Submitted:
20 April 2023
Posted:
21 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Isolation and identification of phytopathogenic fungi
2.3. OMW inhibition effect against phytopathogenic fungi
2.4. Microbial count and water content determination
2.5. Ethyl acetate extract effect against phytopathogenic fungi
2.6. Phenolic and flavonoid compounds analysis using HPLC method
2.7. Statistical Analysis
3. Results
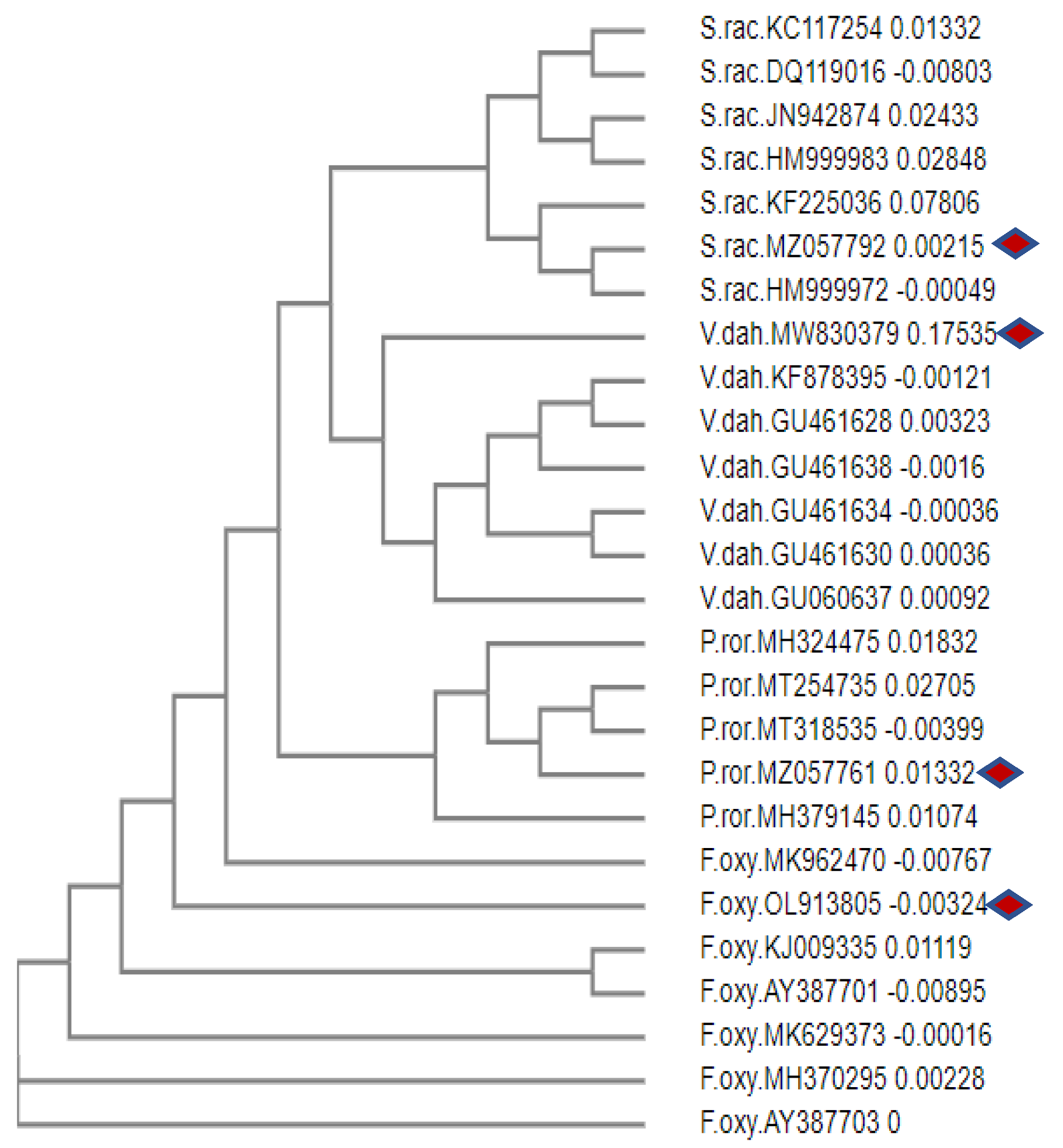
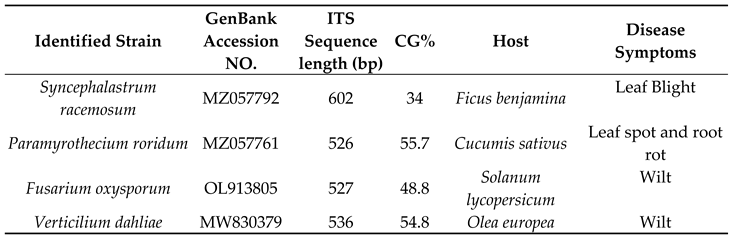
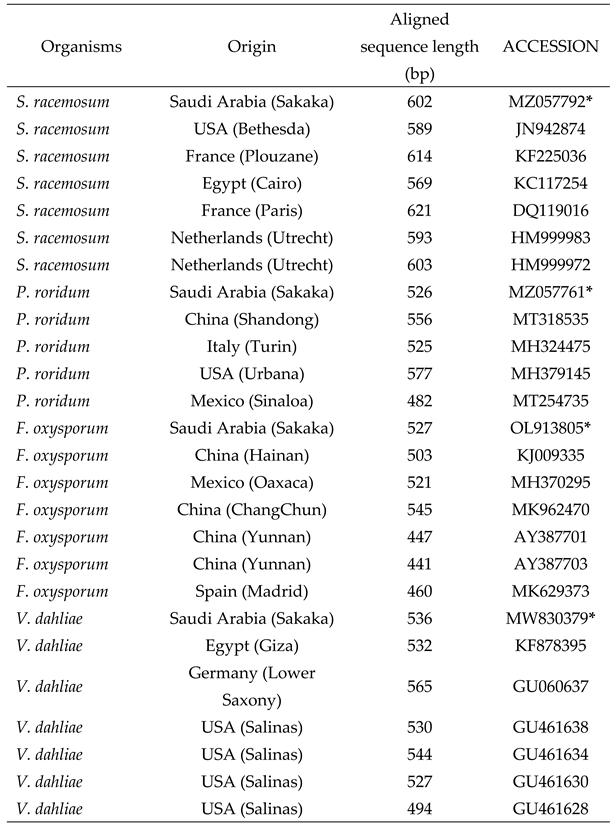
3.1. Molecular identification of phytopathogenic fungi
3.2. Olive mill wastewater physico-chemical characterization
3.3. Effect of OMW against phytopathogenic fungi
3.4. Effect of ethyl acetate extract against phytopathogenic fungi
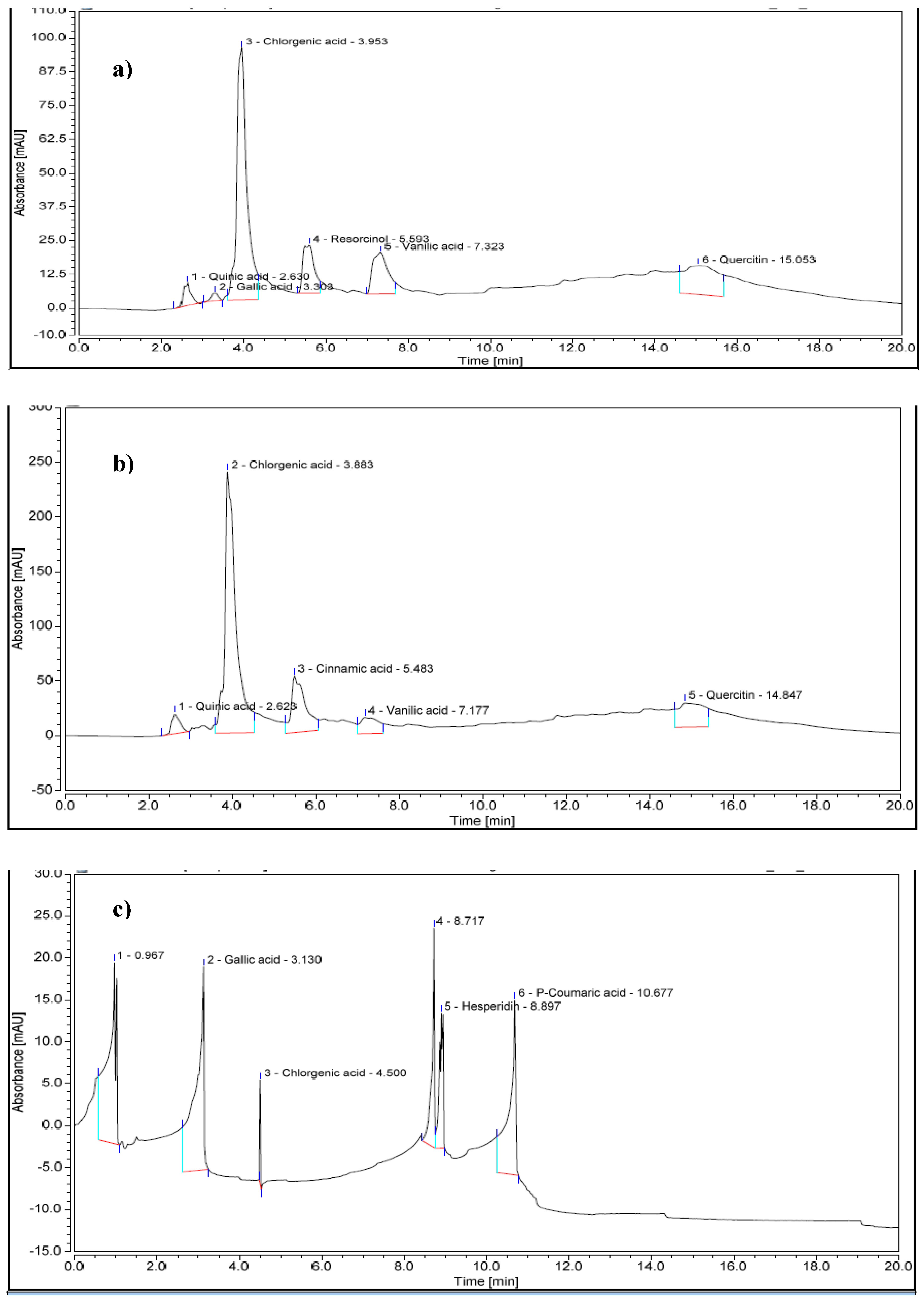
3.5. Identification and quantification of phenolic compounds and flavonoids in fresh and stored OMW
4. Discussion
5. Conclusion
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Food and Agriculture Organization of the United Nations. Crops 2020. Available online: http://www.fao.org/faostat/en/?#data/QC (accessed on 1 January 2021).
- Fraihat, S.; Gilbert-López, B.; Molina-Díaz, A.; Sabouni, I. Physicochemical characterization of olive oil from Al Jouf area of Saudi Arabia. Inter. J. Chem. Tech. Res. 2017, 10, 1004–1010. [Google Scholar]
- Hemida, M.H.; Ibrahim, A.A.E.; Al-Bahnsawy, R.M.; Al-Shathly, M.R. Influence of environmental factors on olive oil production and quality in the Northern region of Kingdom of Saudi Arabia. J. Am. Sci. 2014, 10, 61–66. [Google Scholar]
- Aly, A.A.; Hasan, Y.N.Y.; Al-Farraj, A.S. Olive mill wastewater treatment using a simple zeolite-based low-cost method. J. Environ. Manage. 2014, 145, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Jarboui, R.; Hadrich, B.; Gharsallah, N.; Ammar, E. Olive mill wastewater disposal in evaporation ponds in Sfax (Tunisia) - Moisture content effect on microbiological and physical chemical parameters. Biodegr. 2009, 20, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Vagelas, I.; Kalorizou, H.; Papachatzis, A.; Botu, M. Bioactivity of olive oil mill wastewater against plant pathogens and post-harvest diseases. Biotechnol. Biotechnol. Equip. 2016, 23, 1217–1219. [Google Scholar] [CrossRef]
- Silvan, J.M.; Pinto-Bustillos, M.A.; Vásquez-Ponce, P.; Prodanov, M.; Martinez-Rodriguez, A.J. Olive mill wastewater as a potential source of antibacterial and anti-inflammatory compounds against the food-borne pathogen Campylobacter. Innov. Food Sci. Emerg. Technol. 2019, 51, 177–185. [Google Scholar] [CrossRef]
- Russo, E.; Spallarossa, A.; Comite, A.; Pagliero, M.; Guida, P.; Belotti, V.; Caviglia, D.; Schito, A.M. Valorization and potential antimicrobial use of olive mill wastewater (OMW) from Italian olive oil production. Antioxidants 2022, 11, 903. [Google Scholar] [CrossRef]
- Pannucci, E.; Caracciolo, R.; Romani, A.; Cacciola, F.; Dugo, P.; Bernini, R.; Varvaro, L.; Santi, L. An hydroxytyrosol enriched extract from olive mill wastewaters exerts antioxidant activity and antimicrobial activity on Pseudomonas savastanoi pv. savastanoi and Agrobacterium tumefaciens. Nat. Prod. Res. 2021, 35, 2677–2684. [Google Scholar] [CrossRef]
- Cibelli, F.; Bevilacqua, A.; Raimondo, M.L.; Campaniello, D.; Carlucci, A.; Ciccarone, C.; Sinigaglia, M.; Corbo, M.R. Evaluation of fungal growth on olive-mill wastewaters treated at high temperature and by high-pressure homogenization. Front. Microbiol. 2017, 8, 2515. [Google Scholar] [CrossRef]
- Cayuela, M.L.; Millner, P.D.; Meyer, S.L.F.; Roig, A. Potential of olive mill waste and compost as biobased pesticides against weeds fungi and nematodes. Sci. Total Env. 2008, 399, 11–18. [Google Scholar] [CrossRef]
- El-Abbassi, A.; Saadaoui, N.; Kiai, H.; Raiti, J.; Hafidi, A. Potential applications of olive mill wastewater as biopesticide for crops protection. Sci. Total Env. 2017, 576, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Jarboui, R.; Sellami, F.; Kharroubi, A.; Gharsallah, N.; Ammar, E. Olive mill wastewater stabilization in open-air ponds: Impact on clay–sandy soil. Bioresour. Technol. 2008, 99, 7699–7708. [Google Scholar] [CrossRef] [PubMed]
- Magdich, S.; Jarboui, R.; Ben Rouina, B.; Boukhris, M.; Ammar, E. A yearly spraying of olive mill wastewater on agricultural soil over six successive years: Impact of different application rates on olive production, phenolic compounds, phytotoxicity and microbial counts. Sci. Total Env. 2012, 430, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Bruno, G.; Sparapano, L. Effects of three esca-associated fungi on Vitis vinifera L. changes in the chemical and biological profile of xylem sap from diseased cv. Sangiovese vines. Physiol. Mol. Plant Pathol. 2007, 71, 210–229. [Google Scholar] [CrossRef]
- Xia, E.; Deng, G.; Guo, Y.; Li, H. Biological activities of polyphenols from Grapes. Inter. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef]
- Baydar, N.G.; Sagdic, O.; Ozkan, G.; Cetin, S. Determination of antibacterial effects and total phenolic contents of grape (Vitis vinifera) seed extracts. Int. J. Food Sci. 2006, 41, 799–804. [Google Scholar] [CrossRef]
- Bonanomi, G.; Giorgi, V.; Del Sorbo, G.; Neri, D.; Scala, F. Olive mill residues affect saprophytic growth and disease incidence of foliar and soilborne plant fungal pathogens. Agric. Ecosyst. Environ. 2006, 115, 194–200. [Google Scholar] [CrossRef]
- Quaglia, M.; Moretti, Ch.; Cerri, M.; Linoci, G.; Taticchi, A. Effect of extracts of wastewater from olive milling in postharvest treatments of pomegranate fruit decay caused by Penicillium adametzioides. Postharvest Biol. Technol. 2016, 11, 26–34. [Google Scholar] [CrossRef]
- Japanese Standards Association JIS Handbook 1995. Wastewater Treatment Japanese Standards Association Tokyo.
- Knechtel, R.J. A more economical method for the determination of chemical oxygen demand. J. Water Pollut. Control Fed. 1987, 7, 25–29. [Google Scholar]
- World Health Organization (WHO). Guidelines for Drinking-Water Quality (3rd edn). World Health Organization Geneva. 2004.
- Presidency of Meteorology and Environment (PME). General environmental regulations and rules for implementation. Kingdom of Saudi Arabia 15 October 2001, p. 206.
- Shaima, M. N. M; Rania, H. T.; Hani, M. A. A.; Hassan, A. E. Novel biosynthesis of Ag-nanocomplex for controlling Verticillium wilt disease of olive tree. Arch. Phytopathol. Plant Prot. 2021, 1–19. [Google Scholar]
- Umashankar, V.; Arunkumar, V.; Dorairaj, S. ACUA: A software tool for automated codon usage analysis. Bioinformation 2007, 2, 62–63. [Google Scholar] [CrossRef] [PubMed]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; Mcgettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; Thompson, J.D.; Gibson, T.J.; Higgins, D.G. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed]
- Onaran, A.; Bayram, M. Determination of antifungal activity and phenolic compounds of endemic Muscari aucheri (Boiss.) baker extract. J. Agric. Faculty Gazi. Osman Pasa. Univ. 2018, 35, 60–67. [Google Scholar] [CrossRef]
- International Standardisation Organisation, Règles générales pour les examens microbiologiques (ISO 7218), 1996.
- Association Française de la Normalisation (AFNOR), Microbiologie des aliments – Dénombrement des levures et moisissures par comptage des colonies à 25 °C – Méthode de routine (NF V08-059), 1995.
- Leontopoulos, S.V.; Giavasis, I.; Petrotos, K.; Kokkora, M.; Makridis, Ch. Effect of different formulations of polyphenolic compounds obtained from OMWW on the growth of several fungal plant and food borne pathogens. Studies in vitro and in vivo. Agric. Agric. Sci. Procedia 2015, 4, 327–337. [Google Scholar] [CrossRef]
- Płuciennik-Koropczuk, E.; Myszograj, S. New Approach in COD Fractionation Methods. Water 2019, 11, 1484. [Google Scholar] [CrossRef]
- Saleh, A.R.; Sabouni, I.; Salam, S.A.S. Physico-chemical and microbiological characterization of olive mill wastewater in Sakaka Aljouf region KSA. Int. J. Clim. Chang. Strateg. Manage. 2017, 5, 46–53. [Google Scholar]
- Alhajoj, A.; Alowaiesh, B. Innovative solutions for reduction of olive mill wastewater pollution. Desalin. Water Treat. 2019, 155, 48–54. [Google Scholar] [CrossRef]
- Paredes, C.; Cegarra, J.; Roig, A.; Sánchez-Monedero, M.A.; Berna, M.P. Characterization of olive mill wastewater (alpechin) and its sludge for agricultural purposes. Bioresour. Technol. 1999, 67, 111–115. [Google Scholar] [CrossRef]
- Arabi, M.; Elias, A.; Kamel, Z.; Ait younes, Y.; Mansouri, B.; Toumert, I. Characterization of olive mill wastewater and gamma irradiation effects on some parameters of its composition. J. Radioanal. Nucl. Chem. 2018, 317, 1095–1106. [Google Scholar] [CrossRef]
- Drais, M.I.; Pannucci, E.; Caracciolo, R.; Bernini, R.; Romani, A.; Santi, L.; Varvaro, L. Antifungal activity of hydroxytyrosol enriched extracts from olive mill waste against Verticillium dahliae the cause of Verticillium wilt of olive. Phytopathol. Mediterr. 2021, 60, 139–147. [Google Scholar] [CrossRef]
- Chatzistathis, T.; Kavvadias, V.; Sotiropoulos, T.; Papadakis, I.E. Organic fertilization and tree orchards. Agriculture 2021, 11, 692. [Google Scholar] [CrossRef]
- Misra, A.K.; Garg, N.; Yadav, K.K. First Report of Shell Soft Rot of Bael (Aegle marmelos) Caused by Syncephalastrum racemosum in North India. Plant Disease 2016, 100, 1779–1779. [Google Scholar] [CrossRef]
- Al-Awadi, A.; Al-Judaibi, A. Effects of heating and storage on the antifungal activity of camel urine. Clin. Microbiol. 2014, 3, 1000179. [Google Scholar]
- Jarboui, R.; Hadrich, B.; Gharsallah, N.; Ammar, E. Olive mill wastewater disposal in evaporation ponds in Sfax (Tunisia): moisture content effect on microbiological and physical chemical parameters. Biodegradation 2009, 20, 845–858. [Google Scholar] [CrossRef] [PubMed]
- El-Masry, M.H.; AI Khalil, M.S.; Hassouna, I.H.A.H. In situ and in vitro suppressive effect of agricultural composts and their water extracts on some phytopathogenic fungi. World J. Microbiol. Biotechnol. 2002, 18, 551–558. [Google Scholar] [CrossRef]
- Bess, V.H. Compost Teas and Compost Microbiology Understanding Compost Tea. BioCycle Reprints. 1999.
- Caballero-Guerrero, B.; Garrido-Fernandez, A.; Fermoso, F.G.; Rodríguez-Gutierrez, G.; Fernandez-Prior, M.A.; Reinhard, C.; Nystrom, L.; Benítez-Cabello, A.; Arroyo-Lopez, F.N. Antimicrobial effects of treated olive mill waste on foodborne pathogens. LWT-Food Sci. Technol. 2022, 164, 113628. [Google Scholar] [CrossRef]
- Feki, M.; Allouche, N.; Bouaziz, M.; Gargoubi, A.; Sayadi, S. Effect of storage of olive mill wastewaters on hydroxytyrosol concentration. Eur. J. Lipid Sci. Technol. 2006, 108, 1021–1027. [Google Scholar] [CrossRef]
- Romero, C.; Brenes, M.; Garcia, P.; Garcia, A.; Garrido, A. Polyphenol changes during fermentation of naturally black olives. J. Agric. Food Chem. 2004, 52, 1973–1979. [Google Scholar] [CrossRef]
- Kulik, T.; Stuper-Szablewska, K.; Bilska, K.; Bu´sko, M.; Ostrowska-Kołodziejczak, A.; Załuski, D.; Perkowski, J. Trans-cinnamic and chlorogenic acids affect the secondary metabolic profiles and ergosterol biosynthesis by Fusarium culmorum and F. graminearum sensu stricto. Toxins 2017, 9, 198. [Google Scholar] [CrossRef]
- Oufensou, S.; Balmas, V.; Azara, E.; Fabbri, D.; Dettori, M.A.; Schüller, C.; Zehetbauer, F.; Strauss, J.; Delogu, G.; Migheli, Q. Naturally occurring phenols modulate vegetative growth and deoxynivalenol biosynthesis in Fusarium graminearum. ACS Omega 2020, 5, 29407–29415. [Google Scholar] [CrossRef] [PubMed]
- Boutigny, A.L.; Barreau, C.; Atanasova-Pénichon, V.; Verdal-Bonnin, M.; Pinson-Gadais, L.; Richard-Forget, F. Ferulic acid, an efficient inhibitor of type B trichothecene biosynthesis and Tri gene expression in Fusarium liquid cultures. Mycol. Res. 2009, 113, 746–775. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, J.; Kong, W.; Zhao, G.; Yang, M. Mechanisms of antifungal and anti-aflatoxigenic properties of essential oil derived from turmeric (Curcuma longa L.) on Aspergillus flavus. Food Chem. 2017, 220, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Martínez, G.; Regente, M.; Jacobi, S.; Del Rio, M.; Pinedo, M.; de la Canal, L. Chlorogenic acid is a fungicide active against phytopathogenic fungi. Pest. Biochem. Physiol. 2017, 140, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Calheiros, D.; Dias, M.I.; Calhelha, R.C.; Barros, L.; Ferreira, I.C.F.R.; Fernandes, C.; Gonçalves, T. Antifungal Activity of Spent Coffee Ground Extracts. Microorganisms 2023, 11, 242. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.J.; Liu, M.; Dawuti, G.; Dou, Q.; Ma, Y.; Liu, H.G.; Aibai, S. Antifungal activity of gallic acid in vitro and in vivo. Phytother. Res. 2017, 31, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Bitencourt, T.A.; Komoto, T.T.; Massaroto, B.G.; Miranda, C.E.S.; Beleboni, R.O.; Marins, M.; Fachin, A.L. Trans-chalcone and quercetin down-regulate fatty acid synthase gene expression and reduce ergosterol content in the human pathogenic dermatophyte Trichophyton rubrum. BMC Complement. Altern. Med. 2013, 13, 229. [Google Scholar] [CrossRef]
- Treutter, D. Significance of flavonoids in plant resistance and enhancement of their biosynthesis. Plant Biol. 2005, 7, 581–591. [Google Scholar] [CrossRef]
- Rocha-Parra, D.F; Lanari, M.C.; Zamora, M.C.; Chirife, J. Influence of storage conditions on phenolic compounds stability antioxidant capacity and colour of freeze-dried encapsulated red wine. LWT - Food Sci. Technol. 2016, 70, 162–170. [Google Scholar] [CrossRef]
- Teles, A.S.C.; Chávez, D.W.H.; Gomes, F.D.S.; Cabral, L.M.C.; Tonon, R.V. Effect of temperature on the degradation of bioactive compounds of Pinot Noir grape pomace during drying. Braz. J. Food Technol. Campinas 2018, 21, e2017059. [Google Scholar] [CrossRef]
- Terefe, N.S.; Delon, A.; Buckow, R.; Versteeg, C. Blueberry polyphenol oxidase: characterization and the kinetics of thermal and high-pressure activation and inactivation. Food Chem. 2015, 188, 193–200. [Google Scholar] [CrossRef] [PubMed]
 |
 |
| Parameter | OMW | Maximum limit (WHO) | Maximum limit (KSA) |
| Basic index | |||
| pH | 5.02 ± 0.50 | 6.5-8.0 | 6-9 |
| EC (mS/cm) | 13.52 ± 0.58 | 3000 | ND |
| TS (g/l) | 54.23 ± 1.20 | ND | ND |
| TSS (g/l) | 12.50 ± 0.85 | 500 | 15 |
| COD (g/l) | 76.00 ± 0.15 | ND | 150 |
| CODs (g/l) | 45.40 ± 0.10 | ND | ND |
| BOD5 (g/l) | 19.70 ± 0.05 | 5 | 25 mg/l |
| CODs/BOD5 | 2.30 | ND | ND |
| Organic content | |||
| VS (g/l) | 42.75 ± 1.25 | ND | ND |
| TOC (g/l) | 18.30 ± 1.20 | ND | 50 mg/l |
| Total N (g/l) | 1.50 ± 1.05 | ND | 5 mg/l |
| Total phenols (g/l) | 7.80 ± 0.08 | ND | 0.1 mg/l |
| Inorganic content | |||
| Ash (g/l) | 11.48 ± 0.88 | ND | ND |
| Total P (mg/l) | 76.87 ± 2.30 | < 5 | 1.0 |
| Calcium (mg/l) | 206.70 ± 1.24 | 10 | |
| Potassium (mg/l) | 5500 ± 50 | ND | ND |
| Sodium (mg/l) | 250 ± 2.50 | 919 | ND |
| Magnesium (mg/l) | 128.60 ± 2.00 | 60 | ND |
| Iron (mg/l) | 26.85 ± 2.65 | 5 | ND |
| Zinc (mg/l) | 1.2 ± 0.10 | 3 | 1.0 |
| Barium (mg/l) | 0.29 ± 1.02 | ND | ND |
| Boron (mg/l) | 0.64± 0.40 | ND | ND |
| Chromium (mg/l) | 0.017 ± 0.56 | 0.05 | 0.1 |
| Manganese (mg/l) | 0.166 ± 0.24 | 0.5 | ND |
| Copper (mg/l) | 0.12 ± 0.06 | 0.2 | 0.2 |
| Nickel (mg/l) | 0.087 ± 0.04 | 0.2 | 0.2 |
| Cadmium (mg/l) | 0.012 ± 0.01 | 0.01 | 0.02 |
| Molybdenum (mg/l) | 0.012 ± 0.01 | ND | ND |
| Lead (mg/l) | < 0.001 | 5 | 0.1 |
| Cobalt (mg/l) | < 0.001 | ND | ND |
| Vanadium (mg/l) | < 0.01 | ND | ND |
| OMW | Inhibition percentage of phytopathogenic fungi (%) | Water content (%) | Microbial flora (log10 N (ufc/ml)) |
|||||
| Dilution | S. racemosum | P. roridum | F. oxysporum | V. dahlia | ||||
| Fresh OMW | Raw non-sterile | 1/10 | 80a ± 2 | 100b | 100b | 100b | 94.16 ± 0.50 | TCB: 4.48 ± 0.02 Y&M: 3.50 ± 0.07 |
| 1/5 | 90a ± 4 | 100b | 100b | 100b | ||||
| Centrifuged non-sterile | 1/10 | 40a ± 1 | 94b ± 2 | 100b | 90b ± 2 | 97.25 ± 0.55 | TCB: 3.35 ± 0.04 Y&M: 2.30 ± 0.02 |
|
| 1/5 | 60a ± 3 | 100b | 100b | 100b | ||||
| Raw sterile | 1/10 | 0a | 46b ± 4 | 40b ± 1 | 20b ± 2 | 91.45 ± 0.26 | TCB: nd Y&M: nd |
|
| 1/5 | 0a | 70b ± 2 | 60b ± 2 | 50b ± 3 | ||||
| Stored at 25 °C for 3 months |
Raw non-sterile | 1/10 | 0a | 100b | 90ab± 1 | 90b± 2 | 88.56 ± 0.25 | TCB: 3.30 ± 0.20 Y&M: 4.80 ± 0.12 |
| 1/5 | 0a | 100b | 100b | 94b ± 4 | ||||
| Centrifuged non-sterile | 1/10 | 0a | 70b ± 4 | 80b ± 4 | 70b ± 2 | 90.50 ± 0.50 | TCB: 1.80 ± 0.20 Y&M: 2.80 ± 0.12 |
|
| 1/5 | 0a | 80b ± 3 | 90b ± 2 | 90b ± 4 | ||||
| Raw sterile | 1/10 | 0a | 60c ± 4 | 10b ± 1 | 20c± 2 | 86.35 ± 0.24 | TCB: nd Y&M: nd |
|
| 1/5 | 0a | 70c ± 2 | 36c ± 2 | 30c ± 2 | ||||
| Stored at 45 °C for 3 months |
Raw non-sterile | 1/10 | 0 a | 70b ± 3 | 70b ± 2 | 50b ± 3 | 70.24 ± 0.45 | TCB: nd Y&M: nd |
| 1/5 | 0 a | 80b ± 2 | 80b ± 4 | 60b ± 2 | ||||
| Centrifuged non-sterile | 1/10 | 0a | 60b ± 4 | 40b ± 1 | 40b ± 1 | 71.14 ± 0.24 | TCB: nd Y&M: nd |
|
| 1/5 | 0a | 66b ± 2 | 50b ± 2 | 54b ± 2 | ||||
| Raw sterile | 1/10 | 0a | 40c± 2 | 16c ± 1 | 30c ± 2 | 67.26 ± 0.85 | TCB: nd Y&M: nd |
|
| 1/5 | 0a | 50c ± 4 | 40c ± 1 | 50c ± 3 | ||||
| OMW | S. racemosum | P. roridum | F. oxysporum | V. dahliae |
| Fresh | 40a ± 2 | 70a ± 4 | 88a ± 2 | 80a ± 1 |
| Stored at 25 °C for 3 months |
0ab | 50ab ± 2 | 62ab ± 1 | 56ab ± 2 |
| Stored at 45 °C for 3 months |
0b | 48b ± 2 | 55b ± 2 | 44b ± 1 |
| Peak name | Phenolic and flavonoid compounds concentration (mg/l) ± SD | ||
| Fresh OMW | Stored OMW at 25 °C | Stored OMW at 45 °C | |
| Quinic acid | 141.14 ± 2.35 | 335.10 ± 4.25 | nd |
| Gallic acid | 0.28 ± 0.08 | nd | 5675.54 ± 17.23 |
| Chlorogenic acid | 12.83 ± 0.56 | 35.88 ± 1.25 | 1.87 ± 0.20 |
| Cinnamic acid | nd | 18.14 ± 0.88 | nd |
| Resorcinol | 4.37 ± 0.84 | nd | nd |
| Vanillic acid | 6.04 ± 1.02 | 6.70 ± 0.52 | nd |
| Hesperidin | nd | nd | 1118.00 ± 10.00 |
| p-coumaric acid | nd | nd | 3784.24 ± 12.25 |
| Quercetin | 2.95 ± 0.30 | 4.77 ± 0.66 | nd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





