Submitted:
24 April 2023
Posted:
25 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Advanced oxidation/reduction processes
2.1. ARP
2.2.1. Ultraviolet light (254 nm) systems
2.2.2. Vacuum ultraviolet light (185 nm) systems
2.2. AOP
2.2.1. Ozone
2.2.2. UV degradation techniques
2.2.3. Heterogeneous photocatalysis
3. Nanomaterials based AOP and ARP
3.1. Previous reviews
3.2. Advances in concentration strategies of PFAS
3.3. Advances in PFAS treatment technologies
4. Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Vo, H.N.P.; Ngo, H.H.; Guo, W.; Nguyen, T.M.H.; Li, J.; Liang, H.; Deng, L.; Chen, Z.; Nguyen, T.A.H. Poly-and perfluoroalkyl substances in water and wastewater: A comprehensive review from sources to remediation. J. Water Process. Eng. 2020, 36, 101393. [Google Scholar] [CrossRef]
- Domingo, J.L.; Nadal, M. Human exposure to per- and polyfluoroalkyl substances (PFAS) through drinking water: A review of the recent scientific literature. Environ. Res. 2019, 177, 108648. [Google Scholar] [CrossRef] [PubMed]
- Available online: http://www.pops.int/TheConvention/ThePOPs/tabid/673/Default.aspx (accessed on 19 March 2023).
- Teymoorian, T.; Munoz, G.; Duy, S.V.; Liu, J.; Sauvé, S. Tracking PFAS in Drinking Water: A Review of Analytical Methods and Worldwide Occurrence Trends in Tap Water and Bottled Water. ACS ES&T Water 2023, 3, 246–261. [Google Scholar] [CrossRef]
- Wanninayake, D.M. Comparison of currently available PFAS remediation technologies in water: A review. J. Environ. Manag. 2021, 283, 111977. [Google Scholar] [CrossRef]
- Leung, S.C.E.; Shukla, P.; Chen, D.; Eftekhari, E.; An, H.; Zare, F.; Ghasemi, N.; Zhang, D.; Nguyen, N.-T.; Li, Q. Emerging technologies for PFOS/PFOA degradation and removal: A review. Sci. Total. Environ. 2022, 827, 153669. [Google Scholar] [CrossRef] [PubMed]
- Araújo, R.G.; Rodríguez-Hernandéz, J.A.; González-González, R.B.; Macias-Garbett, R.; Martínez-Ruiz, M.; Reyes-Pardo, H.; Martínez, S.A.H.; Parra-Arroyo, L.; Melchor-Martínez, E.M.; Sosa-Hernández, J.E.; et al. Detection and Tertiary Treatment Technologies of Poly-and Perfluoroalkyl Substances in Wastewater Treatment Plants. Front. Environ. Sci. 2022, 10, 864894. [Google Scholar] [CrossRef]
- Ateia, M.; Alsbaiee, A.; Karanfil, T.; Dichtel, W.R. Efficient PFAS Removal by Amine-Functionalized Sorbents: Critical Review of the Current Literature. Environ. Sci. Technol. Lett. 2019, 6, 688–695. [Google Scholar] [CrossRef]
- Park, M.; Wu, S.; Lopez, I.J.; Chang, J.Y.; Karanfil, T.; Snyder, S.A. Adsorption of perfluoroalkyl substances (PFAS) in groundwater by granular activated carbons: Roles of hydrophobicity of PFAS and carbon characteristics. Water Res. 2020, 170, 115364. [Google Scholar] [CrossRef]
- Militao, I.M.; Roddick, F.A.; Bergamasco, R.; Fan, L. Removing PFAS from aquatic systems using natural and renewable material-based adsorbents: A review. J. Environ. Chem. Eng. 2021, 9, 105271. [Google Scholar] [CrossRef]
- Yadav, S.; Ibrar, I.; Al-Juboori, R.A.; Singh, L.; Ganbat, N.; Kazwini, T.; Karbassiyazdi, E.; Samal, A.K.; Subbiah, S.; Altaee, A. Updated review on emerging technologies for PFAS contaminated water treatment. Chem. Eng. Res. Des. 2022, 182, 667–700. [Google Scholar] [CrossRef]
- Dixit, F.; Dutta, R.; Barbeau, B.; Berube, P.; Mohseni, M. PFAS removal by ion exchange resins: A review. Chemosphere 2021, 272, 129777. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; He, L.; Xue, J.; Ma, Y.; Xie, Z.; Wu, L.; Huang, M.; Zhang, Z. Persulfate-based degradation of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) in aqueous solution: Review on influences, mechanisms and prospective. J. Hazard. Mater. 2020, 393, 122405. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, X.; Pan, Z.; Li, X.; Ling, Y.; Li, L. Efficient degradation of perfluorooctanoic acid (PFOA) by photocatalytic ozonation. Chem. Eng. J. 2016, 296, 329–334. [Google Scholar] [CrossRef]
- Kim, J.; Xin, X.; Mamo, B.T.; Hawkins, G.L.; Li, K.; Chen, Y.; Huang, Q.; Huang, C.-H. Occurrence and Fate of Ultrashort-Chain and Other Per- and Polyfluoroalkyl Substances (PFAS) in Wastewater Treatment Plants. ACS ES&T Water 2022, 2, 1380–1390. [Google Scholar] [CrossRef]
- Manz, K.E.; Kulaots, I.; Greenley, C.A.; Landry, P.J.; Lakshmi, K.; Woodcock, M.J.; Hellerich, L.; Bryant, J.D.; Apfelbaum, M.; Pennell, K.D. Low-temperature persulfate activation by powdered activated carbon for simultaneous destruction of perfluorinated carboxylic acids and 1,4-dioxane. J. Hazard. Mater. 2023, 442, 129966. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Zhu, Y.; Duan, J.; Xia, Y.; Tong, T.; Zhang, L.; Zhao, D. Enhanced photocatalytic degradation of perfluorooctanoic acid using carbon-modified bismuth phosphate composite: Effectiveness, material synergy and roles of carbon. Chem. Eng. J. 2020, 395, 124991. [Google Scholar] [CrossRef]
- Xia, C.; Lim, X.; Yang, H.; Goodson, B.M.; Liu, J. Degradation of per- and polyfluoroalkyl substances (PFAS) in wastewater effluents by photocatalysis for water reuse. J. Water Process. Eng. 2022, 46, 102556. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, D.; Liang, Y. Nanotechnology in remediation of water contaminated by poly- and perfluoroalkyl substances: A review. Environ. Pollut. 2019, 247, 266–276. [Google Scholar] [CrossRef]
- Zhang, J.; Pang, H.; Gray, S.; Ma, S.; Xie, Z.; Gao, L. PFAS removal from wastewater by in-situ formed ferric nanoparticles: Solid phase loading and removal efficiency. J. Environ. Chem. Eng. 2021, 9, 105452. [Google Scholar] [CrossRef]
- Birch, Q.T.; E Birch, M.; Nadagouda, M.N.; Dionysiou, D.D. Nano-enhanced treatment of per-fluorinated and poly-fluorinated alkyl substances (PFAS). Curr. Opin. Chem. Eng. 2022, 35, 100779. [Google Scholar] [CrossRef]
- Yin, S. ; Villagrán. D. Design of nanomaterials for the removal of per- and poly-fluoroalkyl substances (PFAS) in water: Strategies, mechanisms, challenges, and opportunities. Science of the Total Environment 2022, 831, 154939. [Google Scholar]
- Duinslaeger, N.; Radjenovic, J. Electrochemical degradation of per- and polyfluoroalkyl substances (PFAS) using low-cost graphene sponge electrodes. Water Res. 2022, 213, 118148. [Google Scholar] [CrossRef]
- C Fang, C.; Megharaj, M.; Naidu, R. Electrochemical Advanced Oxidation Processes (EAOP) to degrade per- and polyflluoroalkyl substances (PFASs). Journal of Advanced Oxidation Technologies. 2017, 20170014. [Google Scholar]
- Trojanowicza, M.; Bojanowska-Czajkaa, A.; Bartosiewicza, I.; Kulisa, K. Advanced Oxidation/Reduction Processes treatment for aqueous perfluorooctanoate (PFOA) and perfluorooctanesulfonate (PFOS) – A review of recent advances. Chemical Engineering Journal 2018, 336, 170–199. [Google Scholar] [CrossRef]
- Saleh, N.B.; Khalid, A.; Tian, Y.; Ayres, C.; Sabaraya, I.V.; Pietari, J.; Hanigan, D.; Chowdhury, I.; Apul, O.G. Removal of poly- and per-fluoroalkyl substances from aqueous systems by nano-enabled water treatment strategies. Environ. Sci. Water Res. Technol. 2019, 5, 198–208. [Google Scholar] [CrossRef]
- Cui, J.; Gao, P.; Deng, Y. Destruction of Per- and Polyfluoroalkyl Substances (PFAS) with Advanced Reduction Processes (ARPs): A Critical Review. Environ. Sci. Technol. 2020, 54, 3752–3766. [Google Scholar] [CrossRef] [PubMed]
- Leonello, D.; Fendrich, M.A.; Parrino, F.; Patel, N.; Orlandi, M.; Miotello, A. Light-Induced Advanced Oxidation Processes as PFAS Remediation Methods: A Review. Appl. Sci. 2021, 11, 8458. [Google Scholar] [CrossRef]
- Barisci, S.; Suri, R. Occurrence and removal of poly/perfluoroalkyl substances (PFAS) in municipal and industrial wastewater treatment plants. Water Sci. Technol. 2021, 84, 3442–3468. [Google Scholar] [CrossRef]
- Venkatesan, A.K.; Lee, C.-S.; Gobler, C.J. Hydroxyl-radical based advanced oxidation processes can increase perfluoroalkyl substances beyond drinking water standards: Results from a pilot study. Sci. Total. Environ. 2022, 847, 157577. [Google Scholar] [CrossRef]
- Alalm, M.G.; Boffito, D.C. Mechanisms and pathways of PFAS degradation by advanced oxidation and reduction processes: A critical review. Chem. Eng. J. 2022, 450, 138352. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Vaccari, M.; Prasad, S.; Rtimi, S. Recent progress and challenges on the removal of per- and poly-fluoroalkyl substances (PFAS) from contaminated soil and water. Environ. Sci. Pollut. Res. 2022, 29, 58405–58428. [Google Scholar] [CrossRef] [PubMed]
- Eun, H.; Shimamura, K.; Asano, T.; Yamazaki, E.; Taniyasu, S.; Yamashita, N. Removal of perfluoroalkyl substances from water by activated carbons: Adsorption of perfluorooctane sulfonate and perfluorooctanoic acid. Environ. Monit. Contam. Res. 2022, 2, 88–93. [Google Scholar] [CrossRef]
- Riegel, M.; Haist-Gulde, B.; Sacher, F. Sorptive removal of short-chain perfluoroalkyl substances (PFAS) during drinking water treatment using activated carbon and anion exchanger. Environ. Sci. Eur. 2023, 35, 1–12. [Google Scholar] [CrossRef]
- Zango, Z.U.; Khoo, K.S.; Garba, A.; Kadir, H.A.; Usman, F.; Zango, M.U.; Da Oh, W.; Lim, J.W. A review on superior advanced oxidation and photocatalytic degradation techniques for perfluorooctanoic acid (PFOA) elimination from wastewater. Environ. Res. 2023, 221, 115326. [Google Scholar] [CrossRef] [PubMed]
- Vellanki, B.P.; Batchelor, B.; Abdel-Wahab, A.; Jung, B.; Safan, A.; Duan, Y.; Kaushik, V.; Luo, G.; Merino, N.; Qu, Y.; et al. Advanced Reduction Processes: A New Class of Treatment Processes. Environ. Eng. Sci. 2013, 30, 264–271. [Google Scholar] [CrossRef]
- Gu, Y.; Liu, T.; Zhang, Q.; Dong, W. Efficient decomposition of perfluorooctanoic acid by a high photon flux UV/sulfite process: Kinetics and associated toxicity. Chem. Eng. J. 2017, 326, 1125–1133. [Google Scholar] [CrossRef]
- Ren, Z.; Bergmann, U.; Leiviskä, T. Reductive degradation of perfluorooctanoic acid in complex water matrices by using the UV/sulfite process. Water Res. 2021, 205, 117676. [Google Scholar] [CrossRef]
- Cheng, J.-H.; Liang, X.-Y.; Yang, S.-W.; Hu, Y.-Y. Photochemical defluorination of aqueous perfluorooctanoic acid (PFOA) by VUV/Fe3+ system. Chem. Eng. J. 2014, 239, 242–249. [Google Scholar] [CrossRef]
- Gu, Y.; Liu, T.; Wang, H.; Han, H.; Dong, W. Hydrated electron based decomposition of perfluorooctane sulfonate (PFOS) in the VUV/sulfite system. Sci. Total. Environ. 2017, 607-608, 541–548. [Google Scholar] [CrossRef]
- Cardoso, I.M.F.; Cardoso, R.M.F.; da Silva, J.C.G.E. Advanced Oxidation Processes Coupled with Nanomaterials for Water Treatment. Nanomaterials 2021, 11, 2045. [Google Scholar] [CrossRef]
- Cardoso, R.M.F.; Cardoso, I.M.F.; da Silva, L.P.; Esteves da Silva, J.C.G. Copper(II)-Doped Carbon Dots as Catalyst for Ozone Degradation of Textile Dyes. Nanomaterials 2022, 12, 1211. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, I.M.F.; Cardoso, R.M.F.; Pinto da Silva, L.; Esteves da Silva, J.C.G. UV-Based Advanced Oxidation Processes of Remazol Brilliant Blue R Dye Catalyzed by Carbon Dots. Nanomaterials 2022, 12, 2116. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.Y.-C.; Panchangam, S.C.; Chang, C.-Y.; Hong, P.A.; Hsueh, H.-F. Removal of perfluorooctanoic acid and perfluorooctane sulfonate via ozonation under alkaline condition. J. Hazard. Mater. 2012, 243, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Schroder, H.F.; Meesters, R.J.W. Stability of fluorinated surfactants in advanced oxidation processes— A follow up of degradation products using flow injection–mass spectrometry, liquid chromatography–mass spectrometry and liquid chromatography–multiple stage mass spectrometry. Journal of Chromatography A, 2005, 1082, 110–119. [Google Scholar] [CrossRef]
- Franke, V.; Schäfers, M.D.; Lindberg, J.J.; Ahrens, L. Removal of per- and polyfluoroalkyl substances (PFASs) from tap water using heterogeneously catalyzed ozonation. Environ. Sci. Water Res. Technol. 2019, 5, 1887–1896. [Google Scholar] [CrossRef]
- Dai, X.; Xie, Z.; Dorian, B.; Gray, S.; Zhang, J. Comparative study of PFAS treatment by UV, UV/ozone, and fractionations with air and ozonated air. Environ. Sci. Water Res. Technol. 2019, 5, 1897–1907. [Google Scholar] [CrossRef]
- Merényi, G.; Lind, J.; Naumov, S.; von Sonntag, C. Reaction of Ozone with Hydrogen Peroxide (Peroxone Process): A Revision of Current Mechanistic Concepts Based on Thermokinetic and Quantum-Chemical Considerations. Environ. Sci. Technol. 2010, 44, 3505–3507. [Google Scholar] [CrossRef] [PubMed]
- Rekhate, C.V.; Srivastava, J.K. Recent advances in ozone-based advanced oxidation processes for treatment of wastewater- A review. Chem. Eng. J. Adv. 2020, 3, 100031. [Google Scholar] [CrossRef]
- Sauleda, R.; Brillas, E. Mineralization of aniline and 4-chlorophenol in acidic solution by ozonation catalyzed with Fe2+ and UVA light. Appl. Catal. B: Environ. 2001, 29, 135–145. [Google Scholar] [CrossRef]
- Vatankhah, H.; Tajdini, B.; Milstead, R.P.; Clevenger, E.; Murray, C.; Knappe, D.; Remucal, C.K.; Bellona, C. Impact of ozone-biologically active filtration on the breakthrough of Perfluoroalkyl acids during granular activated carbon treatment of municipal wastewater effluent. Water Res. 2022, 223, 118988. [Google Scholar] [CrossRef]
- Javed, H.; Lyu, C.; Sun, R.; Zhang, D.; Alvarez, P.J. Discerning the inefficacy of hydroxyl radicals during perfluorooctanoic acid degradation. Chemosphere 2020, 247, 125883. [Google Scholar] [CrossRef]
- Giri, R.R.; Ozaki, H.; Okada, T.; Taniguchi, S.; Takanami, R. Factors influencing UV photodecomposition of perfluorooctanoic acid in water. Chem. Eng. J. 2012, 180, 197–203. [Google Scholar] [CrossRef]
- Umar, M. Reductive and Oxidative UV Degradation of PFAS—Status, Needs and Future Perspectives. Water 2021, 13, 3185. [Google Scholar] [CrossRef]
- Leonello, D.; Fendrich, M.A.; Parrino, F.; Patel, N.; Orlandi, M.; Miotello, A. Light-Induced Advanced Oxidation Processes as PFAS Remediation Methods: A Review. Appl. Sci. 2021, 11, 8458. [Google Scholar] [CrossRef]
- Hori, H.; Hayakawa, E.; Einaga, H.; Kutsuna, S.; Koike, K.; Ibusuki, T.; Kiatagawa, H.; Arakawa, R. Decomposition of Environmentally Persistent Perfluorooctanoic Acid in Water by Photochemical Approaches. Environ. Sci. Technol. 2004, 38, 6118–6124. [Google Scholar] [CrossRef] [PubMed]
- Hori, H.; Yamamoto, A.; Hayakawa, E.; Taniyasu, S.; Yamashita, N.; Kutsuna, S.; Kiatagawa, H.; Arakawa, R. Efficient Decomposition of Environmentally Persistent Perfluorocarboxylic Acids by Use of Persulfate as a Photochemical Oxidant. Environ. Sci. Technol. 2005, 39, 2383–2388. [Google Scholar] [CrossRef] [PubMed]
- Hori, H.; Murayama, M.; Inoue, N.; Ishida, K.; Kutsuna, S. Efficient mineralization of hydroperfluorocarboxylic acids with persulfate in hot water. Catal. Today 2010, 151, 131–136. [Google Scholar] [CrossRef]
- Liu, C.; Higgins, C.; Wang, F.; Shih, K. Effect of temperature on oxidative transformation of perfluorooctanoic acid (PFOA) by persulfate activation in water. Sep. Purif. Technol. 2012, 91, 46–51. [Google Scholar] [CrossRef]
- Yang, S.; Cheng, J.; Sun, J.; Hu, Y.; Liang, X. Defluorination of Aqueous Perfluorooctanesulfonate by Activated Persulfate Oxidation. PLOS ONE 2013, 8, e74877. [Google Scholar] [CrossRef]
- Park, S.; Lee, L.S.; Medina, V.F.; Zull, A.; Waisner, S. Heat-activated persulfate oxidation of PFOA, 6:2 fluorotelomer sulfonate, and PFOS under conditions suitable for in-situ groundwater remediation. Chemosphere 2016, 145, 376–383. [Google Scholar] [CrossRef]
- Gatto, S.; Sansotera, M.; Persico, F.; Gola, M.; Pirola, C.; Panzeri, W.; Navarrini, W.; Bianchi, C.L. Surface fluorination on TiO2 catalyst induced by photodegradation of perfluorooctanoic acid. Catal. Today 2015, 241, 8–14. [Google Scholar] [CrossRef]
- Chen, M.-J.; Lo, S.-L.; Lee, Y.-C.; Huang, C.-C. Photocatalytic decomposition of perfluorooctanoic acid by transition-metal modified titanium dioxide. J. Hazard. Mater. 2015, 288, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wei, Z.; He, K.; Blaney, L.; Cheng, X.; Xu, T.; Liu, W.; Zhao, D. A concentrate-and-destroy technique for degradation of perfluorooctanoic acid in water using a new adsorptive photocatalyst. Water Res. 2020, 185, 116219. [Google Scholar] [CrossRef] [PubMed]
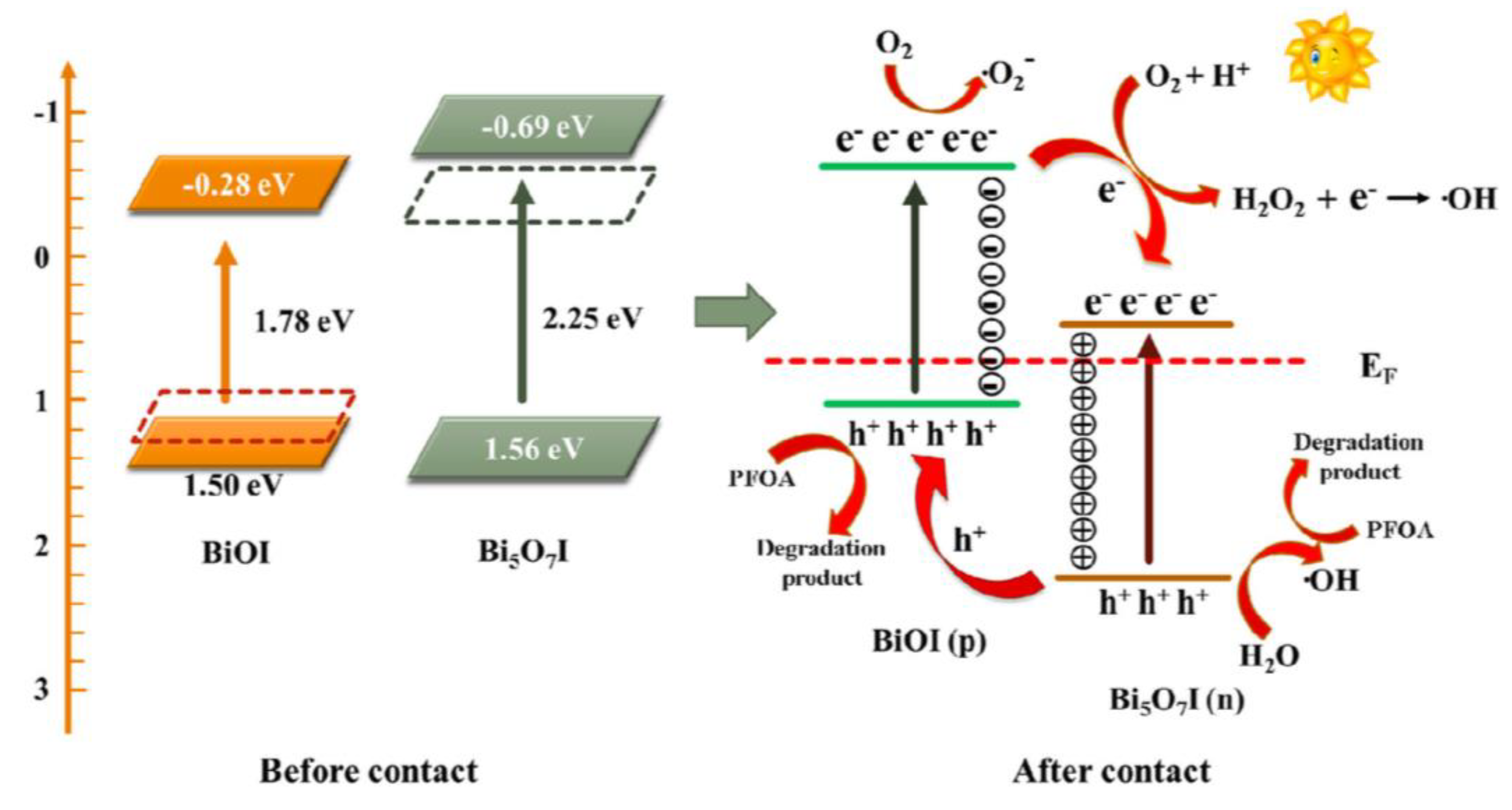
- Wang, J.; Cao, C.; Wang, Y.; Wang, Y.; Sun, B.; Zhu, L. In situ preparation of p-n BiOI@Bi5O7I heterojunction for enhanced PFOA photocatalytic degradation under simulated solar light irradiation. Chemical Engineering Journal 2020, 391, 123530. [Google Scholar] [CrossRef]
- Yang, Y.; Ji, W.; Li, X.; Zheng, Z.; Bi, F.; Yang, M.; Xu, J.; Zhang, X. Insights into the degradation mechanism of perfluorooctanoic acid under visible-light irradiation through fabricating flower-shaped Bi5O7I/ZnO n-n heterojunction microspheres. Chem. Eng. J. 2021, 420, 129934. [Google Scholar] [CrossRef]
- Kurian, M. Advanced oxidation processes and nanomaterials -a review. Clean. Eng. Technol. 2021, 2, 100090. [Google Scholar] [CrossRef]
- Arora, B.; Attri, P. Carbon Nanotubes (CNTs): A Potential Nanomaterial for Water Purification. J. Compos. Sci. 2020, 4, 135. [Google Scholar] [CrossRef]
- Steven, J. Chow, S. J.; Croll, H.C.; Ojeda, N.; Klamerus, J.; Capelle, R.; Oppenheimer, J.; Jacangelo, J.G.; Schwab, K.J; Prasse, C. Comparative investigation of PFAS adsorption onto activated carbon and anion exchange resins during long-term operation of a pilot treatment plant. Water Research 2022, 226, 119198. [Google Scholar]
- Das, R.; Leo, B.F.; Murphy, F. The Toxic Truth About Carbon Nanotubes in Water Purification: a Perspective View. Nanoscale Res. Lett. 2018, 13, 183. [Google Scholar] [CrossRef]
- Esteves da Silva, J.C.G.; Gonçalves, H. Analytical and bioanalytical applications of carbon dots. TrAC - Trends in Analytical Chemistry 2011, 30, 1327–1336. [Google Scholar] [CrossRef]
- Meng, W.; Bai, X.; Wang, B.; Liu, Z.; Lu, S.; Yang, B. Biomass-Derived Carbon Dots and Their Applications. Energy Environ. Mater. 2019, 2, 172–192. [Google Scholar] [CrossRef]
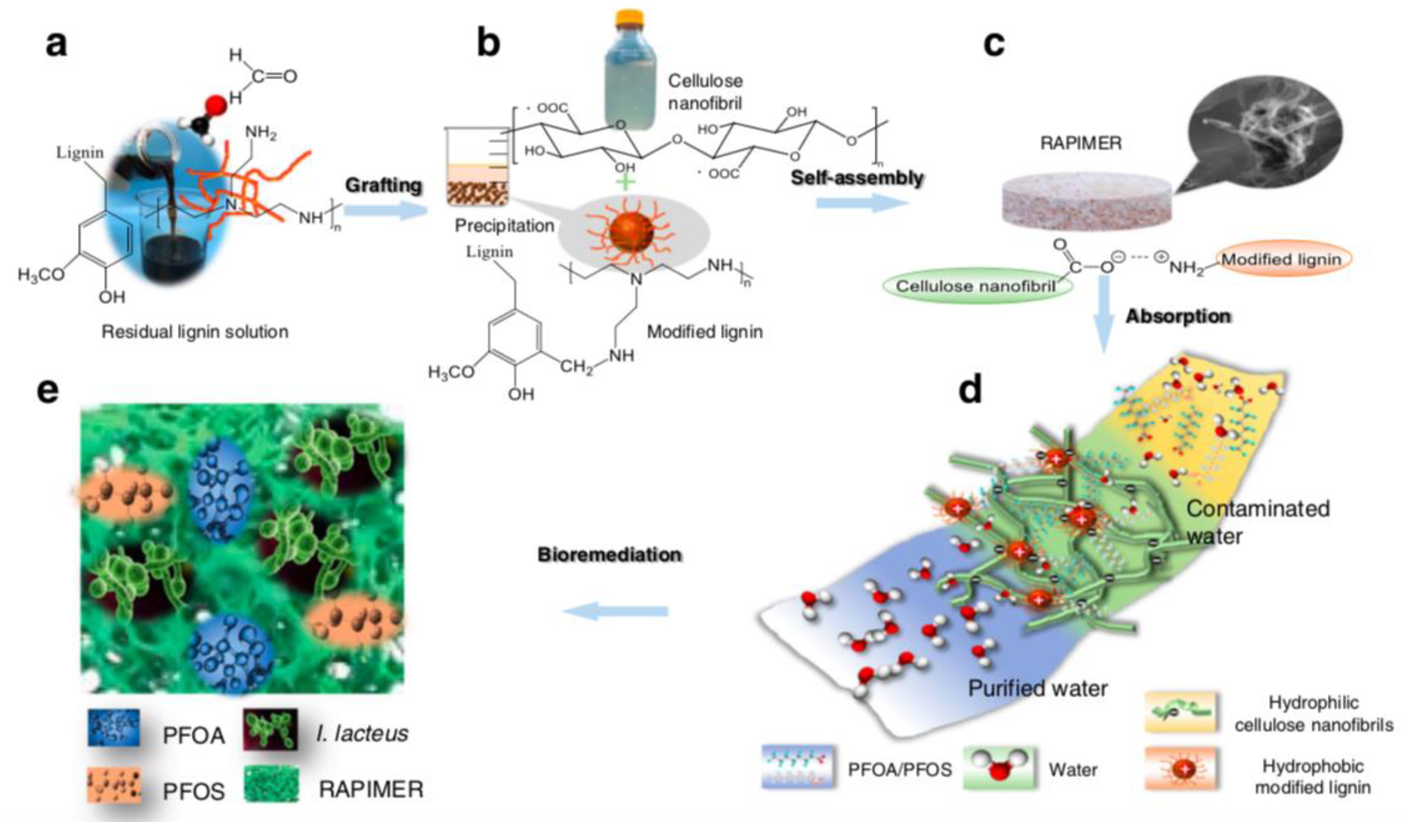
- Li, J.; Li, X.; Da, Y.; Yu, J.; Long, B.; Zhang, P.; Bakker, C.; McCarl, B.A.; Yuan, J.S.; Dai, S.Y. Sustainable environmental remediation via biomimetic multifunctional lignocellulosic nano-framework. Nat. Commun. 2022, 13, 4386. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.R.; Duarte, M.S.; Alves, M.M.; Pereira, L. Bioremediation of Perfluoroalkyl Substances (PFAS) by Anaerobic Digestion: Effect of PFAS on Different Trophic Groups and Methane Production Accelerated by Carbon Materials. Molecules 2022, 27, 1895. [Google Scholar] [CrossRef] [PubMed]
- Douna, B.K.; Yousefi, H. Removal of PFAS by Biological Methods. Asian Pac. J. Environ. Cancer 2023, 6, 53–64. [Google Scholar] [CrossRef]
- Smith, S.J.; Wiberg, K.; McCleaf, P.; Ahrens, L. Pilot-Scale Continuous Foam Fractionation for the Removal of Per- and Polyfluoroalkyl Substances (PFAS) from Landfill Leachate. ACS ES&T Water 2022, 2, 841–851. [Google Scholar] [CrossRef]
- Wang, Y.; Ji, Y.; Tishchenko, V.; Huang, Q. Removing per- and polyfluoroalkyl substances (PFAS) in water by foam fractionation. Chemosphere 2023, 311, 137004. [Google Scholar] [CrossRef]
- Morrison, A.L.; Strezov, V.; Niven, R.K.; Taylor, M.P.; Wilson, S.P.; Wang, J.; Burns, D.J.; Murphy, P.J.C. Impact of Salinity and Temperature on Removal of PFAS Species from Water by Aeration in the Absence of Additional Surfactants: A Novel Application of Green Chemistry Using Adsorptive Bubble Fractionation. Ind. Eng. Chem. Res. 2023, 62, 5635–5645. [Google Scholar] [CrossRef]
- Jia, L.; Liu, W.; Cao, J.; Wu, Z.; Yang, C. Recovery of nanoparticles from wastewater by foam fractionation: Regulating bubble size distribution for strengthening foam drainage. J. Environ. Chem. Eng. 2021, 9, 105383. [Google Scholar] [CrossRef]
- Lei, X.; Lian, Q.; Zhang, X.; Karsili, T.K.; Holmes, W.; Chen, Y.; Zappi, M.E.; Gang, D.D. A review of PFAS adsorption from aqueous solutions: Current approaches, engineering applications, challenges, and opportunities. Environ. Pollut. 2023, 321, 121138. [Google Scholar] [CrossRef]
- Yin, S.; López, J.F.; Solís, J.J.C.; Wong, M.S.; Villagrán, D. Enhanced adsorption of PFOA with nano MgAl2O4@CNTs: influence of pH and dosage, and environmental conditions. Journal of Hazardous Materials Advances 2023, 9, 2023, 100252. [Google Scholar] [CrossRef]
- Karbassiyazdi, E.; Kasula, M.; Modak, S.; Pala, J.; Kalantari, M.; Altaee, A.; Esfahani, M.R.; Razmjou, A. A juxtaposed review on adsorptive removal of PFAS by metal-organic frameworks (MOFs) with carbon-based materials, ion exchange resins, and polymer adsorbents. Chemosphere 2023, 311, 136933. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Rentería-Gómez. ; Day, G.S.; Smith, M.F.; Yan, T.-H.; Ozdemir, R.O.K.; Gutierrez, O.; Sharma, V.K.; Ma, X.; Zhou, H.-C. Integrated Photocatalytic Reduction and Oxidation of Perfluorooctanoic Acid by Metal–Organic Frameworks: Key Insights into the Degradation Mechanisms. J. Am. Chem. Soc. 2022, 144, 11840–11850. [Google Scholar] [CrossRef]
- Chen, C.; Ma, Q.; Liu, F.; Gao, J.; Li, X.; Sun, S.; Yao, H.; Liu, C.; Young, J.; Zhang, W. Photocatalytically reductive defluorination of perfluorooctanoic acid (PFOA) using Pt/La2Ti2O7 nanoplates: Experimental and DFT assessment. J. Hazard. Mater. 2021, 419, 126452. [Google Scholar] [CrossRef]
- Wang, J.; Cao, C.; Zhang, Y.; Zhang, Y.; Zhu, L. Underneath mechanisms into the super effective degradation of PFOA by BiOF nanosheets with tunable oxygen vacancies on exposed (101) facets. Appl. Catal. B: Environ. 2021, 286, 119911. [Google Scholar] [CrossRef]
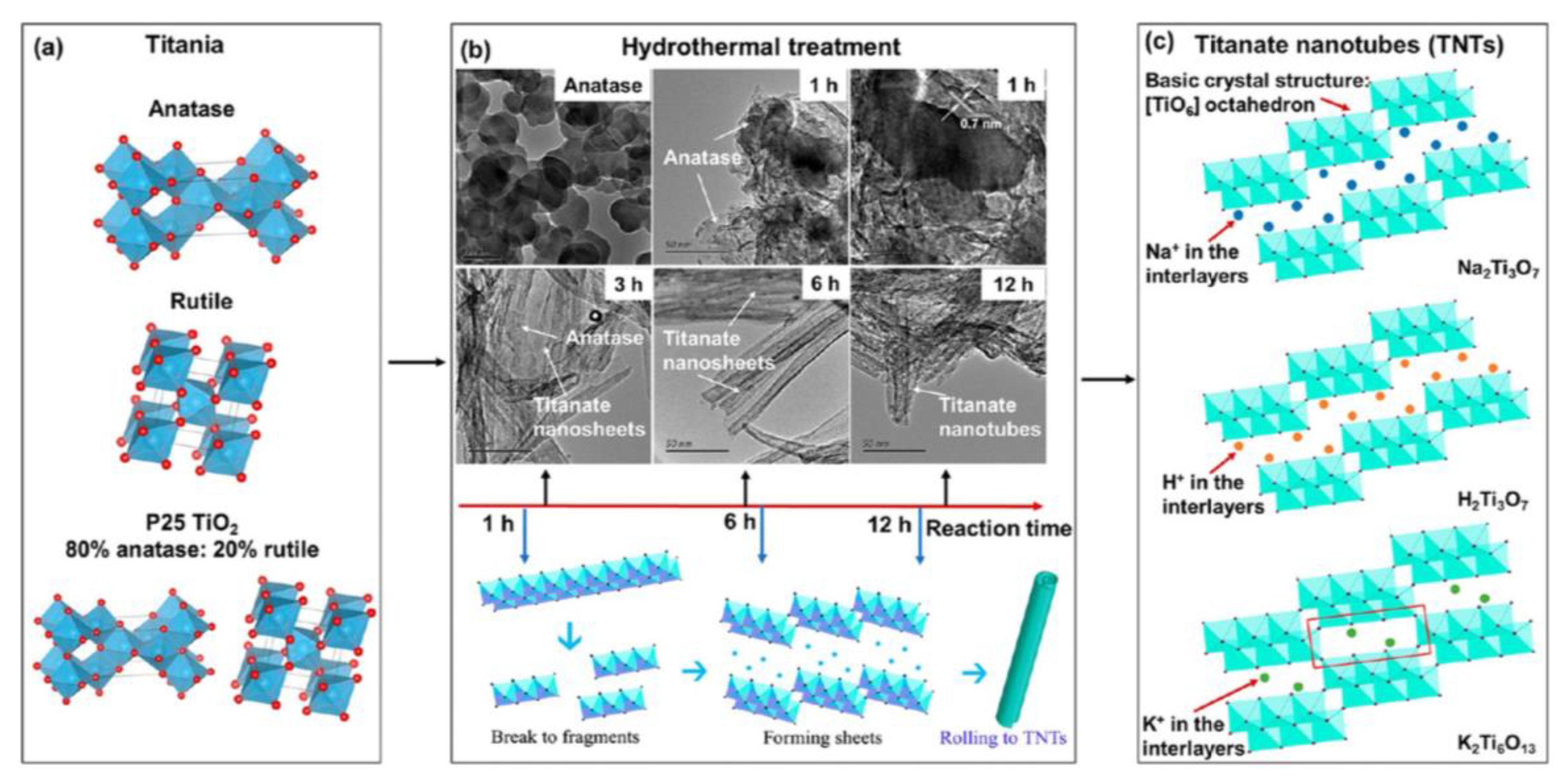
- Ji, H.; Ni, J.; Zhao, D.; Liu, W. Application of Titanate Nanotubes for Photocatalytic Decontamination in Water: Challenges and Prospects. ACS ES&T Eng. 2022, 2, 1015–1038. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, T.; Zhao, D.; Li, F.; Liu, W.; Wang, B.; An, B. Adsorption and solid-phase photocatalytic degradation of perfluorooctane sulfonate in water using gallium-doped carbon-modified titanate nanotubes. Chem. Eng. J. 2021, 421, 129676. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, T.; Zhao, D. Metal-doped carbon-supported/modified titanate nanotubes for perfluorooctane sulfonate degradation in water: Effects of preparation conditions, mechanisms, and parameter optimization. Sci. Total. Environ. 2022, 853, 158573. [Google Scholar] [CrossRef]
- Zhu, Y.; Ji, H.; He, K.; Blaney, L.; Xu, T.; Zhao, D. Photocatalytic degradation of GenX in water using a new adsorptive photocatalyst. Water Res. 2022, 220, 118650. [Google Scholar] [CrossRef]
- Kundu, S.; Radian, A. Surface confinement of per-fluoroalkyl substances on an iron-decorated clay-cyclodextrin composite enables rapid oxidation by hydroxyl radicals. Chem. Eng. J. 2021, 431, 134187. [Google Scholar] [CrossRef]
- Duan, L.; Wang, B.; Heck, K.N.; Clark, C.A.; Wei, J.; Wang, M.; Metz, J.; Wu, G.; Tsai, A.-L.; Guo, S.; et al. Titanium oxide improves boron nitride photocatalytic degradation of perfluorooctanoic acid. Chem. Eng. J. 2022, 448, 137735. [Google Scholar] [CrossRef]
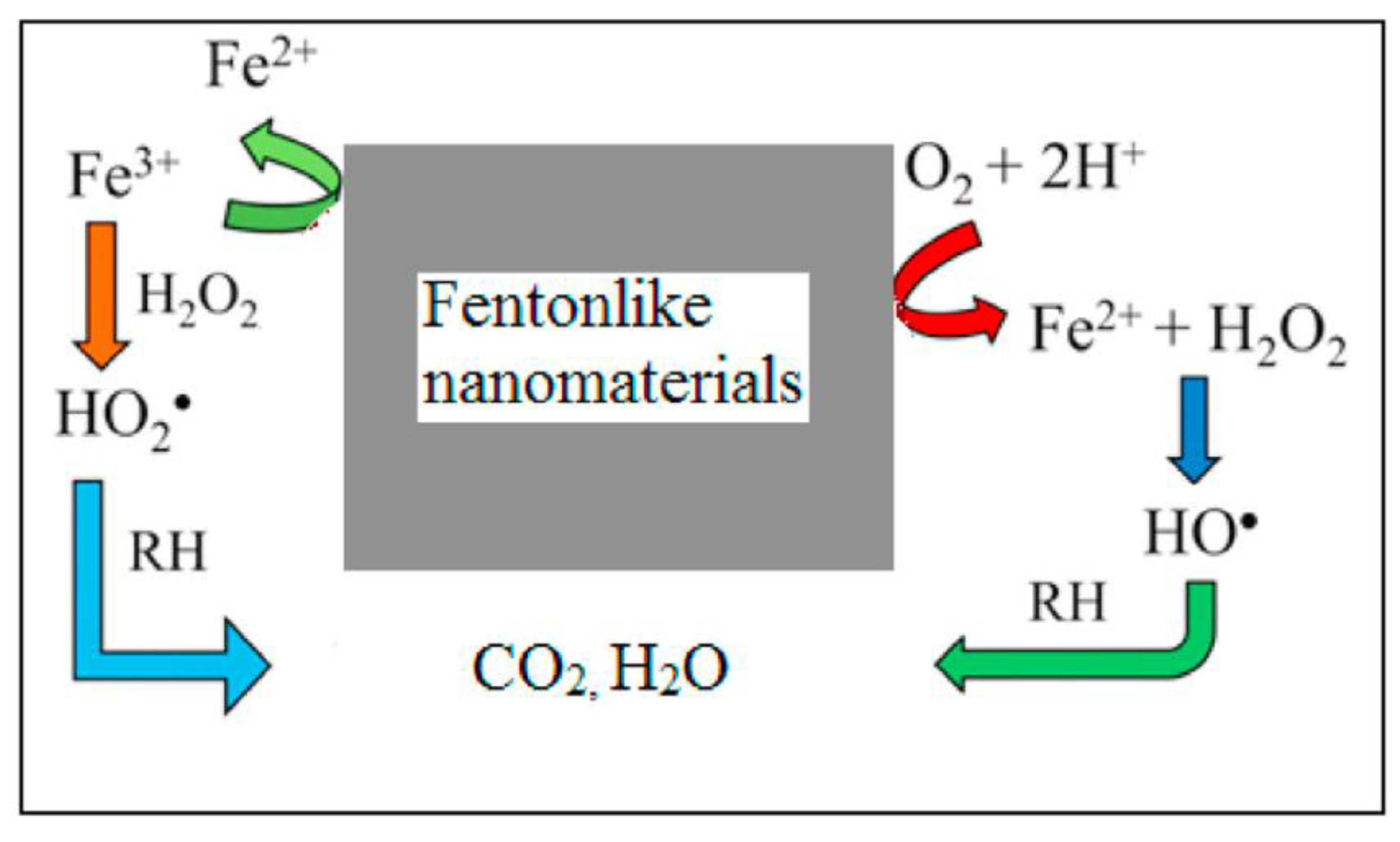
- Schlesinger, D.R.; McDermott, C.; Le, N.Q.; Ko, J.S.; Johnson, J.K.; Demirev, P.A.; Xia, Z. Destruction of per/poly-fluorinated alkyl substances by magnetite nanoparticle-catalyzed UV-Fenton reaction. Environ. Sci. Water Res. Technol. 2022, 8, 2732–2743. [Google Scholar] [CrossRef]
- Metz, J.; Zuo, P.; Wang, B.; Wong, M.S.; Alvarez, P.J.J. Perfluorooctanoic acid Degradation by UV/Chlorine. Environ. Sci. Technol. Lett. 2022, 9, 673–679. [Google Scholar] [CrossRef]
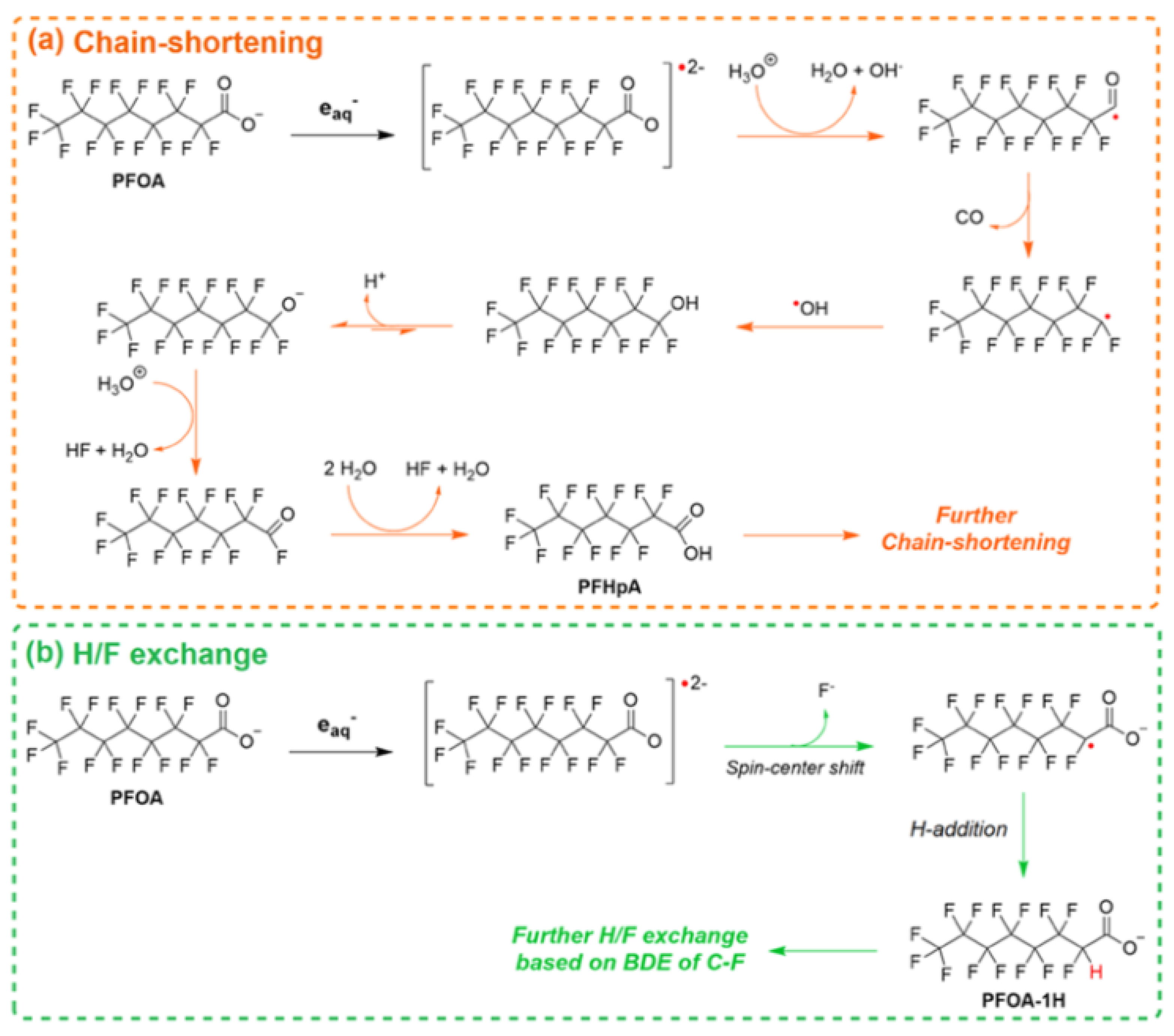
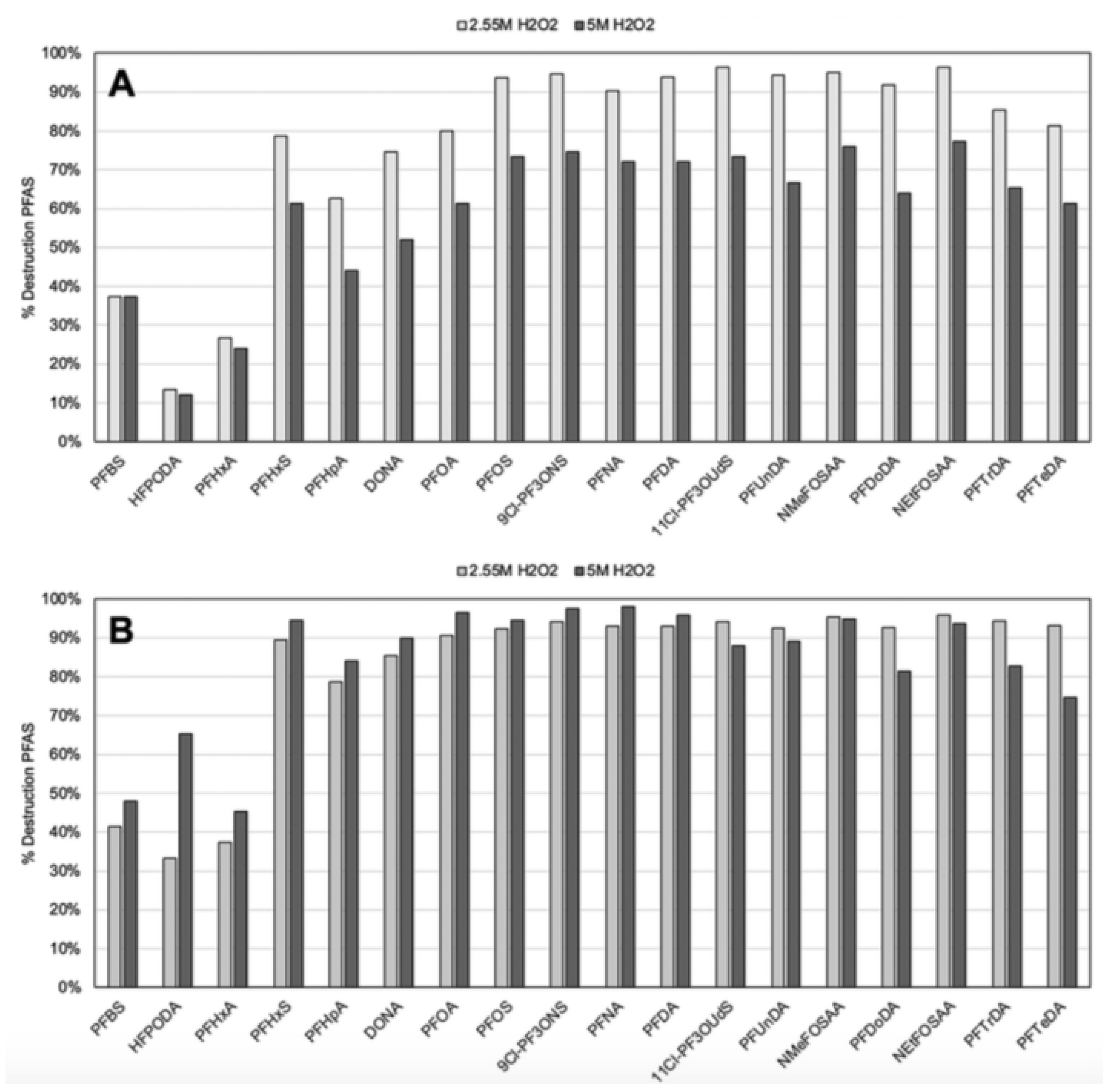
- Trang, B.; Li, Y.; Xue, X.-S.; Ateia, M.; Houk, K.N.; Dichtel, W.R. Low-temperature mineralization of perfluorocarboxylic acids. Science 2022, 377, 839. [Google Scholar] [CrossRef] [PubMed]
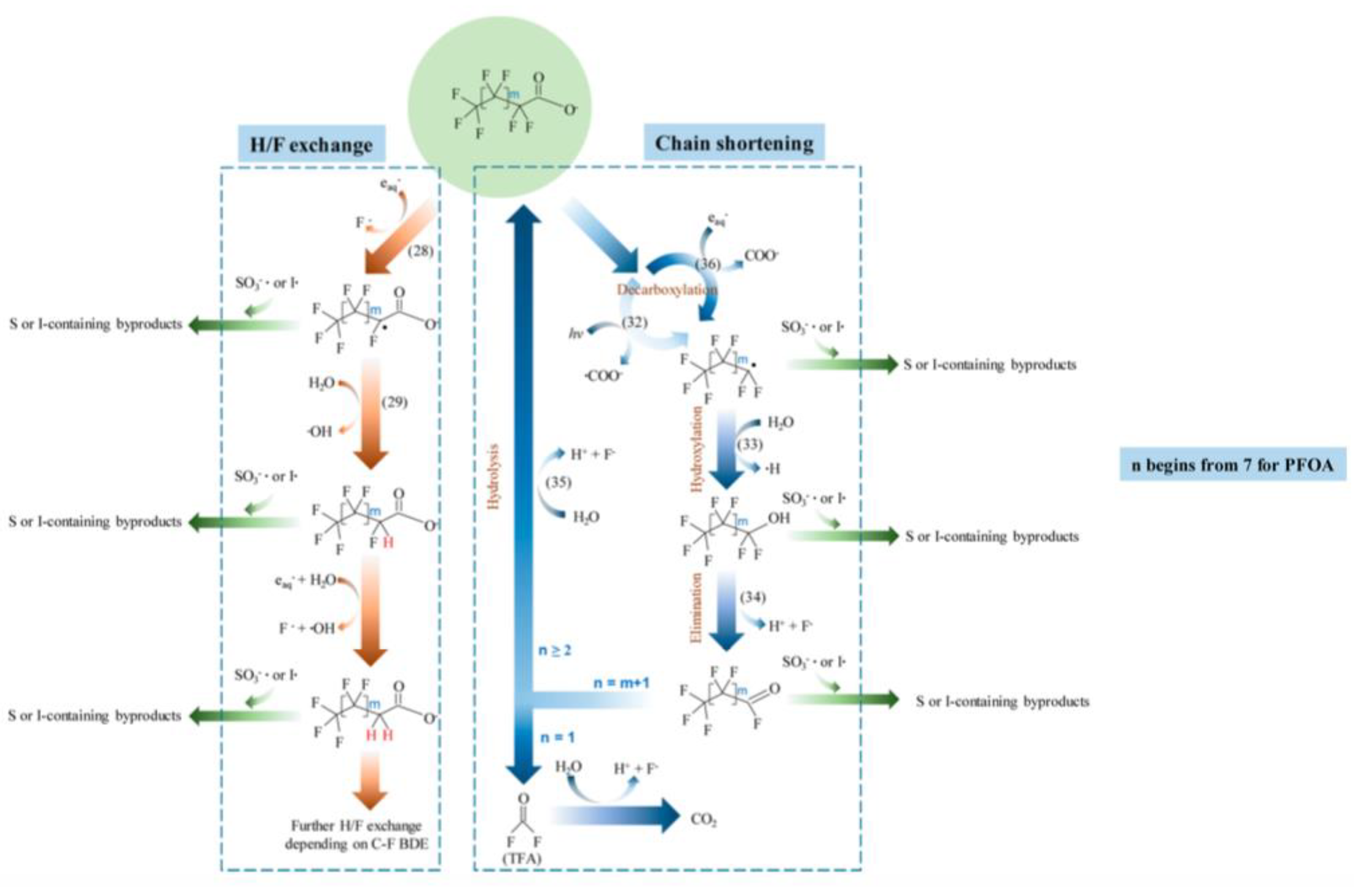
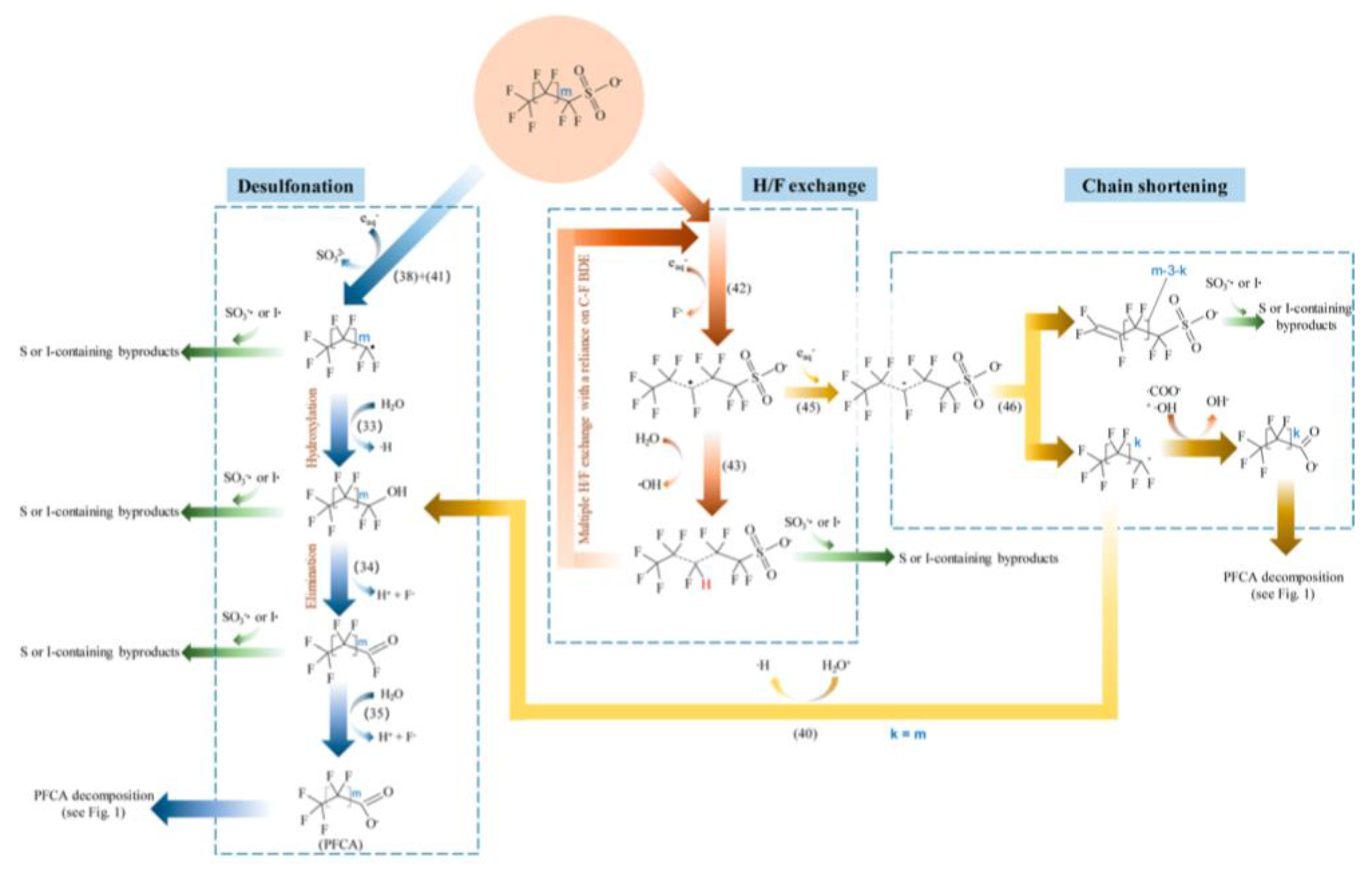
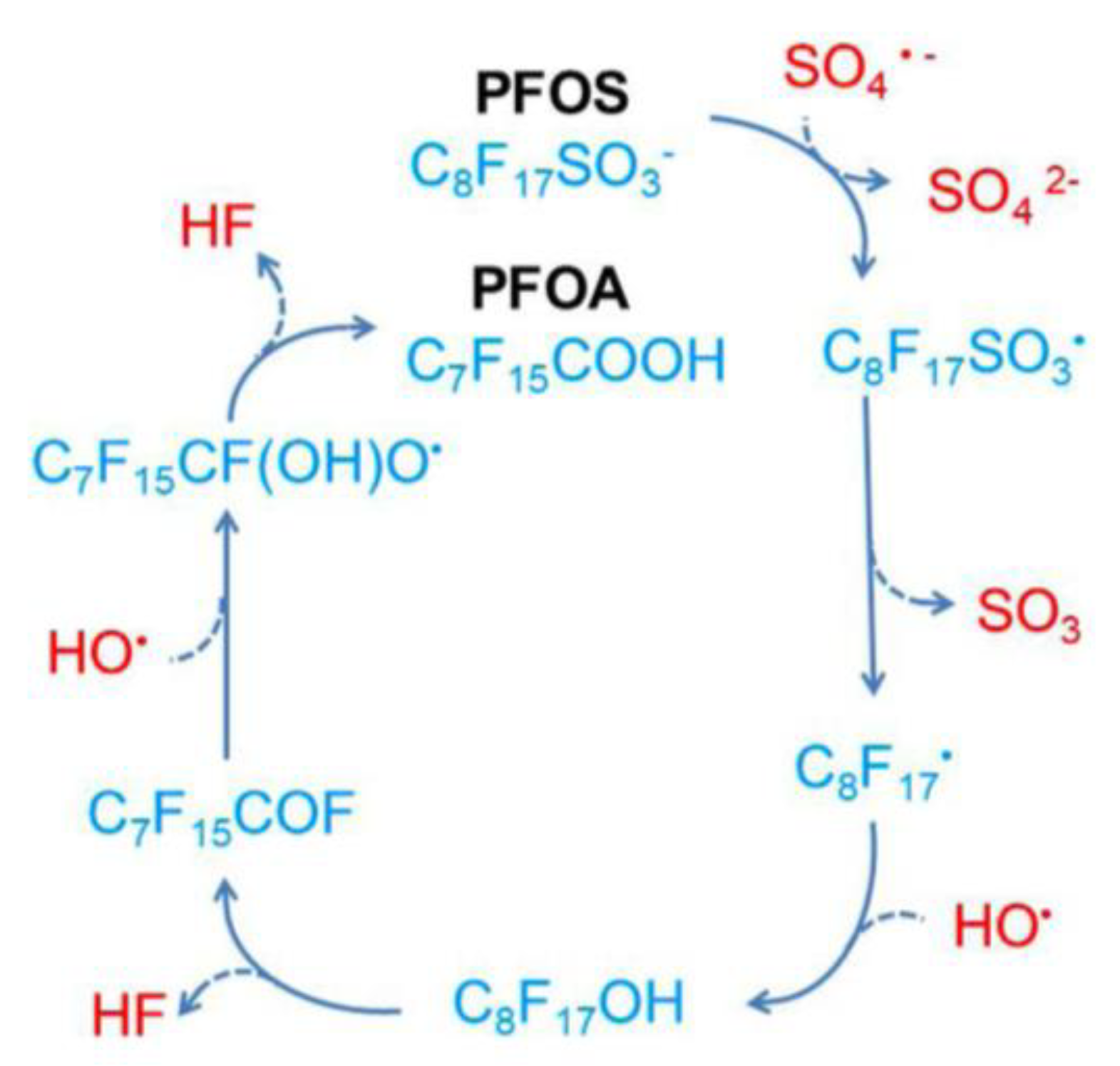
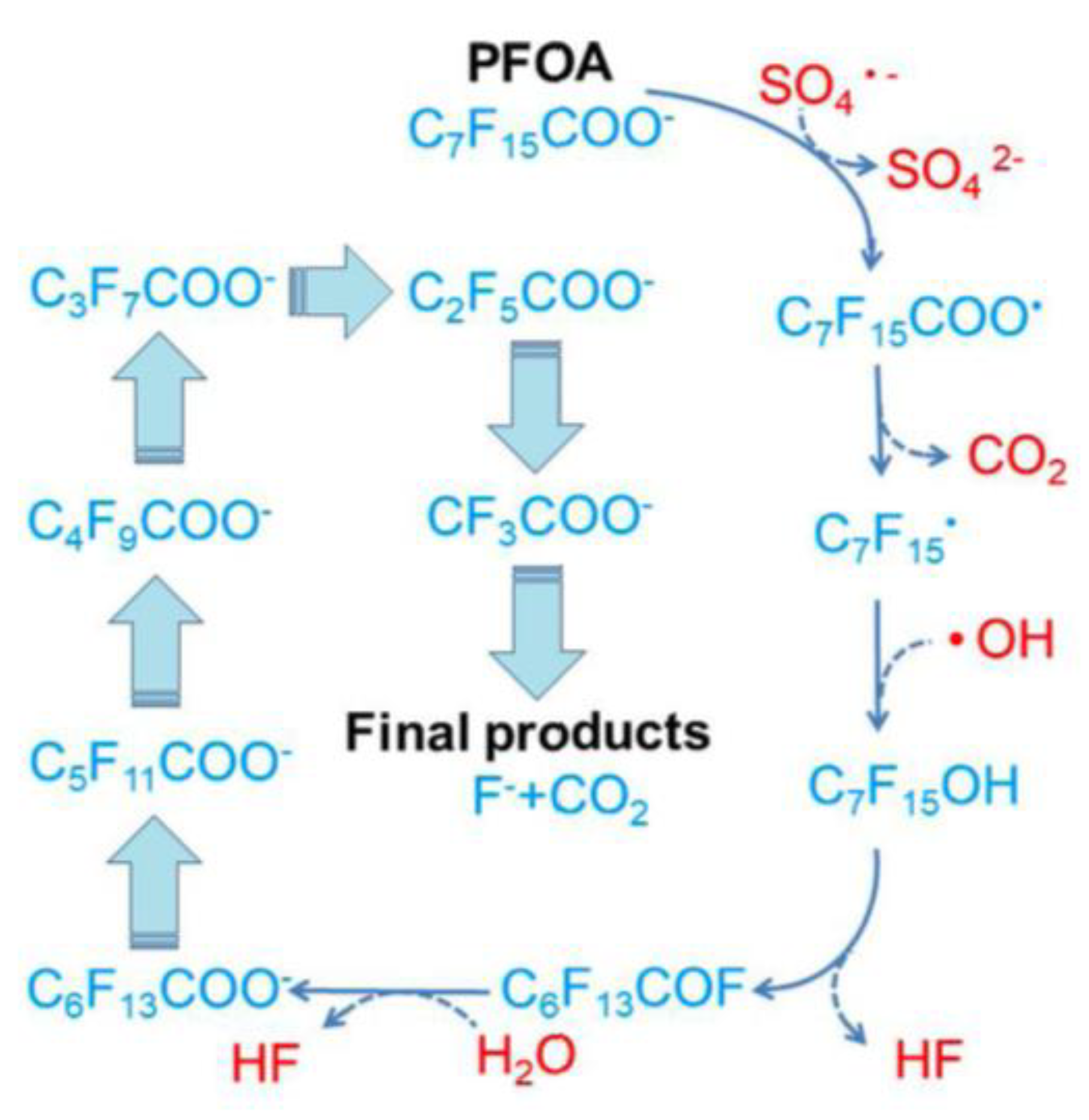
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




