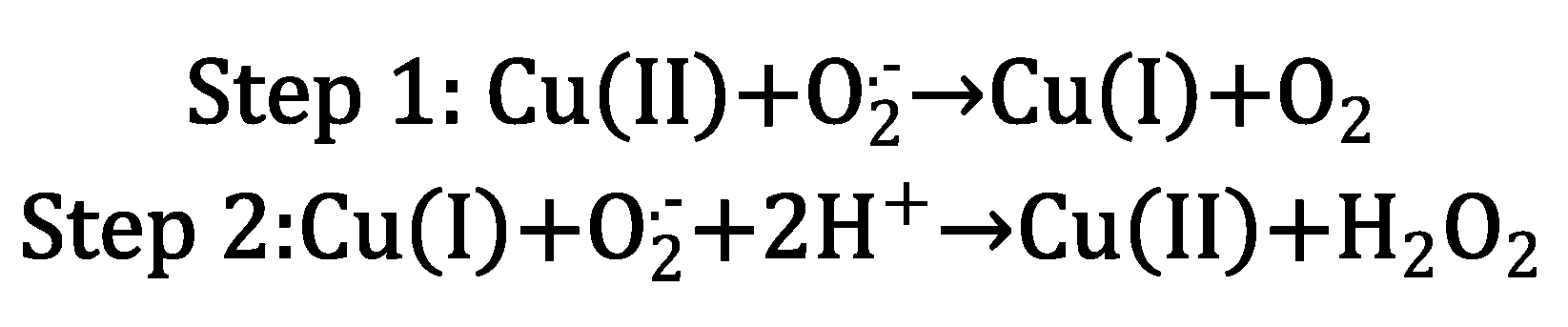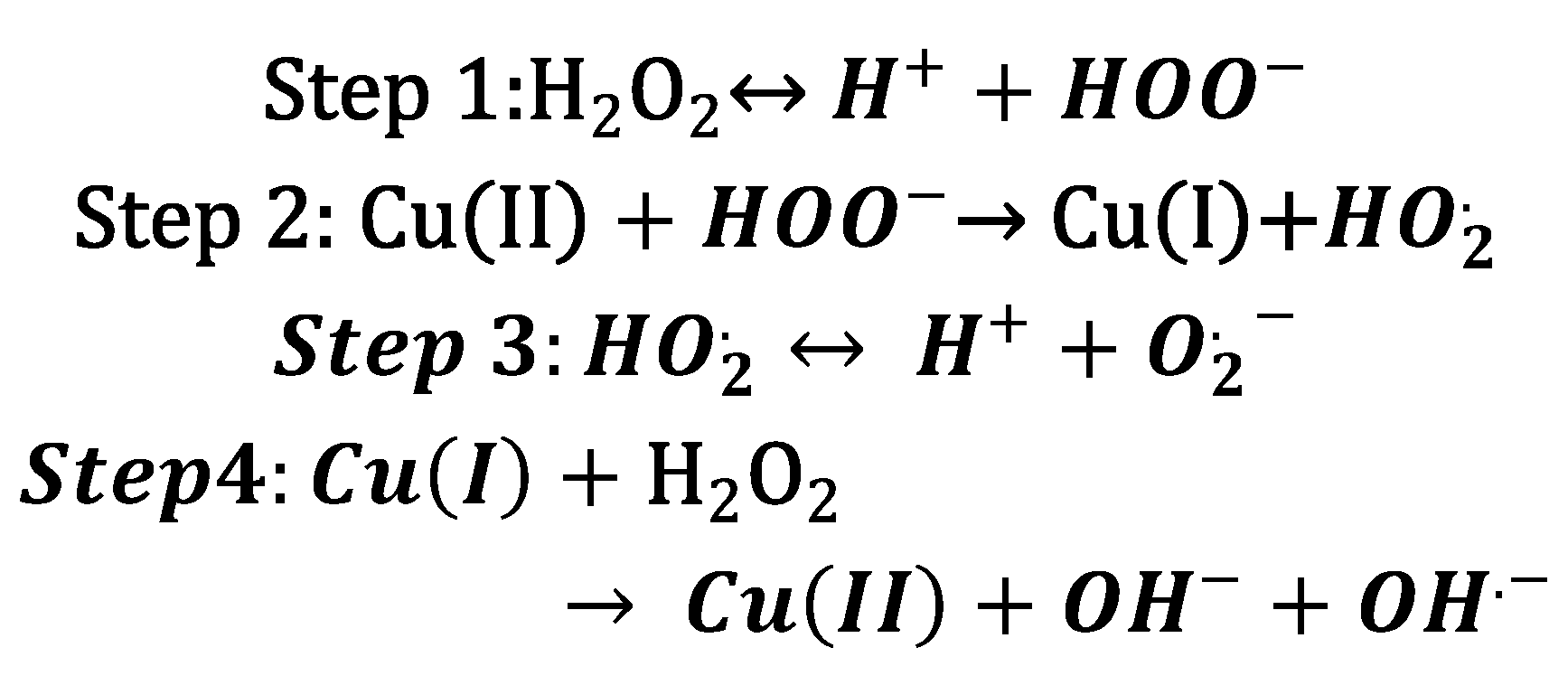1. Introduction
Antibiotics are essential in modern healthcare to treat infectious diseases; however, because of inappropriate use in human health and agriculture, drug-resistant bacteria known as "superbugs" have emerged [
1]. Antimicrobial resistance (AMR) is a leading cause of death, directly resulting in at least 1.27 million deaths worldwide in 2019 [
2]. Out of the 1.27 million, six leading pathogens -
Escherichia coli (E. coli), followed by
Staphylococcus aureus (
S. aureus),
Klebsiella pneumoniae (
K. pneumoniae),
Streptococcus pneumoniae (
S. pneumoniae)
, Acinetobacter baumannii, and
Pseudomonas aeruginosa (
P. aeruginosa)
- were responsible for over 73 % of deaths [
2]. Aggravating this problem, the Center for Disease Control and Prevention (CDC) reported progress loss in combating AMR in the U.S., showing a 15% increase in resistant nosocomial infections and deaths during the first year of the COVID-19 pandemic compared to 2019 [
3]. While the effects of the pandemic on AMR have yet to be established globally, these reports emphasize the urgent need to refocus research and strategies to reduce AMR.
Traditional antibiotics are mostly comprised of organic compounds that target specific bacterial synthesis pathways like: DNA (quinolones)[
4], cell wall (β-lactams)[
5], protein (tetracyclines)[
6], and folate (sulfonamides)[
7]. These drugs even if they’re selective towards bacteria are now limited in their effect, and in some cases inactive when it comes to treating AMR. Developing not just new antibiotics but new classes of antimicrobials that can be effective for multi-drug resistant (MDR)- Gram-negative bacteria must be an essential focus. Frei et al. embarked on a quest to screen new and diverse compounds and, by 2019, they screened over 295,000 compounds that originated from libraries of academic laboratories [
8]. While most fell into the usual conventions for antibacterial drug synthesis, 906 were metal-containing compounds, with 9.9% of these exhibiting antimicrobial activity and no toxicity as measured by their cytotoxicity against human embryonic kidney (HEK293) cells and red blood cells compared to the 0.87% hit rate, including all the compounds tested by the Community for Open Antimicrobial Drug Discovery (CO-ADD)[
8]. This study highlights the diversity and potential of metals and their complexes in antimicrobial research. Additionally, from this work, they also found 13 copper-containing compounds as active against bacteria, and 6 were considered active and non-toxic.
Using copper (Cu) against pathogens has a long medical history traced back to wound treatments and water sanitization by Egyptians around 2600 BC [
9]. Arendsen et al. summarized studies demonstrating Cu's concentration-dependent bactericidal activities for a broad spectrum of bacteria, including multidrug-resistant (MDR)-Gram-negative bacteria like Methicillin Resistant
Staphylococcus aureus (MRSA), on Cu welding coupons and Cu-containing bioactive wound dressings [
9]. Additionally, they highlight Cu's regulatory effects on cell proliferation and wound healing which led them to study and demonstrate how Cu-impregnated sanitary towels significantly reduce postpartum perineal wound infections[
9,
10]. Cu is a tightly regulated metal in bacteria due to toxicity, in the case of
Salmonella, the detectable concentration of Cu has to be 10
-18M in order to be buffered in the bacteria [
11], whereas in normal human serum levels, detectable Cu concentrations are around 10-20*10
-6M [
12]. Several researchers take advantage of Cu toxicity in bacteria in order to expand the antibiotics toolset and overcome current multi-drug resistant bacteria strains.
In this perspective article we will discuss Cu’s utility against bacteria by targeting potential Cu regulatory pathways and new strategies that exploit copper coordination complexes, from the combination of chelators, to the implementation of prochelation strategies. Also, we will be discussing additional advances in the copper nanoparticles (NPs) antibiotic field, especially on Cu surface modifications by using either antibiotics and or biocompatible molecules. One of our main goals is for future researchers to expand metal-based antibiotics in order to overcome current and future AMR.
2. Copper Uses in Hospital Settings
Taking advantage of the known antimicrobial properties of Cu, various recent studies have examined the use of Cu and Cu alloys in hospital bed linens, patient gowns, bed rails, intravenous poles, faucet handles and many other highly touched surfaces in hospitals to decrease bacterial accumulation on these surfaces [
13,
14,
15]. The motivation behind these studies is that one of the many factors that contribute to healthcare-associated infections (HAIs) is the hospital surfaces and medical devices contaminated with pathogenic bacteria [
13]. HAIs are patient acquired infections that were not present when the patient was admitted to the hospital. These types of infections are being constantly monitored by agencies such as the National Healthcare Safety Network (NHSN) of the Center for Disease Control and Prevention (CDC). Among the most common HAIs are the catheter-associated urinary tract infections (CAUTI), Hospital-acquired Pneumonia (HAP), Ventilator-associated Pneumonia (VAP), and
Clostridium difficile infections [
16].
In order to reduce HAIs in Sentara Healthcare Hospitals, regular non-biocidal linens were replaced with copper oxide-impregnated beddings and Butler et al. determined if such replacement had a reduced effect on HAIs [
15]. To do this, they compared the rates of HAIs caused by
Clostridium difficile (CD) and multidrug-resistant organisms (MDROs) in three parallel periods by replacing all the regular non-biocidal linens with the copper oxide-impregnated biocidal linens. CD is a superbug that can causes diarrhea and colitis, and one in 11 people over the age of 65 with this HAI die within one month [
17]. A significant reduction of HAIs caused by combined CD and MDROs, and CD alone was seen, when copper oxide-impregnated linens were used, while the reductions in HAIs caused by MDROs did not reach statistical significance [
15]. A similar study by Arendsen et al., replaced non-Cu wound dressings with 3% copper-oxide impregnated wound dressings to investigate its effect on surgical site infection (SSI) rate after a cesarean section (CS) [
10]. They measured, via a telephone questionnaire, the SSI incidence within a 30-day period from the CS. A significant 38.7% reduction of overall SSI rate and a significant 80.3% reduction of organ/space SSI with the use of a copper impregnated wound dressing was seen. Nevertheless, a significant difference was not seen for the incidence of superficial/deep SSI (24.2% vs. 17.6%) [
10].
Additionally, various studies have shown that Cu alloy surfaces sustain the terminal cleaning bacteria concentration levels of different hospitals [
18,
19]. Terminal cleaning is the process of cleaning and disinfecting all surfaces in the healthcare facility or within an individual hospital room including floors and reusable equipment. This process can be required after an outbreak or increased incidence of infection, and a transfer or death of a patient who has had a known infection [
20]. Schmidt et. al. performed a pragmatic crossover study on the Highpoin
t Health Hospital where five high-touch intensive care unit (ICU) bed surfaces were either fabricated with polyester, or with U.S. Environmental Protection Agency (U.S. EPA) registered antimicrobial Cu, an alloy that contained 91.3% of Cu [
21]
. This study showed that beds with Cu surfaces harbored significantly fewer bacteria than control beds at levels below those considered to increase the likelihood of HAIs, and below the concentrations recommended after terminal cleaning and disinfection of 2.5 aerobic CFU/cm
2 [
18]. Similarly, another study by Hinsa-Leasure et. al. conducted at the Grinnell Regional Medical Center showed that in both occupied and unoccupied rooms, materials fabricated with copper alloys had significantly lower bacteria concentrations compared with control rooms, and these were either at or below the levels prescribed upon terminal cleaning [
19]. In these studies, the Cu surfaces are often referred to as self-disinfecting as these did not require additional cleaning or maintenance after terminal cleaning.
There are different types of Cu alloys used in hospital surfaces, and a study by Bryce
et. al. evaluated whether three different Cu alloys differed from stainless steel (control) in their antimicrobial efficacy, durability, and compatibility with hospital-grade cleaner/disinfectants [
22]. The three Cu -based alloys used in this study were: (a) spray-on alloy coating of 80% Cu–20% Nickel (Ni) applied onto hospital-grade stainless steel, (b) integral 80% Cu–20% Ni, and (c) Cu-impregnated surface that is a 16% copper oxide product embedded in the polymer. These alloys were tested by using 25 × 25 × 3 mm disks that were subjected to a year of cleaning and disinfection with the use of the Wiperator
TM. Three different common hospital disinfectants were used to evaluate the compatibility: accelerated hydrogen peroxide (AHP), quaternary ammonium (QA), or sodium hypochlorite solution (CL). The results of this study showed that all Cu formulations exhibited antimicrobial activity, but integral and spray-on copper alloys showed the greatest efficacy. Additionally, exposure to disinfectants could also affect the antimicrobial efficacy of all the surfaces. In the case of integrated alloy, the antimicrobial efficacy was reduced, they explained that the possible reason behind this reduction of the antimicrobial efficacy was because, as AHP is an oxidizing agent, it can oxidize the Cu on the alloy and create copper oxides, which have been demonstrated to have less Cu ion release. It is important to mention that there was an observed degradation of the copper surfaces throughout the year they analyzed, but it was not significant enough to affect the integrity of the surface. The same trend was seen when CL was used, which is also an oxidizing agent. As for the assessments of durability, integral Cu alloy showed the highest
durability and the least mass loss and abrasion-corrosion rate compared to stainless steel [
22].
Various Cu surface applications exhibit significant antimicrobial activity, and some of these prevent infections caused by the high bacteria burden on highly touched areas. Herein it is observed the importance and the real-life application of Cu antimicrobial activity and how it can be an alternative for, not only the treatment, but also the prevention of HAIs. Cu surfaces have not only been constructed for antimicrobial hospital common area surfaces, as many other applications of these have been investigated, but it has the potential to be incorporated in individual medical equipment. A preliminary study by Wu
et. al. tested the feasibility of an antimicrobial Cu surface for prosthetic joint material. To do this, they coated an ultra-high-molecular-weight polyethylene (UHMWPE) with Cu via aerosol assisted chemical vapor deposition. They used UHMWPE as it is used as a component for hip and knee implants worldwide. The results of this preliminary study showed a significant bactericidal effect of the surface created and, in the absence of bovine serum albumin, a 4.55log and 4.81log reduction of the bacterial number was seen. In addition, after the recovery of the bacteria in PBS, the Cu-coated UHMWPE was stained with live-dead stain and compared with the uncoated UHMWPE, and it was seen that the bacteria left on the copper coated sample were dead, whereas those on the control sample were alive [
23].
3. Copper Chemistry
Cu can be found in organisms in both ionic forms, Cu(I) and Cu(II), and it serves as an important cofactor for several enzymes. Cu’s capability to cycle between both oxidation states is essential because it provides redox (oxidation-reduction) activity to certain enzymes, allowing organisms to regulate reactive oxygen species (ROS) stress. ROS are the highly reactive radical byproducts of O
2 metabolism [
14]. The uncontrolled accumulation of ROS in organisms can damage proteins and DNA, via a generalized stress response [
24]. Cu and Zn are cofactors in the enzyme superoxide dismutase (SOD); this enzyme has the antioxidant capability to transform ROS radicals into molecular oxygen (O
2) [
25,
26]. Cu(II) coordination by the SOD active site promotes a two-step reaction that transforms superoxide into oxygen and hydrogen peroxide (
Scheme 1) [
25].
An excess of Cu ions can also affect organisms in the presence of oxygen. Cu toxicity has the potential to catalyze the production of ROS (
Scheme 2) [
29,
30]. The presence of peroxides is the critical component in promoting the formation of ROS via “Fenton-like” chemistry, leading to lipid peroxidation, protein oxidation, DNA damage, and degrade enzyme cofactors such as iron-sulfur (Fe-S) clusters [
29].
To understand Cu’s activity in organisms, we must also look at its biorelevant coordination chemistry
(Table 1) [
31]. Cu(I), predominant in the reducing intracellular environment, is a soft Lewis acid, meaning that it can bind to soft Lewis bases like thiol groups -RS
- (found in cysteine) and cyano (CN
-) ligands. In contrast, Cu(II), an intermediate acid, prefers binding to, depending on its coordination number and geometry, base ligands such as H
2O, OH, and RNH
2 [
31,
32], but can also bind to intermediate imidazole and soft S-donor groups [
27]. As a point of comparison, the essential metal Fe exists physiologically in the (Fe(II) (intermediate acid) and (Fe(III) (hard acid) forms
(Table 1) [
27]. Different proteins interact with Cu(I/II) through some common modalities, by coordinating to the terminal amine and terminal carboxylate groups, the side groups containing O, N, S coordinating atoms, or the amide backbone. These binding capabilities make Cu(I/II) transport possible in living organisms.
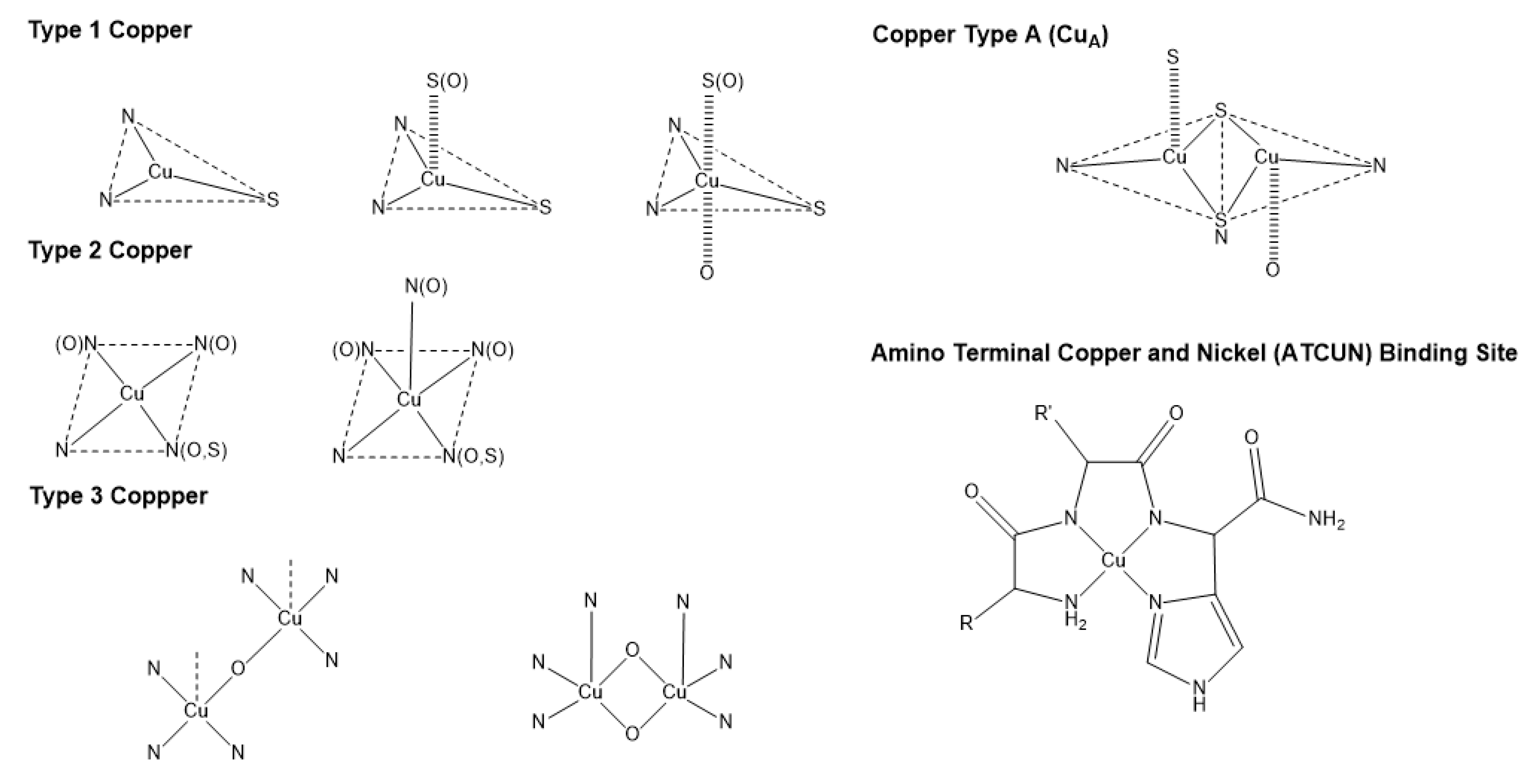
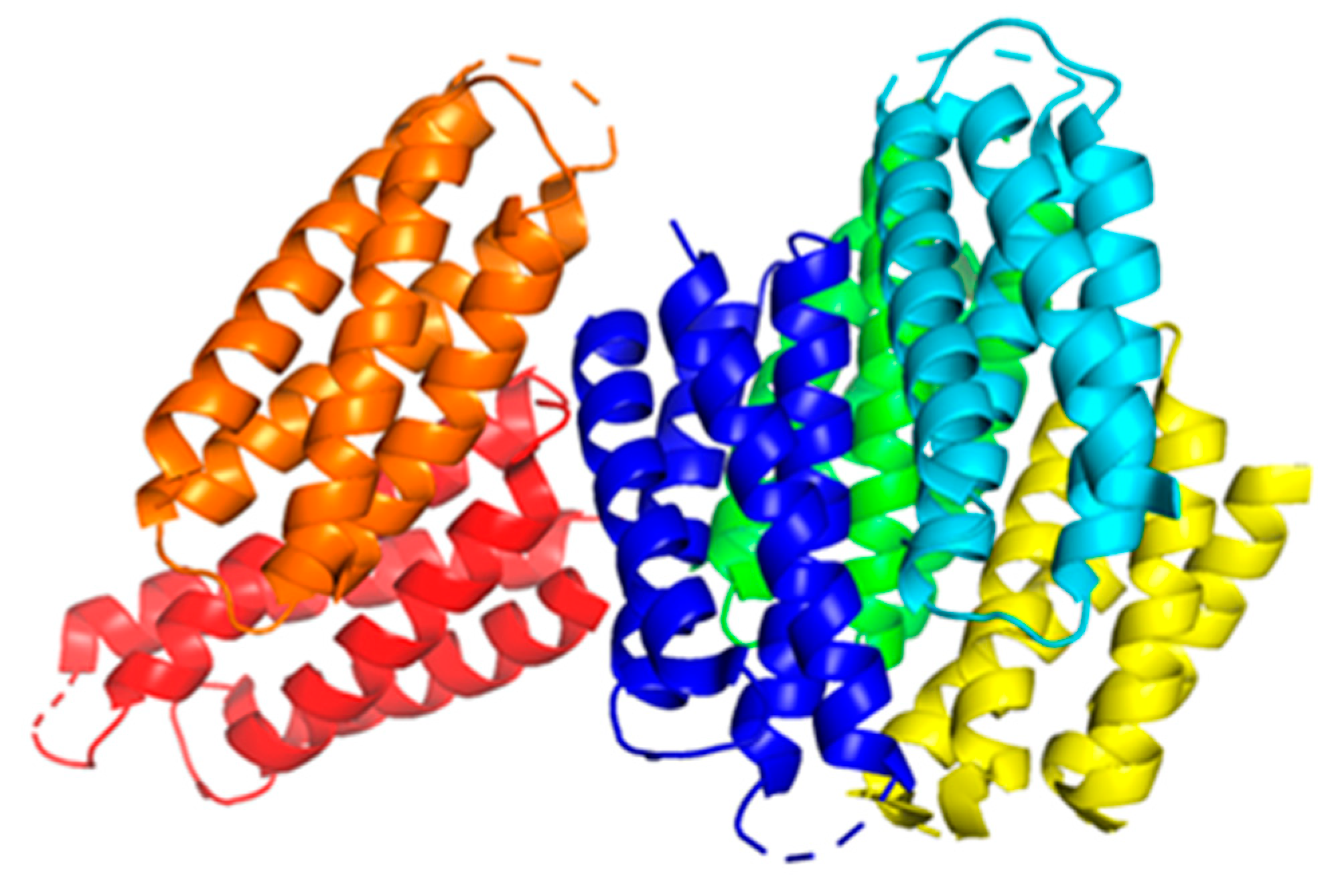
Some examples that highlight Cu coordination in cuproenzymes and copper proteins are the copper binding types (
Figure 1). Type 1 Cu, also known as blue copper, has a characteristic electronic absorption at 600nm and is usually found in plastocyanins, auracyanin, azurin, and other cyanine derivatives [
31]. Type 2 Cu has a characteristic for not having an absorption at 600nm [
33], and is usually found in Cu-Zn SOD enzymes, and some oxidases like amine oxidase [
31]. Similar to type 2 Cu, there’s a motif in some proteins known as amino terminal copper and nickel (ATCUN), this motif found in terminal sites of some proteins has been observed to have antimicrobial properties, and will be discussed later in (
Section 6). Type 3 Cu, known as the binuclear family of copper binding proteins, with a distinct electronic absorption bands at 350 and 600 nm [
31,
34]. Some examples of type 3 Cu are haemocyanins, tyrokinase, and tyrokinase derivatives [
34]. Cu type A (CuA) is a subfamily group from type 3 copper, with the difference that the Cu can bind to cysteines, and are characteristic for being clear when the Cu ions are reduced, and purple when oxidized; cytochrome c oxidase, and nitrous oxide reductases are common examples of CuA [
31,
34].
4. Copper Transport in Eukaryote and Prokaryote Cells
Metalloplasia is recently defined as metal-dependent cell growth and proliferation [
35]. This term, coined by Chang et al., exemplifies the need for metals in cell regulatory pathways and their metabolic functions. Cu is an example of an essential metal, and Chang narrows this definition to cuproplasia, which encompasses both primary and secondary effects of Cu in signaling pathways, including both enzymatic and non-enzymatic Cu modulated activities [
35]. Cu signaling pathways in eukaryotic cells and some prokaryotes is one of the multiple approaches Cu is regulated. Cuproenzymes have evolved to utilize Cu as catalytic cofactors to perform biological functions, many related to oxygen [
36]. To avoid Cu metal loss when redox cycling in cuproenzymes, Cu(II) ions in these groups of proteins are tightly coordinated. However, cuproenzymes are not the only cuproproteins required to regulate cell growth. Because they are metalated at biogenesis, they are not the leading players associated with Cu regulation and exchange. Copper sensors, chaperones, and transporters are thus essential to regulate and maintain Cu related functions.
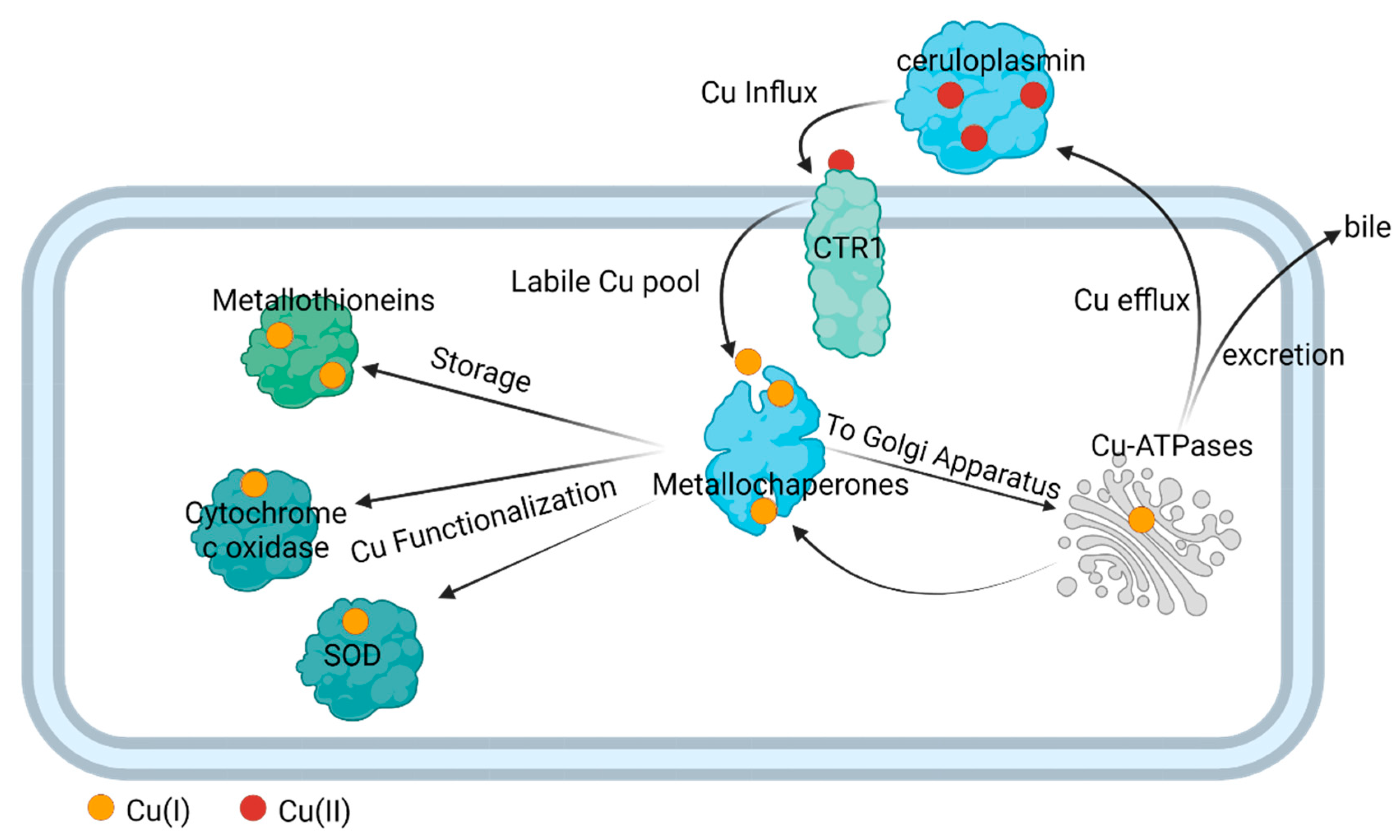
In complex organisms like humans, Cu is transported throughout the plasma via the enzyme ceruloplasmin; this cuproenzyme carries 95% of copper in humans [
37]. Eukaryote cells import Cu (Cu inclux) via active transport using a plasma membrane enzyme Cu(I) importer (CTR1). Once Cu enters the cell, the Cu enters in a dynamic labile pool, which largely consists of small molecular weight Cu(I) complexes [
38,
39,
40,
41,
42]. Recently, the existence of small molecular Cu(II) weight species were also identified to be part of this pool [
43]. There is a rapid turnover of this Cu intracellular population, with metallochaperone proteins quickly binding Cu ions to deliver them to areas where the cell needs it for functionalization [
44,
45,
46]. For instance, the ATOX metallochaperone delivers Cu to Cytochrome c oxidase and metallochaperone for dismutase (CCS) delivers Cu to superoxide dismutase (SOD) [
44]. Metallochaperones also deliver Cu to metallothionein for storage. They also participate in maintaining Cu homeostasis by delivering Cu to exporters to efflux it from cells and enable its bodily redistribution or excretion (
Figure 2). Under normal conditions, ATOX1 transfers Cu(I) to the Cu(I)-transporting ATPases, ATP7A, and ATP7B, primarily localized in the trans Golgi network to provide for systemic Cu supply and maturation of specific cuproproteins; from here, the Cu efflux from cells occurs [
47].
Prokaryotic cells, like bacteria, regulate Cu levels much more than eukaryotic cells. Although Cu is an essential metal for bacteria for redox activities, it’s also very toxic for bacteria and thus they have a much lower requirement for it [
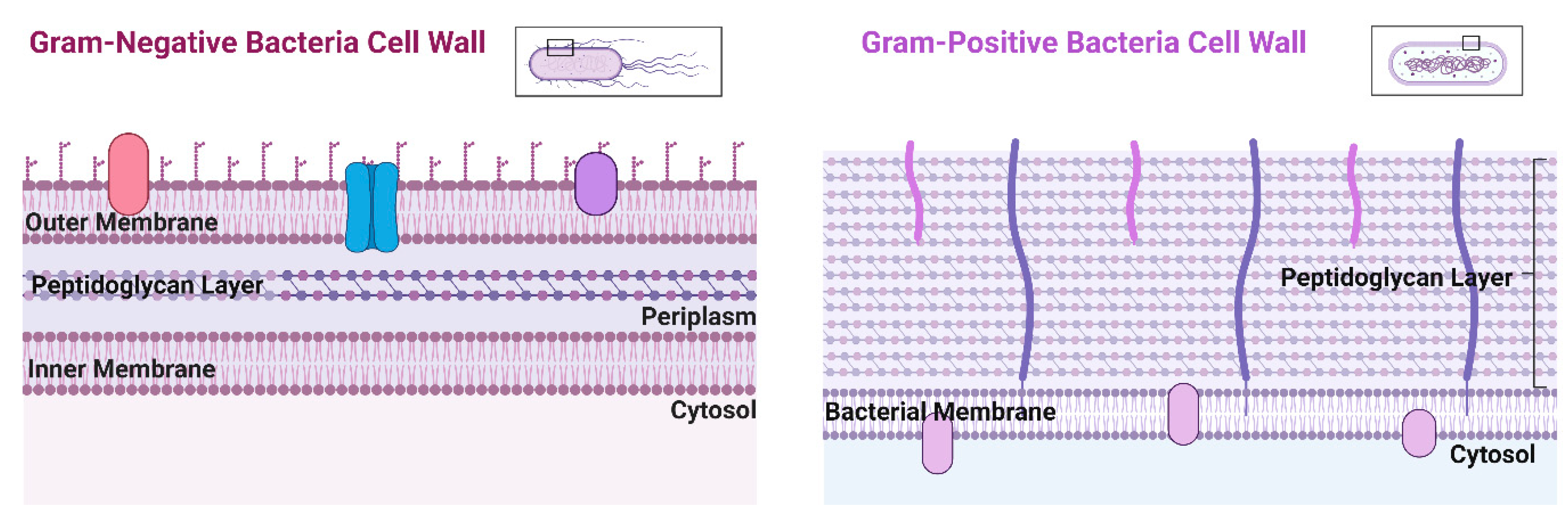
48]. Bacteria can be separated into two groups due to structure and morphological differences: Gram-positive, Gram-negative, and atypical bacteria. Gram-negative bacteria possess two cellular membranes, an inner and outer membrane separated by a peptidoglycan layer (
Figure 3). This outer membrane contains phospholipids, lipoproteins, lipopolysaccharides, and proteins. Unlike Gram-negative bacteria, Gram-positive only possess an inner membrane and do not have a periplasm. To protect the inner membrane, Gram-positive bacteria have a cell wall (
Figure 3) [
49]. When it comes to Cu transport, Gram-positive bacteria export Cu across the plasma membrane and Gram-negative bacteria are exported via the inner membrane and then to the periplasmic space [
50]. Atypical bacteria could be either Gram-positive or Gram-negative, but the thin or lack of peptidoglycan layer makes it impossible to be marked by the Gram stain [
51].
Some bacteria have evolved to lower the effects of Cu toxicity. Bacterial cells use transporters and chaperones proteins/enzymes with high affinity and specificity for Cu(I/II). Some bacteria have a Cu transporters like Cop A, that functions as an ATPase to transport Cu across the cytoplasm back to the periplasm [
29,
52]. In other types of bacteria, like
Mycobacterium tuberculosis, this ATPase is known as CtpV [
53]. Both are Cu transporters for the bacteria and are ATPases like protein. In
S. pneumoniae, a Cu chaperone called CupA guides Cu through the inner membrane from the cytoplasm to the periplasm [
48]. In
M. tuberculosis (which can appear Gram-positive or Gram-negative), the outer membrane uses Msp (porin protein) to make access for Cu(I) and Cu(II) to enter the bacterial periplasm from the extracellular space of the host. While this porin lets Cu enter the bacterial membrane, an outer membrane channel (MctB) allows Cu to permeate through the outer membrane back to the extracellular space. The
M. tuberculosis bacteria use CtpV, which is a Cu exporting ATPase that is required for full virulence of
M. tuberculosis [
29]
, to export Cu out of its cytoplasm to the periplasm, where the
Mycobacterium oxidizes Cu(I) using MmcO, a type of multicopper oxidase that protects bacteria from Cu stress. This MmcO is required for mycobacterial copper resistance [
29].
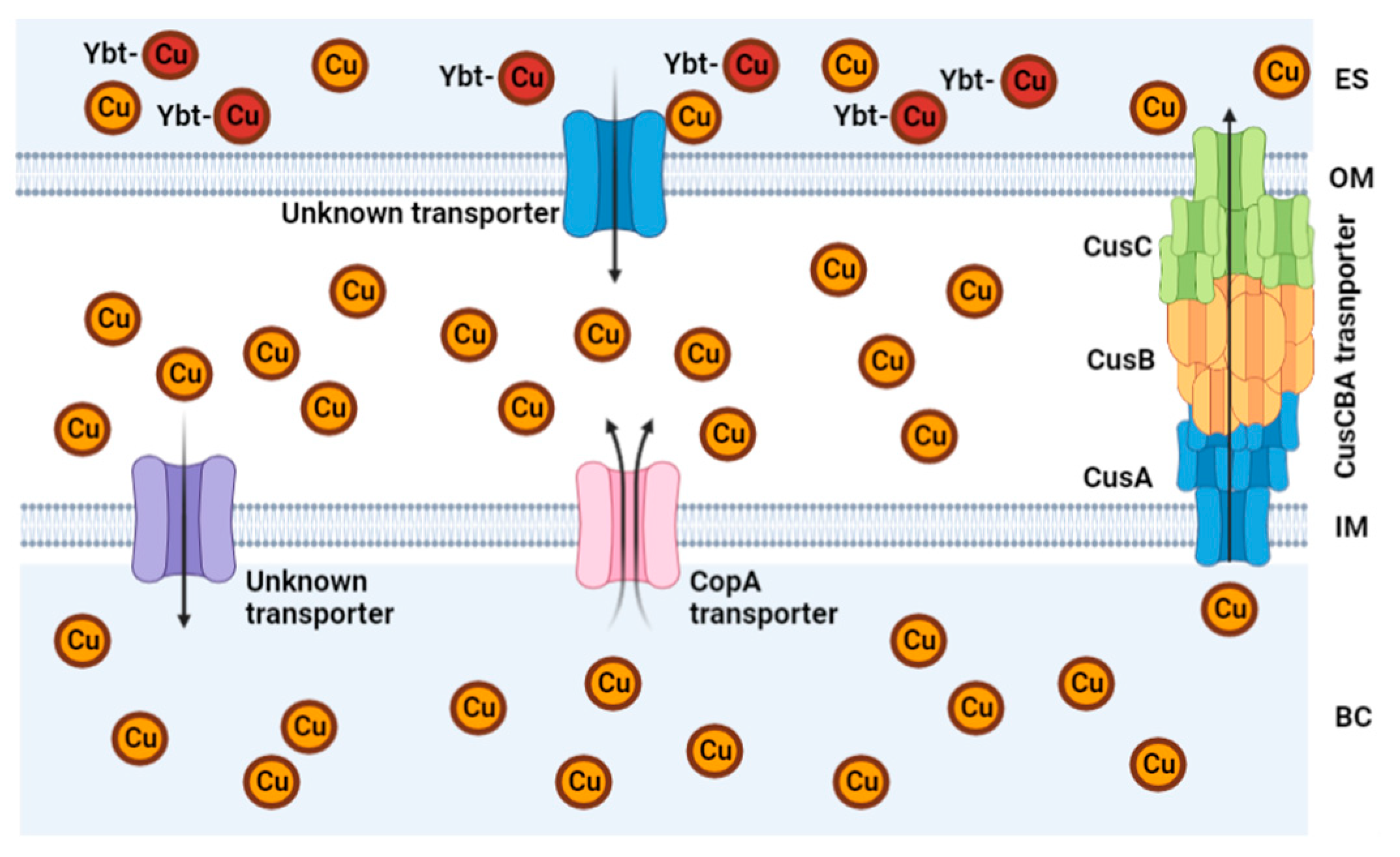
The bacteria
Escherichia coli (Gram-negative bacteria), possess a Cu efflux system known as the CusCBA complex (also refered to as CusFCBA/CusCFBA) (
Figure 4). CusA is a transmembrane efflux pump, CusB is a periplasmic membrane fusion protein, and CusC is an outer membrane; this union produces the CusCBA complex, which allows the Cu(I) to exit out of the periplasm to the extracellular space [
29]. Additionally, a more recent field of interest is the detection of metal chaperones metallothioneins with Cu affinity [
54]. Metallothionein are cysteine-rich proteins. Bacteria that express this type of protein has the advantage of resisting Cu toxicity [
55].
In summary, the main key players that regulate Cu levels, especially in bacteria are transporters that import and export Cu in bacteria. Fu et al. provide a more in-depth description of the Cu transport between eukaryote host and bacteria [
29]. Cu is rarely observed being stored in bacteria compared to eukaryote cells, which means that Cu is believed to constantly be in flux throughout bacteria. With the detection of metallothioneins, there has been an increased interest in detecting them in bacterial strains, since it can lead to Cu resistance [
54].
5. Copper Utility against Bacterial Infections
Due to the increasing evidence of suboptimal Cu levels and disease progression reflected in the low neutrophil count, Cu status is of importance. An imbalance of Cu can occur in our body by genetic mutations, autoimmune diseases, and even pathogenic bacteria that can attack and remove the metal ions from hosts’ proteins that transport them [
29]. However, our body can also sequester available metals as an immune response. The immune system comprises two parts, the acquired immune system, and the innate immune system. The acquired immune system is usually associated with the development of antibodies, whereas the innate immune system is related to a general 1
st line of defense against pathogens [
56].
One of the roles of the innate immune system is achieving nutritional immunity, which is commonly defined as the sequestration of trace metals by the host to avoid bacterial metal acquisition [
57]. This term coined by Hood and Skaar in 2012, highlights the host immune systems’ response of withholding iron (Fe) and Zinc (Zn) from pathogens in an attempt to starve bacteria [
58]. The host will also increase Cu levels, and attempts to circulate Cu in bacteria in order to increase toxicity, creating a Cu stress during nutritional immunity [
59]. Bacteria however, can overcome the immune system’s response by rapidly adapting the capability of (a) overexpressing metal affinity proteins and enzymes to overcome starvation [
58,
60,
61], and (b) increasing expression of Cu exporters [
59]. A key example of the overexpression of Cu transporters was observed by He et al., where CusS-CusR levels increase in
Vibrio alginolyticus during Cu stress, which then leads to an increase in the expression of the CusCFBA complex [
59]. This limits the host response against bacteria, increasing the need for antibiotic treatment options.
With antibiotic resistant bacteria being an increasing threat to global health, Cu has been investigated as a solution due to its antibacterial capabilities in all its biorelevant forms. At lower concentrations Cu is an essential metal for the living organism; nevertheless, it is most toxic to prokaryotic microorganisms. Cu(II) supplementation in salt form against bacteria has been extensively studied. Benhalima et al. [
62] identified the antibacterial effect of Cu(II) sulfate (CuSO
4) against multi-drug resistant nosocomial pathogens isolated from clinical samples. Febré et al. [
63] observed the antibacterial effect of Cu(II) nitrate and Cu(II) acetate against microorganisms obtained from chronic ulcers. While Cu(II) salts display broad spectrum activity, they lack specificity, and require relatively high dosages in order to have a toxic effect against pathogens, for instance, ~1,100 µg/ml of Cu(II) nitrate. This is why there has been an increased interest to develop Cu-based antibiotics that can target bacterial pathways.
5.1. Inhibition of Cu Efflux Pathways
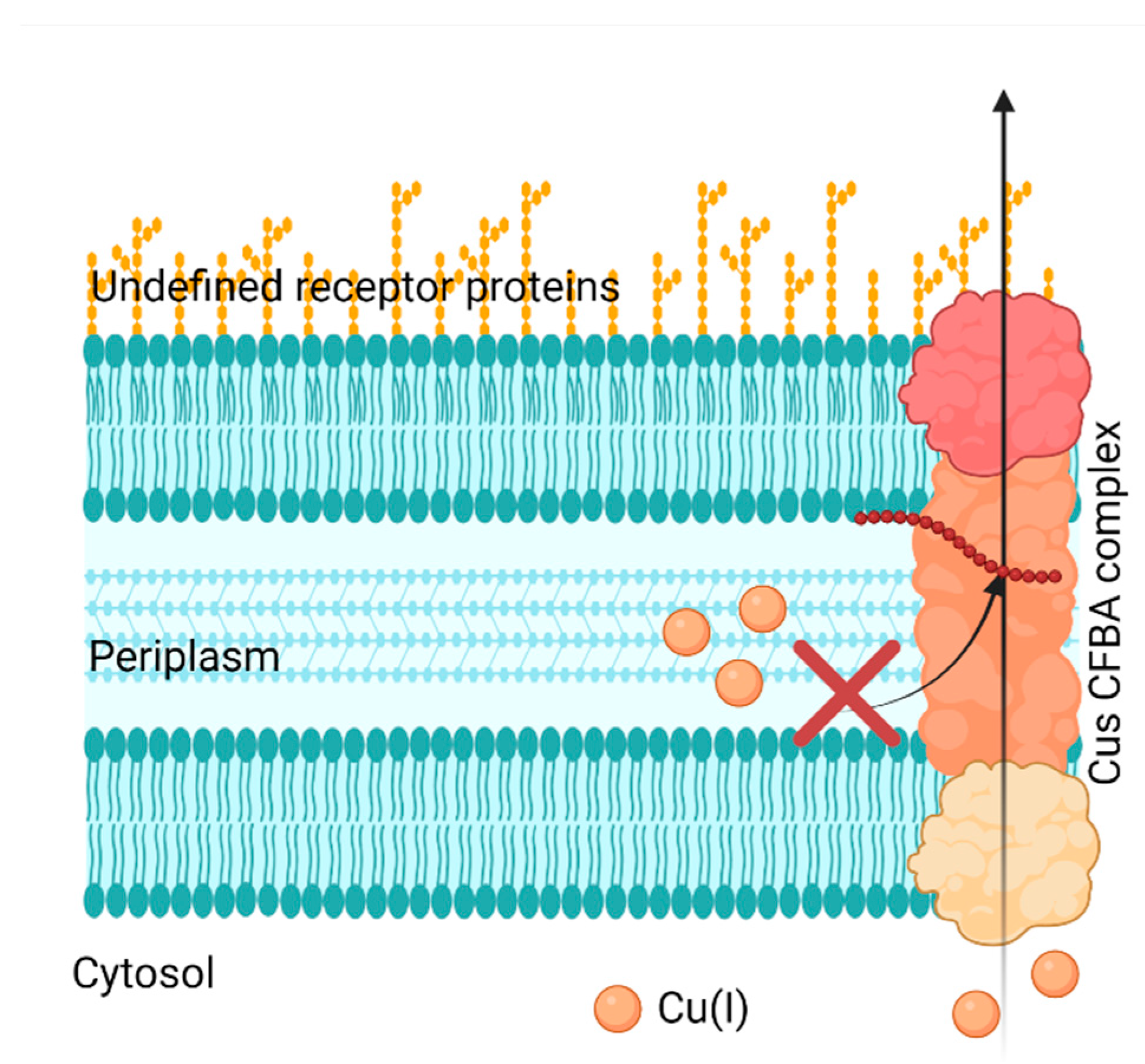
Bacteria can develop Cu resistance; therefore, it’s crucial to target their capability of removing Cu from itself, in other words, by inhibiting the Cu efflux pathways. Cells have four main Cu(I) transport mechanisms: Cytoplasmic CueR-metal sensor (Cu(I) binds and initiates CueO transcription for CopA), CopA transfer (ATPase active transfer of Cu(I)), CusF metallochaperone (Cu(I) transport from the periplasm to extracellular domain), and CueO (oxidation of Cu(I) to Cu(II)) [
64]. Meir et al. took advantage of CusCFBA and CueR systems, characteristic in bacterial Gram-negative Cu(I) efflux system, and designed a method that aimed to inhibit this system. They targeted the CusB protein, which is a channel opener; in other words, it regulates the Cu entering the cell [
64]. Meir et al. expressed the CusB protein in
E. coli cells and identified peptides (via computational docking studies) that could inhibit CusB. They identified peptide Pep5, which exhibited the most significant effect on cell growth and viability, but they failed to prove any interaction with CusB in the in vitro binding studies (
Figure 5). However, they found that the Cu efflux system could be disrupted since Pep5 was much more active in the presence of 5 μM Cu(II) in the growth media at all tested concentrations (
Figure 5). This showed the potential to develop a selective treatment against bacteria. However, this research was limited to identifying the potential peptides that disrupt the Cu efflux pathways present in Gram-negative bacteria, not the feasibility against Gram-positive or superbugs.
Additionally, Wolschendorf et al. have shown that the Cu defense mechanisms present in bacteria like
Mycobacterium tuberculosis can also be inhibited [
65]. An investigation discovered a Cu resistance mechanism that decreases intracellular Cu concentrations using an outer membrane channel protein (Rv1698) in
M. tuberculosis. They tested and concluded the Rv1698 channel activity in-vitro and concluded that the channel could also be another possible efflux mechanism for Cu. Moreover, they mutated
M. tuberculosis lacking the gene expression and presented impaired growth at higher Cu concentrations. A guinea pig model was used to assess Cu resistance. The guinea pigs were infected with wild type
M. tuberculosis and with a
M. tuberculosis mutant lacking the Rv1698 gene (ΔRv1698 mutant). After thirty days, the ΔRv1698 mutant mutant's bacterial burden was reduced compared to the wild-type
M. tuberculosis. These examples show that bacteria can become more sensitive to Cu by targeting genes or peptide modifications associated with the Cu efflux pathways.
5.2. Bacterial Cu Storage Plays a Key Role in Cu Resistance [66,67]
Two different studies have explored the possibility of targeting Cu storage in bacteria. One of the currently identified Cu storage in bacteria is the Cu storage protein (Csp3) (
Figure 6) [
66,
67,
68]. Csp3 is a protein located in bacterial cytosol and has been identified in both Gram-positive bacteria (
Bacillus subtilis) and Gram-negative bacteria (
Methylosinus trichosporium OB3b) [
66,
67]. Lee et al.[
67] analyzed the expression of
Bacillus subtilis Csp3 protein in
E. coli bacteria. This research aimed to analyze the effect of Csp3 on
E. coli bacterial growth. First, they analyzed
E. coli bacterial growth in their native state, wild-type (WT), and
E. coli mutant without Cu transporter Cop A (ΔCopA) by adding dosages of Cu(II) nitrate from up to 3.4 mM. After that, they performed a light scattering optical density (OD) analysis at 600 nm. This technique shows how bacterial growth responds to light; the higher the OD value, the more bacterial proliferation [
69]. When the maximum proliferation signal decreases, the Cu resistance is lost. Using this analogy, Lee et al. [
67] found that WT
E. coli showed a Cu resistance of 2.0 mM and ΔCopA
E. coli had Cu resistance up to 0.5mM. By overexpressing Csp3 in WT and ΔCopA
E. coli, the Cu resistance increased to 2.8mM and 1.5mM, respectively.
In another case study by Vita et al. [
66], they characterized the crystal structures of Csp3 proteins from a Gram-negative bacteria
Methylosinus trichosporium OB3b (
MtCsp3) and a Gram-positive bacteria
Bacillus subtilis (
BsCsp3). They found that up to 80 Cu(I) ions can bind to Csp3, especially
BsCsp3, which showed a greater affinity with the Cu(I) ions compared to
MtCsp3, which could bind around 30 Cu(I) ions. Interestingly, Gram-positive proteins like
BsCsp3 could adapt the bacteria to tolerate Cu in the cytosol, even if it lacks an intermembranal space (periplasm). The bacteria produce a Csp3 with more Cu binding sites to diminish the amount of free Cu(I) ions in the cytosol. Csp3, in both cases, are proteins that contain 4 helices in their structure (
Figure 6) with large amounts of labile cysteines (~SH) that bind to Cu(I) with an affinity of (1–2) × 10
17 M
−1 range [
66]. Cysteines and Cu(I) have a great affinity due to being soft species according to HSAB theory (
Table 1). These results in a stable conformation that favors the accumulation of Cu in bacteria minimizing toxic Cu releasing. These case studies provide great insight into how Cu can make it into bacterial cytosol and develop resistance against the oxidative stress of Cu when Cu levels are elevated. This represents a potential research field that could target bacterial Csp3 activity to overcome Cu resistance. Additionally, it reinforces that targeting CopA also sensitizes bacteria against Cu.
5.3. Cu toxicity by Displacing Iron and Iron-Sulfur Clusters
In contrast to cuproplasia, cuproptosis, the term for copper-mediated cell death, has been coined by Tsvetkov et al. [
70].While this term applies to eukaryotic cells and is an evolutionarily conserved pathway in mitochondrial respiration, it highlights a proposed mechanism for toxicity, Cu mismetallation. Mismetallation occurs when a metal ion coordinates to protein targets it should not, and as a result, interferes with regular protein interactions. This process can happen when Cu directly displaces metals from other sites, such as iron in iron-sulfur clusters, or by interacting with amino acids with chemical affinity to Cu as histidine, methionine, and cysteine.
This creates another possible strategy to increase Cu sensitivity in bacteria by limiting Fe uptake in the cell. Steunou et al.[
71] inactivated Fe-import in Cu(II) efflux mutants in
Rubrivivax gelatinosus. Cu(II) and Fe(II) are interchangeable in coordination between the proteins and enzymes (
Table 1). Steunou et al. observed that Cu displaces Fe in cells but can also damage (degrade) iron-sulfur clusters (Fe-S). Fe-S clusters are also cofactors in several enzymes and proteins and contain more than one type of Fe in the structure; damaging these clusters can release Fe(II) in the cells and trigger Fe-based Fenton chemistry [
71]; in other words, it induces oxidative stress via other pathways. They found that Cu resistance is mainly due to genes that encode the formation of Fe-binding proteins like fpbA and Ftr. One of the highlights of their results using different
Rubrivivax gelatinosus mutant strains was that wild type showed a resistance of Cu(II) sulfate up 400 uM. When they mutated the bacteria by eliminating the copper exporter CopA, the resistance of the bacteria was reduced by 200 uM. When they also eliminated the formation of Fe-binding protein fpbA, the Cu resistance was diminished to 1.6 uM and complete inhibition at a Cu(II) concentration of 50 uM, suggesting that Fe uptake is very important for Cu(I/II) tolerance. This strategy reinforces the importance of Fe for Cu homeostasis and takes advantage of a similar immune response against bacteria: starve bacteria from essential metals for homeostasis.
By 2007 Macomber et al. had already noted that while Fe-driven Fenton reactions led to DNA damage and mutagenesis in
E. coli, Cu overload in sensitive strains resulted in growth defects but no DNA damage. This steered Macomber and Imlay to test the hypothesis that Cu toxicity involves uncontrolled ROS generation and, more importantly, determine primary targets for Cu toxicity [
72]. Their study determined that branched amino acids and their biosynthesis were disrupted due to the inhibition of Fe-S clusters containing dehydratases under Cu toxicity. These results were also seen under anaerobic conditions, implying that this specific toxicity mechanism does not involve ROS, hence occurring through non-oxidative measures. This pivotal study inspired Fung et al. to study how
E. coli protected their Fe-S clusters from Cu degradation [
73]. They highlighted that Cu(I) can detrimentally interfere with Fe-S assembly biosynthesis and that induction of the CusCFBA membrane efflux complex increases Cu(I) tolerance in
E. coli. Additionally, they determined that methionine and cysteine can chelate Cu(I) ions, diminishing free intracellular Cu. Thus, under amino acid limitation
E. coli cells have higher levels of intracellular free Cu ions, especially when coupled with anaerobic conditions where one underlying cause could come from the periplasmic Cu reductase CueO being inhibited. Both of these studies, especially Fung et al.'s, revealed that Cu affects not only solvent-exposed Fe-S clusters in proteins but also the Fe-S cluster biogenesis machinery, which ended overexpressing the alternative sulfur mobilization system [
72,
73].
6. Exploiting Copper Chelation Capabilities for Potential Drug Designs
In the following sections we will discuss a select highlight of the current work on Cu(II) strategies that utilize Cu(II) chelation in order to improve the toxicity against bacteria. As previously discussed in
section 5, Cu(II) salts on their own have two major drawbacks, which are lack of selectivity and high dosage requirement in order to affect bacteria. Before continuing, it is very important to address a common concept discussed by several researchers, which is the minimum inhibitory concentration (MIC). MIC is known as the lowest concentration at which full inhibition of the bacteria is detected, defined as a 80% or greater bacterial growth inhibition [
74].
6.1. Cu(II) Coordination Complexes, a Ligand to Metal Synergistic Effect against Bacteria
Cu(II) complexes have been exploited for having promising antibiotic activity as an alternative to the rising crisis of superbugs [
75]. Cu(II) complexes of Schiff base complexes, in particular, have demonstrated potent antimicrobial and antifungal properties [
76]
. Schiff bases are organic compounds with an imine or azomethine group (R-N=C<R
2) with numerous applications in the synthetic industry [
77], in this section, however, we will focus on some Schiff bases with antimicrobial activities and how such activity can be improved with the combination of Cu(II).
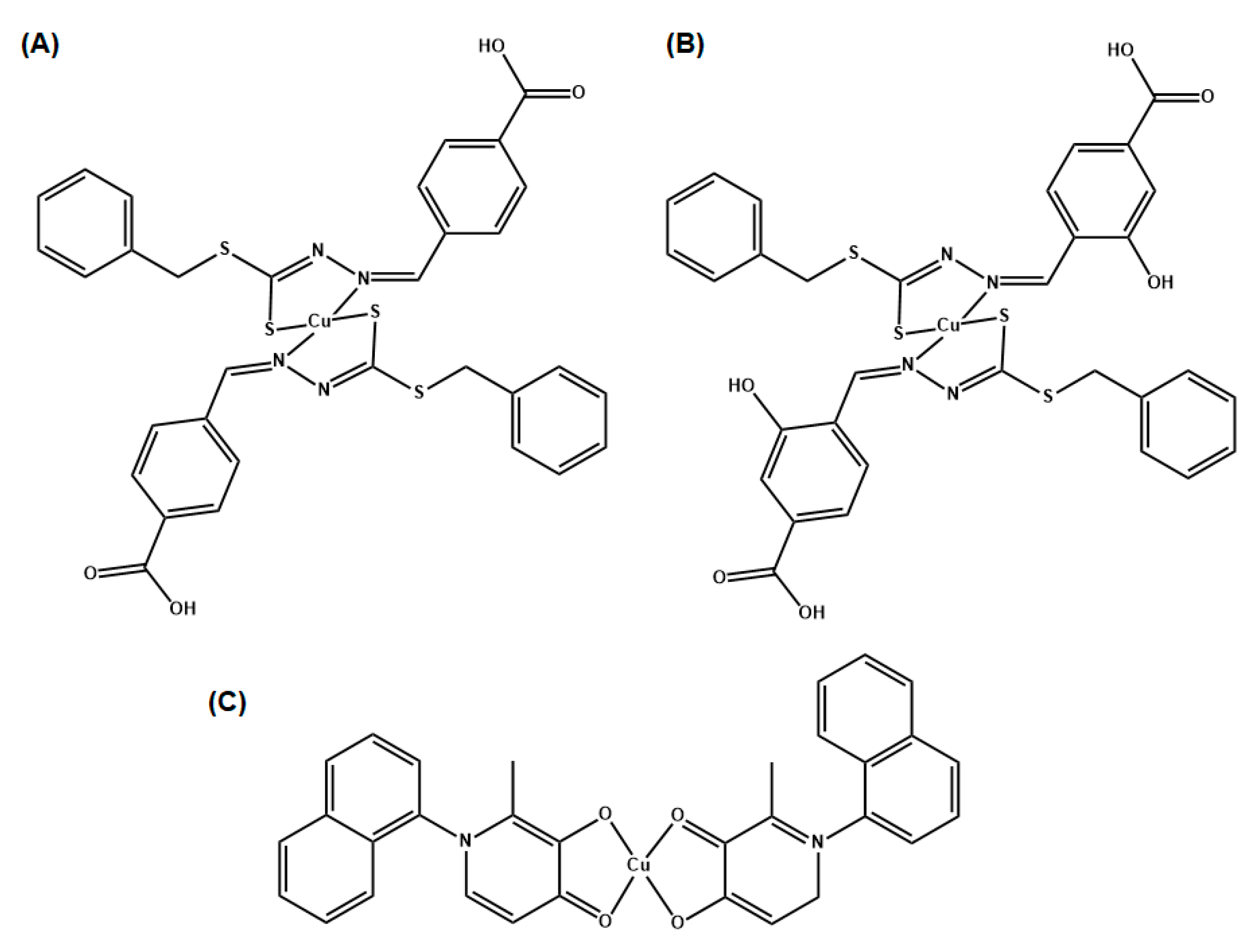
In their study, Chung et al. tested Schiff bases derivative complexes, SBD2 and SBD4 (
Figure 7A and
Figure 7B, respectively) individually and in combination with antibiotics such as oxacillin (a penicillin derivative [
78]) and vancomycin (a glycopeptidic antibiotic [
79]) towards microbes and antibiofilm [
80]. These types of antibiotics target the peptidoglycan bacteria wall synthesis [
81].
The reason for using complexes with antibiotics is to enhance by a synergistic effect the potential of a metallodrug that has biological activity with a defined selectivity towards bacteria. To establish the interaction of every combination that showed inhibition of bacterial growth a checkerboard method (which is used to determine the impact of the combination of antibiotics in comparison with their individual activities) was used. The fractional inhibition (FIC) concentration, which is used to test the interactions between two or more drugs that are intended to be used in combination, was calculated [
82]. E
quation (1) can be used to calculate the FIC value. Where MIC
A and MIC
B represent the minimal inhibitory concentration of compound A and B, C
A and C
B correspond to the concentration of the drugs [
80]
. As a standard measurement for FIC, values ranging from 0.0 ≤ 0.50 are considered a synergistic effect, from 0.50 ≤ 4.0 additive interaction and values above 4.00 are considered to have an antagonist effect.
Chung et al. observed that the compound SBD2 in coordination with Cu(II), exhibited a bactericidal effect against MRSA, with an MIC of 11 uM. For SBD4, concentrations of the complex must increase two-fold when 5 μM oxacillin is used. In the case of vancomycin, an additive effect was observed for both complexes. In MRSA, with oxacillin only showed a synergistic effect with a concentration of SBD2 of 2.8uM with 2.5uM of antibiotic. In the case of vancomycin, a synergistic effect was observed for both complexes. Additionally, they measured the therapeutic window of the synthesized compounds by performing lung cell viability assays. The relatively high selectivity index (SI) values of SBD2 (SI=5.63) and SBD4 (SI=1.63) were an indirect method to determine that the compounds had a net positive therapeutic index against MRSA. In other words, the compounds were able to eliminate MRSA at concentrations below their cytotoxic concentrations in healthy lung cells.
Another type of ligand used to develop Cu(II) complexes against multidrug-resistant bacteria is 3-hydroxy-4-pyridinone chelators. They are characterized by their low toxicity and high and specific metal chelating ability [
83]. Based on these properties, Leite et al explored Cu(II) complexes of three substituted 3-hydroxy-4-pyridinones with naphthyl moieties. [
84]. The reason to use naphthyl substituents is to increase the lipophilicity of ligands and the complex, biological activity, and fluorescence properties. In the case of bacteria strains Gram-positive,
S. aureus and
Enterococcus faecalis (
E. faecalis), and Gram-negative,
E. coli and P. aeruginosa, were used. The complex Cu(naph1pp)
2 (
Figure 7C) showed the strongest activity against Gram-positive strains, with MIC of 0.11 mM and 0.21 mM against
S. aureus and
E. faecalis, respectively. Due to these results, Leite et al. tested Cu(naph1pp)
2 against drug resistant variants of these Gram-positive bacteria, MRSA (MIC=0.11 mM) and vancomycin-resistant
Enterococcus faecalis (MIC=0.21 mM), and the results showed a similar antibacterial activity. Nevertheless, Cu(naph1pp)
2 and other synthesized complexes in this research did not show much activity toward Gram-negative bacteria. It was shown that a concentration of 7 uM of Cu(naph1pp)
2 and 97 uM of ciprofloxacin (a second-generation broad-spectrum fluoroquinolone efficient for Gram-negative and Gram-positive) presented a synergistic effect for
E. faecalis [
84]. For
S. aureus, it was observed an FIC of 0.63, which corresponds to an additive effect when combined with ciprofloxacin. Nonetheless, the fluorescent properties of Cu(naph1pp)
2 could allow tracking of the entrance and distribution inside bacteria cells for future analysis.
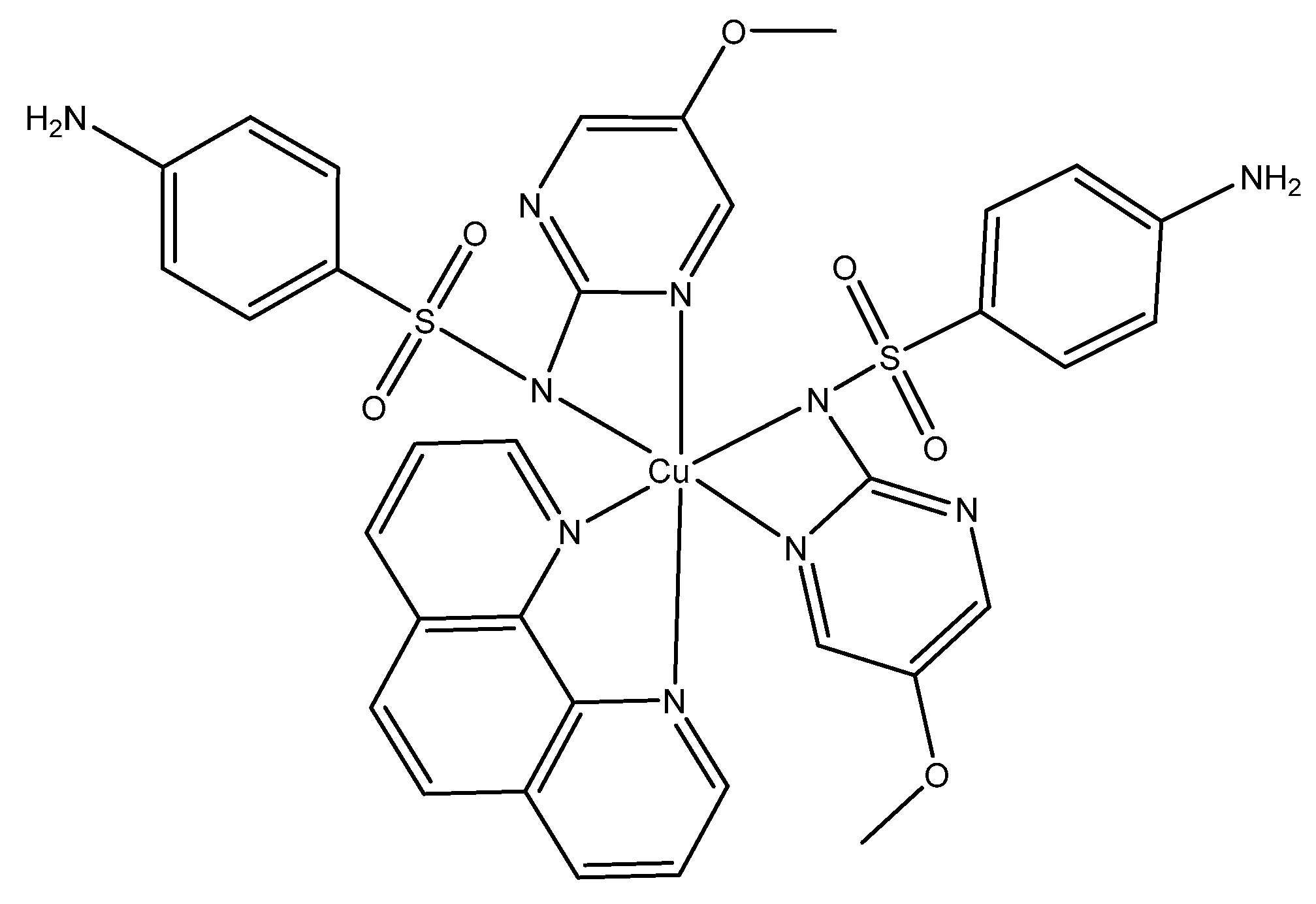
Sulfonamide ligands can also be used as antimicrobials. They have the versatility that they can act as monodentate ligands, bidentate or by bridging two metal ions [
16]. Sulfonamides have been used for treating Gram-negative and Gram-positive bacterial infections. For example, Nakahata et al. evaluated the antimicrobial activity of sulfonamide-containing Cu(II) complexes [
85]
.They observed that complex 1 (Figure 8) had a higher antimicrobial activity compared to sulfonamide ligand and Cu(II) separately. In the case of S. aureus, Nakahata et al. found a decrease of MIC from 20.7 mM (Cu(II) nitrate) to an MIC of 182 uM for complex 1(Figure 8) [
85]
. It was also observed that the ligands alone were not effective against the bacteria, which means that the antibacterial activity is copper-dependent. The researchers stated that this improvement in antimicrobial activity is attributed to the fact that these complexes are (artificial) metallonucleases and is able to generate oxidative damage caused by ROS. The term for (artificial) metallonucleases was previously coined by the authors, as metal complexes with properties to potentially fine-tune the desired nuclease activity [
86]
).
The combination of Schiff bases and other Cu(II) coordinating moieties, introduces a promising outlook for antibacterial drug development, especially when such combinations may have the potential to act synergistically against bacteria with other antibiotics.
6.2. Cu Dependent Inhibitors as Potential Synergistic Treatment with Traditional Antibiotics
An alternative way to the discovery of antibiotics able to restore the sensitivity of antibiotic resistance are Cu dependent inhibitors (CDI) [
87]. This term was coined by a team of researchers of The University of Alabama at Birmingham, and it consists of a series of antibiotics that utilize Cu(II) to inhibit bacteria such
S. aureus,
Mycoplasma spp and
M. tuberculosis. Dalecki et al. provided more detailed examples of CDI antibacterial activity like: disulfiram, 8-hydroxyquinoline, thiosemicarbazones, phenanthroline, and pyrityhione [
88]. At the moment there is not a conclusive mechanism of action in how CDI targets bacteria, other that it needs the presence of Cu in order to induce toxicity in bacteria likely through the formation of unregulated amounts of ROS [
89].
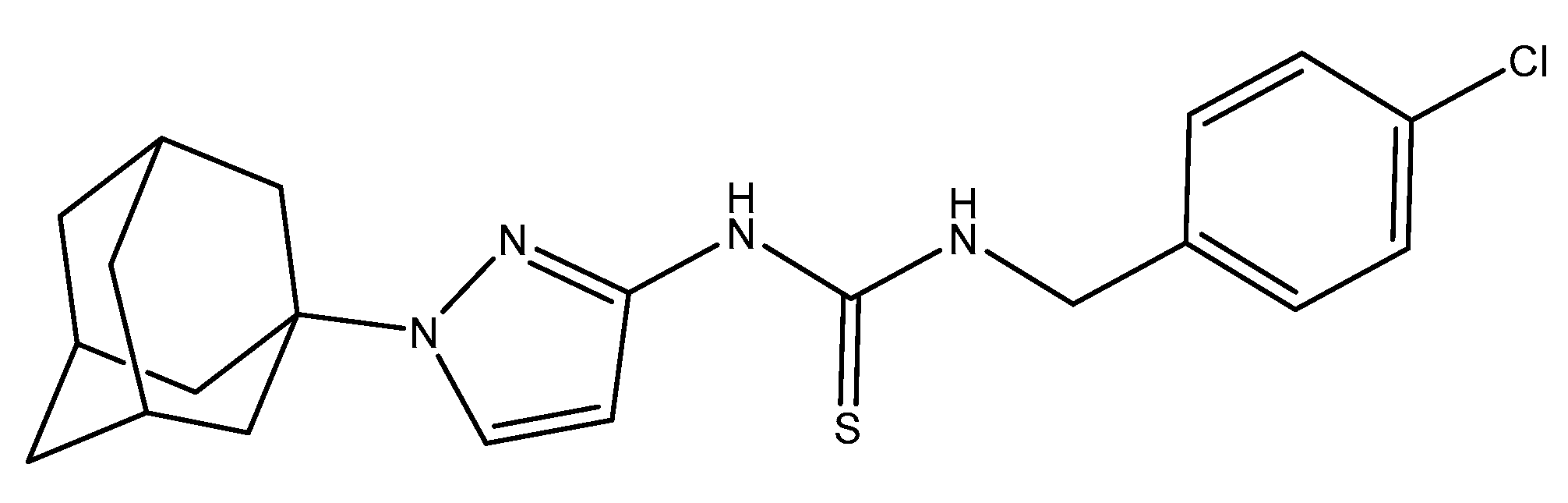
Crawford et al. further contributed to this research field by discussing the second-generation CDI called APT-6K (
Figure 9), which in the presence of 50 uM Cu(II) can affect
S. aureus (strain Newman) with an MIC of 150 nM [
89]. Additionally, ATP-6K was tested against the superbug MRSA. For their work they used four strains, two ATP-6K sensitive MRSA strains and two resistant strains. In order to determine which strain was resistant, they performed a toxicity assay on human monocytic cells THP-1, and they concluded that any concentration over 5 uM could be potentially harmful, therefore any ATP-6K MIC concentration above 5 uM was considered resistant. The key finding was that ATP-6K from 300 to 600 nM, in the presence of 50 uM Cu(II), re-sensitized MRSA strains to ampicillin at physiologically relevant concentrations (4-8 ug/mL) [
89]. This opens up a new research field for CDI as a potential synergistic treatment against superbugs.
6.3. Peptide Based Cu(II) Chelators
Other Cu(II) antibacterial complexes could be formed using motifs generated by protein structures. An example is the research work by Angeles-Boza et al. where they analyzed a peptide moiety known as the Amino Terminal Copper and Nickel (ATCUN) binding motif [
90,
91,
92]. The ATCUN motif is a structural feature present in proteins that bind Cu(II) and Ni(II) ions through a free NH
2- terminus, a histidine, and two other nitrogen residues, (
Figure 1) [
91]. ATCUN has been used in various experiments to form Cu(II) complexes involving antimicrobial peptides (AMP’s) to tackle bacteria activity of natural occurring host-defense peptides (HDP’s). Antimicrobial peptides have membrane solubilizing, cell penetrating, and DNA/RNA binding abilities [
90]. Known HDP’s from the piscidin family are Piscidin-1 (p1) and Piscidin-3 (p3), [
92] discovered in the mast cells of hybrid striped sea bass, are homologous antimicrobial peptides that are active against drug-resistant bacteria [
93]. These two host-defense peptides have a highly similar amino acid sequence and α-helical structure when bound to model membranes [
94].
Angeles-Boza et al. [
92] performed an experiment analyzing Cu(II) binding to p1 and p3, effect when is combined with Cu(II). Results suggested p3 targets bacterial DNA rather than the membranes, thus achieving a strong potency. Both p1 and p3 are ATCUN-containing peptides that have the potential to coordinate Cu(II) [
92]. Structural characterization indicated that in vitro p1 and p3 bind to Cu(II) in a 1:1 fashion using their ATCUN motif, and that no other potential groups in the peptide backbone compete for the Cu(II) ions. Also, p3-Cu(II) cleaved DNA faster than p1-Cu(II) after analyzing nuclease activity after performing a time-dependent cleavage of plasmid pUC19 with the metalated peptides (p3-Cu(II) and p1-Cu(II)). They used the bacteria
E. coli to test the MICs for p1-Cu(II) and p3-Cu(II) and found that there was a strong correlation between DNA damage and antimicrobial efficacy, with of p3-Cu(II) inducing a large magnitude of DNA cleavage by almost three times more than p1-Cu(II)
.
In another study, Angeles-Boza et al. [
90] used two ATCUN motifs selected from a library of ATCUN peptides, LKH (Leu-Lys-His) and RTH (Arg-Thr-His), for their rapid production of ROS when complexed to Cu(II), being third (RTH) and fouth (LKH) fastest ROS-producing compounds but were an order of magnitude faster than any of the remaining ATCUN sequences [
90]. To test if these ATCUNs could increase the activity of AMP’s, they incorporated those sequences to Anoplin (GLLKRIKTLL-NH
2), a peptide purified form the venom of a wasp, and has a membrane lytic activity [
90]. These ATCUN-Anoplin complexes were more active than Anoplin alone against the Gram-positive bacteria
Bacillus subtilis (
B. subtilis), and the Gram-negative bacteria
E. coli. The MIC values of Anoplin were 16 μM for
B. subtilis and 32 μM for
E. coli. For LKH-Anoplin and RTH-Anoplin, results were 8 μM and 4 μM respectively for the
B. subtilis, and 8 μM for both complexes in
E. coli. This led them to hypothesize that ROS formation played an important role in the activity of the complexes [
90].
6.4. Antibacterial Cu(II) Compound Isolated from Bacteria
A study by De Oliveira et al. [
95] extracted, purified and evaluated the antimicrobial activity of secondary metabolites of
P. aeruginosa LV strain produced in vitro against X. citri subsp. citri (strain 306. Xcc 306), to determine the potential of semi-purified secondary metabolites in foliar application to control citrus canker under greenhouse conditions, and to identify the biological activity of semi-purified secondary metabolites inside the leaf, reducing the inoculum potential (the amount of energy available for the fungus to infect the host at the site of infection [
96] inside the citrus canker lesions by electron microscopy). The results showed that the semi-purified secondary metabolites had strong antibiotic activity without phytotoxicity to orange plants, and that activity persisted for many weeks on the phylloplane and inside the leaf, reducing the inoculum potential outside and inside the citrus canker lesions. It is an example of how biomolecules produced by
Pseudomonas species can be utilized to combat other strains of bacteria. However, they were not able to properly characterize the antibacterial extract (F3d) other than it was a compound that contained Cu ions. A review article prepared by Afonso et al. focused on this matter and they found a correlation with Fluopsin C, an organic Cu(II) containing metabolite made by some
Pseudomonas and
Streptomyces bacteria [
97] (
Figure 10). Their review highlights some additional examples of Fluopsin C and different case studies on their antibacterial activity, and the different bacterial strains that can generate this Cu(II)-metabolite (different
Pseusomonas strains, and
Streptomyces 4601). The review covers a timeline from the first isolation of the compound in 1970, to research that analyze the different effects of the compound on bacteria like
Bacillus subtilis and MRSA, and detailed information about the biosynthetic pathway of Fluopsin C.
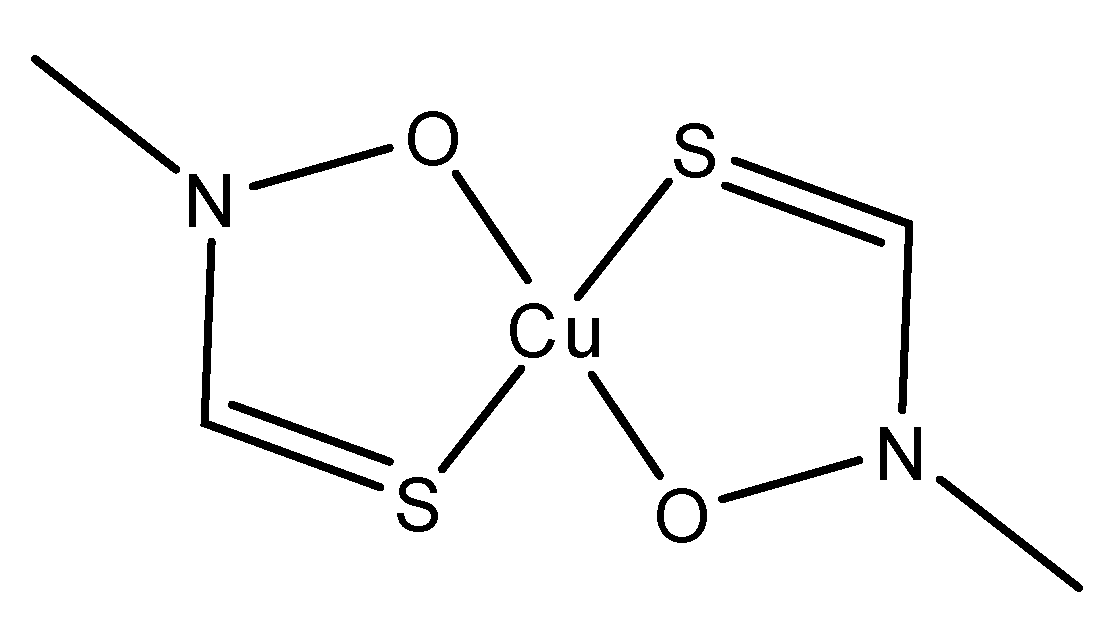
6.5. Cu(II) Prochelators as Potential Multimodal Antibiotic Drugs [98]
Prochelation is defined as the method through which a compound with little to no affinity to a metal ion undergoes a transformation, at a specific condition, that activates the chelation properties of the prochelator. Generally, prochelators do not interact with metal ions unless undergoes a cleavage process of a protecting group that avoids such chelation properties, like elevated levels oxidative stress [
99] or an enzymatic process [
100].
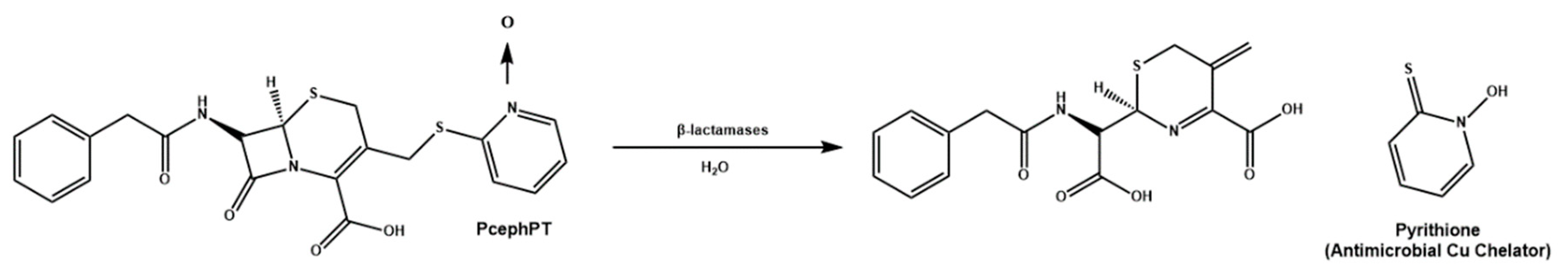
Cu(II) prochelators, have been applied as antibacterial agents for treatment against bacteria developing resistance against some antibiotics, for example penicillins, cephalosporins and carbapenems. In specific, some bacterial strains have gained resistance through β-lactamases, which cleave the antibiotics in a way that decreases their cytotoxicity. Due to this, Franz et al. decided to work on the development of a prochelator, called PcephPT, that was capable of taking advantage of β-lactamases to induce pathogenic cytotoxicity [
98]. The PcephPT prochelator (
phenylacetamido-
cephem-
pyri
thione) gets cleaved by the B-lactamase, producing pyrithione (PT), which had already been shown to be cytotoxic in the presence of Cu(II) (
Scheme 3). In the case of the bacteria
E. coli, strains resistant to cephalosporins (UTI89 CTX-M-1 as an example), PcephPT and PT had an MIC of 17.5 and 35 uM respectively with and without the presence of CuCl
2. Franz et al. demonstrated that the prochelation approach does not affect host cells. PcephPT was virtually nontoxic to human liver epithelial cells even at concentrations of 500 μM. It was slightly less cytotoxic compared to PT. In order for Cu(II) chelators and prochelators to be successfully applied for therapeutic purposes, their ability to selectively bind Cu(II) is one of the most important criteria for its therapeutic applications, in addition to avoiding Cu(II) binding and ROS production in human host cells.
7. Copper Nanoparticles against Pathogens
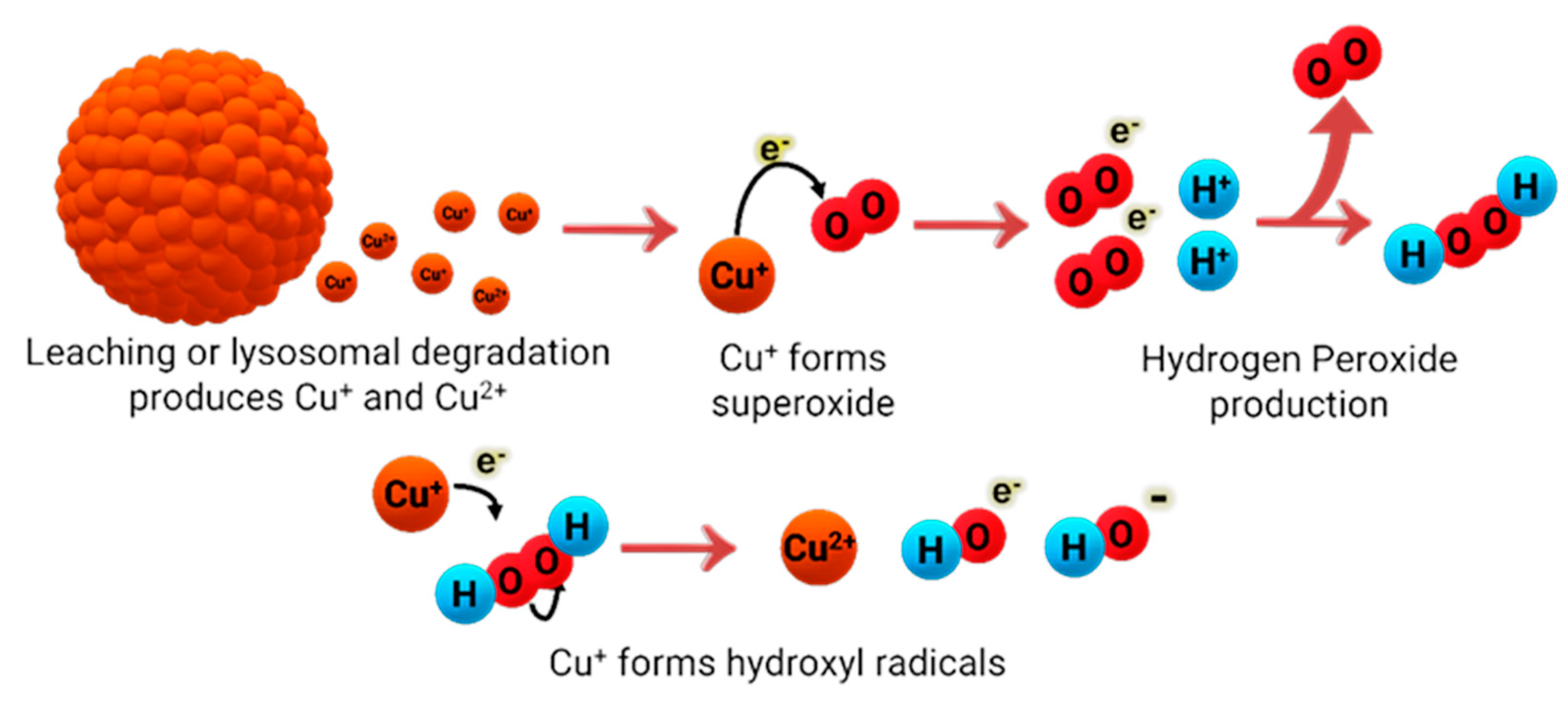
Over the past few years, much attention has been shifted to the antimicrobial abilities of Cu nanoparticles (NPs) and some have even worked on different preparation methods to study its effects on their antimicrobial properties Generally, copper nanoparticles are in spherical shape and with diameter sizes varying in the nanometer range. The most common nanoparticles available are copper (Cu-NPs), copper(I) oxide (Cu
2O-NP), copper(II) oxide (CuO-NPs), and copper(II) sulfide (CuS-NPs). Cu NPs’ antimicrobial activity has been studied and shown to be effective against Gram-positive and Gram-negative bacteria [
101,
102], although the effectiveness has been reported to be dependent on the size of the nanoparticles [
103], and their concentration [
103]. One of the most generally accepted mechanisms of how Cu NPs act against bacteria, is similar to other cytotoxic Cu species, where Cu is oxidized (or reduced, depending on the metal oxidation state) to Cu(I) and via a Fenton-like reaction, it generates ROS that is toxic to bacteria (
Figure 11). Although the antimicrobial effects of Cu NPs had been reported by many, according to Bastos et al. [
104], whether the dispersed or agglomerated forms of Cu NPs were responsible for the antimicrobial effects was not fully understood. Due to this, Bastos et al. decided to work on elucidating the problem because of the importance of Cu NP composition on its antimicrobial properties. Using an
E. coli bacterial strain, they reported that it is unlikely that the bacteria would acquire and internalize ultra-small NPs because of the rigidity of the bacterial cell wall and its incapability of supporting endocytic mechanisms. They showed the dissolution of copper-based particles was responsible for the antimicrobial properties, but not its particulate nature per se. Similarly, Chatterjee et al. [
105], mentioned that continuous release of copper ions into the media is what helps maintain the antimicrobial activity of Cu NPs against
E. Coli.
7.1. Copper Nanoparticles Surface Modification (Capping)
Modification of Cu nanoparticle surfaces has been of interest in order to prevent agglomeration of nanoparticles and/or improving the drug delivery for in-vitro and in-vivo trials. Capping is a strategy commonly described as the adhesion of a molecule to NPs, and in the case of Cu nanoparticles, this extends to more biologically compatible molecules.
Valodkar et al. [
101] observed that uncapped Cu NPs have strong reacting free electrons on their surface that can contribute to nanoparticle aggregation and to their high sensitivity toward environmental factors, such as pH, temperature, electrolytes, and solvents [
101]. Because of this, different preparation and coating methods have been explored to not only enhance antimicrobial activity of Cu NPs, but to protect the nanoparticles from environmental factors that could affect their effectiveness. For example, Valodkar, et al. worked on the synthesis of starch capped water-soluble Cu nanoparticles (CuS-NPs) for the purpose of increasing Cu NP biocompatibility inside a living organism. For their study, they used
S. aureus (Gram-positive),
E. coli (Gram-negative) and
Salmonella typhi (Gram-negative) bacterial strains. The MIC and the minimum bactericidal concentration (MBC) values obtained in their study showed that CuS-NPs were more effective against Gram-negative than Gram-positive bacteria; MIC
Gram+ (µg/mL) = 3.2 ± 0.41, MIC
Gram- (µg/mL) = 1.6 ± 0.22, MBC
Gram+ (µg/mL) = 4.3 ± 0.44, and MBC
Gram- (µg/mL) = 2.1 ± 0.27. They have attributed this result to the higher interaction between the Cu NPs and the negative charge on the cell surface of Gram-negative bacteria.
As previously mentioned, the preparation method can affect the behavior of the nanoparticles against certain bacteria. While the starch-capped preparation of Cu NPs caused the nanoparticles to be effective against Gram-negative
E. coli bacteria, Mehdizadeh et al. [
103] worked on another preparation method that proved to be effective against Gram-positive bacteria. They worked on fixing the Cu nanoparticles on a cellulosic walnut shell (CuNPs-WS) material and investigated its antimicrobial effects. The authors used various sizes of CuNPs-WS, ranging from 15-22 nm (CuNPs-WS1), 60-80 nm (CuNPs-WS2) and an aggregated and non-supported Cu NP size was reported for CuNPs-WS3. In addition, they used
S. aureus,
E. coli and
Listeria monocytogenes (Gram-positive) bacterial strains. They found that the nanoparticles showed a higher antimicrobial effect against the
L. monocytogenes bacteria using CuNPs-WS3 by measuring the inhibition diameter (nm). In addition, the MIC and MBC values supported the previously mentioned result, obtaining a MIC value of 62.5 ppm and an MBC value of 125 ppm for
L. monocytogenes using CuNPs-WS3.
Approaches in therapy towards bacterial infection in plants have been addressed in works like that of Datta et al. [
106]. The researchers synthesized Cu nanoparticles capped with ascorbic acid to combat
Xanthomonas oryzae pvoryzae which causes bacterial leaf blight in rice. These ascorbic acid capped Cu nanoparticles showed decrease in lesions in seedlings comparable to the antibiotic streptomycin sulfate and better activity than the commercial pesticide Bordeaux mixture. The antibacterial activity was due to the release of Cu ions by the ascorbic acid capped Cu nanoparticles upon foliar spray. Although ROS production was confirmed, the plant could withstand the oxidative stress due to a higher production of antioxidants and an increased shoot length and lipid, amino acid, and carbohydrate contents were recorded.
7.2. Synergizing Copper Nanoparticles with Antibiotics
Cu nanoparticles have exhibited synergism with antibiotics, enhancing their therapeutic potential against bacteria. Naqvi et al. [
107] achieved the synthesis of maltol capped Cu nanoparticles with a size range of 50-60 nm and a height of 11 nm. This study, published in 2021, is considered one of the first-ever reported synthesis of maltol capped copper NPs. Their purpose was to evaluate antibacterial and synergistic activity of the maltol capped copper nanoparticles in combination with Ciprofloxacin HCl and Streptomycin sulfate against clinically isolated pathogenic bacterial strains by disc diffusion method. The bacteria strains used were
E. coli,
K. pneumoniae,
Proteus mirabilis (
P. mirabilis),
Klebiscella oxytoca and
P. aeruginosa. The stability of the maltol capped Cu nanoparticles was tested and resulted in being stable for over 1 week at ambient conditions. The maltol capped Cu nanoparticles alone showed an estimated zone of inhibition (ZIH) per tested strain. The most enhanced effect was observed in the combination of the maltol capped Cu NPs in combination with Ciprofloxacin HCl, where there was a ZIH enhancement of 38 mm compared to 2 mm from Ciprofloxacin alone against
E. coli and
K. pneumoniae. However, Streptomycin sulfate adsorbed to the maltol capped NP, showed the highest efficacy with 26 mm ZIH in comparison to Streptomycin alone (2 mm ZIH) against
P. mirabilis.
Selectivity in treatment is an important factor to be able to effectively eradicate an infection and prevent the appearance of resistant variants. Zou et al. made use of NIR light as an activator for the Vancomycin modified Cu sulfide (CuS-Van) nanoparticles [
108] against vancomycin resistant enterococci (VRE). Vancomycin was used as reductant and capping agent for the Cu nanoparticles. Near-infrarred (NIR) irradiation (980 nm, 0.8 W/cm2) for 10 min in combination with CuS-Van NPs showed a bactericidal activity at 64ug/mL. The use of the NIR irradiation was essential for the bactericidal activity, the main purpose of adding the irradiation process was due to CuS nanoparticles, possess very high NIR absorption, making it an attractive approach to treat VRE. Additionally, live/dead fluorescent staining of vancomycin resistant enterococci and in vivo studies in mice showed this therapy combination aided in the mice VRE wound healing process.
Another study explored the effects of Cu oxide nanoparticles and antibiotics is on the partial nitrification process on anaerobic bacteria [
109]. For this specific case study, aerobic ammonia-oxidizing bacteria, usually found in wastewater, transforms ammonia to nitrite for the bacteria survival. This nitrification process serves as a general indicator of the bacteria overall survival. Zhang et al., used sulfamethoxazole as an example as it is one of the most widely used antibiotics [
109]. Four laboratory-scale sequencing batch reactors were utilized along with synthetic wastewater to test the variables of short term and long-term effects, structure of microbial community, expression of antibiotic resistance genes and expression of metal resistance genes and self-recovery of the reactors. The main results of this work showed that at short-term exposure to the combination of copper oxide nanoparticles and sulfamethoxazole, the aerobic ammonia-oxidizing bacteria bioactivity in partial nitrification system decreased by half. Meanwhile, long-term exposure to the previous combination decreased ammonia removal efficiency to 38.2% (versus 62.9% in control). Exposure to lone copper oxide nanoparticles improved the ammonia removal efficiency to 68.9% but exposure to lone Sulfamethoxazole decreased it to 50.1%. The reactors showed bad self-recovery performance after the addition of the Cu oxide nanoparticles and/or Sulfamethoxazole was stopped. Simultaneous addition of Cu oxide nanoparticles and Sulfamethoxazole formed a nanocomposite, which decreased aerobic ammonia-oxidizing bacteria abundance from 38.01% to 28.3% and induced the amplification of preponderant antibiotic resistance genes (sul3 and sulA). This work is important because it considers the inevitable disposal of antibiotics and Cu nanoparticles. As these Cu nanoparticles and antibiotic materials become more used in therapies for infections in a variety of living models, it will bring about an increase in these pollutants in our water sources. This study provides data that may serve as a step to remedy their presence in water.
8. Conclusions
Copper is an essential metal for bacterial survival, in homeostasis, it can protect bacteria from ROS. Compared to eukaryotic cells, bacteria have less resistance against ROS, making Cu a metal with antibiotic activity. The reason that copper is so toxic to bacteria is that an excess of Cu can form ROS, however, bacteria continue to evolve to overcome the oxidative stress that copper can induce by overexpressing Cu binding proteins, an example being Cu storage proteins like Csp3.
Even if the bacterial mutation examples help to increase Cu sensitivity, the feasibility of targeting bacterial pathogens with resistance to Cu poses a challenge. Integrating a peptide to disrupt the CusCFBA efflux system is one of the projects with more feasibility. However, the identification and consequential mutation of bacteria that becomes more sensitive to Cu elucidates the possible pathways to attack pathogenic bacteria. These findings properly address bacterial copper regulation sites for future drug development.
Creative approaches have often been the source of many innovations, like the study of copper metabolite fluopsin C generated by one bacterium to attack other bacteria, or copper-dependent inhibitors (CDIs ) that can increase Cu sensitivity and make a superbug like MRSA more susceptible to ampicillin, a common antibiotic [
89]. Even though the mechanistic details of how these drugs act are still unknown, this proves the feasibility of using Cu against pathogenic bacteria. The copper chelation approach is useful when exploiting copper’s redox potential for therapeutic purposes. It may provide specificity, stability and tuning of redox potentials to more useful values for antibacterial applications. While a recent study sheds light on a new copper induced cell death mechanism called cuproptosis [
110] as a copper-triggered modality of mitochondrial cell death, further studies should be carried out to establish this mechanism and the way it may be exploited for therapeutic purposes.
Copper, and copper oxide, nanoparticles can attack bacteria like
Escherichia coli,
Bacillus subtilis,
Staphylococcus aureus, MRSA and others [
111]. It was observed that the nanoparticle preparation method had a direct effect on which kind of bacteria it was most efficient against. In addition, it is established that the antimicrobial activity of the copper nanoparticles is due to the release of Cu(II) ions instead of its aggregated form. In comparison, many lone copper salts showed little difference in MIC on the same strain yet varied between different strains, this tendency remained in both Gram positive and Gram-negative strains. Most Cu nanoparticles studies that have been done have focused on their use and effectiveness against Gram-positive and Gram-negative bacteria. Results show that these Cu nanoparticles are effective against both types of bacteria, which serves as an advantage when applying these nanoparticles in different settings like, for example, hospital settings. In addition, results also show that modifications in the preparations of these nanoparticles result in changes in the efficiency against certain bacterial strains. This appears to be an important aspect to keep in mind when developing a method for the preparation of such Cu nanoparticles and when applying it in any kind of setting for antibacterial purposes. However, while there are many advantages to using copper nanoparticles, there are some downsides. For example, copper has the potential for ROS production in human hosts, which is another important factor to keep in mind when developing copper nanoparticles, although this ability has been exploited to cause bacterial cell death, and even cause cancer cell death. Another important factor to keep in mind is Cu leakage from the nanoparticles because, while some leaking has been shown to be effective in executing their antibacterial properties, too much leakage could result in the formation of excessive ROS and could cause undesired effects
in vivo.
Cu applications in the health is already moving towards Cu textiles [
112], and by sharing copper-based bacterial target pathways and antimicrobial drug design trends, we hope that further research is developed to expand the toolset against antimicrobial resistance (AMR). The World Health Organization (WHO) lists AMR among top 10 threats for global public health threats, and without effective antimicrobials, treating infections would be at increased risk [
113].
Author Contributions
Conceptualization, software, formal analysis, investigation, writing—original draft preparation, writing—review, editing, visualization, A.M.O.-R., Y.M.-C., N.M.-B., A.M.G.-C., A.R.-R., C. C.-S., C.G.-D., J.L., J.E., J.J.R.-O, J.D.-R., S.C.D.-V., Z.F.-D. and A.D.T.; supervision, project administration, funding acquisition, A.M.O.-R., N.M.-B., Y.M.-C., A.D.T. All authors have read and agreed to the published version of the manuscript.
Funding
A.D.T. was supported by the NIH 1R21CA240997-01A1 grant, MSEIP P120A210035 (which also funded A.M.O.-R., Y.M.-C., and A.R.-R.), and the UPR RP FIPI. A.M.O.-R., N.M.-B., and J.J.R.-O. were supported by the NIH RISE 5R25GM061151-21 grant. N.M.-B. also received funding from NSF BIOXFEL 1231306.
Acknowledgments
We would like to acknowledge Dr. Katherine J. Franz from Duke University for her contribution on antibacterial prochelators.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Sun, D.; Jeannot, K.; Xiao, Y.; Knapp, C.W. Editorial: Horizontal Gene Transfer Mediated Bacterial Antibiotic Resistance. Front. Microbiol. 2019, 10, 1933. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.; Ikuta, K.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E. , et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- COVID-19: U.S. Impact on Antimicrobial Resistance, Special Report 2022. Centers for Disease, C., Prevention, National Center for, E., Zoonotic Infectious, D., Division of Healthcare Quality, P., Eds. https://dx.doi.org/10.15620/cdc:117915: Hyattsville, MD, 2022. [CrossRef]
- Millanao, A.R.; Mora, A.Y.; Villagra, N.A.; Bucarey, S.A.; Hidalgo, A.A. Biological Effects of Quinolones: A Family of Broad-Spectrum Antimicrobial Agents. Molecules 2021, 26. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, P.; Yarlagadda, V.; Ghosh, C.; Haldar, J. A review on cell wall synthesis inhibitors with an emphasis on glycopeptide antibiotics. Med. Chem. Comm. 2017, 8, 516–533. [Google Scholar] [CrossRef] [PubMed]
- Chopra, I.; Roberts, M. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260, second page, table of contents. [Google Scholar] [CrossRef] [PubMed]
- Sköld, O. Sulfonamide resistance: mechanisms and trends. Drug. Resist. Updat. 2000, 3, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Frei, A.; Zuegg, J.; Elliott, A.G.; Baker, M.; Braese, S.; Brown, C.; Chen, F.; C, G.D.; Dujardin, G.; Jung, N. , et al. Metal complexes as a promising source for new antibiotics. Chem. Sci. 2020, 11, 2627–2639. [Google Scholar] [CrossRef] [PubMed]
- Arendsen, L.P.; Thakar, R.; Sultan, A.H. The Use of Copper as an Antimicrobial Agent in Health Care, Including Obstetrics and Gynecology. Clin. Microbiol. Rev. 2019, 32. [Google Scholar] [CrossRef]
- Arendsen, L.P.; Thakar, R.; Bassett, P.; Sultan, A.H. A double blind randomized controlled trial using copper impregnated maternity sanitary towels to reduce perineal wound infection. Midwifery 2021, 92, 102858–102858. [Google Scholar] [CrossRef]
- Hunsaker, E.W.; Franz, K.J. Emerging Opportunities To Manipulate Metal Trafficking for Therapeutic Benefit. Inorg. Chem. 2019, 58, 13528–13545. [Google Scholar] [CrossRef]
- Institute of Medicine Standing Committee on the Scientific Evaluation of Dietary Reference, I.; its Panel on Folate, O.B.V.; Choline. The National Academies Collection: Reports funded by National Institutes of Health. In Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B(6), Folate, Vitamin B(12), Pantothenic Acid, Biotin, and Choline, National Academies Press (US)Copyright © 1998, National Academy of Sciences.: Washington (DC), 1998; 10.17226/6015.
- Montero, D.A.; Arellano, C.; Pardo, M.; Vera, R.; Gálvez, R.; Cifuentes, M.; Berasain, M.A.; Gómez, M.; Ramírez, C.; Vidal, R.M. Antimicrobial properties of a novel copper-based composite coating with potential for use in healthcare facilities. Antimicrob. Resis. Infect. Control 2019, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.G.; Von Dessauer, B.; Benavente, C.; Benadof, D.; Cifuentes, P.; Elgueta, A.; Duran, C.; Navarrete, M.S. Copper surfaces are associated with significantly lower concentrations of bacteria on selected surfaces within a pediatric intensive care unit. Am. J. Infect. Control 2016, 44, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.P. Effect of copper-impregnated composite bed linens and patient gowns on healthcare-associated infection rates in six hospitals. J. Hosp. Infect. 2018, 100, e130–e134. [Google Scholar] [CrossRef] [PubMed]
- Monegro, A.F.; Muppidi, V.; Regunath, H. Hospital Acquired Infections. In StatPearls; StatPearls Publishing: Treasure Island (FL), 2022. [Google Scholar]
- CDC. Could you or your loved one have C. diff? Availabe online: https://www.cdc.gov/cdiff/what-is.html (accessed on April 26, 2023).
- Schmidt, M.G.; Attaway, H.H.; Fairey, S.E.; Howard, J.; Mohr, D.; Craig, S.; Schaffner, D.W. Self-Disinfecting Copper Beds Sustain Terminal Cleaning and Disinfection Effects throughout Patient Care. Appl. Environ. Microbiol. 2019, 86, e01886–01819. [Google Scholar] [CrossRef] [PubMed]
- Hinsa-Leasure, S.M.; Nartey, Q.; Vaverka, J.; Schmidt, M.G. Copper alloy surfaces sustain terminal cleaning levels in a rural hospital. Am. J. Infect. Control 2016, 44, e195–e203. [Google Scholar] [CrossRef] [PubMed]
- Parma, M.; Elli, E.; Terruzzi, E.; Fedele, M.; Doni, E.; Stasia, A.; Pogliani, E.M.; Pioltelli, P. Low dose of Deferasirox treatment in patients actually free of transfusion who present iron overload after bone marrow transplantation. Bone Marrow Transplantation 2015, 50, S421–S421. [Google Scholar]
- Green, J.J. US EPA, Pesticide product label, Antimicrobial copper alloys- Group III. US EPA, Office of Chemical Safety and Pollution Prevention: 2014.
- Bryce, E.A.; Velapatino, B.; Khorami, H.A.; Donnelly-Pierce, T.; Wong, T.; Dixon, R.; Asselin, E. In vitro evaluation of antimicrobial efficacy and durability of three copper surfaces used in healthcare. Biointerphases 2020, 15, 011005. [Google Scholar] [CrossRef]
- Wu, K.; Douglas, S.P.; Wu, G.; MacRobert, A.J.; Allan, E.; Knapp, C.E.; Parkin, I.P. A rugged, self-sterilizing antimicrobial copper coating on ultra-high molecular weight polyethylene: a preliminary study on the feasibility of an antimicrobial prosthetic joint material. J. Mater. Chem. B 2019, 7, 3310–3318. [Google Scholar] [CrossRef]
- Rowe, L.A.; Degtyareva, N.; Doetsch, P.W. DNA damage-induced reactive oxygen species (ROS) stress response in Saccharomyces cerevisiae. Free Radic. Biol. Med. 2008, 45, 1167–1177. [Google Scholar] [CrossRef]
- Robinett, N.G.; Peterson, R.L.; Culotta, V.C. Eukaryotic copper-only superoxide dismutases (SODs): A new class of SOD enzymes and SOD-like protein domains. J. Biol. Chem. 2018, 293, 4636–4643. [Google Scholar] [CrossRef]
- Harris, E.D. Copper as a Cofactor and Regulator of Copper,Zinc Superoxide Dismutase. J. Nutr. 1992, 122, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Barber-Zucker, S.; Shaanan, B.; Zarivach, R. Transition metal binding selectivity in proteins and its correlation with the phylogenomic classification of the cation diffusion facilitator protein family. Sci. Rep. 2017, 7, 16381–16381. [Google Scholar] [CrossRef] [PubMed]
- Bertini, I.; Gray, H.B.; Stiefel, E.I.; Selverstone-Valentine, J. Biological Inorganic Chemistry Structure and Reactivity; University Science Books: Herndon, 2007; pp. 1–3. [Google Scholar]
- Fu, Y.; Chang, F.-M.J.; Giedroc, D.P. Copper Transport and Trafficking at the Host–Bacterial Pathogen Interface. Acc. Chem. Res. 2014, 47, 3605–3613. [Google Scholar] [CrossRef] [PubMed]
- Pham, A.N.; Xing, G.; Miller, C.J.; Waite, T.D. Fenton-like copper redox chemistry revisited: Hydrogen peroxide and superoxide mediation of copper-catalyzed oxidant production. J. Catal. 2013, 301, 54–64. [Google Scholar] [CrossRef]
- Dabrowiak, J.C. , 2nd ed.John Wiley & Sons, Ltd: Hoboken, NJ, 2017.
- Soldatović, T. Correlation between HSAB Principle and Substitution Reactions in Bioinorganic Reactions. In Photophysics, Photochemical and Substitution Reactions - Recent Advances, IntechOpen: 2021. [CrossRef]
- MacPherson, I.S.; Murphy, M.E. Type-2 copper-containing enzymes. Cell. Mol. Life Sci. 2007, 64, 2887–2899. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, F.; McDougall, C.; Degnan, B.M. Origin, evolution and classification of type-3 copper proteins: lineage-specific gene expansions and losses across the Metazoa. BMC Evol. Biol. 2013, 13, 96. [Google Scholar] [CrossRef]
- Ge, E.J.; Bush, A.I.; Casini, A.; Cobine, P.A.; Cross, J.R.; DeNicola, G.M.; Dou, Q.P.; Franz, K.J.; Gohil, V.M.; Gupta, S. , et al. Connecting copper and cancer: from transition metal signalling to metalloplasia. Nat. Rev. Cancer 2022, 22, 102–113. [Google Scholar] [CrossRef]
- Rubino, J.T.; Franz, K.J. Coordination chemistry of copper proteins: how nature handles a toxic cargo for essential function. J Inorg. Biochem. 2012, 107, 129–143. [Google Scholar] [CrossRef]
- Hellman, N.E.; Gitlin, J.D. Ceruloplasmin Metabolism and Function. Annu. Rev. Nutr. 2002, 22, 439–458. [Google Scholar] [CrossRef]
- Chung, C.Y.-S.; Posimo, J.M.; Lee, S.; Tsang, T.; Davis, J.M.; Brady, D.C.; Chang, C.J. Activity-based ratiometric FRET probe reveals oncogene-driven changes in labile copper pools induced by altered glutathione metabolism. Proceedings of the National Academy of Sciences 2019, 116, 18285–18294. [Google Scholar] [CrossRef]
- Banci, L.; Bertini, I.; Ciofi-Baffoni, S.; Kozyreva, T.; Zovo, K.; Palumaa, P. Affinity gradients drive copper to cellular destinations. Nature 2010, 465, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; New, E.J. What has fluorescent sensing told us about copper and brain malfunction? Metallomics 2015, 7, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, C.M.; Lee, S.; Chang, C.J. Analytical Methods for Imaging Metals in Biology: From Transition Metal Metabolism to Transition Metal Signaling. Analytical Chemistry 2017, 89, 22–41. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, C.M.; Chang, C.J. Copper signaling in the brain and beyond. The Journal of biological chemistry 2018, 293, 4628–4635. [Google Scholar] [CrossRef] [PubMed]
- Pezacki, A.T.; Matier, C.D.; Gu, X.; Kummelstedt, E.; Bond, S.E.; Torrente, L.; Jordan-Sciutto, K.L.; DeNicola, G.M.; Su, T.A.; Brady, D.C. , et al. Oxidation state-specific fluorescent copper sensors reveal oncogene-driven redox changes that regulate labile copper(II) pools. Proceedings of the National Academy of Sciences of the United States of America 2022, 119, e2202736119. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, Jack H. ; Maryon, Edward B. How Mammalian Cells Acquire Copper: An Essential but Potentially Toxic Metal. Biophys J 2016, 110, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Pang, W.L.; Kaur, A.; Ratushny, A.V.; Cvetkovic, A.; Kumar, S.; Pan, M.; Arkin, A.P.; Aitchison, J.D.; Adams, M.W.W.; Baliga, N.S. Metallochaperones Regulate Intracellular Copper Levels. PLOS Comput Biol 2013, 9, e1002880. [Google Scholar] [CrossRef] [PubMed]
- Robinson, N.J.; Winge, D.R. Copper metallochaperones. Annu Rev Biochem 2010, 79, 537–562. [Google Scholar] [CrossRef]
- Kaplan, J.H.; Lutsenko, S. Copper Transport in Mammalian Cells: Special Care for a Metal with Special Needs. J. Bio. Chem. 2009, 284, 25461–25465. [Google Scholar] [CrossRef]
- Andrei, A.; Öztürk, Y.; Khalfaoui-Hassani, B.; Rauch, J.; Marckmann, D.; Trasnea, P.-I.; Daldal, F.; Koch, H.-G. Cu Homeostasis in Bacteria: The Ins and Outs. Membranes 2020, 10, 242–242. [Google Scholar] [CrossRef]
- Jiang, W.; Saxena, A.; Song, B.; Ward, B.B.; Beveridge, T.J.; Myneni, S.C.B. Elucidation of Functional Groups on Gram-Positive and Gram-Negative Bacterial Surfaces Using Infrared Spectroscopy. Langmuir 2004, 20, 11433–11442. [Google Scholar] [CrossRef] [PubMed]
- Ladomersky, E.; Petris, M.J. Copper tolerance and virulence in bacteria. Metallomics 2015, 7, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, E.R.; Martin, R.J. Asthma and Atypical Bacterial Infection. Chest 2007, 132, 1962–1966. [Google Scholar] [CrossRef] [PubMed]
- Argüello, J.M.; Raimunda, D.; Padilla-Benavides, T. Mechanisms of copper homeostasis in bacteria. Front. Cell Infect. Microbiol. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Ward, S.K.; Abomoelak, B.; Hoye, E.A.; Steinberg, H.; Talaat, A.M. CtpV: a putative copper exporter required for full virulence of Mycobacterium tuberculosis. Mol. Microbiol. 2010, 77, 1096–1110. [Google Scholar] [CrossRef] [PubMed]
- Dennison, C.; David, S.; Lee, J. Bacterial copper storage proteins. J. Biol. Chem. 2018, 293, 4616–4627. [Google Scholar] [CrossRef] [PubMed]
- Calvo, J.; Jung, H.; Meloni, G. Copper metallothioneins. IUBMB Life 2017, 69, 236–245. [Google Scholar] [CrossRef]
- Kurtz, J. Memory in the innate and adaptive immune systems. Microbes Infect. 2004, 6, 1410–1417. [Google Scholar] [CrossRef]
- Healy, C.; Munoz-Wolf, N.; Strydom, J.; Faherty, L.; Williams, N.C.; Kenny, S.; Donnelly, S.C.; Cloonan, S.M. Nutritional immunity: the impact of metals on lung immune cells and the airway microbiome during chronic respiratory disease. In Respiratory Research; BioMed Central Ltd, 2021; Vol. 22. [Google Scholar]
- Hood, M.I.; Skaar, E.P. Nutritional immunity: transition metals at the pathogen-host interface. Nat. Rev. Microbiol. 2012, 10, 525–537. [Google Scholar] [CrossRef]
- He, R.; Zuo, Y.; Zhao, L.; Ma, Y.; Yan, Q.; Huang, L. Copper stress by nutritional immunity activates the CusS-CusR two-component system that contributes to Vibrio alginolyticus anti-host response but affects virulence-related properties. Aquaculture 2021, 532, 736012. [Google Scholar] [CrossRef]
- Yadav, R.; Noinaj, N.; Ostan, N.; Moraes, T.; Stoudenmire, J.; Maurakis, S.; Cornelissen, C.N. Structural Basis for Evasion of Nutritional Immunity by the Pathogenic Neisseriae. Front. Microbiol. 2019, 10, 2981. [Google Scholar] [CrossRef] [PubMed]
- Cassat, J.E.; Skaar, E.P. Metal ion acquisition in Staphylococcus aureus: overcoming nutritional immunity. Semin. Immunopathol. 2012, 34, 215–235. [Google Scholar] [CrossRef]
- Benhalima, L.; Amri, S.; Bensouilah, M.; Ouzrout, R. Antibacterial effect of copper sulfate against multi-drug resistant nosocomial pathogens isolated from clinical samples: Effect of copper sulfate on clinical isolates. Pak. J. Med. Sci. 2019, 35. [Google Scholar] [CrossRef] [PubMed]
- Febré, N.; Silva, V.; Báez, A.; Palza, H.; Delgado, K.; Aburto, I.; Silva, V. Comportamiento antibacteriano de partículas de cobre frente a microorganismos obtenidos de úlceras crónicas infectadas y su relación con la resistencia a antimicrobianos de uso común. Rev. Med. Chile 2016, 144, 1523–1530. [Google Scholar] [CrossRef] [PubMed]
- Meir, A.; Lepechkin-Zilbermintz, V.; Kahremany, S.; Schwerdtfeger, F.; Gevorkyan-Airapetov, L.; Munder, A.; Viskind, O.; Gruzman, A.; Ruthstein, S. Inhibiting the copper efflux system in microbes as a novel approach for developing antibiotics. PLOS ONE 2019, 14, e0227070–e0227070. [Google Scholar] [CrossRef] [PubMed]
- Wolschendorf, F.; Ackart, D.; Shrestha, T.B.; Hascall-Dove, L.; Nolan, S.; Lamichhane, G.; Wang, Y.; Bossmann, S.H.; Basaraba, R.J.; Niederweis, M. Copper resistance is essential for virulence of Mycobacterium tuberculosis. PNAS 2011, 108, 1621–1626. [Google Scholar] [CrossRef] [PubMed]
- Vita, N.; Landolfi, G.; Baslé, A.; Platsaki, S.; Lee, J.; Waldron, K.J.; Dennison, C. Bacterial cytosolic proteins with a high capacity for Cu(I) that protect against copper toxicity. Sci. Rep. 2016, 6, 39065–39065. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Dennison, C. Cytosolic Copper Binding by a Bacterial Storage Protein and Interplay with Copper Efflux. Int. J. Mol. Sci. 2019, 20, 4144–4144. [Google Scholar] [CrossRef]
- Sehnal, D.; Bittrich, S.; Deshpande, M.; Svobodová, R.; Berka, K.; Bazgier, V.; Velankar, S.; Burley, S.K.; Koča, J.; Rose, A.S. Mol* Viewer: modern web app for 3D visualization and analysis of large biomolecular structures. Nucleic Acids Res. 2021, 49, W431–W437. [Google Scholar] [CrossRef]
- Brown, H.L. Microbial Growth. In Encyclopedia of Infection and Immunity; Elsevier, 2022; pp. 324–335. [Google Scholar] [CrossRef]
- Tsvetkov, P.; Coy, S.; Petrova, B.; Dreishpoon, M.; Verma, A.; Abdusamad, M.; Rossen, J.; Joesch-Cohen, L.; Humeidi, R.; Spangler, R.D. , et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science (New York, N.Y.) 2022, 375, 1254–1261. [Google Scholar] [CrossRef]
- Steunou, A.S.; Bourbon, M.L.; Babot, M.; Durand, A.; Liotenberg, S.; Yamaichi, Y.; Ouchane, S. Increasing the copper sensitivity of microorganisms by restricting iron supply, a strategy for bio-management practices. Microb. Biotech. 2020, 13, 1530–1545. [Google Scholar] [CrossRef] [PubMed]
- Macomber, L.; Imlay, J.A. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. PNAS 2009, 106, 8344–8349. [Google Scholar] [CrossRef] [PubMed]
- Fung, D.K.; Lau, W.Y.; Chan, W.T.; Yan, A. Copper efflux is induced during anaerobic amino acid limitation in Escherichia coli to protect iron-sulfur cluster enzymes and biogenesis. J. Bacteriol. 2013, 195, 4556–4568. [Google Scholar] [CrossRef] [PubMed]
- Kowalska-Krochmal, B.; Dudek-Wicher, R. The Minimum Inhibitory Concentration of Antibiotics: Methods, Interpretation, Clinical Relevance. Pathogens 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Claudel, M.; Schwarte, J.V.; Fromm, K.M. New Antimicrobial Strategies Based on Metal Complexes. Chemistry 2020, 2. [Google Scholar] [CrossRef]
- Ejidike, I.P. Cu(II) Complexes of 4-[(1E)-N-{2-[(Z)-Benzylidene-amino]ethyl}ethanimidoyl]benzene-1,3-diol Schiff Base: Synthesis, Spectroscopic, In-Vitro Antioxidant, Antifungal and Antibacterial Studies. Molecules 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Ceramella, J.; Iacopetta, D.; Catalano, A.; Cirillo, F.; Lappano, R.; Sinicropi, M.S. A Review on the Antimicrobial Activity of Schiff Bases: Data Collection and Recent Studies. Antibiotics (Basel) 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.L. The penicillins: A review and update. J. Midwifery Womens Health 2002, 47, 426–434. [Google Scholar] [CrossRef]
- Bruniera, F.R.; Ferreira, F.M.; Saviolli, L.R.; Bacci, M.R.; Feder, D.; da Luz Gonçalves Pedreira, M.; Sorgini Peterlini, M.A.; Azzalis, L.A.; Campos Junqueira, V.B.; Fonseca, F.L. The use of vancomycin with its therapeutic and adverse effects: a review. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 694–700. [Google Scholar]
- Chung, P.Y.; Khoo, R.E.Y.; Liew, H.S.; Low, M.L. Antimicrobial and antibiofilm activities of Cu(II) Schiff base complexes against methicillin-susceptible and resistant Staphylococcus aureus. Ann. Clin. Microbiol. 2021, 20, 67–67. [Google Scholar] [CrossRef]
- Kapoor, G.; Saigal, S.; Elongavan, A. Action and resistance mechanisms of antibiotics: A guide for clinicians. J. Anaesthesiol. Clin. Pharmacol. 2017, 33, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Meletiadis, J.; Pournaras, S.; Roilides, E.; Walsh Thomas, J. Defining Fractional Inhibitory Concentration Index Cutoffs for Additive Interactions Based on Self-Drug Additive Combinations, Monte Carlo Simulation Analysis, and In Vitro-In Vivo Correlation Data for Antifungal Drug Combinations against Aspergillus fumigatus. Antimicrob. Agents Chemother. 2010, 54, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Irto, A.; Cardiano, P.; Chand, K.; Cigala, R.M.; Crea, F.; De Stefano, C.; Gano, L.; Gattuso, G.; Sammartano, S.; Santos, M.A. New bis-(3-hydroxy-4-pyridinone)-NTA-derivative: Synthesis, binding ability towards Ca2+, Cu2+, Zn2+, Al3+, Fe3+ and biological assays. J. Mol. Liq. 2018, 272, 609–624. [Google Scholar] [CrossRef]
- Leite, A.; Bessa, L.J.; Silva, A.M.G.; Gameiro, P.; de Castro, B.; Rangel, M. Antibacterial activity of naphthyl derived bis-(3-hydroxy-4-pyridinonate) copper(II) complexes against multidrug-resistant bacteria. J. Inorg. Biochem. 2019, 197, 110704. [Google Scholar] [CrossRef] [PubMed]
- Nakahata, D.H.; de Paiva, R.E.F.; Lustri, W.R.; Corbi, P.P. Sulfonamide-containing copper(ii) complexes: new insights on biophysical interactions and antibacterial activities. New J. Chem. 2020, 44, 17236–17244. [Google Scholar] [CrossRef]
- Nakahata, D.H.; de Paiva, R.E.F.; Lustri, W.R.; Ribeiro, C.M.; Pavan, F.R.; da Silva, G.G.; Ruiz, A.; de Carvalho, J.E.; Corbi, P.P. Sulfonamide-containing copper(II) metallonucleases: Correlations with in vitro antimycobacterial and antiproliferative activities. J. Inorg. Biochem. 2018, 187, 85–96. [Google Scholar] [CrossRef]
- Dalecki, A.G.; Haeili, M.; Shah, S.; Speer, A.; Niederweis, M.; Kutsch, O.; Wolschendorf, F. Disulfiram and Copper Ions Kill Mycobacterium tuberculosis in a Synergistic Manner. Antimicrob. Agents Chemother. 2015, 59, 4835–4844. [Google Scholar] [CrossRef]
- Dalecki, A.G.; Crawford, C.L.; Wolschendorf, F. Chapter Six - Copper and Antibiotics: Discovery, Modes of Action, and Opportunities for Medicinal Applications. In Advances in Microbial Physiology; Poole, R.K., Ed.; Academic Press, 2017; Vol. 70, pp. 193–260. [Google Scholar]
- Crawford, C.L.; Dalecki, A.G.; Perez, M.D.; Schaaf, K.; Wolschendorf, F.; Kutsch, O. A copper-dependent compound restores ampicillin sensitivity in multidrug-resistant Staphylococcus aureus. Sci. Rep. 2020, 10, 8955–8955. [Google Scholar] [CrossRef]
- Libardo, M.D.; Nagella, S.; Lugo, A.; Pierce, S.; Angeles-Boza, A.M. Copper-binding tripeptide motif increases potency of the antimicrobial peptide Anoplin via Reactive Oxygen Species generation. Biochem. Biophys. Res. Comm. 2015, 456, 446–451. [Google Scholar] [CrossRef]
- Libardo, M.D.; Gorbatyuk, V.Y.; Angeles-Boza, A.M. Central Role of the Copper-Binding Motif in the Complex Mechanism of Action of Ixosin: Enhancing Oxidative Damage and Promoting Synergy with Ixosin B. ACS Infect. Dis. 2016, 2, 71–81. [Google Scholar] [CrossRef]
- Libardo, M.D.J.; Bahar, A.A.; Ma, B.; Fu, R.; McCormick, L.E.; Zhao, J.; McCallum, S.A.; Nussinov, R.; Ren, D.; Angeles-Boza, A.M. , et al. Nuclease activity gives an edge to host-defense peptide piscidin 3 over piscidin 1, rendering it more effective against persisters and biofilms. Febs. J. 2017, 284, 3662–3683. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Cotten, M.L. Expression, purification, and micelle reconstitution of antimicrobial piscidin 1 and piscidin 3 for NMR studies. Protein. Expr. Purif. 2014, 102, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Hayden, R.M.; Goldberg, G.K.; Ferguson, B.M.; Schoeneck, M.W.; Libardo, M.D.; Mayeux, S.E.; Shrestha, A.; Bogardus, K.A.; Hammer, J.; Pryshchep, S. , et al. Complementary Effects of Host Defense Peptides Piscidin 1 and Piscidin 3 on DNA and Lipid Membranes: Biophysical Insights into Contrasting Biological Activities. J. Phys. Chem. B 2015, 119, 15235–15246. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, A.G.; Spago, F.R.; Simionato, A.S.; Navarro, M.O.P.; da Silva, C.S.; Barazetti, A.R.; Cely, M.V.T.; Tischer, C.A.; San Martin, J.A.B.; de Jesus Andrade, C.G.T. , et al. Bioactive Organocopper Compound from Pseudomonas aeruginosa Inhibits the Growth of Xanthomonas citri subsp. citri. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.S.K.V.; Rao, A.S. Inoculum potential, disease development and penetration of host byAlternaria triticina. Incitant of leaf blight of wheat. Proc. Indian Acad. Sci. 1979, 88, 359–365. [Google Scholar] [CrossRef]
- Afonso, L.; Andreata, M.F.; Chryssafidis, A.L.; Alarcon, S.F.; das Neves, A.P.; da Silva, J.V.; Gonçalves, G.D.; Abussafi, L.D.; Simionato, A.S.; Cely, M.V.; et al. Fluopsin C: A Review of the Antimicrobial Activity against Phytopathogens. Agronomy 2022, 12. [Google Scholar] [CrossRef]
- Zaengle-Barone, J.M.; Jackson, A.C.; Besse, D.M.; Becken, B.; Arshad, M.; Seed, P.C.; Franz, K.J. Copper Influences the Antibacterial Outcomes of a β-Lactamase-Activated Prochelator against Drug-Resistant Bacteria. ACS Infect. Dis. 2018, 4, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Hyman, L.M.; Stephenson, C.J.; Dickens, M.G.; Shimizu, K.D.; Franz, K.J. Toward the development of prochelators as fluorescent probes of copper-mediated oxidative stress. Dalton Trans. 2010, 39, 568–576. [Google Scholar] [CrossRef]
- Folk, D.S.; Franz, K.J. A Prochelator Activated by β-Secretase Inhibits Aβ Aggregation and Suppresses Copper-Induced Reactive Oxygen Species Formation. J. Amer. Chem. Soc. 2010, 132, 4994–4995. [Google Scholar] [CrossRef]
- Valodkar, M.; Rathore, P.S.; Jadeja, R.N.; Thounaojam, M.; Devkar, R.V.; Thakore, S. Cytotoxicity evaluation and antimicrobial studies of starch capped water soluble copper nanoparticles. J. Hazard. Mater. 2012, 201-202, 244–249. [Google Scholar] [CrossRef]
- Tao, B.; Lin, C.; Deng, Y.; Yuan, Z.; Shen, X.; Chen, M.; He, Y.; Peng, Z.; Hu, Y.; Cai, K. Copper-nanoparticle-embedded hydrogel for killing bacteria and promoting wound healing with photothermal therapy. J. Mat. Chem. B 2019, 7, 2534–2548. [Google Scholar] [CrossRef] [PubMed]
- Mehdizadeh, T.; Zamani, A.; Abtahi Froushani, S.M. Preparation of Cu nanoparticles fixed on cellulosic walnut shell material and investigation of its antibacterial, antioxidant and anticancer effects. Heliyon 2020, 6, e03528. [Google Scholar] [CrossRef] [PubMed]
- Bastos, C.A.P.; Faria, N.; Wills, J.; Malmberg, P.; Scheers, N.; Rees, P.; Powell, J.J. Copper nanoparticles have negligible direct antibacterial impact. NanoImpact 2020, 17, 100192. [Google Scholar] [CrossRef]
- Chatterjee, A.K.; Chakraborty, R.; Basu, T. Mechanism of antibacterial activity of copper nanoparticles. Nanotech. 2014, 25, 135101. [Google Scholar] [CrossRef] [PubMed]
- Datta Majumdar, T.; Ghosh, C.K.; Mukherjee, A. Dual Role of Copper Nanoparticles in Bacterial Leaf Blight-Infected Rice: A Therapeutic and Metabolic Approach. ACS Agric. Sci. Technol. 2021, 1, 160–172. [Google Scholar] [CrossRef]
- Naqvi, S.S.; Anwer, H.; Siddiqui, A.; Zohra, R.R.; Ali, S.A.; Shah, M.R.; Hashim, S. Novel Synthesis of Maltol Capped Copper Nanoparticles and Their Synergistic Antibacterial Activity with Antibiotics. Plasmonics 2021, 16, 1915–1928. [Google Scholar] [CrossRef]
- Zou, Z.; Sun, J.; Li, Q.; Pu, Y.; Liu, J.; Sun, R.; Wang, L.; Jiang, T. Vancomycin modified copper sulfide nanoparticles for photokilling of vancomycin-resistant enterococci bacteria. Colloids Surf. B 2020, 189, 110875. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, Z.; Ma, Y.; Zhang, N.; Wei, D.; Zhang, H.; Zhang, H. Response of partial nitrification sludge to the single and combined stress of CuO nanoparticles and sulfamethoxazole antibiotic on microbial activity, community and resistance genes. Sci. Total Environ. 2020, 712, 135759. [Google Scholar] [CrossRef]
- Tang, D.; Chen, X.; Kroemer, G. Cuproptosis: a copper-triggered modality of mitochondrial cell death. Cell Res. 2022, 32, 417–418. [Google Scholar] [CrossRef]
- Mahmoodi, S.; Elmi, A.; Hallaj Nezhadi, S. Copper Nanoparticles as Antibacterial Agents. J. Mol. Pharm. Org. Process Res. 2018, 06. [Google Scholar] [CrossRef]
- Qian, J.; Dong, Q.; Chun, K.; Zhu, D.; Zhang, X.; Mao, Y.; Culver, J.N.; Tai, S.; German, J.R.; Dean, D.P.; et al. Highly stable, antiviral, antibacterial cotton textiles via molecular engineering. Nature Nanotech. 2022. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial resistance. Availabe online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 02-15-2023).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).