Submitted:
27 April 2023
Posted:
28 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Case selection
2.2. Quantification of TILs
2.3. Immunohistochemistry
2.4. Statistical analysis
3. Results
3.1. Patient and tumor characteristics
| All Cases | ER+/HER2- | TNBC | HER2+ | p | |
|---|---|---|---|---|---|
| Total number n (%) | 66 | 54 (82) | 5 (8) | 7 (10) | |
| Age (years) | 0.61 | ||||
| Mean | 58 | 58 | 61 | 62 | |
| Median | 59 | 59.5 | 57 | 62 | |
| Tumor size n (%) | 0.58 | ||||
| pT1 | 22 (33) | 16 (30) | 2 (40) | 4 (57) | |
| pT2 | 33 (50) | 29 (54) | 2 (40) | 2 (29) | |
| pT3 | 11 (17) | 9 (6) | 1 (20) | 1 (14) | |
| Tumor size (mm) | 34 | 35 | 42 | 26 | |
| Graden (%) | 0.17 | ||||
| G2 | 30 (45) | 27 (50) | 2 (40) | 1 (15) | |
| G3 | 36 (55) | 27 (50) | 3 (60) | 6 (85) | |
| LVSI n (%) | 0.33 | ||||
| Negative | 40 (61) | 35 (65) | 2 (40) | 3 (43) | |
| Positive | 26 (39) | 19 (35) | 3 (60) | 4 (57) | |
| Lymph node metastases n (%) | 0.014 | ||||
| pN0 | 38 (60) | 31 (57) | 1 (20) | 6 (85) | |
| pN1 | 17 (25) | 15 (28) | 2 (40) | 0 | |
| pN2 | 7 (10) | 7 (13) | 0 | 0 | |
| pN3 | 4 (5) | 1 (2) | 2 (40) | 1 (15) | |
| Ki67 n (%) | 0.21 | ||||
| <15 | 18 (37) | 17 (42) | 0 (0) | 1 (16) | |
| ³15 | 31 (63) | 23 (58) | 3 (100) | 5 (84) | |
| Missing | 17 |
3.2. sTIL distribution and PD-L1 expression (22C3 – SP142)
| All Cases | ER+/HER2- | TNBC | HER2+ | p | |
|---|---|---|---|---|---|
| sTILs n (%) | 0.26 | ||||
| 0 | 24 (36) | 18 (33) | 3 (60) | 3 (43) | |
| <5 | 19 (29) | 18 (33) | 0 | 1 (14) | |
| 9-May | 11 (17) | 10 (18) | 1 (20) | 0 | |
| ≥ 10 | 12 (18) | 8 (16) | 1 (20) | 3 (43) | |
| PD-L1 SP142 ICs n (%) | 0.48 | ||||
| <1% | 38 (64) | 32 (68) | 3 (60) | 3 (43) | |
| ≥1% | 21 (36) | 15 (32) | 2 (40) | 4 (57) | |
| Non-assessable | 7 | ||||
| PD-L1 22C3 CPS n (%) | 0.58 | ||||
| <1 | 42 (72) | 35 (76) | 3 (60) | 4 (57) | |
| ≥1 | 13 (23) | 9 (20) | 2 (40) | 2 (29) | |
| ≥10 | 3 (5) | 2 (4) | 0 | 1 (14) | |
| Non-assessable | 8 |
3.3. sTIL distribution and PD-L1 (22C3 - SP142) expression according to patient characteristics
| sTILs | PD-L1 SP142 | PD-L1 22C3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0% | 1-4% | 5-9% | ≥10% | <1% | ≥1% | <1 | ≥1 | ≥10 | |
| Age (years) | |||||||||
| Mean | 57 | 61 | 57 | 58.5 | 57 | 60 | 58 | 56 | 66 |
| Tumor (mm) | 33 | 33 | 35 | 36 | 34 | 34.3 | 36 | 30 | 3 |
| Stage n (%) | |||||||||
| pT1 | 9 (37) | 6 (32) | 4 (36) | 3 (25) | 13 (34) | 8 (38) | 14 (33) | 6 (46) | 0 |
| pT2 | 12 (50) | 9 (47) | 5 (46) | 7 (58) | 19 (50) | 9 (43) | 20 (48) | 5 (38) | 3 (100) |
| pT3 | 3 (13) | 4 (21) | 2 (18) | 2 (17) | 6 (16) | 4 (19) | 8 (19) | 2 (15) | 0 |
| Grade n (%) | |||||||||
| G2 | 12 (50) | 9 (47) | 3 (27) | 6 (50) | 19 (50) | 6 (28) | 18 (43) | 5 (38) | 1 (33) |
| G3 | 12 (50) | 10 (53) | 8 (73) | 6 (50) | 19 (50) | 15 (72) | 24 (57) | 8 (62) | 2 (67) |
| LVSI n (%) | |||||||||
| Negative | 16 (66) | 11 (58) | 7 (64) | 6 (50) | 24 (63) | 11 (52) | 27 (64) | 5 (38) | 2 (66) |
| Positive | 8 (34) | 8 (42) | 4 (36) | 6 (50) | 14 (37) | 10 (48) | 15 (34) | 8 (63) | 1 (34) |
| Lymph node metastasis n (%) | |||||||||
| pN0 | 16 (67) | 10 (53) | 6 (60) | 5 (42) | 22 (58) | 11 (55) | 25 (60) | 5 (42) | 2 (66) |
| pN1 | 4 (17) | 6 (32) | 3 (30) | 4 (33) | 9 (24) | 6 (30) | 10 (24) | 4 (33) | 1 (34) |
| pN2 | 3 (12) | 2 (10) | 1 (10) | 1 (8) | 5 (13) | 1 (5) | 5 (12) | 1 (8) | 0 |
| pN3 | 1 (4) | 1 (5) | 0 | 2 (17) | 2 (5) | 2 (10) | 2 (5) | 2 (17) | 0 |
| HER2 n (%) | |||||||||
| negative | 21 (87) | 18 (95) | 11 (100) | 9 (75) | 35 (92) | 17 (81) | 38 (90) | 11 (85) | 2 (67) |
| positive | 3 (13) | 1 (5) | 0 | 3 (25) | 3 (8) | 4 (19) | 4 (10) | 2 (15) | 1 (33) |
| Ki67 n (%) | |||||||||
| <15 | 7 (50) | 7 (41) | 2 (18) | 2 (25) | 13 (45) | 2 (12)* | 13 (41) | 3 (18) | 0 |
| ≥15 | 7 (50) | 10 (59) | 8 (82) | 6 (75) | 16 (55) | 15 (88)* | 19 (59) | 9 (82) | 3 (100) |
3.4. Correlation of sTILs with PD-L1 expression (22C3 - SP142)
| < 1% | ≥ 1% | ||
| n (%) | n (%) | ||
| sTILS | 0% | 21 (55) | 0 |
| <5% | 16 (42) | 2 (10) | |
| 5-9% | 1 (3) | 9 (43) | |
| ≥10% | 0 | 10 (47) |
| < 1 | ≥ 1 | ≥10 | ||
| n (%) | n (%) | n (%) | ||
| sTILS | 0% | 20 (48) | 0 | 0 |
| <5% | 16 (38) | 2 (15) | 0 | |
| 5-9% | 4(9) | 5 (39) | 1 (34) | |
| ≥10% | 2(5) | 6 (46) | 2 (66) |
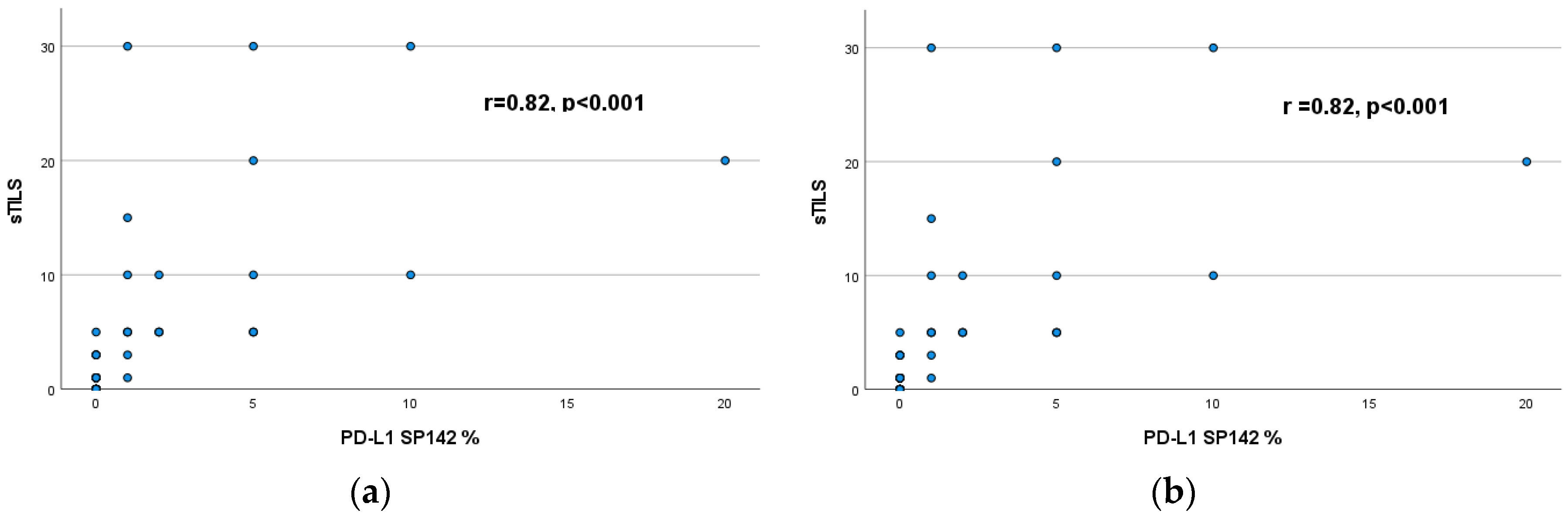
3.5. Correlation of sTILs and PD-L1 (22C3 - SP142) expression with patient survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Pramod N, Nigam A, Basree M, Mawalkar R, Mehra S, Shinde N, et al. Comprehensive Review of Molecular Mechanisms and Clinical Features of Invasive Lobular Cancer. Oncologist 2021, 26, 6. https://doi.org/10.1002/onco.13734. [CrossRef]
- Delpech Y, Coutant C, Hsu L, Barranger E, Iwamoto T, Barcenas CH, et al. Clinical benefit from neoadjuvant chemotherapy in oestrogen receptor-positive invasive ductal and lobular carcinomas. Br J Cancer 2013, 108, 285–91. https://doi.org/10.1038/bjc.2012.557. [CrossRef]
- Marmor S, Hui JYC, Huang JL, Kizy S, Beckwith H, Blaes AH, et al. Relative effectiveness of adjuvant chemotherapy for invasive lobular compared with invasive ductal carcinoma of the breast. Cancer 2017, 123, 3015–21. https://doi.org/10.1002/cncr.30699. [CrossRef]
- Pestalozzi BC, Zahrieh D, Mallon E, Gusterson BA, Price KN, Gelber RD, et al. Distinct clinical and prognostic features of infiltrating lobular carcinoma of the breast: combined results of 15 International Breast Cancer Study Group clinical trials. J Clin Oncol 2008, 26, 3006–14. https://doi.org/10.1200/JCO.2007.14.9336. [CrossRef]
- Adachi Y, Ishiguro J, Kotani H, Hisada T, Ichikawa M, Gondo N, et al. Comparison of clinical outcomes between luminal invasive ductal carcinoma and luminal invasive lobular carcinoma. BMC Cancer 2016, 16, 248. https://doi.org/10.1186/s12885-016-2275-4. [CrossRef]
- Korhonen T, Kuukasjärvi T, Huhtala H, Alarmo EL, Holli K, Kallioniemi A, et al. The impact of lobular and ductal breast cancer histology on the metastatic behavior and long term survival of breast cancer patients. Breast 2013, 22, 1119–24. https://doi.org/10.1016/J.BREAST.2013.06.001. [CrossRef]
- Desmedt C, Salgado R, Fornili M, Pruneri G, Van den Eynden G, Zoppoli G, et al. Immune Infiltration in Invasive Lobular Breast Cancer. J Natl Cancer Inst 2018, 110, 768–76. https://doi.org/10.1093/jnci/djx268. [CrossRef]
- Tille J-C, Vieira AF, Saint-Martin C, Djerroudi L, Furhmann L, Bidard • Francois-Clement, et al. Tumor-infiltrating lymphocytes are associated with poor prognosis in invasive lobular breast carcinoma. Mod Pathol 2020, 33, 2198–207. https://doi.org/10.1038/s41379-020-0561-9. [CrossRef]
- Denkert C, von Minckwitz G, Darb-Esfahani S, Lederer B, Heppner BI, Weber KE, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol 2018, 19, 40–50. https://doi.org/10.1016/S1470-2045(17)30904-X. [CrossRef]
- Loi S, Drubay D, Adams S, Pruneri G, Francis PA, Lacroix-Triki M, et al. Tumor-Infiltrating Lymphocytes and Prognosis: A Pooled Individual Patient Analysis of Early-Stage Triple-Negative Breast Cancers. J Clin Oncol 2019, 37, 559. https://doi.org/10.1200/JCO.18.01010. [CrossRef]
- G G, Z W, X Q, Z Z. Prognostic value of tumor-infiltrating lymphocytes in patients with triple-negative breast cancer: a systematic review and meta-analysis. BMC Cancer 2020, 20. https://doi.org/10.1186/S12885-020-6668-Z. [CrossRef]
- Salgado R, Denkert C, Campbell C, Savas P, Nuciforo P, Aura C, et al. Tumor-Infiltrating Lymphocytes and Associations With Pathological Complete Response and Event-Free Survival in HER2-Positive Early-Stage Breast Cancer Treated With Lapatinib and Trastuzumab: A Secondary Analysis of the NeoALTTO Trial. JAMA Oncol 2015, 1, 448–55. https://doi.org/10.1001/JAMAONCOL.2015.0830. [CrossRef]
- Loi S, Michiels S, Salgado R, Sirtaine N, Jose V, Fumagalli D, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol Off J Eur Soc Med Oncol 2014, 25, 1544–50. https://doi.org/10.1093/ANNONC/MDU112. [CrossRef]
- Narendra S, Jenkins SM, Khoor A, Nassar A. Clinical outcome in pleomorphic lobular carcinoma: A case-control study with comparison to classic invasive lobular carcinoma. Ann Diagn Pathol 2015, 19, 2. https://doi.org/10.1016/j.anndiagpath.2015.01.005. [CrossRef]
- Sahin S, Karatas F, Erdem GU, Hacioglu B, Altundag K. Invasive Pleomorphic Lobular Histology Is an Adverse Prognostic Factor on Survival in Patients with Breast Cancer. Am Surg 2017;83:359–64.
- Segar JM, Pandey R, Farr KJ, Nagle R, Lebeau L, Gonzalez VJ, et al. Clinicopathological and Molecular Characteristics of Pleomorphic Invasive Lobular Carcinoma. Int J Breast Cancer 2020, 2020. https://doi.org/10.1155/2020/8816824. [CrossRef]
- Haque W, Arms A, Verma V, Hatch S, Brian Butler E, Teh BS. Outcomes of pleomorphic lobular carcinoma versus invasive lobular carcinoma. Breast 2019, 43, 67–73. https://doi.org/10.1016/J.BREAST.2018.11.007. [CrossRef]
- Kythreotou A, Siddique A, Mauri FA, Bower M, Pinato DJ. PD-L1. J Clin Pathol 2018, 71, 189–94. https://doi.org/10.1136/jclinpath-2017-204853. [CrossRef]
- Stovgaard ES, Dyhl-Polk A, Roslind A, Balslev E, Nielsen D. PD-L1 expression in breast cancer: expression in subtypes and prognostic significance: a systematic review. Breast Cancer Res Treat 2019, 174, 571–84. https://doi.org/10.1007/s10549-019-05130-1. [CrossRef]
- Sabatier R, Finetti P, Mamessier E, Adelaide J, Chaffanet M, Raza Ali H, et al. Prognostic and predictive value of PDL1 expression in breast cancer. Oncotarget 2015, 6, 5449-64.
- Davey MG, Ryan É, Davey MS, Lowery AJ, Miller N, Kerin MJ. Clinicopathological and prognostic significance of programmed cell death ligand 1 expression in patients diagnosed with breast cancer: meta-analysis. Br J Surg 2021, 108, 622–31. https://doi.org/10.1093/BJS/ZNAB103. [CrossRef]
- Cirqueira MB, Rodrigues Mendonça C, Noll M, Soares LR, Auxiliadora M, Cysneiros PC, et al. Prognostic Role of PD-L1 Expression in Invasive Breast Cancer: A Systematic Review and Meta-Analysis. Cancers (Basel) 2021, 13,23, 60. https://doi.org/10.3390/cancers13236090. [CrossRef]
- Dill EA, Gru AA, Atkins KA, Friedman LA, Moore ME, Bullock TN, et al. PD-L1 Expression and Intratumoral Heterogeneity Across Breast Cancer Subtypes and Stages: An Assessment of 245 Primary and 40 Metastatic Tumors. Am J Surg Pathol 2017, 41, 334–42. https://doi.org/10.1097/PAS.0000000000000780. [CrossRef]
- Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, et al. The evaluation of tumor-infiltrating lymphocytes (TILS) in breast cancer: Recommendations by an International TILS Working Group 2014. Ann Oncol 2015, 26, 259–71. https://doi.org/10.1093/annonc/mdu450. [CrossRef]
- Droeser R, Zlobec I, Kilic E, Güth U, Heberer M, Spagnoli G, et al. Differential pattern and prognostic significance of CD4+, FOXP3+ and IL-17+ tumor infiltrating lymphocytes in ductal and lobular breast cancers. BMC Cancer 2012, 12. https://doi.org/10.1186/1471-2407-12-134. [CrossRef]
- Iorfida M, Maiorano E, Orvieto E, Maisonneuve P, Bottiglieri L, Rotmensz N, et al. Invasive lobular breast cancer: subtypes and outcome. Breast Cancer Res Treat 2012, 133, 713–23. https://doi.org/10.1007/s10549-012-2002-z. [CrossRef]
- Loi S, Michiels S, Adams S, Loibl S, Budczies J, Denkert C, et al. The journey of tumor-infiltrating lymphocytes as a biomarker in breast cancer: clinical utility in an era of checkpoint inhibition. Ann Oncol 2021, 32, 1236–44. https://doi.org/10.1016/J.ANNONC.2021.07.007. [CrossRef]
- Park JH, Jonas SF, Bataillon G, Criscitiello C, Salgado R, Loi S, et al. Prognostic value of tumor-infiltrating lymphocytes in patients with early-stage triple-negative breast cancers (TNBC) who did not receive adjuvant chemotherapy. Ann Oncol Off J Eur Soc Med Oncol 2019, 30, 1941–9. https://doi.org/10.1093/ANNONC/MDZ395. [CrossRef]
- Ali HR, Provenzano E, Dawson SJ, Blows FM, Liu B, Shah M, et al. Association between CD8+ T-cell infiltration and breast cancer survival in 12 439 patients. Ann Oncol 2014, 25, 1536–43. https://doi.org/10.1093/annonc/mdu191. [CrossRef]
- Sanft T, Berkowitz A, Schroeder B, Hatzis C, Schnabel CA, Brufsky A, et al. A prospective decision-impact study incorporating Breast Cancer Index into extended endocrine therapy decision-making. Breast Cancer Manag 2019, 8. https://doi.org/10.2217/bmt-2019-0001. [CrossRef]
- Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol 2013, 31, 860–7. https://doi.org/10.1200/JCO.2011.41.0902. [CrossRef]
- Sobral-Leite M, Van de Vijver K, Michaut M, van der Linden R, Hooijer GKJ, Horlings HM, et al. Assessment of PD-L1 expression across breast cancer molecular subtypes, in relation to mutation rate, BRCA1-like status, tumor-infiltrating immune cells and survival. Oncoimmunology 2018, 7. https://doi.org/10.1080/2162402X.2018.1509820. [CrossRef]
- Ades F, Zardavas D, Bozovic-Spasojevic I, Pugliano L, Fumagalli D, De Azambuja E, et al. Luminal B breast cancer: Molecular characterization, clinical management, and future perspectives. J Clin Oncol 2014, 32, 2794–803. https://doi.org/10.1200/JCO.2013.54.1870. [CrossRef]
- Uhercik M, Sanders AJ, Owen S, Davies EL, Sharma AK, Jiang WG, et al. Clinical significance of PD1 and PDL1 in human breast cancer. Anticancer Res., vol. 37, International Institute of Anticancer Research; 2017, p. 4249–54. https://doi.org/10.21873/anticanres.11817. [CrossRef]
- Beckers RK, Selinger CI, Vilain R, Madore J, Wilmott JS, Harvey K, et al. Programmed death ligand 1 expression in triple-negative breast cancer is associated with tumour-infiltrating lymphocytes and improved outcome. Histopathology 2016, 69, 25–34. https://doi.org/10.1111/his.12904. [CrossRef]
- Ali HR, Glont S-E, Blows FM, Provenzano E, Dawson S-J, Liu B, et al. PD-L1 protein expression in breast cancer is rare, enriched in basal-like tumours and associated with infiltrating lymphocytes. Ann Oncol 2015, 26, 1488–93. https://doi.org/10.1093/annonc/mdv192. [CrossRef]
- Bae SB, Cho HD, Oh MH, Lee JH, Jang SH, Hong SA, et al. Expression of programmed death receptor ligand 1 with high tumor-infiltrating lymphocytes is associated with better prognosis in breast cancer. J Breast Cancer 2016, 19, 242–51. https://doi.org/10.4048/jbc.2016.19.3.242. [CrossRef]
- Qin T, Zeng YD, Qin G, Xu F, Lu J Bin, Fang WF, et al. High PD-L1 expression was associated with poor prognosis in 870 Chinese patients with breast cancer. Oncotarget 2015, 6, 33972–81. https://doi.org/10.18632/oncotarget.5583. [CrossRef]
- Mori H, Kubo M, Yamaguchi R, Nishimura R, Osako T, Arima N, et al. The combination of PD-L1 expression and decreased tumorinfiltrating lymphocytes is associated with a poor prognosis in triple-negative breast cancer. Oncotarget 2017, 8, 15584–92. https://doi.org/10.18632/oncotarget.14698. [CrossRef]
- Thompson ED, Taube JM, Asch-Kendrick RJ, Ogurtsova A, Xu H, Sharma R, et al. PD-L1 expression and the immune microenvironment in primary invasive lobular carcinomas of the breast. Mod Pathol 2017, 30, 1551–60. https://doi.org/10.1038/modpathol.2017.79. [CrossRef]
- Ahn SG, Kim SK, Shepherd JH, Cha YJ, Bae SJ, Kim C, et al. Clinical and genomic assessment of PD-L1 SP142 expression in triple-negative breast cancer. Breast Cancer Res Treat 2021, 188, 165–78. https://doi.org/10.1007/S10549-021-06193-9/FIGURES/5. [CrossRef]
- Badve SS, Ed Erique Penault-Llorca F, Reis-Filho JS, Deurloo R, Siziopikou KP, D’arrigo C, et al. Determining PD-L1 Status in Patients With Triple-Negative Breast Cancer: Lessons Learned From IMpassion130. J Natl Cancer Inst 2021, 114, 5. https://doi.org/10.1093/jnci/djab121. [CrossRef]
- Huang X, Ding Q, Guo H, Gong Y, Zhao J, Zhao M, et al. Comparison of three FDA-approved diagnostic immunohistochemistry assays of PD-L1 in triple-negative breast carcinoma. Hum Pathol 2021, 108, 42–50. https://doi.org/10.1016/J.HUMPATH.2020.11.004. [CrossRef]
- Shah AN, Flaum L, Helenowski I, Santa-Maria CA, Jain S, Rademaker A, et al. Phase II study of pembrolizumab and capecitabine for triple negative and hormone receptor-positive, HER2-negative endocrine-refractory metastatic breast cancer. J Immunother Cancer 2020, 8. https://doi.org/10.1136/JITC-2019-000173. [CrossRef]
- Tolaney SM, Barroso-Sousa R, Keenan T, Li T, Trippa L, Vaz-Luis I, et al. Effect of Eribulin With or Without Pembrolizumab on Progression-Free Survival for Patients With Hormone Receptor-Positive, ERBB2-Negative Metastatic Breast Cancer: A Randomized Clinical Trial. JAMA Oncol 2020, 6, 1598–605. https://doi.org/10.1001/JAMAONCOL.2020.3524. [CrossRef]
- Schmid P, Cortes J, Dent R, Pusztai L, McArthur H, Kümmel S, et al. Event-free Survival with Pembrolizumab in Early Triple-Negative Breast Cancer. N Engl J Med 2022, 386, 556–67. https://doi.org/10.1056/NEJMOA2112651/SUPPL_FILE/NEJMOA2112651_DATA-SHARING.PDF. [CrossRef]
- Nanda R, Liu MC, Yau C, Shatsky R, Pusztai L, Wallace A, et al. Effect of Pembrolizumab Plus Neoadjuvant Chemotherapy on Pathologic Complete Response in Women With Early-Stage Breast Cancer: An Analysis of the Ongoing Phase 2 Adaptively Randomized I-SPY2 Trial. JAMA Oncol 2020, 6, 676–84. https://doi.org/10.1001/JAMAONCOL.2019.6650. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





