Introduction
A person’s ability to flexibly deploy their cognitive resources to offset the effects of natural age-related decline or neurodegenerative disease is described as cognitive reserve (CR) (Stern 2009; Cabeza et al. 2018; Stern et al. 2020). There are important inter-individual differences in cognitive ability emerging in younger adults that are shaped by lifetime experiences, some of which may offer protection from neuropathology later in life (Livingston et al. 2020). The main proxies of CR are socio-behavioural variables including educational status, occupational attainment, intelligence quotient (IQ), and cognitive efficiency measured using standardized neuropsychological tests (Stern et al. 2020). However, several findings (Cuttler and Graf 2007; Franchow et al. 2013; Ihle et al. 2019; Karsazi et al. 2021) suggest that a particular personality trait – openness to experience (OE) – may also enhance resilience to cognitive impairment in older adults. A possible explanation is that because high openness promotes a receptivity to novel experiences, which are pursued with high interest, this necessitates flexible cognitive engagement with the world. Conversely, a person who scores lower on the openness trait is more inflexible in their daily routine and more fixed in their beliefs and attitudes.
Scoring higher on the OE trait is also associated with greater IQ levels (fluid and crystallized/verbal) in both younger and older adults (Rammsted et al. 2018; Schretlen et al. 2010; Ackerman and Heggestad 1997) and is inversely related to Alzheimer’s disease (AD) biomarkers (Tautvydaite et al. 2017), suggesting that this personal disposition might be related to better cognitive and biological health outcomes. Similarly, a study of 845 older adults found that, over a period of twenty years, OE was found to be protective against cognitive slowdown (Sharp et al. 2010). Openness is also not entirely fixed in its expression over time, and it has been demonstrated that thirty weeks of a cognitive training intervention can increase scores on the OE scale (Jackson et al. 2012). Taken together, these findings suggest that openness is an important dispositional factor that is related to key cognitive reserve proxies and has a role in shaping the resilience trajectory in the course of healthy and pathological ageing.
The extent to which the personal disposition of openness and the construct of reserve are related through a common neurobiological mechanism is not currently known. However, Robertson (Roberston 2013&2014) has postulated that the continuous activation (and the related integrity) of the Locus Coeruleus – noradrenergic system (the main source of noradrenaline -NA- in the brain) could be a key candidate affecting cognitive reserve and resilience capabilities. Specifically, it is hypothesized that the continuous upregulation of the noradrenergic system through cognitive stimulation and exposure to novelty might be one of the key neurobiological components for building cognitive reserve, and therefore resilience to neurodegenerative pathologies across the lifespan. In the last decades several studies reported that the LC-NA system integrity was related to greater brain and cognitive health (Wilson et al. 2013; Elman et al. 2021; Jacobs et al. 2021; Plini et al. 2021; Dahl et al. 2022), particularly to better attentive and mnemonic functions both in healthy and clinical populations (Clewett et al. 2016; Dahl et al. 2019; Dutt et al. 2021; Plini et al. 2021).
There are two potential reasons why OE may be associated with repeated activation of the LC-NA system. Firstly, openness promotes exploration of our environment in pursuit of novelty (
https://dictionary.apa.org/openness-to-experience). The LC appears to underlie novelty signaling patterns, which project to new network configurations within the hippocampus when encoding new vs. familiar experiential episodes (Grella et al. 2019). It has also been demonstrated in animal studies that exposure to repeated novel stimuli is a key factor underpinning the benefits of environmental enrichment upon memory function (Naka et al. 2002; Grilli et al. 2009). Blocking noradrenergic transmission pharmacologically disrupts the benefits of this novelty recognition (Veyrac et al. 2009). In the longer term, repeated phasic NA responding causes greater LC innervation and metabolic health, increasing autophagic factors, which are linked to reduced AD pathology (Omulabi et al. 2021). Secondly, a receptive and open mind depends on cognitive flexibility (Robertson 2013&2014; Chen et al. 2019). The LC-NA system is hypothesized to underlie an antagonistic relationship between exploitative vs. explorative states. The adaptive gain theory (Aston-Jones & Cohen, 2005) proposes that phasic LC activity is driven by an exploit mode for prioritising goal- or task-related information. When the utility of the current goal wanes, a tonic pattern of LC activity emerges that is associated with an exploratory mode promoting search for other alternative behaviours. Flexibly shifting between explore and exploit modes is a neurobehavioural mechanism that could plausibly support the trait of openness, which also varies along an exploration-exploitation dimension. Narrowing of openness is associated with exploiting familiar routines and behaviours whereas an expansion of openness is an exploratory mode promoting curiosity-led behaviours (Herz et al. 2020).
The aim of the current study is to test the noradrenergic theory of cognitive reserve by investigating whether the integrity of the LC in humans (measured by voxel-based morphometry - Ashburner and Friston 2000) is related to the expression of the OE trait measured by using the NEO - Five Factor Personality Inventory - NEO-FFI (Costa & McCrae 1992). Specifically, it is hypothesized that people with higher expression of the OE trait will have greater LC integrity (as a proxy of volume) because they are more likely to engage with a set of noradrenergically-mediated behaviours that promote mechanisms such as neurogenesis and synaptogenesis within the LC-NA system (Robertson 2013&2014; Mather 2021). Personality, as a relatively stable construct across the lifetime that shapes behaviour on a daily basis (Sutin et al. 2011; Fleeson and Gallagher 2009; Martin et al. 2007; Rhodes et al. 2006), is well positioned to build reserve and neuroprotective effects against disease from a young age. Indeed, it is known that AD pathology is found in healthy brains even from the second and third decades of life (Braak et al. 2011). A secondary aim of this study was to investigate whether LC signal intensity IQ are related, and whether the mediation of the LC-NA system may help explain the relationship between the OE trait and IQ, which is already reported in literature ((Moutafi et al. 2006; Anglim et al. 2022; De Young et al. 2020; Bartles et al. 2012; Rammsted et al. 2018; Schretlen et al. 2010; Ackerman and Heggestad 1997).
The current study will apply Voxel Based Morphometry (VBM) analyses utilizing 3Tesla T1-weighted MRI scans from 135 healthy young subjects (age range 20-35; 96 males, 39 females) derived from the LEMON (Leipzig Study for Mind-Body-Emotion Interactions) dataset (Bayaban et al. 2019). The relationship between the structural integrity of the LC and the OE trait was examined, and as a control procedure, the relationships between the LC and the other four dimensions of the Big Five personality inventory were also tested. Finally, to assess the differential contribution of neuromodulators to the OE trait, the specificity of the noradrenergic hypothesis was tested using VBM to measure the relative involvement of the other main neuromodulatory subcortical nuclei projecting to the cortex (Dorsal Raphe [DR] and Median Raphe [MR] 5-HT; Ventral Tegmental Area [VTA] DA; and Nucleus Basalis of Meynert [NBM] Ach) to the expression of the OE trait. The same models were repeated while investigating the relationship between LC and IQ.
Methods:
The data were provided by Max Plank Institute (
https://www.mpg.de/en) from the LEMON dataset by Babayan et al. (2019). The behavioural data were downloaded from nitrc.org (
https://fcon_1000.projects.nitrc.org/indi/retro/MPI_LEMON.html accessed in July 2020), and the raw MRI data were downloaded from the following source between July and September 2020
https://fcon_1000.projects.nitrc.org/indi/retro/MPI_LEMON/downloads/download_MRI.html. The behavioural data including neuropsychological, psychological and medical variables were manually merged as to build a comprehensive sheet for all the subjects comprised in the LEMON initiative. The analyses were carried out only on the subjects between 20 to 35 years old age range (n.39 females, n.96 males). Subjects with current psychological and psychiatric disorders, such anorexia, depression and substance abuse, were excluded.
Personality Measure and IQ:
Personality traits were assessed using the NEO-FFI questionnaire by Costa & McCrae 1992 (Costa & McCrae 1992). The NEO-FFI is a 44 items self-report measures on Likert scale (1 to 5) investigating the five main components of personality: Openness to Experience (OE), Conscientiousness (C), Extraversion (E), Agreeableness (A) and Neuroticism (N). Each trait mirrors different personological attitudes, and the interaction between these five dimensions defines personality profiles. Greater values reported reflect greater adherence to the items of the five facets. For example, people who scores high in C, are more likely to be well organized and not impulsive, while people that scores high in E are more prone to be assertive and highly sociable. On the other hand, individuals with high scores in N tend to be irritable, shy and moody while low scores A indicate antagonism, lack of compassion and stubbornness. As described earlier, high scores in OE are informative about the disposition to be open minded, prone to creativity and willing to engage new experiences. Conversely, people who score low in OE are generally disposed to routine, to conservative thinking and tend to avoid changes and challenges.
Intelligence Quotient (IQ) was measured using the WST - Wortschatztest – vocabulary test (Schmid et al. 1992), which is a multiple-choice vocabulary test capable to estimate verbal “crystalized” intelligence on the base of vocabulary knowledge. The test consists of discriminating non-existing words among real words. In
Table 1 the average personality trait scale levels and IQ values together with TIV are reported.
MRI data processing:
3Tesla high-res T1-weighted images in Nifti format underwent a preliminary manual quality control to detect major motion or reconstruction artefacts. This procedure was carried out referring to the rating scale guidelines (1= poor, 2= fair, 3= good, 4= excellent) of the Human Connectome Project (HCP) (
https://www.humanconnectome.org/). Subjects with low definition (excessive blurriness) and/or marked ringing, inhomogeneities and motion artefacts were removed from the dataset. In order to perform volumetric analyses, the images were processed using CAT12 (Computational Anatomy Toolbox -
http://www.neuro.uni-jena.de/cat/) implemented in SPM12. The segmentation was run following the default CAT12 settings, except for the voxel size that was settled at 1mm isotropic voxel size. On completion of this process, 135 young subjects (96 males, 39 females) out of 153 were considered suitable for the analyses. The processed and modulated whole brain images (MNI grey matter + white matter) were smoothed using SPM12 interface with a 2 mm^3 FWHM kernel. Lastly, to better account for individual volumetric variability in the Voxel Based Morphometry (VBM) analyses, the Total Intracranial Volume (TIV) was calculated for each subject using CAT12 interface (Statistical Analyses – Estimate TIV).
Neuromodulator’s region of interest (ROI) masks:
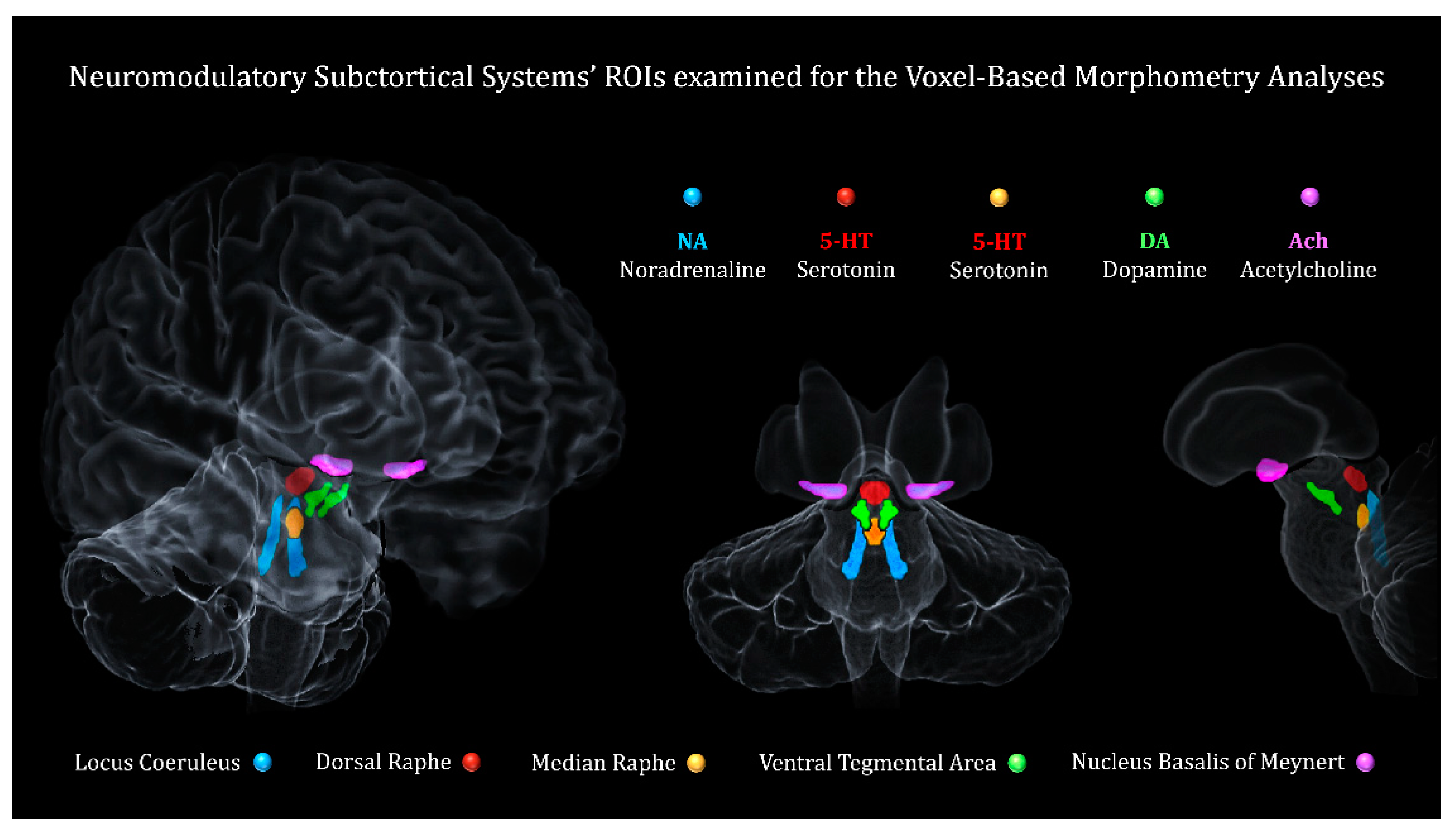
As described in detail in our previous work (Plini et al. 2021), the ROIs were developed on the base of previously published atlases. The ROIs were symmetrically corrected from the original atlases in order to avoid overlapping borders with other structures and to avoid possible biases of “induced lateralization”. All the ROIs were 1mm3 isotropic voxel size and oriented in the Montreal Neurological Institute – MNI – space as the processed images of CAT12. See
Figure 1 for ROI’s volumetric info. For the current study it was used the LC “omini-comprehensive” probabilistic mask we developed earlier (Plini et al. 2021). The “omni-comprehensive” LC mask solves the inconsistent LC spatial localization reported by previous works (Keren et al. 2009 & 2015; Tona et al. 2017; Betts et al. 2017; Dahl et al. 2019; Liu et al. 2019; Rong Ye et al. 2020; Dahl et al. 2021), while enabling to comprise the whole anatomical LC regions defined across lifespan, without encroaching other pontine and cerebellar regions, and without crossing the walls of the 4th ventricles (further details can be found in supplementary materials of Plini et al. 2021 and at this link:
https://www.youtube.com/watch?v=90bsA6Jqxs4). The MR and DR ROIs were provided by Beliveau et al. (Beliveau et al. 2015), the VTA mask was obtained by downloading the VTA MNI probabilistic map from the atlas made by Pauli et al. 2018 (Pauli et al. 2018) from the NeuroVault website (
https://neurovault.org/ accessed on 15 December 2018). The NMB was developed on the base of the probabilistic MNI maps of the acetylcholine cells of the Forebrain are provided by SPM Anatomy Toolbox 2.2c (
https://www.fzjuelich.de/inm/inm1/EN/Forschung/_docs/SPMAnatomyToolbox/SPMAnatomyToolbox_node.html accessed on 15 December 2018) by Zaborszky et al. 2008 and George et al. 2011, Schulz et al. 2018, Liu et al. 2015, Kilimann et al. 2014, Koulousakis et al. 2019.
Figure 1.
Neuromodulatory subcortical system. The five ROIs considered for the voxel-based morphometry analyses. In blue is shown the Locus Coeruleus (LC - noradrenaline), in orange the Median Raphe (MR - Serotonin), in red the Dorsal Raphe (DR - Serotonin), in geen the Ventral Tegmental Area (VTA - Dopamine), in purple the Nucleus Basalis of Meynert (NBM - Acetylcholine).
Figure 1.
Neuromodulatory subcortical system. The five ROIs considered for the voxel-based morphometry analyses. In blue is shown the Locus Coeruleus (LC - noradrenaline), in orange the Median Raphe (MR - Serotonin), in red the Dorsal Raphe (DR - Serotonin), in geen the Ventral Tegmental Area (VTA - Dopamine), in purple the Nucleus Basalis of Meynert (NBM - Acetylcholine).
VBM analyses:
Using the processed and smoothed whole brain images, the VBM analyses were carried out in CAT12 (basic models) implementing multiple regression models. The analyses were divided into two main branches. The first one investigated the LC-NA system and the five personality traits of NEO-FFI in five different statistical models (Openness to Experience, Conscientiousness, Extraversion, Agreeableness, Neuroticism). The second one investigated the relationship between the LC-NA system and I.Q (WST - Wortschatztest – vocabulary). All the analyses followed the same procedures and had the same set of continuous covariates (age, TIV, gender). After the estimation of the statistical models (“estimating the statistical model”) and after checking for the design orthogonality (“checking for design orthogonality”), the “results” were checked using the following contrast in the SPM12 “contrast interface”: 0 0 0 0 1 (for positive relationship) and 0 0 0 0 -1 (for negative relationship). Both relationships were always tested as to control the reliability of the findings. A further step in the analyses pipeline was to indicate the LC binary mask as inclusive mask to isolate the LC involvement in the models. Eventually the statistical threshold was settled at P<0.01, and later increased progressively until the results disappeared (namely: P<0.001, P<0.05 family wise error corrected – FWE). Each set of analyses, after having examined the LC, was systematically repeated considering the other ROIs’ binary masks in this order: DR, MR, VTA and NBM as controlling assurance for the LC findings.
In order to perform Bayesian analyses, the average signal intensity of the ROIs was extracted in FSL by using the binary masks on the smoothed (2 mm^3 FWHM kernel) whole brain images. In FSL terminal, the flags of “fslstats” “-k” (mask) and “-m” (output mean) were used to gather the average voxel intensities for each ROI subject by subject. This procedure was also carried out to extract the average signal intensity from clusters of significant voxels outcoming from the VBM analyses in order to calculate more accurate Bayes Factors (BF) and to perform mediation analyses.
Bayesian modelling and correlation matrices and partial correlations
In JASP (
https://jasp-stats.org/), a Bayesian model was performed to compare within the same model the differential strengths of the neuromodulators predicting OE (linear regression model, OE entered as dependent variable, and the five ROIs as covariates). TIV, age and gender were also included and added to the ‘null model’. The differential relationship of the five ROIs were compared with the null model, and the analyses were run with default parameters with the only exception of Bayesian information criteria (BIC) selected in the advanced option of JASP interface. The same models were run for the other personality traits.
Both Bayesian and Pearson’s correlation matrices were built considering the following continuous variables: Openness to Experience, Conscientiousness, Extraversion, Agreeableness, Neuroticism, WST-IQ, and the five personality traits with the STAI-G-X2 anxiety scale and Perceived Stress Questionnaire (PSQ). Similarly STAI-G-X2 and PSQ were entered in a correlation matrix with LC, MR, DR, MR, VTA, NBM to explore possible relationship with the neuromodulatory subcortical system. BF were generated in the JASP interface (Bayesian correlation model) on the base of the average signal intensity of the significant clusters outcoming from the VBM analyses (when the ROIs were surviving the statistical thresholds settled). The average signal intensity of the whole ROI was considered in the Bayesian correlation matrices only for the ROIs, which showed no significant cluster of voxels in the VBM analyses. Finally, partial correlations were performed in SPSS 25 (
https://www.ibm.com/products/spss-statistics). The relationships between LC and IQ was controlled for OE in two different batches, first from the LC-IQ relationship OE was regressed out, then from the LC-OE relationship the effect of IQ was regressed outThese analyses had the aim to better differentiate the LC variance across these different domains.
Results
1st branch of VBM analyses: relationship between neuromodulators’ signal intensity and BIG-5 personality traits - Does the LC signal intensity predict Openness to Experience values relative to other neuromodulator seed regions?
As can be observed in
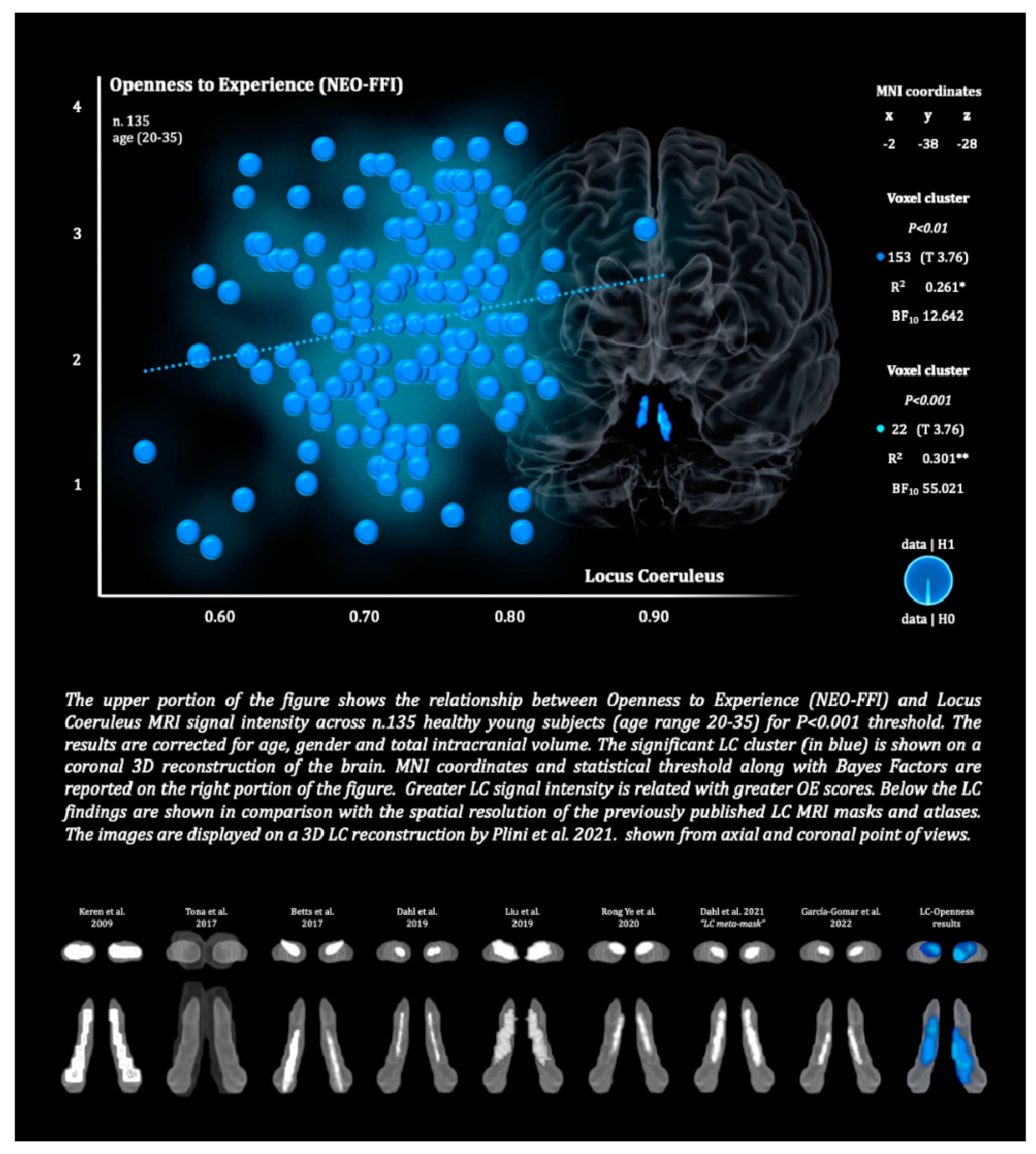
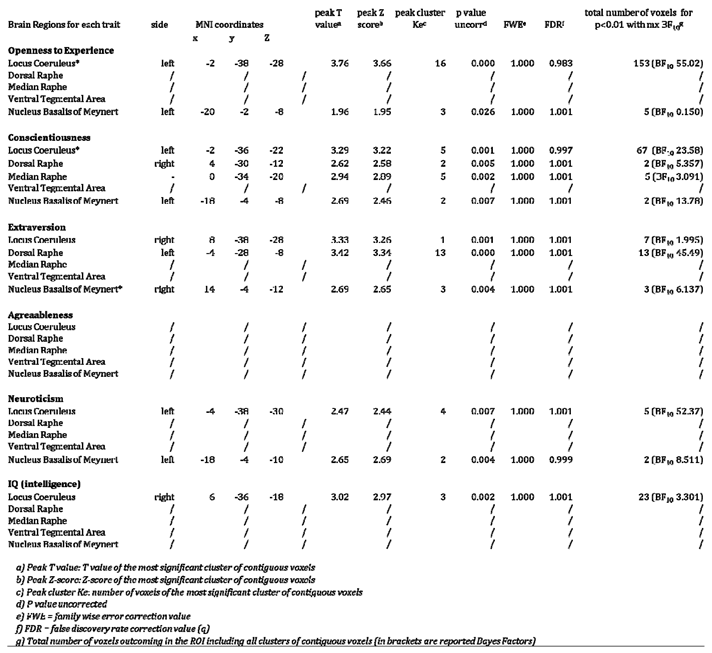
Table 2, across the 135 healthy subjects, LC signal intensity was the strongest predictor of the OE personality trait compared to the other neuromodulators, while controlling for age, gender and TIV. Two large bilateral clusters, for a total of 153 voxels within the LC region, positively related to greater OE values for p<0.01 threshold. As shown in
Figure 2, the spatial localizations of the LC results overlapped the LC region defined by the previously published LC atlases and masks (Keren et al. 2009 & 2015; Tona et al. 2017; Betts et al. 2017; Dahl et al. 2019; Liu et al. 2019; Rong Ye et al. 2020; Dahl et al. 2021). By increasing the statistical threshold up to p<0.001 only a cluster of 22 voxels survived in the LC core. However, no LC voxels remained when FWE correction was applied.
Regarding other personality traits, 67 voxels within the LC region were associated with Conscientiousness. Only 5 voxels of the LC clusters survived when more conservative thresholds were set (p<0.001), however the cluster did not survive FWE correction. In table n.2, the minor and negligible associations between the five ROIs and the other personality traits are reported in detail.
As control procedure, when the opposite relationships were tested, no associations were found between the LC and five personality traits, neither for the other ROIs. The only exception (not reported in tables) was the negative relationship between the VTA and Conscientiousness. Lower VTA signal intensity (68 voxels) related to greater Conscientiousness values. However, this result did not survive the p<0.001 threshold.
Figure 2.
The upper portion of the figure shows the relationship between Openness to Experience (NEO-FFI) and Locus Coeruleus MRI signal intensity across n.135 healthy young subjects (age range 20-35) for P<0.001 threshold. The results are corrected for age, gender and total intracranial volume. The significant LC cluster (in blue) is shown on a coronal 3D reconstruction of the brain. MNI coordinates and statistical threshold along with Bayes Factors are reported on the right portion of the figure. Greater LC signal intensity is related with greater OE scores. Below the LC findings are shown in comparison with the spatial resolution of the previously published LC MRI masks and atlases. The images are displayed on a 3D LC reconstruction by Plini et al. 2021. shown from axial and coronal point of views.
Figure 2.
The upper portion of the figure shows the relationship between Openness to Experience (NEO-FFI) and Locus Coeruleus MRI signal intensity across n.135 healthy young subjects (age range 20-35) for P<0.001 threshold. The results are corrected for age, gender and total intracranial volume. The significant LC cluster (in blue) is shown on a coronal 3D reconstruction of the brain. MNI coordinates and statistical threshold along with Bayes Factors are reported on the right portion of the figure. Greater LC signal intensity is related with greater OE scores. Below the LC findings are shown in comparison with the spatial resolution of the previously published LC MRI masks and atlases. The images are displayed on a 3D LC reconstruction by Plini et al. 2021. shown from axial and coronal point of views.
2nd branch of VBM analyses: relationship between neuromodulators’ signal intensity and IQ - Does the LC predict IQ relative to other neuromodulator seed regions?
As reported in
Table 2, 23 LC voxels (for p<0.01) mostly left lateralised rostrally were related to greater IQ. The LC spatial localization overlaps the LC core defined in the previously published LC atlases. When the statistical threshold was increased to p<0.001 the cluster disappeared. No associations between IQ and the other ROIs were found, demonstrating specificity for the relationship between IQ and LC. No significant clusters across the five ROIs were found when the opposite relationships were tested.
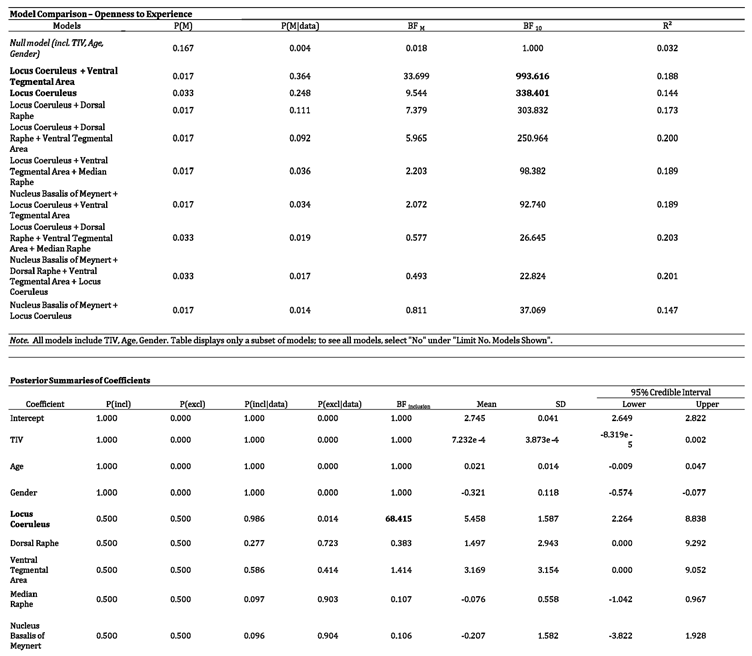
Using Bayesian modelling to examine single and combined contributions of different neuromodulalators
The abovementioned VBM analysis found that the LC was a key predictor of the OE trait. However, a further Bayesian approach provides a means to compare the strength of evidence for different neuromodulator seeds in predicting OE trait scores. Here, we employed a model in which each neuromodulator seed is examined as a standalone predictor and also in a combined way, which compares the predictive strength when different neuromodulator seeds also examined in combination if they explain more variance. The Bayesian linear regression model showed that as standalone variable the LC signal intensity has the strongest relationship with OE against the null model (BF
10 338.401 – BF
M 9.544). The combined effect for LC and VTA signal intensity has the strongest relationship among single and combined models (BF
10 993.661 – BF
M 33.699) suggesting a disproportionate catecholaminergic involvement in OE trait accounting for 14% (LC alone) - 18% (LC + VTA) of its variance. Moreover, the combined effect of LC and serotoninergic DR was the third strongest model related to OE accounting for 17% of variance. A summary of these analyses is reported in detail in
Table 3.
Exploring the relationships between personality, IQ and the Locus Coeruleus
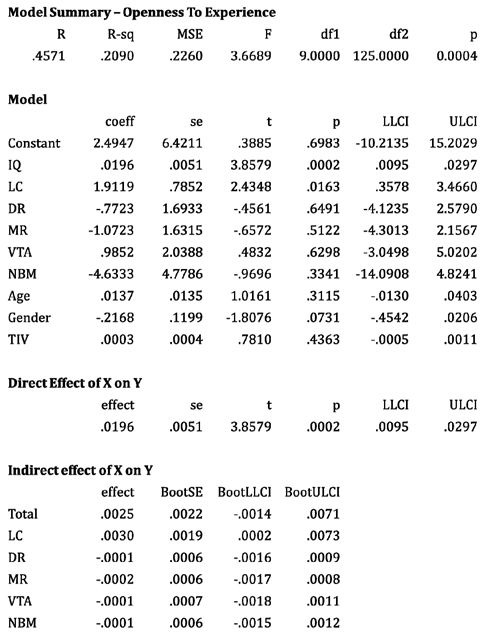
Correlation matrices revealed that among the five personality traits only the OE scale related significantly with IQ. Specifically, greater OE values related to greater IQ scores (Pearson’s R 0.391, - BF10 3305.75 – for p<0.001). For greater detail refer to the correlation table in the supplementary materials.
Partial correlations revealed that the relationship between LC and IQ disappeared when the effect of OE trait was regressed out in the model. On the other hand, the relationship between OE trait and LC remained significant when controlling for IQ. These two partial correlations together are suggestive that the relationship between OE and LC might be more stable than the relationship between LC and IQ. For greater detail refer to the partial correlation table in the supplementary materials.
Exploring the relationships between personality, IQ and the Locus Coeruleus
Correlation matrices revealed that among the five personality traits only the OE scale related significantly with IQ. Specifically, greater OE values related to greater IQ scores (Pearson’s R 0.391, - BF10 3305.75 – for p<0.001). For greater detail refer to the correlation table in the supplementary materials.
Partial correlations revealed that the relationship between LC and IQ disappeared when the effect of OE trait was regressed out in the model On the other hand, the relationship between OE trait and LC remained significant when controlling for IQ. These two partial correlations together are suggestive that the relationship between OE and LC might be more stable than the relationship between LC and IQ. For greater detail refer to the partial correlation table in the supplementary materials.
Mediation analyses with parallel multiple mediators - Does the LC mediate the relationship between the OE trait level and IQ over the other neuromodulators?
Mediation analyses with multiple parallel mediators were carried out in order to clarify possible mediation effects of the neuromodulators. While controlling for age, TIV and gender, bootstrap (10000) confidence intervals were used to examine the role of the five subcortical ROIs in mediating the relationship between the OE trait and IQ. Only LC alone significantly mediates the relationship between OE (Y) and IQ (X) indicating that greater OE was predictive of greater IQ score and that this relationship was mediated by the LC and not by the other neuromodulators, which did not mediate this relationship. Indeed, the total indirect effect of the parallel mediators was not significant. These results suggest that the strong relationship found between OE and IQ is disproportionally influenced by the noradrenergic system over the other neuromodulators, as previously observed in the VBM analyses (see
Table 4 for details).
Discussions
The present study investigated the relationship between MRI signal intensity of different neuromodulators’ seeds and the Five Factor Personality Inventory score in 135 healthy young participants. As hypothesized, greater structural integrity of the Locus Coeruleus (LC) was found to be related to higher scores in the Openness to Experience (OE) trait, and with a greater Intelligence Quotient (IQ) score. When neuromodulator seeds were considered together in a Bayesian model, catecholamines (LC+VTA) were the strongest predictor of OE, followed by the LC as stand-alone predictor of OE, and thirdly the LC combined with the serotoninergic DR. These findings are the first evidence linking neuromodulatory MRI signal intensity to personality traits.
Given the OE trait is commonly associated with intelligence (both fluid and crystalized/verbal) and is frequently named as the “intellect” trait in the literature (Moutafi et al. 2006; Anglim et al. 2022; De Young et al. 2020; Bartles et al. 2012; Rammsted et al. 2018; DeYoung et al. 2013; Schretlen et al. 2010; DeYoung et al. 2005; Ackerman and Heggestad 1997), we examined the extent to which LC-NA integrity might account for this relationship. A mediation analyses revealed that, in comparison with the other neuromodulatory nuclei, only the LC accounted for the relationship between OE and IQ, supporting the assertion that the LC-NA system may be a key neurobiological substrate mediating the relationship between openness to experience and intellectual expansion. We also established that the relationship between the LC and OE remained when the effect of IQ was regressed out, demonstrating that unique variance in the openness trait is associated with LC-NA system integrity. Finally, it was apparent that the LC was not exclusively associated with the OE trait. We also observed a weaker relationship between LC and the Conscientiousness (C) scale.
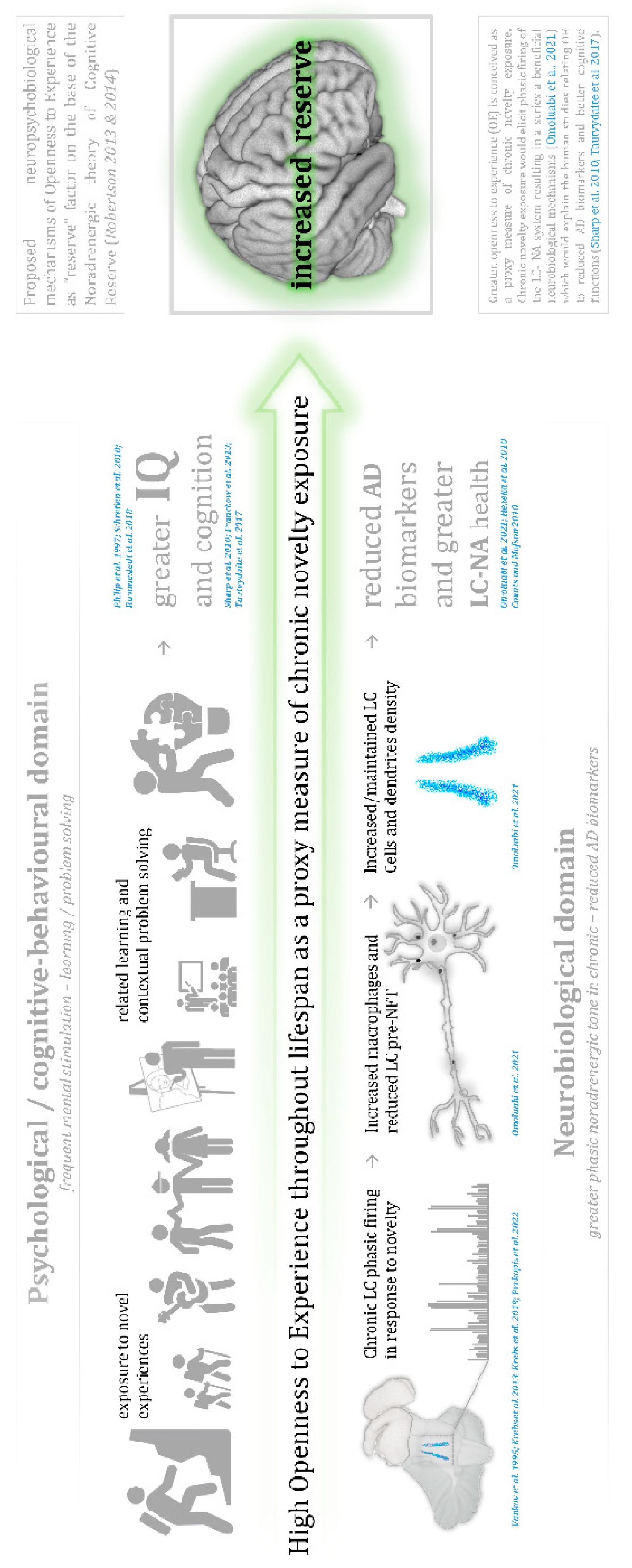
We interpret the key findings of the current study in the context of the Noradrenergic Theory of Cognitive Reserve (Robertson 2013&2014) proposing that more protracted responses to novelty and chronic exposure to enriched environments in high-OE trait individuals is underpinned by the activation of the LC-NA system. Why might novelty be an important factor for increasing LC signal intensity? One potential underlying mechanism could be increased frequency of LC phasic firing in response to novel events. Omulabi and colleagues examined single unit recordings from the LC during novelty exposure in rats. After six-weeks of LC stimulation (LC phasic activation for 20 minutes five days per week) rats showed increased response to novel stimuli, and greater LC health (increased macrophage activation and greater LC fiber density), along with increased cognitive functions and reduced AD biomarkers in comparison with rats which did not undergo the LC phasic activation. Rats not exposed to novelty showed greater LC neurodegeneration with increased AD biomarkers and poorer cognitive outcomes. Omulabi et al. (2021) concluded that LC-phasic firing in response to novelty was protective against the detrimental effect of AD biomarkers (pre-tangle tau production in the LC), preventing LC fiber loss resulting in greater LC axonal integrity and more preserved cognition.
Consistent with the aforementioned animal study, a recent fMRI study in humans carried on 128 healthy individuals from the Harvard aging study (Prokopiou et al. 2022) found LC activity significantly increased while novel stimuli were presented. They also reported that lower novelty-related LC activity was associated with greater cognitive decline related to AD biomarkers levels (beta-amyloid PET). Krebs et al. 2018 have also demonstrated that phasic LC activity when a contextually-unexpected stimulus occurs, namely, a novel stimulus that is incongruent to context.
Although the VBM analyses revealed that the LC was the key predictor of OE, a Bayesian multiple regression model explored the combined effects of LC with the other subcortical nuclei. This analysis revealed that LC + VTA was a stronger predictor of OE than the LC alone. An additive involvement of the dopaminergic system in shaping personality may explain this relationship: exploratory behaviour that characterizes openness will be further reinforced by the reward value of novel experiences via the mesolimbic dopamine system. Alternatively, the role of DA in supporting the OE trait may have a genetic basis as catalyst for the conversion of DA to NA. (Barnes et al. 2011). Furthermore, the role of the DR nucleus, while only a negligible contributor to the model on its own, also combined with the LC to predict OE trait variation. Serotoninergic involvement in OE trait expression may involve increased sensitivity to stress during exploration of novel environments. Previous work has found that cerebral 5-HTT levels are associated with OE (Kalbitzer et al. 2008; Ren et al. 2021). However, 30% of DR is composed by catecholaminergic neurons (Farley et al. 1977; Baker et al. 1991; Ordway et al. 1997; Kirby et al. 2003), therefore this association might reflect common dopaminergic and noradrenergic involvement in OE expression. Overall, the synergic involvement of these three main neuromodulatory nuclei may contribute to shaping the nature of openness via different pathways – through VTA-DA mediated reward sensitivity, DR-5-HT potentiation of stress-related responses during exposure to novelty, as well as overarching explore-exploit behavioral patterns promoted by the LC-NA system (Tochigi et al. 2005; Zmorzyński et al. 2021; Ren et al. 2021; Deyoung et al. 2008; Deyoung et al. 2011; Deyoung 2013). We observed positive relationships between the integrity of such nuclei and OE, therefore these interpretations are based on the assumption that greater structural integrity of these nuclei would ensure an adequate neuromodulatory functioning building the ground for a more developed OE trait. Conversely, lower integrity of these nuclei would undermine the normal neuromodulator biosynthesis underlying OE trait expression. This interpretation is supported by the literature reporting how variations of neuromodulators’ concentrations can bidirectionally affect personality traits expression (Tochigi et al. 2005; Narita et al. 2015; Ward et al. 2017; Fischer et al. 2018; Käckenmester et al. 2019; Kanen et al. 2021). By contrast, reduced integrity of the neuromodulatory subcortical system can result in poorer bioavailability of such neuromodulators (May and Paxinos 2012).
An unique contribution of the LC-NA system in this study was its mediatory role in accounting for a relationship between OE and IQ. This intercorrelation highlights LC-NA system centrality in higher order cognition. Greater IQ and higher OE scores are both conceived as Reserve proxies that reduce the risk of cognitive decline and dementia (Cuttler and Graf 2007; Franchow et al. 2013; Ihle et al. 2019; Karsazi et al. 2021; Rammsted et al. 2018; Schretlen et al. 2010; Ackerman and Heggestad 1997; Tautvydaite et al. 2017; Sharp et al. 2010). The integrity of the LC-NA system, given its role in neurodegeneration, might be a common factor contributing to the expression of these two constructs in terms of brain and cognitive health (DeYoung et al. 2013; Robertson 2013&2014). These findings are therefore suggestive that the LC integrity, even at young age, can significantly affect cognition and personality expression with the potential to build resilience to neurodegeneration in later life. Although the current study is the first to link LC-NA integrity to OE, there is previous evidence that the noradrenergic system is associated with greater cognitive performance and intelligence (Tsukahara and Engle 2021; Zhao et al. 2014; Clewett et al. 2016; Liu et al. 2020; Wilson et al. 2013; Elman et al. 2021; Jacobs et al. 2021; Plini et al. 2021; Dahl et al. 2022; Dahl et al. 2019; Dutt et al. 2021).
Although OE showed the strongest relationship to the LC, a secondary VBM association was also observed between the C trait, conscientiousness, and the LC. The C trait, which reflects perseverance and focus, is associated with increased attention performance and reduced speed of processing (Yoneda et al. 2022; Chapman et al. 2017; Sutin et al. 2019) and improved memory (Lucchetti et al. 2016; Allen et al. 2018; Sutin et al. 2019 b). These cognitive domains also implicate LC-NA system integrity and functioning across several studies (Aston-Jones et al. 2000; Aston-Jones and Cohen 2005; Bari et al. 2022; Grueschow et al. 2022; Dahl et al. 2022; Unsworth et al. 2017; Plini et al. 2021). Moreover, further work has found that greater C scores are associated with both greater resistance to Dementia (Yoneda et al. 2022; Kaup et al. 2019). and lower risk of mild cognitive impairment and Alzheimer’s Disease (Wilson et el. 2007; Terracciano et al. 2017; Sutin et al. 2018; Aschenbrenner et al. 2020). It should be noted that the role of additional variables needs further investigation. For example, it is documented that individuals who are high in C are more prone to engage regular physical activity (Sutin et al. 2016), which is also known to be related to better brain and cognitive health and to the LC-NA system. Clearer dissociation of the role these different protective factors is warranted.
The current findings demonstrates that the C trait combined with greater OE expression in healthy individuals is associated with greater signal intensity in the LC. It remains to be seen whether these personality traits, through interaction with LC-NA system over the lifespan, provide greater resilience to neurodegeneration. However, these significant clinical implications warrant longitudinal investigation.
Limitations
The main limitation of the current argumentation lays on the cross-sectional nature of the study. A longitudinal study would provide more accurate casual relationships among these variables and would enable to understand possible trajectories. However, personality traits measured by NEO-FFI tend to be stable throughout lifetime (McCrae et al. 2011; Gnambs et al. 2014). This gives us more confidence in the mediation analyses and in the partial correlations, which may help us understand the actual nature of these relationships.
Another potential limitation is selection bias - people who were recruited via the LEMON study might be, by nature of their voluntary participation, individuals who are “highly open” to engage in new experiences and this might limit the representativeness of the sample to general population. This selection bias may also have be exacerbated by excluding individuals with ongoing psychological or psychiatric symptoms. The current sample reported low levels of anxiety, and had a low level of neuroticism compared to other scales such as OE or C. Therefore, the possible detrimental effect of “high tonic” LC firing commonly observed in response to stress and anxiety could affect or reduce the LC-OE relationship and their possible neuroprotective outcomes (Omulabi et al. 2021). Nonetheless, it is worth mentioning we found no relationships between LC signal intensity and anxiety scales which could affect the present set of findings.
Other minor limitations are the relatively small size of the sample, which can explain why the results did not survive multiple comparison corrections despite the very strong Bayesian Factors, and the constraints of the self-report personality questionnaires with forced choices, along with the IQ measured on the base of vocabulary only. However, within these limitations, we made the attempt to account for the confounding variables, controlling for age, gender and TIV. In the same vein, we also systematically tested with numerous control analyses different relationships, and we also tested the antithetic hypothesis to consider the alternative direction of effects. A total of 60 VBM control analyses contrasted to our main analyses yielding greater confidence in the validity of our key findings.
Clinical implications and future directions
Together, these findings are suggestive that personal attitudes to life, captured by the personality of openness (and Conscientiousness), might be influenced, in part, by activation of the noradrenergic system. These findings may provide explanatory ground concerning the neuropsychological dynamics underlining the association between OE and resilience to neurodegenerative diseases while supporting Robertson’s theory (Robertson 2013&2014; Tautvydaite et al. 2017; Sharp et al. 2010; Cuttler and Graf 2007; Franchow et al. 2013; Ihle et al. 2019; Karsazi et al. 2021). Indeed, as outlined by Robertson (Robertson 2013&2014), LC-NA system plays an important role underpinning all the attentional processes, and in particular the exposure to novelty along with managing and developing problem-solving skills. This interacting with greater Conscientiousness may ensure consistent cognitive control eliciting optimal noradrenergic tone (Robertson 2013&2014; Aston-Jones et al. 2000; Aston-Jones and Cohen 2005). In keeping with this, a dispositional ‘openness’ trait might drive a greater noradrenergic tone throughout lifetime, leading to more frequent phasic activation of LC-NA system yielding to greater LC integrity and greater brain and cognitive health (Robertson 2013&2014; Omulabi et al. 2021; Clewett et al. 2016; Dutt et al. 2021; Mather et al. 2021; Plini et al. 2021; Prokopiou et al. 2022). This ‘openness’ trait throughout lifespan might interact with other reserve variables, such as I.Q. via the mediation of the LC-NA system. Indeed, several studies reported greater OE is linked to greater crystallized intelligence (Rammsted et al. 2018; Schretlen et al. 2010; Ackerman and Heggestad 1997). Alternatively, it is possible that LC-NA characteristics could contribute to the ‘openness’ personality characteristic, or that the relationship is explained by other variables. However, the newly established link between personality traits, LC-NA system, and cognition outlines the intercorrelation between neuropsychological variables and opens to potential psychological interventions targeting the LC-NA system. Cognitive and behavioral approaches (Cognitive Behavioral Therapy – CBT) which focus on OE, in people who score low in OE and C, might be considered among the preventing strategies for neurodegenerative diseases (Forgeard et al. 2019). This might be beneficial improving people’s well-being both in enhancing the executive/attentional domain (Forgeard et al. 2019; Jackson et al. 2012) and in building the resilience ground to neurodegenerative diseases. Personality traits (OE especially) might be considered as critical component to focus on for brain health potentially influencing the neuroprotective role of LC-NA system particularly in the face of neurodegeneration. Future studies should replicate these findings and design longitudinal investigations considering biological biomarkers, personological and cognitive variables using high-res MRI to better understand the neuropsychological dynamics which underpin our novel findings.
Figure 3.
- Graphical discussion: Proposed neuropsychobiological mechanisms of Openness to Experience as “reserve” factor on the base of the Noradrenergic theory of Cognitive Reserve (Robertson 2013 & 2014). Greater openness to experience (OE) is conceived as a proxy measure of chronic novelty exposure. Chronic novelty exposure would elicit phasic firing of the LC- NA system resulting in a series a beneficial neurobiological mechanisms (Omoluabi et al. 2021) which would explain the human studies relating OE to reduced AD biomarkers and better cognitive functions (Sharp et al. 2010, Tautvydaite et al. 2017).
Figure 3.
- Graphical discussion: Proposed neuropsychobiological mechanisms of Openness to Experience as “reserve” factor on the base of the Noradrenergic theory of Cognitive Reserve (Robertson 2013 & 2014). Greater openness to experience (OE) is conceived as a proxy measure of chronic novelty exposure. Chronic novelty exposure would elicit phasic firing of the LC- NA system resulting in a series a beneficial neurobiological mechanisms (Omoluabi et al. 2021) which would explain the human studies relating OE to reduced AD biomarkers and better cognitive functions (Sharp et al. 2010, Tautvydaite et al. 2017).
While the nature of the observed relationship between LC and OE is correlational, it should be considered that the other experiments reported causational effects of “novelty-like” LC activations and reduced biomarkers of neurodegeneration. However, it should be taken into account that the proposed underlining mechanisms might be explained by other variables and potentially reside also within other neurobiological/neuropsychological pathways.
Author Contributions
ERGP: conceptualization, study design, statistical analyses, results interpretation and visualization, manuscript writing. IHR: conceptualization and supervision. MB: statistical analyses design and manuscript editing. PMD: supervision, result interpretation and manuscript editing. All authors approved the final version of the manuscript.
Funding
Project founded by the Irish Research Council—Irish Research Council Laureate Consolidator Award (2018-23) IRCLA/2017/306 to Paul Dockree.
Acknowledgments
Thanks are extended to Francesca Fabbricatore for proofreading the manuscript and for the thoughtful comments provided throughout the course.
Conflict of Interest
The authors declare no conflict of interest
References
- Ackerman, P.L.; Heggestad, E.D. Intelligence, personality, and interests: Evidence for overlapping traits. Psychol. Bull. 1997, 121, 219–245. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.S.; Laborde, S.; Walter, E.E. Health-Related Behavior Mediates the Association Between Personality and Memory Performance in Older Adults. J. Appl. Gerontol. 2017, 38, 232–252. [Google Scholar] [CrossRef] [PubMed]
- Aschenbrenner, A.J.; Petros, J.; McDade, E.; Wang, G.; Balota, D.A.; Benzinger, T.L.; Cruchaga, C.; Goate, A.; Xiong, C.; Perrin, R.; et al. Relationships between big-five personality factors and Alzheimer's disease pathology in autosomal dominant Alzheimer's disease. Alzheimer's Dementia: Diagn. Assess. Dis. Monit. 2020, 12, e12038. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, J.; Friston, K.J.; Ashburner, J.; Friston, K.J.; Ashburner, J.; Friston, K.J. Voxel-Based Morphometry—The Methods. NeuroImage 2000, 11, 805–821. [Google Scholar] [CrossRef]
- Aston-Jones, G.; Cohen, J.D. AN INTEGRATIVE THEORY OF LOCUS COERULEUS-NOREPINEPHRINE FUNCTION: Adaptive Gain and Optimal Performance. Annu. Rev. Neurosci. 2005, 28, 403–450. [Google Scholar] [CrossRef] [PubMed]
- Aston-Jones G, Rajkowski J, Cohen J. Locus coeruleus and regulation of behavioral flexibility and attention. Prog Brain Res. 2000;126:165-82. [CrossRef]
- Babayan, A.; Erbey, M.; Kumral, D.; Reinelt, J.D.; Reiter, A.M.F.; Röbbig, J.; Schaare, H.L.; Uhlig, M.; Anwander, A.; Bazin, P.-L.; et al. A mind-brain-body dataset of MRI, EEG, cognition, emotion, and peripheral physiology in young and old adults. Sci. Data 2019, 6, 180308. [Google Scholar] [CrossRef]
- Baker, K.; Halliday, G.; Hornung, J.-P.; Geffen, L.; Cotton, R.; To¨rk, I. Distribution, morphology and number of monoamine-synthesizing and substance P-containing neurons in the human dorsal raphe nucleus. Neuroscience 1991, 42, 757–775. [Google Scholar] [CrossRef]
- Bari, A.; Xu, S.; Pignatelli, M.; Takeuchi, D.; Feng, J.; Li, Y.; Tonegawa, S. Differential attentional control mechanisms by two distinct noradrenergic coeruleo-frontal cortical pathways. Proc. Natl. Acad. Sci. 2020, 117, 29080–29089. [Google Scholar] [CrossRef]
- Barnes, J.J.; Dean, A.J.; Nandam, L.S.; O'Connell, R.G.; Bellgrove, M.A. The Molecular Genetics of Executive Function: Role of Monoamine System Genes. Biol. Psychiatry 2011, 69, e127–e143. [Google Scholar] [CrossRef]
- Bartels, M.; van Weegen, F.I.; van Beijsterveldt, C.E.; Carlier, M.; Polderman, T.J.; Hoekstra, R.A.; Boomsma, D.I. The five factor model of personality and intelligence: A twin study on the relationship between the two constructs. Pers. Individ. Differ. 2012, 53, 368–373. [Google Scholar] [CrossRef]
- Beliveau, V.; Svarer, C.; Frokjaer, V.G.; Knudsen, G.M.; Greve, D.N.; Fisher, P.M. Functional connectivity of the dorsal and median raphe nuclei at rest. NeuroImage 2015, 116, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Betts, M.J.; Cardenas-Blanco, A.; Kanowski, M.; Jessen, F.; Düzel, E. In vivo MRI assessment of the human locus coeruleus along its rostrocaudal extent in young and older adults. NeuroImage 2017, 163, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Thal, D.R.; Ghebremedhin, E.; Del Tredici, K. Stages of the pathologic process in Alzheimer disease: Age categories from 1 to 100 years. J. Neuropathol. Exp. Neurol. 2011, 70, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Cabeza, R.; Albert, M.; Belleville, S.; Craik, F.I.M.; Duarte, A.; Grady, C.L.; Lindenberger, U.; Nyberg, L.; Park, D.C.; Reuter-Lorenz, P.A.; et al. Maintenance, reserve and compensation: the cognitive neuroscience of healthy ageing. Nat. Rev. Neurosci. 2018, 19, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Chapman, B.P.; Benedict, R.H.; Lin, F.; Roy, S.; Federoff, H.J.; Mapstone, M. Personality and Performance in Specific Neurocognitive Domains Among Older Persons. Am. J. Geriatr. Psychiatry 2017, 25, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; He, J.; Fan, X. Relationships between openness to experience, cognitive flexibility, self-esteem, and creativity among bilingual college students in the U.S. Int. J. Biling. Educ. Biling. 2019, 25, 342–354. [Google Scholar] [CrossRef]
- Clewett, D.V.; Lee, T.-H.; Greening, S.; Ponzio, A.; Margalit, E.; Mather, M. Neuromelanin marks the spot: identifying a locus coeruleus biomarker of cognitive reserve in healthy aging. Neurobiol. Aging 2016, 37, 117–126. [Google Scholar] [CrossRef]
- Costa PT, McCrae RR (1985). The NEO Personality Inventory manual. Odessa, FL: Psychological Assessment Resources.
- Costa, P. T. & McCrae, R. R. Revised NEO Personality Inventory (NEO PI-R) and NEO Five Factor Inventory (NEO-FFI) Professional Manual. (Psychological Assessment Resources Inc., 1992).
- Cuttler, C.; Graf, P. Personality predicts prospective memory task performance: An adult lifespan study. Scand. J. Psychol. 2007, 48, 215–231. [Google Scholar] [CrossRef]
- Dahl, M.J.; Mather, M.; Werkle-Bergner, M.; Kennedy, B.L.; Guzman, S.; Hurth, K.; Miller, C.A.; Qiao, Y.; Shi, Y.; Chui, H.C.; et al. Locus coeruleus integrity is related to tau burden and memory loss in autosomal-dominant Alzheimer's disease. Neurobiol. Aging 2021, 112, 39–54. [Google Scholar] [CrossRef]
- Dahl, M.J.; Mather, M.; Werkle-Bergner, M.; Kennedy, B.L.; Guzman, S.; Hurth, K.; Miller, C.A.; Qiao, Y.; Shi, Y.; Chui, H.C.; et al. Locus coeruleus integrity is related to tau burden and memory loss in autosomal-dominant Alzheimer's disease. Neurobiol. Aging 2021, 112, 39–54. [Google Scholar] [CrossRef]
- Dahl, M.J.; Mather, M.; Werkle-Bergner, M. Noradrenergic modulation of rhythmic neural activity shapes selective attention. Trends Cogn. Sci. 2021, 26, 38–52. [Google Scholar] [CrossRef]
- Dahl, M.J.; Mather, M.; Düzel, S.; Bodammer, N.C.; Lindenberger, U.; Kühn, S.; Werkle-Bergner, M. Rostral locus coeruleus integrity is associated with better memory performance in older adults. Nat. Hum. Behav. 2019, 3, 1203–1214. [Google Scholar] [CrossRef]
- Devilbiss, D.M.; Waterhouse, B.D.; Marzo, A.; Totah, N.K.; Neves, R.M.; Logothetis, N.K.; Eschenko, O. Phasic and Tonic Patterns of Locus Coeruleus Output Differentially Modulate Sensory Network Function in the Awake Rat. J. Neurophysiol. 2011, 105, 69–87. [Google Scholar] [CrossRef]
- DeYoung, C.G.; Cicchetti, D.; Rogosch, F.A.; Gray, J.R.; Eastman, M.; Grigorenko, E.L. Sources of cognitive exploration: Genetic variation in the prefrontal dopamine system predicts Openness/Intellect. J. Res. Pers. 2011, 45, 364–371. [Google Scholar] [CrossRef] [PubMed]
- DeYoung, C.G.; Peterson, J.B.; Higgins, D.M. Sources of Openness/Intellect: Cognitive and Neuropsychological Correlates of the Fifth Factor of Personality. J. Pers. 2005, 73, 825–858. [Google Scholar] [CrossRef] [PubMed]
- DeYoung, C.G.; Peterson, J.B.; Séguin, J.R.; Tremblay, R.E. Externalizing behavior and the higher order factors of the Big Five. J. Abnorm. Psychol. 2008, 117, 947–953. [Google Scholar] [CrossRef] [PubMed]
- DeYoung, C.G.; Quilty, L.C.; Peterson, J.B.; Gray, J.R. Openness to Experience, Intellect, and Cognitive Ability. J. Pers. Assess. 2013, 96, 46–52. [Google Scholar] [CrossRef] [PubMed]
- DeYoung, C.G. The neuromodulator of exploration: A unifying theory of the role of dopamine in personality. Front. Hum. Neurosci. 2013, 7, 762. [Google Scholar] [CrossRef]
- DeYoung, C. G. (2020). Intelligence and personality. In R. J. Sternberg (Ed.), The Cambridge handbook of intelligence (pp. 1011–1047). Cambridge University Press. [CrossRef]
- Dutt, S. ; Alzheimer’s Disease Neuroimaging Initiative; Li, Y.; Mather, M.; Nation, D.A. Brainstem substructures and cognition in prodromal Alzheimer’s disease. Brain Imaging Behav. [CrossRef]
- Elman, J.A.; Puckett, O.K.; Beck, A.; Fennema-Notestine, C.; Cross, L.K.; Dale, A.M.; Eglit, G.M.L.; Eyler, L.T.; Gillespie, N.A.; Granholm, E.L.; et al. MRI-assessed locus coeruleus integrity is heritable and associated with multiple cognitive domains, mild cognitive impairment, and daytime dysfunction. Alzheimer's Dement. 2021, 17, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Naka, F. , Shiga, T., Yaguchi, M. and Okado, N. An enriched environment increases noradrenaline concentration in the mouse brain. Brain research 2002, 92, 124–126. [Google Scholar] [CrossRef] [PubMed]
- Rector, N.A.; Bagby, R.M.; Huta, V.; Ayearst, L.E. Examination of the trait facets of the five-factor model in discriminating specific mood and anxiety disorders. Psychiatry Res. 2012, 199, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Farley, I.J.; Hornykiewicz, O. Noradrenaline distribution in subcortical areas of the human brain. Brain Res. 1977, 126, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.; Lee, A.; Verzijden, M.N. Dopamine genes are linked to Extraversion and Neuroticism personality traits, but only in demanding climates. Sci. Rep. 2018, 8, 1733. [Google Scholar] [CrossRef] [PubMed]
- Fleeson, W.; Gallagher, P. The implications of Big Five standing for the distribution of trait manifestation in behavior: Fifteen experience-sampling studies and a meta-analysis. J. Pers. Soc. Psychol. 2009, 97, 1097–1114. [Google Scholar] [CrossRef] [PubMed]
- Forgeard, M.; Herzhoff, K.; Jayawickreme, E.; Tsukayama, E.; Beard, C.; Björgvinsson, T. Changes in daily manifestations of Openness to Experience during intensive cognitive-behavioral treatment. J. Pers. 2018, 87, 856–870. [Google Scholar] [CrossRef]
- Williams, S.R.T.P. More than Education: Openness to Experience Contributes to Cognitive Reserve in Older Adulthood. J. Aging Sci. 2013, 01. [Google Scholar] [CrossRef]
- George, S.; Mufson, E.; Leurgans, S.; Shah, R.; Ferrari, C.; Detoledo-Morrell, L. MRI-based volumetric measurement of the substantia innominata in amnestic MCI and mild AD. Neurobiol. Aging 2011, 32, 1756–1764. [Google Scholar] [CrossRef]
- Gnambs, T. A meta-analysis of dependability coefficients (test–retest reliabilities) for measures of the Big Five. J. Res. Pers. 2014, 52, 20–28. [Google Scholar] [CrossRef]
- Grella, S.L.; Neil, J.M.; Edison, H.T.; Strong, V.D.; Odintsova, I.V.; Walling, S.G.; Martin, G.M.; Marrone, D.F.; Harley, C.W. Locus Coeruleus Phasic, But Not Tonic, Activation Initiates Global Remapping in a Familiar Environment. J. Neurosci. 2018, 39, 445–455. [Google Scholar] [CrossRef]
- Grueschow, M.; Kleim, B.; Ruff, C.C. Functional Coupling of the Locus Coeruleus Is Linked to Successful Cognitive Control. Brain Sci. 2022, 12, 305. [Google Scholar] [CrossRef]
- Herz, N.; Baror, S.; Bar, M. Overarching States of Mind. Trends Cogn. Sci. 2020, 24, 184–199. [Google Scholar] [CrossRef] [PubMed]
- Ihle, A.; Zuber, S.; Gouveia. R.; Gouveia, B.R.; Mella, N.; Desrichard, O.; Cullati, S.; Oris, M.; Maurer, J.; Kliegel, M. Cognitive Reserve Mediates the Relation between Openness to Experience and Smaller Decline in Executive Functioning. Dement. Geriatr. Cogn. Disord. 2019, 48, 39–44. [Google Scholar] [CrossRef]
- Jackson, J.J.; Hill, P.L.; Payne, B.R.; Roberts, B.W.; Stine-Morrow, E.A.L. Can an old dog learn (and want to experience) new tricks? Cognitive training increases openness to experience in older adults. Psychol. Aging 2012, 27, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, H.I.L.; Becker, J.A.; Kwong, K.; Engels-Domínguez, N.; Prokopiou, P.C.; Papp, K.V.; Properzi, M.; Hampton, O.L.; Uquillas, F.D.; Sanchez, J.S.; et al. In vivo and neuropathology data support locus coeruleus integrity as indicator of Alzheimer’s disease pathology and cognitive decline. Sci. Transl. Med. 2021, 13, eabj2511–eabj2511. [Google Scholar] [CrossRef]
- Käckenmester, W.; Bott, A.; Wacker, J. Openness to experience predicts dopamine effects on divergent thinking. Pers. Neurosci. 2019, 2, e3. [Google Scholar] [CrossRef] [PubMed]
- Kalbitzer, J.; Frokjaer, V.G.; Erritzoe, D.; Svarer, C.; Cumming, P.; Nielsen, F. .; Hashemi, S.H.; Baaré, W.F.; Madsen, J.; Hasselbalch, S.G.; et al. The personality trait openness is related to cerebral 5-HTT levels. NeuroImage 2009, 45, 280–285. [Google Scholar] [CrossRef]
- Kanen, J.W.; Arntz, F.E.; Yellowlees, R.; Cardinal, R.N.; Price, A.; Christmas, D.M.; Apergis-Schoute, A.M.; Sahakian, B.J.; Robbins, T.W. Serotonin depletion amplifies distinct human social emotions as a function of individual differences in personality. Transl. Psychiatry 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Karsazi, H.; Rezapour, T.; Kormi-Nouri, R.; Mottaghi, A.; Abdekhodaie, E.; Hatami, J. The moderating effect of neuroticism and openness in the relationship between age and memory: Implications for cognitive reserve. Pers. Individ. Differ. 2021, 176. [Google Scholar] [CrossRef]
- Kaup, A.R.; Harmell, A.L.; Yaffe, K. Conscientiousness Is Associated with Lower Risk of Dementia among Black and White Older Adults. Neuroepidemiology 2019, 52, 86–92. [Google Scholar] [CrossRef]
- Keren, N.I.; Lozar, C.T.; Harris, K.C.; Morgan, P.S.; Eckert, M.A. In vivo mapping of the human locus coeruleus. NeuroImage 2009, 47, 1261–1267. [Google Scholar] [CrossRef]
- Keren, N.I.; Taheri, S.; Vazey, E.M.; Morgan, P.S.; Granholm, A.-C.E.; Aston-Jones, G.S.; Eckert, M.A. Histologic validation of locus coeruleus MRI contrast in post-mortem tissue. NeuroImage 2015, 113, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Kilimann, I.; Grothe, M.; Heinsen, H.; Alho, E.J.L.; Grinberg, L.; Jr. , E.A.; dos Santos, G.A.B.; da Silva, R.E.; Mitchell, A.J.; Frisoni, G.B.; et al. Subregional Basal Forebrain Atrophy in Alzheimer's Disease: A Multicenter Study. J. Alzheimer's Dis. 2014, 40, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Kirby, L.; Pernar, L.; Valentino, R.; Beck, S. Distinguishing characteristics of serotonin and non-serotonin-containing cells in the dorsal raphe nucleus: electrophysiological and immunohistochemical studies. Neuroscience 2003, 116, 669–683. [Google Scholar] [CrossRef] [PubMed]
- Koulousakis, P.; Andrade, P.; Visser-Vandewalle, V.; Sesia, T. The Nucleus Basalis of Meynert and Its Role in Deep Brain Stimulation for Cognitive Disorders: A Historical Perspective. J. Alzheimer's Dis. 2019, 69, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Krebs, R.M.; Fias, W.; Achten, E.; Boehler, C.N. Picture novelty attenuates semantic interference and modulates concomitant neural activity in the anterior cingulate cortex and the locus coeruleus. NeuroImage 2013, 74, 179–187. [Google Scholar] [CrossRef]
- Krebs, R.M.; Park, H.R.P.; Bombeke, K.; Boehler, C.N. Modulation of locus coeruleus activity by novel oddball stimuli. Brain Imaging Behav. 2017, 12, 577–584. [Google Scholar] [CrossRef]
- Liu, A.K.L.; Chang, R.C.-C.; Pearce, R.K.B.; Gentleman, S.M. Nucleus basalis of Meynert revisited: anatomy, history and differential involvement in Alzheimer’s and Parkinson’s disease. Acta Neuropathol. 2015, 129, 527–540. [Google Scholar] [CrossRef]
- Liu, K.Y.; Acosta-Cabronero, J.; Cardenas-Blanco, A.; Loane, C.; Berry, A.J.; Betts, M.J.; Kievit, R.A.; Henson, R.N.; Düzel, E.; Howard, R.; et al. In vivo visualization of age-related differences in the locus coeruleus. Neurobiol. Aging 2018, 74, 101–111. [Google Scholar] [CrossRef]
- Liu, K.Y.; Kievit, R.A.; Tsvetanov, K.A.; Betts, M.J.; Düzel, E.; Rowe, J.B.; Tyler, L.K.; Brayne, C.; Bullmore, E.T.; Calder, A.C.; et al. Noradrenergic-dependent functions are associated with age-related locus coeruleus signal intensity differences. Nat. Commun. 2020, 11, 1–9. [Google Scholar] [CrossRef]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Luchetti, M.; Terracciano, A.; Stephan, Y.; Sutin, A.R. Personality and Cognitive Decline in Older Adults: Data From a Longitudinal Sample and Meta-Analysis. Journals Gerontol. Ser. B 2015, 71, 591–601. [Google Scholar] [CrossRef]
- Grilli, M. , Zappettini, S., Zanardi, A., Lagomarsino, F., Pittaluga, A., Zoli, M. and Marchi, M. Exposure to an enriched environment selectively increases the functional response of the pre-synaptic NMDA receptors which modulate noradrenaline release in mouse hippocampus. Journal of neurochemistry 2009, 110, 1598–1606. [Google Scholar]
- Mai, J.K. , Paxinos G. The Human Nervous System. 3rd ed. 2: Academic Press; Cambridge, MA, USA, 2012. [Google Scholar]
- Martin, L.R.; Friedman, H.S.; Schwartz, J.E. Personality and mortality risk across the life span: The importance of conscientiousness as a biopsychosocial attribute. Heal. Psychol. 2007, 26, 428–436. [Google Scholar] [CrossRef]
- Matchett, B.J.; Grinberg, L.T.; Theofilas, P.; Murray, M.E. The mechanistic link between selective vulnerability of the locus coeruleus and neurodegeneration in Alzheimer’s disease. Acta Neuropathol. 2021, 141, 631–650. [Google Scholar] [CrossRef] [PubMed]
- Mather, M.; Harley, C.W. The Locus Coeruleus: Essential for Maintaining Cognitive Function and the Aging Brain. Trends Cogn. Sci. 2016, 20, 214–226. [Google Scholar] [CrossRef]
- Mather, M. Noradrenaline in the aging brain: Promoting cognitive reserve or accelerating Alzheimer's disease? Semin. Cell Dev. Biol. 2021, 116, 108–124. [Google Scholar] [CrossRef] [PubMed]
- McCrae, R.R.; Kurtz, J.E.; Yamagata, S.; Terracciano, A. Internal Consistency, Retest Reliability, and Their Implications for Personality Scale Validity. Pers. Soc. Psychol. Rev. 2010, 15, 28–50. [Google Scholar] [CrossRef] [PubMed]
- Moutafi, J.; Furnham, A.; Crump, J. What facets of openness and conscientiousness predict fluid intelligence score? Learn. Individ. Differ. 2005, 16, 31–42. [Google Scholar] [CrossRef]
- Anglim, J.; Dunlop, P.D.; Wee, S.; Horwood, S.; Wood, J.K.; Marty, A. Personality and intelligence: A meta-analysis. Psychol. Bull. 2022, 148, 301–336. [Google Scholar] [CrossRef]
- Narita, S.; Iwahashi, K.; Nagahori, K.; Numajiri, M.; Yoshihara, E.; Ohtani, N.; Ishigooka, J. Analysis of Association between Norepinephrine Transporter Gene Polymorphisms and Personality Traits of NEO-FFI in a Japanese Population. Psychiatry Investig. 2015, 12, 381–387. [Google Scholar] [CrossRef]
- Omoluabi, T.; Torraville, S.E.; Maziar, A.; Ghosh, A.; Power, K.D.; Reinhardt, C.; Harley, C.W.; Yuan, Q. Novelty-like activation of locus coeruleus protects against deleterious human pretangle tau effects while stress-inducing activation worsens its effects. Alzheimer's Dementia: Transl. Res. Clin. Interv. 2021, 7, e12231. [Google Scholar] [CrossRef] [PubMed]
- Ordway, G.A.; Stockmeier, C.A.; Cason, G.W.; Klimek, V. Pharmacology and Distribution of Norepinephrine Transporters in the Human Locus Coeruleus and Raphe Nuclei. J. Neurosci. 1997, 17, 1710–1719. [Google Scholar] [CrossRef] [PubMed]
- Pauli, W.M.; Nili, A.N.; Tyszka, J.M. A high-resolution probabilistic in vivo atlas of human subcortical brain nuclei. Sci. Data 2018, 5, 180063. [Google Scholar] [CrossRef] [PubMed]
- Plini, E.R.G.; O’hanlon, E.; Boyle, R.; Sibilia, F.; Rikhye, G.; Kenney, J.; Whelan, R.; Melnychuk, M.C.; Robertson, I.H.; Dockree, P.M. Examining the Role of the Noradrenergic Locus Coeruleus for Predicting Attention and Brain Maintenance in Healthy Old Age and Disease: An MRI Structural Study for the Alzheimer’s Disease Neuroimaging Initiative. Cells 2021, 10, 1829. [Google Scholar] [CrossRef]
- Prokopiou, P.C.; Engels-Domínguez, N.; Papp, K.V.; Scott, M.R.; Schultz, A.P.; Schneider, C.; Farrell, M.E.; Buckley, R.F.; Quiroz, Y.T.; El Fakhri, G.; et al. Lower novelty-related locus coeruleus function is associated with Aβ-related cognitive decline in clinically healthy individuals. Nat. Commun. 2022, 13, 1–14. [Google Scholar] [CrossRef]
- Rammstedt, B.; Lechner, C.M.; Danner, D. Relationships between Personality and Cognitive Ability: A Facet-Level Analysis. J. Intell. 2018, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Liu, C.; Meng, J.; Liu, Q.; Shi, L.; Wu, X.; Song, L.; Qiu, J. Effects of the Openness to Experience Polygenic Score on Cortical Thickness and Functional Connectivity. Front. Neurosci. 2021, 14. [Google Scholar] [CrossRef]
- E Rhodes, R.; E I Smith, N. Personality correlates of physical activity: a review and meta-analysis. Br. J. Sports Med. 2006, 40, 958–965. [Google Scholar] [CrossRef]
- Robertson, I.H. A noradrenergic theory of cognitive reserve: implications for Alzheimer's disease. Neurobiol. Aging 2013, 34, 298–308. [Google Scholar] [CrossRef]
- Robertson, I.H. A right hemisphere role in cognitive reserve. Neurobiol. Aging 2014, 35, 1375–1385. [Google Scholar] [CrossRef]
- Rong, Y.; Rua, C.; O’Callaghan, C.; Jones, P.S.; Hezemans, F.; Kaalund, S.S.; Tsvetanov, K.A.; Rodgers, C.T.; Williams, G.; Passamonti, L.; et al. An in vivo Probabilistic Atlas of the Human Locus Coeruleus at Ultra-high Field. bioRxiv 2020. [Google Scholar] [CrossRef]
- Schmidt, K. H. & Metzler, P. WST: Wortschatztest. 1992. [Google Scholar]
- Schretlen, D.J.; van der Hulst, E.-J.; Pearlson, G.D.; Gordon, B. A neuropsychological study of personality: Trait openness in relation to intelligence, fluency, and executive functioning. J. Clin. Exp. Neuropsychol. 2010, 32, 1068–1073. [Google Scholar] [CrossRef]
- Schulz, J.; Pagano, G.; Bonfante, J.A.F.; Wilson, H.; Politis, M. Nucleus basalis of Meynert degeneration precedes and predicts cognitive impairment in Parkinson’s disease. Brain 2018, 141, 1501–1516. [Google Scholar] [CrossRef] [PubMed]
- Sharp, E.S.; Reynolds, C.A.; Pedersen, N.L.; Gatz, M. Cognitive engagement and cognitive aging: Is openness protective? Psychol. Aging 2010, 25, 60–73. [Google Scholar] [CrossRef]
- Stern, Y. Cognitive reserve. Neuropsychologia 2009, 47, 2015–2028. [Google Scholar] [CrossRef] [PubMed]
- Stern, Y.; Arenaza-Urquiljo, E.M.; Bartrés-Faz, D.; Belleville, S.; Cantillon, M.; Chetelat, G.; Ewers, M.; Franzmeier, N.; Kempermann, G.; Kremen, W.S.; et al. Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimer's Dement. 2020, 16, 1305–1311. [Google Scholar] [CrossRef]
- Sutin, A.R.; Stephan, Y.; Damian, R.I.; Luchetti, M.; Strickhouser, J.E.; Terracciano, A. Five-factor model personality traits and verbal fluency in 10 cohorts. Psychol. Aging 2019, 34, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Sutin, A.R.; Ferrucci, L.; Zonderman, A.B.; Terracciano, A. Personality and obesity across the adult life span. J. Pers. Soc. Psychol. 2011, 101, 579–592. [Google Scholar] [CrossRef]
- Sutin, A.R.; Stephan, Y.; Luchetti, M.; Artese, A.; Oshio, A.; Terracciano, A. The five-factor model of personality and physical inactivity: A meta-analysis of 16 samples. J. Res. Pers. 2016, 63, 22–28. [Google Scholar] [CrossRef]
- Sutin, A.R.; Stephan, Y.; Terracciano, A. Facets of Conscientiousness and risk of dementia. Psychol. Med. 2017, 48, 974–982. [Google Scholar] [CrossRef]
- Sutin, A.R.; Stephan, Y.; Luchetti, M.; Terracciano, A. Five-factor model personality traits and cognitive function in five domains in older adulthood. BMC Geriatr. 2019, 19, 1–10. [Google Scholar] [CrossRef]
- Tautvydaitė, D.; Kukreja, D.; Antonietti, J.-P.; Henry, H.; von Gunten, A.; Popp, J. Interaction between personality traits and cerebrospinal fluid biomarkers of Alzheimer’s disease pathology modulates cognitive performance. Alzheimer's Res. Ther. 2017, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Terracciano, A.; Stephan, Y.; Luchetti, M.; Albanese, E.; Sutin, A.R. Personality traits and risk of cognitive impairment and dementia. J. Psychiatr. Res. 2017, 89, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Tochigi, M.; Otowa, T.; Hibino, H.; Kato, C.; Otani, T.; Umekage, T.; Utsumi, T.; Kato, N.; Sasaki, T. Combined analysis of association between personality traits and three functional polymorphisms in the tyrosine hydroxylase, monoamine oxidase A, and catechol-O-methyltransferase genes. Neurosci. Res. 2006, 54, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Tona, K.-D.; Keuken, M.C.; de Rover, M.; Lakke, E.; Forstmann, B.U.; Nieuwenhuis, S.; van Osch, M.J.P. In vivo visualization of the locus coeruleus in humans: quantifying the test–retest reliability. Anat. Embryol. 2017, 222, 4203–4217. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, J.S.; Engle, R.W. Fluid intelligence and the locus coeruleus–norepinephrine system. Proc. Natl. Acad. Sci. 2021, 118. [Google Scholar] [CrossRef]
- Unsworth, N.; Robison, M.K. A locus coeruleus-norepinephrine account of individual differences in working memory capacity and attention control. Psychon. Bull. Rev. 2017, 24, 1282–1311. [Google Scholar] [CrossRef]
- Veyrac, A. , Sacquet, J., Nguyen, V., Marien, M., Jourdan, F. and Didier, A. Novelty determines the effects of olfactory enrichment on memory and neurogenesis through noradrenergic mechanisms. Neuropsychopharmacology 2009, 34, 786–795. [Google Scholar] [CrossRef]
- Ward, R.; Sreenivas, S.; Read, J.; Saunders, K.E.A.; Rogers, R.D. The role of serotonin in personality inference: tryptophan depletion impairs the identification of neuroticism in the face. Psychopharmacol. 2017, 234, 2139–2147. [Google Scholar] [CrossRef]
- Wilson, R.S.; Nag, S.; Boyle, P.A.; Hizel, L.P.; Yu, L.; Buchman, A.S.; Schneider, J.A.; Bennett, D.A. Neural reserve, neuronal density in the locus ceruleus, and cognitive decline. Neurology 2013, 80, 1202–1208. [Google Scholar] [CrossRef]
- Wilson, R.S.; Schneider, J.A.; Arnold, S.E.; Bienias, J.L.; Bennett, D.A. Conscientiousness and the Incidence of Alzheimer Disease and Mild Cognitive Impairment. Arch. Gen. Psychiatry 2007, 64, 1204–1212. [Google Scholar] [CrossRef]
- Yoneda, T.; Graham, E.; Lozinski, T.; Bennett, D.A.; Mroczek, D.; Piccinin, A.M.; Hofer, S.M.; Muniz-Terrera, G. Personality traits, cognitive states, and mortality in older adulthood. J. Pers. Soc. Psychol. 2023, 124, 381–395. [Google Scholar] [CrossRef] [PubMed]
- Zaborszky, L.; Hoemke, L.; Mohlberg, H.; Schleicher, A.; Amunts, K.; Zilles, K. Stereotaxic probabilistic maps of the magnocellular cell groups in human basal forebrain. NeuroImage 2008, 42, 1127–1141. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Kong, L.; Qu, H. A systems biology approach to identify intelligence quotient score-related genomic regions and pathways relevant to potential therapeutic treatments. Sci. Rep. 2014, 4, 4176. [Google Scholar] [CrossRef] [PubMed]
- Zmorzyński, S.; Styk, W.; Klinkosz, W.; Iskra, J.; Filip, A.A. Personality traits and polymorphisms of genes coding neurotransmitter receptors or transporters: review of single gene and genome-wide association studies. Ann. Gen. Psychiatry 2021, 20, 1–6. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).