1. Introduction
Rheumatoid arthritis (RA) is associated with progressive disability, early death and requires lifelong therapy with disease-modifying drugs (DMARDs), ultimately inflicting a high socioeconomic burden [
1]. Over the years, treatment of RA substantially evolved and many RA patients experience remission of disease due to earlier intervention, frequent monitoring and treatment-adjustment guided by stringent disease activity targets, and the development of highly effective targeted treatments. These achievements, along with the changing disease definition [
2], led to a considerable RA patient population who does not show symptoms of disease but are taking continuous DMARD treatment [
2]. This growing population of RA patients under remission poses an important challenge since the need to broaden the evidence for the management of remission in RA becomes more apparent.
We have previously reported analyses from the RETRO study showing that tapering and stopping DMARDs in RA patients in sustained remission is feasible [
3] and lowers costs [
4]. However, flares of RA are more frequent when tapering and stopping DMARDs [
3]. While remission or low disease activity can still be restored after re-starting the previous DMARD treatment [
5], flares are hard to predict and may lead to joint damage and worsening functional status over time [
6], [
7]. Therefore, it is necessary to understand the impact of treatment reduction or cessation not only on disease activity but also on functional status in order to better inform the patient and to develop better strategies for remission management. To this end, we performed a post-hoc analysis of data from the RETRO trial to characterize the change in functional status during RETRO trial interventions.
2. Methods
2.1. Study design and patients
RETRO was a phase 3, open label, parallel-group, multicentre, randomized-controlled study (EudraCT number: 2009-015740-42) described elsewhere [
3],[
8],. The primary objective of the study was to assess the possibility of treatment reduction and discontinuation in RA patients under sustained remission. The study was approved by the local ethics committee and conducted according to the guidelines of the Declaration of Helsinki. Written informed consent was obtained from each participant before screening.
2.2. Outcome
Health Assessment Questionnaire (HAQ) data was collected at baseline, 3, 6, 9 and 12 months. The outcomes for this analysis were HAQ at study visits, HAQ change from baseline and functional worsening defined as a HAQ increase of ≥ 0.25 corresponding to a commonly used minimal clinically important change threshold 9.
2.3. Statistical analysis
We tabulated baseline patient characteristics using appropriate summary statistics for continuous and categorical data. We used the Kaplan-Meier method to summarize the risk of HAQ worsening over time in study groups and used the log-rank test of trend for an overall comparison of HAQ-worsening-free survival. Cox regression was used to estimate the hazard ratio (HR) of HAQ worsening over time. A linear mixed effects regression was fit to analyse the mean HAQ across study groups. This analysis used HAQ measurements obtained across all visits as the dependent variable, a categorical variable indicating the visit and study group and an interaction term for visits and study groups. A categorical participant identifier variable was used as the cluster identifier for random intercepts. A 95% confidence interval for the visit-study group interaction term excluding zero was considered to indicate a significant difference of mean HAQ between groups for a given visit. Otherwise two-tailed P values less than 0.05 were considered significant. Analyses were conducted using the open source R software v. 4.0.1 (R Foundation for Statistical Computing, Vienna, Austria) working under the GUI RStudio v. 1.2.1 (Rstudio, Boston MA). No specific procedure was utilized for handling missing HAQ data.
3. Results
3.1. Patients
One-year follow-up data of 282 patients enrolled in the RETRO study were available. The patients were randomized 1:1:1 in the 3 different treatment arms: arm 1 (control, n = 93), arm 2 (tapering, n = 93), arm 3 (tapering and stopping, n = 96).
Table 1 shows the baseline characteristics of the patients. Mean age was 56.5 (± 13.0) years, mean disease duration 7.4 (± 7.3) years, and 59.4% (n = 167) were female. All patients were in sustained clinical remission with a mean DAS28-ESR score of 1.7 (± 0.6) at baseline. The mean HAQ at baseline over all three groups was 0.2 (± 0,4) and identical among the three groups. The low HAQ score at baseline indicated good function of RA patients in sustained remission. There were 75.9% of patients taking csDMARD treatment with methotrexate (n = 214), while 43.6% (n = 123) of patients were taking bDMARD, most of them TNF inhibitors.
3.2. Physical function over time
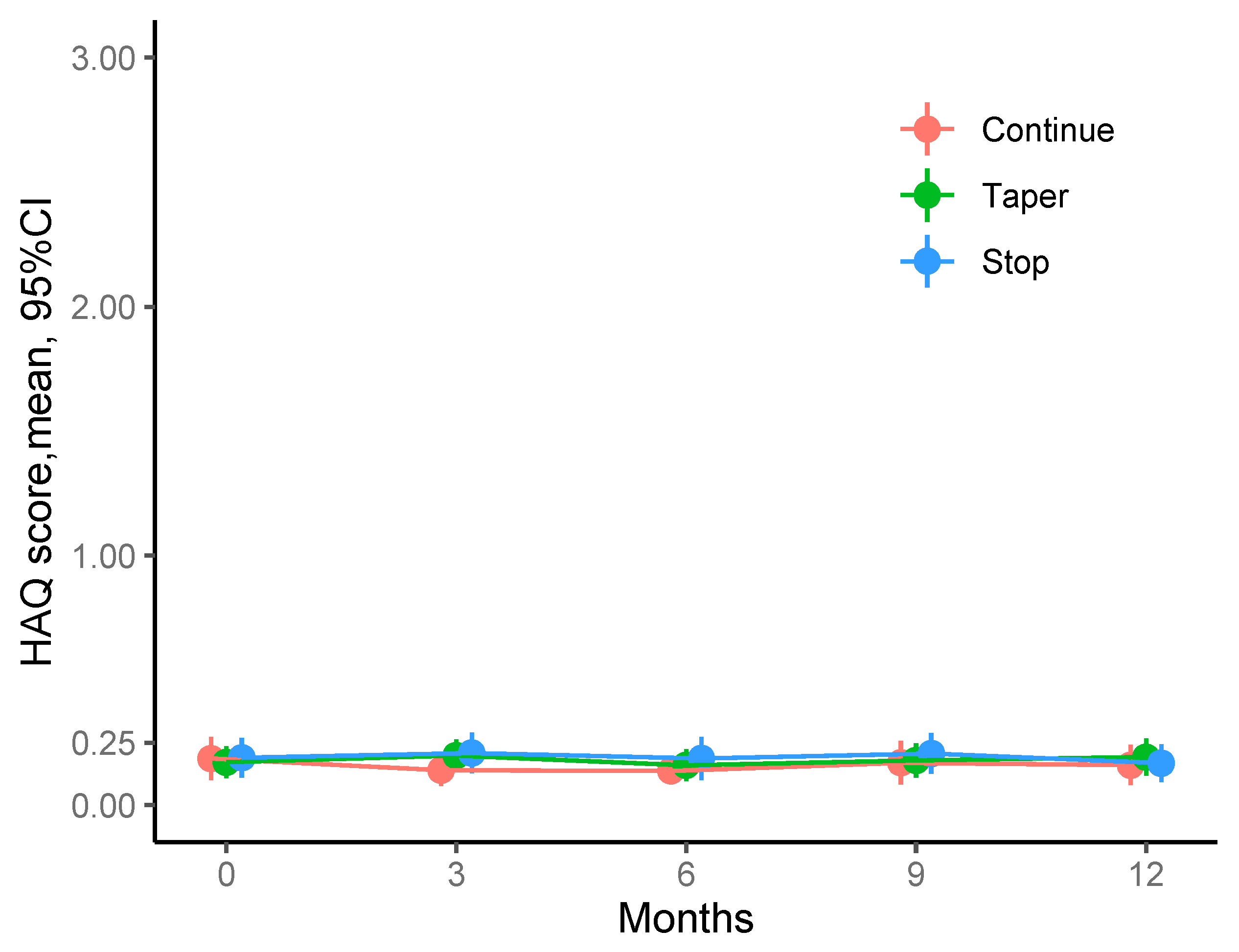
When analysing mean HAQ values at baseline and 3, 6, 9 and 12 months after the intervention we found that physical function was equally good throughout the observation time and virtually identical among the three study arms. Thus, mean HAQ at study visits ranged between 0.14 (0.30) and 0.21 (0.38) units among the three study groups across all visits. Notanly, values were well below HAQ values of 0.5, which means that there is no relevant disability.
The change from baseline HAQ at study visits ranged between -0.052 (0.292) and 0.032 (0.201) and all mean change values were below the minimal clinically important change threshold of 0.25.
Figure 1 summarizes the mean HAQ values and changes from baseline for each study group and visit.
Mean HAQ values for each study group (continuation, tapering, stopping) and each visit. Mean HAQ scores were below 0.25 units throughout the study.
3.3. Incident decline in physical function
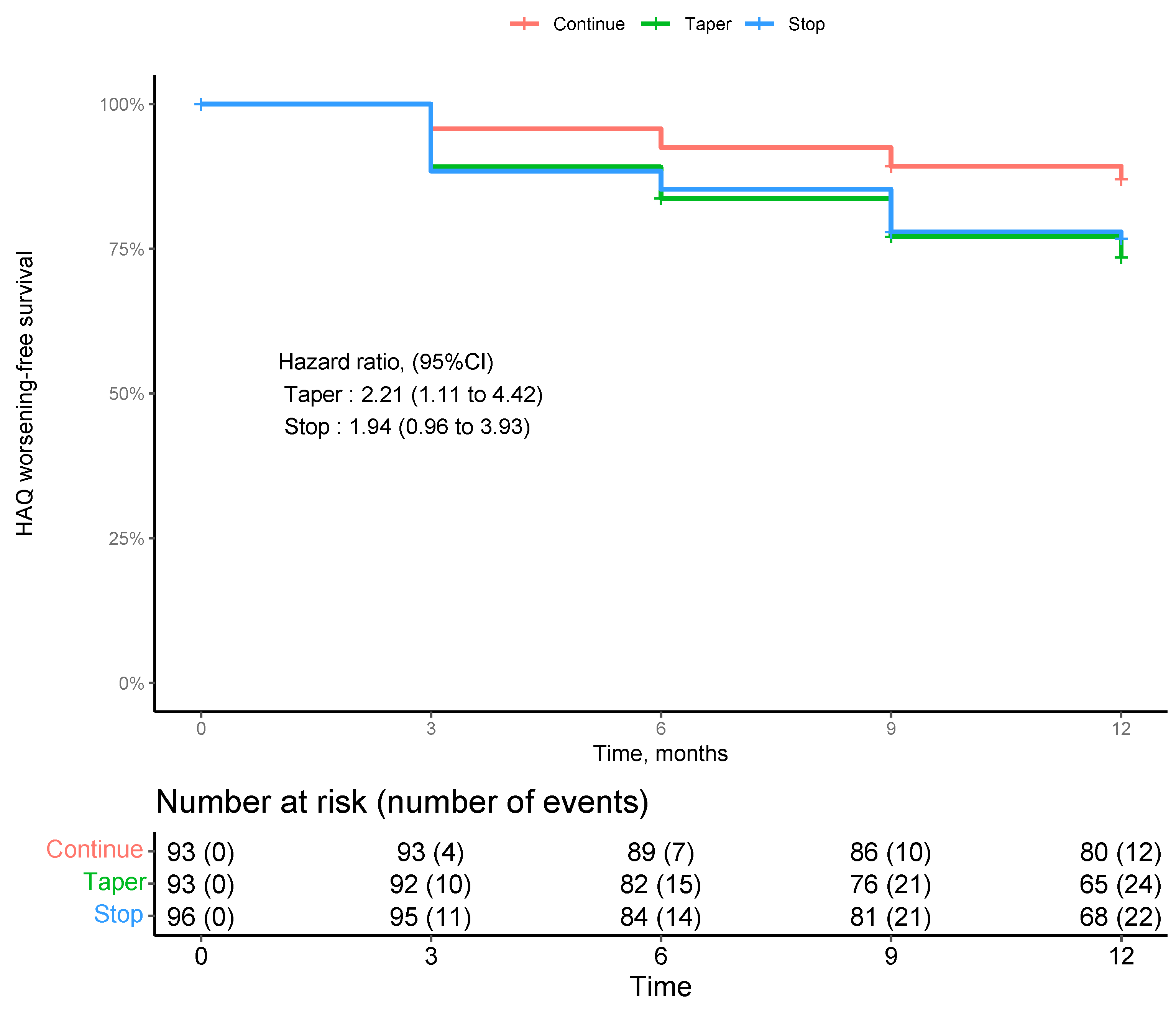
Although the mean HAQ values and change values were low, we performed a survival analysis for the risk of functional decline by study intervention. 282 patients with at least one post-baseline follow-up were included in this analysis. Overall, incident functional impairment was rare as shown in
Figure 2. Survival curves showed a numerically higher incident decline in physical function in the tapering and stopping groups. However, this difference was not across study groups (p=0.87 by log rank test of trend).
Survival analysis for the risk of functional decline by study group (continuation, tapering, stopping) . 282 patients with at least one post-baseline follow-up were included in this analysis. We did not find a significant difference of functional decline over time across study groups, while there was a trend to higher incident functional worsening in those patients tapering DMARDs.
4. Discussion
Reducing DMARD therapy becomes an option for RA patients in sustained remission. The possibility for reducing treatment in stable remission has been included into the ACR and EULAR criteria of management of RA [
2],[
10]. Still, the question is whether and under which conditions a patient with RA in stable remission could taper or stop DMARD therapy or whether treatment should be continued. Stopping DMARD therapy, of course, has the advantage of reducing adverse events and costs [
11],[
4]. While relapse rates were higher in patients tapering or stopping DMARDs, RETRO showed that more than half of them stayed in remission despite DMARD tapering [
3],[
8]. This suggested defining the accurate patient profile for tapering or even stopping DMARDs. Firstly tapering or stopping therapy should only be considered in patients with very low or complete absence of disease symptoms [
12]. Additionally, our previous data revealed that serological examination can help in predicting the relapse risk in patients tapering DMARD[
13]. J. Symptom duration at presentation and the absence of RA-specific autoantibodies are associated with sustained DMARD-free remission[
14].
Keeping good physical function is key to RA treatment and in interest for the patient. Hence, tapering or even stopping DMARD treatment should not put the patients at risk for deteriorating physical function. If tapering or stopping DMARD therapy does not worsen the physical function of the patient it might help to advocate the reduction of DMARDs in stable remission and in consequence reduce potential overtreatment and adverse events. Herein, we show that physical function does not deteriorate with the tapering of DMARDs. This observation can be explained by the fact that in case of flare of RA during DMARD tapering, effective DMARD therapy was re-installed and the patients responded well to this treatment and thereby improved in function. Hence, overall physical function remained good during the follow-ups. Flares may indeed have been the reason for spurious impairment of function during the trial, which was evident from the survival curves, showing that the tapering arms, which are characterized by higher flare rates, were more prone to lose, though spuriously, their functional remission status.
Limitations of the study are due to the fact that the data on function are limited to one year. Hence it cannot be completely rules out that after one year, there might be still a loss in physical function. However, we think this is unlikely as patients who had flared, were exposed to effective treatment and regained functional remission. Another limitation is that we used stable DAS28 of less than 2.6 units as an inclusion criterion allowing tapering or stopping DMARDs. ACR guidelines state that patients‘ medication should not be tapered unless the patient is in ACR/EULAR remission [
10]. However, most of these patients also fulfilled ACR/EULAR remission and were in deep remission for over a year upon inclusion [
3]. Hence, we think that this population is a representative RA population, in which treatment DMARD tapering is considered.
5. Conclusions
Point estimates and survival curves shows no decline in physical function after tapering or stopping DMARDs in RA patients with stable remission. While there is a tendency to a higher incidence of impaired physical function due to flares in the tapering groups, no overall decline in physical function was observed as RA patients experiences flares returned to their activate drug treatment and regained functional remission.
Author Contributions
SF, AJH, JR, and GS conceived, conceptualised and designed the trial, ME, JR, and GS developed the protocol and statistical analysis plan oft he RETRO study, AK, DS, AJH, BM, SF, H-PT, FS, SK, CPF, JFC, JW, MRo, MFe, MFl, KM, WO, MS-H, H-ML, HN, RA, KK, and JH recruited the patients and collected patient data, MRe oversaw trial sites, monitored data collection, and supervised data management. GS, KT, MS and JR verified the data, KT, MS, GS, and JR analysed and interpreted the data, KT did the statistical analyses, MS and JR wrote the first draft of the manuscript, and KT, MS, GS, and JR edited and developed the final version. Corresponding author JR.
Funding
This research received no external funding.
Informed Consent Statement
The study was approved by the Ethics Committee of the Medical University Erlangen-Nuremberg (Bearbeitungs Nr. 01_2010). Written informed consent was obtained from each patient, and the study was conducted in accordance with the declaration of Helsinki.
Acknowledgments
The present work was performed in fulfillment (by co-author Marlene Stephan) of the requirements for obtaining the degree “Dr. med.” at the Friedrich-Alexander-University Erlangen-Nuremberg.
Conflicts of Interest
The authors declare no conflict of interest.
References
- McInnes, I. B.; Schett, G. The Pathogenesis of Rheumatoid Arthritis. N. Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar] [CrossRef] [PubMed]
- Aletaha, D.; Neogi, T.; Silman, A. J.; Funovits, J.; Felson, D. T.; Bingham, C. O.; Birnbaum, N. S.; Burmester, G. R.; Bykerk, V. P.; Cohen, M. D.; Combe, B.; Costenbader, K. H.; Dougados, M.; Emery, P.; Ferraccioli, G.; Hazes, J. M. W.; Hobbs, K.; Huizinga, T. W. J.; Kavanaugh, A.; Kay, J.; Kvien, T. K.; Laing, T.; Mease, P.; Ménard, H. A.; Moreland, L. W.; Naden, R. L.; Pincus, T.; Smolen, J. S.; Stanislawska-Biernat, E.; Symmons, D.; Tak, P. P.; Upchurch, K. S.; Vencovský, J.; Wolfe, F.; Hawker, G. 2010 Rheumatoid Arthritis Classification Criteria: An American College of Rheumatology/European League Against Rheumatism Collaborative Initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef] [PubMed]
- Tascilar, K.; Hagen, M.; Kleyer, A.; Simon, D.; Reiser, M.; Hueber, A. J.; Manger, B.; Englbrecht, M.; Finzel, S.; Tony, H.-P.; Schuch, F.; Kleinert, S.; Wendler, J.; Ronneberger, M.; Figueiredo, C. P.; Cobra, J. F.; Feuchtenberger, M.; Fleck, M.; Manger, K.; Ochs, W.; Schmitt-Haendle, M.; Lorenz, H.-M.; Nuesslein, H.; Alten, R.; Kruger, K.; Henes, J.; Schett, G.; Rech, J. Treatment Tapering and Stopping in Patients with Rheumatoid Arthritis in Stable Remission (RETRO): A Multicentre, Randomised, Controlled, Open-Label, Phase 3 Trial. Lancet Rheumatol. 2021, 3, e767–e777. [Google Scholar] [CrossRef]
- Hagen, M.; Englbrecht, M.; Haschka, J.; Reiser, M.; Kleyer, A.; Hueber, A.; Manger, B.; Figueiredo, C.; Cobra, J. F.; Tony, H.-P.; Finzel, S.; Kleinert, S.; Wendler, J.; Schuch, F.; Ronneberger, M.; Feuchtenberger, M.; Fleck, M.; Manger, K.; Ochs, W.; Lorenz, H.-M.; Nüsslein, H.; Alten, R.; Henes, J.; Krüger, K.; Schett, G.; Rech, J. Cost-Effective Tapering Algorithm in Patients with Rheumatoid Arthritis: Combination of Multibiomarker Disease Activity Score and Autoantibody Status. J. Rheumatol. 2019, 46, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Verhoef, L. M.; Tweehuysen, L.; Hulscher, M. E.; Fautrel, B.; den Broeder, A. A. BDMARD Dose Reduction in Rheumatoid Arthritis: A Narrative Review with Systematic Literature Search. Rheumatol. Ther. 2017, 4, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Bechman, K.; Tweehuysen, L.; Garrood, T.; Scott, D. L.; Cope, A. P.; Galloway, J. B.; Ma, M. H. Y. Flares in Rheumatoid Arthritis Patients with Low Disease Activity: Predictability and Association with Worse Clinical Outcomes. J. Rheumatol. 2018, 45, 1515–1521. [Google Scholar] [CrossRef] [PubMed]
- Markusse, I. M.; Dirven, L.; Gerards, A. H.; van Groenendael, J. H. L. M.; Ronday, H. K.; Kerstens, P. J. S. M.; Lems, W. F.; Huizinga, T. W. J.; Allaart, C. F. Disease Flares in Rheumatoid Arthritis Are Associated with Joint Damage Progression and Disability: 10-Year Results from the BeSt Study. Arthritis Res. Ther. 2015, 17, 232. [Google Scholar] [CrossRef] [PubMed]
- Haschka, J.; Englbrecht, M.; Hueber, A. J.; Manger, B.; Kleyer, A.; Reiser, M.; Finzel, S.; Tony, H.-P.; Kleinert, S.; Feuchtenberger, M.; Fleck, M.; Manger, K.; Ochs, W.; Schmitt-Haendle, M.; Wendler, J.; Schuch, F.; Ronneberger, M.; Lorenz, H.-M.; Nuesslein, H.; Alten, R.; Demary, W.; Henes, J.; Schett, G.; Rech, J. Relapse Rates in Patients with Rheumatoid Arthritis in Stable Remission Tapering or Stopping Antirheumatic Therapy: Interim Results from the Prospective Randomised Controlled RETRO Study. Ann. Rheum. Dis. 2016, 75, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Li, N.; Peterson, S. Minimal Important Difference in HAQ: A Validation from Health Economic Perspectives in Patient with Rheumatoid Arthritis Using Real-World Data from Adelphi Database. Ann. Rheum. Dis. 2016, 75 (Suppl 2), 193–193. [Google Scholar] [CrossRef]
- Singh, J. A.; Saag, K. G.; Bridges, S. L.; Akl, E. A.; Bannuru, R. R.; Sullivan, M. C.; Vaysbrot, E.; McNaughton, C.; Osani, M.; Shmerling, R. H.; Curtis, J. R.; Furst, D. E.; Parks, D.; Kavanaugh, A.; O’Dell, J.; King, C.; Leong, A.; Matteson, E. L.; Schousboe, J. T.; Drevlow, B.; Ginsberg, S.; Grober, J.; St. Clair, E. W.; Tindall, E.; Miller, A. S.; McAlindon, T. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatol. 2016, 68, 1–26. [Google Scholar] [CrossRef]
- Klarenbeek, N. B.; Kooij, S. M. van der; Güler-Yüksel, M.; Groenendael, J. H. L. M. van; Han, K. H.; Kerstens, P. J. S. M.; Huizinga, T. W. J.; Dijkmans, B. a. C.; Allaart, C. F. Discontinuing Treatment in Patients with Rheumatoid Arthritis in Sustained Clinical Remission: Exploratory Analyses from the BeSt Study. Ann. Rheum. Dis. 2011, 70, 315–319. [Google Scholar] [CrossRef]
- Schett, G.; Emery, P.; Tanaka, Y.; Burmester, G.; Pisetsky, D. S.; Naredo, E.; Fautrel, B.; van Vollenhoven, R. Tapering Biologic and Conventional DMARD Therapy in Rheumatoid Arthritis: Current Evidence and Future Directions. Ann. Rheum. Dis. 2016, 75, 1428–1437. [Google Scholar] [CrossRef] [PubMed]
- Rech, J.; Hueber, A. J.; Finzel, S.; Englbrecht, M.; Haschka, J.; Manger, B.; Kleyer, A.; Reiser, M.; Cobra, J. F.; Figueiredo, C.; Tony, H.-P.; Kleinert, S.; Wendler, J.; Schuch, F.; Ronneberger, M.; Feuchtenberger, M.; Fleck, M.; Manger, K.; Ochs, W.; Schmitt-Haendle, M.; Lorenz, H.-M.; Nuesslein, H.; Alten, R.; Henes, J.; Krueger, K.; Schett, G. Prediction of Disease Relapses by Multibiomarker Disease Activity and Autoantibody Status in Patients with Rheumatoid Arthritis on Tapering DMARD Treatment. Ann. Rheum. Dis. 2016, 75, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
- van der Woude, D.; Young, A.; Jayakumar, K.; Mertens, B. J.; Toes, R. E. M.; van der Heijde, D.; Huizinga, T. W. J.; van der Helm-van Mil, A. H. M. Prevalence of and Predictive Factors for Sustained Disease-Modifying Antirheumatic Drug-Free Remission in Rheumatoid Arthritis: Results from Two Large Early Arthritis Cohorts. Arthritis Rheum. 2009, 60, 2262–2271. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).