Submitted:
26 April 2023
Posted:
27 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Inclusion criteria
2.2. Exclusion criteria
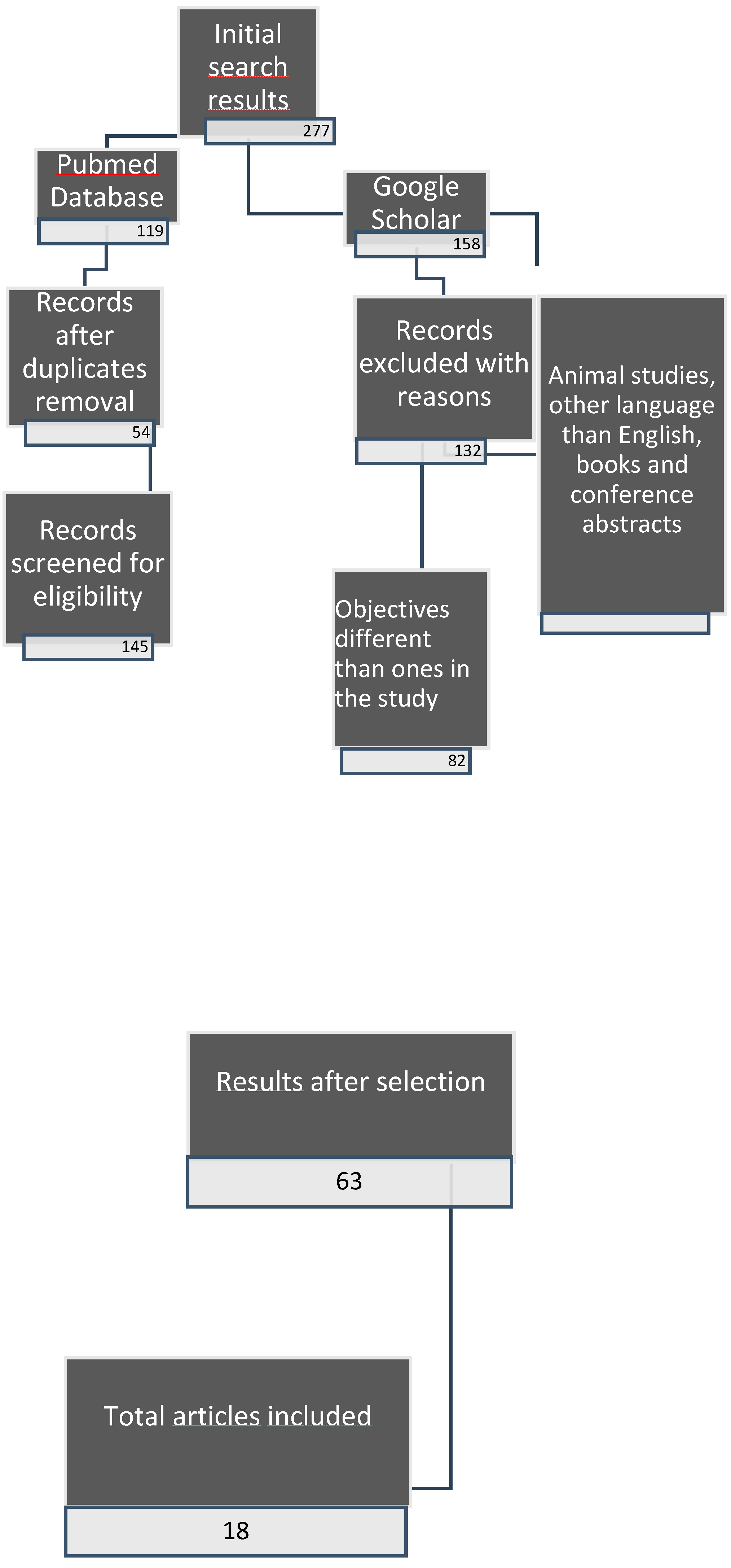
2.3. Data selection protocol
2.4. Study selection
2.5. Statistical analysis
3. Results
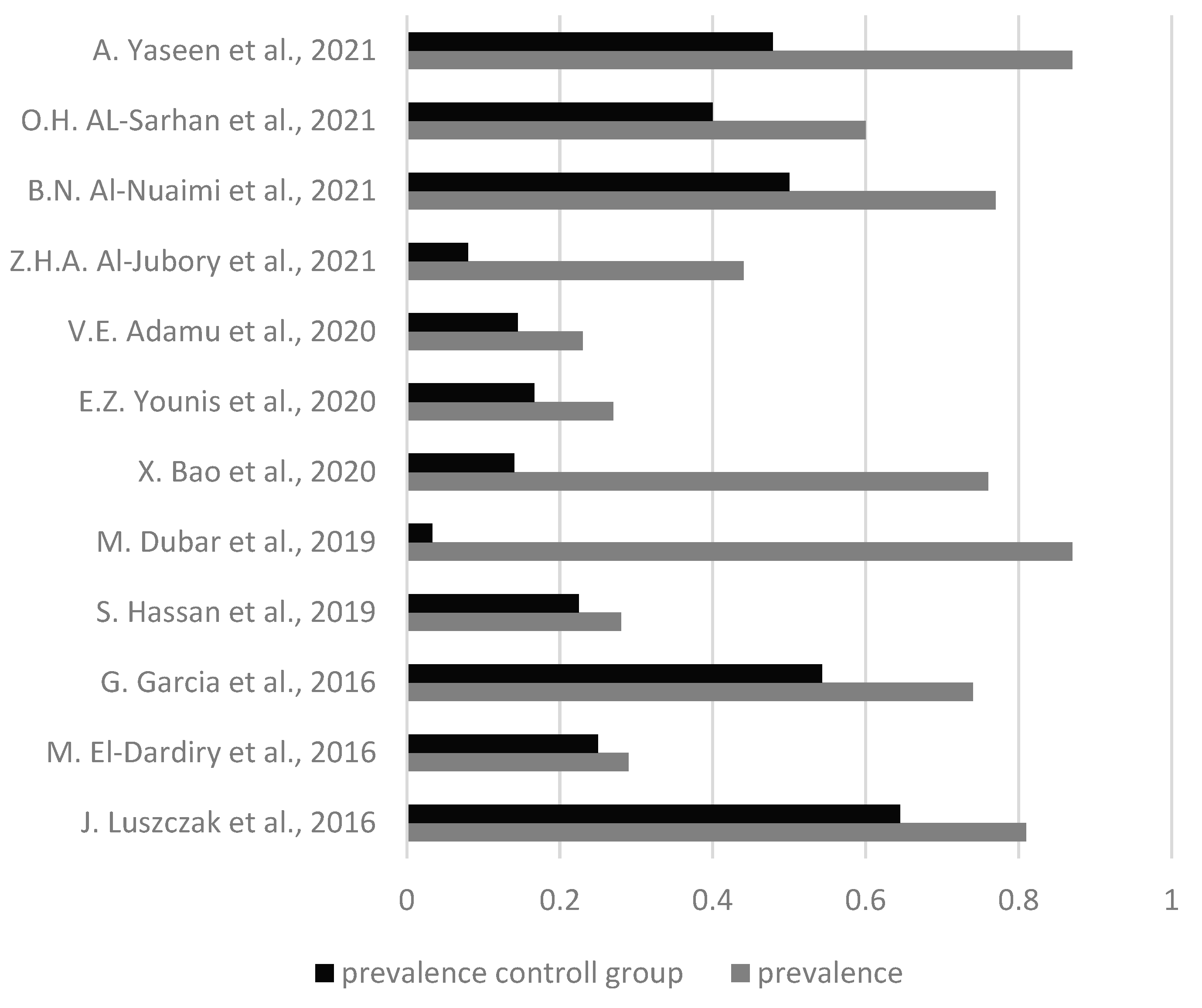
3.1. Results based on Periodontal disease
3.2. Results based on Dental health
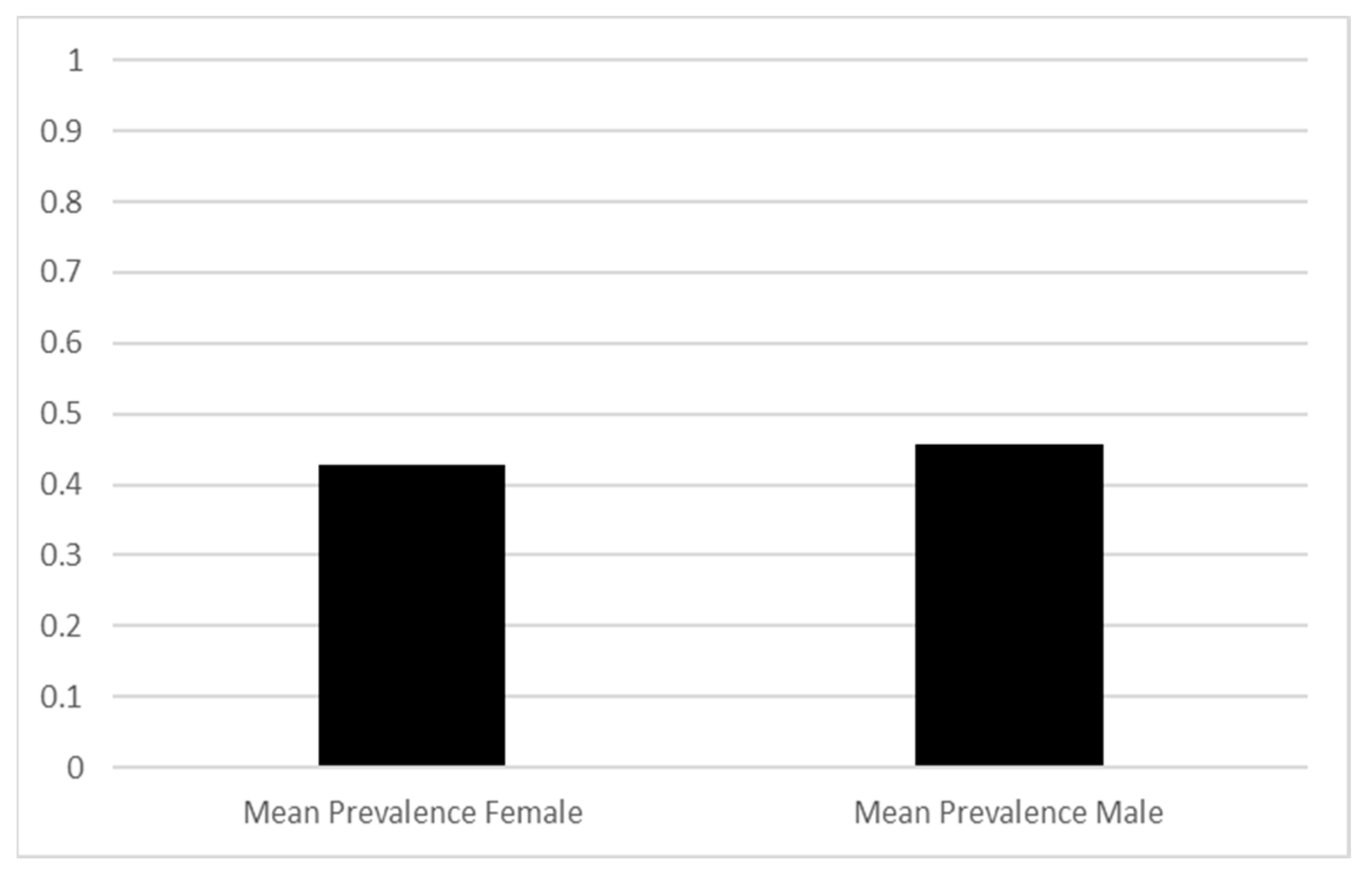
3.3. Results based on the gender of the patients
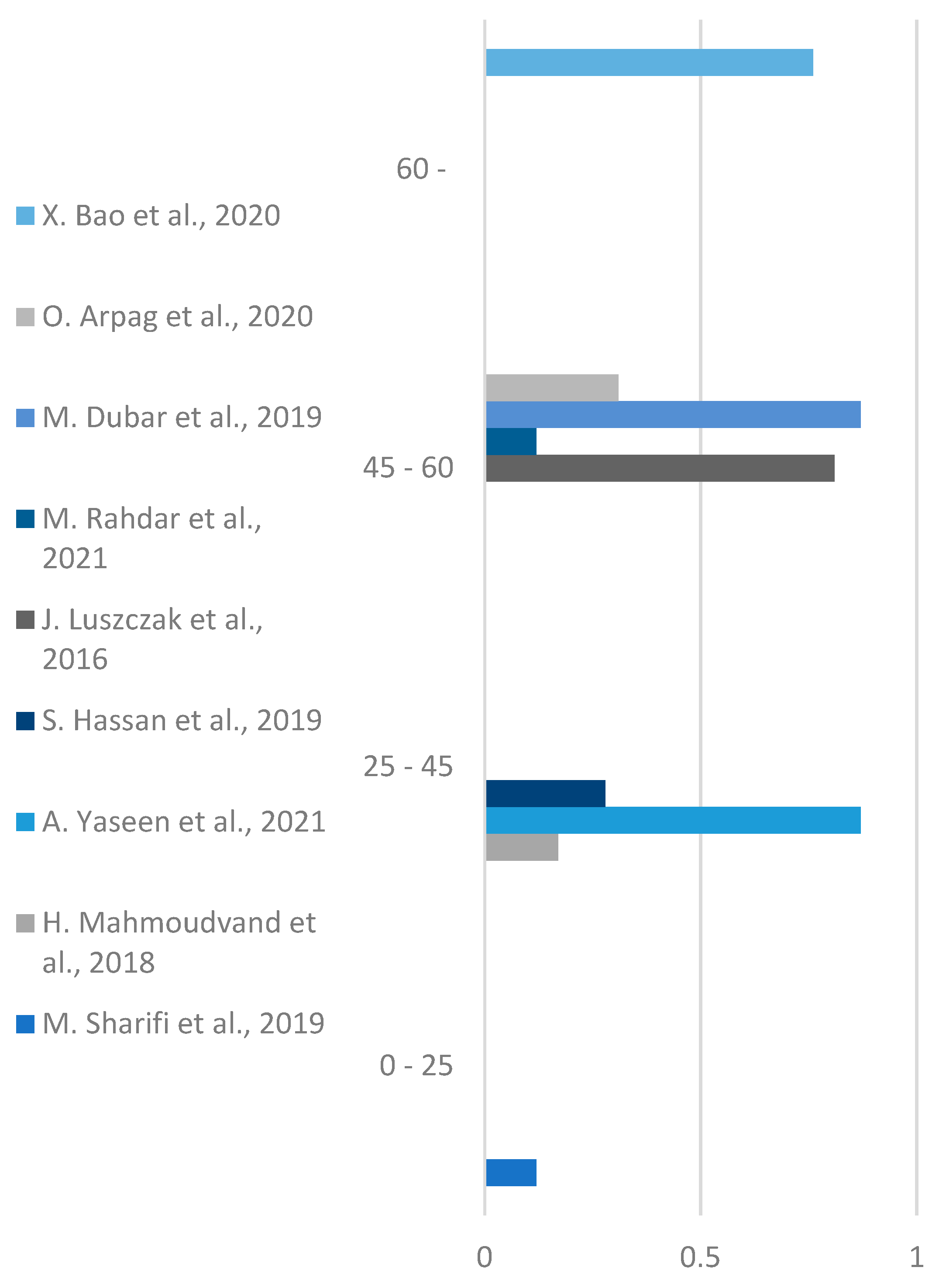
Results based on Age
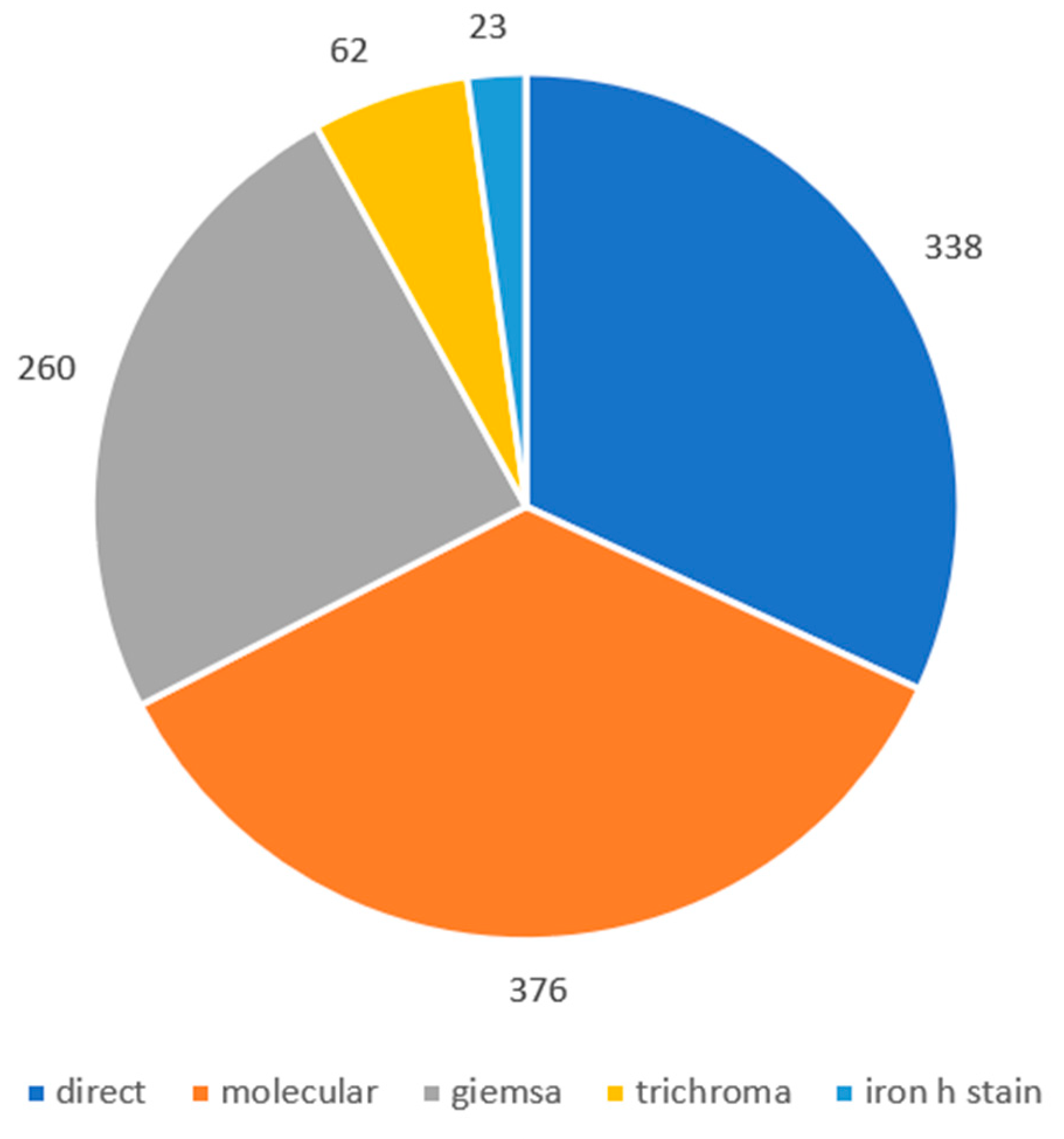
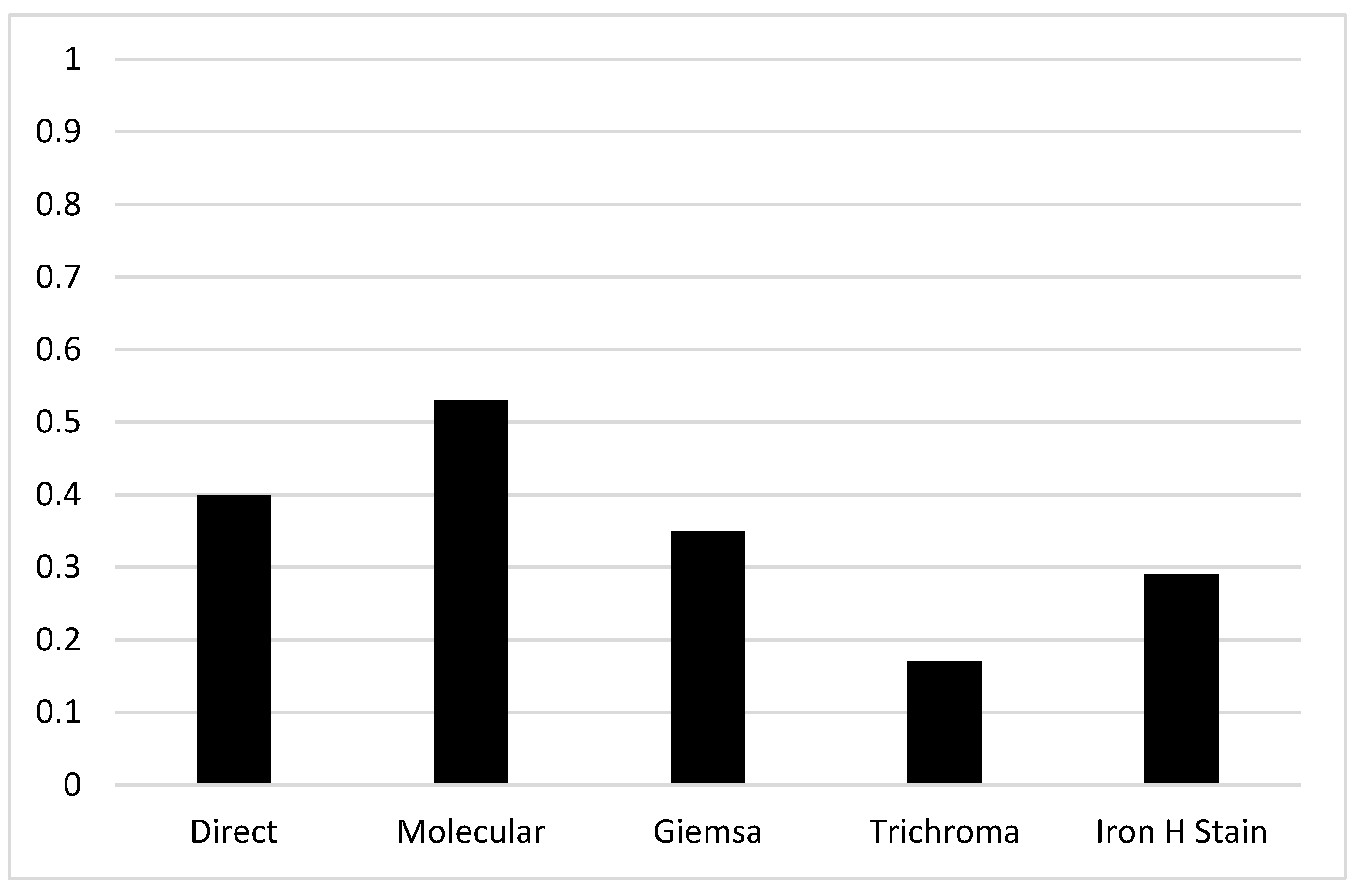
3.4. Results based on the method used
3.5. Results based on Sample collected
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hijryana, M.; MacDougall, M.; Ariani, N.; Kusdhany, L.S.; Walls, A.W.G. Impact of Periodontal Disease on the Quality of Life of Older People in Indonesia: A Qualitative Study. JDR Clin Trans Res, 2021, 22:23800844211041911. [CrossRef]
- The CDC overview of Periodontal disease Available online: https://www.cdc.gov/oralhealth/conditions/periodontal-disease.html (accessed on May 30,2022).
- Chen, C.; Hemme, C.; Beleno, J.; Shi, Z.J.; Ning, D. Oral microbiota of periodontal health and disease and their changes after nonsurgical periodontal therapy. ISME J, 2018, 12: 1210-1224. [CrossRef]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: dynamic communities and host interactions. Nat Rev Microbiol, 2018, 16(12): 745-759. [CrossRef]
- Kilian, M.; Chapple, I.; Hannig, M. The oral microbiome—an update for oral healthcare professionals, Br Dent J, 2016, 221: 657-666. [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation, Cell, 2014, 157(1): 121-41. [CrossRef]
- Newman, M.G.; Takei, H.H.; Klokkevold, P.R.; Carranza, A. Carranza’s Clinical Periodontology Twelfth edition, Elsevier, St. Louis, Missouri, USA, 2015, 138-146.
- Suzuki, N.; Yoneda, M.; Hirofuji, T. Mixed red-Complex bacterial infection in periodontitis, Int J Dent, 2013, 2013:587279. [CrossRef]
- Bao, X.; Wiehe, R.; Dommisch, H.; Schaefer, A.S. Entamoeba gingivalis causes Oral inflammation and tissue destruction, J Dent Res, 2020, 99(5): 561-567. [CrossRef]
- El-Dib, N.A.; Khater, M.M. Entamoeba. In: Rezaei N., editor. Encyclopedia of Infection and Immunity. Elsevier; Oxford, UK: 2022. 492–512.
- Bonner, M.; Fresno, M.; Gironès, N.; Guillén, N.; Santi-Rocca, J. Reassessing the Role of Entamoeba gingivalis in Periodontitis. Front Cell Infect Microbiol, 2018, 8:379. [CrossRef]
- Mielnik-Błaszczak, M.; Rzymowska, J.; Michałowski, A.; Skawińska-Bednarczyk, A.; Błaszczak, J. Entamoeba gingivalis—prevalence and correlation with dental caries in children from rural and urban regions of Lublin Province, Eastern Poland. Ann Agric Environ Med, 2018, 25(4): 656-658. [CrossRef]
- Luszczak, J.; Bartosik, M.; Rzymowska, J.; Sochaczewska-Dolecka, A.; Tomaszek, E. The occurrence of Entamoeba gingivalis among patients with periodontal disease, Curr. Issues Pharm. Med Sci, 2016, 29(2):86-89. [CrossRef]
- El-Dardiry, M.A.; Shabaan, S.H. Detection of Entamoeba gingivalis trophozoites in patients suffering from gingivitis versus healthy subjects, AENSI J, 2016, 10(12): 222-226.
- Garcia, G.; Ramos, F.; Maldonado, J.; Fernandez, A.; Yáñez, J.; Hernandez, L. Prevalence of two Entamoeba gingivalis ST1 and ST2-kamaktli subtypes in the human oral cavity under various conditions, Parasitol Res, 2018, 117(9): 2941-2948. [CrossRef]
- Mahmoudvand, H.; Saedi Dezaki, S.; Soleimani, S.; Baneshi, M.R. Seroprevalence and risk factors of Toxoplasma gondii infection among healthy blood donors in south-east of Iran. Parasite Immunology, 2016, 37 (7), 362-367. [CrossRef]
- Hassan, S.S.; Madkour, G.G.; Henin, R.W.; Gad, S.W.F.; Abd El-Aal, A.A. Is Entamoeba Gingivalis a Risk Factor for Periodontal Diseases? A Case-Control Study. Perio J, 2019, 3(1): 18–28. [CrossRef]
- Sharifi, M.; Jahanimoghadam, F.; Babaei, Z.; Mohammadi, M.A.; Sharifi, A. Prevalence and Associated-Factors for entamoeba gingivalis in Adolescents in Southeastern Iran by Culture and PCR. Iran J pub Health, 2020, 49(2): 351-359. [CrossRef]
- Dubar, M.; Zaffino, M.L.; Remen, T.; Thilly, N.; Cunat, L.; Machouart, M.C.; Bisson, C. Protozoans in subgingival biofilm: clinical and bacterial associated factors and impact of scaling and root planning treatment. J Oral Microbiol, 2019, 12(1): 1693222. [CrossRef]
- Arpag, O.F. Presence of Trichomonas tenax and Entamoeba gingivalis in peri-implantitis lesions. Quintessence Intrnational ,2020, 51(3). [CrossRef]
- Younis, E.Z.; Khater, H.F.; Hayam Elawamy, A.; Aldinali, A.; Rabia El Ghazal, B. Biochemical Assays and Epidemiological Status of Visceral Leishmaniasis among Patient Attending to Benghazi Children’s Hospital. Ann Microbiol Immunol, 2020, 3(1): 1022.
- Adamu, V.E.; Eneojo, N.I.F.; Amaechi, A.A.; Nwoke, B.E.B.; Ajaero, C.M.U. Periodontal health and human oral protozoa in parts of Enugu State, Nigeria. Orap J, 2020, 1(1): 701.
- Ani, O. C.; Agbo, E. E.; Nnamonu, E. I.; Onyeidu, S. O.; Onyeidu, B. U.; Okwerekwu, N. J. Rising Profile of Oral Cavity Protozoa amongst Dental Patients in South Eastern Nigeria. LIFE: International Journal of Health and Life-Sciences, 2020,6(2), 34-42. [CrossRef]
- Al-Jubory, Z.H.; Al-Hamairy, A.K. Molecular Study of entamoeba gingivalis and trichomonas tenax among plaque-induced gingivitis patients in Babylon Province. Annals of R.S.C.B, 2021, 25(6): 14012-14027.
- Rahdar, M.; Abolfazli-Karizi, S.; Pedram, H. The Comparison of Entamoeba gingivalis Presence in Healthy and Periodontitis Patients by Using Direct Examination and PCR Methods. Jundishapur J Health. Sci, 2019, 11(1). [CrossRef]
- Al-Nuaimi, B.N.; Al-Taee, A.F.; Al-Kattan, M.M. Conventional and Molecular Identification of Entamoeba gingivalis from Periodontitis Patients in Nineveh Governorate /Iraq. Ann. Romanian Soc. Cell Biol, 2021, 25(4): 1293-1306.
- AL-Sarhan, O.H.; Mohamed, A.A.; Saeed, A.Y. Detection of entamoeba gingivalis by PCI technology and its association with oral diseases. Parasite,2021, 21: 30. [CrossRef]
- Stensvold, C.R.; Lebbad, M.; Victory, E.L.; Verweij, J.J.; Tannich, E.; Alfellani, M.; Legarraga, P.; Clark, C.G. Increased sampling reveals novel lineages of Entamoeba: Consequences of genetic diversity and host specificity for taxonomy and molecular detection. Protist, 2011, 162, 525–541. [CrossRef]
- Yaseen, A.; Mahafzah, A.; Dababseh, D.; Taim, D.; Hamdan, A.A.; Al-Fraihat, E. Oral Colonization by Entamoeba gingivalis and Trichomonas tenax: A PCR-Based Study in Health, Gingivitis, and Periodontitis. Front Cell Infect Microbiol, 2021, 11: 782805. [CrossRef]
- Meyer, M.S.; Joshipura, K.; Giovannucci, E.; Michaud, D.S. A review of the relationship between tooth loss, periodontal disease, and cancer. Cancer Causes Control, 2008, 19(9): 895-907. [CrossRef]
- Winning, L.; Kinden, G.J. Periodontitis and systemic disease. BDJ team, 2015, 2(10). [CrossRef]
- Irani, S.; Barati, I.; Badiei, M. Periodontitis and oral cancer—current concepts of the etiopathogenesis. Oncol Rev, 2020, 14(1): 465. [CrossRef]
- Tonetti, M.S.; Jepsen, S.; Jin, L.; Otoma-Corgel, J. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: A call for global action. J Clin Periodontol, 2017, 44(5): 456-462. [CrossRef]
- Sedghi, L.M.; Bacino, M.; Kapila, Y.L. Periodontal Disease: The Good, The Bad, and the Unknown, Jundishapur. J. Health. Sci, 2021, 11: 766944. [CrossRef]
- Di Stefano, M.; Polizzi, A.; Santonocito, S.; Romano, A.; Lombardi, T.; Isola, G. Impact of oral microbiome in periodontal health and Periodontitis: A critical review on prevention and treatment. Int. J. Mol. Sci., 2022, 23(9): 5142. [CrossRef]
- Badri, M.; Olfatifar, M.; Abdoli, A.; Houshmand, E.; Zarabadipour, M.; Abadi, P.A. Current Global Status and the Epidemiology of Entamoeba gingivalis in Humans: A Systematic Review and Meta-analysis. Acta Parasitol, 2021, 66(4): 1102-1113. [CrossRef]
- Santi-Rocca, J. The Protozoome of the Periodontal Sulcus: From Health to Disease. Springer, 2020, 113-131.
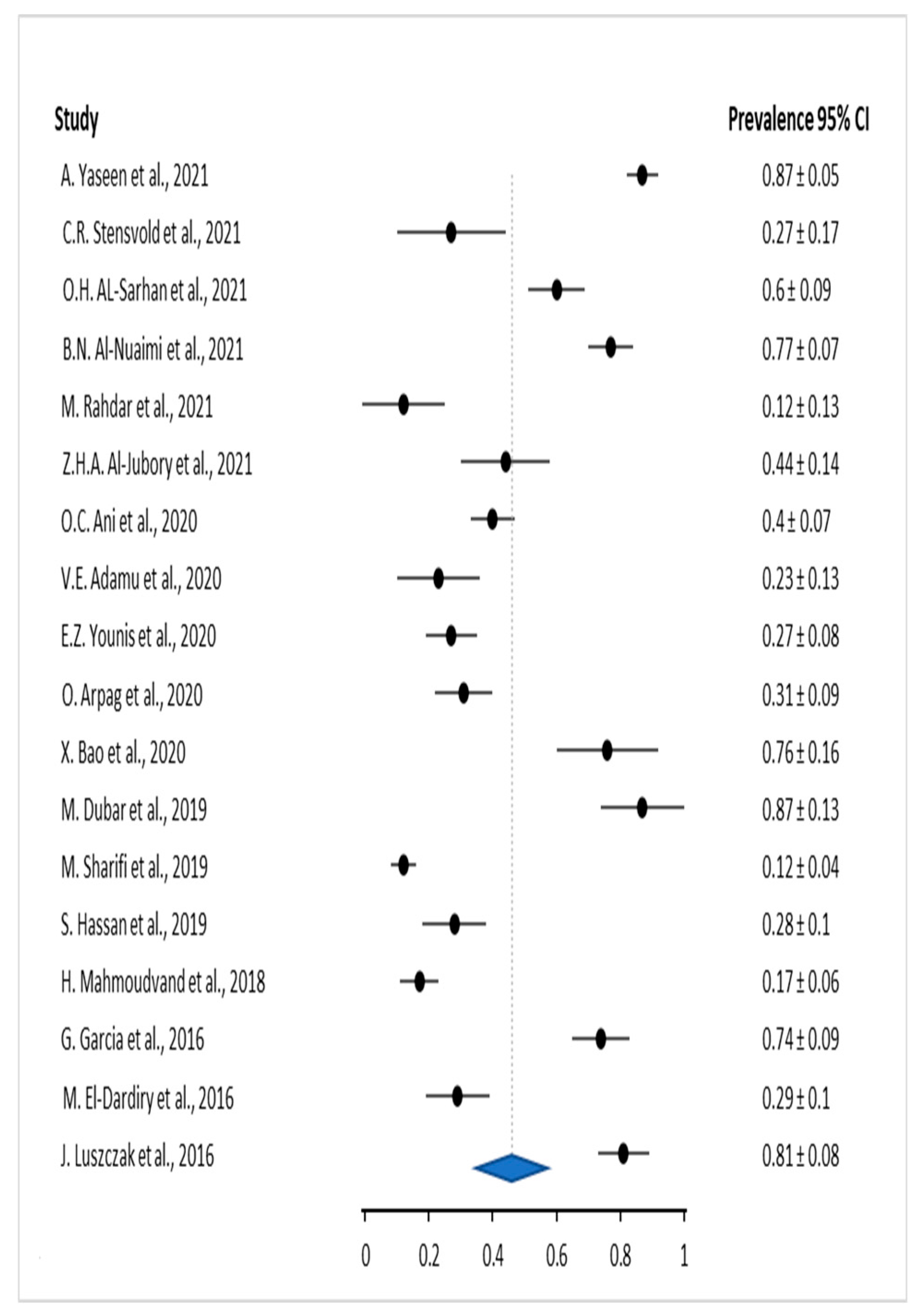
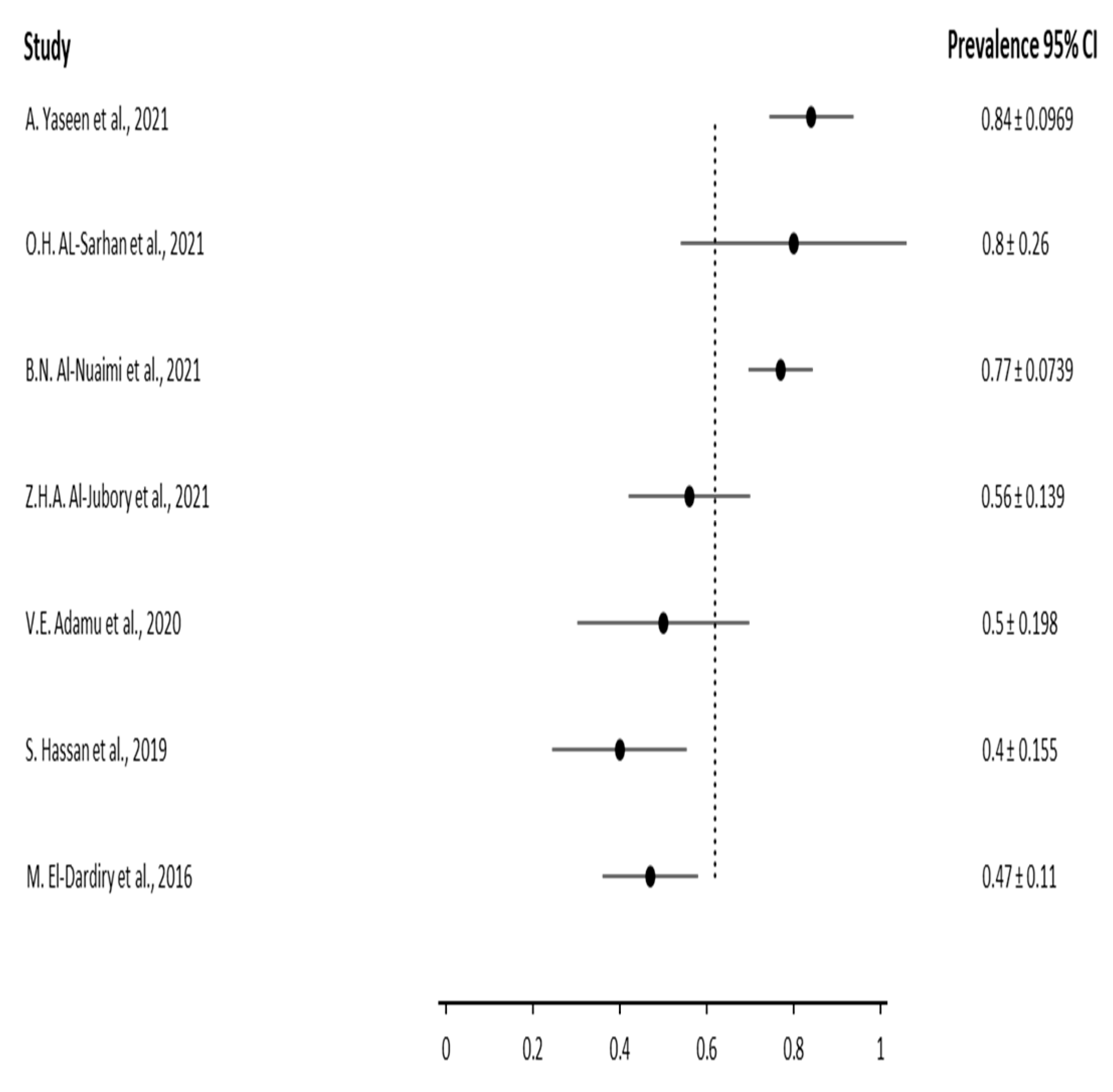
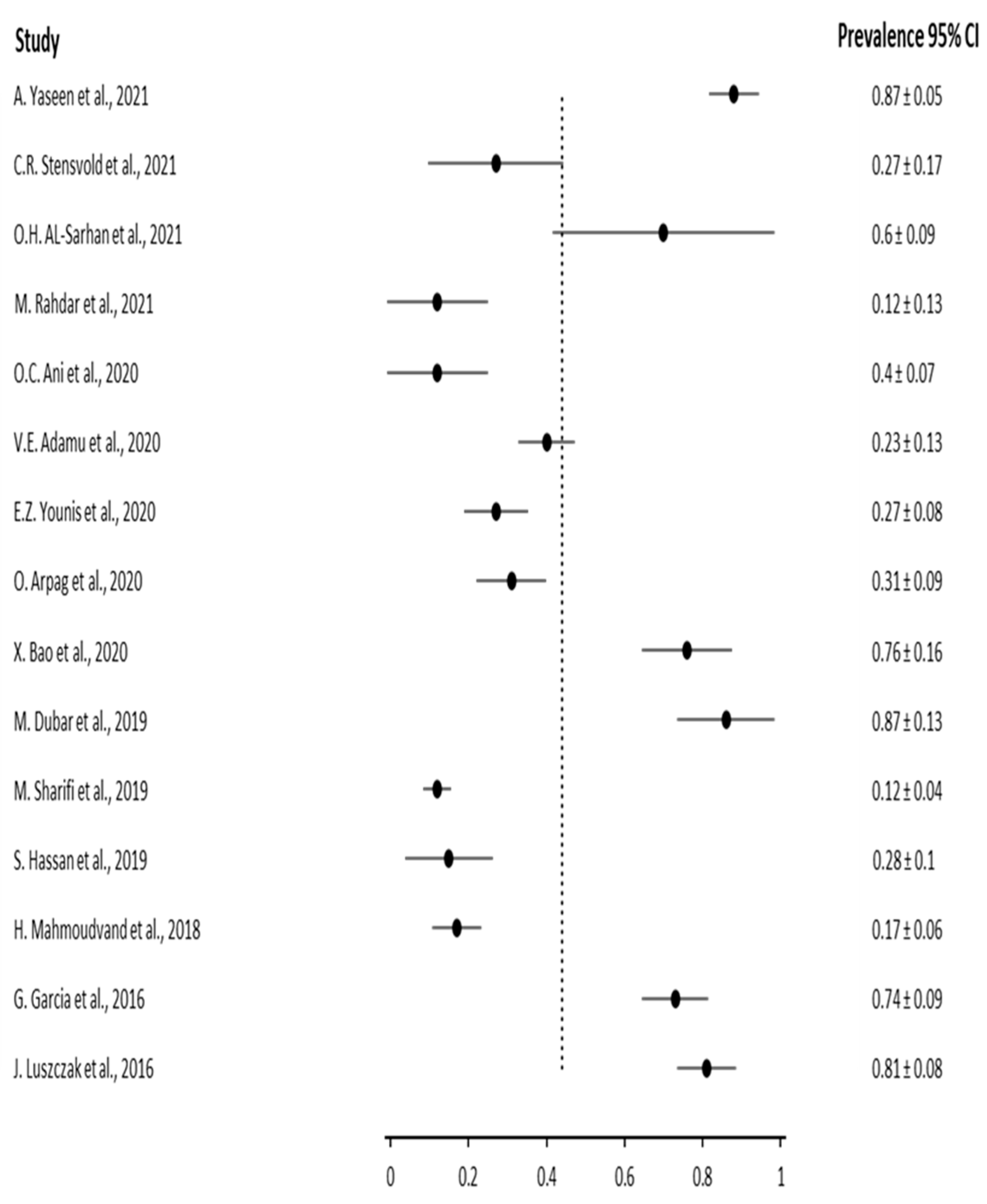
| Author | Year | Sample size (number of patients) | Infected patients | |
|---|---|---|---|---|
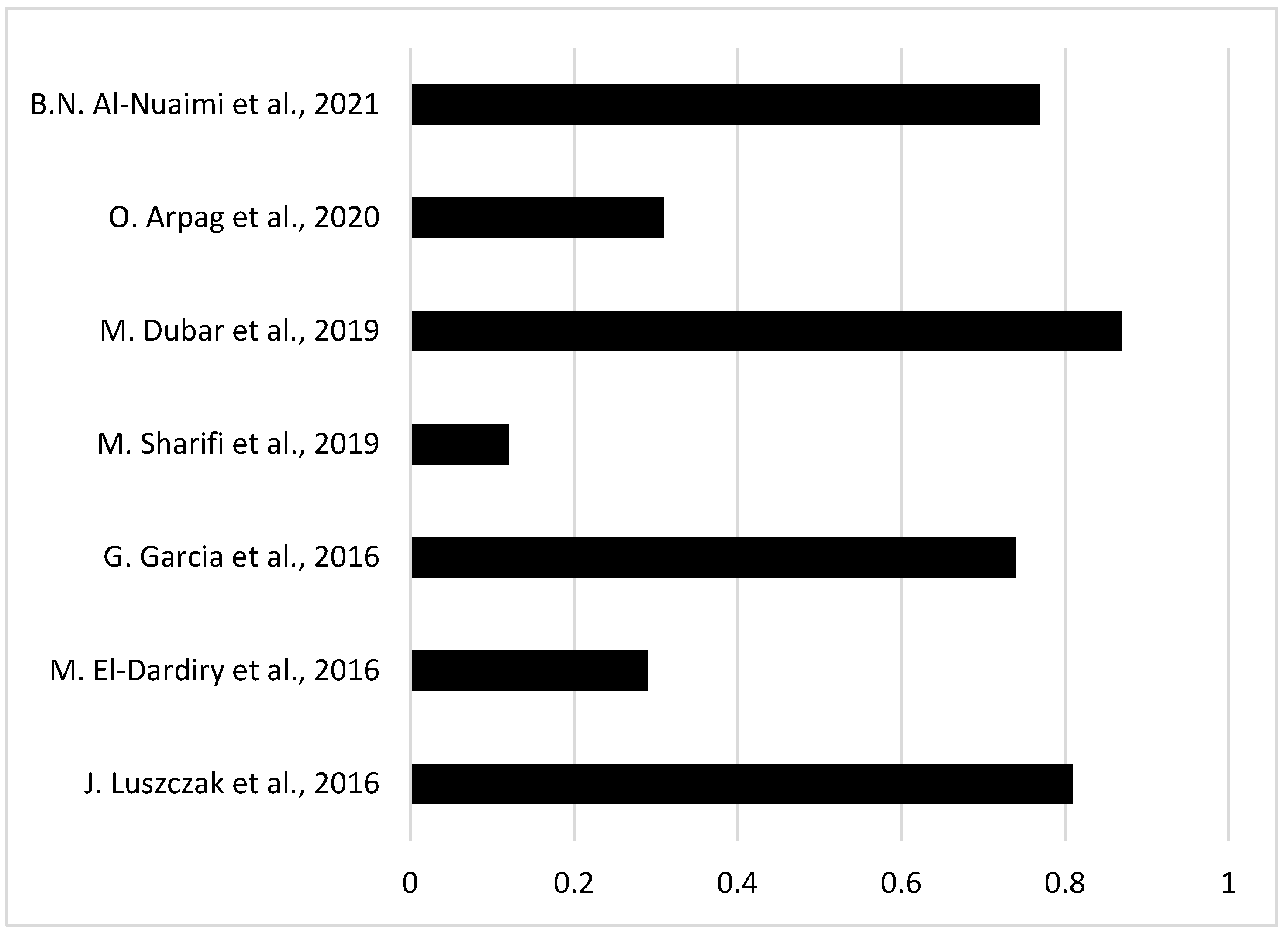
| 1 | J. Luszczak et. al. [13] | 2016 | 102 | 83 |
| 2 | M. El-Dardiry et. al. [14] | 2016 | 80 | 23 |
| 3 | G. Garcia et. al. [15] | 2018 | 102 | 75 |
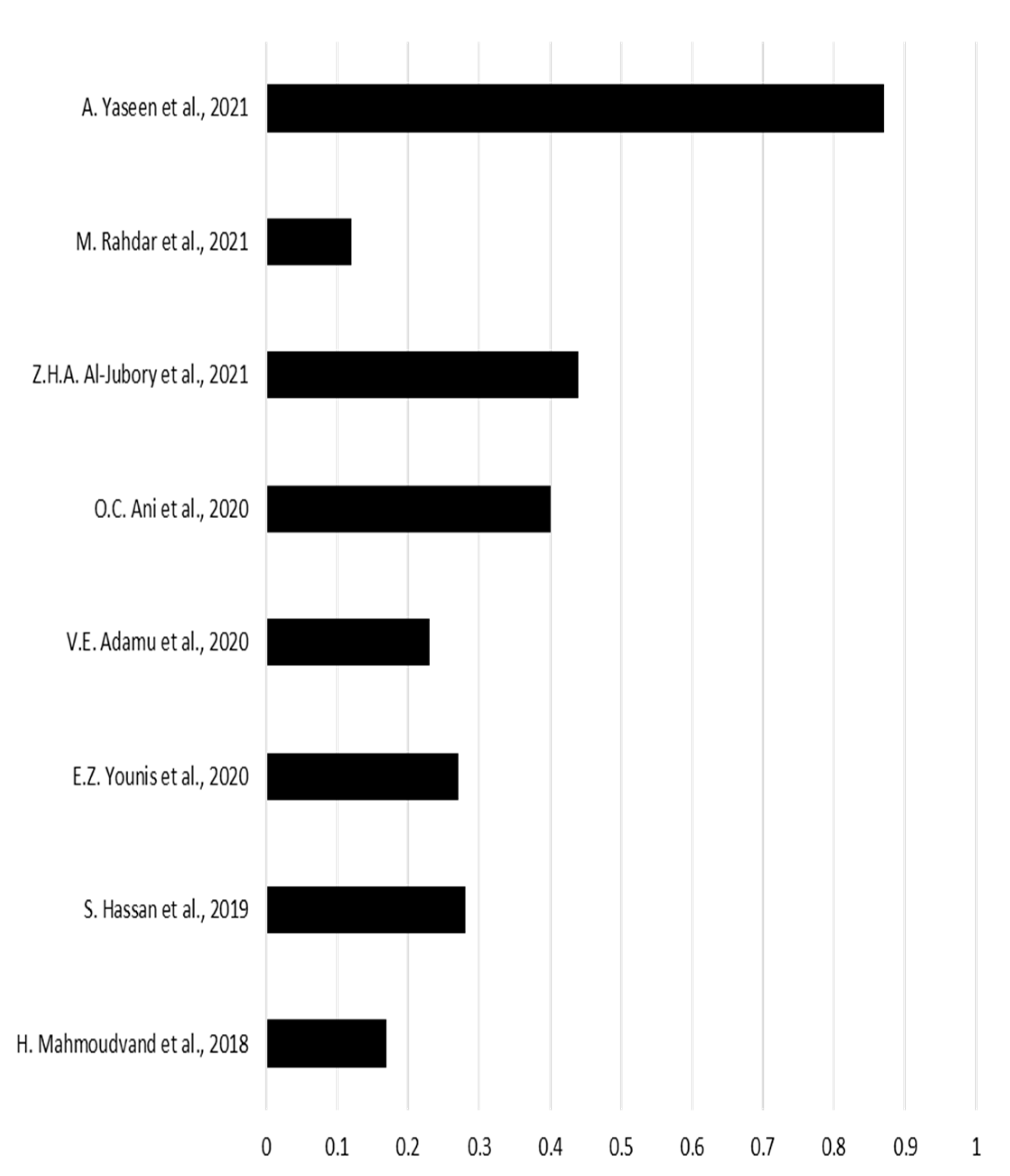
| 4 | H. Mahmoudvand et al. [16] | 2019 | 140 | 24 |
| 5 | S. Hassan et. al. [17] | 2019 | 80 | 22 |
| 6 | M. Sharifi et. al. [18] | 2019 | 315 | 37 |
| 7 | M. Dubar et. al. [19] | 2019 | 30 | 26 |
| 8 | X. Bao et. al. [9] | 2020 | 51 | 39 |
| 9 | O. Arpag et. al. [20] | 2020 | 101 | 31 |
| 10 | E.Z. Younis et.al. [21] | 2020 | 70 | 19 |
| 11 | V.E. Adamu et. al. [22] | 2020 | 40 | 9 |
| 12 | O.C. Ani et.al. [23] | 2020 | 180 | 72 |
| 13 | Z.H.A. Al-Jubory et. al. [24] | 2021 | 50 | 22 |
| 14 | M. Rahdar et. al. [25] | 2021 | 25 | 3 |
| 15 | B.N. Al-Nuaimi et. al. [26] | 2021 | 124 | 96 |
| 16 | O.H. Al-Sarhan et. al. [27] | 2021 | 70 | 42 |
| 17 | C.R. Stensvold et al. [28] | 2021 | 26 | 7 |
| 18 | A.Yaseen et.al. [29] | 2021 | 143 | 125 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





