Lower dealyed memory due to reduced memory consolidation
4.2. Lower delayed memory due to impaired attentional filtering
4.3. Structural correlates of lower delayed memory in PD
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Albin, R.L.; Young, A.B.; Penney, J.B. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989, 12, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, J. & Friston, K. (2000). Voxel-based morphometry - The methods. NeuroImage, 11, 805-821. [CrossRef]
- Atkinson, R. C. , & Shiffrin, R. M. (1968). Human memory: A proposed system and its control processes. In K. W. Spence & J. T. Spence (Eds.). The psychology of learning and motivation: Advances in research and theory (Vol. 2, pp. 89–195). New York: Academic Press. [CrossRef]
- Awh, E.; Barton, B.; Vogel, E.K. Visual Working Memory Represents a Fixed Number of Items Regardless of Complexity. Psychol. Sci. 2007, 18, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Awh & Vogel (2008). The bouncer in the brain. Nature Neuroscience, 11(1), 5-6. [CrossRef]
- Baddeley, A.D. , & Hitch, G.J. (1974).Working memory. In G. Bower (Ed.), The psychology of learning and motivation (Vol. VIII, pp. 47-90). New York: Academic Press.
- Baddeley, A. Working memory: looking back and looking forward. Nat. Rev. Neurosci. 2003, 4, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Bennett, K.M.B.; Waterman, C.; Scarpa, M.; Castiello, U. Covert visuospatial attentional mechanisms in Parkinson's disease. Brain 1995, 118, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Bradley, A.V.; Welch, J.L.; Dick, D.J. Visuospatial working memory in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 1989, 52, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Del Tredici, K.; Rüb, U.; de Vos, R.A.; Steur, E.N.J.; Braak, E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Buchel, C.; Friston, K.J. Modulation of connectivity in visual pathways by attention: cortical interactions evaluated with structural equation modelling and fMRI. Cereb. Cortex 1997, 7, 768–778. [Google Scholar] [CrossRef]
- Cameron, I. G. , Watanabe, M., Pari, G., & Munoz, D. P. (2010). Executive impairment in Parkinson's disease: response automaticity and task switching. Neuropsychologia, 48(7), 1948-57. [CrossRef]
- Case, R.; Kurland, D.; Goldberg, J. Operational efficiency and the growth of short-term memory span. J. Exp. Child Psychol. 1982, 33, 386–404. [Google Scholar] [CrossRef]
- Chang, C.; Crottaz-Herbette, S.; Menon, V. Temporal dynamics of basal ganglia response and connectivity during verbal working memory. NeuroImage 2007, 34, 1253–1269. [Google Scholar] [CrossRef]
- Cohen, J. Statistical power analysis for the behavioral sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Cohen, M.X.; Frank, M.J. Neurocomputational models of basal ganglia function in learning, memory and choice. Behav. Brain Res. 2009, 199, 141–156. [Google Scholar] [CrossRef]
- function in learning, memory and choice. Behav Brain Res, 199(1), 141-156. [CrossRef]
- Cools, R.; Gibbs, S.E.; Miyakawa, A.; Jagust, W.; D'Esposito, M. Working Memory Capacity Predicts Dopamine Synthesis Capacity in the Human Striatum. J. Neurosci. 2008, 28, 1208–1212. [Google Scholar] [CrossRef] [PubMed]
- Cools, R.; Barker, R.A.; Sahakian, B.J.; Robbins, T.W. Enhanced or Impaired Cognitive Function in Parkinson's Disease as a Function of Dopaminergic Medication and Task Demands. Cereb. Cortex 2001, 11, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Cools, R.; Ivry, R.B.; D'Esposito, M. The Human Striatum is Necessary for Responding to Changes in Stimulus Relevance. J. Cogn. Neurosci. 2006, 18, 1973–1983. [Google Scholar] [CrossRef] [PubMed]
- Cools, R.; Miyakawa, A.; Sheridan, M.; D'Esposito, M. Enhanced frontal function in Parkinson's disease. Brain 2009, 133, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J. A. , Sagar, H. J., Jordan, N., Harvey, N. S., & Sullivan, E. V. (1991). Cognitive impairment in early, untreated Parkinson's disease and its relationship to motor disability. Brain, 114 (5), 2095-2122. [CrossRef]
- Cowan, N. The magical number 4 in short-term memory: A reconsideration of mental storage capacity. Behav. Brain Sci. 2001, 24, 87–114. [Google Scholar] [CrossRef] [PubMed]
- Cowan, N. , Elliott, E. M., Saults, J. S., Morey, C. C., Mattox, S., Hismjatullina, A.
- Cowan, N.; Elliott, E.M.; Saults, J.S.; Morey, C.C.; Mattox, S.; Hismjatullina, A.; Conway, A.R. On the capacity of attention: Its estimation and its role in working memory and cognitive aptitudes. Cogn. Psychol. 2005, 51, 42–100. [Google Scholar] [CrossRef]
- Cowan, N.; Li, D.; Moffitt, A.; Becker, T.M.; Martin, E.A.; Saults, J.S.; Christ, S.E. A Neural Region of Abstract Working Memory. J. Cogn. Neurosci. 2011, 23, 2852–2863. [Google Scholar] [CrossRef]
- Conway, A.R.A.; Kane, M.J.; Bunting, M.F.; Hambrick, D.Z.; Wilhelm, O.; Engle, R.W. Working memory span tasks: A methodological review and user’s guide. Psychon. Bull. Rev. 2005, 12, 769–786. [Google Scholar] [CrossRef]
- Dalrymple-Alford, J.C.; Kalders, A.S.; Jones, R.D.; Watson, R.W. A central executive deficit in patients with Parkinson's disease. J. Neurol. Neurosurg. Psychiatry 1994, 57, 360–367. [Google Scholar] [CrossRef]
- Daneman, M.; Carpenter, P.A. Individual differences in working memory and reading. J. Verbal Learn. Verbal Behav. 1980, 19, 450–466. [Google Scholar] [CrossRef]
- Daneman, M.; Merikle, P.M. Working memory and language comprehension: A meta-analysis. Psychon. Bull. Rev. 1996, 3, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Eimer, M.; Kiss, M. An electrophysiological measure of access to representations in visual working memory. Psychophysiology 2009, 47, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Erdfelder, E.; Faul, F.; Buchner, A. GPOWER: A general power analysis program. Behav. Res. Methods Instrum. Comput. 1996, 28, 1–11. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-Mental State”. A Practical Method for Grading the Cognitive State of Patients for the Clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.J. Dynamic Dopamine Modulation in the Basal Ganglia: A Neurocomputational Account of Cognitive Deficits in Medicated and Nonmedicated Parkinsonism. J. Cogn. Neurosci. 2005, 17, 51–72. [Google Scholar] [CrossRef] [PubMed]
- Friston, K.J.; Frith, C.D.; Frackowiak, R.S.J. Time-dependent changes in effective connectivity measured with PET. Hum. Brain Mapp. 1993, 1, 69–79. [Google Scholar] [CrossRef]
- Friston, K.J. Functional and effective connectivity in neuroimaging: A synthesis. Hum. Brain Mapp. 1994, 2, 56–78. [Google Scholar] [CrossRef]
- Friston, K.; Buechel, C.; Fink, G.; Morris, J.; Rolls, E.; Dolan, R.; Friston, K.; Buechel, C.; Fink, G.; Morris, J.; et al. Psychophysiological and Modulatory Interactions in Neuroimaging. NeuroImage 1997, 6, 218–229. [Google Scholar] [CrossRef]
- Friston, K.J. , Buchel, C. (2000). Attentional modulation of effective connectivity from V2 to V5/MT in humans. Proc Natl Acad Sci U S A, 97, 7591–7596. [CrossRef]
- Friston, K.J., Harrison, L., Penny, W. (2003). Dynamic causal modelling. NeuroImage, 19, 1273–1302. [CrossRef]
- Gabrieli, J.D.E.; Singh, J.; Stebbins, G.T.; Goetz, C.G. Reduced working memory span in Parkinson's disease: Evidence for the role of frontostriatal system in working and strategic memory. Neuropsychology 1996, 10, 322–332. [Google Scholar] [CrossRef]
- Gerfen, C.R. Molecular effects of dopamine on striatal-projection pathways. Trends Neurosci. 2000, 23, S64–S70. [Google Scholar] [CrossRef]
- Geweke, J. Measurement of Linear Dependence and Feedback Between Multiple Time Series. J. Am. Stat. Assoc. 1982, 77, 304. [Google Scholar] [CrossRef]
- Gilbert, B. , Belleville, S., Bherer, L., & Chouinard, S. (2005). Study of verbal working memory in patients with Parkinson's disease. Neuropsychology, 19, 106-114. [CrossRef]
- Goebel, R. , Roebroeck, A., Kim, D.S., Formisano, E. (2004). Directed cortical interactions during dynamic sensory-motor mapping. In: Duncan, J. (Ed.), Attention and Performance XX. Oxford University Press, New York, pp. 439–462.
- Good, C.; Johnsrude, I.; Ashburner, J.; Henson, R.; Friston, K.; Frackowiak, R. A voxel-based morphometric study of ageing in 465 normal adult human brains. 5th IEEE EMBS International Summer School on Biomedical Imaging, 2002.. LOCATION OF CONFERENCE, FranceDATE OF CONFERENCE; p. 16 pp.
- Gruber, A.J.; Dayan, P.; Gutkin, B.S.; Solla, S.A. Dopamine modulation in the basal ganglia locks the gate to working memory. J. Comput. Neurosci. 2006, 20, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Gouttard, S.; Styner, M.; Joshi, S.; Smith, R.G.; Hazlett, H.C.; Gerig, G. Subcortical structure segmentation using probabilistic atlas priors. Medical Imaging. LOCATION OF CONFERENCE, COUNTRYDATE OF CONFERENCE; pp. 65122J–65122J-11.
- Harrison, A.; Jolicoeur, P.; Marois, R. "What" and "Where" in the Intraparietal Sulcus: An fMRI Study of Object Identity and Location in Visual Short-Term Memory. Cereb. Cortex 2010, 20, 2478–2485. [Google Scholar] [CrossRef] [PubMed]
- Cereb Cortex, 20(10): 2478–2485. [CrossRef]
- Harrison, L.; Penny, W.; Friston, K.; Harrison, L.; Penny, W.; Friston, K.; Harrison, L.; Penny, W.; Friston, K. Multivariate autoregressive modeling of fMRI time series. NeuroImage 2003, 19, 1477–1491. [Google Scholar] [CrossRef] [PubMed]
- Hochstadt, J.; Nakano, H.; Lieberman, P.; Friedman, J. The roles of sequencing and verbal working memory in sentence comprehension deficits in Parkinson’s disease. Brain Lang. 2006, 97, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Hoehn M, Yahr M. (1967). Parkinsonism: onset, progression and mortality. Neurology, 17: 427–42.
- Hsieh, S.; Hwang, W.-J.; Tsai, J.-J.; Tsai, C.-Y. Visuospatial Orienting of Attention in Parkinson's Disease. Percept. Mot. Ski. 1996, 82, 1307–1315. [Google Scholar] [CrossRef]
- Jellinger, K. A. (2001). The pathology of Parkinson’s disease. Advances in Neurology, 86, 55-72.
- Jolicœur, P.; Brisson, B.; Robitaille, N. Dissociation of the N2pc and sustained posterior contralateral negativity in a choice response task. Brain Res. 2008, 1215, 160–172. [Google Scholar] [CrossRef]
- Joshi, S.; Davis, B.; Jomier, M.; Gerig, G. Unbiased diffeomorphic atlas construction for computational anatomy. NeuroImage 2004, 23, S151–S160. [Google Scholar] [CrossRef]
- Just, M.A.; Carpenter, P.A. A capacity theory of comprehension: Individual differences in working memory. Psychol. Rev. 1992, 99, 122–149. [Google Scholar] [CrossRef]
- Kay, S.M. (1988). Modern Spectral Estimation: Theory and Application. Prentice Hall, Englewood Cliffs, NJ.
- Kennett, S.; van Velzen, J.; Eimer, M.; Driver, J. Disentangling gaze shifts from preparatory ERP effects during spatial attention. Psychophysiology 2006, 44, 69–78. [Google Scholar] [CrossRef]
- Kensinger, E. A. , Shearer, D. K., Locascio, J. J., Growdon, J. H., & Corkin, S. (2003). Working memory in mild Alzheimer's disease and early Parkinson's disease. Neuropsychology, 17(2), 230-239. [CrossRef]
- Lee, E. Y. , Cowan, N., Vogel, E. K., Rolan, T., Valle-Inclan, F., & Hackley, S. A. (2010). Visual working memory deficits in patients with Parkinson's disease are due to both reduced storage capacity and impaired ability to filter out irrelevant information. Brain, 133(9), 2677-2689. [CrossRef]
- Lewis, S.J.G.; Dove, A.; Robbins, T.W.; Barker, R.A.; Owen, A.M. Cognitive impairments in early Parkinson’s disease are accompanied by reductions in activity in frontostriatal neural circuitry. J. Neurosci. 2003, 23, 6351–6356. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S. J. , Slabosz, A., Robbins, T. W., Barker, R. A., & Owen, A. M. (2005). Dopaminergic basis for deficits in working memory but not attentional set-shifting in Parkinson's disease. Neuropsychologia, 43(6), 823-832. [CrossRef]
- Linden, D.E.; Bittner, R.A.; Muckli, L.; Waltz, J.A.; Kriegeskorte, N.; Goebel, R.; Singer, W.; Munk, M.H. Cortical capacity constraints for visual working memory: dissociation of fMRI load effects in a fronto-parietal network. NeuroImage 2003, 20, 1518–1530. [Google Scholar] [CrossRef]
- Lu, Z.-L.; Neuse, J.; Madigan, S.; Dosher, B.A. Fast decay of iconic memory in observers with mild cognitive impairments. 102, 1797. [Google Scholar] [CrossRef]
- Luck, S. J. , & Hillyard, S. A. (1994). Spatial filtering during visual search: evidence from human electrophysiology. J Exp Psychol Hum Percept Perform, 20(5), 1000-1014. [CrossRef]
- Luck, S.J.; Vogel, E.K. The capacity of visual working memory for features and conjunctions. Nature 1997, 390, 279–281. [Google Scholar] [CrossRef]
- Matsui, H.; Nishinaka, K.; Oda, M.; Niikawa, H.; Komatsu, K.; Kubori, T.; Udaka, F. Wisconsin Card Sorting Test in Parkinson's disease: diffusion tensor imaging. Acta Neurol. Scand. 2007, 116, 108–112. [Google Scholar] [CrossRef] [PubMed]
- McNab, F.; Klingberg, T. Prefrontal cortex and basal ganglia control access to working memory. Nat. Neurosci. 2007, 11, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Miller, G. A. (1956). The magical number seven, and or minus two: Some limits on our capacity for processing information. Psychological Review, 63, 81–97. [CrossRef]
- Miyake, A. , & Shah, P. (Eds.). (1999). Models of Working Memory: Mechanisms of active maintenance and executive control. Cambridge, UK: Cambridge University Press.
- Morris, R.G.; Downes, J.J.; Sahakian, B.J.; Evenden, J.L.; Heald, A.; Robbins, T.W. Planning and spatial working memory in Parkinson's disease. J. Neurol. Neurosurg. Psychiatry 1988, 51, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, A.A.; Sherman, S.J.; Frank, M.J. A dopaminergic basis for working memory, learning and attentional shifting in Parkinsonism. Neuropsychologia 2008, 46, 3144–3156. [Google Scholar] [CrossRef] [PubMed]
- O'Reilly, R.C.; Frank, M.J. Making Working Memory Work: A Computational Model of Learning in the Prefrontal Cortex and Basal Ganglia. Neural Comput. 2006, 18, 283–328. [Google Scholar] [CrossRef]
- Owen, A.M.; Iddon, J.L.; Hodges, J.R.; A Summers, B.; Robbins, T.W. Spatial and non-spatial working memory at different stages of Parkinson's disease. Neuropsychologia 1997, 35, 519–532. [Google Scholar] [CrossRef]
- Owen, A.M. Cognitive Dysfunction in Parkinson’s Disease: The Role of Frontostriatal Circuitry. Neurosci. 2004, 10, 525–537. [Google Scholar] [CrossRef]
- Pashler, H. Familiarity and visual change detection. Percept. Psychophys. 1988, 44, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Patenaude, B.; Smith, S.M.; Kennedy, D.N.; Jenkinson, M. A Bayesian model of shape and appearance for subcortical brain segmentation. NeuroImage 2011, 56, 907–922. [Google Scholar] [CrossRef] [PubMed]
- Petit, L.; Courtney, S.M.; Ungerleider, L.G.; Haxby, J.V. Sustained Activity in the Medial Wall during Working Memory Delays. J. Neurosci. 1998, 18, 9429–9437. [Google Scholar] [CrossRef] [PubMed]
- Postle, B.R., Ferrarelli, F., Hamidi, M., Feredoes, E., Massimini, P. M., Alexander, A., and Tononi, G. (2006). Repetitive transcranial magnetic stimulation dissociates working memory manipulation from retention functions in prefrontal, but not posterior parietal cortex. Journal of Cognitive Neuroscience 2006, 18, 1712–1722. [PubMed]
- Praamstra, P. , & Plat, F. M. (2001). Failed suppression of direct visuomotor activation in Parkinson's disease. J Cogn Neurosci, 13(1), 31-43.
- Praamstra, P. , Stegeman, D. F., Cools, A. R., & Horstink, M. W. (1998). Reliance on external cues for movement initiation in Parkinson's disease. Evidence from movement-related potentials. Brain, 121, 167-177. [CrossRef]
- Roebroeck, A.; Formisano, E.; Goebel, R. Mapping directed influence over the brain using Granger causality and fMRI. NeuroImage 2005, 25, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Ruchkin, D.S.; Grafman, J.; Cameron, K.; Berndt, R.S. Working memory retention systems: A state of activated long-term memory. Behav. Brain Sci. 2003, 26, 709–728. [Google Scholar] [CrossRef] [PubMed]
- Salimi-Khorshidi, G.; Smith, S.M.; Nichols, T.E. Adjusting the effect of nonstationarity in cluster-based and TFCE inference. NeuroImage 2011, 54, 2006–2019. [Google Scholar] [CrossRef]
- Samuel, M.; O Ceballos-Baumann, A.; Blin, J.; Uema, T.; Boecker, H.; E Passingham, R.; Brooks, D.J. Evidence for lateral premotor and parietal overactivity in Parkinson's disease during sequential and bimanual movements. A PET study. Brain 1997, 120, 963–976. [Google Scholar] [CrossRef]
- Sen, S. , Flynn M., Du G., Tröster A., An H., Huang X. (2011). Manganese accumulation in the olfactory bulbs and other brain regions of "asymptomatic" welders. Toxicol Sci. 121(1):160-7. [CrossRef]
- Sheikh, J. I. & Yesavage, J. A. (1986).Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. Clinical Gerontology: A Guide to Assessment and Intervention. NY: The Haworth Press, 165-173.
- Skeel, R. L. , Crosson, B., Nadeau, S. E., Algina, J., Bauer, R. M., & Fennell, E. B. (2001). Basal ganglia dysfunction, working memory, and sentence comprehension in patients with Parkinson's disease. Neuropsychologia, 39(9), 962-971. [CrossRef]
- Smith, S.M.; Jenkinson, M.; Woolrich, M.W.; Beckmann, C.F.; Behrens, T.E.; Johansen-Berg, H.; Bannister, P.R.; De Luca, M.; Drobnjak, I.; Flitney, D.E.; et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 2004, 23, S208–S219. [Google Scholar] [CrossRef]
- Smith, S.M. & Nichols, T.E. (2009).Threshold-freeclusterenhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage, 44, 83-98. [CrossRef]
- Sperling, G. The information available in brief visual presentations. Psychol. Monogr. Gen. Appl. 1960, 74, 1–29. [Google Scholar] [CrossRef]
- Surmeier, D.J.; Ding, J.; Day, M.; Wang, Z.; Shen, W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007, 30, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Turner, M. L. , & Engle, R. W. (1989). Is working memory capacity task dependent? Journal of Memory and Language, 28, 127–154. [CrossRef]
- Unsworth, N.; Redick, T.S.; Heitz, R.P.; Broadway, J.M.; Engle, R.W. Complex working memory span tasks and higher-order cognition: A latent-variable analysis of the relationship between processing and storage. Memory 2009, 17, 635–654. [Google Scholar] [CrossRef] [PubMed]
- Verleger, R.; Hagenah, J.; Weiß, M.; Ewers, T.; Heberlein, I.; Pramstaller, P.P.; Siebner, H.R.; Klein, C. Responsiveness to distracting stimuli, though increased in Parkinson's disease, is decreased in asymptomatic PINK1 and Parkin mutation carriers. Neuropsychologia 2010, 48, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Vogel, E.K.; Woodman, G.F.; Luck, S.J. Pushing around the Locus of Selection: Evidence for the Flexible-selection Hypothesis. J. Cogn. Neurosci. 2005, 17, 1907–1922. [Google Scholar] [CrossRef]
- Vogel, E.K.; McCollough, A.W.; Machizawa, M.G. Neural measures reveal individual differences in controlling access to working memory. Nature 2005, 438, 500–503. [Google Scholar] [CrossRef]
- West, R. , Ergis, A. M., Winocur, G., & Saint-Cyr, J. (1998). The contribution of impaired working memory monitoring to performance of the self-ordered pointing task in normal aging and Parkinson’s disease. Neuropsychology, 12, 546-554. [CrossRef]
- Wiecki, T. V. , & Frank, M. J. (2010). Neurocomputational models of motor and cognitive deficits in Parkinson's disease. Prog Brain Res, 183, 275-297. [CrossRef]
- Woodward, T.S.; Bub, D.N.; A Hunter, M. Task switching deficits associated with Parkinson’s disease reflect depleted attentional resources. Neuropsychologia 2002, 40, 1948–1955. [Google Scholar] [CrossRef]
- with Parkinson’s disease reflect depleted attentional resources. Neuropsychologia, 40(12), 1948–1955.
- Woolrich, M.W.; Jbabdi, S.; Patenaude, B.; Chappell, M.; Makni, S.; Behrens, T.; Beckmann, C.F.; Jenkinson, M.; Smith, S.M. Bayesian analysis of neuroimaging data in FSL. NeuroImage 2009, 45, S173–S186. [Google Scholar] [CrossRef]
- Wu, T. , & Hallett, M. (2005). A functional MRI study of automatic movements in patients with Parkinson's disease, Brain, 128 (10), 2250-2259. [CrossRef]
- Zgaljardic, D.J.; Borod, J.C.; Foldi, N.S.; Mattis, P. A Review of the Cognitive and Behavioral Sequelae of Parkinson's Disease: Relationship to Frontostriatal Circuitry. Cogn. Behav. Neurol. 2003, 16, 193–210. [Google Scholar] [CrossRef]
- Aarsland D, Kurz MW. 2010. The epidemiology of dementia associated with parkinson's disease. Brain pathology. 20(3):633-639. [CrossRef]
- Anang JB, Gagnon J-F, Bertrand J-A, Romenets SR, Latreille V, Panisset M, Montplaisir J, Postuma RB. 2014. Predictors of dementia in parkinson disease: A prospective cohort study. Neurology. 83(14):1253-1260. [CrossRef]
- Bäckman L, Jones S, Berger A-K, Laukka EJ, Small BJ. 2005. Cognitive impairment in preclinical alzheimer's disease: A meta-analysis. Neuropsychology. 19(4):520. [CrossRef]
- Bäckman L, Small BJ, Fratiglioni L. 2001. Stability of the preclinical episodic memory deficit in alzheimer's disease. Brain : a journal of neurology. 124(1):96-102. [CrossRef]
- Breen KC, Drutyte G. 2013. Non-motor symptoms of parkinson’s disease: The patient’s perspective. Journal of neural transmission. 120:531-535. [CrossRef]
- Brookmeyer, R.; Evans, D.A.; Hebert, L.; Langa, K.M.; Heeringa, S.G.; Plassman, B.L.; Kukull, W.A. National estimates of the prevalence of Alzheimer's disease in the United States. Alzheimer's Dement. 2011, 7, 61–73. [Google Scholar] [CrossRef]
- Douaud G, Menke RA, Gass A, Monsch AU, Rao A, Whitcher B, Zamboni G, Matthews PM, Sollberger M, Smith S. 2013. Brain microstructure reveals early abnormalities more than two years prior to clinical progression from mild cognitive impairment to alzheimer's disease. Journal of Neuroscience. 33(5):2147-2155. [CrossRef]
- Eichenbaum, H.; Lipton, P.A. Towards a functional organization of the medial temporal lobe memory system: Role of the parahippocampal and medial entorhinal cortical areas. Hippocampus 2008, 18, 1314–1324. [Google Scholar] [CrossRef]
- Fellgiebel, A.; Dellani, P.R.; Greverus, D.; Scheurich, A.; Stoeter, P.; Müller, M.J. Predicting conversion to dementia in mild cognitive impairment by volumetric and diffusivity measurements of the hippocampus. Psychiatry Res. Neuroimaging 2006, 146, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Fischl, B.; Salat, D.H.; Busa, E.; Albert, M.; Dieterich, M.; Haselgrove, C.; van der Kouwe, A.; Killiany, R.; Kennedy, D.; Klaveness, S.; et al. Whole Brain Segmentation: Automated Labeling of Neuroanatomical Structures in the Human Brain. Neuron 2002, 33, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Fischl, B.; Salat, D.H.; van der Kouwe, A.J.; Makris, N.; Ségonne, F.; Quinn, B.T.; Dale, A.M. Sequence-independent segmentation of magnetic resonance images. NeuroImage 2004, 23 (Suppl. 1), S69–S84. [Google Scholar] [CrossRef] [PubMed]
- Holm, S. 1979. A simple sequentially rejective multiple test procedure. Scandinavian journal of statistics.65-70.
- Lancaster, M.A.; Seidenberg, M.; Smith, J.C.; Nielson, K.A.; Woodard, J.L.; Durgerian, S.; Rao, S.M. Diffusion Tensor Imaging Predictors of Episodic Memory Decline in Healthy Elders at Genetic Risk for Alzheimer’s Disease. J. Int. Neuropsychol. Soc. 2016, 22, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Le Bihan, D.; Mangin, J.; Poupon, C.; Clark, C.A.; Pappata, S.; Molko, N.; Chabriat, H. Diffusion tensor imaging: Concepts and applications. J. Magn. Reson. Imaging 2001, 13, 534–546. [Google Scholar] [CrossRef]
- Leff, A.P.; Schofield, T.M.; Crinion, J.T.; Seghier, M.L.; Grogan, A.; Green, D.W.; Price, C.J. The left superior temporal gyrus is a shared substrate for auditory short-term memory and speech comprehension: evidence from 210 patients with stroke. Brain 2009, 132, 3401–3410. [Google Scholar] [CrossRef]
- Lim, Y.Y.; Maruff, P.; Pietrzak, R.H.; Ames, D.; Ellis, K.A.; Harrington, K.; Lautenschlager, N.T.; Szoeke, C.; Martins, R.N.; Masters, C.L.; et al. Effect of amyloid on memory and non-memory decline from preclinical to clinical Alzheimer’s disease. Brain 2014, 137, 221–231. [Google Scholar] [CrossRef]
- Müller, M.J.; Greverus, D.; Weibrich, C.; Dellani, P.R.; Scheurich, A.; Stoeter, P.; Fellgiebel, A. Diagnostic utility of hippocampal size and mean diffusivity in amnestic MCI. Neurobiol. Aging 2007, 28, 398–403. [Google Scholar] [CrossRef]
- Nani, A.; Manuello, J.; Mancuso, L.; Liloia, D.; Costa, T.; Cauda, F. The Neural Correlates of Consciousness and Attention: Two Sister Processes of the Brain. Front. Neurosci. 2019, 13, 1169. [Google Scholar] [CrossRef]
- Ségonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B. 2004. A hybrid approach to the skull stripping problem in mri. Neuroimage. 22(3):1060-1075. [CrossRef]
- Squire LR, Genzel L, Wixted JT, Morris RG. 2015. Memory consolidation. Cold Spring Harbor perspectives in biology. 7(8):a021766.
- Sugar, J.; Moser, M.-B. Episodic memory: Neuronal codes for what, where, and when. Hippocampus 2019, 29, 1190–1205. [Google Scholar] [CrossRef]
- Tulving, E. Précis of Elements of episodic memory. Behav. Brain Sci. 1984, 7, 223–238. [Google Scholar] [CrossRef]
- Weintraub D, Simuni T, Caspell-Garcia C, Coffey C, Lasch S, Siderowf A, Aarsland D, Barone P, Burn D, Chahine LM. 2015. Cognitive performance and neuropsychiatric symptoms in early, untreated parkinson's disease. Movement Disorders. 30(7):919-927. [CrossRef]
- Yarnall, A.J.; Breen, D.P.; Duncan, G.W.; Khoo, T.K.; Coleman, S.Y.; Firbank, M.J.; Nombela, C.; Winder-Rhodes, S.; Evans, J.R.; Rowe, J.B.; et al. Characterizing mild cognitive impairment in incident Parkinson disease: The ICICLE-PD Study. Neurology 2013, 82, 308–316. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





