Submitted:
20 April 2023
Posted:
21 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Viral Vectors for Gene Therapy
3. Gene Therapy Applications using Viral Vectors
3.1. Cancer Therapy
3.2. Cardiovascular and Metabolic Diseases
3.3. Hematological Diseases
3.4. Neurological Disorders
3.5. Muscular Diseases
3.6. Immunodeficiency
3.7. Infectious Diseases
3.8. Other Diseases
4. Challenges for Viral Vector-Based Gene Therapy
5. Conclusions and Perspectives
Funding
Conflicts of Interest
References
- Lundstrom, K. New era in gene therapy. In Novel Approaches and Strategies for Biologics, Vaccines and CancerTherapies; Elsevier: San Diego. California, USA, 2015; pp. 15–37. [Google Scholar]
- Ramirez-Montagut, T. Cancer vaccines. In Novel Approaches and Strategies for Biologics, Vaccines and Cancer Therapies; Elsevier: California, USA, 2015; pp. 365–88. [Google Scholar]
- Martinez, T.; Wright, N.; López-Fraga, M.; et al. Silencing human genetic diseases with oligonucleotide-based therapies. Hum. Genet. 2013, 132, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Bobbin, M.L.; Rossi, J.J. RNA interference (RNAi)-based therapeutics: delivering on the promise? Annu. Rev. Pharmacol. Toxicol. 2016, 56, 103–122. [Google Scholar] [CrossRef] [PubMed]
- Anurogo, D.; Yuli Prasetyo Budi, N.; Thi Ngo, M.H.; et al. Cell and Gene Therapy for Anemia: Hematopoietic Stem Cells and Gene Editing. Int.J Mol. Sci. 2021, 22, 6275. [Google Scholar] [CrossRef] [PubMed]
- Sermer, D.; Brentjens, R. CAR-T cell therapy: full speed ahead. Hematol. Oncol. 2019, 37, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Lino, C.A.; Harper, J.C.; Carney, J.P.; et al. Delivering CRISPR: a review of the challenges and approaches. Drug Delivery 2018, 25, 1234–1257. [Google Scholar] [CrossRef] [PubMed]
- Lundstrom, K. Gene Therapy Cargoes Based on Viral Vector Delivery. Curr Gene Ther. 2023, 23, 111–134. [Google Scholar] [CrossRef] [PubMed]
- Lundstrom, K. Viral Vectors in Gene Therapy: Where Do We Stand in 2023? Viruses 2023, 15, 698. [Google Scholar] [CrossRef] [PubMed]
- Samulski, R.; Muzycka, N. AAV-mediated gene therapy for research and therapeutic purposes. Annu. Rev. Virol. 2014, 1, 427–451. [Google Scholar] [CrossRef]
- Epstein, A.L.; Marconi, P.; Argnani, R.; Manservigi, A. HSV-1 derived recombinant and amplicon vectors for gene transfer and gene therapy. Curr. Gene Ther. 2005, 5, 445–458. [Google Scholar] [CrossRef]
- Lesbats, P.; Engelman, A.N.; Cherepanov, P. Retroviral DNA Integration. Chem. Rev. 2016, 116, 12730–12757. [Google Scholar] [CrossRef]
- Kay, M.A.; Glorioso, J.C.; Naldini, L. Viral vectors for gene therapy: The art of turning infectious agents into vehicles of therapeutics. Nat. Med. 2001, 7, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Brunetti-Pierri, N.; Ng, T.; Iannitti, D.; Cioffi, W.; Stapleton, G.; Law, M.; Breinholt, J.; Palmer, D.; Grove, N.; Rice, K.; et al. Transgene expression up to 7 years in nonhuman primates following hepatic transduction with helper-dependent adenoviral vectors. Hum. Gene Ther. 2013, 24, 761–765. [Google Scholar] [CrossRef] [PubMed]
- Deyle, D.R.; Russell, D.W. Adeno-associated virus integration. Curr. Opin. Mol. Ther. 2009, 11, 442–447. [Google Scholar] [PubMed]
- Wang, Z.; Lisowski, L.; Finegold, M.J.; Nakai, H.; Kay, M.A.; Grompe, M. AAV Vectors Containing rDNA Homology Increased Chromosomal Integration and Transgene Persistence. Mol. Ther. 2012, 20, 1902–1911. [Google Scholar] [CrossRef] [PubMed]
- Cartier, N.; Hacein-Bey-Abina, S.; Bartholomae, C.C.; Veres, G.; Schmidt, M.; Kutschera, I.; Vidaud, M.; Abel, U.; Dal-Cortivo, L.; Caccavelli, L.; et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science 2009, 326, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Strauss, J.H.; Strauss, E.G. The alphaviruses; gene expression, replication and evolution. Microbiol. Rev. 1994, 58, 491–562. [Google Scholar] [CrossRef]
- Pijlman, G.P.; Suhrbier, A.; Khromykh, A.A. Kunjin virus replicons: An RNA-based, non-cytopathic viral vector system for protein production, vaccine and gene therapy applications. Exp. Opin. Biol. Ther. 2006, 6, 134–145. [Google Scholar] [CrossRef]
- Zhao, D.; Chen, P.; Yang, H.; Wu, Y.; Zeng, X.; Zhao, Y.; Wen, Y.; Zhao, X.; Liu, X.; Wei, Y.; et al. Live attenuated measles virus vaccine induces apoptosis and promotes tumor regression in lung cancer. Oncol. Rep. 2013, 29, 199–204. [Google Scholar] [CrossRef]
- Finke, S.; Conzelmann, K.K. Recombinant rhabdoviruses: Vectors for vaccine development and gene therapy. Curr. Top. Microbiol. Immunol. 2005, 292, 165–200. [Google Scholar]
- Ganar, K.; Das, M.; Sinha, S.; Kumar, S. Newcastle disease virus: Current status and our understanding. Virus Res. 2014, 184, 71–81. [Google Scholar] [CrossRef]
- Kwak, H.; Honig, H.; Kaufman, H.L. Poxviruses as vectors for cancer immunotherapy. Curr. Opin. Drug Discov. Devel. 2003, 6, 161–168. [Google Scholar] [PubMed]
- Shafren, D.R.; Au, G.G.; Nguyen, T.; Newcombe, N.G.; Haley, E.S.; Beagley, L.; Johansson, E.S.; Hersey, P.; Barry, R.D. Systemic therapy of malignant human melanoma tumors by a common cold-producing enterovirus, coxsackievirus a21. Clin. Cancer Res. 2014, 10, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Hadac, E.M.; Kelly, E.J.; Russell, S.J. Myeloma xenograft destruction by a nonviral vector delivering oncolytic infectious nucleic acid. Mol. Ther. 2011, 19, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Clements, D.; Helson, E.; Gujar, S.A.; Lee, P.W. Reovirus in cancer therapy: An evidence-based review. Oncol. Virother. 2014, 3, 69–82. [Google Scholar]
- Cordelier, P.; Bienvenu, C.; Lulka, H.; Marrache, F.; Bouisson, M.; Openheim, A.; Strayer, D.S.; Vaysse, N.; Pradayrol, L.; Buscail, L. Replication-deficient rSV40 mediate pancreatic gene transfer and long-term inhibition of tumor growth. Cancer Gene Ther. 2007, 14, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Fan, J.; Liao, J.; Zou, Y.; Song, D.; Liu, J.; Cui, J.; Liu, F.; Ma, C.; Hu, X.; et al. Engineering the rapid adenovirus production and amplification (RAPA) cell line to expedite the generation of recombinant adenoviruses. Cell Physiol. Biochem. 2017, 41, 2383–2398. [Google Scholar] [CrossRef] [PubMed]
- Robert, M.A.; Chahal, P.S.; Audy, A.; Kamen, A.; Gilbert, R.; Gaillet, B. Manufacturing of recombinant adeno-associated viruses using mammalian expression platforms. Biotechnol. J. 2017, 12. [Google Scholar] [CrossRef]
- Berg, K.; Schäfer, V.N.; Barthnicki, N.; Eggenschwiler, R.; Cantz, T.; Stitz, J. Rapid establishment of stable retroviral packaging cells and recombinant susceptible target cell lines employing novel transposon vectors derived from Sleeping Beauty. Virology 2019, 531, 192–202. [Google Scholar] [CrossRef]
- Ferreira, M.V.; Cabral, E.T.; Coroadinha, A.S. Progress and Perspectives in the Development of Lentiviral Vector Producer. Cells Biotechnol. J. 2021, 16, e2000017. [Google Scholar] [CrossRef]
- Gherke, R.; Ecker, M.; Aberle, S.W.; Allison, S.L.; Heinz, F.X.; Mandl, C.W. Incorporation of tick-borne encephalitis virus replicons into virus-like particles by a packaging cell line. J. Virol. 2003, 77, 8924–8933. [Google Scholar] [CrossRef]
- Khromykh, A.A.; Varnavski, A.N.; Westaway, E.G. Encapsidation of the flavivirus Kunjin replicon RNA by using a complementation system providing Kunjin virus structural proteins in trans. J. Virol. 1998, 72, 5967–5977. [Google Scholar] [CrossRef] [PubMed]
- Lal, G.; Rajala, M. Engineering of measles virus to target cancer cells, an attempt. Intl. J. Infect. Dis. 2016, 45, 333–334. [Google Scholar] [CrossRef]
- Ito, N.; Takayama-Ito, M.; Yamada, K.; Hosokawa, J.; Sugiyama, M.; Minamoto, N. Improved recovery of rabies virus from cloned cDNA using a vaccinia virus-free reverse genetics system. Microbiol. Immunol. 2003, 47, 613–677. [Google Scholar] [CrossRef] [PubMed]
- Toscano, M.G.; van der Velden, J.; van der Werf, S.; Odijk, M.; Roque, A.; Camacho-Garcia, R.J.; Herrera-Gomez, I.G.; Mancini, I.; de Haan, P. Generation of a Vero-based packaging cell line to produce SV40 gene delivery vectors for use in clinical gene therapy studies. Mol. Ther. Methods Clin. Dev. 2017, 6, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Liljeström, P.; Garoff, H. A new generation of animal cell expression vectors based on the Semliki Forest virus replicon. Biotechnology 1991, 9, 1356–1361. [Google Scholar] [CrossRef] [PubMed]
- DiCiommo, P.D.; Bremner, R. Rapid, high level protein production using DNA-based Semliki Forest virus vectors. J. Biol. Chem. 1998, 273, 18060–18066. [Google Scholar] [CrossRef]
- Wadwha, A.; Aljabbari, A.; Lokras, A.; Foged, C.; Takur, A. Opportunities and Challenges in the Delivery of mRNA-Based Vaccines. Pharmaceutics 2020, 12, 102. [Google Scholar] [CrossRef]
- Raouane, M.; Desmaele, D.; Urbinati, G.; Massaad-Massade, L.; Couvreur, P. Lipid conjugated oligonucleotides: a useful strategy for delivery. Bioconjug. Chem. 2012, 23, 1091–1104. [Google Scholar] [CrossRef]
- Bishop, C.J.; Majewski, R.L.; Guiriba, T.-R.M.; Wilson, D.R.; Bhise, N.S.; Quinones-Hinojosa, A.; Green, J.J. Quantification of Cellular and Nuclear Uptake Rates of Polymeric Gene Delivery Nnaoparticles and DNA Plasmids via Flow Cytometry. Acta Biomater. 2016, 37, 120–130. [Google Scholar] [CrossRef]
- Kaufman, H.L.; Kim, D.W.; DeRaffele, G.; Mitcham, J.; Coffin, R.S.; Kim-Schulze, S. Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage IIIc and IV melanoma. Ann. Surg. Oncol. 2010, 17, 718–730. [Google Scholar] [CrossRef]
- Li, J.M.; Kao, K.C.; Li, L.F. Micro-RNA-145 regulates oncolytic herpes simplex virus-1 for selective killing of human non-small lung cancer cells. Virol. J. 2013, 10, 241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Y.; Tan, J.; Zhang, Y.; Wong, C.-W.; Lin, Z.; Liu, X.; Sander, M.; Yang, X.; Liang, L.; et al. Necroptotic virotherapy of oncolytic alphavirus M1 cooperated with Doxorubicin displays promising therapeutic efficacy in TNBC. Oncogene 2021, 40, 4783–4795. [Google Scholar] [CrossRef] [PubMed]
- Boisgerault, N.; Guillerme, J.-B.; Pouliquen, D.; Mesel-Lemoine, M.; Achard, C.; Combredet, C.; Fontenau, J.F.; Tangy, F.; Gré- goire, M. Natural oncolytic activity of live-attenuated measles virus against human lung and colorectal adenocarcinomas. Biomed. Res. Int. 2013, 2013, 387362. [Google Scholar] [CrossRef] [PubMed]
- Urbiola, C.; Santer, F.R.; Petersson, M.; van der Pluijm, G.; Horninger, W.; Erlmann, P.; Wollmann, G.; Kimpel, J.; Culig, Z.; von Laer, D. Oncolytic activity of the rhabdovirus VSV-GP against prostate cancer. Int. J. Cancer 2018, 143, 1786–1796. [Google Scholar] [CrossRef]
- Le Boeuf, F.; Selman, M.; Son, H.H.; Bergeron, A.; Chen, A.; Tsang, J.; Butterwick, D.; Arulanandam, R.; Forbes, N.E.; Tzelepis, F.; et al. Oncolytic Maraba Virus MG1 as a Treatment for Sarcoma. Int. J. Cancer 2017, 141, 1257–1264. [Google Scholar] [CrossRef]
- Liu, Y.; Deisseroth, A. Tumor vascular targeting therapy with viral vectors. Blood 2006, 107, 3027–3033. [Google Scholar] [CrossRef]
- Montaño-Samaniego, M.; Bravo-Estupiñan, D.M.; Méndez-Guerrero, O.; Alarcon-Hernandez, E.; Ibanez-Hernandez, M. Strategies for Targeting Gene Therapy in Cancer Cells with Tumor-Specific Promoters. Front. Oncol. 2020, 10, 605380. [Google Scholar] [CrossRef]
- Shimada, H.; Shimizu, T.; Ochiai, T.; Liu, T.L.; Sashiyama, H.; Nakamura, A.; Matusbara, H.; Gunji, Y.; Kobayashi, S.; Tagawa, M.; et al. Preclinical study of adenoviral p53 gene therapy for esophageal cancer. Surg. Today 2001, 3, 597–604. [Google Scholar] [CrossRef]
- von Grueningen, V.E.; Santoso, J.T.; Coleman, R.L.; Muller, C.Y.; Miller, D.S.; Mathis, J.M. In vivo studies of adenovirus-based p53 gene therapy for ovarian cancer. Gynecol. Oncol. 1998, 69, 197–204. [Google Scholar] [CrossRef]
- Tang, R.; Xu, Z. Gene therapy: a double-edged sword with great powers. Mol. Cell Biochem. 2020, 474, 73–81. [Google Scholar] [CrossRef]
- Räty, J.K.; Pikkarainen, J.T.; Wirth, T.; Ylä-Herttuala, S. Gene therapy: The first approved gene-based medicines, molecular mechanisms and clinical indications. Curr. Mol. Pharmacol. 2008, 1, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.W.; Li, L.; Li, D.; Liu, J.; Li, X.; Li, W.; Xu, X.; Zhang, M.J.; Chandler, L.A.; Lin, H.; et al. The first approved gene therapy product for cancer ad-p53 (Gendicine): 12 years in the clinic. Hum. Gene Ther. 2018, 29, 160–179. [Google Scholar] [CrossRef] [PubMed]
- Toda, M.; Martuza, R.L.; Rabkin, S.D. Tumor growth inhibition by intratumoral inoculation of defective herpes simplex virus vectors expressing granulocyte-macrophage colony-stimulating factor. Mol Ther. 2000, 2, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B.; Puzanov, I.; Kelley, M.C. Talimogene laherparevec (T-VEC) for the treatment of advanced melanoma. Immunotherapy 2015, 7, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Rehman, H.; Silk, A.W.; Kane, M.P.; Kaufman, H.L. Into the clinic: Talimigene laherparevec (T-VEC), a first-in-class intratumoral oncolytic viral therapy. J. Immunother. Ther. Cancer 2016, 4, 53. [Google Scholar] [CrossRef]
- Huang, T.T.; Parab, S.; Burnett, R.; Diago, O.; Ostertag, D.; Hofman, F.M.; Lopez Espinoza, F.; Martin, B.; Ibanez, C.E.; Kasahara, N.; et al. Intravenous administration of retroviral replicating vector, Toca 511, demonstrates efficacy in orthotopic immune-competent mouse glioma model. Hum. Gene Ther. 2015, 26, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Cloughesy, T.F.; Landolfi, J.; Hogan, D.J.; Bloomfield, S.; Carter, B.; Chen, C.C.; Elder, J.B.; Kalkanis, S.N.; Kesari, S.; Lai, A.; et al. Phase I trial of vocimagine amiroretrorepvec and 5-fluorocytosine for recurrent high-grade glioma. Sci. Transl. Med. 2016, 8, 341ra75. [Google Scholar] [CrossRef]
- Tocagen Reports Results of Toca 5 Phase 3 Trial in Recurrent Brain Cancer. Tocagen. Published 12 September 2019. https://bit.ly/2lPm19v (accessed on 21 March 2023).
- Hingorani, P.; Zhang, W.; Lin, J.; Liu, L.; Guha, C.; Kolb, E.A. Systemic administration of reovirus (Reolysin) inhibits growth of human sarcoma xenografts. Cancer 2011, 117, 1764–1774. [Google Scholar] [CrossRef]
- Gollamudi, R.; Ghalib, M.H.; Desai, K.K.; Chaudhary, I.; Wong, B.; Einstein, M.; Coffey, M.; Gill, G.M.; Mettinger, K.; Mariadason, J.M.; et al. Intravenous administration of Reolysin, a live replication competent RNA virus is safe in patients with solid tumors. Invest. New Drugs 2010, 28, 641–649. [Google Scholar] [CrossRef]
- Mahalingam, D.; Fountzilas, C.; Moseley, J.; Noronha, N.; Tran, H.; Chakrabarty, R.; Selvaggi, G.; Coffey, M.; Thompson, B.; Sarantopoulos, J. A phase II study of REOLYSIN® (pelareorep) in combination with carboplatin and paclitaxel for patients with advanced malignant melanoma. Cancer Chemother Pharmacol. 2017, 79, 697–703. [Google Scholar] [CrossRef]
- Oncolytics Biotech (ONCY) Announces Receipt of FDA Orphan Drug Designation for REOLYSIN. April 2015. www.streetinsider.com (accessed on 21 March 2023).
- Oncolytics Biotech Inc. Announces FDA Fast Track Designation for REOLYSIN in Metastatic Breast Cancer”. www.newswire.ca (accessed on 21 March 2023).
- Msaouel, P.; Iankov, I.D.; Allen, C.; Morris, J.C.; von Messling, V.; Cattaneo, R.; Koutsilieris, M.; Russell, S.J.; Galanis, E. Engi- neered measles virus as a novel oncolytic therapy against prostate cancer. Prostate 2009, 69, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Galanis, E.; Hartmann, L.C.; Cliby, W.A.; Long, H.J.; Peethambaram, P.P.; Barrette, B.A.; Kaur, J.S.; Haluska, P.J. Jr.; Aderca, I.; Zollman, P.J.; et al. Phase I trial of intraperitoneal administration of an oncolytic measles virus strain engineered to express carcinoembryonic antigen for recurrent ovarian cancer. Cancer Res. 2010, 70, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Durso, R.J.; Andjelic, S.; Gardner, J.P.; Margitich, D.J.; Donovan, G.P.; Arrigale, R.R.; Wang, X.; Maughan, M.F.; Talarico, T.L.; Olmsted, R.A.; et al. A novel alphavirus vaccine encoding prostate-specific membrane antigen elicits potent cellular and humoral immune responses. Clin. Cancer Res. 2017, 13, 3999–4008. [Google Scholar] [CrossRef] [PubMed]
- Slovin, S.F.; Kehoe, M.; Durso, R.; Fernandez, C.; Olson, W.; Gao, J.P.; Israel, R.; Scher, H.I.; Morris, S. A phase I dose escalation trial of vaccine replicon particles (VRP) expressing prostate-specific membrane antigen (PSMA) in subjects with prostate cancer. Vaccine 2013, 31, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Gianni, D.; Chan, J.; Gwathmey, J.K.; del Monte, F.; Hajjar, R.J. SERCA2a in heart failure: role and therapeutic prospects. J. Bioenerg. Biomembr. 2005, 37, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, M.I.; del Monte, F.; Schmidt, U.; DiSalvo, T.S.; Kang, Z.B.; Matsui, T.; Guerrero, J.L.; Gwathmey, J.K.; Rosenzweig, A.; Hajjar, R.J. Adenoviral gene transfer of SERCa2a improves left-ventricular function in aortic-banded rats in transition to heart failure. Proc. Natl. Acad. Sci. USA 2000, 97, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Hadri, L.; Bobe, R.; Kawase, Y.; Ladage, D.; Ishikawa, K.; Atassi, F.; Lebeche, D.; Kranisa, E.G.; Leopold, J.A.; Lompre, A.-M-; et al. SERCA2a gene transfer enhances eNOS expression and activity in endothelial cells. Mol. Ther. 2010, 18, 1284–1292. [Google Scholar] [CrossRef]
- Niwano, K.; Arai, M.; Koitabashi, N.; Watanabe, A.; Ikeda, Y.; Miyoshi, H.; Kurabayashi, M. Lentiviral vector–mediated SERCA2 gene transfer protects against heart failure and left ventricular remodeling after myocardial infarction in rats. Mol. Ther. 2008, 16, 1026–1032. [Google Scholar] [CrossRef]
- Jaski, B.E.; Jessup, M.L.; Mancini, D.M.; Cappola, T.P.; Pauly, D.F.; Greenberg, B.; Borrow, K.; Dittrich, H.; Zsebo, K.M.; Hajjar, R.J. Calcium Up-Regulation by Percutaneous Administration of Gene Therapy In Cardiac Disease (CUPID) Trial Investigators. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID Trial), a first-in-human phase 1/2 clinical trial. J. Card. Fail. 2009, 15, 171–181. [Google Scholar]
- Jessup, M.; Greenberg, B.; Mancini, D.; Cappola, T.; Pauly, D.F.; Jaski, B.; Yaroshinsky, A.; Zsebo, K.M.; Dittrich, H.; Haijjar, H. Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID) Investigators. Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID): A phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum Ca2+-ATPase in patients with advanced heart failure. Circulation 2011, 124, 304–313. [Google Scholar]
- Zsebo, K.; Yaroshinsky, A.; Rudy, J.J.; Wagner, K.; Greenberg, B.; Jessup, M.; Hajjar, R.J. Long-term effects of AAV1/SERCA2a gene transfer in patients with severe heart failure: Analysis of recurrent cardiovascular events and mortality. Circ. Res. 2014, 114, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Goudy, K.; Campbell-Thompson, M.; Wasserfall, C.; Scott-Jorgensen, M.; Wang, J.; Tang, Q.; Crawford, J.M.; Ellis, T.M.; Atkinson, M.A.; et al. Recombinant adeno-associated virus-mediated alpha-1 antitrypsin gene therapy prevents type I diabetes in NOD mice. Gene Ther. 2004, 11, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Brantly, M.L.; Chulay, J.D.; Wang, L.; Muller, C.; Humphries, M.; Spencer, L.T.; Rouhani, F.; Conlon, T.J.; Calcedo, R.; Betts, M.R.; et al. Sustained transgene expression despite Tlymphocyte responses in a clinical trial of rAAV1-AAT gene therapy. Proc. Natl. Acad. Sci. USA 2009, 106, 16363–16368. [Google Scholar] [CrossRef]
- Flotte, T.R.; Trapnell, B.C.; Humphries, M.; Carey, B.; Calcedo, R.; Rouhani, F.; Campbell-Thompson, M.; Yachnis, A.T.; Sandhaus, R.A.; McElvaney, N.G.; et al. Phase 2 clinical trial of a recombinant adeno-associated viral vector expressing alphal- antitrypsin: Interim results. Hum. Gene Ther. 2011, 22, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Lundstrom, K. Gene Therapy in Hematology. In Comprehensive Hematology and Stem Cell Research; Elsevier: Chennai, India, 2023; in press. [Google Scholar]
- Gale, A.J.; Pellequer, J.L.; Getzoff, E.D.; Griffin, J.H. Structural basis for hemophilia A caused by mutations in the C domains of blood coagulation factor VIII. Thromb. Haemost. 2000, 83, 78–85. [Google Scholar] [CrossRef]
- Ludwig, M.; Sabharwal, A.K.; Brackmann, H.H.; Olek, K.; Smith, K.J.; Birktoft, J.J.; Bajaj, S.P. Hemophilia B caused by five different nondeletion mutations in the protease domain of factor IX. Blood 1992, 79, 1225–1232. [Google Scholar] [CrossRef]
- Balagué, C.; Zhou, J.; Dai, Y.; Alemany, R.; Josephs, S.F.; Andreason, G.; Hariharan, M.; Sethi, E.; Prokopenko, E.; Jan, H.Y.; et al. Sustained high-level expression of full-length human factor VIII and a restoration of clotting activity in hemophilic mice using a minimal adenovirus vector. Blood 2000, 95, 820–828. [Google Scholar] [CrossRef]
- Wang, L.; Herzog, R.W. AAV-mediated gene transfer for treatment of hemophilia. Curr. Gene Ther. 2005, 5, 349–360. [Google Scholar] [CrossRef]
- Sarkar, R.; Xiao, W.; Kazazian, H.H., Jr. A single adenoassociated virus (AAV)-murine factor FVIII. J. Thromb. Haemost. 2003, 1, 220–226. [Google Scholar] [CrossRef]
- Sarkar, R.; Tetreault, R.; Gao, G.; Wang, L.; Bell, P.; Chandler, R.; Wilson, J.M.; Kazazian, H.H., Jr. Total correction of hemophilia A mice with canine FVIII using an AAV 8 serotype. Blood 2004, 103, 1253–1260. [Google Scholar] [CrossRef]
- Jiang, H.; Lillicrap, D.; Patarroyo-White, S.; Liu, T.; Qian, X.; Scallan, C.D.; Powell, S.; Keller, T.; McMurray, M.; Labelle, A.; et al. Multiyear therapeutic benefit of AAV serotypes 2, 6, and 8 delivering factor VIII to hemophilia A mice and dogs. Blood 2006, 108, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Callan, M.B.; Haskins, M.E.; Wang, P.; Zhou, S.; High, K.A.; Arruda, V.R. Successful Phenotype Improvement following Gene Therapy for Severe Hemophilia A in Privately Owned Dogs. PLoS ONE 2016, 11, e0151800. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, G.N.; Everett, J.K.; Kafle, S.; Roche, A.M.; Raymond, H.E.; Leiby, J.; Wood, C.; Assenmacher, C.-A.; Merricks, E.P.; Long, C.T.; et al. A long-term study of AAV gene therapy in dogs with hemophilia A identifies clonal expansions of transduced liver cells. Nat. Biotechnol. 2021, 39, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Wilcox, D.A.; Fahs, S.A.; Fang, J.; Johnson, B.D.; Du, L.M.; Desai, D.; Montgomery, R.R. Lentivirus-mediated platelet-derived factor VIII gene therapy in murine haemophilia A. J. Tromb. Haemost. 2007, 5, 352–361. [Google Scholar] [CrossRef]
- Nathwani, A.C. Gene therapy for hemophilia. Hematol. Am. Soc. Hematol. Educ. Program 2019, 2019, 1–8. [Google Scholar] [CrossRef]
- Pasi, K.J.; Rangarajan, S.; Mitchell, N.; Lester, W.; Symington, E.; Madan, B.; Laffan, M.; Russell, C.B.; Li, M.; Pierce, G.F.; Wong, W.Y. Multiyear Follow-up of AAV5-hFVIII-SQ Gene Therapy for Hemophilia A. N. Engl. J. Med. 2020, 382, 29–40. [Google Scholar] [CrossRef]
- VandenDriessche, T.; Pipe, S.W.; Pierce, G.F.; Kaczmarek, R. First conditional marketing authorization approval in the European Union for hemophilia “A” gene therapy. Mol. Ther. 2022, 30, 3335–3336. [Google Scholar] [CrossRef]
- Dai, Y.; Schwarz, E.M.; Gu, D.; Zhang, W.W.; Sarvetnick, N.; Verma, I.M. Cellular and humoral immune responses to adenoviral vectors containing factor IX gene: Tolerization of factor IX and vector antigens allows long-term expression. Proc. Natl. Acad. Sci. USA 1995, 92, 1401–1405. [Google Scholar] [CrossRef]
- Kay, M.A.; Landen, C.N.; Rothenberg, S.R.; Taylor, L.A.; Leland, F.; Wiehle, S.; Fang, B.; Bellinger, D.; Finegold, M.; Thompson, A.R.; et al. In vivo hepatic gene therapy: Complete albeit transient correction of factor IX deficiency in hemophilia B dogs. Proc. Natl. Acad. Sci. USA 1994, 91, 2353–2357. [Google Scholar] [CrossRef]
- Fang, B.; Eisensmith, R.C.; Wang, H.; Kay, M.A.; Cross, R.E.; Landen, C.N.; Gordon, G.; Bellinger, D.A.; Read, M.S.; Hu, P.C.; et al. Gene therapy for hemophilia B: Host immunosuppression prolongs the therapeutic effect of adenovirus-mediated factor IX expression. Hum. Gene Ther. 1995, 6, 1039–1044. [Google Scholar] [CrossRef]
- Crudele, J.M.; Finn, J.D.; Siner, J.I.; Martin, N.B.; Niemeyer, G.P.; Zhou, S.; Mingozzi, F.; Lothrop, C.D. Jr.; Arruda, V.R. AAV liver expression of FIXPadua prevents and eradicates FIX inhibitor without increasing thrombogenicity in hemophilia B dogs and mice. Blood 2015, 125, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Nathwani, A.C.; Reiss, U.M.; Tuddenham, E.G.; Rosales, C.; Chowdary, P.; McIntosh, J.; Della Peruta, M.; Lheriteau, E.; Patel, N.; Raj, D.; Riddell, A.; et al. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N. Engl. J. Med. 2014, 371, 1994–2004. [Google Scholar] [CrossRef]
- McCarty, D.M.; Monahan, P.E.; Samulski, R.J. Self-complimentary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther. 2001, 8, 1248–1254. [Google Scholar] [CrossRef]
- Wu, Z.; Sun, J.; Zhang, T.; Yin, C.; Yin, F.; Van Dyke, T.; Samulski, R.J.; Monahan, R.E. Optimization of self-complementary AAV vectors for liver-directed expression results in sustained correction of hemophilia B at low vector dose. Mol. Ther. 2008, 16, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhu, L.; Zhang, J.; Guo, L.; Sun, X.; Huang, C.; Xu, K.; Zhang, Y.; Li, W.; Zhou, X. Rational engineering of adeno-associated virus capsid enhances human hepatocyte tropismm and reduced immunogenicity. Cell Prolif. 2022, 55, e13339. [Google Scholar] [CrossRef] [PubMed]
- Chowdary, P.; Shapiro, S.; Makris, M.; Evans, G.; Boyce, S.; Talks, K.; Dolan, G.; Reiss, U.; Phillips, M.; Riddell, A.; et al. Phase 1-2 Trial of AAVS3 Gene Therapy in Patients with Hemophilia B. N. Engl. J. Med. 2022, 387, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Cantore, A.; Ranzani, M.; Bartholomae, C.C.; Volpin, M.; Della Valle, P.; Sanvito, F.; Sergi Sergi, L.; Gallina, P.; Benedicenti, F.; Bellinger, D.; et al. Liver-directed lentiviral gene therapy in a dog model of hemophilia B. Sci. Transl. Med. 2015, 7, 277ra28. [Google Scholar] [CrossRef]
- Cavazzana, M.; Mavilio, F. Gene Therapy for Hemoglobinopathies. Hum. Gene Ther. 2018, 29, 1106–1113. [Google Scholar] [CrossRef]
- Lal, A.; Locatelli, F.; Kwiatkowski, J.L.; Porter, J.B.; Trasher, A.J.; Homgeng, S.; Sauer, M.G.; Thuret, I.; Lal, A.; Algeri, M.; et al. Northstar-3: Interim results from a phase 3 study evaluating lentiglobin gene therapy in patients with transfusion-dependent β-thalassemia and either a β0 or IVS-I-110 mutation at both alleles of the HBB gene. Blood 2019, 134, 815. [Google Scholar] [CrossRef]
- Shangaris, P.; Loukogeorkakis, S.P.; Subramaniam, S.; Flouri, C.; Jackson, L.H.; Wang, W.; Blundell, M.P.; Liu, S.; Eaton, S.; Bakhamis, N.; et al. In Utero Gene Therapy (IUGT) Using GLOBE Lentiviral Vector Phenotypically Corrects the Heterozygous Humanised Mouse Model and Its Progress Can Be Monitored Using MRI Techniques. Sci. Rep. 2019, 9, 11592. [Google Scholar] [CrossRef]
- Marktel, S.; Scaramuzza, S.; Cicalese, M.P.; Giglio, F.; Galimberti, S.; Lidonnici, M.R.; Calbi, V.; Assanelli, A.; Bernardo, M.E.; Rossi, C.; et al. Intrabone hematopoietic stem cell gene therapy for adult and pediatric patients affected by transfusion-depend- ent ss-thalassemia. Nat. Med. 2019, 25, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Pawliuk, R.; Westerman, K.A.; Fabry, M.E.; Payen, E.; Tighe, R.; Bouhassira, E.E.; Acharya, S.A.; Ellis, J.; London, I.M.; Eaves, C.J.; et al. Correction of sickle cell disease in transgenic mouse models by gene therapy. Science 2001, 294, 2368–2371. [Google Scholar] [CrossRef] [PubMed]
- Ribeil, J.-A.; Hacein-Bey-Abina, S.; Payen, E.; Magnani, A.; Semeraro, M.; Magrin, E.; Caccavelli, L.; Neven, B.; Bourget, P.; El Nemer, W.; et al. Gene therapy in a patient with sickle cell disease. N. Engl. J. Med. 2017, 376, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Kanter, J.; Walters, M.C.; Krishnamurti, L.; Mapara, M.Y.; Kwiatkowski, J.L.; Rifkin-Zenenberg, S.; Aygun, B.; Kasow, K.A.; Pierciey Jr., F.J.; Bonner, M.; et al. Biologic and Clinical Efficacy of LentiGlobin for Sickle Cell Disease. N. Engl. J. Med. 2022, 386, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Gambari, R. Alternative options for DNA-based experimental therapy of β-thalassemia. Expert Opin. Biol. Ther. 2012, 12, 443–462. [Google Scholar] [CrossRef] [PubMed]
- Ingram, V. A specific chemical difference between the globins of normal human and sickle cell anemia hemoglobin. Nature 1956, 178, 792–794. [Google Scholar] [CrossRef]
- Kim, J.; Yoon, Y.S.; Lee, H.; Chang, W. AAV-GAD gene for rat models of neuropathic pain and Parkinson’s disease. Acta Neurochir. Suppl. 2008, 101, 99–105. [Google Scholar]
- Kaplitt, M.G.; Feigin, A.; Tang, C.; Fitzsimons, H.L.; Mattis, P.; Lawlor, P.A.; Bland, R.J.; Young, D.; Strybing, K.; Eidelberg, D.; et al. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson’s disease: an open label, phase I trial. Lancet 2007, 369, 2097–2105. [Google Scholar] [CrossRef]
- Björklund, A.; Kirik, D.; Rosenblad, C.; Georgievska, B.; Lundberg, C.; Mandel, R.J. Towards a neuroprotective gene therapy for Parkinson’s disease: Use of adenovirus, AAV and lentivirus vectors for gene transfer of GDNF to the nigrostriatal system in the rat Parkinson model. Brain Res. 2000, 886, 82–98. [Google Scholar] [CrossRef]
- Kordower, J.H.; Emborg, M.E.; Bloch, J.; Ma, S.Y.; Chu, Y.; Leventhal, L.; McBride, J.; Chen, E.Y.; Palfi, S.; Roitberg, B.Z.; et al. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate model of Parkinson’s disease. Science 2000, 290, 767–773. [Google Scholar] [CrossRef]
- Forsayeth, J.R.; Eberling, J.L.; Sanftner, L.M.; Zhen, Z.; Pivirotto, P.; Bringas, J.; Cunningham, J.; Bankiewicz, K.S. A dose-ranging study of AAV-hAADCtherapy in Parkinsonian monkeys. Mol. Ther. 2006, 14, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Eberling, J.L.; Jagust, W.J.; Christine, C.W.; Starr, P.; Larson, P.; Bankiewicz, K.S.; Aminoff, M.J. Results from a phase I safety trial of hAADC gene therapy for Parkinson disease. Neurology 2008, 70, 1980–1983. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Muramatsu, S.I.; Ikeguchi, K.; Fujimoto, K.I.; Fan, D.S.; Ogawa, M.; Mizukami, H.; Urabe, M.; Kume, A.; Nagatsu, I.; et al. Triple transduction with adeno-associated virus vectors expressing tyrosine hydroxylase, aromatic-L-amino acid decarboxylase, and GTP cyclohydrolase I for gene therapy for Parkinson’s disease. Hum. Gene Ther. 2000, 11, 1509–1519. [Google Scholar] [CrossRef]
- Palfi, S.; Gurruchaga, J.M.; Ralph, G.S.; Lepetit, H.; Lavisse, S.; Buttery, P.C.; Watts, C.; Miskin, J.; Kelleher, M; Deeley, S.; et al. Long-term safety and tolerability of ProSavin, a lentiviral vector-based gene therapy for Parkinson’s disease: A dose escalation, open-label, phase 1/2 trial. Lancet 2014, 383, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- Palfi, S.; Gurruchaga, J.M.; Lepetit, H.; Howard, K; Ralph, G.S.; Mason, S.; Gouello, G.; Domenech, P.; Buttery, P.C.; Hantraye, P.; et al. Long-Term Follow-up of a Phase I/II of ProSavin, a Lentiviral Vector Gene Therapy for Parkinson’s Disease. Hum. Gene Ther. Clin. Dev. 2018, 29, 148–155. [Google Scholar] [CrossRef]
- Miniarikova, J.; Zimmer, V.; Martier, R.; Bouwers, C.C.; Pythoud, C.; Richetin, K.; Rey, M.; Lubelski, J.; Evers, M.M.; van Deventer, S.J.; et al. AAV5-miHTT gene therapy demonstrates suppression of huntingtin aggregation and neuronal dysfunction in a rat model of Huntington’s disease. Gene Ther. 2017, 24, 630–639. [Google Scholar] [CrossRef]
- Evers, M.M.; Miniarikova, J.; Juhas, S.; Vallès, A.; Bohuslavova, B.; Juhasova, J.; Skalnikova, H.K.; Vodicka, P.; Valekova, I.; Brouwers, C.; et al. AAV5-miHTT gene therapy demonstrates broad distribution and strong human mutant huntingtin lowering in Huntington’s disease minipig model. Mol. Ther. 2018, 26, 2163–2177. [Google Scholar] [CrossRef]
- Rodrigues, F.B.; Wild, E.J. Huntington’s Disease Clinical Trials Corner: April 2020. J. Huntington’s Dis. 2020, 9, 185–197. [Google Scholar] [CrossRef]
- Passini, M.A.; Bu, J.; Roskelley, E.M.; Richards, A.M.; Pablo Sardi, S.; O’Riordan, C.R.; Klinger, K.W.; Shihabuddin, L.S.; Cheng, S.H. CNS-targeted gene therapy improves survival and motor function in a mouse model of spinal muscular atrophy. J. Clin. Invest. 2010, 120, 1253–1264. [Google Scholar] [CrossRef]
- Pattali, R.; Mou, Y.; Li, X.-J. AAV9 vector: A novel modality in gene therapy for spinal muscular atrophy. Gene Ther. 2019, 26, 287–295. [Google Scholar] [CrossRef]
- Mendell, J.R.; Al-Zaidy, S.; Shell, R.; Arnold, W.D.; Rodino-Klapac, L.R.; Prior, T.W.; Lowes, L.; Alfano, L.; Berry, K.; Church, K.; et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N. Engl. J. Med. 2017, 377, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Hoy, S.M. Onasemnogene Abeparvovec First Global Approval. Drugs 2019, 79, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, J.R.; Chamberlain, J.S. Progress toward gene therapy for Duchenne muscular dystrophy. Mol. Ther. 2017, 25, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, M.; Yuasa, K.; Yoshimura, M.; Yokota, T.; Ikemoto, T.; Suzuki, M.; Dickson, G.; Miyagoe-Suzuki, Y.; Takeda, S. Micro- dystrophin cDNA ameliorates dystrophic phenotypes when introduced into mdx mice as a transgene. Biochem. Biophys. Res. Comm. 2002, 293, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Gregorevic, P.; Allen, J.M.; Minami, E.; Blankinship, M.J.; Haraguchi, M.; Meuse, L.; Finn, E.; Adams, M.E.; Froehner, S.C.; Murry, C.E.; et al. rAAV6-microdystrophin preserves muscle function and extends lifespan in severely dystrophic mice. Nat. Med. 2006, 12, 787–789. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Storb, R.; Halbert, C.L.; Banks, G.B.; Butts, T.M.; Finn, E.E.; Allen, J.M.; Miller, A.D.; Chamberlain, J.S.; Tapscott, S.J. Successful regional delivery and long-term expression of a dystrophin gene in canine muscular dystrophy: A preclinical model for human therapies. Mol. Ther. 2012, 20, 1501–1507. [Google Scholar] [CrossRef] [PubMed]
- Mendell, J.R.; Sahenk, Z.; Lehman, K.; Nease, C.; Lowes, L.P.; Miller, N.F.; Iammarino, M.A.; Alfano, L.N.; Nicholl, A.; Al-Zaidy, S.; et al. Assessment of systemic delivery of rAAVrh74. MHCK7.micro-dystrophin in children with Duchenne muscular dystrophy. JAMA Neurol. 2020, 77, 1122–1131. [Google Scholar] [CrossRef]
- Butterfield, R.; Shieh, P.; Geffen, D.; Yong, F.; Binks, M.; McDonnell, T.G.; Ryan, K.A.; Belluscio, B.; Neelakanten, S.; Levy, D.; et al. One year data from ambulatory boys in a phase 1b, open-label study of fordadistrogene movaparvovec (PF-06939926) for Duchenne muscular dystrophy (DMD). MDA Conference, Poster 53. www.mdaconference.org/abstract-library/one-year-data-from-ambulatory-boys-in-a-phase-1b-open-label-study-of-fordadistrogene-movaparvovec-pf-06939926-for-duchenne- muscular-dystrophy-dmd/.
- Goedeker, N.L.; Dharia, S.D.; Griffin, D.A.; Coy, J.; Truesdale, T.; Parikh, R.; Whitehouse, K.; Santra, S.; Asher, D.R.; Zaidman, C.M. Evaluation of rAAVrh74 gene therapy vector seroprevalence by measurement of total binding antibodies in patients with Duchenne muscular dystrophy. Ther. Adv. Neurol. Disord. 2023, 16, 17562864221149781. [Google Scholar] [CrossRef]
- Cavazzana-Calvo, M.; Hacein-Bey, S.; de Saint Basile, G.; Gross, F.; Yvon, E.; Nusbaum, P.; Selz, F.; Hue, C.; Certain, S.; Casa- nova, J.L.; Bousso, P.; et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science 2000, 28, 669–672. [Google Scholar] [CrossRef]
- Hacein-Bey-Abina, S.; Garrigue, A.; Wang, G.P.; Soulier, J.; Lim, A.; Morillon, E.; Clappier, E.; Caccavelli, L.; Delabesse, E.; Beldjord, K.; et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J. Clin. Investig. 2008, 118, 3132–3142. [Google Scholar] [CrossRef]
- Fischer, A.; Hacein-Bey-Abina, S. Gene therapy for severe combined immunodeficiencies and beyond. J. Exp. Med. 2020, 217, e20190607. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, H.B.; Parsley, K.L.; Howe, S.; King, D.; Gilmour, K.C.; Sinclair, J.; Brouns, G.; Schmidt, M.; Von Kalle, C.; Barington, T.; et al. Gene therapy of X-linked severe combined immunodeficiency by use of a pseudotyped gammaretroviral vector. Lancet 2004, 364, 2181–2187. [Google Scholar] [CrossRef] [PubMed]
- Hacein-Bey-Abina, S.; Pai, S.Y.; Gaspar, H.B.; Armant, M.; Berry, C.C.; Blanche, S.; Bleesing, J.; Blondeau, J.; de Boer, H.; Buck- land, K.F.; et al. A modified γ-retrovirus vector for X-linked severe combined immunodeficiency. N. Engl. J. Med. 2014, 371, 1407–1417. [Google Scholar] [CrossRef] [PubMed]
- Howe, S.J.; Mansour, M.R.; Schwarzwaelder, K.; Bartholomae, C.; Hubank, M.; Kempski, H.; Brugman, M.H.; Pike-Overzet, K.; Chatters, S.J.; de Ridder, D.; et al. Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients. J. Clin. Investig. 2008, 118, 3143–3150. [Google Scholar] [CrossRef] [PubMed]
- Kohn, D.B.; Hershfield, M.S.; Puck, J.M.; Aiuti, A.; Blincoe, A.; Gaspar, H.B.; Notarangelo, L.D.; Grunebaum, E. Consensus approach for the management of severe combined immune deficiency caused by adenosine deaminase deficiency. J. Allergy Clin. Immunol. 2019, 143, 852–863. [Google Scholar] [CrossRef]
- Kohn, D.B.; Booth, C.; Shaw, K.L.; Xu-Bayford, J.; Garabedian, E.; Trevisan, V.; Carbonaro-Sarracino, D.A.; Soni, K.; Terrazas, D.; Snell, K.; et al. Autologous Ex Vivo Lentiviral Gene Therapy for Adenosine Deaminase Deficiency. N. Engl. J. Med. 2021, 384, 2002–2013. [Google Scholar] [CrossRef]
- Ramasamy, M.N.; Minassian, A.M.; Ewer, K.J.; Flaxman, A.L.; Folegatti, P.M.; Owens, D.R.; Voysey, M.; Aley, P.K.; Angus, B.; Babbage, G.; et al. Safety and immunogenicity of ChAdOx1 nCov-19 vaccine adminsitered in a prime-boost regimen in young and old adults (COV002): A singe-blind, randomised, controlled phase 2/3 trial. Lancet 2020, 336, 1979–1993. [Google Scholar] [CrossRef]
- Zhu, F.C.; Guan, X.H.; Li, Y.H.; Huang, J.Y.; Jiang, T.; Hou, L.H.; Li, J.X.; Yang, B.F.; Wang, L.; Wang, W.J.; et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: A random- ised, double-blind, placebo-controlled, phase 2 trial. Lancet 2020, 396, 479–488. [Google Scholar] [CrossRef]
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhvatulin, A.I.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19: An interim analysis of a randomised controlled phase 3 in Russia. Lancet 2021, 397, 671–681. [Google Scholar] [CrossRef]
- Stephenson, K.E.; Le Gars, M.; Sadoff, J.; de Groot, A.M.; Heerwegh, D.; Truyers, C.; Atyeo, C.; Loos, C.; Chandrashekar, A.; McMahan, K.; et al. Immunogenicity of the Ad26.COV2.S vaccine for COVID-19. JAMA 2021, 325, 1535–1544. [Google Scholar] [CrossRef]
- Lundstrom, K. Viral Vectors for COVID-19 Vaccine Development. Viruses 2021, 13, 317. [Google Scholar] [CrossRef] [PubMed]
- Callaway, E. Russia’s fast-track coronavirus vaccine draws outrage over safety. Nature 2020, 584, 334–335. [Google Scholar] [CrossRef] [PubMed]
- Henao-Restrepo, A.M.; Longini, I.M.; Egger, M.; Dean, N.E.; Edmunds, W.J.; Camacho, A.; Carroll, M.W.; Doumbia, M.; Draguez, B.; Duraffour, S.; et al. Efficacy and effectiveness of an rVSV-vectored vaccine expressing Ebola surface glycoprotein: Interim results from the Guinea ring vaccination cluster-randomised trial. Lancet 2015, 386, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Henao-Restrepo, A.M.; Camacho, A.; Longini, I.M.; Watson, C.H.; Edmunds, W.J.; Egger, M.; Carroll, M.W.; Dean, N.E.; Diatta, I.; Doumbia, M.; et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: Final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ca Suffit!). Lancet 2017, 389, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Ollmann Saphire, E. A vaccine against Ebola virus. Cell 2020, 181, 6. [Google Scholar] [CrossRef]
- Maclachlan, T.K.; Lukason, M.; Collins, M.; Munger, R.; Isenberger, E.; Rogers, C.; Malatos, S.; Dufresne, E.; Morris, J.; Calcedo, R.; et al. Preclinical safety evaluation of AAV2-sFFLT101- a gene therapy for age-related macular degeneration. Mol Ther. 2011, 19, 326–334. [Google Scholar] [CrossRef]
- Heier, J.S.; Kherani, S.; Desai, S.; Dugel, P.; Kaushal, S.; Cheng, S.H.; Delacono, C.; Purvis, A.; Richards, S.; Le-Halpere, A.; et al. Intravitreous injection of AAV2-sFLT01 in patients with advanced neovascular age-related macular degeneration: A phase I, open-label trial. Lancet 2017, 390, 50–61. [Google Scholar] [CrossRef]
- Constable, I.J.; Pierce, C.M.; Lai, C.-M.; Magno, A.L.; Degli-Esposti, M.A.; French, M.A.; McAllister, I.L.; Butler, S.; Barone, S.B.; Schwartz, S.D.; et al. Phase 2a randomized clinical trial: Safety and post hoc analysis of subretinal rAAV.sFLT-1 for wet age-related macular degeneration. EBioMedicine 2016, 14, 168–175. [Google Scholar] [CrossRef]
- Guy, J.; Feuer, W.J.; Davis, J.L.; Porciatti, V.; Gonzalez, P.J.; Koilkonda, R.D.; Yuan, H.; Hauswirth, W.W.; Lam, B.L. Gene therapy for Leder hereditary optic neuropathy: Low and medium-dose visual results. Ophthalmology 2017, 124, 1621–1634. [Google Scholar] [CrossRef]
- Vignal, C.; Uretsky, S.; Fitoussi, S.; Galy, A.; Blouin, L.; Girmens, J.-F.; Bidot, S.; Thomasson, N.; Bouquet, C.; Valero, S.; et al. Safety of rAAV2/2-ND4 gene therapy. Ophthalmology 2018, 125, 945–947. [Google Scholar] [CrossRef]
- Russell, S.; Bennett, J.; Wellman, J.A.; Chung, D.C.; Yu, Z.F.; Tillman, A.; Wittes, J.; Pappas, J.; Elci, O.; McCague, S.; et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: A randomised, controlled, open-label, phase 3 trial. Lancet 2017, 390, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Maguire, A.M.; Bennett, J.; Aleman, E.M.; Leroy, B.P.; Aleman, T.S. Clinical Perspective: Treating RPE65-Associated Retinal Dystrophy. Mol. Ther. 2021, 29, 442–463. [Google Scholar] [CrossRef]
- Flotte, T.R.; Afione, S.A.; Conrad, C.; McGrath, S.A.; Solow, R.; Oka, H.; Zeitlin, P.L.; Guggino, W.B.; Carter, B.J. Stable in vivo expression of the cystic fibrosis transmembrane conductance regulator with an adeno-associated virus vector. Proc. Natl. Acad. Sci. USA 1993, 90, 10613–10617. [Google Scholar] [CrossRef] [PubMed]
- Conrad, C.K.; Allen, S.S.; Afione, S.A.; Reynolds, T.C.; Beck, S.E.; Fee-Maki, M.; Barazza-Ortiz, X.; Adams, R.; Askin, F.B.; Carter, B.J.; et al. Safety of single-dose administration of an adeno-associated virus (AAV)-CFTR vector in the primate lung. Gene Ther. 1996, 3, 658–668. [Google Scholar] [PubMed]
- Limberis, M.; Anson, D.S.; Fuller, M.; Parsons, D.W. Recovery of airway cystic fibrosis transmembrane conductance regulator function in mice with cystic fibrosis after single-dose lentivirus-mediated gene transfer. Hum. Gene Ther. 2002, 13, 1961–1970. [Google Scholar] [CrossRef] [PubMed]
- Cooney, A.L.; Abou Alaiwa, M.H.; Shah, V.S.; Bouzek, D.C.; Stroik, M.R.; Powers, L.S.; Gansemer, N.D.; Meyerholz, D.K.; Welsh, M.J.; Stoltz, D.A.; et al. Lentiviral-mediated phenotypic correction of cystic fibrosis pigs. JCI Insight 2016, 1, e88730. [Google Scholar] [CrossRef] [PubMed]
- Agapov, E.V.; Frolov, I.; Lindenbach, D.B.; Pragai, B.M.; Schlesinger, S.; Rice, C.M. Noncytopathic Sindbis virus RNA vectors for heterologous gene expression. Proc. Natl. Acad. Sci. USA 1998, 95, 12989–12994. [Google Scholar] [CrossRef] [PubMed]
- Lundstrom, K.; Abenavoli, A.; Malgaroli, A.; Ehrengruber, M.U. Novel Semliki Forest virus vectors with reduced toxicity and temperature-sensitivity for long-term enhancement of transgene expression. Mol. Ther. 2003, 7, 202–209. [Google Scholar] [CrossRef]
- Perkovic, M.; Gawletta, S.; Hempel, T.; Brill, S.; Nett, E.; Sahin, U.; Beissert, T. A trans-amplifying RNA simplified to essential elements is highly replicative and robustly immunogenic in mice. Mol. Ther. 2023. [Google Scholar] [CrossRef]
- Lundstrom, K. Therapeutic Applications for Oncolytic Self-Replicating RNA Viruses. Int. J. Mol. Sci. 2022, 23, 15622. [Google Scholar] [CrossRef]
- Xiang, Q.; Li, L.; Wu, J.; Tian, M.; Fu, Y. Application of pseudovirus system in the development of vaccine, antiviral-drugs, and neutralizing antibodies. Microbiol. Res. 2022, 258, 126993. [Google Scholar] [CrossRef]
- Morse, M.A.; Crosby, E.J.; Force, J.; Osada, T.; Hobeika, A.C.; Hartman, Z.C.; Berglund, P.; Smith, J.; Lyerly, H.K. Clinical trials of self-replicating RNA-based cancer vaccines. Cancer Gene Ther. 2023, 10, 1–9. [Google Scholar]
- Chen, L.; Ewing, D.; Subramanian, H.; Block, K.; Rayner, J.; Alterson, K.D.; Sedegah, M.; Hayes, C.; Porter, K.; Raviprakash, K. A heterologous DNA prime-Venezuelan equine encephalitis virus replicon particle boost dengue vaccine regimen affords complete protection from virus challenge in cynomolgus macaques. J. Virol. 2007, 81, 11634–11639. [Google Scholar] [CrossRef] [PubMed]
- Gan, H.K.; Burgess, A.W.; Clayton, A.H.; Scott, A.M. Targeting of a conformationally exposed, tumor-specific epitope of EGFR as a strategy for cancer therapy. Cancer Res. 2012, 72, 2924–2930. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, H.; Zou, H.; Tian, X.; Hu, J.; Qiu, P.; Ferreira, L. Liposome Encapsulation of Oncolytic Virus M1 To Reduce Immunogenicity and Immune Clearance in Vivo. Mol. Pharm. 2019, 16, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Boulikas, T.; Lundstrom, K.; Soling, P.C.; Warnke, P.C.; Rainov, N.G. Immunogene therapy of recurrent glioblastoma multiforme with a liposomally encapsulated replication-incompetent Semliki Forest virus vector carrying the human interleukin-12 gene—a phase I/II clinical protocol. J. Neuro-Oncol. 2003, 64, 147–154. [Google Scholar] [CrossRef]
- Van der Loo, J.C.; Wright, J.F. Progress and challenges in viral vector manufacturing. Hum. Mol. Genet. 2016, 25, R42–52. [Google Scholar] [CrossRef]
- Saeki, Y.; Fraefel, C.; Ichikawa, T.; Breakfield, X.O.; Chiocca, E.A. Improved helper virus-free packaging system for HSV amplicon vectors using an ICP27-deleted, oversized HSV-1 DNA in a bacterial artificial chromosome. Mol. Ther. 2001, 3, 591–601. [Google Scholar] [CrossRef]
- Polo, J.M.; Belli, B.A.; Driver, D.A.; Frolov, I.; Sherill, S.; Hariharan, M.J.; Townsend, K.; Perri, S.; Mento, S.J.; Jolly, D.J.; et al. Stable alphavirus packaging cell lines for Sindbis virus and Semliki Forest virus-derived vectors. Proc. Natl. Acad. Sci. USA 1999, 96, 4598–4603. [Google Scholar] [CrossRef]
- Guerriaud, M.; Kohli, E. RNA-based drugs and regulation: Toward a necessary evolution of the definitions issued from the European Union legislation. Front. Med. 2022, 9, 1012497. [Google Scholar] [CrossRef]
- Food and Drug Administration. Personal Communication: Questions About mRNA Drugs; Food and Drug Administration,: Silver Spring, MD:, 2022. [Google Scholar]
- Celis, P. Personal Communication: mRNA Drugs Regulation and Categorization.; European Medicines Agency,: ATMP Office of EMA Amsterdam, 2022. [Google Scholar]
- Li, C.; Wang, H.; Georgakopoulou, A.; Gil, S.; Yannaki, E.; Lieber, A. In Vivo HSC Gene Therapy Using a Bi-modular HDAd5/35++ Vector Cures Sickle Cell Disease in a Mouse Model. Mol. Ther. 2021, 29, 822–837. [Google Scholar] [CrossRef] [PubMed]
- Tarone, L.; Barutello, G.; Iussich, S.; Giacobino, D.; Quaglino, E.; Buracco, P.; Cavallo, F.; Riccardo, F. Naturally occurring cancers in pet dogs as pre-clinical models for cancer immunotherapy. Cancer Immunol. Immunother. 2019, 68, 1839–1853. [Google Scholar] [CrossRef] [PubMed]
- Barthélémy, F.; Wein, N. Personalized gene and cell therapy for Duchenne Muscular Dystrophy. Neuromusc. Disorders 2018, 28, 803–824. [Google Scholar] [CrossRef] [PubMed]
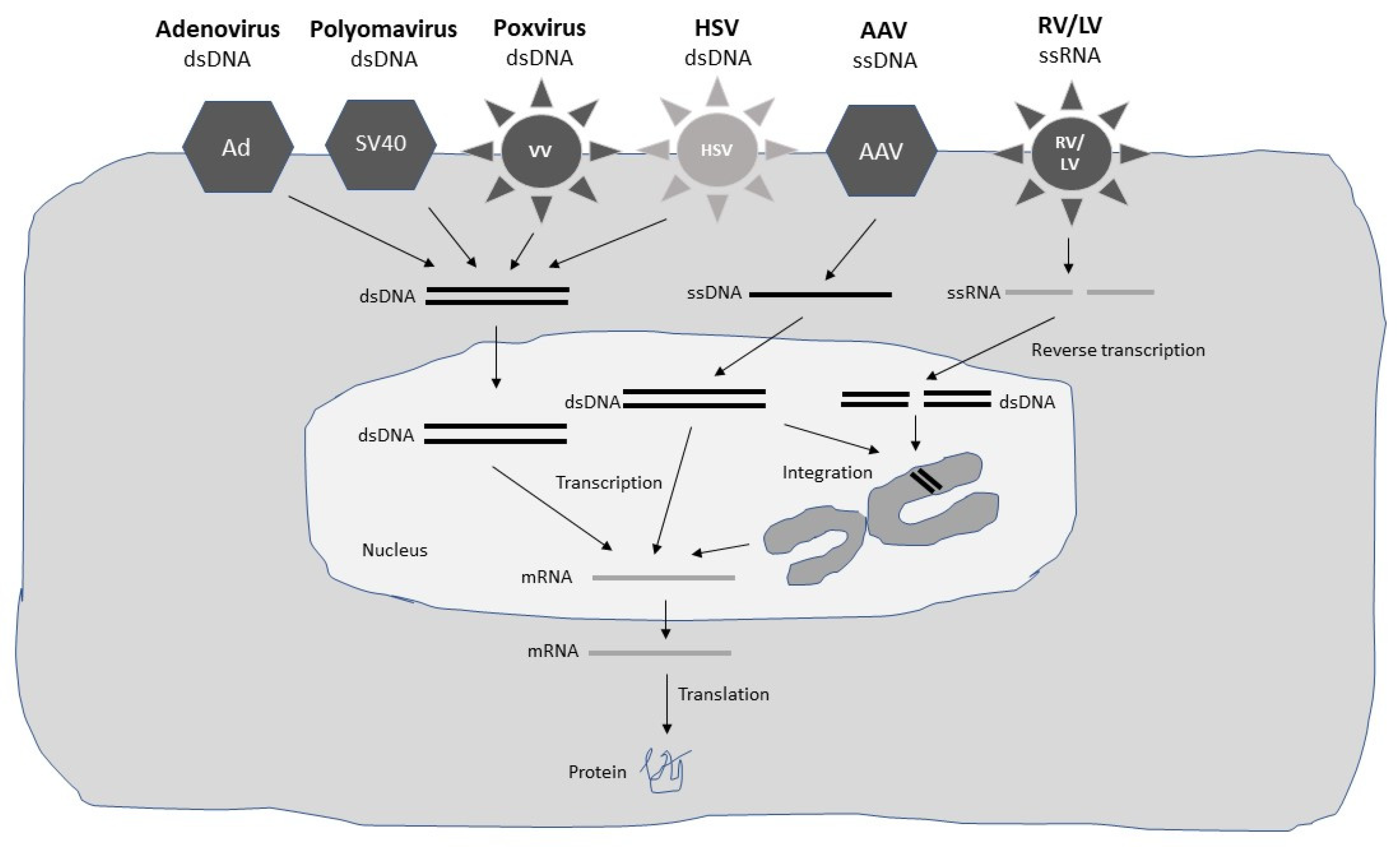
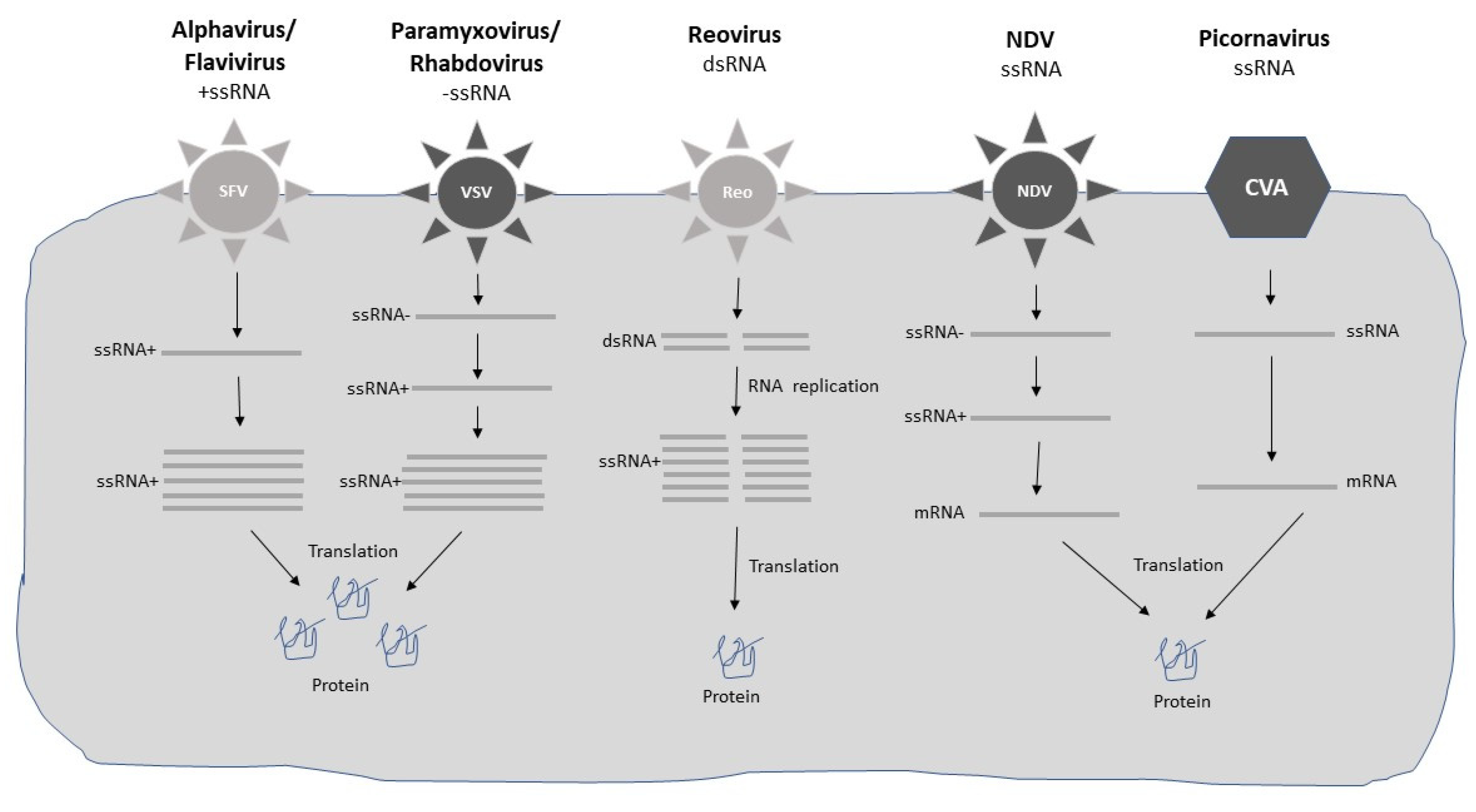
| Virus | Genome | Capacity | Features |
|---|---|---|---|
| Ad | dsDNA | 7.5-37 kb | Extrachromosomal, short- and long-term expression [14] |
| AAV HSV RV LV Alphavirus Flavivirus Paramyxovirus Rhabdovirus NDV Poxvirus Picornavirus Reovirus Polyomavirus |
ssDNA dsDNA ssRNA ssRNA ssRNA ssRNA ssRNA ssRNA ssRNA dsDNA ssRNA dsRNA dsDNA |
4 kb 30-150 kb 8 kb 8 kb 8 kb 6 kb 6 kb 6 kb 4 kb > 30 kb 6 kb ND 17.7 kb |
Extrachromosomal, integration for long-term expression [10] Extrachromosomal, long-term expression, latency [11] Integration, long-term expression [12] Integration, long-term expression, transduction of non-dividing cells [13] Extreme short-term expression, RNA degradation [18] Extreme short-term expression, RNA degradation [19] Short-term expression, RNA degradation [20] Short-term expression, RNA degradation [21] Short-term expression, oncolytic activity [22] Extrachromosomal expression [23] Extrachromosomal expression [24,25] Extrachromosomal expression [24] Extrachromosomal expression [23] |
| Cancer | Vector | Target | Findings |
|---|---|---|---|
| Esophageal | Ad | p53 | Significant tumor suppression of tumor growth in nude mice [50] |
| Ovarian HNSCC Head & neck Melanoma Melanoma Melanoma HGG HGG HGG Sarcoma Solid tumors Melanoma Glioma Breast Prostate Ovarian Prostate Prostate |
Ad Ad Ad HSV T-VEC HSV T-VEC HSV T-VEC RRV Toca 511 RRV Toca 511 RRV Toca 511 Reovirus Reovirus Reovirus Reovirus Reovirus MV MV VEE VEE |
p53 p53 p53 GM-CSF GM-CSF GM-CSF yCD yCD yCD Reolysin Reolysin Reolysin Reolysin Reolysin CEA CEA PSMA PSMA |
Prolonged survival in mice with implanted tumors [51] Safe and promising results in clinical trials [52] Approved as GendicineTM for head & neck cancer in China [53] Enhanced tumor growth inhibition, prolonged survival in mice [55] Minor adverse events, good therapeutic efficacy in Phase II and III [56] Approval for melanoma treatment in the US, Europa, and Australia [57] Extended survival in mice with orthotopic gliomas [58] Prolonged survival of 13.6 months in HGG patients in phase I [59] No overall extension of survival in HGG patients in phase II/III [60] Inhibition of tumor growth in nude mice [61] Clinical benefits in phase I: 1 PR, 7 SD [62] Good safety and clinical efficacy in melanoma patients in phase II [63] Orphan drug designation for malignant glioma [64] Fast Track designation for metastatic breast cancer [65] Delayed tumor growth, prolonged survival in mice [66] SD in all 9 patients, prolonged OS survival of 12.15 months in phase I [67] Robust PSMA-specific immune responses in mice [68] Good safety, and tolerability, but weak immunogenicity in phase I [69] |
| Disease | Vector | Target | Findings |
|---|---|---|---|
| Heart failure | Ad | SERCa2a | Restored systolic & diastolic heart functions rat heart model [71] |
| Heart failure Heart failure Heart failure Heart failure Heart failure DMT1 AAT AAT |
AAV1 LV AAV1 AAV1 AAV1 AAV2 AAV2 AAV2 |
SERCa2a SERCa2a SERCa2a SERCa2a SERCa2a hAAT hAAT hAAT |
Enhanced coronary blood flow in pig heart failure model [72] Improved systolic & diastolic functions, reduced mortality in rats [73] Improved functional, symptomatic and ventricular activity in phase I [74] Improved walking, peak oxygen consumption in phase II [75] Reduced cardiovascular events and deaths in Phase II [76] Reduced insulitis, insulin autoantibodies, and DTM1 in mice [77] Sustained AAT expression > 1 year in AAT patients in phase I [78] Immunostaining of AAT in AAT patients in phase II [79] |
| Disease | Vector | Target | Findings |
|---|---|---|---|
| Hemophilia A | Ad | FVIII | Expression of physiological levels of FVIII in mice [83] |
| Hemophilia A Hemophilia A Hemophilia A Hemophilia A Hemophilia A Hemophilia A Hemophilia A Hemophilia B Hemophilia B Hemophilia B Hemophilia B Hemophilia B Hemophilia B Hemophilia B Hemophilia B Hemophilia B β-thalassemia β-thalassemia β-thalassemia β-thalassemia SCD SCD SCD |
AAV6/AAV8 AAV8 AAV8/AAV9 LV-BM AAV AAV5 AAV5 Ad Ad Ad +CsA AAV8 AAV8 scAAV2 scAAV2 AAVS3 SIN-LV LentiGlobin LentiGlobin GLOBE LV GLOBE LV LV-HSC LentiGlobin LentiGlobin |
FVIII FVIII FVIII FVIII FVIII FVIII-SQ FVIII FIX cFIX cFIX FIX FIX FIX FIX FIX FIX HbAT87Q HbAT87Q Mini-β Mini-β βA globin HbAT87Q HbAT87Q |
Therapeutic FVIII levels lasting for > 3 years in dogs [87] 1-2% of normal FVIII levels, 90% reduction of bleeding in dogs [88] 1.9-11.3% of normal FVIII levels in dogs [89] Sustained FVIII activity, hemophilia A phenotype correction in mice [90] 8-60% of normal FVIII levels in hemophilia A patients in phase II [91] Clinical benefits: less bleeding, no need for prophylactic FVIII [92] Conditional EMA marketing approval for severe hemophilia A [93] Expression of FIX for > 300 days in mice [94] Complete correction of hemophilia B phenotype in dogs [95] Restored therapeutic FIX for 6 months with CsA in dogs [96] 25-200% FIX activity, correction of hemophilic phenotype in dogs [97] 1-6% of normal FIX, reduced bleeding episodes in patients in phase I [98] Correction of coagulation function in FIX-deficient mice [100] Significant reduction of bleeding episodes in hemophilia B patients [91] FIX expression for 27 months in hemophilia B patients in phase I/II [102] Stable long-term FIX expression in dogs [103] Blood transfusions terminated in β-thalassemia patients in phase I [104] Independence of transfusions in β-thalassemia patients in phase III [105] Normalized phenotype after in utero gene therapy [106] Reduced or no need for transfusion in β-thalassemia patients [107] Anti-sickling protein expression for 10 months in mice [108] Case report of complete remission of SCD patient [109] Complete resolution of vaso-occlusive events in SCD patients [110] |
| Disease | Vector | Target | Findings |
|---|---|---|---|
| PD PD PD PD PD PD PD PD PD PD PD PD HD HD HD SMA SMA SMA SMA |
AAV AAV AAV LV AAV LV LV AAV AAV AAV LV LV AAV5 AAV5 AAV5 AAV8 AAV8 AAV8 AAV9 |
GAD65 GAD GDNF GDNF GDNF GDNF GDNF hAADC hAADC TH, GCH, hAADC ProSavin ProSavin miHTT miHTT miHTT hSMN hSMN hSMN hSMN1 |
Reduced PD symptoms and relief of pain in rat models [113] Safe, improved motor neuron functions in PD patients in phase I [114] Regeneration, functional activity in 6-OHDA-lesioned rats [115] Regeneration, functional activity in 6-OHDA-lesioned rats [115] Regeneration, functional activity in MTTP-lesioned primates [115] Regeneration, functional activity in MTTP-lesioned primates [115] Reversed functional and motor deficits in macaques [116] 50% improvement in L-Dopa responsiveness in primates [117]. Significant improvement in PD patients for 2 years [118] Enhanced BH4 and dopamine production, improved rotational behavior in rats [119] Significant motor function improvement in PD patients [120] Long-term (4 years) motor function improvement in PD patients [121] Prevention of mutant HTT, decrease in neuronal dysfunction in rats [122] Reduced HTT mRNA and protein levels in transgenic minipigs [123] Good safety and tolerability in HD patients in phase I/II [124] Improved muscle strength, coordination and locomotion in mice [125] Improved motor function and prolonged survival in SMA patients [126] Improved motor function and prolonged survival in SMA patients [127] Approval for SMA patients in the US, the EU and Canada [128] |
| Disease | Vector | Target | Findings | |
|---|---|---|---|---|
| Muscular | ||||
| Dystrophy | AAV6 | µDys | Restored Dys expression, reduced muscle pathology in mice [131] | |
| Dystrophy | AAV6 | µDys | Efficient Dys distribution in skeletal muscles in a canine model [132] | |
| DMD | rAAVrh74 | µDys | Therapeutic Dys levels, improved NSAA scores in 4 DMD patients [133] | |
| DMD | AAV9 | mini-Dys | Phase I in progress in 4-12-year-old DMD patients [134] | |
| Immunodeficiency | ||||
| SCID-X1 | γRV | IL2RG | SCID-X1 correction in pediatric patients [136], few leukemia cases [137] | |
| SCID-X1 | γRV | IL2RG | Long-term clinical benefits in 8 out of 10 patients [136] | |
| SCID-X1 | γRV | IL2RG | Normal growth, and protection against infections after 18 years [138] | |
| SCID-X1 | γRV | IL2RG | Unfavroable chromosomal integration causing T-ALL [139] | |
| SCID-X1 | SIN-γRV | IL2RG | No cases of leukemia in 9 treated SCID-X1 patients [141] | |
| SCID-X1 | SIN-LV | IL2RG | No cases of leukemia in 44 treated SCID-X1 patients [141] | |
| ALD | SIN-LV | ABCD1 | Prevention of cerebral demyelination, clinical benefits [140] | |
| ALD | SIN-γRV | ADA | Metabolic correction, high OS in ADA-SCID patients [142] | |
| ADA-SCID | SIN-LV | ADA | Metabolic correction, high OS in ADA-SCID patients [143] |
| Disease | Vector | Target | Findings |
|---|---|---|---|
| COVID-19 | ChAdOx1 nCoV-19 | SARS-CoV-2 S | Good safety and 62-90% efficacy in Phase III [144] |
| COVID-19 COVID-19 COVID-19 COVID-19 COVID-19 COVID-19 COVID-19 EVD EVD EVD |
Ad5-S-nb2 rAd26-S/rAd5-S Ad26.COV2.S ChAdOx1 nCoV-19 Ad5-S-nb2 rAd26-S/rAd5-S Ad26.COV2.S VSV-ZEBOV VSV-ZEBOV VSV.ZEBOV |
SARS-CoV-2 S SARS-CoV-2 S SARS-CoV-2 S SARS-CoV-2 SARS-CoV-2 SARS-CoV-2 SARS-CoV-2 EBOV GP EBOV GP EBOV GP |
Good safety and efficacy in Phase III [145] Good safety and efficacy in Phase III [146] Good vaccine efficacy after a single dose [147] EUA in the UK in December 2020 [148] EUA in China in February 2021 [148] Approval in Russia in August 2020 [149] EUA in the US in February 2021 [148] Good safety and efficacy in Phase III [150] Good safety and efficacy in Phase III [151] Approval for EVD in 2020 [152] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




