Submitted:
20 April 2023
Posted:
21 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
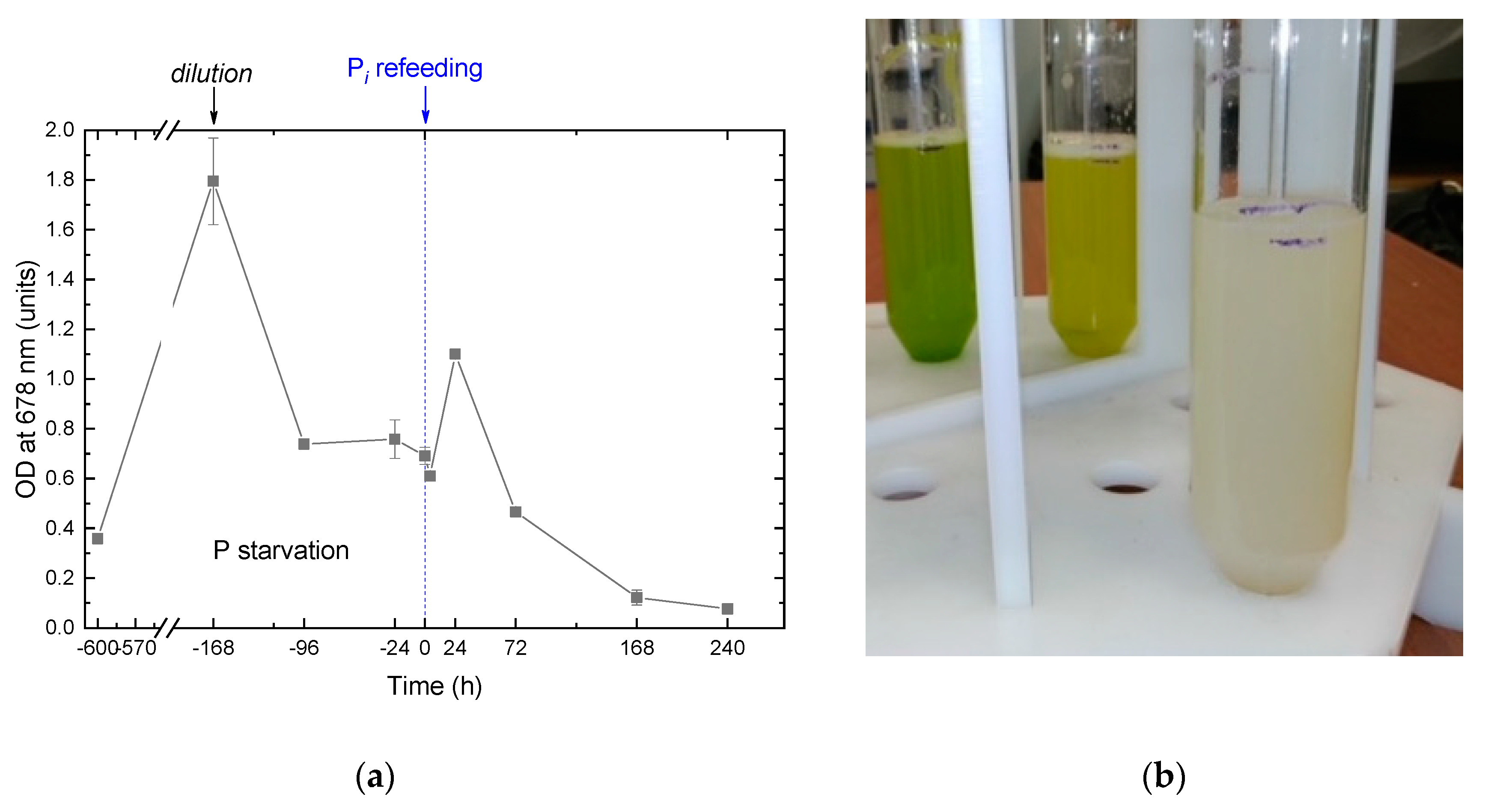
2.1. The Dynamics of the Culture Condition during Phosphorus Starvation and Re-Feeding
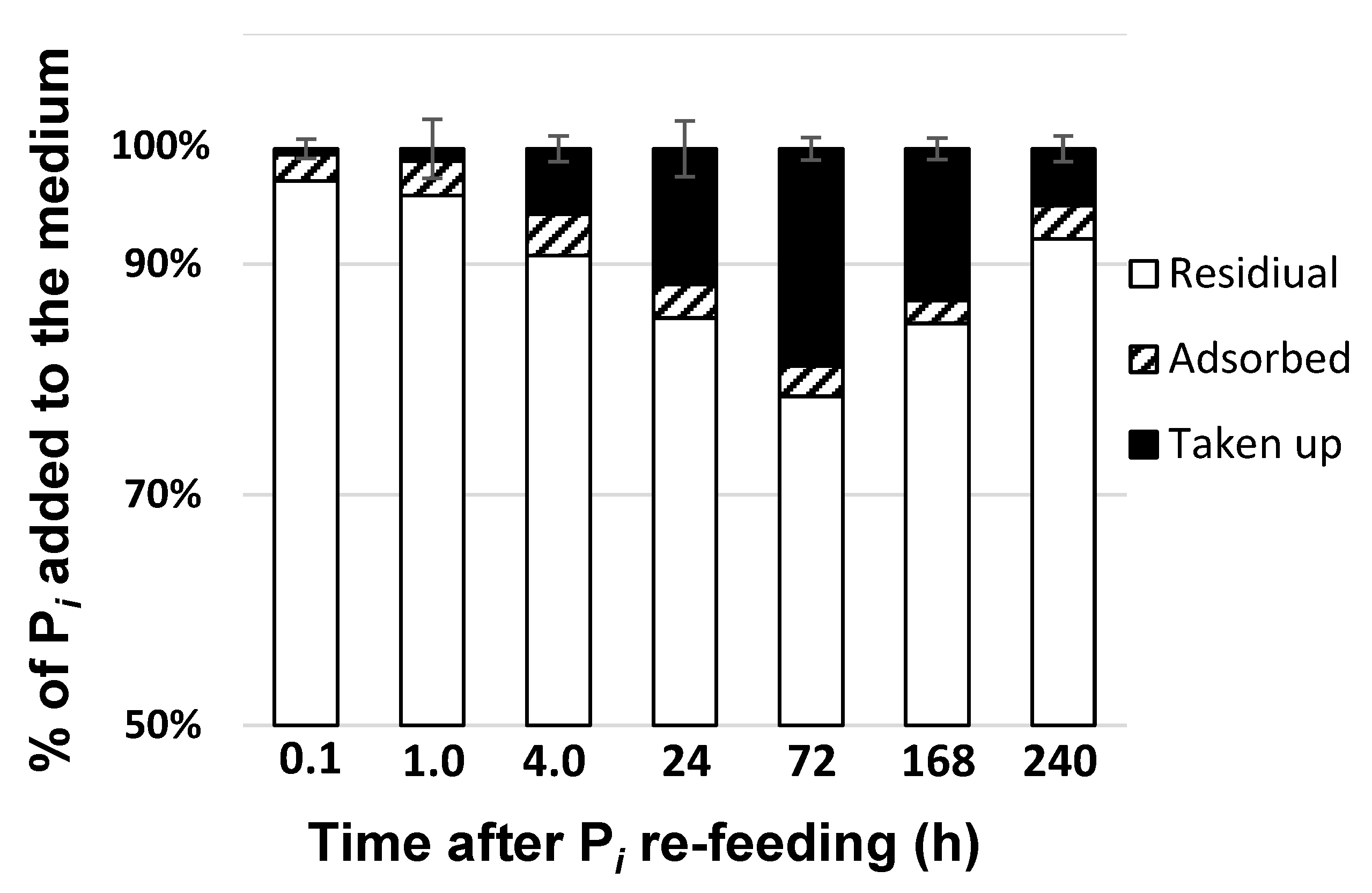
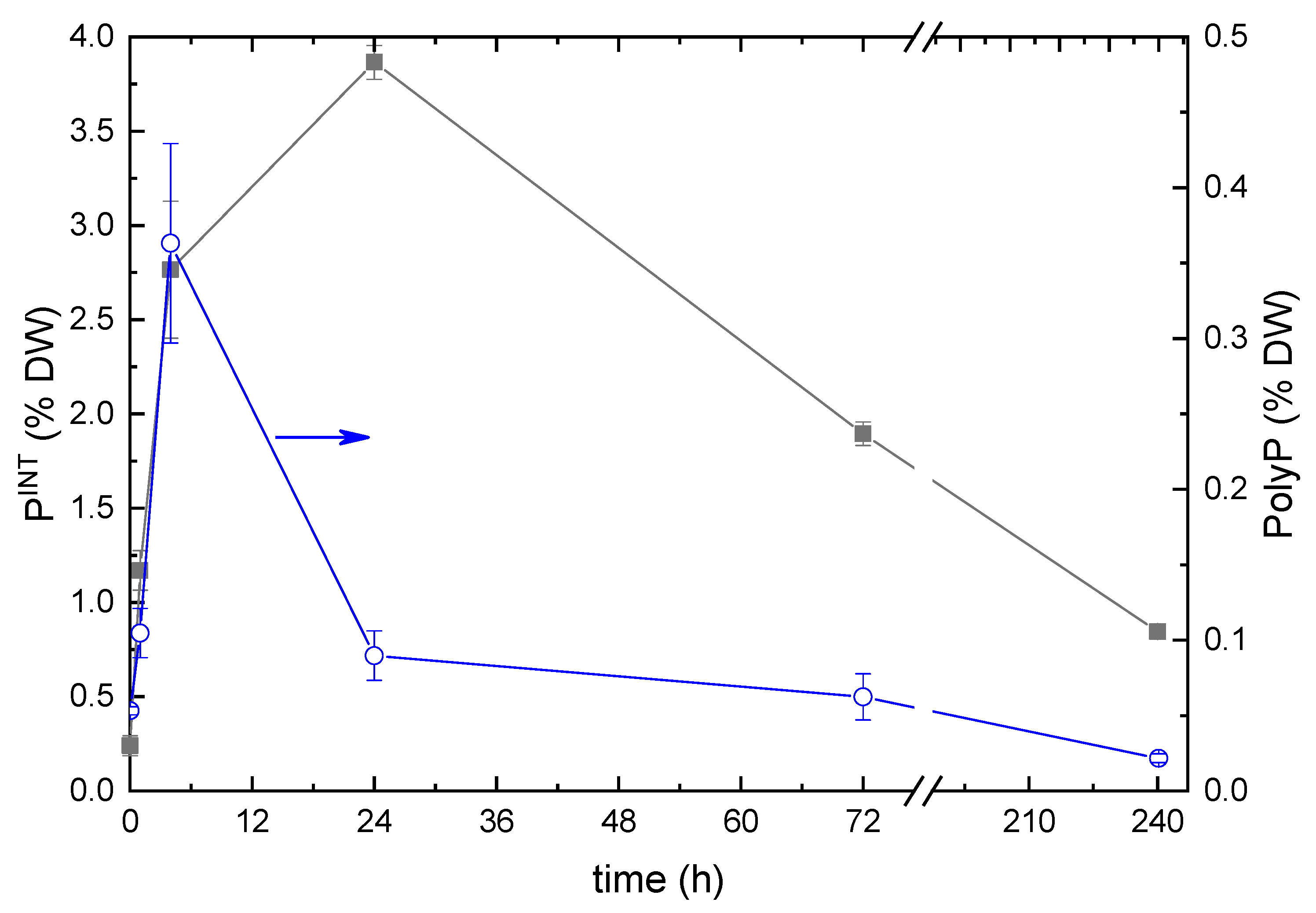
2.2. Phosphorus Removal from the Medium and Its Uptake by the Cells
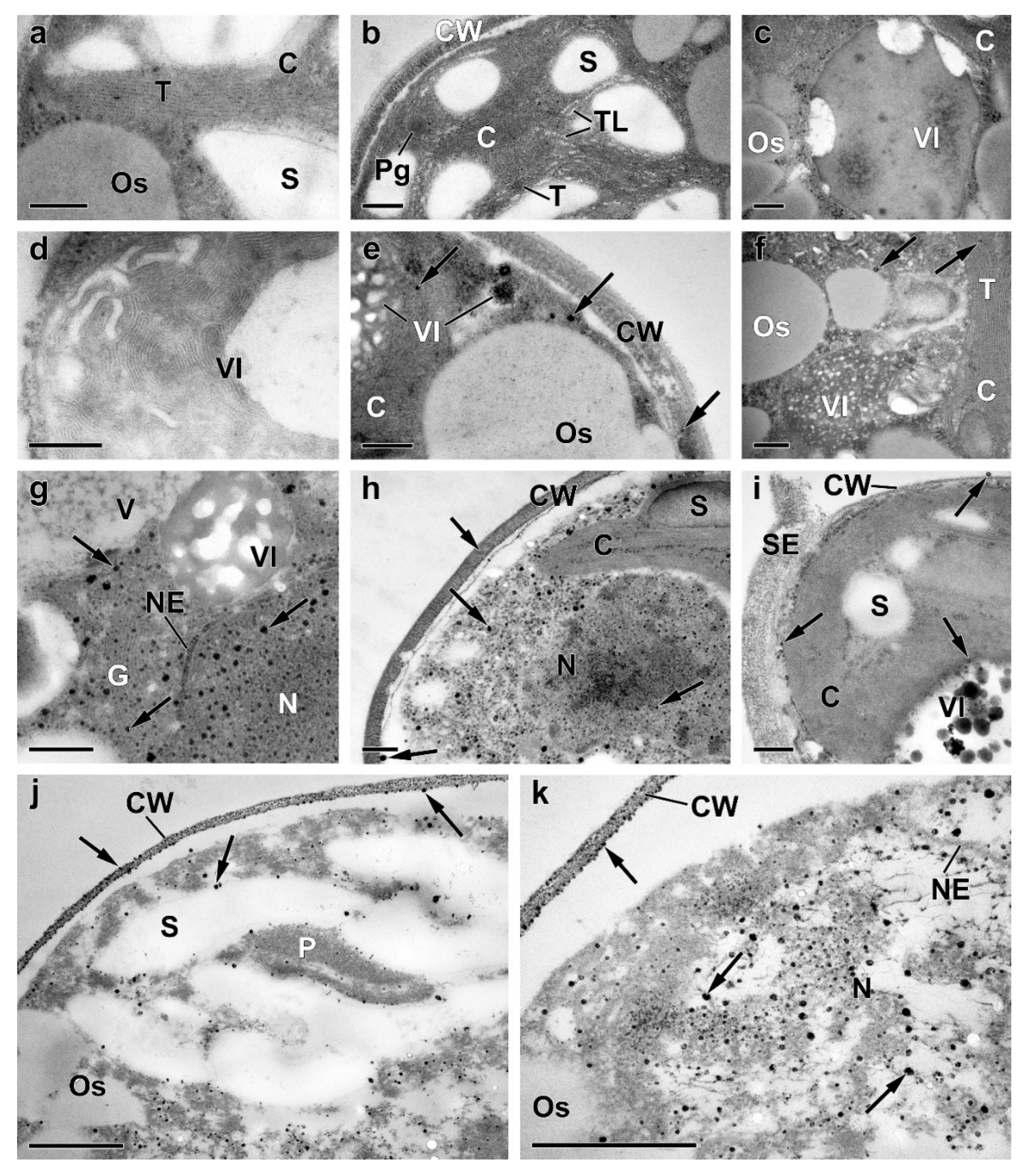
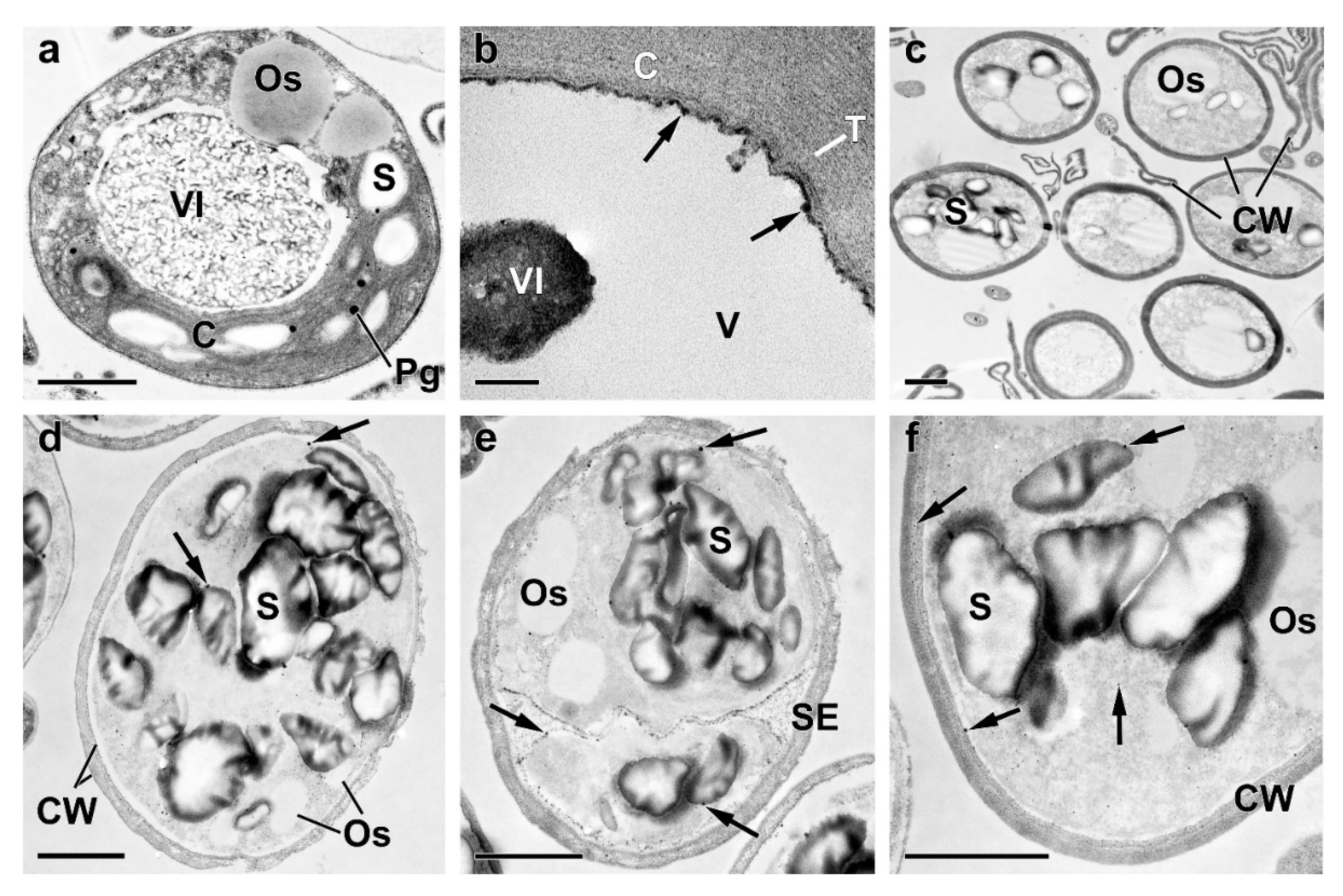
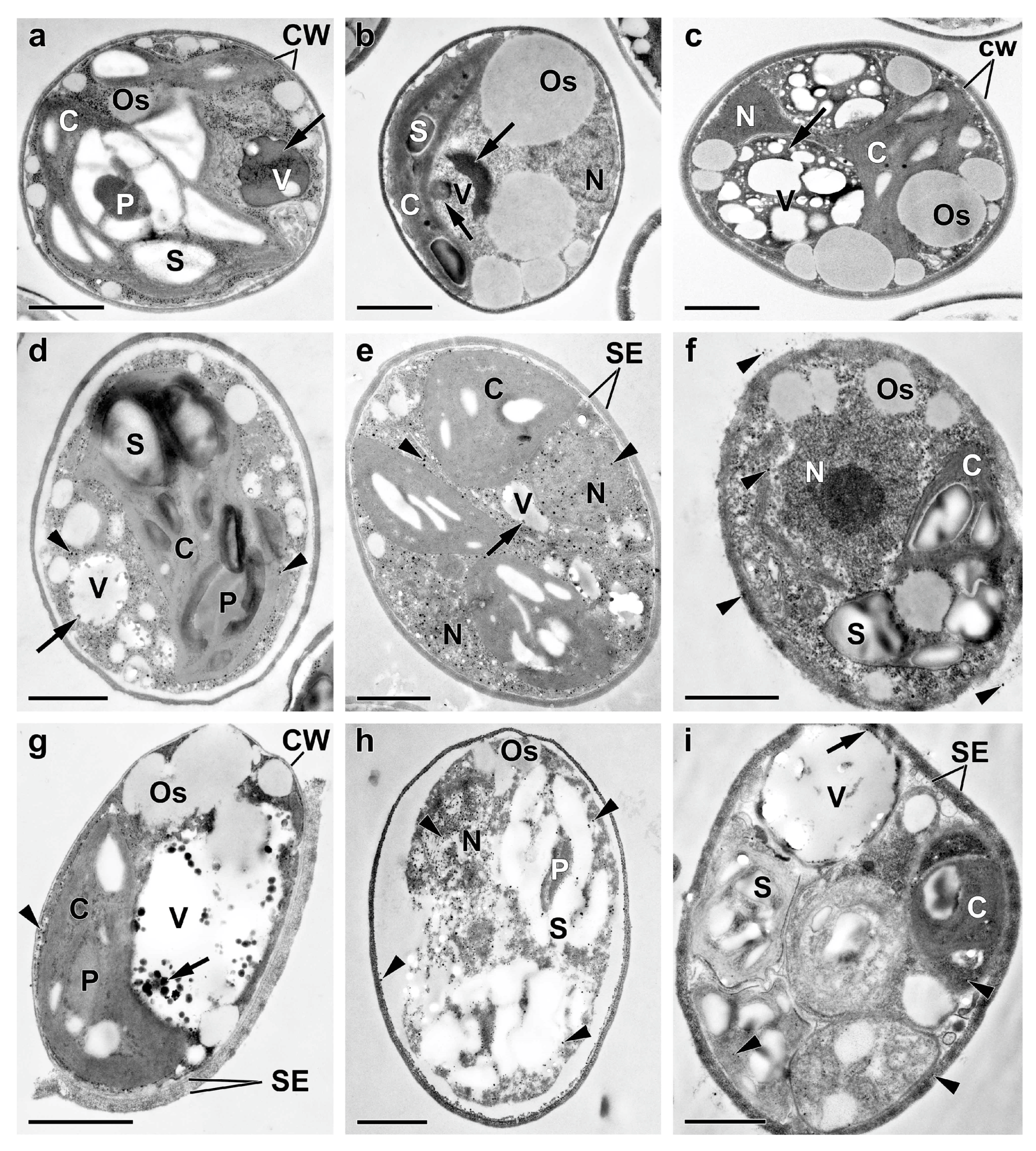
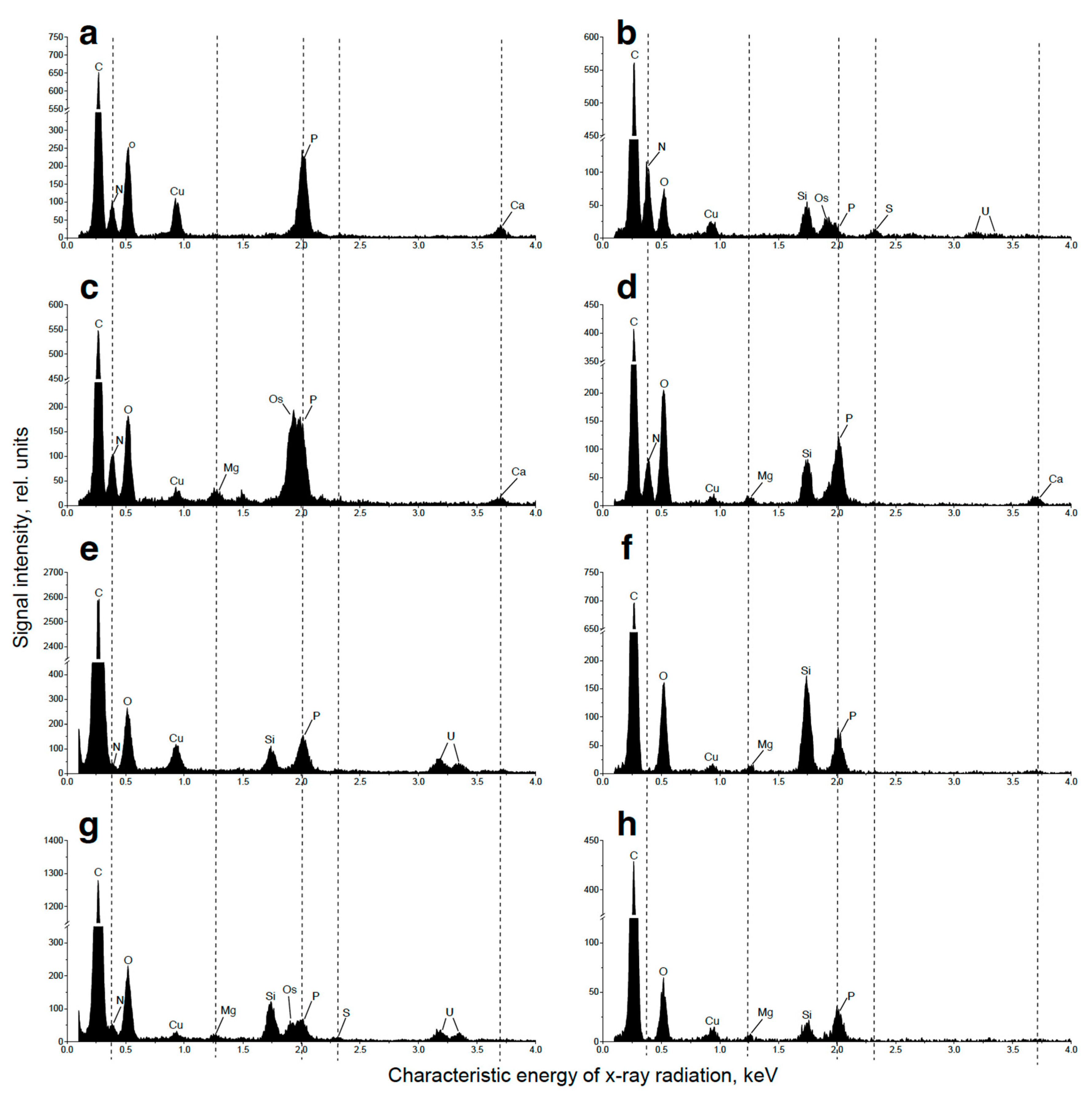
2.3. Ultrastructural View of the Changes in the Cell P-Pools
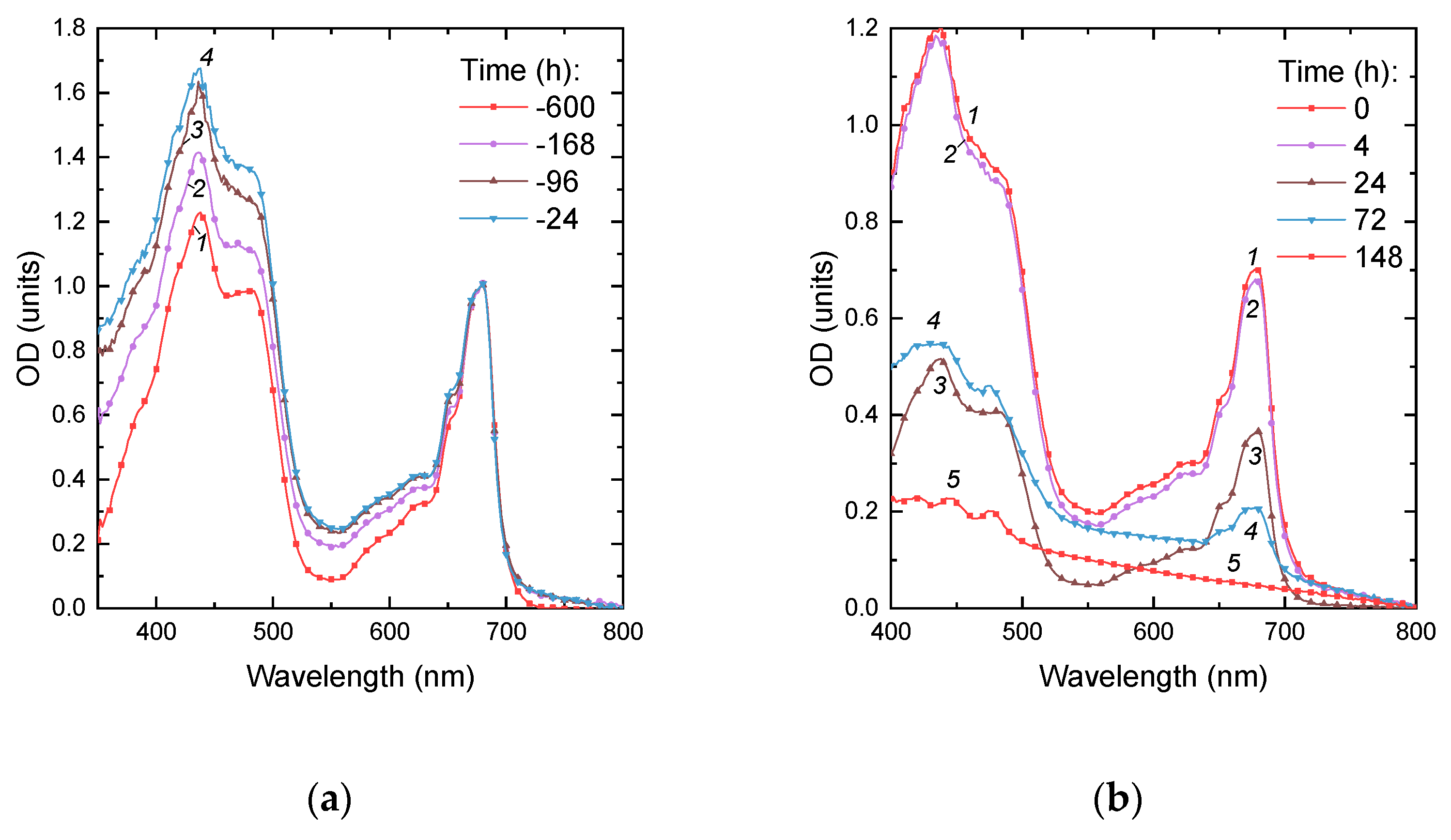
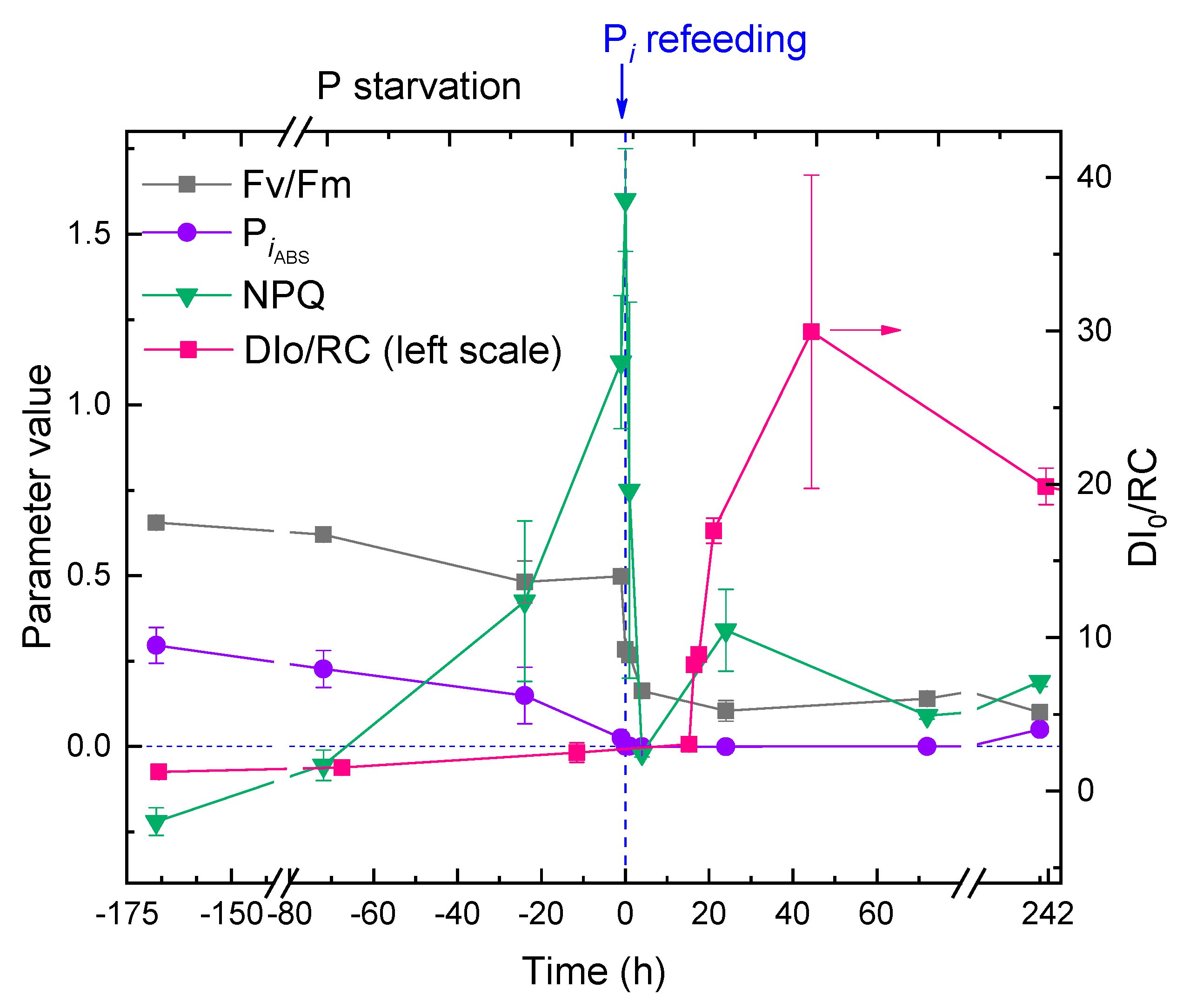
2.4. Responses of the Photosynthetic Apparatus of the Cells to P-Starvation and Re-Feeding
3. Discussion
4. Materials and Methods
4.1. Strain and Cultivation Conditions
4.2. Suspension Absorption Spectra and Pigment Assay
4.3. Biomass Content Determination
4.4. Assay of External and Intracellular Content of Different P Species
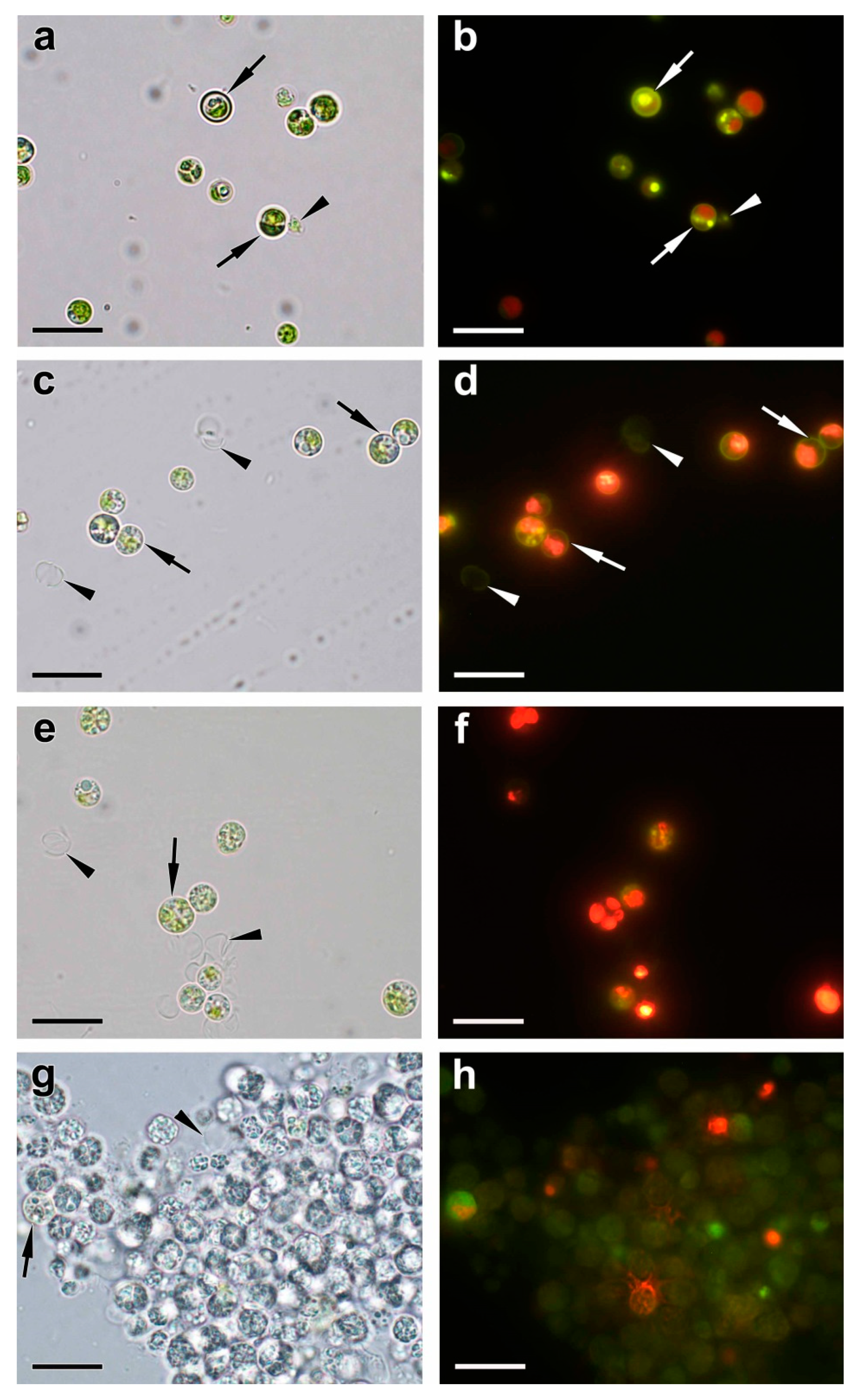
4.5. Light Microscopy
4.6. Transmission Electron Microscopy
4.7. Analytical Electron Microscopy
4.8. Photosynthetic Activity and Photoprotective Mechanism Assessment
4.9. Statistical Treatment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Medium type or time after Pi re-feeding | Cell wall | Cytosol | Vacuole | Chloroplast stroma | Nucleus | |
|---|---|---|---|---|---|---|
| Small spherules | Large globules | |||||
| BG-11K | **P†, Fe |
P-N, P† P-Ca† |
P / P† | P-N-Ca†, P-N-Mg-Ca |
P-N†, P-Mg† |
P-N* |
| P-free medium | P / P†, Fe, P-S, P-Mg-Ca†, N-S† |
P-N-Mg-Ca-Na† | P-N†, P-Mg-Ca†, P-N-Mg-Ca†, P |
N-P-S, N-P†, P-N-Mg-Ca† |
P / P† | P-N*, P-N-Mg* |
| 4 h* | Fe-S, P-N-S, S-N, N-P-S-Mg P-S-Mg-Ca-U |
P-N-Ca-Mg† P-Mg† P-N |
P-Mg-Ca, P-N-Mg-Ca†, P-N-Ca, P-Mg†, P-N-Mg† P / P† |
P-N-Na-Ca, P-N-Mg-Ca, P-N / P-N †, P-N-Ca P-Mg-Ca† |
P-N†, P |
P-N*, P-S*, P-N-S-Mg* |
| 24 h | Fe, P-Fe, P-N-S-Fe, P-Ca / P-Ca †, P-N† P-Mg-Ca†, P-Mg-K-Ca P-S-Ca† P-Mg |
P-Mg, P-Ca P-Mg-Ca, P-N-S-Mg-Ca |
P-Mg P-Mg-Ca† P-N-Ca-Mg† P-N-Ca P-N-Mg |
P-N-Mg | P-N-S†, P† Fe |
P-N* P-Mg-Ca P-K-Mg-Ca |
| 72 h | P-Ca-Fe-Mg Fe |
P | P-N-Mg-Na-Ca† P-N-Mg-Ca† P-N-Mg† P-Mg-Ca† |
P-N-Mg-Ca | P-Mg-K†, P-Ca† P-Na |
P |
| 168 – 240 h | P-Ca | P-N-Mg, P-N, P |
P-N-Mg-Ca, P-N-Mg |
ND | P†, P-S†, P-S-Ca† P-N-Mg P-Ca† |
ND |
Appendix B
References
- Solovchenko, A.E.; Ismagulova, T.T.; Lukyanov, A.A.; Vasilieva, S.G.; Konyukhov, I.V.; Pogosyan, S.I.; Lobakova, E.S.; Gorelova, O.A. Luxury phosphorus uptake in microalgae. J. Appl. Phycol. 2019, 31, 2755–2770. [Google Scholar] [CrossRef]
- Solovchenko, A.; Verschoor, A.M.; Jablonowski, N.D.; Nedbal, L. Phosphorus from wastewater to crops: An alternative path involving microalgae. Biotechnol. Adv. 2016, 34, 550–564. [Google Scholar] [CrossRef]
- Dyhrman, S.T. Nutrients and their acquisition: Phosphorus physiology in microalgae. In The physiology of microalgae; Springer: Dordrecht, The Netherlands, 2016; pp. 155–183. [Google Scholar] [CrossRef]
- de Mazancourt, C.; Schwartz, M.W. Starve a competitor: Evolution of luxury consumption as a competitive strategy. Theor. Ecol. 2012, 5, 37–49. [Google Scholar] [CrossRef]
- Watanabe, M.; Kohata, K.; Kunugi, M. Phosphate accumulation and metabolism by Heterosigma akashiwo (Raphidophyceae) during diel vertical migration in a stratified microcosm. J. Phycol. 1988, 24, 22–28. [Google Scholar] [CrossRef]
- Cembella, A.; Antia, N.; Harrison, P.; Rhee, G.-Y. The Utilization of Inorganic and Organic Phosphorous Compounds as Nutrients by Eukaryotic Microalgae: A Multidisciplinary Perspective: Part 2. Crit. Rev. Microbiol. 1984, 11, 13–81. [Google Scholar] [CrossRef]
- Cembella, A.D.; Antia, N.J.; Harrison, P.J. The utilization of inorganic and organic phosphorous compounds as nutrients by eukaryotic microalgae: A multidisciplinary perspective: Part I. Crit. Rev. Microbiol. 1982, 10, 317–391. [Google Scholar] [CrossRef]
- Haneklaus, S.; Bloem, H.; Schnug, E. Hungry Plants—A Short Treatise on How to Feed Crops under Stress. Agriculture 2018, 8, 43. [Google Scholar] [CrossRef]
- Rhee, G.-Y. A continuous culture study of phosphate uptake, growth rate and polyphosphate in Scenedesmus sp. J. Phycol. 1973, 9, 495–506. [Google Scholar] [CrossRef]
- Lobakova, E.S.; Selyakh, I.O.; Semenova, L.R.; Scherbakov, P.N.; Fedorenko, T.A.; Chekanov, K.A.; Chivkunova, O.B.; Baulina, O.I.; Vasilieva, S.G.; Solovchenko, A.E.; et al. Hints for understanding microalgal phosphate-resilience from Micractinium simplicissimum IPPAS C-2056 (Trebouxiophyceae) isolated from a phosphorus-polluted site. J. Appl. Phycol. 2022, 34, 2409–2422. [Google Scholar] [CrossRef]
- Brown, N.; Shilton, A. Luxury uptake of phosphorus by microalgae in waste stabilisation ponds: Current understanding and future direction. Rev. Environ. Sci. Bio/Technol. 2014, 13, 321–328. [Google Scholar] [CrossRef]
- Smil, V. Phosphorus in the environment: Natural flows and human interferences. Annu. Rev. Energy Environ. 2000, 25, 53–88. [Google Scholar] [CrossRef]
- Ketchum, B.H. The absorption of phosphate and nitrate by illuminated cultures of Nitzschia closterium. Am. J. Bot. 1939, 399–407. [Google Scholar] [CrossRef]
- Eixler, S.; Karsten, U.; Selig, U. Phosphorus storage in Chlorella vulgaris (Trebouxiophyceae, Chlorophyta) cells and its dependence on phosphate supply. Phycologia 2006, 45, 53–60. [Google Scholar] [CrossRef]
- Cordell, D.; White, S. Life’s Bottleneck: Implications of Global Phosphorus Scarcity and Pathways for a Sustainable Food System. Annu. Rev. Environ. Resour. 2014, 39. [Google Scholar]
- Ronga, D.; Biazzi, E.; Parati, K.; Carminati, D.; Carminati, E.; Tava, A. Microalgal Biostimulants and Biofertilisers in Crop Productions. Agronomy 2019, 9, 192. [Google Scholar] [CrossRef]
- de Siqueira Castro, J.; Calijuri, M.L.; Ferreira, J.; Assemany, P.P.; Ribeiro, V.J. Microalgae based biofertilizer: A life cycle approach. Sci. Total Environ. 2020, 724, 138138. [Google Scholar] [CrossRef]
- Mulbry, W.; Westhead, E.K.; Pizarro, C.; Sikora, L. Recycling of manure nutrients: Use of algal biomass from dairy manure treatment as a slow release fertilizer. Bioresour. Technol. 2005, 96, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Slocombe, S.P.; Zúñiga-Burgos, T.; Chu, L.; Wood, N.J.; Camargo-Valero, M.A.; Baker, A. Fixing the Broken Phosphorus Cycle: Wastewater Remediation by Microalgal Polyphosphates. Front. Plant Sci. 2020, 11, 982. [Google Scholar] [CrossRef] [PubMed]
- Pittman, J.K.; Dean, A.P.; Osundeko, O. The potential of sustainable algal biofuel production using wastewater resources. Bioresource Technology 2011, 102, 17–25. [Google Scholar] [CrossRef]
- Kulaev, I.; Vagabov, I.; Kulakovskaya, T. The Biochemistry of Inorganic Polyphosphates, 2nd ed.; John Wiley & Sons, Ltd: Chichester, UK, 2004. [Google Scholar]
- Xie, L.; Jakob, U. Inorganic polyphosphate, a multifunctional polyanionic protein scaffold. J. Biol. Chem. 2019, 294, 2180–2190. [Google Scholar] [CrossRef]
- Li, Q.; Fu, L.; Wang, Y.; Zhou, D.; Rittmann, B.E. Excessive phosphorus caused inhibition and cell damage during heterotrophic growth of Chlorella regularis. Bioresour. Technol. 2018, 268, 266–270. [Google Scholar] [CrossRef]
- Fu, L.; Li, Q.; Yan, G.; Zhou, D.; Crittenden, J.C. Hormesis effects of phosphorus on the viability of Chlorella regularis cells under nitrogen limitation. Biotechnol. Biofuels 2019, 12, 121. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, L.; Abramchuk, I.; Wafy, G.; Denoncourt, A.; Lavallée-Adam, M.; Downey, M. Ddp1 Cooperates with Ppx1 to Counter a Stress Response Initiated by Nonvacuolar Polyphosphate. mBio 2022, 13, e0039022. [Google Scholar] [CrossRef]
- Solovchenko, A.; Khozin-Goldberg, I.; Selyakh, I.; Semenova, L.; Ismagulova, T.; Lukyanov, A.; Mamedov, I.; Vinogradova, E.; Karpova, O.; Konyukhov, I.; et al. Phosphorus starvation and luxury uptake in green microalgae revisited. Algal Res. 2019, 43, 101651. [Google Scholar] [CrossRef]
- Vasilieva, S.; Lobakova, E.; Gorelova, O.; Baulina, O.; Scherbakov, P.; Chivkunova, O.; Semenova, L.; Selyakh, I.; Lukyanov, A.; Solovchenko, A. Photosynthetic and ultrastructural responses of the chlorophyte Lobosphaera to the stress caused by a high exogenic phosphate concentration. Photochem. Photobiol. Sci. 2022, 21, 2035–2051. [Google Scholar] [CrossRef] [PubMed]
- Kokabi, K.; Gorelova, O.; Ismagulova, T.; Itkin, M.; Malitsky, S.; Boussiba, S.; Solovchenko, A.; Khozin-Goldberg, I. Metabolomic foundation for differential responses of lipid metabolism to nitrogen and phosphorus deprivation in an arachidonic acid-producing green microalga. Plant Sci. 2019, 283, 95–115. [Google Scholar] [CrossRef] [PubMed]
- Gorelova, O.; Baulina, O.; Ismagulova, T.; Kokabi, K.; Lobakova, E.; Selyakh, I.; Semenova, L.; Chivkunova, O.; Karpova, O.; Scherbakov, P.; et al. Stress-induced changes in the ultrastructure of the photosynthetic apparatus of green microalgae. Protoplasma 2019, 256, 261–277. [Google Scholar] [CrossRef] [PubMed]
- Shebanova, A.; Ismagulova, T.; Solovchenko, A.; Baulina, O.; Lobakova, E.; Ivanova, A.; Moiseenko, A.; Shaitan, K.; Polshakov, V.; Nedbal, L.; et al. Versatility of the green microalga cell vacuole function as revealed by analytical transmission electron microscopy. Protoplasma 2017, 254, 1323–1340. [Google Scholar] [CrossRef]
- Ismagulova, T.; Shebanova, A.; Gorelova, O.; Baulina, O.; Solovchenko, A. A new simple method for quantification and locating P and N reserves in microalgal cells based on energy-filtered transmission electron microscopy (EFTEM) elemental maps. PLoS ONE 2018, 13, e0208830. [Google Scholar] [CrossRef]
- Miyachi, S.; Kanai, R.; Mihara, S.; Miyachi, S.; Aoki, S. Metabolic roles of inorganic polyphosphates in Chlorella cells. Biochim. Biophys. Acta 1964, 93, 625–634. [Google Scholar] [CrossRef]
- Werner, T.P.; Amrhein, N.; Freimoser, F.M. Specific localization of inorganic polyphosphate (poly P) in fungal cell walls by selective extraction and immunohistochemistry. Fungal Genet. Biol. 2007, 44, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Werner, T.P. , Amrhein, N., Freimoser, F.M. Inorganic polyphosphate occurs in the cell wall of Chlamydomonas reinhardtii and accumulates during cytokinesis. BMC Plant Biol. 2007, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, F.A.; Marchesini, N.; Seufferheld, M.; Govindjee; Docampo, R. The Polyphosphate bodies of Chlamydomonas reinhardtii possess a proton-pumping pyrophosphatase and are similar to acidocalcisomes. J. Biol. Chem. 2001, 276, 46196–46203. [Google Scholar] [CrossRef] [PubMed]
- Docampo, R.; de Souza, W.; Miranda, K.; Rohloff, P.; Moreno, S.N. Acidocalcisomes? Conserved from bacteria to man. Nat. Rev. Microbiol. 2005, 3, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Docampo, R.; Huang, G. Acidocalcisomes of eukaryotes. Curr. Opin. Cell Biol. 2016, 41, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Geyer, G. Ultrahistochemie: Histochemische Arbeitsvorschriften für die Elektronenmikroskopie. Jena, Stuttgart: VEB Gustav Fischer Verlag, 1973.
- Solovchenko, A.E.; Vasilieva, S.G.; Zaitsev, P.; Lukyanov, A.A.; Skripnikova, E.V.; Antal, T.K. Approaches to rapid screening of pharmaceutical xenobiotic effects on microalgae via monitoring of photosynthetic apparatus condition. J. Appl. Phycol. 2022, 34, 353–361. [Google Scholar] [CrossRef]
- Gerasimaite, R.; Sharma, S.; Desfougeres, Y.; Schmidt, A.; Mayer, A. Coupled synthesis and translocation restrains polyphosphate to acidocalcisome-like vacuoles and prevents its toxicity. J. Cell Sci. 2014, 127, 5093–5104. [Google Scholar] [CrossRef]
- Desfougeres, Y.; Gerasimaite, R.U.; Jessen, H.J.; Mayer, A. Vtc5, a Novel Subunit of the Vacuolar Transporter Chaperone Complex, Regulates Polyphosphate Synthesis and Phosphate Homeostasis in Yeast. J. Biol. Chem. 2016, 291, 22262–22275. [Google Scholar] [CrossRef]
- Gerasimaitė, R.; Mayer, A. Enzymes of yeast polyphosphate metabolism: Structure, enzymology and biological roles. Biochem. Soc. Trans. 2016, 44, 234–239. [Google Scholar] [CrossRef]
- Aitchison, P.; Butt, V. The relation between the synthesis of inorganic polyphosphate and phosphate uptake by Chlorella vulgaris. J. Exp. Bot. 1973, 24, 497–510. [Google Scholar] [CrossRef]
- Arriola, M.B.; Velmurugan, N.; Zhang, Y.; Plunkett, M.H.; Hondzo, H.; Barney, B.M. Genome sequences of Chlorella sorokiniana UTEX 1602 and Micractinium conductrix SAG 241.80: Implications to maltose excretion by a green alga. Plant J. 2018, 93, 566–586. [Google Scholar] [CrossRef]
- Achbergerová, L.; Nahálka, J. Polyphosphate-an ancient energy source and active metabolic regulator. Microb. Cell Fact. 2011, 10, 14170–14175. [Google Scholar] [CrossRef]
- Stanier, R.; Kunisawa, R.; Mandel, M.; Cohen-Bazire, G. Purification and properties of unicellular blue-green algae (order Chroococcales). Microbiol. Mol. Biol. Rev. 1971, 35, 171–205. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Naqvi, K.R. On recording the true absorption spectrum and the scattering spectrum of a turbid sample: Application to cell suspensions of the cyanobacterium Anabaena variabilis. J. Photochem. Photobiol. B 2000, 58, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Solovchenko, A.; Merzlyak, M.; Khozin-Goldberg, I.; Cohen, Z.; Boussiba, S. Coordinated carotenoid and lipid syntheses induced in Parietochloris incisa (Chlorophyta, Trebouxiophyceae) mutant deficient in Δ5 desaturase by nitrogen starvation and high light. J. Phycol. 2010, 46, 763–772. [Google Scholar] [CrossRef]
- Solovchenko, A.; Pogosyan, S.; Chivkunova, O.; Selyakh, I.; Semenova, L.; Voronova, E.; Scherbakov, P.; Konyukhov, I.; Chekanov, K.; Kirpichnikov, M.; et al. Phycoremediation of alcohol distillery wastewater with a novel Chlorella sorokiniana strain cultivated in a photobioreactor monitored on-line via chlorophyll fluorescence. Algal Res.-Biomass Biofuels Bioprod. 2014, 6, 234–241. [Google Scholar] [CrossRef]
- Ota, S.; Kawano, S. Extraction and Molybdenum Blue-based Quantification of Total Phosphate and Polyphosphate in Parachlorella. Bio-Protocol. 2017, 7, e2539. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, I.; Wilkinson, J. Chapter IV Chemical Extraction Methods of Microbial Cells. In Methods in microbiology; Elsevier: Amsterdam, The Netherlands, 1971; Volume 5, pp. 345–383. [Google Scholar]
- Gorelova, O.; Baulina, O.; Solovchenko, A.; Selyakh, I.; Chivkunova, O.; Semenova, L.; Scherbakov, P.; Burakova, O.; Lobakova, E. Coordinated rearrangements of assimilatory and storage cell compartments in a nitrogen-starving symbiotic chlorophyte cultivated under high light. Arch. Microbiol. 2015, 197, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, E. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 1963, 17, 208. [Google Scholar] [CrossRef] [PubMed]
- Strasser, R.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescence transient. In Chlorophyll a Fluorescence: A Signature of Photosynthesis; Papageorgiou, G., Govindjee, Eds.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 321–362. [Google Scholar]
- Solovchenko, A.; Lukyanov, A.; Vasilieva, S.; Lobakova, E. Chlorophyll fluorescence as a valuable multitool for microalgal biotechnology. Biophys. Rev. 2022, 14, 973–983. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





