Submitted:
25 April 2023
Posted:
26 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
Plant Material and Extraction of Essential Oils
Isolation of Alternaria Alternata Fungus and Preparation of Inoculum
Analysis of Essential Oils by GC-FID and GC-MS
Inhibition of Alternaria Alternata Fungus In Vitro
Preventive and Curative Control on Detached Leaves
Assessments and Data Analysis
3. Results and Discussion
Chemical Composition of Essential Oils
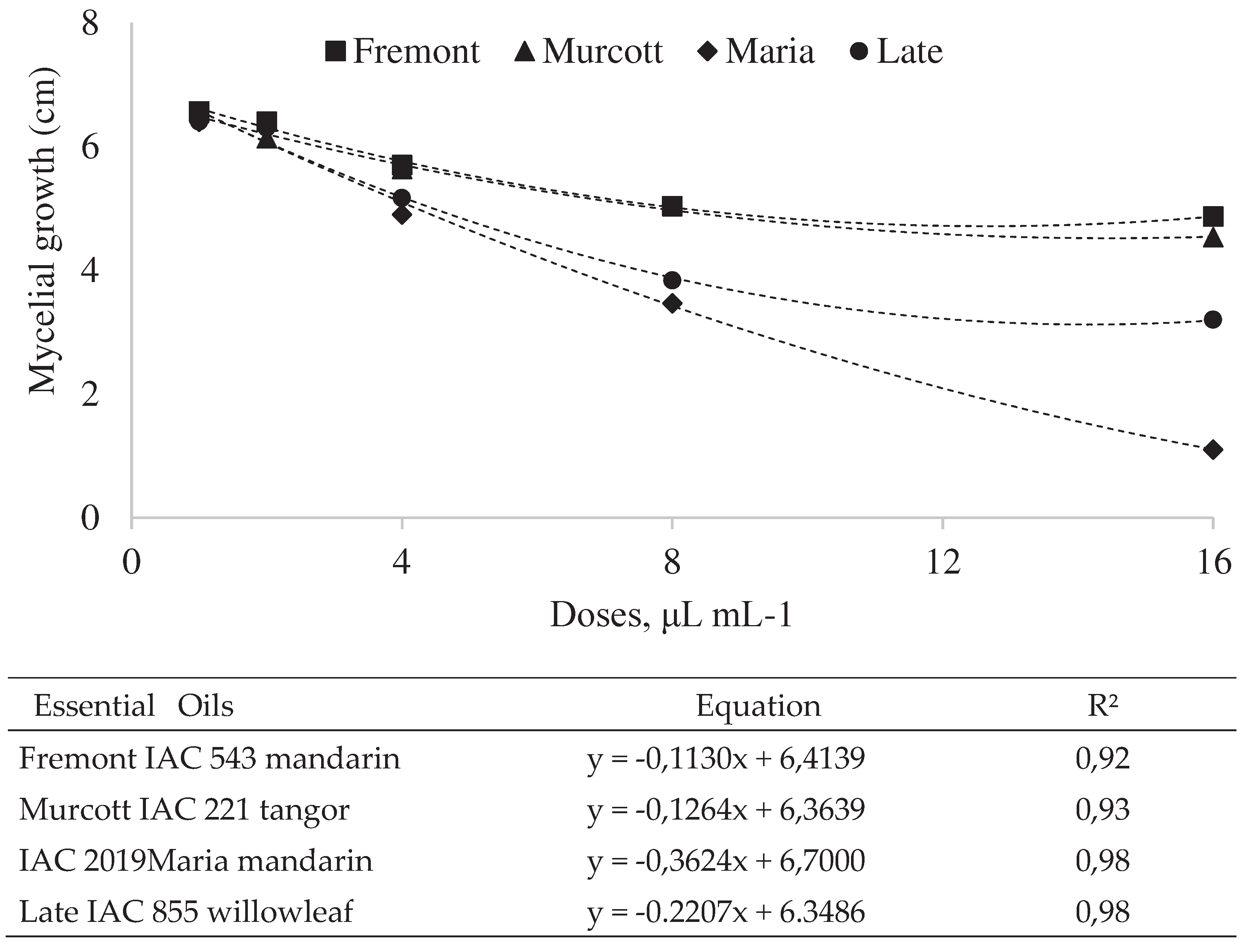
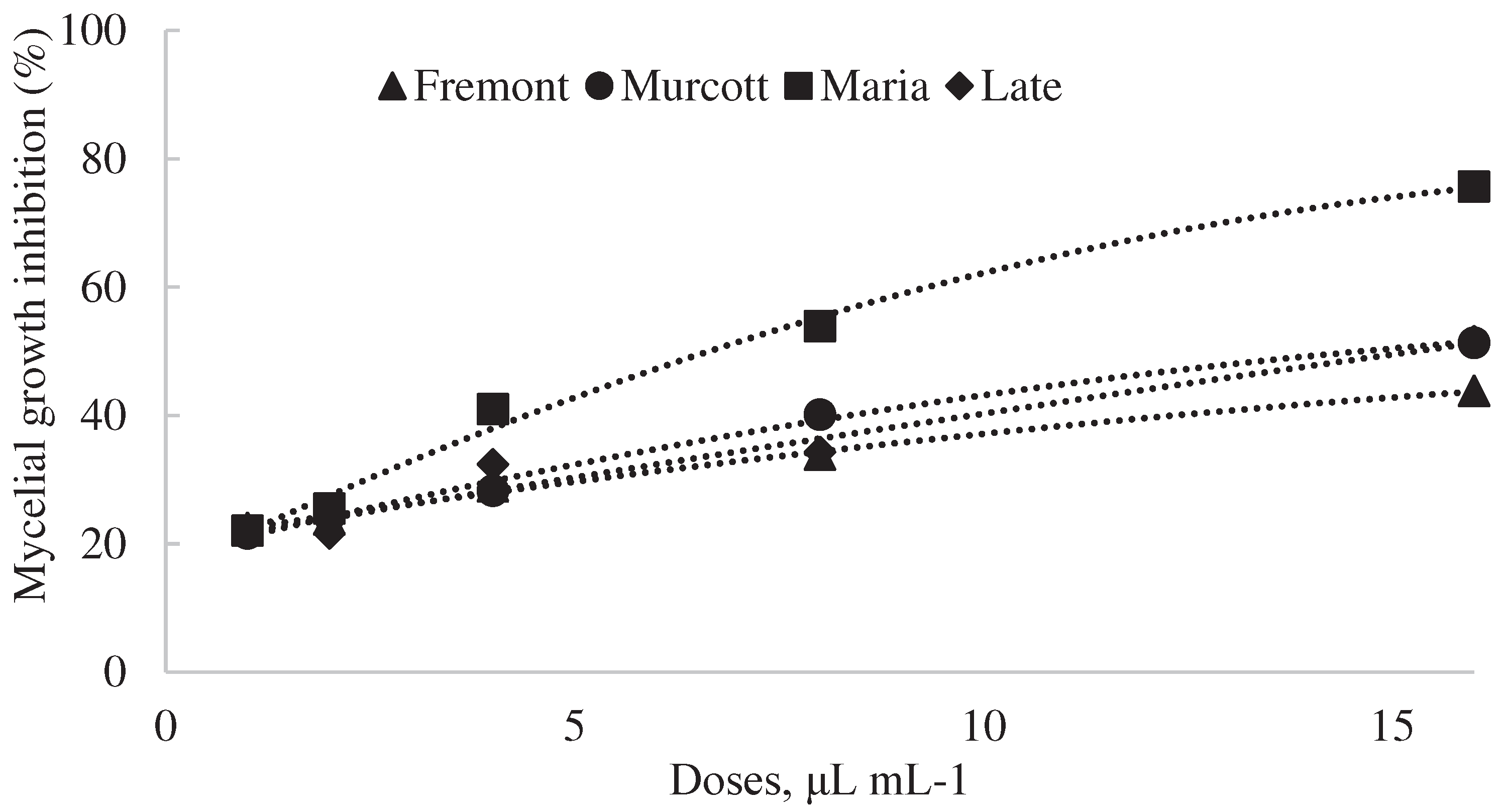
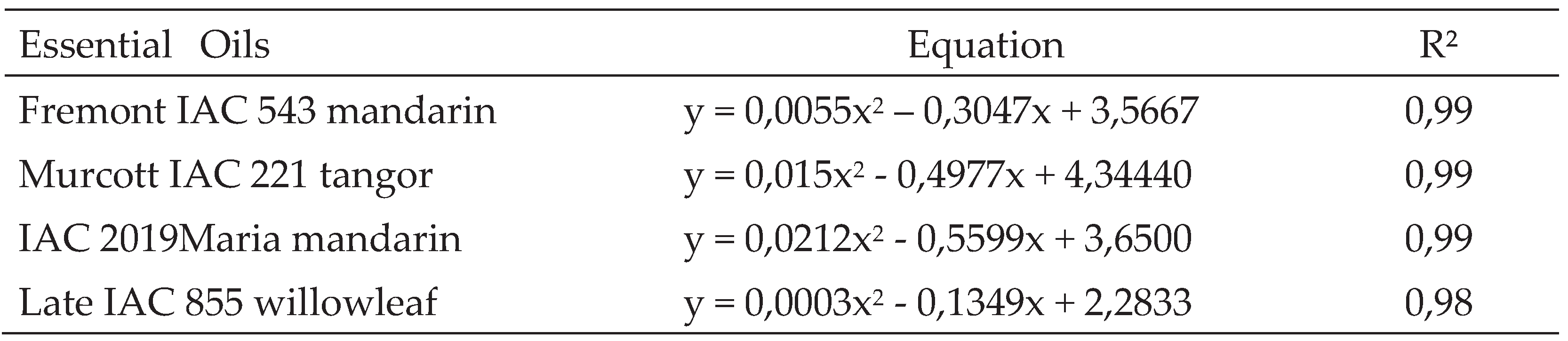
In Vitro Inhibition of Alternaria Alternata Fungus
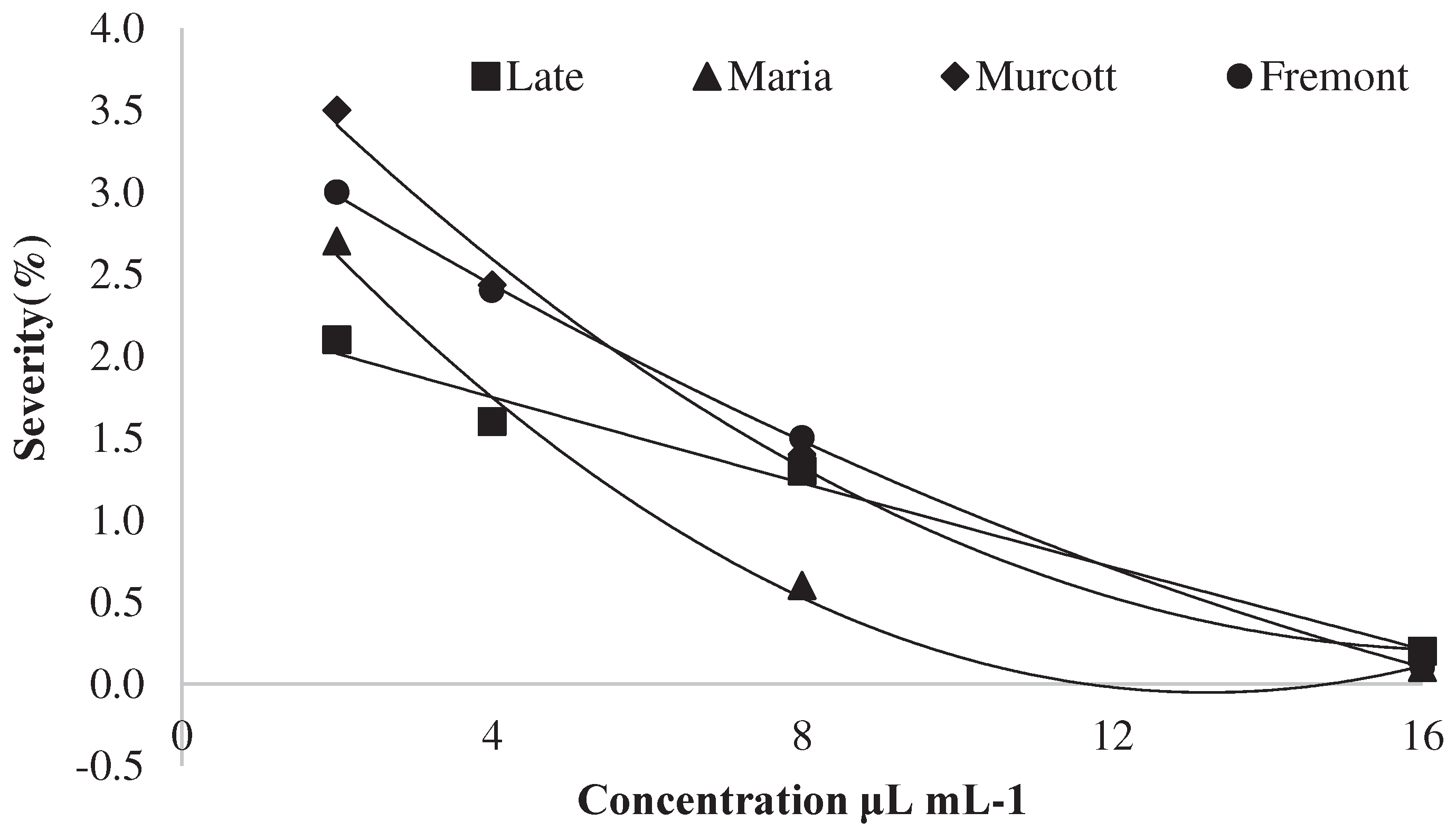
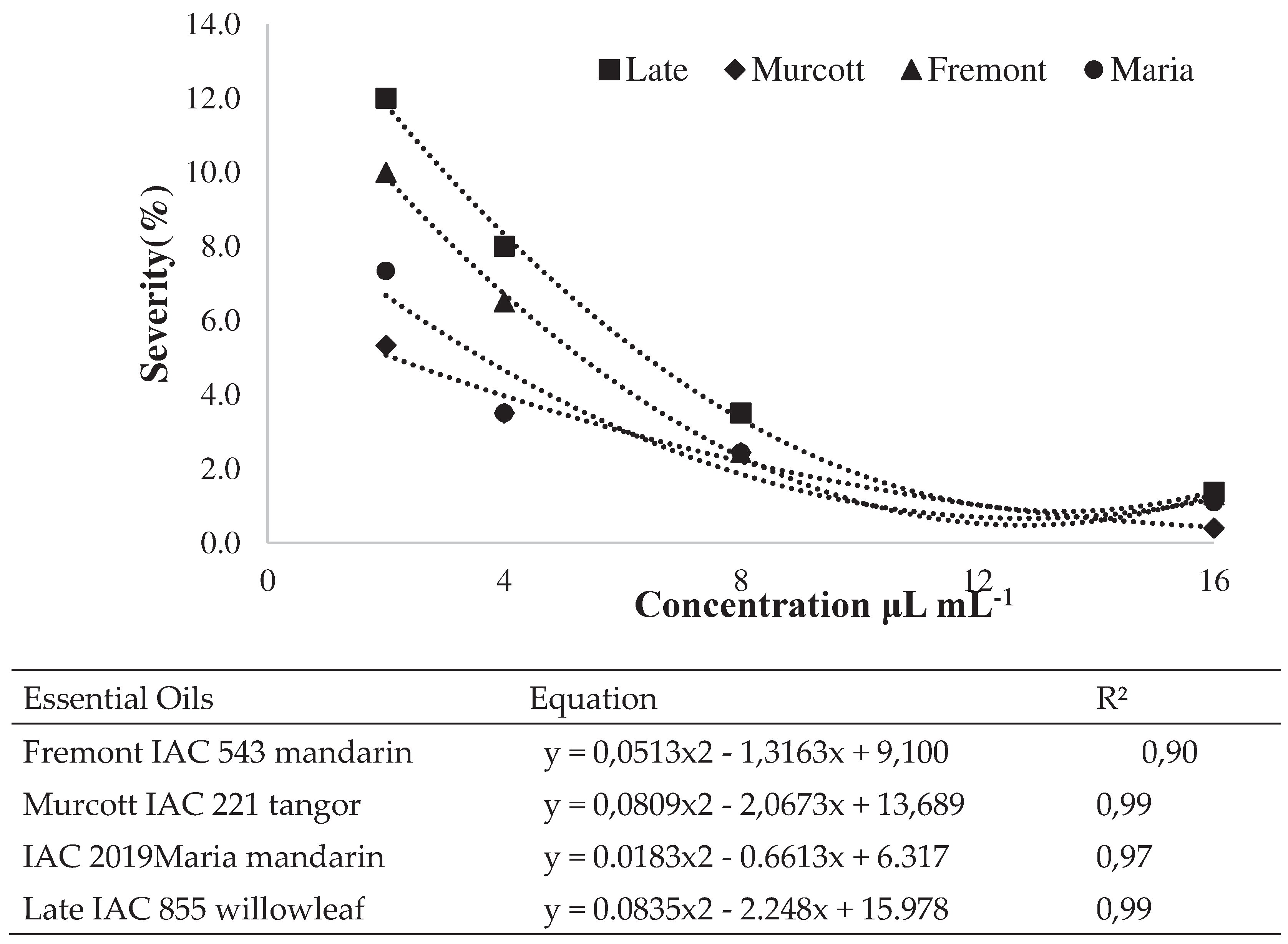
Preventive and Curative Control on Detached Leaves
5. Conclusions
Author Contributions
Funding
References
- Food and Agriculture Organization: FAO. Database results. Available online: http://www.fao.org/faostat/en/#search/mandarin (accessed on 10 August 2021).
- Fundecitrus - Citrus Industry Defense Fund. Tree inventory and estimate of the orange crop of the citrus belt of São Paulo and Triângulo/Sudoeste Mineiro, 2019/20. Fundecitrus 2019, 30. [Google Scholar]
- Bassanezi, R.B.; Silva, G.J.; Feichtenberger, E.; Belasque, J.; Behlau, F.; Wulff, N.A. Citrus Diseases. In Manual de Fitopatologia; Amorim, C.L., Rezende, J.A.M., Filho, A.B., Eds.; Brazil, 2016; pp. 292–293.
- Azevedo, F.A.; Polydoro, D.A.; Bastianel, M.; Kupper, K.C.; Stuart, R.M.; Costa, F.P.; Pio, R.M. Response of different mandarin genotypes and their hybrids to in vitro and in vivo inoculation of Alternaria alternata. Rev. Bras. Frutic. 2010, 32, 1–10. [Google Scholar] [CrossRef]
- Brazilian Institute of Geography and Statistics - IBGE. World agricultural production. Available online: https://www.ibge.gov.br/estatisticas-novoportal/economicas/agricultura-e-pecuaria/9201-levantamento-sistematico-da-producao-agricola.html?=&t=o-que-e (accessed on 10 August 2021).
- Schubert, T.S.; Dewdney, M.M.; Peres, N.A.; Palm, M.E.; Jeyaprakash, A. First Report of Guignardia citricarpa Associated with Citrus Black Spot on Sweet Orange (Citrus sinensis) in North America. Plant Dis. 2012, 96, 1225. [Google Scholar] [CrossRef] [PubMed]
- Chitolina, G.M.; Silva-Junior, G.J.; Feichtenberger, E.; Pereira, R.G.; Amorim, L. First report on quinone outside inhibitor resistance of Alternaria alternata causing Alternaria brown spot in mandarins in São Paulo, Brazil. Plant Health Prog. 2019, 20, 94. [Google Scholar] [CrossRef]
- Silva, A.D.; Sales, N.D.L.P.; Araujo, A.V.; Júnior, C.F.C. In vitro effect of plant compounds on the fungus Colletotrichum gloeosporioides Penz. Isolated from passion fruit tree. Ciência Agrotecnologia 2008, 33, 1853–1860. [Google Scholar] [CrossRef]
- Neto, A.C.A.A.; Araújo, P.C.; Souza, W.C.O.; Medeiros, J.G.F.; de Aguiar, A.V.M. Essential oil in the incidence and control of pathogens in fennel seeds (Foeniculum vulgare Mill.). Revista Verde de Agroecologia e Desenvolvimento Sustentável. Mossoró 2012, 7, 170–176. [Google Scholar]
- Sarmento-Brum, R.B.C.; Castro, H.G.; Silva, M.L.; Sarmento, R.A.; Nascimento, I.R.; Santos, G.R. Effect of plant oils inhibiting the mycelial growth of pathogenic fungi. J. Biotechnol. Biodivers. 2014, 5, 63–70. [Google Scholar] [CrossRef]
- Bigaton, D.; Bacchi, L.M.A.; Formagio, A.S.N.; Gavassoni, W.L.; Zanella, C.S. Evaluation of fungicidal activity of extracts and essential oils on Asian soybean rust. Rev. Ciência Agronômica 2013, 44, 757–763. [Google Scholar] [CrossRef]
- Costa, A.R.T.; Amaral, M.F.Z.J.; Martins, P.M.; Paula, J.A.M.; Fiuza, T.S. Action of the essential oil of Syzygium aromaticum (L.) Merr. & L. M. Perry on hyphae of some phytopathogenic fungi. Rev. Bras. Plantas Med. Botucatu 2011, 13, 240–245. [Google Scholar]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; de Moraes, G.; Leonardo, J.; Sparovek, G. Köppen's climate classification map for Brazil. Meteorol 2012, 22, 711–728. [Google Scholar] [CrossRef]
- Maia, T.F.; Donato, A.; Fraga, M.E. Antifungal Activity of Essential Oils of Plants. Braz. J. Agroindustrial Prod. 2014, 17, 105–116. [Google Scholar]
- Canihos, Y.; Timmer, L.W. Temperature, leaf wetness, and isolate effects on infection of Minneola tangelo leaves by Alternaria spp. Plant Disease 1999, 83, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Frizzo, C.D.; Lorenzo, D.; Dellacassa, E. Composition and Seasonal Variation of the Essential Oils from Two Mandarin Cultivars of Southern Brazil. J. Agric. Food Chem. 2004, 52, 3036–3041. [Google Scholar] [CrossRef] [PubMed]
- Freire, I.C.; Pérez, A.L.; Cardoso, A.M.; Mariz, B.A.; Almeida, L.F.; Cavalcanti, Y.W. Antibacterial activity of essential oils on Streptococcus mutans and Staphylococcus aureus. Med. Plants 2014, 16. [Google Scholar] [CrossRef]
- Martelli, I.B.; Pacheco, C.A.; Bastianel, M.; Schinor, E.H.; Conceição, P.M.; Azevedo, F.A. Diagramatic scale for assessing foliar symptoms of alternaria brown spot in citrus. Agron. Sci. Biotechnol. 2016, 2, 56–61. [Google Scholar]
- Ferreira, D.F. Sisvar: A computer statistical analysis system. Ciência e Agrotecnologia (UFLA) 2011, 35, 1039–1042. [Google Scholar] [CrossRef]
- Asikin, Y.; Kawahira, S.; Goki, M.; Hirose, N.; Kyoda, S.; Wada, K. Extended aroma extract dilution analysis profile of Shiikuwasha (Citrus depressa Hayata) pulp essential oil. J. Food Drugs Anal. 2018, 26, 268–276. [Google Scholar] [CrossRef]
- Yi, F.; Jin, R.; Sun, J.; Ma, B.; Bao, X. Evaluation of mechanical-pressed essential oil from Nanfeng mandarin (Citrus reticulata Blanco cv. Kinokuni) as a food preservative based on antimicrobial and antioxidant activities. LWT - Food Sci. Technol. 2018, 95, 346–353. [Google Scholar]
- Martins, A.P.; Nogueira, M.T.; Costa, M.C.; Salgueiro, L. Quality requirements in essential oils: The importance of European Pharmacopoeia monographs and ISO Standards. Rev Fitoter. 2011, 11, 35–50. [Google Scholar]
- Pauletti, G.F.; Silvestre, W.P. Citrus essential oil: Production, composition and fractionation. In: Citricultura do Rio Grande do Sul:Indicações Técnicas. 1ed.: P.V.D. Souza (Eds) SEAPI 2018, 245-268.
- Maia, T.F.; Donato, A.; Fraga, M.E. Antifungal activity of essential oils of plants. Rev Bras Prod Agroind. 2014, 17, 105–116. [Google Scholar]
- Smith-Palmer, A.; Stewart, J.; Fyfe, L. The potential application of plant essential oils as natural food preservatives in soft cheese. Food Microbiol. 2001, 18, 463–470. [Google Scholar] [CrossRef]
- Viuda-martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Álvarez, P. Antibacterial activity of lemon (Citrus lemon L.), mandarin (Citrus reticulata L.), grapefruit (Citrus paradisi L.) and orange (Citrus sinensis L.) essential oils. J. Food Saf. 2008, 28, 567–576. [Google Scholar] [CrossRef]
- Gomes, M.S. Chemical characterization and antifungal activity of essential oils from five species of Citrus genus. 98 f. Dissertation (Master in agrochemistry) - Federal University of Lavras, Lavras (2011).
- Russo, M.; Suraci, F.; Postorino, S.; Serra, D.; Roccotelli, A.; Agosteo, G.E. Essential oil Chemical composition and antifungal effects on Sclerotium cepivorum of Thymus capitatus wild populations from Calabria, southern Italy. Rev. Bras. Farm. 2013, 23, 239–248. [Google Scholar] [CrossRef]
- Santos, M.S. Molecular Hybrids Derived from Peryl Alcohol and Borneol: Antifungal Evaluation; Dissertation (Master in Natural Products and Bioactive Synthetics) - Universidade Federal da Paraíba (2020).
- Bem-Miri, Y.; Ariño, A.; Djenane, D. Study of antifungal, anti-aflatoxigenic, antioxidant activity and phytotoxicity of Algerian Citrus limon var. Eureka and Citrus sinensis var. Valencia essential oils. J. Essent. Oil Bear. Plants 2018, 21, 345–361. [Google Scholar] [CrossRef]
- Brand, S.C. Isolation and identification of substances from the orange tree "Valencia" (Citrus sinensis) involved in the stimulation and/or break of dormancy of quiescent structures of Colletotrichum acutatum, causal agent of citrus flower rot. 104f. Dissertation (Master of Science) - School of Agriculture "Luiz de Queiroz", University of São Paulo, Piracicaba (2012).
- Bosquez-Molina, E.; de Jesús, E.S.R.; Bautista-Banos; Verde-Calvo, J. R.; Morales-Lopez, J. Inhibitory effect of essential oils against Colletotrichum gloeosporioides and Rhizopus stolonifer in stored papaya fruit and their possible application in coatings. Postharvest Biol. Tecnonologia 2010, 57, 132–137. [Google Scholar] [CrossRef]
- Stevic, T.; Beric, T.; Savikin, K.; Sokovic, M.; Godevac, D.; Dimkic, I.; Stankovic, S. Antifungal activity of selected essential oils against fungi isolated from medicinal plants. Ind. Crops Production 2014, 55, 116–122. [Google Scholar] [CrossRef]
- Belletti, N.; Kamdem, S.S.; Tabanelli, G.; Lanciotti, R.; Gardini, F. Modeling of combined effects of citral, linalool and B-pinene used against Saccharomyces cerevisiae in citrus-based beverages subjected to a mild heat treatment. Food Microbiol. 2010, 136, 283–289. [Google Scholar] [CrossRef]
- Bem-Miri, Y.; Ariño, A.; Djenane, D. Study of antifungal, anti-aflatoxigenic, antioxidant activity and phytotoxicity of Algerian Citrus limon var. Eureka and Citrus sinensis var. Valencia essential oils. J. Essent. Oil Bear. Plants 2018, 21, 345–361. [Google Scholar] [CrossRef]
- Sikkema, J.; de Bont, J.A.M.; Poolman, B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 1995, 59, 201–222. [Google Scholar] [CrossRef]
- Marei, G.I.K.; Rasoul, M.A.; Abdelgalei, L. Comparative antifungal activities and biochemical effects of monoterpenes on plant pathogenic fungi. Pestic. Biochem. Physiol. 2012, 103, 56–61. [Google Scholar] [CrossRef]
- Guimarães, L.G.L.; Cardoso, M.G.; Sousa, P.E.; Andrade, J.; Vieira, S.S. Antioxidant and fungitoxic activities of the lemongrass essential oil and citral. Rev. Ciência Agronômica 2011, 42, 464–472. [Google Scholar] [CrossRef]
- Chutia, M.; Bhuyan, P.D.; Pathak, M.G.; Sarma, T.C.; Boruah, P. Antifungal activity and chemical composition of Citrus reticulata Blanco essential oil against phytopathogens from North East India. LWT - Food Sci. Technol. 2009, 42, 777–780. [Google Scholar] [CrossRef]
- Lopes, D.; Bizzo, H.R.; Oliveira, D.R.; Lima, M.F.; Pimentel, F. Avaliação química dos óleos essenciais de exemplares de pimenta longa (Piper hispidinervum C. DC.) do Estado do Acre Rio Branco: Embrapa-CPAF/AC.226 2002, 75.
- Sharma, K.; Mahato, N.; Cho, M.H.; Lee, Y.R. Converting citrus wastes into valueadded products: Economic and environmentally friendly approaches. Nutrition 2017, 34, 29–46. [Google Scholar] [CrossRef]
- Hani, U.; Shivakumar, H.G.; Vaghela, R. Candidiasis: A fungal infection- current challenges and progress in prevention and treatment. Infect. Disord. - Drug Targets 2015, 15, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, F.; Averbeck, S.; Idaomar, M. Biological effects of essential oils - A review. Food and Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Antunes, M.D.; Cavaco, A.M. Phenols and antioxidant activity of hydro-alcoholic extracts of propolis from Algarve, South of Portugal. Food Chem. Toxicol. 2010, 48, 3418–3423. [Google Scholar]
- Pacheco, C.A.; Martelli, I.B.; Polydoro, D.A.; Schinor, E.H.; Pio, R. Resistance and susceptibility of mandarins and their hybrids to Alternaria alternata. Sci. Agric. 2012, 69, 347–403. [Google Scholar] [CrossRef]
- Perini, V.B.M.; Castro, H.G.; Santos, G.R.; Aguiar, R.W.S.; Leão, E.U.; Seixas, P.T. Evaluation of the curative and preventive effect of citronella grass essential oil in the control of Pyricularia grisea. J. Biotechnol. Biodivers. 2011, 2, 23–27. [Google Scholar] [CrossRef]
- Fonseca, A.C.C.; Rotili, E.A.; Ferreira, T.P.S.; Mourão, D.S.C.; Dias, B.L.; Oliveira, G.R.A.S.; Santos, G.R. Potential of noni essential oil in preventive and curative control of mango anthracnose. J. Biotechnol. Biodivers. 2019, 7, 356–362. [Google Scholar] [CrossRef]
- Derbalah, A.S.; Dewir, Y.H.; El-Sayed, A.E. Antifungal activity of some plant extracts against sugar beet damping-off caused by Sclerotium rolfsii. Ann. Microbiol. 2011, 62, 1021–1029. [Google Scholar] [CrossRef]
- Dellavalle, P.D.; Cabrera, A.; Alem, D.; Larrañaga, P.; Ferreira, F.; Rizza, M.D. Antifungal activity of medicinal plant extractsagainst phytopathogenic fungus Alternarias spp. Chil. J. Agric. Res. 2011, 71, 231–239. [Google Scholar] [CrossRef]
- Gachkar, L.; Yadegari, D.; Rezaei, M.B.; Taghizadeh, M.; Astaneh, S.A.; Rasooli, I. Chemical and biological characteristics of Cuminum cyminum and Rosmarinus officinalis essential oils. Food Chem. 2007, 102, 898–904. [Google Scholar] [CrossRef]
- Fialho, R.O.; Papa, M.F.S.; Pereira, D.A.S. Efeito fungitóxico de óleos essenciais sobre Phakopsora euvitis, agente causal da ferrugem da vinha. Arquivos do Instituto Biológico. São Paulo-SP 2015, 82, 1–7. [Google Scholar]
| Volatile compounds | Relative Percentage (%)* | |||||||
|---|---|---|---|---|---|---|---|---|
| Fremont IAC 543 mandarin | Late mandarin IAC 855 | mandarin IAC 2019Maria |
tangor Murcott IAC 221 | |||||
| U | R | U | R | U | R | U | R | |
| Acids | ||||||||
| benzoic acid | - | - | - | 1,03 | - | - | - | - |
| formic acid | - | - | - | - | - | 0,06 | - | - |
| Alcohols | ||||||||
| 3,7-Dimethyloct-7-en-1-ol | - | - | - | - | - | 0,41 | - | - |
| cis-homomenthol | - | - | - | - | 0,02 | 0,02 | 0,05 | - |
| citronellol | - | 0,16 | 0,34 | 0,2 | - | - | 0,32 | 0,14 |
| isocarveol | - | - | - | - | 0,6 | 0,87 | 0,04 | - |
| Linalool | 3,04 | 3,39 | 1,05 | 0,41 | 13,13 | 9,7 | 2,89 | 1,37 |
| octanol | 0,15 | 0,22 | - | - | 0,49 | - | - | - |
| p-menth-2-en-1-ol | - | - | 0,04 | - | - | - | - | - |
| trans-isocarveol | - | - | 0,32 | - | 1,3 | 1,5 | 0,49 | - |
| terpinen-4-ol | 0,05 | 0,23 | 2,74 | 0,63 | 0,92 | 0,44 | 0,09 | 0,06 |
| terpineol | - | - | 2,65 | - | 0,09 | 0,04 | - | - |
| trans-p-mentha-dien-2-ol | - | 0,05 | - | - | 0,66 | - | 0,28 | - |
| α-Terpineol | 0,25 | 0,45 | - | 1,06 | 1,03 | 0,71 | 0,61 | 0,21 |
| Aldehydes | ||||||||
| citronellal | 0,19 | 0,24 | 0,08 | 0,07 | 0,37 | 0,45 | 0,02 | 0,3 |
| decanal | 0,32 | 0,72 | 0,07 | 0,18 | 0,45 | 0,82 | 0,03 | 0,44 |
| neral | - | - | 0,11 | 0,1 | 0,08 | - | - | - |
| octanal | 1,45 | 0,98 | 0,22 | - | - | - | 1,01 | - |
| perillaldehyde | 0,16 | 0,16 | 0,29 | 0,15 | 0,15 | 0,18 | 0,18 | - |
| Ketones | ||||||||
| carvone | - | 0,02 | - | - | - | - | 0,02 | 0,3 |
| Monoterpenes | ||||||||
| ∆-carene | 0,02 | - | 0,75 | 0,55 | 0,15 | 0,02 | 0,14 | |
| 1,3,6-Heptatriene, 2,5,5-trimethyl- | 0,01 | - | - | - | - | - | 0,02 | 0,4 |
| 1,3,8-p-Menthatriene | - | - | - | - | - | 0,02 | - | 0,03 |
| bornanone | - | - | 0,04 | - | - | - | - | - |
| carvacrol | - | 0,03 | 2,71 | 2,06 | - | - | - | - |
| citronellol | - | - | 0,34 | |||||
| felandrene | - | 0,01 | 0,13 | 0,11 | - | 0,01 | - | 0,11 |
| isolimonene | - | 0,05 | 0,39 | 0,25 | - | - | - | - |
| Isoterpinolene | - | - | 1,3 | 1,12 | - | 0,04 | 0,07 | 0,03 |
| limonene | 90,37 | 88,86 | 58,89 | 66,19 | 77,18 | 80,62 | 88,77 | 90,89 |
| ocimene | - | - | - | - | 0,09 | 0,04 | - | 2,79 |
| p-Cymeno | - | 0,03 | 0,29 | 0,12 | - | - | - | 0,44 |
| sabinene | 0,5 | 0,71 | 0,27 | - | - | 0,87 | 1,01 | 0,54 |
| Terpinene | - | 0,02 | - | - | - | - | 0,02 | - |
| tujeno | - | 0,01 | 0,79 | 0,74 | 0,01 | - | - | - |
| α-Pinene | 0,75 | 0,73 | 1,98 | 1,95 | 0,6 | 0,63 | 0,68 | 0,92 |
| α-terpinene | - | 0,06 | - | - | 0,02 | 0,12 | 0,02 | - |
| β-myrcene | 2,47 | 2,52 | 1,89 | 2,03 | 2,13 | 2,02 | 2,5 | 0,08 |
| β-ocimene | - | 0,02 | 0,02 | - | 0,09 | - | - | - |
| β-pinene | 0,06 | 0,08 | 1,75 | 1,47 | 0,07 | 0,07 | 0,04 | - |
| γ-terpinene | 0,06 | 0,17 | 19,88 | 18,93 | 0,22 | 0,13 | 0,06 | 0,08 |
| Sesquiterpenes | ||||||||
| caryophyllene | - | - | 0,39 | 0,38 | - | - | - | - |
| farneceno | - | - | 0,11 | 0,12 | - | - | - | 0,15 |
| germacrene | 0,07 | 0,02 | - | - | - | - | - | 0,03 |
| selinene | - | - | 0,11 | 0,1 | - | - | - | - |
| valencene | - | 0,01 | - | - | - | 0,2 | 0,11 | 0,23 |
| α-copaene | 0,04 | 0,02 | 0,02 | 0,02 | 0,02 | - | 0,56 | - |
| β-cadinene | 0,04 | 0,03 | 0,04 | 0,03 | 0,04 | 0,03 | 0,09 | 0,21 |
| β-Copaene | - | - | - | - | - | - | - | 0,02 |
| TOTAL | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





