Submitted:
20 April 2023
Posted:
21 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Seed Germination
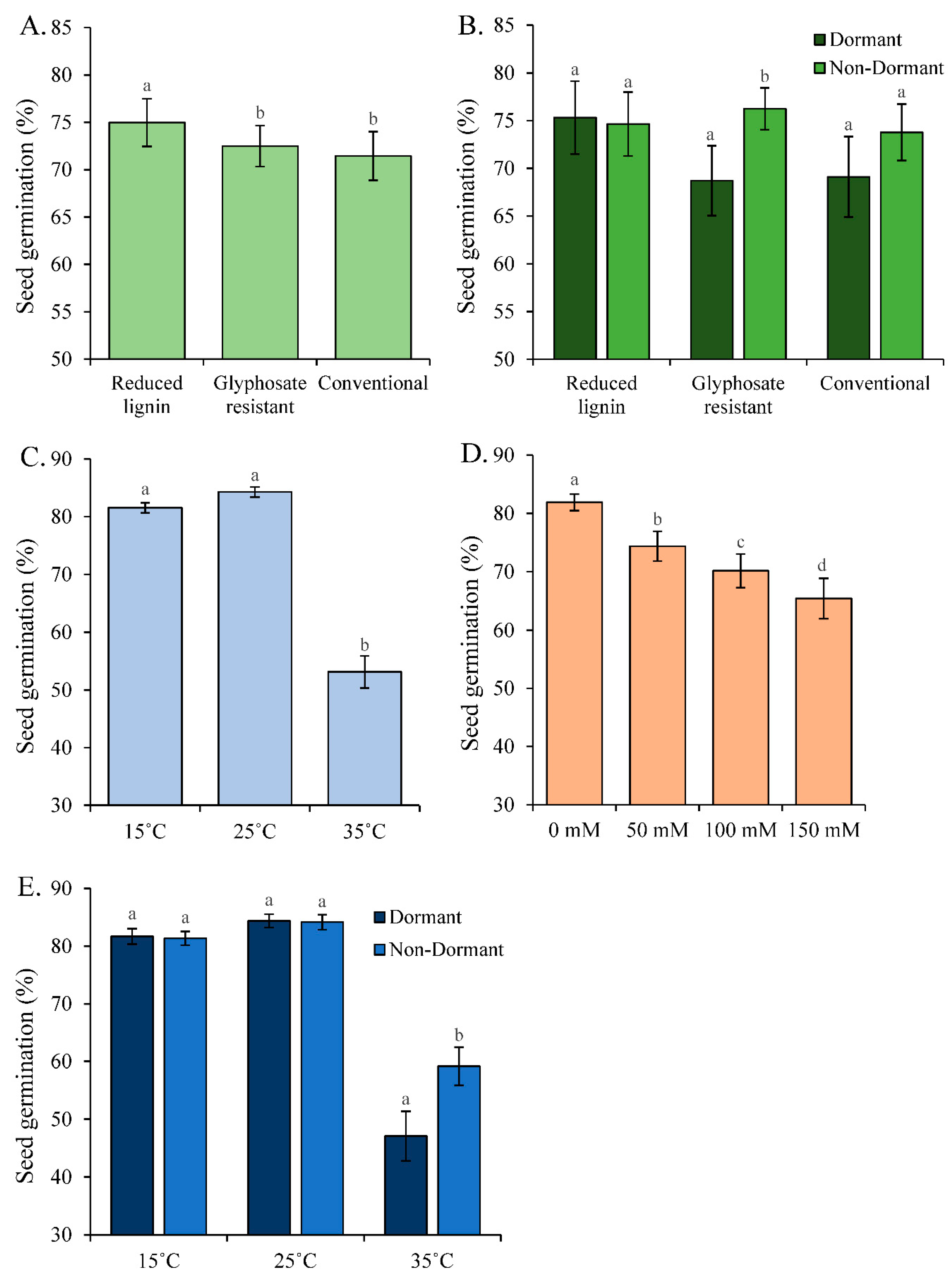
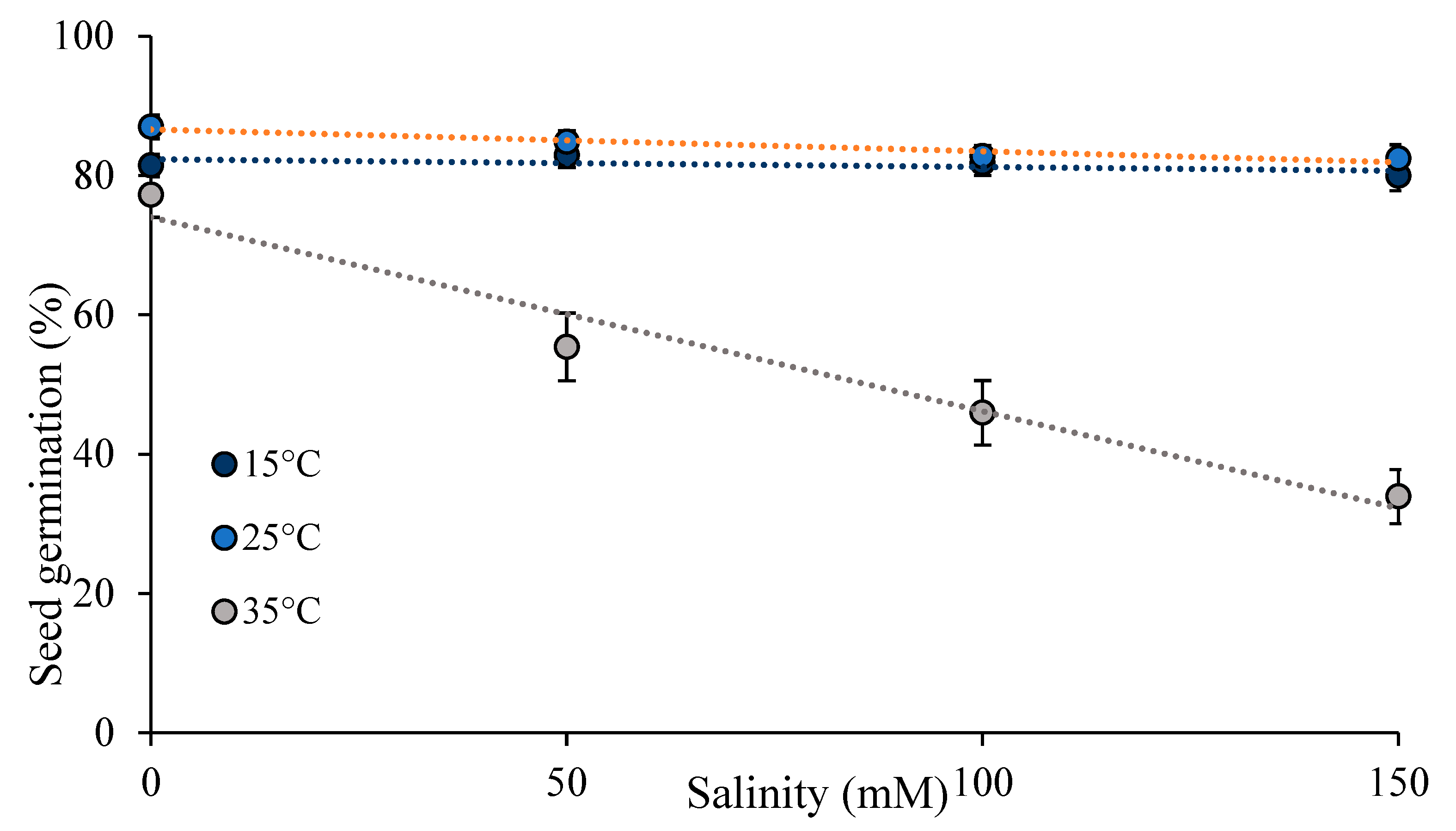
2.2. Radicle Length
| Effect | Num df | Den df | F value | Pr > F |
|---|---|---|---|---|
| Temperature | 2 | 144 | 299.58 | < 0.0001 |
| Category | 2 | 144 | 2.44 | 0.0911 |
| Dormancy | 1 | 144 | 5.69 | 0.0183 |
| Salinity | 3 | 144 | 74.59 | <.0001 |
| Temperature X Category | 4 | 144 | 0.62 | 0.6473 |
| Temperature X Dormancy | 2 | 144 | 0.78 | 0.4605 |
| Temperature X Salinity | 6 | 144 | 3.92 | 0.0012 |
| Category X Dormancy | 2 | 144 | 14.84 | <.0001 |
| Category X Salinity | 6 | 144 | 2.02 | 0.0673 |
| Dormancy X Salinity | 3 | 144 | 0.15 | 0.9281 |
| Temperature X Category X Salinity | 4 | 144 | 1.71 | 0.1512 |
| Temperature X Dormancy X Salinity | 6 | 144 | 0.72 | 0.6334 |
| Category X Dormancy X Salinity | 6 | 144 | 1.84 | 0.0957 |
| Temperature X Category X Dormancy X Salinity | 24 | 144 | 1.03 | 0.4352 |
3. Discussion
3.1. Seed Germination
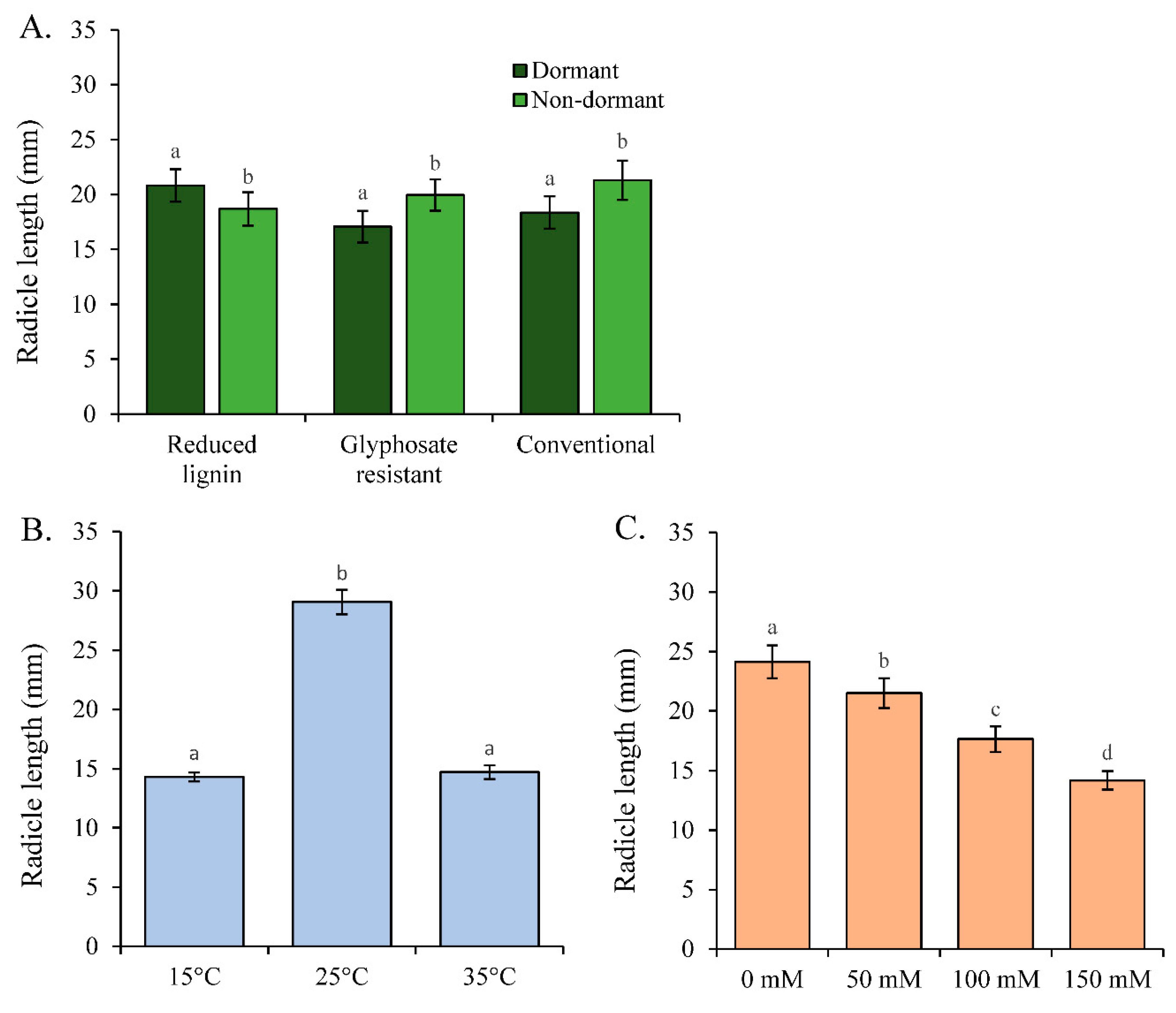
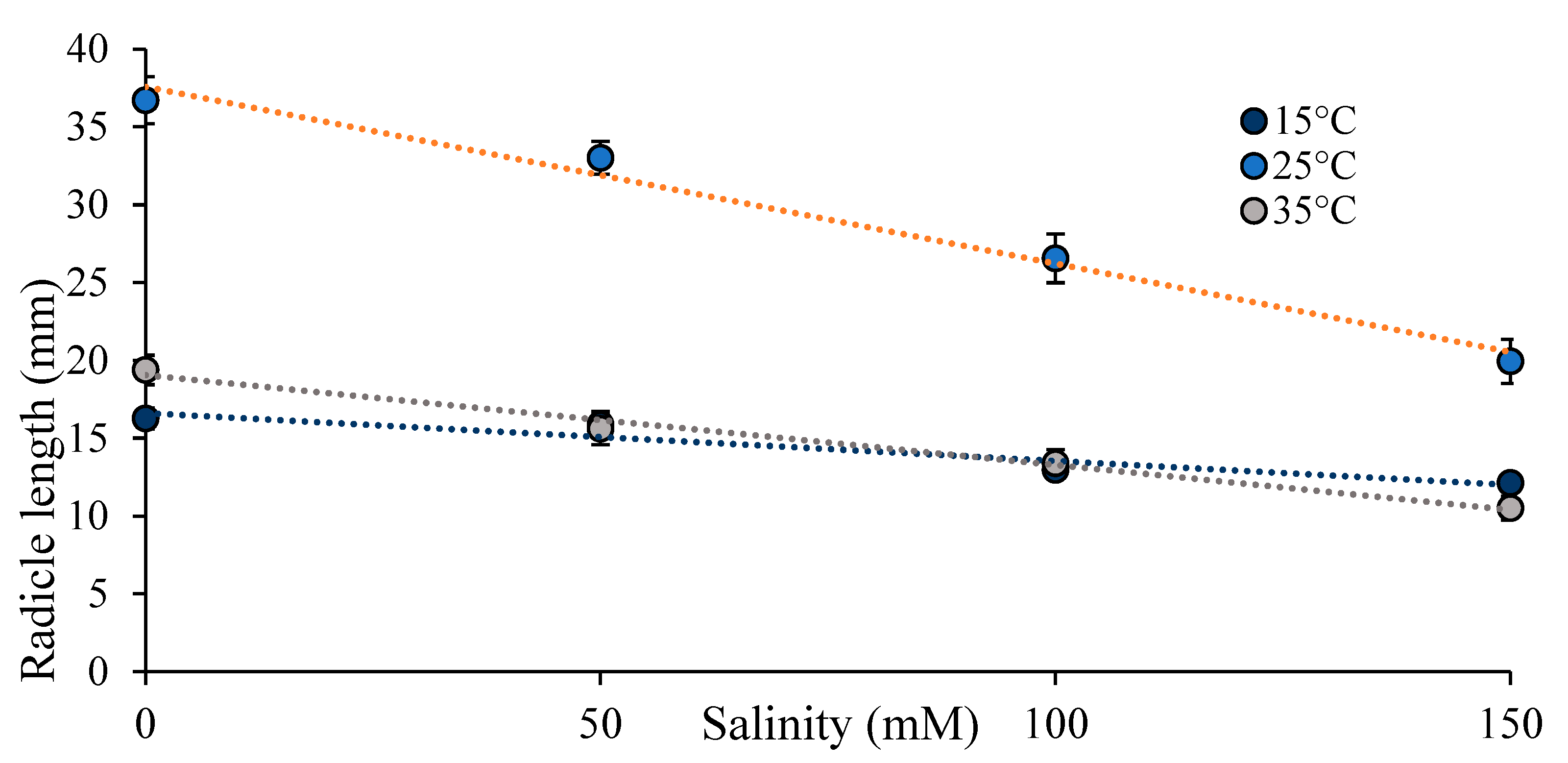
3.2. Radicle Length
3.3. Alfalfa under Climate Change
4. Materials and Methods
4.1. Plant Material
4.2. Experimental Design
| Category | Dormancy Type | Variety Name | Dormancy Level | Number |
|---|---|---|---|---|
| Conventional | Dormant | AmeriStand 427TQ | 4.3 | 1 |
| FSG 424 | 4 | 2 | ||
| Lightning Bolt | 4 | 3 | ||
| Non- Dormant | Fertillac 10 | 10 | 4 | |
| Salado | 9.5 | 5 | ||
| Sun Quest | 9 | 6 | ||
| Glyphosate | Dormant | 428RR | 4 | 7 |
| Resistant | Heritage RR | 4 | 8 | |
| WL 336HQ.RR | 3.2 | 9 | ||
| Non-Dormant | 6015R | 10 | 10 | |
| RRALF 9R100 | 9 | 11 | ||
| AmeriStand 835NTS RR | 8 | 12 | ||
| Reduced lignin | Dormant | HX FD-1 | NA | 13 |
| HX FD-2 | NA | 14 | ||
| HX FD-3 | NA | 15 | ||
| Non-Dormant | HX ND-1 | NA | 16 | |
| HX ND-2 | NA | 17 | ||
| HX ND-3 | NA | 18 |
4.3. Seed Germination and Radicle length Measurements
| Temperature 15˚C | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Conventional | Glyphosate resistant | Reduced lignin | |||||||||||||||||||||
| Dormant | Non-Dormant | Dormant | Non-Dormant | Dormant | Non-Dormant | ||||||||||||||||||
| Salinity level (mM) | |||||||||||||||||||||||
| 0 | 50 | 100 | 150 | 0 | 50 | 100 | 150 | 0 | 50 | 100 | 150 | 0 | 50 | 100 | 150 | 0 | 50 | 100 | 150 | 0 | 50 | 100 | 150 |
| 1 | 1 | 1 | 1 | 4 | 4 | 4 | 4 | 7 | 7 | 7 | 7 | 10 | 10 | 10 | 10 | 13 | 13 | 13 | 13 | 16 | 16 | 16 | 16 |
| 2 | 2 | 2 | 2 | 5 | 5 | 5 | 5 | 8 | 8 | 8 | 8 | 11 | 11 | 11 | 11 | 14 | 14 | 14 | 14 | 17 | 17 | 17 | 17 |
| 3 | 3 | 3 | 3 | 6 | 6 | 6 | 6 | 9 | 9 | 9 | 9 | 12 | 12 | 12 | 12 | 15 | 15 | 15 | 15 | 18 | 18 | 18 | 18 |
| Temperature 25˚C | |||||||||||||||||||||||
| Conventional | Glyphosate resistant | Reduced lignin | |||||||||||||||||||||
| Dormant | Non-Dormant | Dormant | Non-Dormant | Dormant | Non-Dormant | ||||||||||||||||||
| Salinity level | |||||||||||||||||||||||
| 0 | 50 | 100 | 150 | 0 | 50 | 100 | 150 | 0 | 50 | 100 | 150 | 0 | 50 | 100 | 150 | 0 | 50 | 100 | 150 | 0 | 50 | 100 | 150 |
| 1 | 1 | 1 | 1 | 4 | 4 | 4 | 4 | 7 | 7 | 7 | 7 | 10 | 10 | 10 | 10 | 13 | 13 | 13 | 13 | 16 | 16 | 16 | 16 |
| 2 | 2 | 2 | 2 | 5 | 5 | 5 | 5 | 8 | 8 | 8 | 8 | 11 | 11 | 11 | 11 | 14 | 14 | 14 | 14 | 17 | 17 | 17 | 17 |
| 3 | 3 | 3 | 3 | 6 | 6 | 6 | 6 | 9 | 9 | 9 | 9 | 12 | 12 | 12 | 12 | 15 | 15 | 15 | 15 | 18 | 18 | 18 | 18 |
| Temperature 35˚C | |||||||||||||||||||||||
| Conventional | Glyphosate resistant | Reduced lignin | |||||||||||||||||||||
| Dormant | Non-Dormant | Dormant | Non-Dormant | Dormant | Non-Dormant | ||||||||||||||||||
| Salinity level | |||||||||||||||||||||||
| 0 | 50 | 100 | 150 | 0 | 50 | 100 | 150 | 0 | 50 | 100 | 150 | 0 | 50 | 100 | 150 | 0 | 50 | 100 | 150 | 0 | 50 | 100 | 150 |
| 1 | 1 | 1 | 1 | 4 | 4 | 4 | 4 | 7 | 7 | 7 | 7 | 10 | 10 | 10 | 10 | 13 | 13 | 13 | 13 | 16 | 16 | 16 | 16 |
| 2 | 2 | 2 | 2 | 5 | 5 | 5 | 5 | 8 | 8 | 8 | 8 | 11 | 11 | 11 | 11 | 14 | 14 | 14 | 14 | 17 | 17 | 17 | 17 |
| 3 | 3 | 3 | 3 | 6 | 6 | 6 | 6 | 9 | 9 | 9 | 9 | 12 | 12 | 12 | 12 | 15 | 15 | 15 | 15 | 18 | 18 | 18 | 18 |
4.4. Statistical Analyses
| Factor | Interpretation |
|---|---|
| Temperature | Phenotypic plasticity to temperature |
| Salinity | Phenotypic plasticity to salinity |
| Category | Genetic differentiation in trait among categories |
| Dormancy | Genetic differentiation in trait among dormancy types |
| Temperature X Category | Genetic differentiation (category) in plastic response to temperature |
| Temperature X Dormancy | Genetic differentiation (dormancy) in plastic response to temperature |
| Temperature X Salinity | Phenotypic response to salinity varies with temperature |
| Category X Dormancy | Genetic differentiation in trait among dormancy types varies between categories |
| Salinity X Category | Genetic differentiation (category) in plastic response to salinity |
| Salinity X Dormancy | Genetic differentiation (dormancy) in plastic response to salinity |
| Temperature X Salinity X Category | Genetic differentiation (category) in plastic response to temperature X salinity |
| Temperature X Salinity X Dormancy | Genetic differentiation (dormancy) in plastic response to temperature X salinity |
| Salinity X Category X Dormancy | Genetic differentiation (category X dormancy) in plastic response to salinity |
| Temperature X Salinity X Category X Dormancy | Genetic differentiation (Category X dormancy) in plastic response to temperature X salinity |
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brunet, J.; Van Etten, M.L. The response of floral traits associated with pollinator attraction to environmental changes expected under anthropogenic climate change in high-altitude habitats. Int. J. Plant Sciences, 2019, 180, 929-933. https://www.journals.uchicago.edu/doi/pdfplus/10.1086/705591.
- Mooney, H.; Larigauderie, A.; Cesario, M.; Elmquis, T.; Hoegh-Guldberg, O.; Lavorel, S.; Mace, G.M.; Palmer, M.; Robert Scholes, R.; Tetsukazu Yahara, T. Biodiversity, climate change, and ecosystem services. COSUST .2009, 1, 46–54. [CrossRef]
- Trivedi, M.R.; Morecroftc, M.D.; Berrya, P.M.; Dawsond, T.P. Potential effects of climate change on plant communities in three montane nature reserves in Scotland, UK. Biol. Conserv. 2008, 141, 1665–1675.
- Christensen, J.H.; Hewitson, B.; Busuioc, A.; Chen, A.; Gao, X.; Held, R.; Jones, R. et al. Regional climate projections. In Climate change 2007: the physical science basis—contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change. Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L. eds. Cambridge University Press, New York. 2007. pp 847–940.
- Bita, C.E.; Gerats, T. Plant tolerance to high temperature in a changing environment: scientific fundamentals and production of heat stress-tolerant crops. Front. Plant Sci. 2013, 4, 273. [CrossRef]
- Ullah, A.; Bano, A.; Khan, N. Climate Change and Salinity Effects on Crops and Chemical Communication Between Plants and Plant Growth-Promoting Microorganisms Under Stress Front. Sustain. Food Syst., 2021, 5, 2021. | . [CrossRef]
- Li, J.; Ma, M.; Sun, Y.; Lu, P.; Shi, H.; Guo, Z.; Xhu, H. Comparative physiological and transcriptome profiles uncover salt tolerance mechanisms in alfalfa. Frontiers in Plant Science 2022, 13, 931619. [CrossRef]
- Porter, J.R. Rising temperatures are likely to reduce crop yields. Nature 2005, 436, 174. [CrossRef]
- Jagadish, S.V.K.; Way, D.A.; Sharkey, T.D. Scaling plant responses to high temperature from cell to ecosystem. Plant Cell Environ. 2021, 44, 1987–1991.
- Rising, J.; Devineni, N. Crop switching reduces agricultural losses from climate change in the United States by half under RCP 8.5. Nature Communications, 2020. 11, 4991. [CrossRef]
- Potters, G.; Pasternak, T.P.; Guisez, Y.; Palme, K.J.; Jansen, M.A.K. Stress-induced morphogenic responses: growing out of trouble? Trends Plant Sci. 2007, 12, 98–105.
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat Tolerance in Plants: An Overview. Environ. Exp. Bot. 2007, 61, 3.
- Ungar, I.A. Influence of Salinity and Temperature on Seed Germination. Ohio J. Sci. 1967, 67, 120–123.
- Esechie, H.A. Interaction of Salinity and Temperature on the Germination of Alfalfa CV CUF-101. Agronomie 1993, 13, 301–306.
- Putnam, D.; Meccage, E. Profitable alfalfa production sustains the environment. In Proceedings World Alfalfa Congress, San Diego, CA. 14-17 November 2022.
- NASS U.S. Department of Agriculture’s National Agricultural Statistics Service (NASS) 2017. https://www.nass.usda.gov/Publications/AgCensus/2017/index.php.
- Zhanwu, G.; Hui, Z.; Jicai, G.; Chunwu, Y.; Chunsheng, M.; Deli, W. Germination responses of Alfalfa (Medicago sativa L.) seeds to various salt-alkaline mixed stress. J. Agric. Res. 2001, 6, 3793–3803.
- Johnson, D.; Smith, S.; Dobrenz, A. Genetic and phenotypic relationships in response to NaC1 at different developmental stages in alfalfa. Theor Appl Genet. 1992, 83, 833–838.
- Scasta, J.D.; Trostle, C.L.; Foster, M.A. Evaluating Alfalfa (Medicago sativa L.) Cultivars for Salt Tolerance.
- Using Laboratory, Greenhouse and Field Methods. J. Agric. Sci. 2012, 4. [CrossRef]
- Waissman Assadian, N., Miyamoto, S. Salt Effects on alfalfa seedling emergence. Agron. J. 1987, 79, 710–714.
- Sandhu, D.; Cornacchione, M.V.; Ferreira J.F.S.; Suarez D.L. Variable salinity responses of 12 alfalfa genotypes and comparative expression analyses of salt-response genes. Sci. Rep. 2017, 7, 42958. [CrossRef]
- Stone, J.E.; Marx, D.B.; Dobrenz, A.K. Interaction of sodium chloride and temperature on germination of two alfalfa cultivars. Agron. J. 1979, 71, 425–427.
- Flajoulot, S.; Ronfort, J.; Baudouin, P.; Barre, P.; Huguet, T.; Huyghe C.; Julier, B. Genetic diversity among alfalfa (Medicago sativa) cultivars coming from a breeding program, using SSR markers. Theor Appl Genet 2005, 111, 1420–1429.
- Qiang, H.; Chen, Z.; Zhang, Z.; Wang, X.; Gao, H.; Wang, Z. Molecular diversity and population structure of a worldwide collection of cultivated tetraploid alfalfa (Medicago sativa subsp. sativa L.) germplasm as revealed by microsatellite markers. PLoS ONE 2015, 10, e0124592. [CrossRef]
- Annicchiarico, P.; Nazicari, N., Ananta, A.; Carelli, M.; Wei, Y.; Brummer, E.C. Assessment of cultivar distinctness in alfalfa: a comparison of genotyping-by-sequencing, simple-sequence repeat marker, and morphophysiological observations. Plant Genome, 2016, 9. [CrossRef]
- Yu, L.X.; Liu, X.; Boge, W.; Liu, X.P. Genome-wide association study identifies loci for salt tolerance during germination in autotetraploid alfalfa (Medicago sativa L.) using genotyping-by-sequencing. Front. Plant Sci. 2016, 7, 956. [CrossRef]
- Rogan, G.; Fitzpatrick, S. Petition for determination of nonregulated status: Roundup Ready alfalfa (Medicago sativa L.) events J101 and J163. USDA Petition 2004, (04-110), 101.
- Barros, J.; Temple, S.; Dixon, R.A. Development and commercialization of reduced lignin alfalfa. Curr. Opin.Biotechnol. 2019, 56, 48–54.
- Liu, Z.; Li, X.; Wang, Z.; Sun, Q. 2015. Contrasting Strategies of Alfalfa Stem Elongation in Response to Fall Dormancy in Early Growth Stage: The Tradeoff between Internode Length and Internode Number. PLOS ONE 2015, 10, e0135934. [CrossRef]
- Ghalambor, C.K.; McKay, J.K.; Carroll, S.P.; Reznick, D.N. Adaptive versus non-adaptive phenotypic plasticity and the pote tial for contemporary adaptation in new environments. Functional Ecology 2007, 21, 394‒407.
- Lande, R. Adaptation to an extraordinary environment by evolution of phenotypic plasticity and genetic assimilation. J. Evol. Biol. 2009, 22, 1435‒1446.
- Chevin, L.M.; Lande, R.; Mace, G.M. Adaptation, plasticity, and extinction in a changing environment: towards a predictive theory. PLOS Biology 2010, 8, 1‒8.
- Fox, R.J.; Donelson, J.M.; Schunter,C.R; Timothy, R.; Gaitan-Espitia, J.D. Beyond buying time: the role of plasticity in phenotypic adaptation to rapid environmental changes. Philos. Trans. R. Soc. B. 2019, 374, 20180174. [CrossRef]
- Laitinen, R.A.E.; Nikoloski, Z. Genetic basis of plasticity in plants. J. Exp. Bot. 2019, 70, 739–745.
- Chevin, L.M; Lande, R. When do adaptive plasticity and genetic evolution prevent extinction of a density-regulated popul tion? Evolution 2009, 64, 1143‒1150.
- Al-Farsi, S.M.; Nadaf, S.K.; Al-Sadi, A.M.; Ullah, A.; Farooq, M. Evaluation of indigenous Omani alfalfa landraces for morphology and forage yield under different levels of salt stress. Physiol Mol Biol Plants, 2020, 26, 1763–1772.
- Scott, K. High salinity soils take special considerations to raise alfalfa. High Plains Journal, 2020. https://www.hpj.com/crops/high-salinity-soils-take-special-considerations-to-raise-alfalfa/article_07d6fec8-a73a-11ea-baf9-9b539fd9730f.html, viewed 03-16-2023.
- Irish, B.M.; Greene, S.L. Germplasm collection, genetic resources, and gene pools in alfalfa. In: The Alfalfa Genome. Compendium of Plant Genomes. Yu LX., Kole C., Eds.; Springer. Cham, Switzerland. 2021. pp. 43–64.
- Bohart, G.E. Pollination of alfalfa and red clover. Annu Rev Entomol. 1957, 2, 355–389.
- Dieterich Mabin, M.E.; Brunet, J.; Riday, H.; Lehmann, L. Self-fertilization, inbreeding and yield in alfalfa seed production fields. Front.Plant Sci. 2021, 12, 700708. [CrossRef]
- Abramoff, M.D.; Magalhaes, P.J.; Ram, S.J. 2014. Image Processing with ImageJ. Biophotonics Int., 2014, 11, 36–42.
| Effect | Num df | Den df | F value | Pr > F |
|---|---|---|---|---|
| Temperature | 2 | 144 | 190.27 | <0.0001 |
| Category | 2 | 144 | 4.26 | 0.0159 |
| Dormancy | 1 | 144 | 5.98 | 0.0157 |
| Salinity | 3 | 144 | 23.44 | <0.0001 |
| Temperature X Category | 4 | 144 | 1.23 | 0.299 |
| Temperature X Dormancy | 2 | 144 | 8.01 | 0.0005 |
| Temperature X Salinity | 6 | 144 | 14.54 | <0.0001 |
| Category X Dormancy | 2 | 144 | 3.14 | 0.0462 |
| Category X Salinity | 6 | 144 | 1.25 | 0.287 |
| Dormancy X Salinity | 3 | 144 | 0.22 | 0.882 |
| Temperature X Category X Salinity | 4 | 144 | 1.02 | 0.399 |
| Temperature X Dormancy X Salinity | 6 | 144 | 1.10 | 0.366 |
| Category X Dormancy X Salinity | 6 | 144 | 0.58 | 0.749 |
| Temperature X Category X Dormancy X Salinity | 24 | 144 | 0.55 | 0.957 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




