Submitted:
21 April 2023
Posted:
23 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Patients with Acute Myocardial Infarction
| SGLT2 inhibitor | Population | Timing | Primary outcomes | Results | |
|---|---|---|---|---|---|
| EMMY | Empagliflozin | AMI with creatinine kinase>800 iu/L | Within 3 days of PCI | Change in NTproBNP | NTproBNP reduction and improvement in echocardiographic parameters in the empagliflozin group |
| EMPACT-MI | Empagliflozin | AMI with or at high risk of HF | Within 14 days of admission | Time to first hospitalization for HF or all-cause mortality | Ongoing trial |
| DAPA-MI | Dapagliflozin | AMI (stable) | Within 7-10 days | Time to first hospitalization for HF or cardiovascular death | Ongoing trial |
3. Patients with Acute Heart Failure
4. Patients with Isolated Right Ventricular Failure
5. Patients with LVAD
6. Type 1 Diabetes
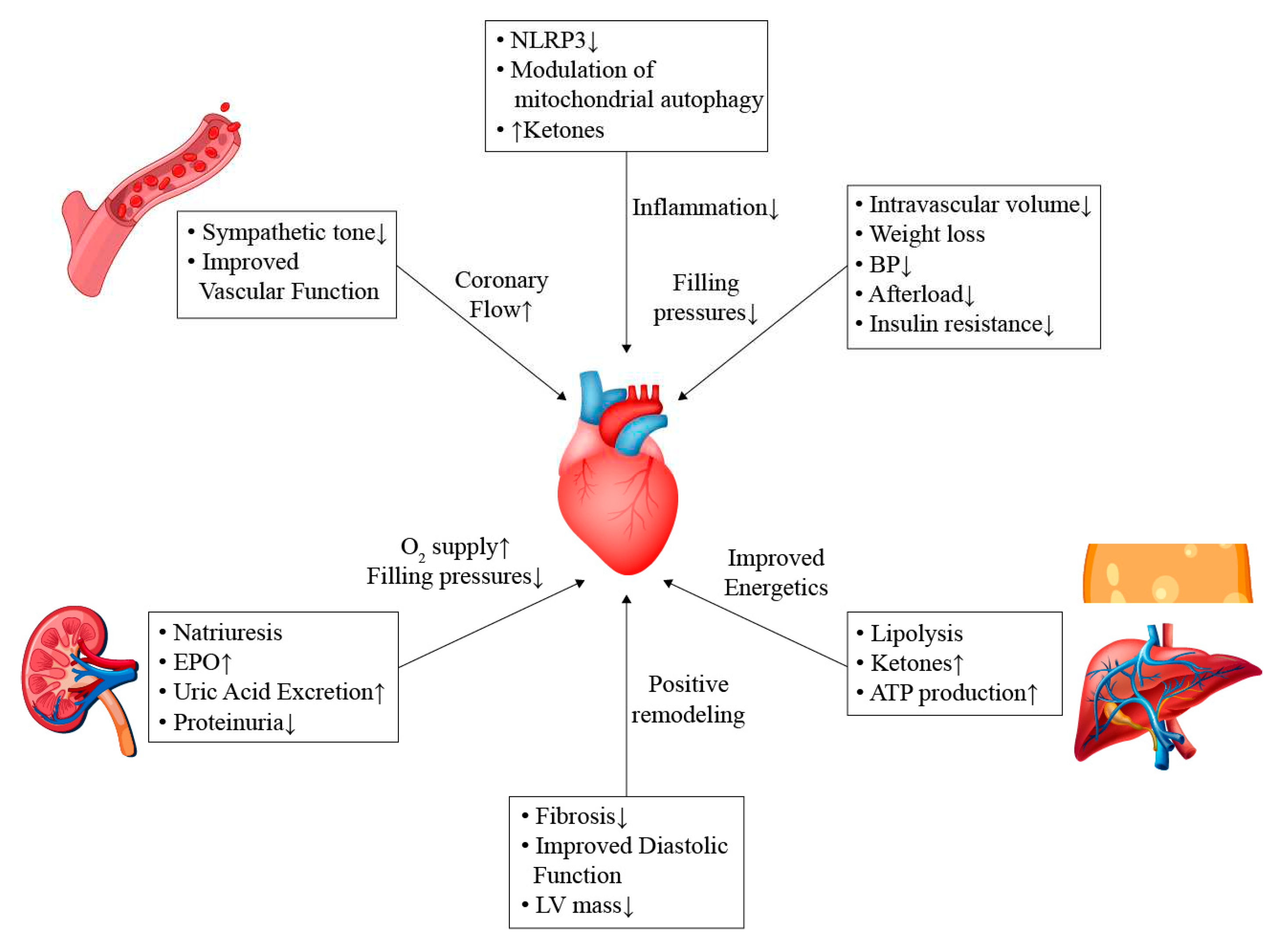
7. Potential Mechanisms
7.1. General Effects
7.2. Effects on Sympathetic Pathways
7.3. Cardio-Renal Pathways
7.4. Modulation of Energy Sources and Inflammatory Process
7.5. Microvascular Function
7.6. Effect on Lipid Profile
7.7. Effect on Remodeling and Fibrosis
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed consent statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Xu, B.; Li, S.; Kang, B.; Zhou, J. The current role of sodium-glucose cotransporter 2 inhibitors in type 2 diabetes mellitus management. Cardiovasc Diabetol. 2022, 21, 83. [Google Scholar] [CrossRef]
- Clar, C.; Gill, J.A.; Court, R.; Waugh, N. Systematic review of SGLT2 receptor inhibitors in dual or triple therapy in type 2 diabetes. BMJ Open. 2012, 2, e001007. [Google Scholar] [CrossRef] [PubMed]
- Neumiller, J.J.; White, J.R., Jr.; Campbell, R.K. Sodium-glucose co-transport inhibitors: progress and therapeutic potential in type 2 diabetes mellitus. Drugs. 2010, 70, 377–85. [Google Scholar] [CrossRef] [PubMed]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; Broedl, U.C.; Inzucchi, S.E. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015, 373, 2117–28. [Google Scholar] [CrossRef] [PubMed]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; Bhatt, D.L.; Leiter, L.A.; McGuire, D.K.; Wilding, J.P.H.; Ruff, C.T.; Gause-Nilsson, I.A.M.; Fredriksson, M.; Johansson, P.A.; Langkilde, A.M.; Sabatine, M.S.; DECLARE–TIMI 58 Investigators. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes.
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R.; CANVAS Program Collaborative Group. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017, 377, 644–657. [Google Scholar] [CrossRef] [PubMed]
- Cannon, C.P.; Pratley, R.; Dagogo-Jack, S.; Mancuso, J.; Huyck, S.; Masiukiewicz, U.; Charbonnel, B.; Frederich, R.; Gallo, S.; Cosentino, F.; Shih, W.J.; Gantz, I.; Terra, S.G.; Cherney, D.Z.I.; McGuire, D.K.; VERTIS CV Investigators. Cardiovascular Outcomes with Ertugliflozin in Type 2 Diabetes. N Engl J Med. 2020, 383, 1425–1435. [Google Scholar] [CrossRef]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; Jamal, W.; Kimura, K.; Schnee, J.; Zeller, C.; Cotton, D.; Bocchi, E.; Böhm, M.; Choi, D.J.; Chopra, V.; Chuquiure, E.; Giannetti, N.; Janssens, S.; Zhang, J.; Gonzalez Juanatey, J.R.; Kaul, S.; Brunner-La Rocca, H.P.; Merkely, B.; Nicholls, S.J.; Perrone, S.; Pina, I.; Ponikowski, P.; Sattar, N.; Senni, M.; Seronde, M.F.; Spinar, J.; Squire, I.; Taddei, S.; Wanner, C.; Zannad, F.; EMPEROR-Reduced Trial Investigators. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N Engl J Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; Böhm, M.; Chiang, C.E.; Chopra, V.K.; de Boer, R.A.; Desai, A.S.; Diez, M.; Drozdz, J.; Dukát, A.; Ge, J.; Howlett, J.G.; Katova, T.; Kitakaze, M.; Ljungman, C.E.A.; Merkely, B.; Nicolau, J.C.; O’Meara, E.; Petrie, M.C.; Vinh, P.N.; Schou, M.; Tereshchenko, S.; Verma, S.; Held, C.; DeMets, D.L.; Docherty, K.F.; Jhund, P.S.; Bengtsson, O.; Sjöstrand, M.; Langkilde, A.M.; DAPA-HF Trial Committees and Investigators. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner-La Rocca, H.P.; Choi, D.J.; Chopra, V.; Chuquiure-Valenzuela, E.; Giannetti, N.; Gomez-Mesa, J.E.; Janssens, S.; Januzzi, J.L.; Gonzalez-Juanatey, J.R.; Merkely, B.; Nicholls, S.J.; Perrone, S.V.; Piña, I.L.; Ponikowski, P.; Senni, M.; Sim, D.; Spinar, J.; Squire, I.; Taddei, S.; Tsutsui, H.; Verma, S.; Vinereanu, D.; Zhang, J.; Carson, P.; Lam, C.S.P.; Marx, N.; Zeller, C.; Sattar, N.; Jamal, W.; Schnaidt, S.; Schnee, J.M.; Brueckmann, M.; Pocock, S.J.; Zannad, F.; Packer, M.; EMPEROR-Preserved Trial Investigators. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N Engl J Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; Shah, S.J.; Desai, A.S.; Jhund, P.S.; Belohlavek, J.; Chiang, C.E.; Borleffs, C.J.W.; Comin-Colet, J.; Dobreanu, D.; Drozdz, J.; Fang, J.C.; Alcocer-Gamba, M.A.; Al Habeeb, W.; Han, Y.; Cabrera Honorio, J.W.; Janssens, S.P.; Katova, T.; Kitakaze, M.; Merkely, B.; O’Meara, E.; Saraiva, J.F.K.; Tereshchenko, S.N.; Thierer, J.; Vaduganathan, M.; Vardeny, O.; Verma, S.; Pham, V.N.; Wilderäng, U.; Zaozerska, N.; Bachus, E.; Lindholm, D.; Petersson, M.; Langkilde, A.M.; DELIVER Trial Committees and Investigators. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N Engl J Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; Sjöström, C.D.; Toto, R.D.; Langkilde, A.M.; Wheeler, D.C.; DAPA-CKD Trial Committees and Investigators. Dapagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef] [PubMed]
- The EMPA-KIDNEY Collaborative Group; Herrington, W.G.; Staplin, N.; Wanner, C.; Green, J.B.; Hauske, S.J.; Emberson, J.R.; Preiss, D.; Judge, P.; Mayne, K.J.; Ng, S.Y.A.; Sammons, E.; Zhu, D.; Hill, M.; Stevens, W.; Wallendszus, K.; Brenner, S.; Cheung, A.K.; Liu, Z.H.; Li, J.; Hooi, L.S.; Liu, W.; Kadowaki, T.; Nangaku, M.; Levin, A.; Cherney, D.; Maggioni, A.P.; Pontremoli, R.; Deo, R.; Goto, S.; Rossello, X.; Tuttle, K.R.; Steubl, D.; Petrini, M.; Massey, D.; Eilbracht, J.; Brueckmann, M.; Landray, M.J.; Baigent, C.; Haynes, R. Empagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2023, 388, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Gibb, A.A.; Hill, B.G. Metabolic Coordination of Physiological and Pathological Cardiac Remodeling. Circ Res. 2018, 123, 107–128. [Google Scholar] [CrossRef] [PubMed]
- Daud, E.; Ertracht, O.; Bandel, N.; Moady, G.; Shehadeh, M.; Reuveni, T.; Atar, S. The impact of empagliflozin on cardiac physiology and fibrosis early after myocardial infarction in non-diabetic rats. Cardiovasc Diabetol. 2021, 20, 132. [Google Scholar] [CrossRef]
- von Lewinski, D.; Kolesnik, E.; Tripolt, N.J.; Pferschy, P.N.; Benedikt, M.; Wallner, M.; Alber, H.; Berger, R.; Lichtenauer, M.; Saely, C.H.; Moertl, D.; Auersperg, P.; Reiter, C.; Rieder, T.; Siller-Matula, J.M.; Gager, G.M.; Hasun, M.; Weidinger, F.; Pieber, T.R.; Zechner, P.M.; Herrmann, M.; Zirlik, A.; Holman, R.R.; Oulhaj, A.; Sourij, H. Empagliflozin in acute myocardial infarction: the EMMY trial. Eur Heart J. 2022, 43, 4421–4432. [Google Scholar] [CrossRef]
- Damman, K.; Beusekamp, J.C.; Boorsma, E.M.; Swart, H.P.; Smilde, T.D.J.; Elvan, A.; van Eck, J.W.M.; Heerspink, H.J.L.; Voors, A.A. Randomized, double-blind, placebo-controlled, multicentre pilot study on the effects of empagliflozin on clinical outcomes in patients with acute decompensated heart failure (EMPA-RESPONSE-AHF). Eur J Heart Fail. 2020, 22, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Szarek, M.; Steg, P.G.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Voors, A.A.; Metra, M.; Lund, L.H.; Komajda, M.; Testani, J.M.; Wilcox, C.S.; Ponikowski, P.; Lopes, R.D.; Verma, S.; Lapuerta, P.; Pitt, B.; SOLOIST-WHF Trial Investigators. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. N Engl J Med. 2021, 384, 117–128. [Google Scholar] [CrossRef]
- Voors, A.A.; Angermann, C.E.; Teerlink, J.R.; Collins, S.P.; Kosiborod, M.; Biegus, J.; Ferreira, J.P.; Nassif, M.E.; Psotka, M.A.; Tromp, J.; Borleffs, C.J.W.; Ma, C.; Comin-Colet, J.; Fu, M.; Janssens, S.P.; Kiss, R.G.; Mentz, R.J.; Sakata, Y.; Schirmer, H.; Schou, M.; Schulze, P.C.; Spinarova, L.; Volterrani, M.; Wranicz, J.K.; Zeymer, U.; Zieroth, S.; Brueckmann, M.; Blatchford, J.P.; Salsali, A.; Ponikowski, P. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: a multinational randomized trial. Nat Med. 2022, 28, 568–574. [Google Scholar] [CrossRef]
- Salah, H.M.; Al’Aref, S.J.; Khan, M.S.; Al-Hawwas, M.; Vallurupalli, S.; Mehta, J.L.; Mounsey, J.P.; Greene, S.J.; McGuire, D.K.; Lopes, R.D.; Fudim, M. Efficacy and safety of sodium-glucose cotransporter 2 inhibitors initiation in patients with acute heart failure, with and without type 2 diabetes: a systematic review and meta-analysis. Cardiovasc Diabetol. 2022, 21, 20. [Google Scholar] [CrossRef]
- Schulze, P.C.; Bogoviku, J.; Westphal, J.; Aftanski, P.; Haertel, F.; Grund, S.; von Haehling, S.; Schumacher, U.; Möbius-Winkler, S.; Busch, M. Effects of Early Empagliflozin Initiation on Diuresis and Kidney Function in Patients With Acute Decompensated Heart Failure (EMPAG-HF). Circulation. 2022, 146, 289–298. [Google Scholar] [CrossRef]
- Cox, Z.L.; Collins, S.P.; Aaron, M.; Hernandez, G.A.; Iii, A.T.M.; Davidson, B.T.; Fowler, M.; Lindsell, C.J.; Jr, F.E.H.; Jenkins, C.A.; Kampe, C.; Miller, K.F.; Stubblefield, W.B.; Lindenfeld, J. Efficacy and safety of dapagliflozin in acute heart failure: Rationale and design of the DICTATE-AHF trial. Am Heart J. 2021, 232, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Nassif, M.E.; Qintar, M.; Windsor, S.L.; Jermyn, R.; Shavelle, D.M.; Tang, F.; Lamba, S.; Bhatt, K.; Brush, J.; Civitello, A.; Gordon, R.; Jonsson, O.; Lampert, B.; Pelzel, J.; Kosiborod, M.N. Empagliflozin Effects on Pulmonary Artery Pressure in Patients With Heart Failure: Results From the EMBRACE-HF Trial. Circulation. 2021, 143, 1673–1686. [Google Scholar] [CrossRef] [PubMed]
- Wolsk, E.; Jürgens, M.; Schou, M.; Ersbøll, M.; Hasbak, P.; Kjær, A.; Zerahn, B.; Brandt, N.H.; Gæde, P.H.; Rossing, P.; Faber, J.; Inzucchi, S.E.; Kistorp, C.M.; Gustafsson, F. Randomized Controlled Trial of the Hemodynamic Effects of Empagliflozin in Patients With Type 2 Diabetes at High Cardiovascular Risk: The SIMPLE Trial. Diabetes. 2022, 71, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Kayano, H.; Koba, S.; Hirano, T.; Matsui, T.; Fukuoka, H.; Tsuijita, H.; Tsukamoto, S.; Hayashi, T.; Toshida, T.; Watanabe, N.; Hamazaki, Y.; Geshi, E.; Murakami, M.; Aihara, K.; Kaneko, K.; Yamada, H.; Kobayashi, Y.; Shinke, T. Dapagliflozin Influences Ventricular Hemodynamics and Exercise-Induced Pulmonary Hypertension in Type 2 Diabetes Patients - A Randomized Controlled Trial. Circ J. 2020, 84, 1807–1817. [Google Scholar] [CrossRef]
- Sarak, B.; Verma, S.; David Mazer, C.; Teoh, H.; Quan, A.; Gilbert, R.E.; Goodman, S.G.; Bami, K.; Coelho-Filho, O.R.; Ahooja, V.; Deva, D.P.; Garg, V.; Gandhi, S.; Connelly, K.A.; Yan, A.T. Impact of empagliflozin on right ventricular parameters and function among patients with type 2 diabetes. Cardiovasc Diabetol. 2021, 20, 200. [Google Scholar] [CrossRef]
- Çamcı, S.; Yılmaz, E. Effects of Sodium-Glucose Co-Transporter-2 Inhibition on Pulmonary Arterial Stiffness and Right Ventricular Function in Heart Failure with Reduced Ejection Fraction. Medicina (Kaunas). 2022, 58, 1128. [Google Scholar] [CrossRef]
- Fang, J.C.; Ewald, G.A.; Allen, L.A.; Butler, J.; Westlake Canary, C.A.; Colvin-Adams, M.; Dickinson, M.G.; Levy, P.; Stough, W.G.; Sweitzer, N.K.; Teerlink, J.R.; Whellan, D.J.; Albert, N.M.; Krishnamani, R.; Rich, M.W.; Walsh, M.N.; Bonnell, M.R.; Carson, P.E.; Chan, M.C.; Dries, D.L.; Hernandez, A.F.; Hershberger, R.E.; Katz, S.D.; Moore, S.; Rodgers, J.E.; Rogers, J.G.; Vest, A.R.; Givertz, M.M.; Heart Failure Society of America Guidelines Committee. Advanced (stage D) heart failure: a statement from the Heart Failure Society of America Guidelines Committee. J Card Fail. 2015, 21, 519–34. [Google Scholar] [CrossRef]
- Mullan, C.W.; Chouairi, F.; Sen, S.; Mori, M.; Clark, K.A.A.; Reinhardt, S.W.; Miller, P.E.; Fuery, M.A.; Jacoby, D.; Maulion, C.; Anwer, M.; Geirsson, A.; Mulligan, D.; Formica, R.; Rogers, J.G.; Desai, N.R.; Ahmad, T. Changes in Use of Left Ventricular Assist Devices as Bridge to Transplantation With New Heart Allocation Policy. JACC Heart Fail. 2021, 9, 420–429. [Google Scholar] [CrossRef]
- Cagliostro, M.; Hundal, P.; Ting, P.; Patel, S.; Sudarshan, S.; Thomas, J.; Morris, K.; Mancini, D.M.; Moss, N.; Lala, A.; Ravichandran, A.; Mitter, S.S. Safety and effects of SGLT-2 inhibitor use among LVAD patients with type 2 diabetes mellitus. Am Heart J Plus. 2022, 18, 100154. [Google Scholar] [CrossRef]
- Aburjania, N.; Hay, C.M.; Sohail, M.R. Continuous-flow left ventricular assist device systems infections: current outcomes and management strategies. Ann Cardiothorac Surg. 2021, 10, 233–239. [Google Scholar] [CrossRef]
- Lega, I.C.; Bronskill, S.E.; Campitelli, M.A.; Guan, J.; Stall, N.M.; Lam, K.; McCarthy, L.M.; Gruneir, A.; Rochon, P.A. Sodium glucose cotransporter 2 inhibitors and risk of genital mycotic and urinary tract infection: A population-based study of older women and men with diabetes. Diabetes Obes Metab. 2019, 21, 2394–2404. [Google Scholar] [CrossRef]
- Colacci, M.; Fralick, J.; Odutayo, A.; Fralick, M. Sodium-Glucose Cotransporter-2 Inhibitors and Risk of Diabetic Ketoacidosis Among Adults With Type 2 Diabetes: A Systematic Review and Meta-Analysis. Can J Diabetes. 2022, 46, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Ata, F.; Yousaf, Z.; Khan, A.A.; Razok, A.; Akram, J.; Ali, E.A.H.; Abdalhadi, A.; Ibrahim, D.A.; Al Mohanadi, D.H.S.H.; Danjuma, M.I. SGLT-2 inhibitors associated euglycemic and hyperglycemic DKA in a multicentric cohort. Sci Rep. 2021, 11, 10293. [Google Scholar] [CrossRef]
- Pieber, T.R.; Famulla, S.; Eilbracht, J.; Cescutti, J.; Soleymanlou, N.; Johansen, O.E.; Woerle, H.J.; Broedl, U.C.; Kaspers, S. Empagliflozin as adjunct to insulin in patients with type 1 diabetes: a 4-week, randomized, placebo-controlled trial (EASE-1). Diabetes Obes Metab. 2015, 17, 928–35. [Google Scholar] [CrossRef] [PubMed]
- Dandona, P.; Mathieu, C.; Phillip, M.; Hansen, L.; Tschöpe, D.; Thorén, F.; Xu, J.; Langkilde, A.M.; DEPICT-1 Investigators. Efficacy and Safety of Dapagliflozin in Patients With Inadequately Controlled Type 1 Diabetes: The DEPICT-1 52-Week Study. Diabetes Care. 2018, 41, 2552–2559. [Google Scholar] [CrossRef] [PubMed]
- Groop, P.H.; Dandona, P.; Phillip, M.; Gillard, P.; Edelman, S.; Jendle, J.; Xu, J.; Scheerer, M.F.; Thoren, F.; Iqbal, N.; Repetto, E.; Mathieu, C. Effect of dapagliflozin as an adjunct to insulin over 52 weeks in individuals with type 1 diabetes: post-hoc renal analysis of the DEPICT randomised controlled trials. Lancet Diabetes Endocrinol. 2020, 8, 845–854. [Google Scholar] [CrossRef]
- Henry, R.R.; Thakkar, P.; Tong, C.; Polidori, D.; Alba, M. Efficacy and Safety of Canagliflozin, a Sodium-Glucose Cotransporter 2 Inhibitor, as Add-on to Insulin in Patients With Type 1 Diabetes. Diabetes Care. 2015, 38, 2258–65. [Google Scholar] [CrossRef]
- Garg, S.K.; Henry, R.R.; Banks, P.; Buse, J.B.; Davies, M.J.; Fulcher, G.R.; Pozzilli, P.; Gesty-Palmer, D.; Lapuerta, P.; Simó, R.; Danne, T.; McGuire, D.K.; Kushner, J.A.; Peters, A.; Strumph, P. Effects of Sotagliflozin Added to Insulin in Patients with Type 1 Diabetes. N Engl J Med. 2017, 377, 2337–2348. [Google Scholar] [CrossRef]
- Scheen, A.J. Effect of SGLT2 Inhibitors on the Sympathetic Nervous System and Blood Pressure. Curr Cardiol Rep. 2019, 21, 70. [Google Scholar] [CrossRef]
- Sano, M. A new class of drugs for heart failure: SGLT2 inhibitors reduce sympathetic overactivity. J Cardiol. 2018, 71, 471–476. [Google Scholar] [CrossRef]
- Rahman, A.; Fujisawa, Y.; Nakano, D.; Hitomi, H.; Nishiyama, A. Effect of a selective SGLT2 inhibitor, luseogliflozin, on circadian rhythm of sympathetic nervous function and locomotor activities in metabolic syndrome rats. Clin Exp Pharmacol Physiol. 2017, 44, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Herat, L.Y.; Magno, A.L.; Rudnicka, C.; Hricova, J.; Carnagarin, R.; Ward, N.C.; Arcambal, A.; Kiuchi, M.G.; Head, G.A.; Schlaich, M.P.; Matthews, V.B. SGLT2 Inhibitor-Induced Sympathoinhibition: A Novel Mechanism for Cardiorenal Protection. JACC Basic Transl Sci. 2020, 5, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, W.; Kubota, Y.; Hoshika, Y.; Mozawa, K.; Tara, S.; Tokita, Y.; Yodogawa, K.; Iwasaki, Y.K.; Yamamoto, T.; Takano, H.; Tsukada, Y.; Asai, K.; Miyamoto, M.; Miyauchi, Y.; Kodani, E.; Ishikawa, M.; Maruyama, M.; Ogano, M.; Tanabe, J.; EMBODY trial investigators. Effects of empagliflozin versus placebo on cardiac sympathetic activity in acute myocardial infarction patients with type 2 diabetes mellitus: the EMBODY trial. Cardiovasc Diabetol. 2020, 19, 148. [Google Scholar] [CrossRef] [PubMed]
- Onishi, A.; Fu, Y.; Patel, R.; Darshi, M.; Crespo-Masip, M.; Huang, W.; Song, P.; Freeman, B.; Kim, Y.C.; Soleimani, M.; Sharma, K.; Thomson, S.C.; Vallon, V. A role for tubular Na+/H+ exchanger NHE3 in the natriuretic effect of the SGLT2 inhibitor empagliflozin. Am J Physiol Renal Physiol. 2020, 319, F712–F728. [Google Scholar] [CrossRef] [PubMed]
- Sano, M.; Goto, S. Possible Mechanism of Hematocrit Elevation by Sodium Glucose Cotransporter 2 Inhibitors and Associated Beneficial Renal and Cardiovascular Effects. Circulation. 2019, 139, 1985–1987. [Google Scholar] [CrossRef] [PubMed]
- Mazer, C.D.; Hare, G.M.T.; Connelly, P.W.; Gilbert, R.E.; Shehata, N.; Quan, A.; Teoh, H.; Leiter, L.A.; Zinman, B.; Jüni, P.; Zuo, F.; Mistry, N.; Thorpe, K.E.; Goldenberg, R.M.; Yan, A.T.; Connelly, K.A.; Verma, S. Effect of Empagliflozin on Erythropoietin Levels, Iron Stores, and Red Blood Cell Morphology in Patients With Type 2 Diabetes Mellitus and Coronary Artery Disease. Circulation. 2020, 141, 704–707. [Google Scholar] [CrossRef] [PubMed]
- Swedberg, K.; Young, J.B.; Anand, I.S.; Cheng, S.; Desai, A.S.; Diaz, R.; Maggioni, A.P.; McMurray, J.J.; O’Connor, C.; Pfeffer, M.A.; Solomon, S.D.; Sun, Y.; Tendera, M.; van Veldhuisen, D.J.; RED-HF Committees; RED-HF Investigators. Treatment of anemia with darbepoetin alfa in systolic heart failure. N Engl J Med. 2013, 368, 1210–9. [Google Scholar] [CrossRef]
- Cowie, M.R.; Fisher, M. SGLT2 inhibitors: mechanisms of cardiovascular benefit beyond glycaemic control. Nat Rev Cardiol. 2020, 17, 761–772. [Google Scholar] [CrossRef]
- Ferrannini, E.; Baldi, S.; Frascerra, S.; Astiarraga, B.; Heise, T.; Bizzotto, R.; Mari, A.; Pieber, T.R.; Muscelli, E. Shift to Fatty Substrate Utilization in Response to Sodium-Glucose Cotransporter 2 Inhibition in Subjects Without Diabetes and Patients With Type 2 Diabetes. Diabetes. 2016, 65, 1190–5. [Google Scholar] [CrossRef]
- Verma, S.; Rawat, S.; Ho, K.L.; Wagg, C.S.; Zhang, L.; Teoh, H.; Dyck, J.E.; Uddin, G.M.; Oudit, G.Y.; Mayoux, E.; Lehrke, M.; Marx, N.; Lopaschuk, G.D. Empagliflozin Increases Cardiac Energy Production in Diabetes: Novel Translational Insights Into the Heart Failure Benefits of SGLT2 Inhibitors. JACC Basic Transl Sci. 2018, 3, 575–587. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Verma, S. Mechanisms of Cardiovascular Benefits of Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitors: A State-of-the-Art Review. JACC Basic Transl Sci. 2020, 5, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Youm, Y.H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.D.; Kang, S.; Horvath, T.L.; Fahmy, T.M.; Crawford, P.A.; Biragyn, A.; Alnemri, E.; Dixit, V.D. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015, 21, 263–9. [Google Scholar] [CrossRef] [PubMed]
- Dewanjee, S.; Vallamkondu, J.; Kalra, R.S.; John, A.; Reddy, P.H.; Kandimalla, R. Autophagy in the diabetic heart: A potential pharmacotherapeutic target in diabetic cardiomyopathy. Ageing Res Rev. 2021, 68, 101338. [Google Scholar] [CrossRef] [PubMed]
- Packer, M. Autophagy stimulation and intracellular sodium reduction as mediators of the cardioprotective effect of sodium-glucose cotransporter 2 inhibitors. Eur J Heart Fail. 2020, 22, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Adingupu, D.D.; Göpel, S.O.; Grönros, J.; Behrendt, M.; Sotak, M.; Miliotis, T.; Dahlqvist, U.; Gan, L.M.; Jönsson-Rylander, A.C. SGLT2 inhibition with empagliflozin improves coronary microvascular function and cardiac contractility in prediabetic ob/ob-/- mice. Cardiovasc Diabetol. 2019, 18, 16. [Google Scholar] [CrossRef] [PubMed]
- Suhrs, H.E.; Nilsson, M.; Bové, K.B.; Zander, M.; Prescott, E. Effect of empagliflozin on coronary microvascular function in patients with type 2 diabetes mellitus-A randomized, placebo-controlled cross-over study. PLoS One. 2022, 17, e0263481. [Google Scholar] [CrossRef] [PubMed]
- Leccisotti, L.; Cinti, F.; Sorice, G.P.; D’Amario, D.; Lorusso, M.; Guzzardi, M.A.; Mezza, T.; Gugliandolo, S.; Cocchi, C.; Capece, U.; Indovina, L.; Ferraro, P.M.; Iozzo, P.; Crea, F.; Giordano, A.; Giaccari, A. Dapagliflozin improves myocardial flow reserve in patients with type 2 diabetes: the DAPAHEART Trial: a preliminary report. Cardiovasc Diabetol. 2022, 21, 173. [Google Scholar] [CrossRef]
- Osto, E.; Bonacina, F.; Pirillo, A.; Norata, G.D. Neutral effect of SGLT2 inhibitors on lipoprotein metabolism: From clinical evidence to molecular mechanisms. Pharmacol Res. 2023, 188, 106667. [Google Scholar] [CrossRef]
- Sánchez-García, A.; Simental-Mendía, M.; Millán-Alanís, J.M.; Simental-Mendía, L.E. Effect of sodium-glucose co-transporter 2 inhibitors on lipid profile: A systematic review and meta-analysis of 48 randomized controlled trials. Pharmacol Res. 2020, 160, 105068. [Google Scholar] [CrossRef]
- Storgaard, H.; Gluud, L.L.; Bennett, C.; Grøndahl, M.F.; Christensen, M.B.; Knop, F.K.; Vilsbøll, T. Benefits and Harms of Sodium-Glucose Co-Transporter 2 Inhibitors in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. PLoS One. 2016, 11, e0166125. [Google Scholar] [CrossRef]
- Langslet, G.; Zinman, B.; Wanner, C.; Hantel, S.; Espadero, R.M.; Fitchett, D.; Johansen, O.E. Cardiovascular outcomes and LDL-cholesterol levels in EMPA-REG OUTCOME®. Diab Vasc Dis Res. 2020, 17, 1479164120975256. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhu, D.; Wang, S.; Jiang, A.; Li, F. Dapagliflozin Attenuates Cardiac Remodeling in Mice Model of Cardiac Pressure Overload. Am J Hypertens. 2019, 32, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lin, X.; Chu, Y.; Chen, X.; Du, H.; Zhang, H.; Xu, C.; Xie, H.; Ruan, Q.; Lin, J.; Liu, J.; Zeng, J.; Ma, K.; Chai, D. Dapagliflozin: a sodium-glucose cotransporter 2 inhibitor, attenuates angiotensin II-induced cardiac fibrotic remodeling by regulating TGFβ1/Smad signaling. Cardiovasc Diabetol. 2021, 20, 121. [Google Scholar] [CrossRef] [PubMed]
- Salah, H.M.; Verma, S.; Santos-Gallego, C.G.; Bhatt, A.S.; Vaduganathan, M.; Khan, M.S.; Lopes, R.D.; Al’Aref, S.J.; McGuire, D.K.; Fudim, M. Sodium-Glucose Cotransporter 2 Inhibitors and Cardiac Remodeling. J Cardiovasc Transl Res. 2022, 15, 944–956. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Zhang, M.; Suo, M.; Liu, D.; Wang, X.; Liu, M.; Pan, J.; Jin, T.; An, F. Dapagliflozin alleviates cardiac fibrosis through suppressing EndMT and fibroblast activation via AMPKα/TGF-β/Smad signalling in type 2 diabetic rats. J Cell Mol Med. 2021, 25, 7642–7659. [Google Scholar] [CrossRef] [PubMed]
- Pabel, S.; Hamdani, N.; Luedde, M.; Sossalla, S. SGLT2 Inhibitors and Their Mode of Action in Heart Failure-Has the Mystery Been Unravelled? Curr Heart Fail Rep. 2021, 18, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Mazer, C.D.; Yan, A.T.; Mason, T.; Garg, V.; Teoh, H.; Zuo, F.; Quan, A.; Farkouh, M.E.; Fitchett, D.H.; Goodman, S.G.; Goldenberg, R.M.; Al-Omran, M.; Gilbert, R.E.; Bhatt, D.L.; Leiter, L.A.; Jüni, P.; Zinman, B.; Connelly, K.A. Effect of Empagliflozin on Left Ventricular Mass in Patients With Type 2 Diabetes Mellitus and Coronary Artery Disease: The EMPA-HEART CardioLink-6 Randomized Clinical Trial. Circulation. 2019, 140, 1693–1702. [Google Scholar] [CrossRef]
- Lee, M.M.Y.; Brooksbank, K.J.M.; Wetherall, K.; Mangion, K.; Roditi, G.; Campbell, R.T.; Berry, C.; Chong, V.; Coyle, L.; Docherty, K.F.; Dreisbach, J.G.; Labinjoh, C.; Lang, N.N.; Lennie, V.; McConnachie, A.; Murphy, C.L.; Petrie, C.J.; Petrie, J.R.; Speirits, I.A.; Sourbron, S.; Welsh, P.; Woodward, R.; Radjenovic, A.; Mark, P.B.; McMurray, J.J.V.; Jhund, P.S.; Petrie, M.C.; Sattar, N. Effect of Empagliflozin on Left Ventricular Volumes in Patients With Type 2 Diabetes, or Prediabetes, and Heart Failure With Reduced Ejection Fraction (SUGAR-DM-HF). Circulation. 2021, 143, 516–525. [Google Scholar] [CrossRef]
- Dhingra, N.K.; Mistry, N.; Puar, P.; Verma, R.; Anker, S.; Mazer, C.D.; Verma, S. SGLT2 inhibitors and cardiac remodelling: a systematic review and meta-analysis of randomized cardiac magnetic resonance imaging trials. ESC Heart Fail. 2021, 8, 4693–4700. [Google Scholar] [CrossRef]
- Gamaza-Chulián, S.; Díaz-Retamino, E.; González-Testón, F.; Gaitero, J.C.; Castillo, M.J.; Alfaro, R.; Rodríguez, E.; González-Caballero, E.; Martín-Santana, A. Effect of sodium-glucose cotransporter 2 (SGLT2) inhibitors on left ventricular remodelling and longitudinal strain: a prospective observational study. BMC Cardiovasc Disord. 2021, 21, 456. [Google Scholar] [CrossRef]
- Tanaka, H.; Soga, F.; Tatsumi, K.; Mochizuki, Y.; Sano, H.; Toki, H.; Matsumoto, K.; Shite, J.; Takaoka, H.; Doi, T.; Hirata, K.I. Positive effect of dapagliflozin on left ventricular longitudinal function for type 2 diabetic mellitus patients with chronic heart failure. Cardiovasc Diabetol. 2020, 19, 6. [Google Scholar] [CrossRef] [PubMed]
| SGLT2 inhibitor | Population | Timing | Primary outcomes | Results | |
|---|---|---|---|---|---|
| SOLOIST-WHF | Sotagliflozin 200-400 mg | Acute HF and diabetes | In hospital- within 3 days of discharge | Cardiovascular death and urgent visit/hospitalization for HF | Lower urgent visit/hospitalization for HF in the sotagliflozin group |
| EMPA-RESPONSE | Empagliflozin 10 mg | Acute HF | Within 24h of presentation | Dyspnea score, diuretic response, NTproBNP, LOS | Empagliflozin had no effect on primary outcome* |
| Empagliflozin 10 mg | Acute HF | In hospital | Clinical benefit** | Clinical benefit favors empagliflozin | |
| EMPAG-HF | Empagliflozin 25 mg | Acute HF | Within 12h | Urine output | Empagliflozin increased urine output |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




