Submitted:
19 April 2023
Posted:
20 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Plant-derived metabolites (PDM)
2.2. Therapeutic targets (TTs)
2.3. Co-crystallized ligand
2.4. Ligand and target interaction
2.5. Molecular Docking Analysis
2.6. Selection of best candidates
2.7. Molecular dynamics (MDs) simulations
2.8. In vitro tests
2.8.1. Cell line
2.8.2. Resazurin assay
2.8.3. Scratch wound migration assay
2.9. In vivo experiments
2.9.1. Animals and ethical considerations
2.9.2. Formulation preparation
2.9.3. Wound model
2.9.4. Histological analysis
2.9.5. Tissue-protein and IL-6, IL-1β, VEGF, and TNFα determination
2.9.6. Statistical analysis
3. Results and discussion
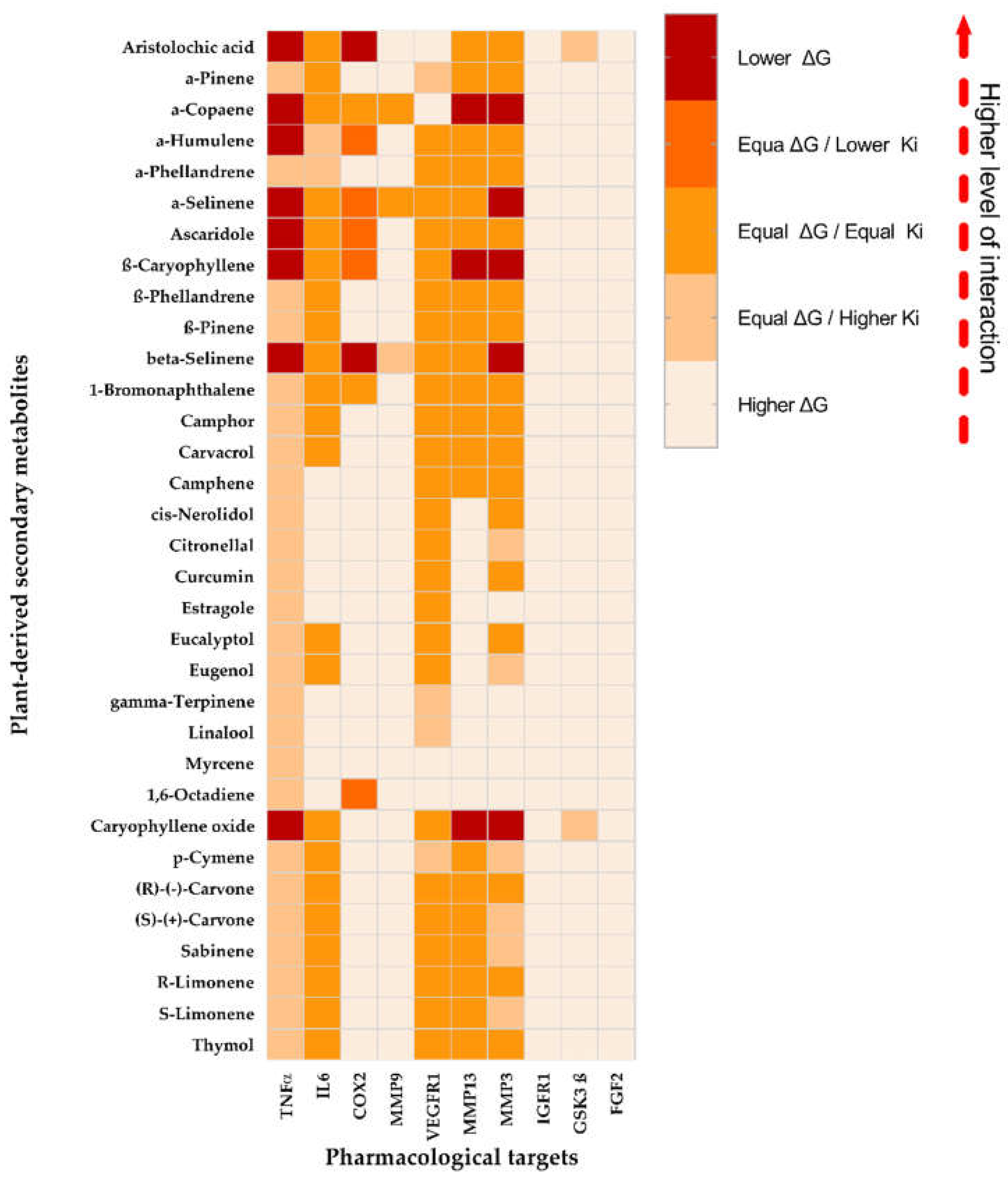
3.1. PDMs versus recognized inhibitors of WH-targets
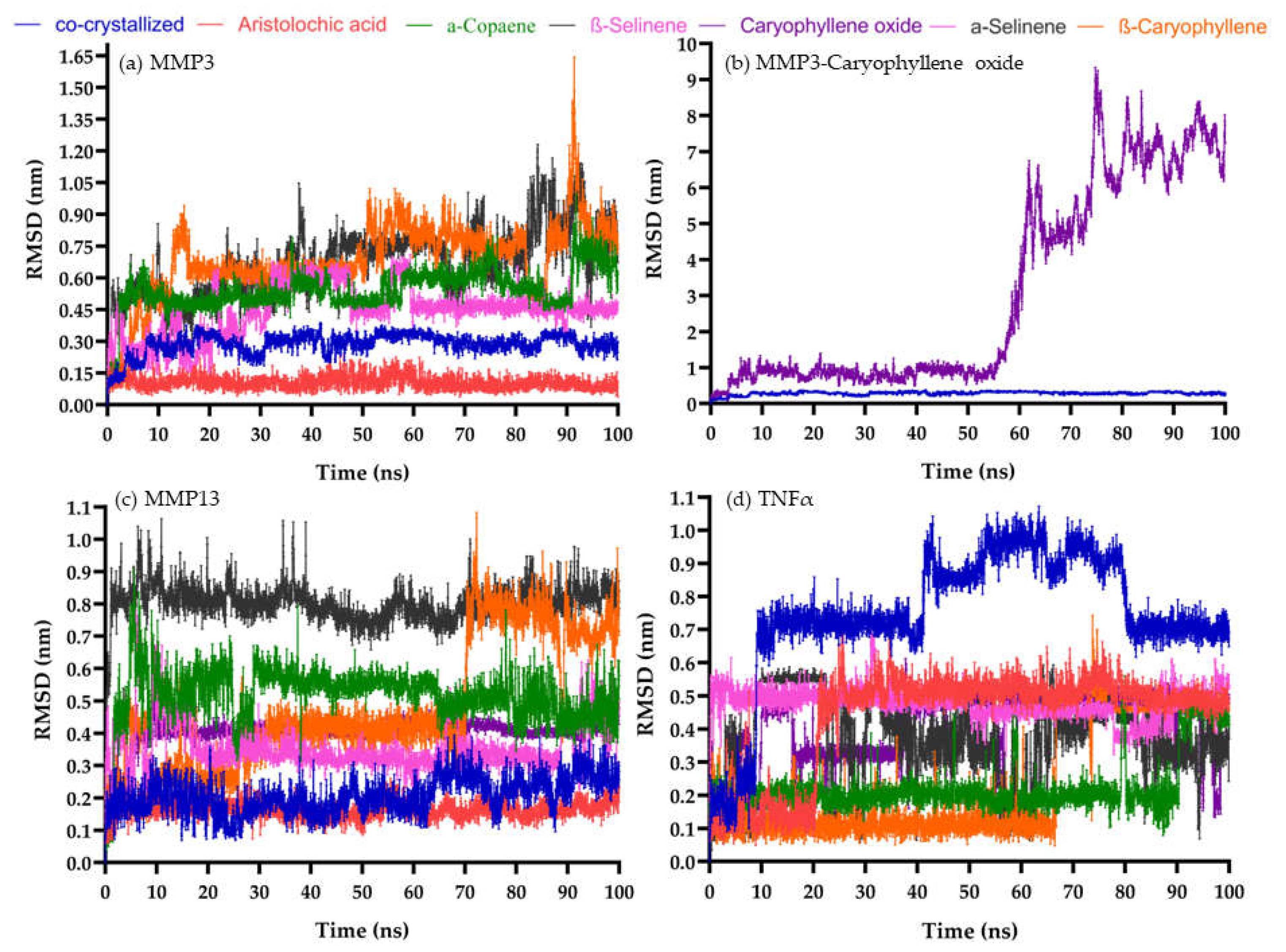
3.2. Molecular dynamics between PDMs inhibitors and MMP-3, MMP-13, and TNFα targets
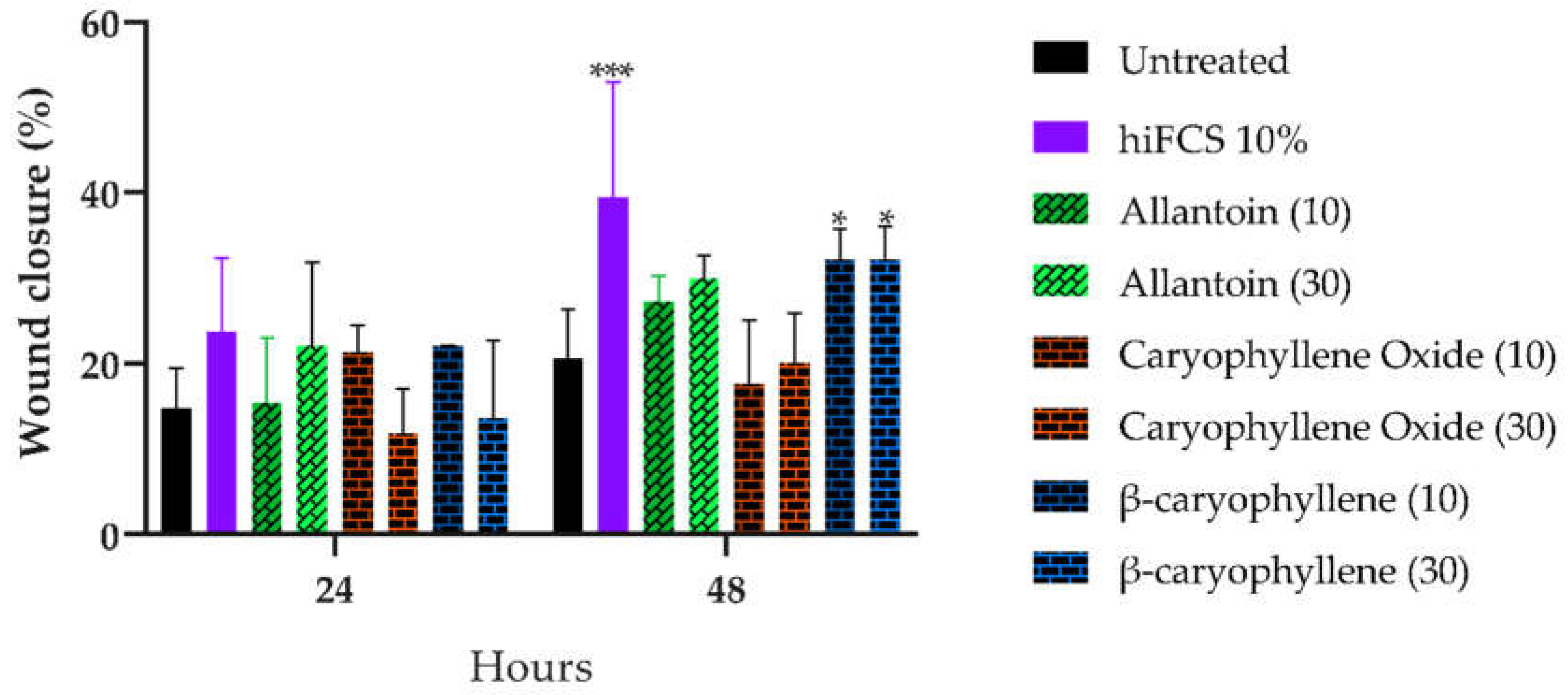
3.3. BC and BCoxide activities on HaCat cells
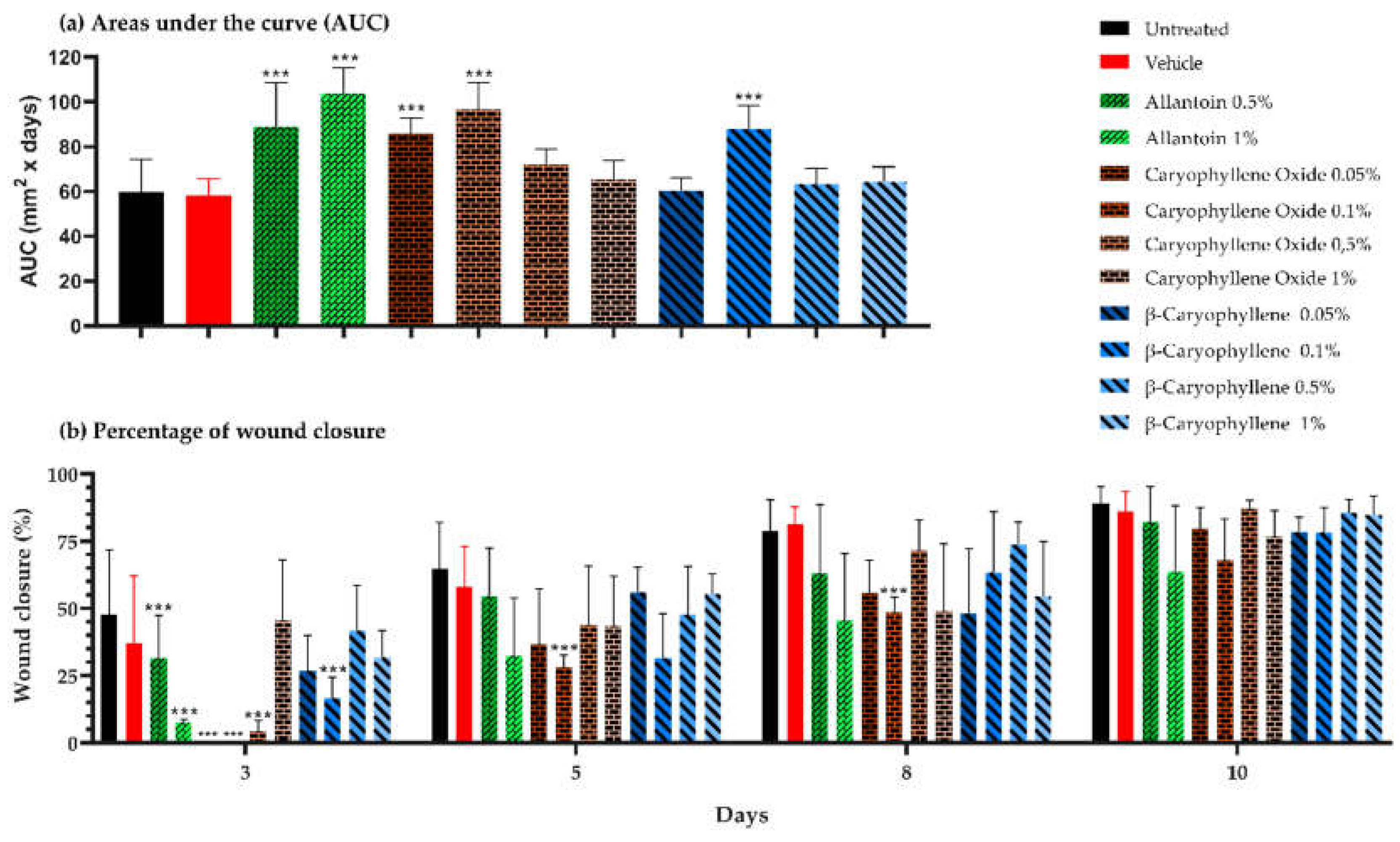
3.3. BC and BCoxide activities on BALB/c excisional acute wound model
3.3.1. Initial delay of wound closure was induced by BC and BCoxide treatments.
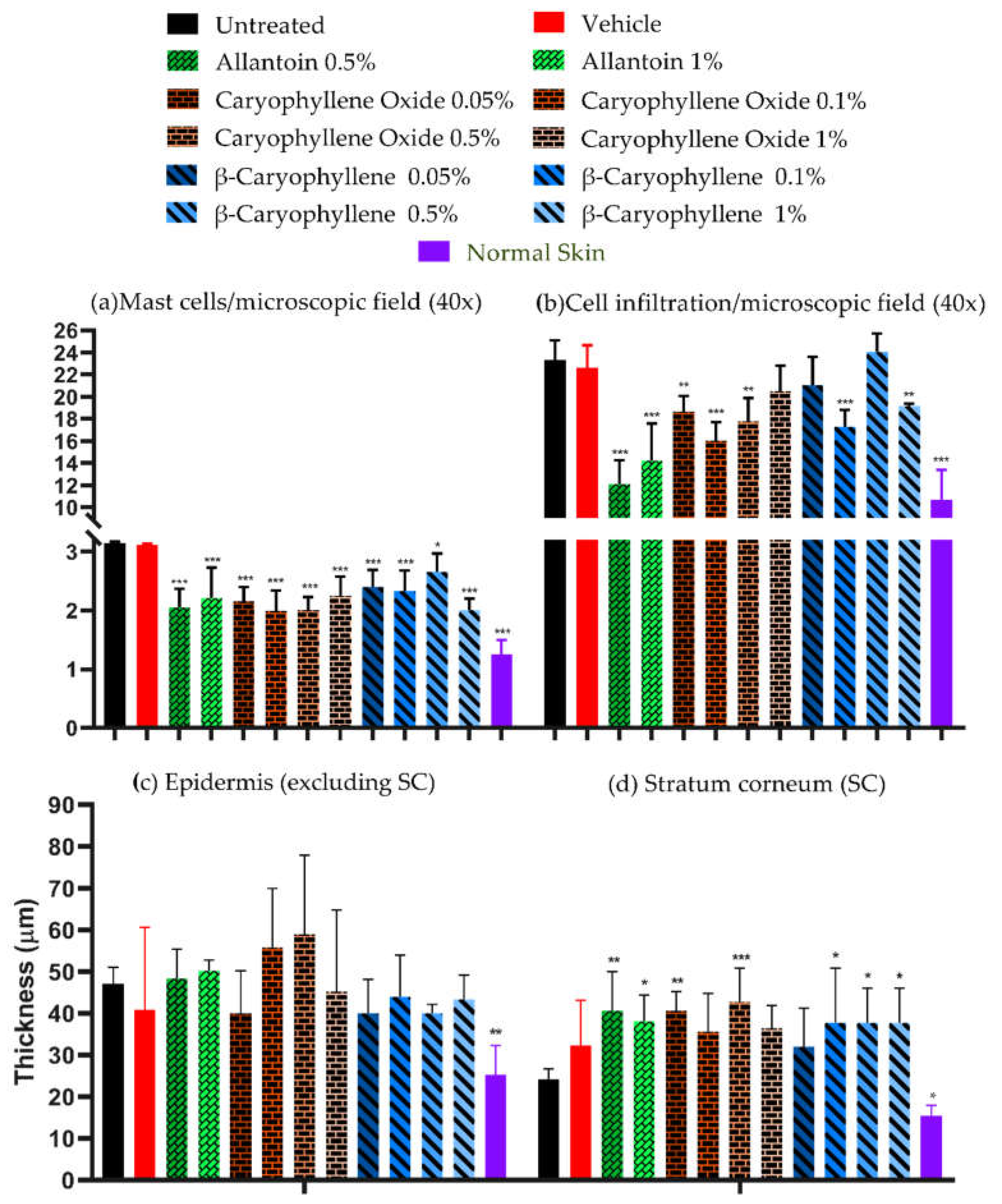
3.3.2. Morphometric analysis at the end of treatment
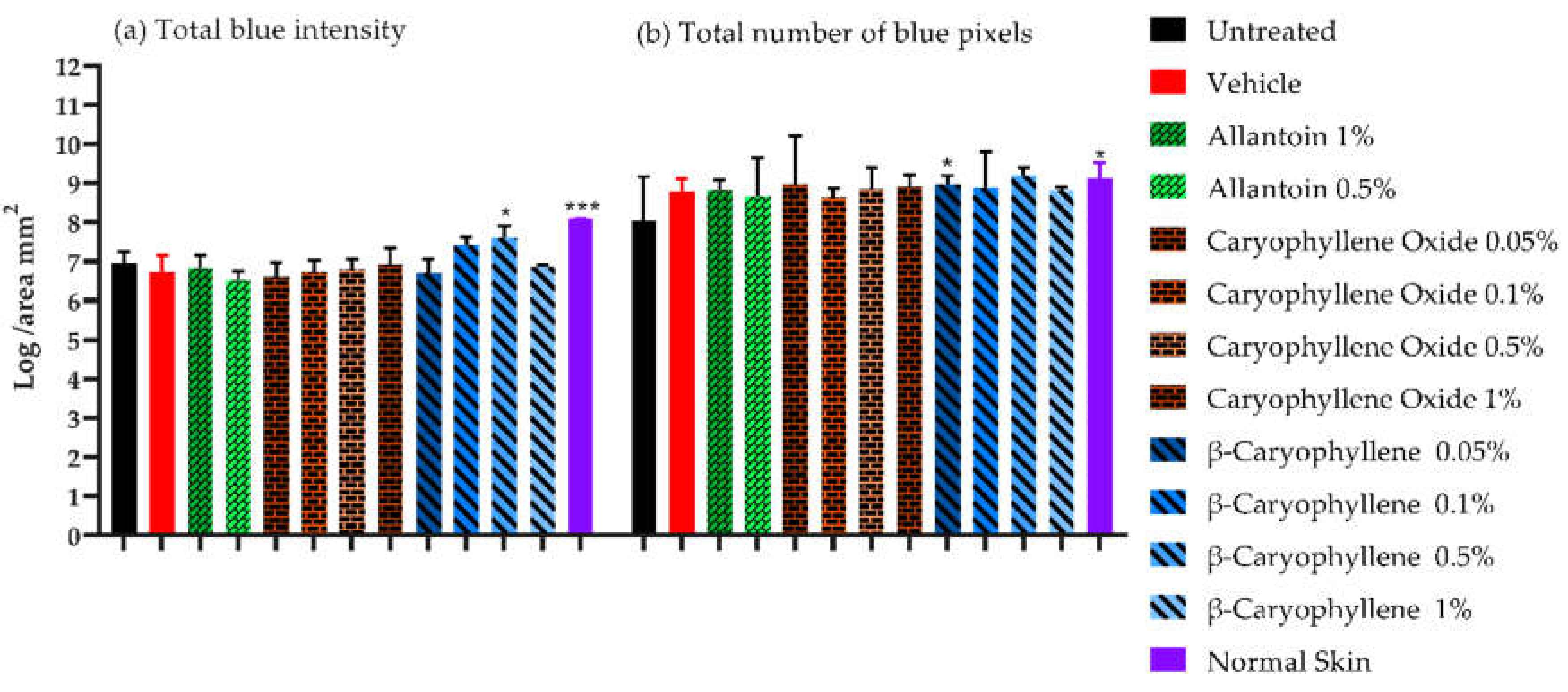
3.3.3. Collagen deposition in Masson's trichrome-stained slides
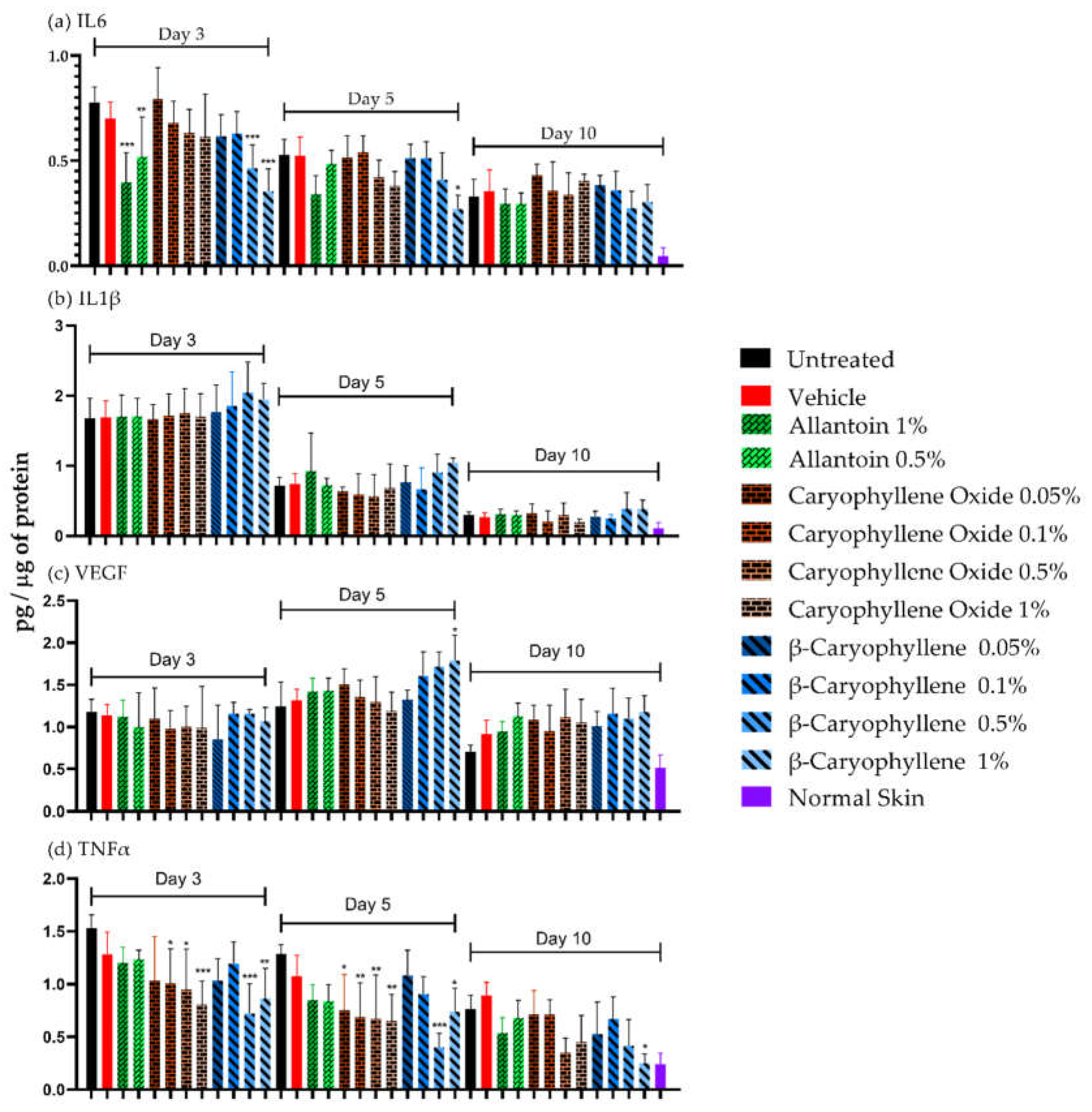
3.3.4. Tissue-protein and IL-6, IL-1, VEGF and TNFα determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Smigiel, K.S.; Parks, W.C. Macrophages, wound healing, and fibrosis: Recent insights. Curr. Rheumatol. Rep. 2018, 20, 17. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Yang, F.; Zhou, W.; Xiao, N.; Luo, M.; Tang, Z. The initiation of oxidative stress and therapeutic strategies in wound healing. Biomed. Pharmacother. 2023, 157, 114004. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound healing: A cellular perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef] [PubMed]
- Ashcroft, G.S.; Jeong, M.J.; Ashworth, J.J.; Hardman, M.; Jin, W.; Moutsopoulos, N.; Wild, T.; McCartney-Francis, N.; Sim, D.; McGrady, G.; Song, X.Y.; Wahl, S.M. Tumor necrosis factor-alpha (TNF-α) is a therapeutic target for impaired cutaneous wound healing. Wound Repair Regen. 2012, 20, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Frykberg, R.G.; Banks, J. Challenges in the treatment of chronic wounds. Adv. Wound Care (New Rochelle) 2015, 4, 560–582. [Google Scholar] [CrossRef]
- Yadav, E.; Yadav, P.; Verma, A. In Silico study of Trianthema portulacastrum embedded iron oxide nanoparticles on glycogen synthase kinase-3β: A Possible contributor to its enhanced in vivo wound healing potential. Front Pharmacol. 2021, 12, 664075. [Google Scholar] [CrossRef]
- Moses, R.L.; Prescott, T.A.K.; Mas-Claret, E.; Steadman, R.; Moseley, R.; Sloan, A.J. Evidence for natural products as alternative wound-healing therapies. Biomolecules 2023, 13, 444. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Amorim, J.L.; Figueiredo, J.B.; Amaral, A.C.F.; Barros, E.G.O.; Palmero, C.; Palantinos, M.A.; Ramos, A.S.; Ferreira, J.LP.; Silva, J.R.A.; Benjamim, C.F.; Basso, S.L.; Nasciutti, L.E.; Fernandes, P.D. Wound healing properties of Copaifera paupera in diabetic mice. PLoS ONE 2017, 12, e0187380. [Google Scholar] [CrossRef]
- Sousa Da Silva, A.W.; Vranken, W.F. ACPYPE-AnteChamber PYthon Parser interfacE. BMC Research Notes 2012, 5, 1–8. [Google Scholar] [CrossRef]
- Rosenthal, M.D.; Vishwanath, B.S.; Franson, R.C. Effects of aristolochic acid on phospholipase A2 activity and arachidonate metabolism of human neutrophils. Biochim. Biophys. Acta 1989, 23, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Murugan, D.; Mangathayaru, K.; Rajasekaran, S.; Preeth, J.; Rangarao, S. Aristolochia bracteolata enhances wound healing through anti-inflammatory and proliferative effect on human dermal fibroblasts and keratinocytes. Pharmacogn. J. 2017, 9, 129–136. [Google Scholar] [CrossRef]
- Bolla, S.R.; Mohammed Al-Subaie, A.; Yousuf Al-Jindan, R.; Papayya Balakrishna, J.; Kanchi Ravi, P.; Veeraraghavan, V.P.; Arumugam Pillai, A.; Gollapalli, S.S.R.; Palpath Joseph, J.; Surapaneni, K.M. In Vitro wound healing potency of methanolic leaf extract of Aristolochia saccata is possibly mediated by its stimulatory effect on collagen-1 expression. Heliyon 2019, 5, e01648. [Google Scholar] [CrossRef] [PubMed]
- Shirwaikar, A.; Somashekar, A.P.; Udupa, A.L.; Udupa, S.L.; Somashekar, S. Wound healing studies of Aristolochia bracteolata Lam. with supportive action of antioxidant enzymes. Phytomedicine 2003, 10, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Souza, B.E.; Ottoni, M.H.F.; de Alvarenga, P.G.M.; Meireles, A.B.; Silveira, J.V.W.; Almeida, V.G.; Dos Santos, M.G.; González-Torres, L.A.; Fuzer Grael, C.F.; Alvim Brito-Melo, G.E.; Avelar-Freitas, B.A. Effect of essential oil from Ageratum fastigiatum on beta-integrin (CD18) expression on human lymphocytes stimulated with phorbol myristate acetate in vitro. Nat. Prod. Res. 2020, 34, 3409–3413. [Google Scholar] [CrossRef]
- Seyed Ahmadi, S.G.; Farahpour, M.R.; Hamishehkar, H. Topical application of Cinnamon verum essential oil accelerates infected wound healing process by increasing tissue antioxidant capacity and keratin biosynthesis. Kaohsiung J. Med. Sci. 2019, 35, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Soares, K.D.; Bordignon, S.A.L.; Apel, M.A. Chemical composition and anti-inflammatory activity of the essential oils of Piper gaudichaudianum and Piper mikanianum. J. Ethnopharmacol. 2022, 297, 115533. [Google Scholar] [CrossRef] [PubMed]
- Andjić, M.; Božin, B.; Draginić, N.; Kočović, A.; Jeremić, J.N.; Tomović, M.; Milojević Šamanović, A.; Kladar, N.; Čapo, I.; Jakovljević, V.; Bradić, J.V. Formulation and evaluation of Helichrysum italicum essential oil-based topical formulations for wound healing in diabetic rats. Pharmaceuticals 2021, 14, 813. [Google Scholar] [CrossRef]
- Perini, J.A.; Angeli-Gamba, T.; Alessandra-Perini, J.; Ferreira, L.C.; Nasciutti, L.E.; Machado, D.E. Topical application of Acheflan on rat skin injury accelerates wound healing: a histopathological.; immunohistochemical and biochemical study. BMC Complement Altern. Med. 2015, 15, 203. [Google Scholar] [CrossRef] [PubMed]
- Mazutti da Silva, S.M.; Rezende Costa, C.R.; Martins Gelfuso, G.; Silva Guerra, E.N.; de Medeiros Nóbrega, Y.K.; Gomes, S.M.; Pic-Taylor, A.; Fonseca-Bazzo, Y.M.; Silveira, D.; Magalhães, P.O. Wound healing effect of essential oil extracted from Eugenia dysenterica DC (Myrtaceae) leaves. Molecules 2018, 24, 2. [Google Scholar] [CrossRef] [PubMed]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. β-caryophyllene and β-caryophyllene oxide-natural compounds of anticancer and analgesic properties. Cancer Med. 2016, 10, 3007–3017. [Google Scholar] [CrossRef] [PubMed]
- Parks, W.C.; Wilson, C.L.; López-Boado, Y.S. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat. Rev. Immunol. 2004, 8, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Lazaro, J.L.; Izzo, V.; Meaume, S.; Davies, A.H.; Lobmann, R.; Uccioli, L. Elevated levels of matrix metalloproteinases and chronic wound healing: An updated review of clinical evidence. J. Wound Care 2016, 25, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Lockmann, A.; Schill, T.; Hartmann, F.; Grönemeyer, L.L.; Holzkamp, R.; Schön, M.P.; Thoms, K.M. Testing elevated protease activity: Prospective analysis of 160 wounds. Adv. Skin Wound Care. 2018, 31, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.W.; Kim, M.M. β-Caryophyllene oxide inhibits metastasis by downregulating MMP-2, p-p38 and p-ERK in human fibrosarcoma cells. J. Food Biochem. 2022, 46, e14468. [Google Scholar] [CrossRef]
- Liu, S.; Liu, J.; Wang, Y.; Deng, L.; Chen, S.; Wang, X.; Zuo, T.; Hu, Q.; Rao, J.; Wang, Q.; Dong, Z. Differentially expressed genes induced by β-caryophyllene in a rat model of cerebral ischemia-reperfusion injury. Life Sci. 2021, 273, 119293. [Google Scholar] [CrossRef]
- Kishi, C.; Higashihara, M.; Takemoto, Y.; Kamei, M.; Yoshioka, Y.; Matsumura, S.; Yamada, K.; Kobayashi, T.; Matahira, Y.; Moriyama, T.; Zaima, N. Inhaled volatile β-caryophyllene is incorporated into the aortic wall and attenuates nicotine-induced aorta degeneration via a CB2 receptor-dependent pathway. Biomed. Pharmacother. 2022, 153, 113423. [Google Scholar] [CrossRef]
- Fathima, J.S.; Selvaraj, J.; Sivabalan, V.; Rekha, U.V.; Ponnulakshmi, R.; Vishnupriya, V.; Kullappan, M.; Sreekandan, R.N.; Mohan, S.K. Molecular docking of alkaloid compounds with the matrix metalloproteinase 2. Bioinformation 2021, 17, 206–211. [Google Scholar] [CrossRef]
- Kant, V.; Jangir, B.L.; Sharma, M.; Kumar, V.; Joshi, V.G. Topical application of quercetin improves wound repair and regeneration in diabetic rats. Immunopharmacol. Immunotoxicol. 2021, 43, 536–553. [Google Scholar] [CrossRef]
- Chen, L.Y.; Cheng, H.L.; Kuan, Y.H.; Liang, T.J.; Chao, Y.Y.; Lin, H.C. Therapeutic potential of luteolin on impaired wound healing in streptozotocin-induced rats. Biomedicines 2021, 9, 761. [Google Scholar] [CrossRef]
- Cao, G.; Xiang, C.; Zhou, R.; Zhang, Y.; Xu, H.; Yang, H.; Zhang, J. Notoginsenoside R1 facilitated wound healing in high-fat diet/streptozotocin-induced diabetic rats. Oxid. Med. Cell. Longev. 2022, 2022, 2476493. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wu, S.; Mao, X.; Wang, W.; Tai, H. Inhibition effect of curcumin on TNF-α and MMP-13 expression induced by advanced glycation end products in chondrocytes. Pharmacology 2013, 91, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Jiao, Y.; Yu, X.; Li, X.; Wang, W.; Ding, L.; Liu, L. Resveratrol inhibits the IL-1β-induced expression of MMP-13 and IL-6 in human articular chondrocytes via TLR4/MyD88-dependent and -independent signaling cascades. Int. J. Mol. Med. 2017, 39, 734–740. [Google Scholar] [CrossRef]
- Han, J.; Xian, Z.; Zhang, Y.; Liu, J.; Liang, A. Systematic overview of aristolochic acids: Nephrotoxicity.; carcinogenicity, and underlying mechanisms. Front. Pharmacol. 2019, 10, 648. [Google Scholar] [CrossRef] [PubMed]
- Koyama, S.; Purk, A.; Kaur, M.; Soini, H.A.; Novotny, M.V.; Davis, K.; Kao, C.C.; Matsunami, H.; Mescher, A. Beta-caryophyllene enhances wound healing through multiple routes. PLoS ONE 2019, 14, e0216104. [Google Scholar] [CrossRef]
- Delgado, C.; Mendez-Callejas, G.; Celis, C. Caryophyllene oxide, the active compound isolated from leaves of Hymenaea courbaril L. (Fabaceae) with antiproliferative and apoptotic effects on pc-3 androgen-independent prostate cancer cell line. Molecules 2021, 26, 6142. [Google Scholar] [CrossRef]
- Parisotto-Peterle, J.; Bidone, J.; Lucca, L.G.; Araújo, G.M.S.; Falkembach, M.C.; da Silva Marques, M.; Horn, A.P.; Dos Santos, M.K.; da Veiga, V.F., Jr.; Limberger, R.P.; Teixeira, H.F.; Dora, C.L.; Koester, L.S. Healing activity of hydrogel containing nanoemulsified β-caryophyllene. Eur. J. Pharm. Sci. 2020, 148, 105318. [Google Scholar] [CrossRef]
- Gushiken, L.F.S.; Beserra, F.P.; Hussni, M.F.; Gonzaga, M.T.; Ribeiro, V.P.; de Souza, P.F.; Campos, J.C.L.; Massaro, T.N.C.; Hussni, C.A.; Takahira, R.K.; Marcato, P.D.; Bastos, J.K.; Pellizzon, C.H. Beta-caryophyllene as an antioxidant, anti-inflammatory and re-epithelialization activities in a rat skin wound excision model. Oxid. Med. Cell. Longev. 2022, 2022, 9004014. [Google Scholar] [CrossRef] [PubMed]
- Araújo, L.U.; Grabe-Guimarães, A.; Mosqueira, V.C.F.; Carneiro, C.M.; Silva-Barcellos, N.M. Profile of wound healing process induced by allantoin. Acta Cir. Bras. 2010, 25, 460–466. [Google Scholar] [CrossRef]
- Komi, D.E.A.; Khomtchouk, K.; Santa Maria, P.L. A review of the contribution of mast cells in wound healing: Involved molecular and cellular mechanisms. Clin. Rev. Allergy. Immunol. 2020, 58, 298–312. [Google Scholar] [CrossRef]
- Syed, F.; Bagabir, R.A.; Paus, R.; Bayat, A. Ex Vivo evaluation of antifibrotic compounds in skin scarring: EGCG and silencing of PAI-1 independently inhibit growth and induce keloid shrinkage. Lab. Invest. 2013, 93, 946–960. [Google Scholar] [CrossRef]
- Gertsch, J.; Leonti, M.; Raduner, S.; Racz, I.; Chen, J.Z.; Xie, X.Q.; Altmann, K.H.; Karsak, M.; Zimmer, A. Beta-caryophyllene is a dietary cannabinoid. Proc. Natl. Acad. Sci. USA 2008, 105, 9099–9104. [Google Scholar] [CrossRef]
- Shan, J.; Chen, L.; Lu, K. Protective effects of trans-caryophyllene on maintaining osteoblast function. IUBMB Life 2017, 69, 22–29. [Google Scholar] [CrossRef]
- Han, X.; Beaumont, C.; Rodriguez, D.; Bahr, T. Black pepper (Piper nigrum) essential oil demonstrates tissue remodeling and metabolism modulating potential in human cells. Phytother. Res. 2018, 32, 1848–1852. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, D.; Chen, Y.; Yang, M. β-Caryophyllene inhibits high glucose-induced oxidative stress, inflammation and extracellular matrix accumulation in mesangial cells. Int. Immunopharmacol. 2020, 84, 106556. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.Z.; Stevenson, A.W.; Prêle, C.M.; Fear, M.W.; Wood, F.M. The role of IL-6 in skin fibrosis and cutaneous wound healing. Biomedicines 2020, 8, 101. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.W.; Cachianes, G.; Kuang, W.J.; Goeddel, D.V.; Ferrara, N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 1989, 246, 1306–1309. [Google Scholar] [CrossRef] [PubMed]
| ID Code | Ligand Name | ID Code | Ligand Name |
|---|---|---|---|
| 2236 | Aristolochic acid | 11672 | Curcumin |
| 15573 | α-Pinene | 8815 | Estragole |
| 70678558 | α-Copaene | 2758 | Eucalyptol |
| 5281520 | α-Humulene | 09086 | Eugenol |
| 7460 | α-Phellandrene | 7461 | γ-Terpinene |
| 10856614 | α-Selinene | 6549 | Linalool |
| 10545 | Ascaridole | 31253 | Myrcene |
| 5281515 | (E)-β-caryophyllene | 319084028 | 1,6-Octadiene |
| 11142 | β-Phellandrene | 71742210 | Caryophyllene oxide |
| 14896 | β-Pinene | 7463 | ρ-Cymene |
| 442393 | β-Selinene | 439570 | (R)- (-)-Carvone |
| 7001 | 1-Bromonaphthalene | 16724 | (S)- (+)-Carvone |
| 01744 | Camphor | 440917 | R-Limonene |
| 10364 | Carvacrol | 439250 | S-Limonene |
| 6616 | Camphene | 18818 | Sabinene |
| 5320128 | cis-Nerolidol | 02513 | Thymol |
| 7794 | Citronellal |
| ID PDB | Therapeutic target | Coordinates (x, y, z) |
|---|---|---|
| 2AZ5 | Tumor necrosis factor-α (TNFα) | -19.259, 74.79, 34.032 |
| 1ALU | Interleukin-6 (IL6) | -3.934, -18.113, 3.394 |
| 5IKQ | Cyclooxygenase-2 (COX2) | 22.222, 52.346, 18.018 |
| 4OEE | Fibroblast growth factor-2 (FGF2) | -11.744, -6.027, -3.03 |
| 1Q5K | Glycogen synthase kinase-3β (GSK3β) | 23.992, 22.827, 8.884 |
| 2ZM3 | Insulin-like growth factor 1 (IGFR1) | 37.061, 79.097, 58.051 |
| 2D1O | Matrix metalloproteinases (MMP3) | 28.762, 6.733, 14.320 |
| 1GKC | MMP9 | 48.145, 64.140, 42.896 |
| 2PJT | MMP13 | 9.323, 27.639, 141.777 |
| 3HNG | Vascular endothelial growth factor receptor-1 (VEGFR1) | 4.729, 17.729, 33.352 |
| Ligand | Target | ||
|---|---|---|---|
| MMP-3 | MMP-13 | TNFα | |
| Co-crystallized ligand | -51.755 +/- 4.118 | -29.563 +/- 3.255 | -48.432+/- 3.139 |
| Aristolochic acid | -57.820 +/- 5.573 * | -56.075 +/- 5.712 * | -25.796+/- 2.176 * |
| α-Copaene | -17.447 +/- 1.967 * | -21.081 +/- 2.193 * | -26.874 +/- 2.083 * |
| α-Selinene | -28.741 +/- 2.063 * | -20.222 +/- 4.226 * | -19.417 +/- 1.808 * |
| β-Caryophyllene | -17.432 +/- 2.554 * | -23.419 +/- 2.948 * | -26.386 +/- 1.686 * |
| β-Selinene | -17.107 +/- 2.168 * | -24.646 +/- 2.489 * | -24.303 +/- 2.188 * |
| Caryophyllene oxide | no interaction | -27.980 +/- 1.989 * | -25.709 +/- 2.216 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





