Submitted:
27 April 2023
Posted:
27 April 2023
You are already at the latest version
Abstract
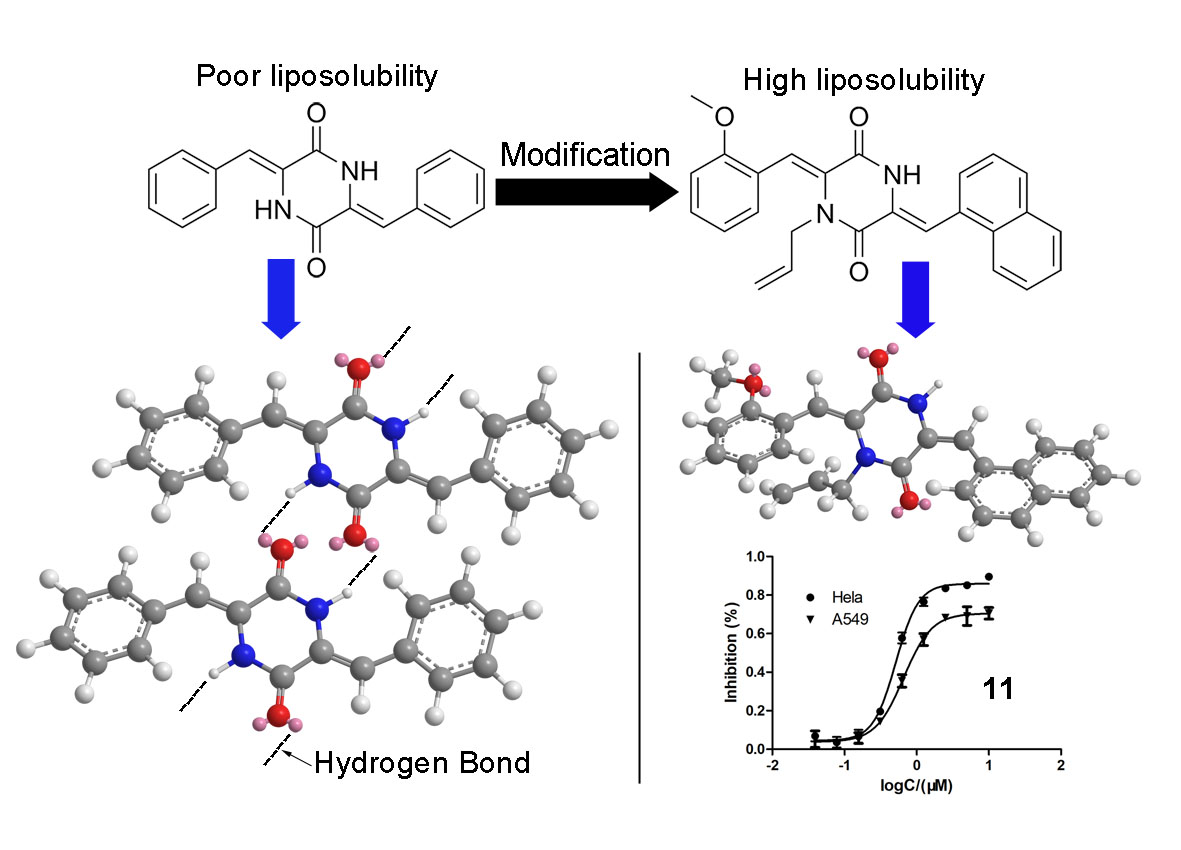
Keywords:
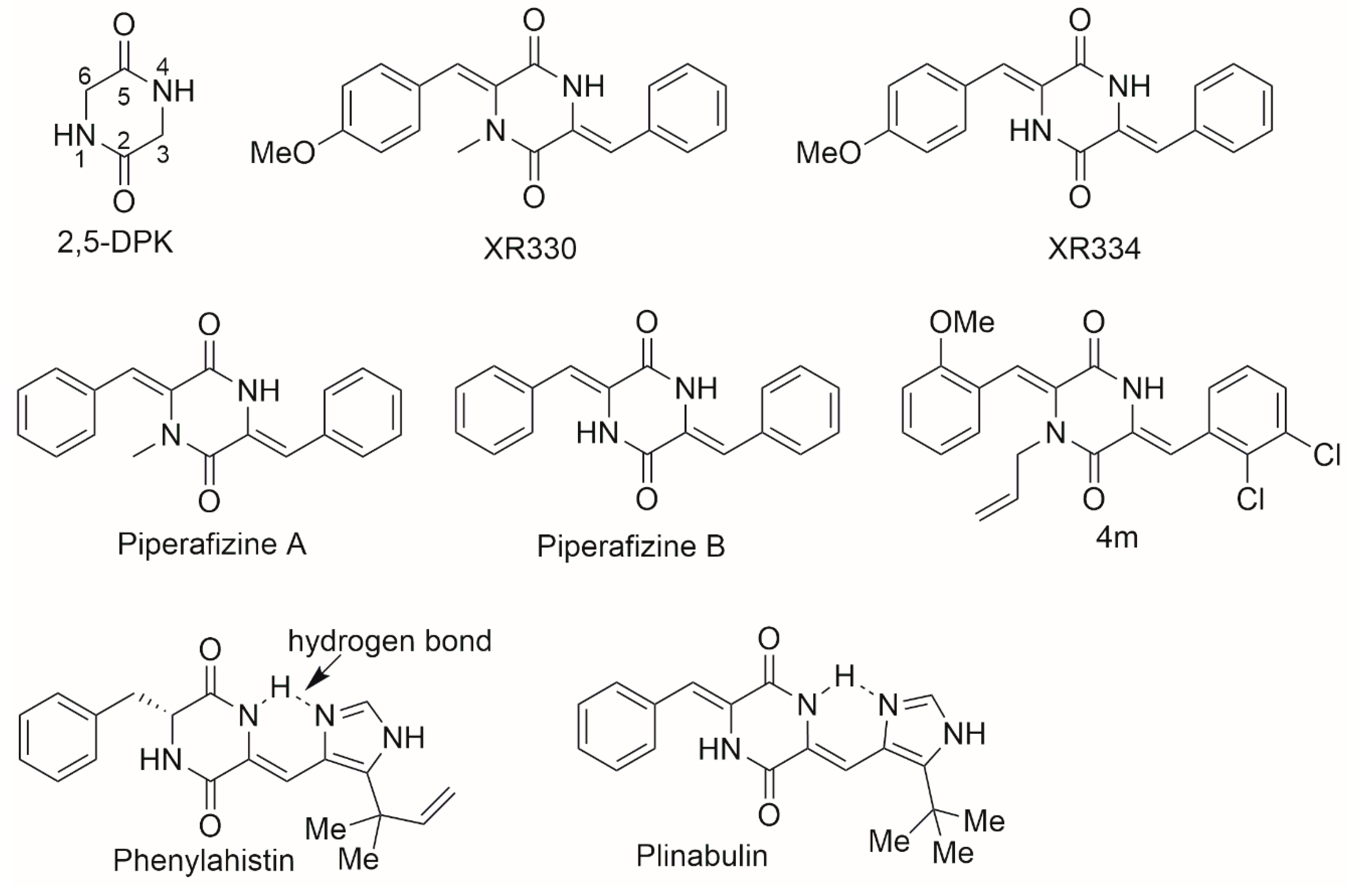
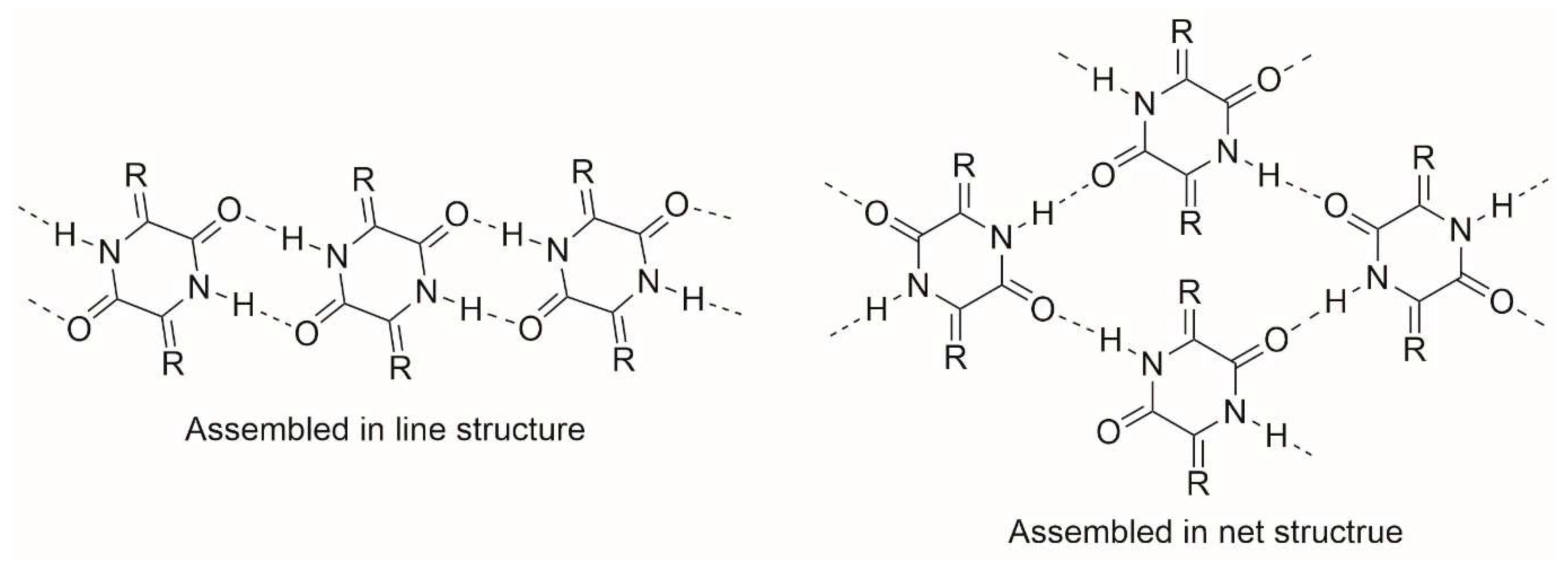
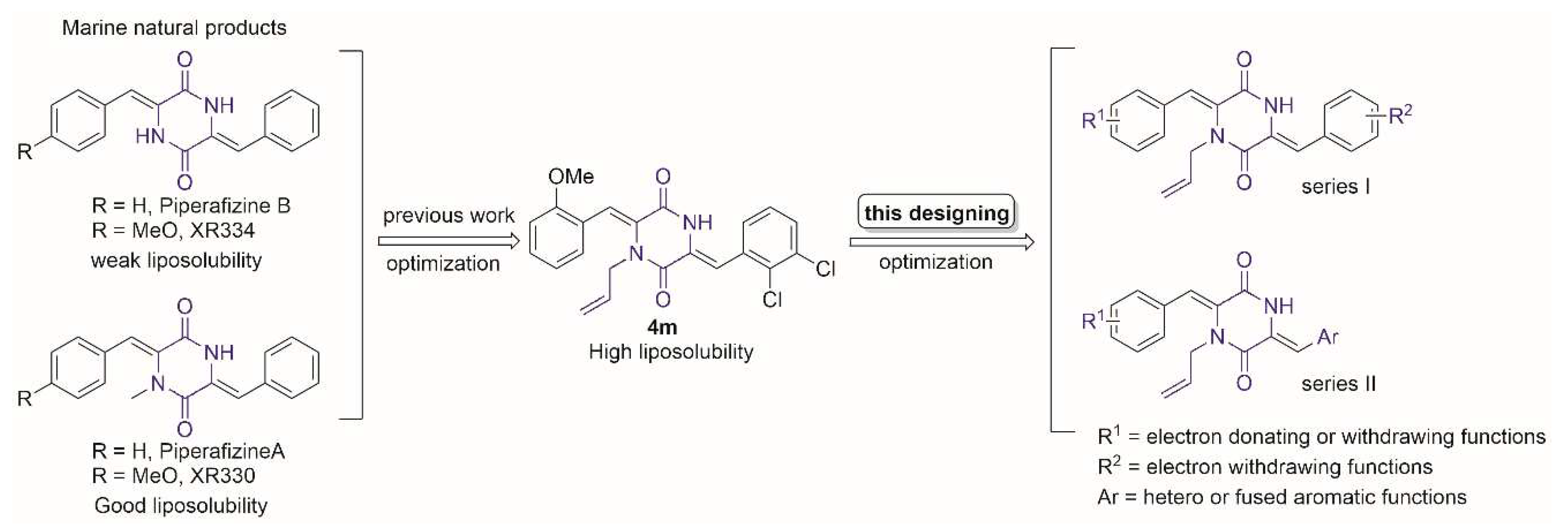
1. Introduction
2. Results and Discussion
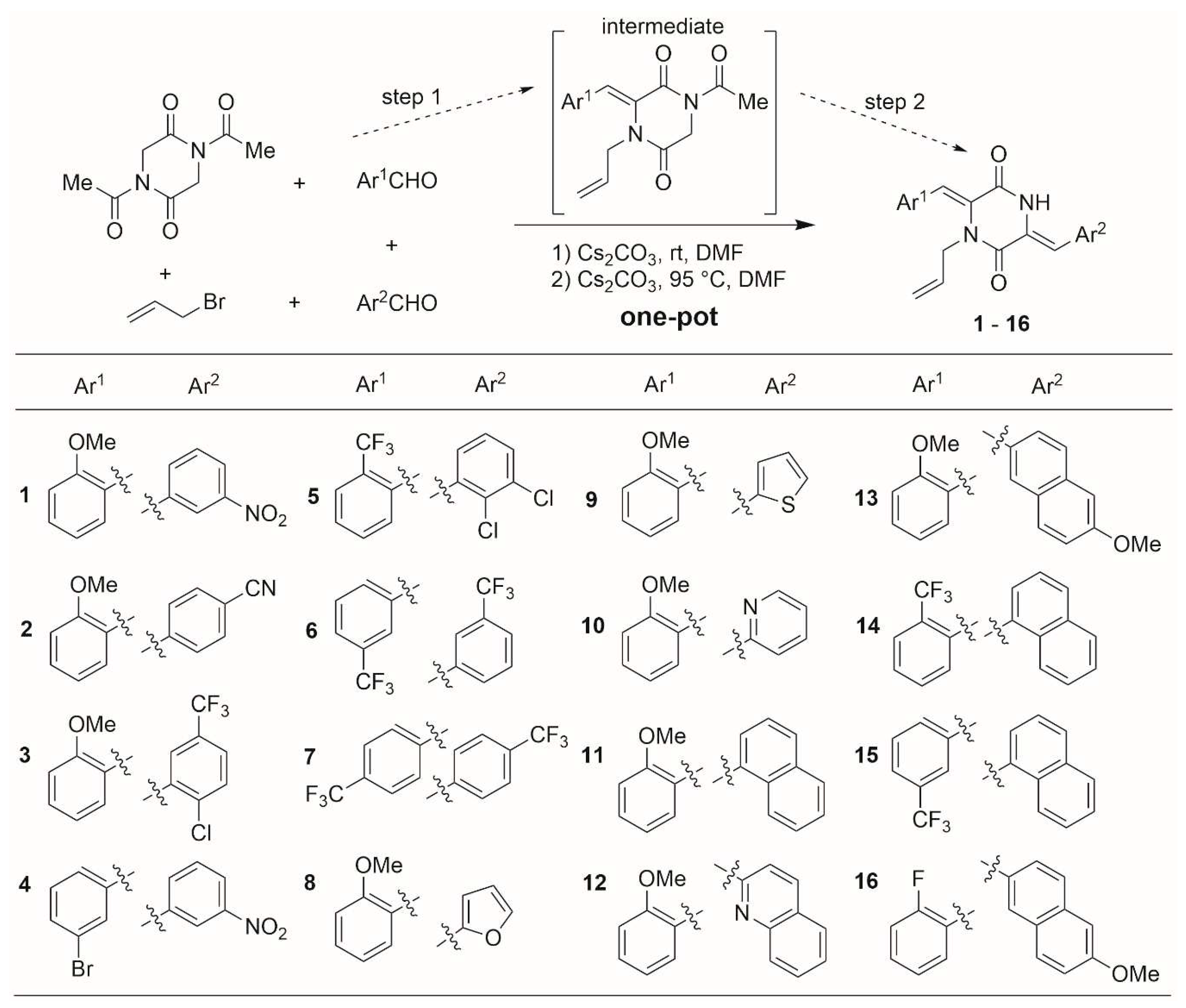
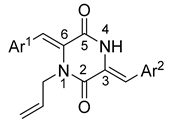
2.1. Synthesis of compound
2.2. Biological evaluation of derivatives
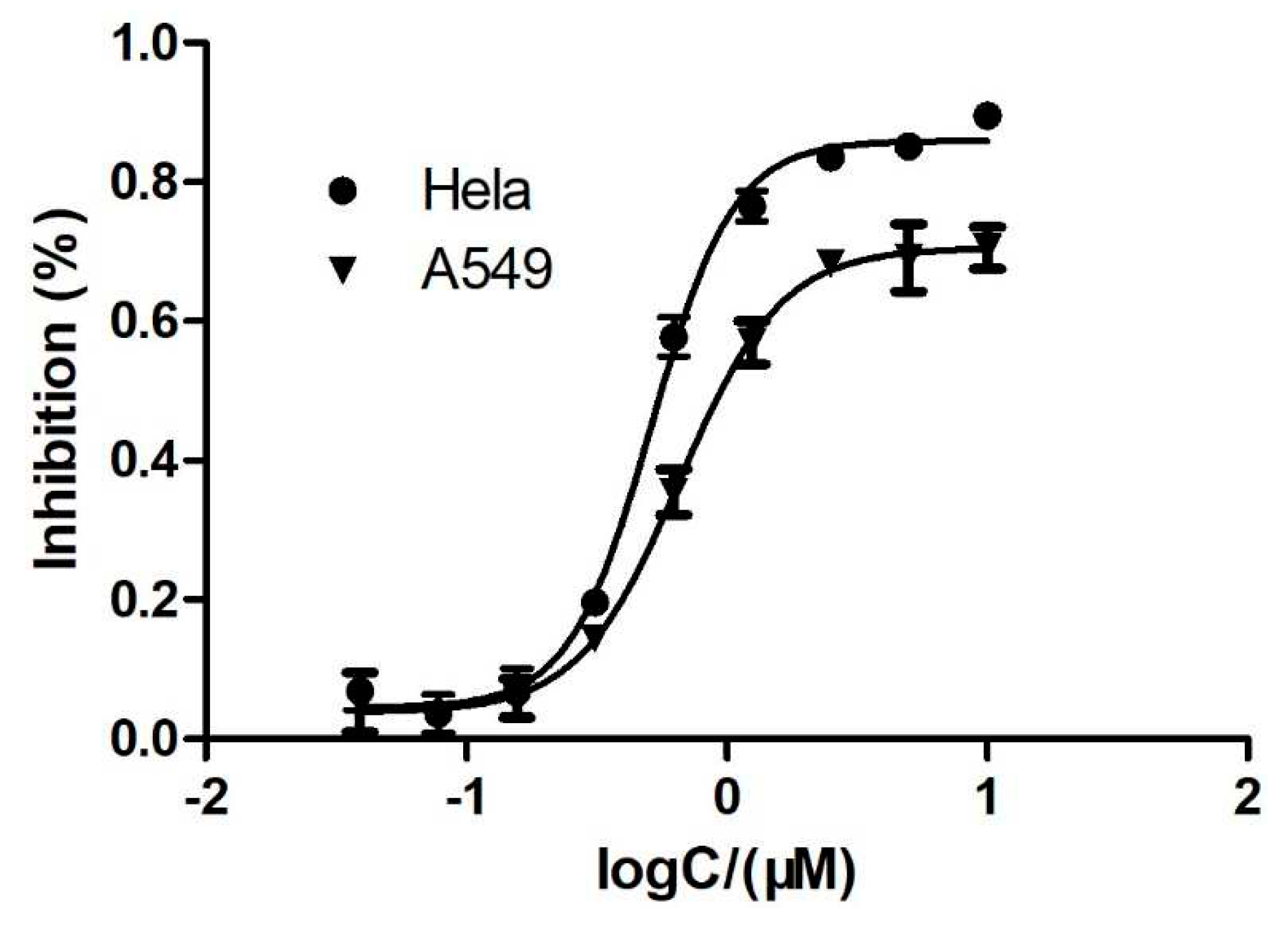
2.2.1. Inhibiting proliferation of cancer cells
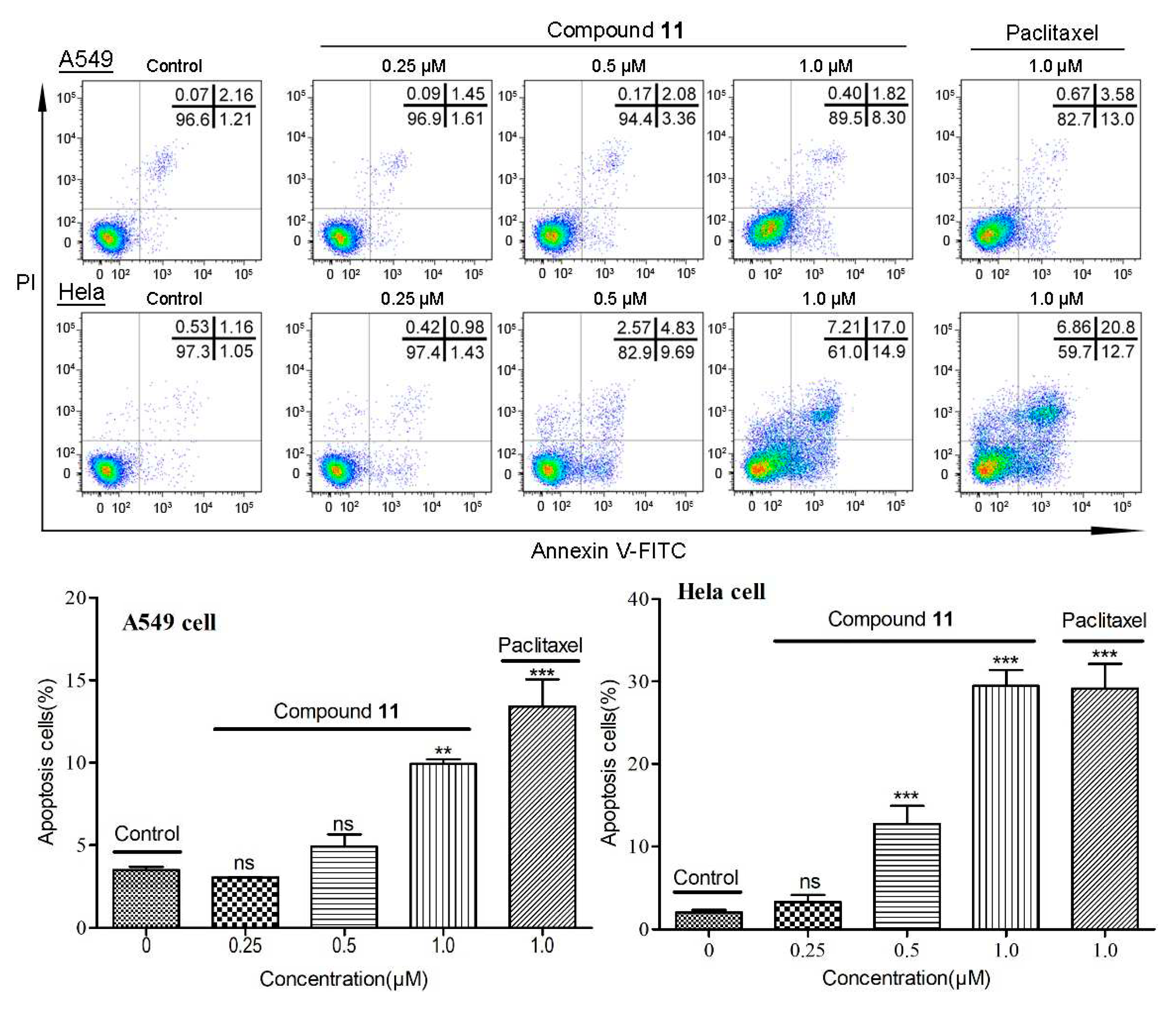
2.2.2. Apoptosis in cancer cell lines induced by compound 11
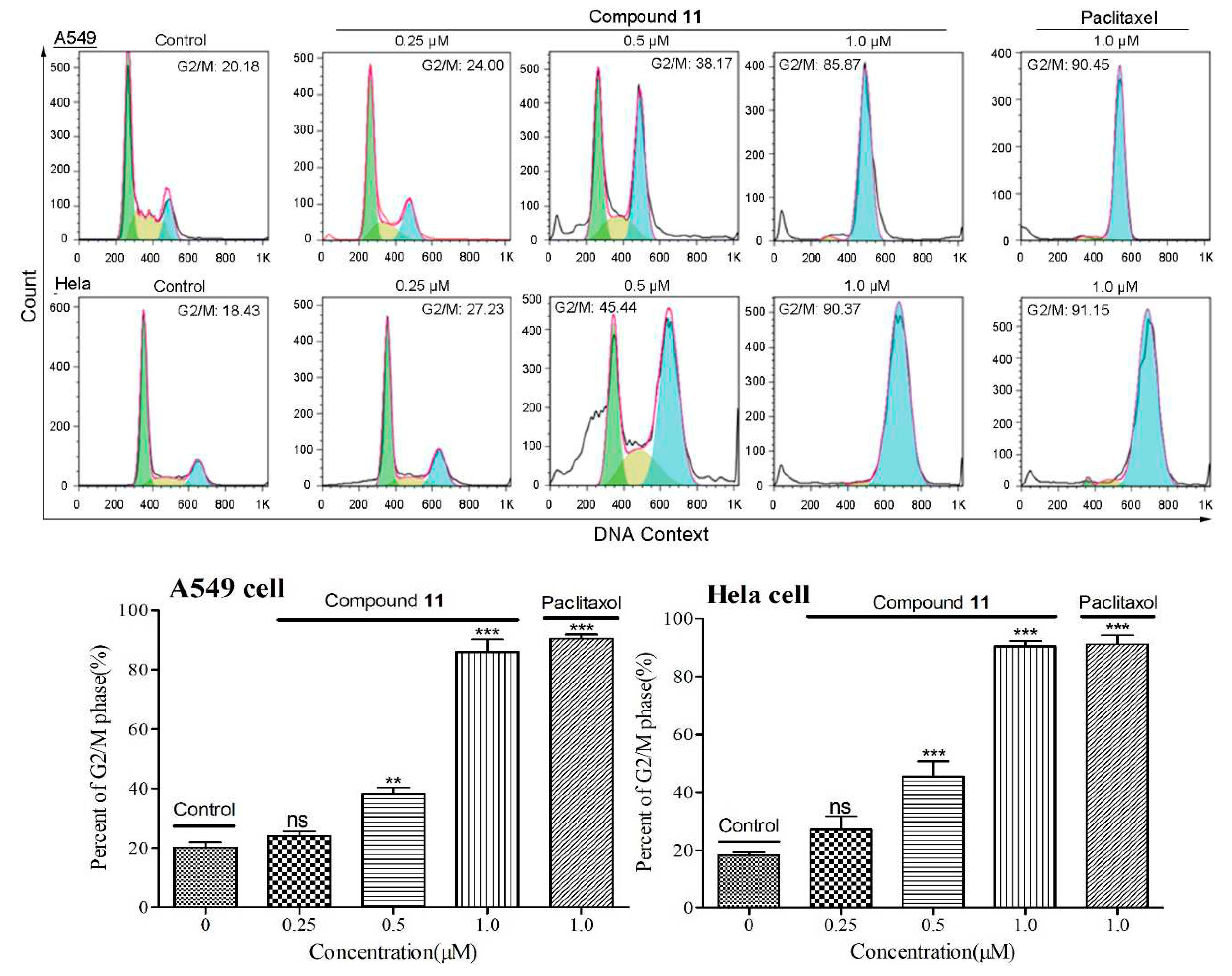
2.2.3. Cell cycle arrest induced by compound 11
3. Materials and Methods
3.1. Chemicals
3.1.1. Synthesis of compounds
General procedure for the synthesis of products 1-16
1-allyl-6-(2-methoxybenzylidene)-3-(3-nitrobenzylidene)piperazine-2,5-dione (1)
4-((Z)-(4-allyl-5-((Z)-2-methoxybenzylidene)-3,6-dioxopiperazin-2-ylidene)methyl)benzonitrile (2)
(3Z,6Z)-1-allyl-6-(3-bromobenzylidene)-3-(3-nitrobenzylidene)piperazine-2,5-dione (4)
1-allyl-3-((Z)-2,3-dichlorobenzylidene)-6-((Z)-2-(trifluoromethyl)benzylidene)piperazine-2,5-dione (5)
1-allyl-3,6-bis((Z)-3-(trifluoromethyl)benzylidene)piperazine-2,5-dione (6)
1-allyl-3-(furan-2-ylmethylene)-6-(2-methoxybenzylidene)piperazine-2,5-dione (8)
1-allyl-6-(2-methoxybenzylidene)-3-(thiophen-2-ylmethylene)piperazine-2,5-dione (9)
(3Z,6Z)-1-allyl-6-(2-methoxybenzylidene)-3-(pyridin-2-ylmethylene)piperazine-2,5-dione (10)
1-allyl-6-(2-methoxybenzylidene)-3-(naphthalen-1-ylmethylene)piperazine-2,5-dione (11)
1-allyl-6-(2-methoxybenzylidene)-3-(quinolin-2-ylmethylene)piperazine-2,5-dione (12)
(Z)-1-allyl-6-((Z)-2-methoxybenzylidene)-3-((6-methoxynaphthalen-2-yl)methylene)piperazine-2,5-dione (13)
(Z)-1-allyl-3-(naphthalen-1-ylmethylene)-6-((Z)-2-(trifluoromethyl)benzylidene)piperazine-2,5-dione (14)
(Z)-1-allyl-3-(naphthalen-1-ylmethylene)-6-((Z)-3-(trifluoromethyl)benzylidene)piperazine-2,5-dione (15)
(Z)-1-allyl-6-((Z)-2-fluorobenzylidene)-3-((6-methoxynaphthalen-2-yl)methylene)piperazine-2,5-dione (16)
3.1.2. liposolubility tests
3.2. Biological evaluations
3.2.1. Cytotoxicity bioassay
3.2.2. Apoptosis and cell cycle assay
4. Conclusion
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Huang, R. M.; Yi, X. X.; Zhou, Y.; Su, X.; Peng, Y.; Gao, C. H. An update on 2,5-diketopiperazines from marine organisms. Mar. Drugs 2014, 12, 6213–6235. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Hou, Y.; Yang, Q.; Li, X.; Wu, S. Structures and Biological Activities of Diketopiperazines from Marine Organisms: A Review. Mar. Drugs 2021, 19, 403. [Google Scholar] [CrossRef] [PubMed]
- Borthwick, A. D. 2,5-Diketopiperazines: Synthesis, Reactions, Medicinal Chemistry, and Bioactive Natural Products. Chem. Rev. 2012, 112, 3641–3716. [Google Scholar] [CrossRef]
- Huber, E. M. Epipolythiodioxopiperazine-Based Natural Products: Building Blocks, Biosynthesis and Biological Activities. Chembiochem 2022, 23, e202200341. [Google Scholar] [CrossRef] [PubMed]
- Borgman, P.; Lopez, R. D.; Lane, A. L. The expanding spectrum of diketopiperazine natural product biosynthetic pathways containing cyclodipeptide synthases. Org. Biomol. Chem. 2019, 17, 2305–2314. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A. K.; Choi, J.; Choi, S. J.; Baek, K. H. Cyclodipeptides: An Overview of Their Biosynthesis and Biological Activity. Molecules 2017, 22, 1796. [Google Scholar] [CrossRef] [PubMed]
- Winyakul, C.; Phutdhawong, W.; Tamdee, P.; Sirirak, J.; Taechowisan, T.; Phutdhawong, W. S. 2,5-Diketopiperazine Derivatives as Potential Anti-Influenza (H5N2) Agents: Synthesis, Biological Evaluation, and Molecular Docking Study. Molecules 2022, 27, 4200. [Google Scholar] [CrossRef]
- Fu, Z. Y.; Hou, Y. W.; Ji, C. P.; Ma, M. X.; Tian, Z. H.; Deng, M. Y.; Zhong, L. L.; Chu, Y. Y.; Li, W. B. Design, synthesis and biological evaluation of anti-pancreatic cancer activity of plinabulin derivatives based on the co-crystal structure. Bioorgan. Med. Chem. 2018, 26, 2061–2072. [Google Scholar] [CrossRef]
- Ding, Z.P.; Ma, M.X.; Zhong, C.J.; Wang, S.X.; Fu, Z.Y.; Hou, Y.W.; Liu, Y.Q.; Zhong, L.L.; Chu, Y.Y.; Li, F.; et al. Development of novel phenoxy-diketopiperazine-type plinabulin derivatives as potent antimicrotubule agents based on the co-crystal structure. Bioorgan. Med. Chem. 2020, 28, 115186. [Google Scholar] [CrossRef]
- Ganesher, A.; Chaturvedi, P.; Karkara, B. B.; Chatterjee, I.; Datta, D.; Panda, G. One pot synthesis of N-monoalkylated plinabulin derivatives via multicomponent protocol and their application as anticancer agents. J. Mol. Struct. 2021, 1229, 129830. [Google Scholar] [CrossRef]
- Liao, S.; Qin, X.; Li, D.; Tu, Z.; Li, J.; Zhou, X.; Wang, J.; Yang, B.; Lin, X.; Liu, J.; et al. Design and synthesis of novel soluble 2,5-diketopiperazine derivatives as potential anticancer agents. Euro. J. Med. Chem. 2014, 83, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Tanaka, K.; Nicholson, B.; Deyanat-Yazdi, G.; Potts, B.; Yoshida, T.; Oda, A.; Kitagawa, T.; Orikasa, S.; Kiso, Y.; et al. Synthesis and structure-activity relationship study of antimicrotubule agents phenylahistin derivatives with a didehydropiperazine-2,5-dione structure. J. Med. Chem. 2012, 55, 1056–1071. [Google Scholar] [CrossRef] [PubMed]
- Labriere, C.; Cervin, G.; Pavia, H.; Hansen, J.H.; Svenson, J. Structure-Activity Relationship Probing of the Natural Marine Antifoulant Barettin. Mar. Biotechnol. 2021, 23, 904–916. [Google Scholar] [CrossRef]
- Sjogren, M.; Johnson, A. L.; Hedner, E.; Dahlstrom, M.; Goransson, U.; Shirani, H.; Bergman, J.; Jonsson, P. R.; Bohlin, L. Antifouling activity of synthesized peptide analogs of the sponge metabolite barettin. Peptides 2006, 27, 2058–2064. [Google Scholar] [CrossRef] [PubMed]
- Labriere, C.; Andersen, J. H.; Albrigtsen, M.; Hansen, J. H.; Svenson, J. Heterocyclic cellular lipid peroxidation inhibitors inspired by the marine antioxidant barettin. Bioorgan. Chem. 2019, 84, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Bryans, J.; Charlton, P.; Chicarelli-Robinson, I.; Collins, M.; Faint, R.; Latham, C.; Shaw, I.; Trew, S. Inhibition of plasminogen activator inhibitor-1 activity by two diketopiperazines, XR330 and XR334 produced by Streptomyces sp. J. Antibio. 1996, 49, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.M.; Golec, J.; Miller, W.; Milutinovic, S.; Folkes, A.; Williams, S.; Brooks, T.; Hardman, K.; Charlton, P.; Wren, S.; et al. Novel inhibitors of plasminogen activator inhibitor-1: Development of new templates from diketopiperazines. Bioorgan. Med. Chem. Lett. 2002, 12, 2367–2370. [Google Scholar] [CrossRef]
- Kamei, H.; Oka, M.; Hamagishi, Y.; Tomita, K.; Konishi, M.; Oki, T. Piperafizines A and B, potentiators of cytotoxicity of vincristine. J. Antibio. 1990, 43, 1018–1020. [Google Scholar] [CrossRef]
- Kanoh, K.; Kohno, S.; Asari, T.; Harada, T.; Katada, J.; Muramatsu, M.; Kawashima, H.; Sekiya, H.; Uno, I. (-)-phenylahistin: A new mammalian cell cycle inhibitor produced by Aspergillus ustus. Bioorgan. Med. Chem. Lett 1997, 7, 2847–2852. [Google Scholar] [CrossRef]
- Nicholson, B.; Lloyd, G. K.; Miller, B. R.; Palladino, M. A.; Kiso, Y.; Hayashi, Y.; Neuteboom, S. T. C. NPI-2358 is a tubulin-depolymerizing agent: in-vitro evidence for activity as a tumor vascular-disrupting agent. Anti-Cancer Drug. 2006; 17, 25–31. [Google Scholar]
- Ma, M.X.; Ding, Z.P.; Wang, S.X.; Ma, L.L.; Wang, Y.X.; Zhong, L.L.; Li, Z.P.; Yang, J.L.; Li, W.B. Polymorphs, co-crystal structure and pharmacodynamics study of MBRI-001, a deuterium-substituted plinabulin derivative as a tubulin polymerization inhibitor. Bioorgan. Med. Chem. 2019, 27, 1836–1844. [Google Scholar] [CrossRef]
- Chen, X.; Chen, X.Y.; Steimbach, R.R.; Wu, T.; Li, H.M.; Dan, W.J.; Shi, P.D.; Cao, C.Y.; Li, D.; Miller, A.K.; et al. Novel 2, 5-diketopiperazine derivatives as potent selective histone deacetylase 6 inhibitors: Rational design, synthesis and antiproliferative activity. Euro. J. Med. Chem. 2020, 187, 111950. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.Y.; Chen, Z.A.; Shen, Q.K.; Quan, Z.S. Application of triazoles in the structural modification of natural products. J. Enzym. Inhib. Med. Chem. 2021, 36, 1115–1144. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.H.; Zhang, H.R.; Tian, M.; Tian, Z.Z.; Xu, Y.J.; Yang, Y.L.; Peng, H.W.; Liu, P.; Liu, Z. Electronic effects on reactivity and anticancer activity by half-sandwich N,N-chelated iridium(III) complexes. New. J. Chem. 2018, 42, 16183–16192. [Google Scholar] [CrossRef]
- Labriere, C.; Kondori, N.; Caous, J.S.; Boomgaren, M.; Sandholm, K.; Ekdahl, K.N.; Hansen, J.H.; Svenson, J. Development and evaluation of cationic amphiphilic antimicrobial 2,5-diketopiperazines. J. Pept. Sci. 2018, 24, e3090. [Google Scholar] [CrossRef]
- Liao, S.R.; Du, L.J.; Qin, X.C.; Xu, L.; Wang, J.F.; Zhou, X.F.; Tu, Z.C.; Li, J.; Liu, Y.H. Site selective synthesis of cytotoxic 1,3,6-trisubstituted 3,6-diunsaturated (3Z,6Z)-2,5-diketopiperazines via a one-pot multicomponent method. Tetrahedron 2016, 72, 1051–1057. [Google Scholar] [CrossRef]
- Zhang, C.C.; Li, C.G.; Wang, Y.F.; Xu, L.H.; He, X.H.; Zeng, Q.Z.; Zeng, C.Y.; Mai, F.Y.; Hu, B.; Ouyang, D.Y. Chemotherapeutic paclitaxel and cisplatin differentially induce pyroptosis in A549 lung cancer cells via caspase-3/GSDME activation. Apoptosis 2019, 24, 312–325. [Google Scholar] [CrossRef]
- Liao, S. R.; Xu, Y.; Tang, Y.; Wang, J.F.; Zhou, X.F.; Xu, L.; Liu, Y.H. Design, synthesis and biological evaluation of soluble 2,5-diketopiperazines derivatives as potential antifouling agents. Rsc. Adv. 2015, 5, 51020–51026. [Google Scholar] [CrossRef]
- Cai, J.; Wang, X.N.; Gan, X.; Zhou, Q.; Luo, X.W.; Yang, B.; Liu, Y.H.; Ratnasekera, D.; Zhou, X.F. New Chlorinated Metabolites and Antiproliferative Polyketone from the Mangrove Sediments-Derived Fungus Mollisia sp. SCSIO41409. Mar. Drugs 2023, 21, 32. [Google Scholar] [CrossRef]
| Compound | IC50/μMa | |
| A549 | Hela | |
| 4m | 1.6 ± 0.2 | 2.4 ± 0.2 |
| 1 | >10 | >10 |
| 2 | >10 | >10 |
| 3 | >10 | >10 |
| 4 | >10 | >10 |
| 5 | >10 | >10 |
| 6 | >10 | 8.9 ± 0.7 |
| 7 | >10 | >10 |
| 8 | 7.3 ± 1.2 | 5.9 ± 1.6 |
| 9 | 3.7 ± 0.4 | 4.8 ± 1.4 |
| 10 | 5.6 ± 1.0 | 4.7 ± 2.8 |
| 11 | 1.2 ± 0.1 | 0.7 ± 0.2 |
| 12 | >10 | 6.2 ± 0.4 |
| 13 | >10 | >10 |
| 14 | >10 | 3.9 ± 0.2 |
| 15 | >10 | >10 |
| 16 | >10 | >10 |
| Paclitaxol | 0.004 ± 0.2 | 0.004 ± 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





