Submitted:
28 April 2023
Posted:
28 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
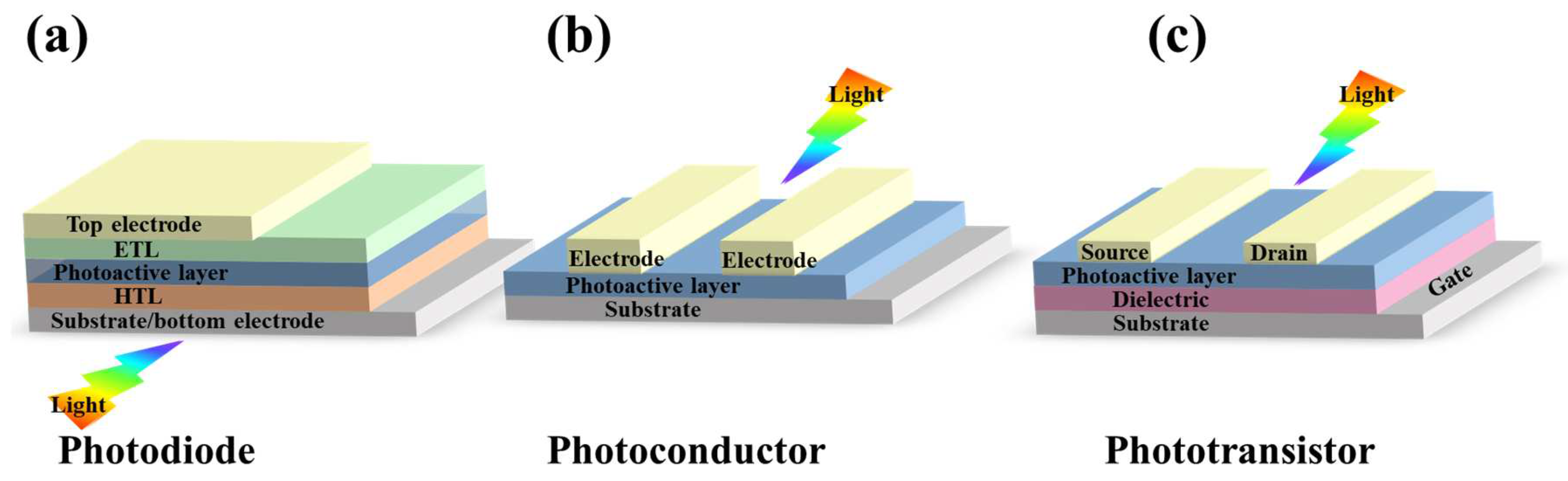
2. Categories and Key Performance Parameters of Semiconductor-Based PDs
- Responsivity (R): It is a key parameter to quantify the response efficiency of photodetectors to an optical signal and is defined as the photocurrent generated by the incident light of the unit power per unit area. Its unit is A W-1.
- 2.
- Detectivity (D*): It describes the ability of detector materials to detect weak light. D* is determined by the responsivity and noise of the PD and is defined as follows:
- 3.
- Response time (rise/decay time): Response time reflects the response speed of the detector and is a key parameter to evaluate the performance of PDs. Generally, we can use the square wave test method to measure the optical response time of the detector. Where rise time (τr)/decay time (τf) is defined as the rise (fall) time from 10% (90%) to 90% (10%) of the maximum current, respectively. Its unit is s.
- 4.
- On-off ratio: The On-off ratio is the ratio of the photocurrent (Ip) and the dark current (Id), reflecting the photosensitivity of PDs. The higher the on-off current ratio, the higher the accuracy of the detector in detecting weak light signals.
- 5.
- EQE: It is defined as the ratio of output carriers to the number of incident photons per unit time under specific wavelength radiation, which reflects the luminous efficiency of the whole detector.
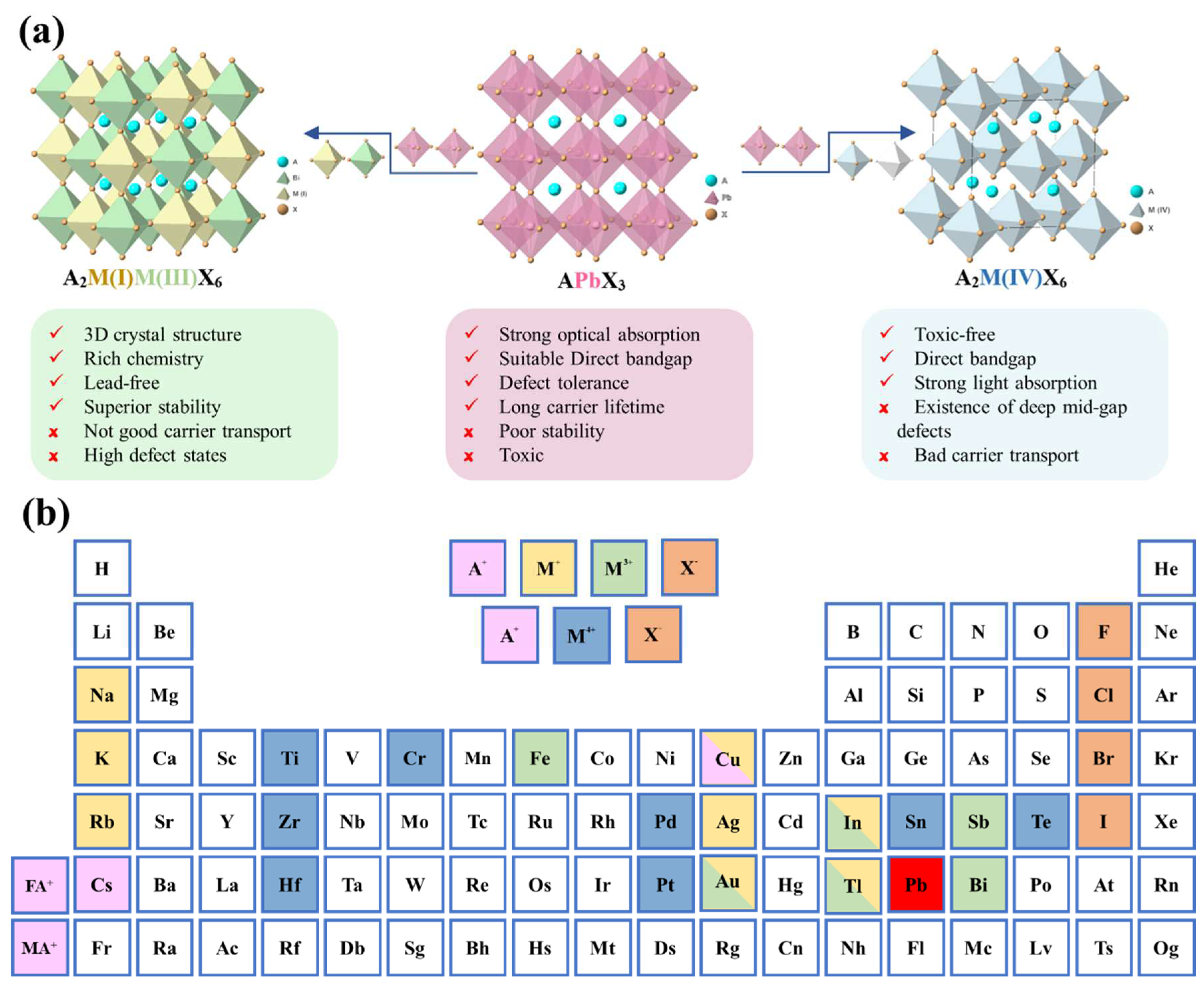
3. Design Principle for Lead-Free Double Perovskite Materials
4. A2M(I)M(III)X6-based double perovskite photodetector
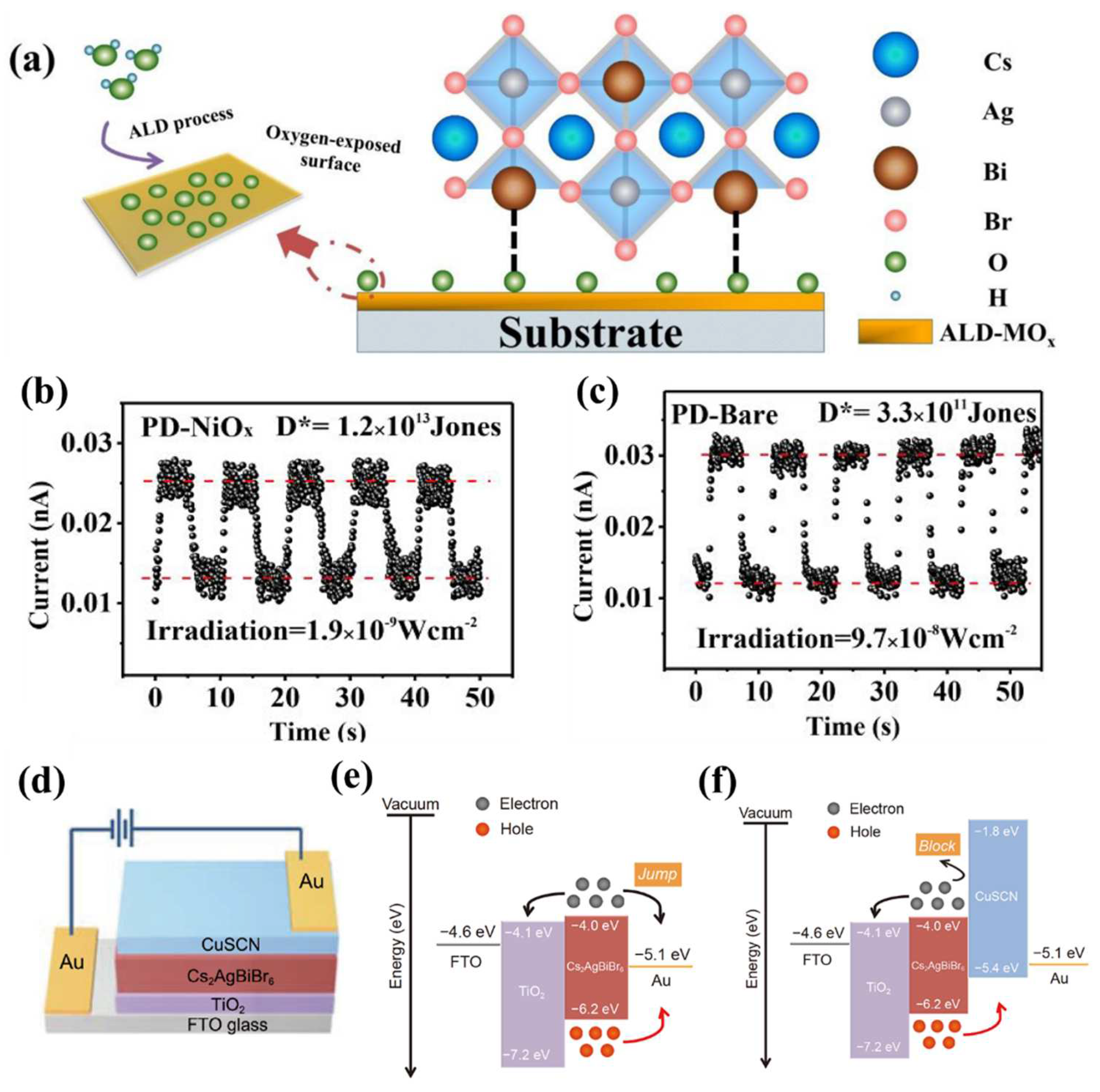
4.1. Bi-based double perovskite photodetector
4.2. Other (Sb3+, Fe3+, In3+, Tl3+, Au3+-based) double perovskite photodetector
5. A2M(Ⅳ)X6-based double perovskite photodetector
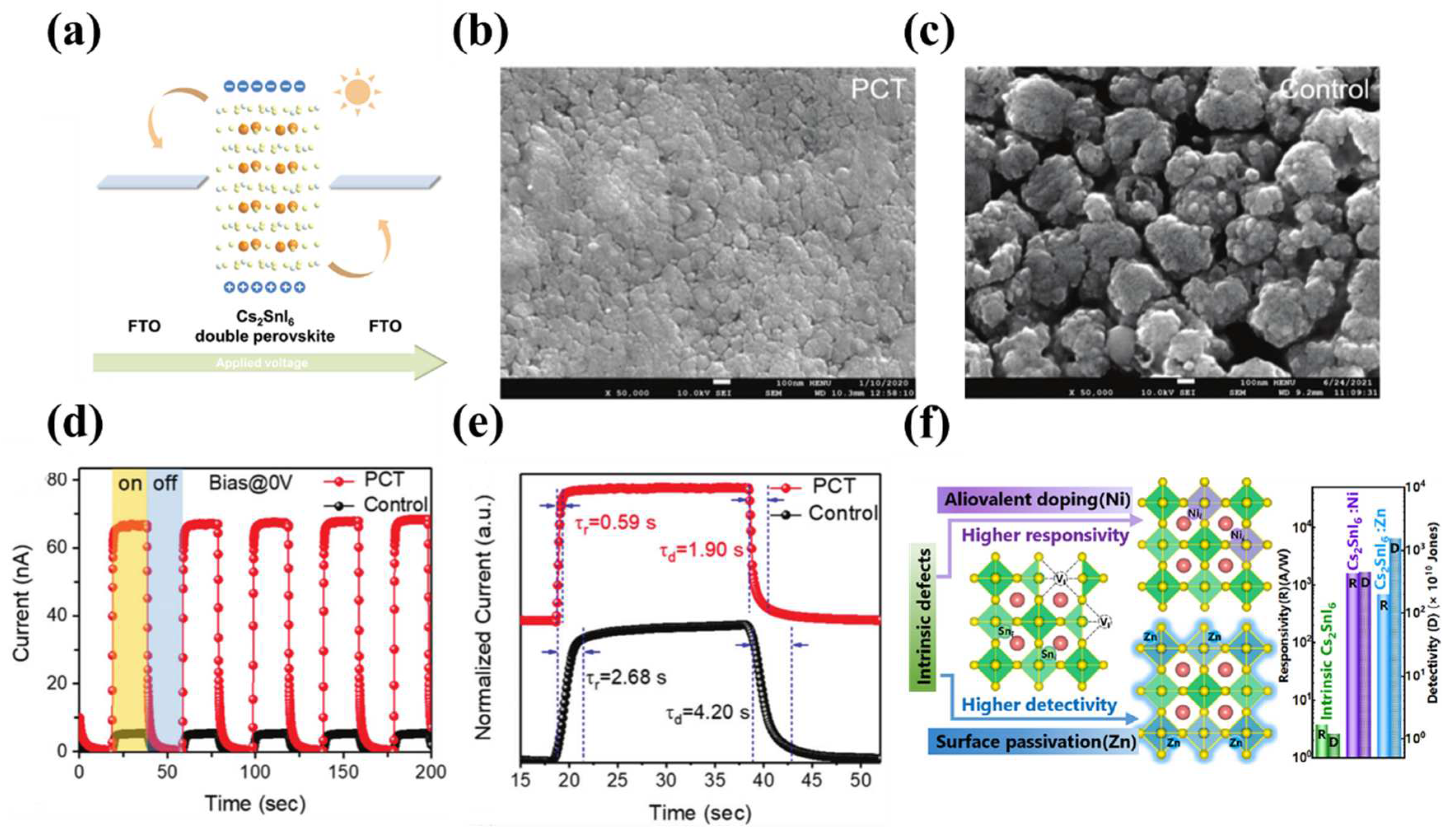
5.1. Sn-based double perovskite photodetector
5.2. Other (Ti4+, Pd4+, Hf4+, Te4+, Cr4+, Zr4+-based) double perovskite photodetector
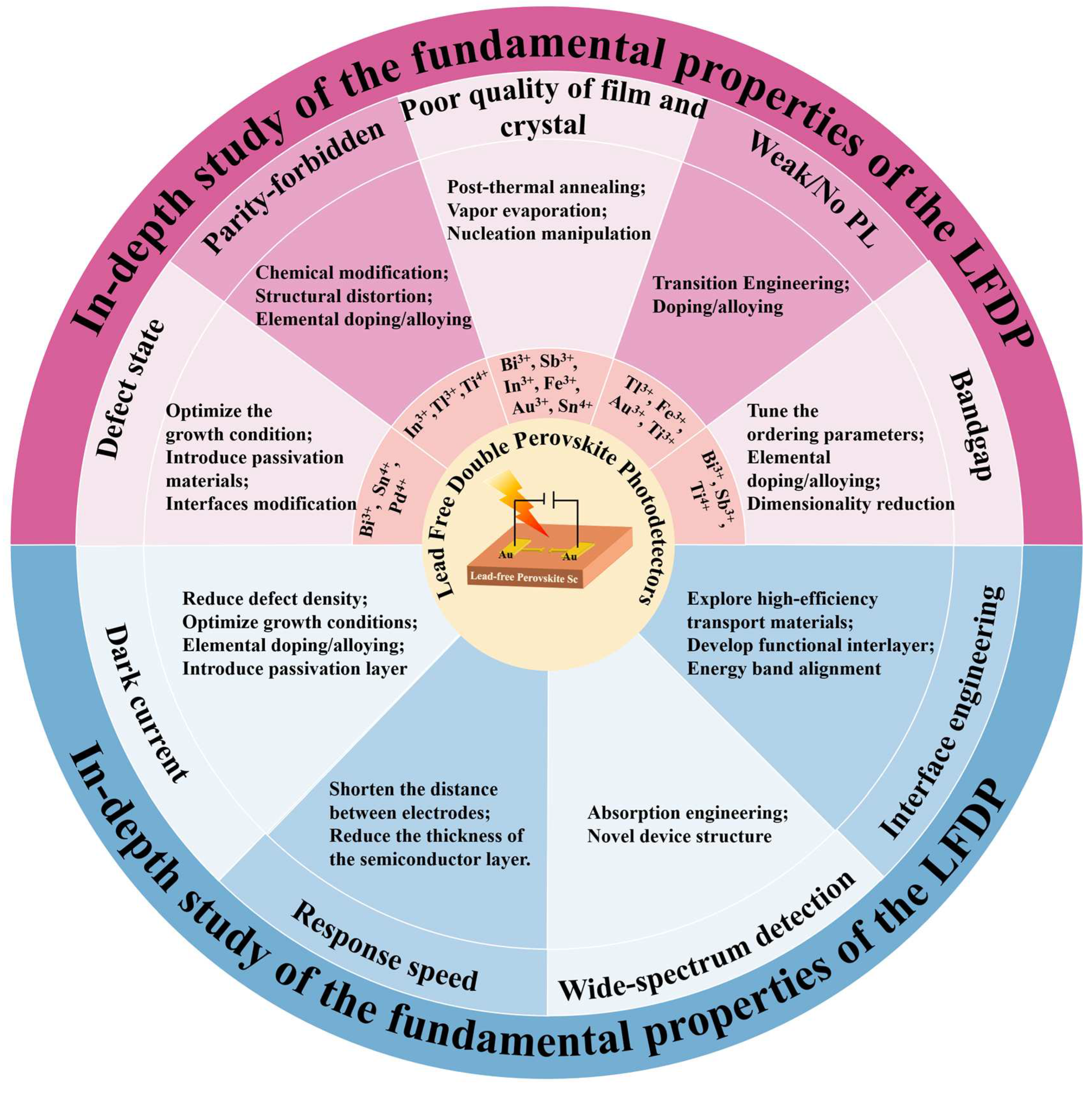
6. Challenges and Perspective for Lead-Free Double Perovskite-based PDs
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Li J, Duan J, Yang X, et al. Review on recent progress of lead-free halide perovskites in optoelectronic applications[J]. Nano Energy 2021, 80, 105526. [Google Scholar] [CrossRef]
- Akkerman Q A, Rainò G, Kovalenko M V, et al. Genesis, challenges and opportunities for colloidal lead halide perovskite nanocrystals[J]. Nature materials 2018, 17, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Zhang Z, Yang G, Zhou C, et al. Optical and electrical properties of all-inorganic Cs2AgBiBr6 double perovskite single crystals[J]. RSC advances 2019, 9, 23459–23464. [Google Scholar] [CrossRef] [PubMed]
- Volonakis G, Haghighirad A A, Milot R L, et al. Cs2InAgCl6: a new lead-free halide double perovskite with direct band gap[J]. The journal of physical chemistry letters 2017, 8, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Singh A, Chaurasiya R, Bheemaraju A, et al. Strain-Induced Band-Edge Modulation in Lead-Free Antimony-Based Double Perovskite for Visible-Light Absorption[J]. ACS Applied Energy Materials 2022, 5, 3926–3932. [Google Scholar] [CrossRef]
- Zhang L, Wang K, Zou B. Bismuth Halide Perovskite-Like Materials: Current Opportunities and Challenges[J]. ChemSusChem 2019, 12, 1612–1630. [Google Scholar] [CrossRef] [PubMed]
- Longo G, Mahesh S, Buizza L R V, et al. Understanding the performance-limiting factors of Cs2AgBiBr6 double-perovskite solar cells[J]. ACS Energy Letters 2020, 5, 2200–2207. [Google Scholar] [CrossRef]
- Lei L Z, Shi Z F, Li Y, et al. High-efficiency and air-stable photodetectors based on lead-free double perovskite Cs2AgBiBr6 thin films[J]. Journal of Materials Chemistry C 2018, 6, 7982–7988. [Google Scholar] [CrossRef]
- Yan G, Jiang B, Yuan Y, et al. Importance of Bi–O Bonds at the Cs2AgBiBr6 Double-Perovskite/Substrate Interface for Crystal Quality and Photoelectric Performance[J]. ACS applied materials & interfaces 2020, 12, 6064–6073. [Google Scholar]
- Arora N, Dar M I, Hinderhofer A, et al. Perovskite solar cells with CuSCN hole extraction layers yield stabilized efficiencies greater than 20%[J]. Science 2017, 358, 768–771. [Google Scholar] [CrossRef]
- Qin P, Tanaka S, Ito S, et al. Inorganic hole conductor-based lead halide perovskite solar cells with 12.4% conversion efficiency[J]. Nature communications 2014, 5, 3834. [Google Scholar] [CrossRef]
- Wijeyasinghe N, Regoutz A, Eisner F, et al. Copper(I) thiocyanate (CuSCN) hole-transport layers processed from aqueous precursor solutions and their application in thin-film transistors and highly efficient organic and organometal halide perovskite solar cells. Adv Funct Mater 2017, 27, 1701818. [Google Scholar] [CrossRef]
- Yan G, Ji Z, Li Z, et al. All-inorganic Cs2AgBiBr6/CuSCN-based photodetectors for weak light imaging[J]. Sci. China Mater. 2020. [Google Scholar]
- Shen, Wenhu, et al. “Enhanced device performance of Cs2AgBiBr6 double perovskite photodetector by SnO2/ZnO double electron transport layer.”. Journal of Alloys and Compounds 2022, 929, 167329. [Google Scholar] [CrossRef]
- Lee H J, Na S I. Investigation of PCBM/ZnO and C60/BCP-based electron transport layer for high-performance pin perovskite solar cells[J]. Journal of Alloys and Compounds 2022, 921, 166007. [Google Scholar] [CrossRef]
- Liu G, Zhong Y, Mao H, et al. Highly efficient and stable ZnO-based MA-free perovskite solar cells via overcoming interfacial mismatch and deprotonation reaction[J]. Chemical Engineering Journal 2022, 431, 134235. [Google Scholar] [CrossRef]
- Ruankham P, Wongratanaphisan D, Gardchareon A, et al. Full coverage of perovskite layer onto ZnO nanorods via a modified sequential two-step deposition method for efficiency enhancement in perovskite solar cells[J]. Applied Surface Science 2017, 410, 393–400. [Google Scholar] [CrossRef]
- Sivashanmugan K, Lin C H, Hsu S H, et al. Interfacial engineering of ZnO surface modified with poly-vinylpyrrolidone and p-aminobenzoic acid for high-performance perovskite solar cells[J]. Materials Chemistry and Physics 2018, 219, 90–95. [Google Scholar] [CrossRef]
- Yang Z, Babu B H, Wu S, et al. Review on practical interface engineering of perovskite solar cells: From efficiency to stability[J]. Solar Rrl 2020, 4, 1900257. [Google Scholar] [CrossRef]
- Niu H, Fang C, Wei X, et al. Magnetron sputtered ZnO electron transporting layers for high performance perovskite solar cells[J]. Dalton Transactions 2021, 50, 6477–6487. [Google Scholar] [CrossRef]
- Yuan, Ye, et al. UV soaking for enhancing the photocurrent and response speed of Cs2AgBiBr6-based all-inorganic perovskite photodetectors. Science China Materials 2022, 65, 442–450. [Google Scholar] [CrossRef]
- Tress W, Yavari M, Domanski K, et al. Interpretation and evolution of open-circuit voltage, recombination, ideality factor and subgap defect states during reversible light-soaking and irreversible degradation of perovskite solar cells[J]. Energy & Environmental Science 2018, 11, 151–165. [Google Scholar]
- Yamada Y, Endo M, Wakamiya A, et al. Spontaneous defect annihilation in CH3NH3PbI3 thin films at room temperature revealed by time-resolved photoluminescence spectroscopy[J]. The journal of physical chemistry letters 2015, 6, 482–486. [Google Scholar] [CrossRef]
- Tian Y, Merdasa A, Unger E, et al. Enhanced organo-metal halide perovskite photoluminescence from nanosized defect-free crystallites and emitting sites[J]. The Journal of Physical Chemistry Letters 2015, 6, 4171–4177. [Google Scholar] [CrossRef]
- Tian Y, Peter M, Unger E, et al. Mechanistic insights into perovskite photoluminescence enhancement: light curing with oxygen can boost yield thousandfold[J]. Physical Chemistry Chemical Physics 2015, 17, 24978–24987. [Google Scholar] [CrossRef]
- Chen S, Wen X, Huang S, et al. Light illumination induced photoluminescence enhancement and quenching in lead halide perovskite[J]. Solar Rrl 2017, 1, 1600001. [Google Scholar] [CrossRef]
- Steele J A, Pan W, Martin C, et al. Photophysical pathways in highly sensitive Cs2AgBiBr6 double-perovskite single-crystal X-ray detectors[J]. Advanced Materials 2018, 30, 1804450. [Google Scholar] [CrossRef]
- Xu Q, Shao W, Li Y, et al. High-sensitivity X-ray imaging of a lead halide perovskite single-crystal scintillator[J]. Optics Letters 2020, 45, 355–358. [Google Scholar] [CrossRef]
- Douissard P A, Cecilia A, Rochet X, et al. A versatile indirect detector design for hard X-ray microimaging[J]. Journal of Instrumentation 2012, 7, P09016. [Google Scholar]
- Lian L, Zheng M, Zhang W, et al. Efficient and Reabsorption-Free Radioluminescence in Cs3Cu2I5 Nanocrystals with Self-Trapped Excitons[J]. Advanced Science 2020, 7, 2000195. [Google Scholar] [CrossRef]
- Chen Q, Wu J, Ou X, et al. All-inorganic perovskite nanocrystal scintillators[J]. Nature 2018, 561, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Zhang X, Yu S, Meng X, et al. A Review on Lead-Free Perovskites for X-Ray Detection and Imaging[J]. Crystal Research and Technology.
- Wu Y, Feng J, Yang Z, et al. Halide Perovskite: A Promising Candidate for Next-Generation X-Ray Detectors[J]. Advanced Science 2023, 10, 2205536. [Google Scholar] [CrossRef]
- Yuan W, Niu G, Xian Y, et al. In situ regulating the order–disorder phase transition in Cs2AgBiBr6 single crystal toward the application in an X-ray detector[J]. Advanced Functional Materials 2019, 29, 1900234. [Google Scholar] [CrossRef]
- Pan W, Wu H, Luo J, et al. Cs2AgBiBr6 single-crystal X-ray detectors with a low detection limit[J]. Nature photonics 2017, 11, 726–732. [Google Scholar] [CrossRef]
- Steele J A, Pan W, Martin C, et al. Photophysical pathways in highly sensitive Cs2AgBiBr6 double-perovskite single-crystal X-ray detectors[J]. Advanced Materials 2018, 30, 1804450. [Google Scholar] [CrossRef]
- Shao Q, Yan J, Gong W, et al. Dopant-compensated Cs2AgBiBr6−xClx single crystals for photo-imaging and X-ray detection[J]. Journal of Materials Chemistry C 2022, 10, 18366–18374. [Google Scholar] [CrossRef]
- Tie S, Dong S, Yuan R, et al. Halide perovskites for sensitive, stable and scalable X-ray detection and imaging[J]. Chemical Communications 2023. [Google Scholar]
- Wang M, Zeng P, Bai S, et al. High-quality sequential-vapor-deposited Cs2AgBiBr6 thin films for lead-free perovskite solar cells[J]. Solar Rrl 2018, 2, 1800217. [Google Scholar] [CrossRef]
- Yang B, Hong F, Chen J, et al. Colloidal Synthesis and Charge-Carrier Dynamics of Cs2AgSb1− yBiyX6 (X: Br, Cl; 0≤ y≤ 1) Double Perovskite Nanocrystals[J]. Angewandte Chemie 2019, 131, 2300–2305. [Google Scholar] [CrossRef]
- Rodrigues J E F S, Escanhoela Jr C A, Fragoso B, et al. Experimental and theoretical investigations on the structural, electronic, and vibrational properties of Cs2AgSbCl6 double perovskite[J]. Industrial & Engineering Chemistry Research 2021, 60, 18918–18928. [Google Scholar]
- Wei F, Deng Z, Sun S, et al. Enhanced visible light absorption for lead-free double perovskite Cs2AgSbBr6[J]. Chemical Communications 2019, 55, 3721–3724. [Google Scholar] [CrossRef]
- Wang C F, Li H, Ji Q, et al. Discovery of a 2D Hybrid Silver/Antimony-Based Iodide Double Perovskite Photoferroelectric with Photostrictive Effect and Efficient X-Ray Response[J]. Advanced Functional Materials 2022, 32, 2205918. [Google Scholar] [CrossRef]
- Yin H, Xian Y, Zhang Y, et al. An Emerging Lead-Free Double-Perovskite Cs2AgFeCl6: In Single Crystal[J]. Advanced Functional Materials 2020, 30, 2002225. [Google Scholar] [CrossRef]
- Volonakis G, Haghighirad A A, Milot R L, et al. Cs2InAgCl6: a new lead-free halide double perovskite with direct band gap[J]. The journal of physical chemistry letters 2017, 8, 772–778. [Google Scholar] [CrossRef]
- Luo J, Li S, Wu H, et al. Cs2AgInCl6 double perovskite single crystals: parity forbidden transitions and their application for sensitive and fast UV photodetectors[J]. Acs Photonics 2018, 5, 398–405. [Google Scholar] [CrossRef]
- Liao Q, Chen J, Zhou L, et al. Bandgap engineering of lead-free double perovskite Cs2AgInCl6 nanocrystals via Cu2+-doping[J]. The Journal of Physical Chemistry Letters 2020, 11, 8392–8398. [Google Scholar] [CrossRef]
- Locardi F, Cirignano M, Baranov D, et al. Colloidal synthesis of double perovskite Cs2AgInCl6 and Mn-doped Cs2AgInCl6 nanocrystals[J]. Journal of the American Chemical Society 2018, 140, 12989–12995. [Google Scholar] [CrossRef]
- Jia H, Zhang X, Feng Z, et al. Na+-doped lead-free double perovskite Cs2AgInCl6 for broadband solar-blind UV detection[J]. Optical Materials 2022, 128, 112365. [Google Scholar] [CrossRef]
- Slavney A H, Leppert L, Saldivar Valdes A, et al. Small-band-gap halide double perovskites[J]. Angewandte Chemie 2018, 130, 12947–12952. [Google Scholar] [CrossRef]
- Roy M, Borkar H, Alam A, et al. Spontaneous anion-exchange synthesis of optically active mixed-valence Cs2Au2I6 perovskites from layered CsAuCl4 perovskites[J]. Chemical Communications 2021, 57, 1478–1481. [Google Scholar] [CrossRef]
- Kangsabanik J, Ghorui S, Aslam M, et al. Optoelectronic Properties and Defect Physics of Lead-Free Photovoltaic Absorbers Cs2AuIAuIIIX6 (X= I, Br)[J]. Physical Review Applied 2020, 13, 014005. [Google Scholar] [CrossRef]
- Ghosh B, Febriansyah B, Harikesh P C, et al. Direct Band Gap Mixed-Valence Organic–inorganic Gold Perovskite as Visible Light Absorbers[J]. Chemistry of Materials 2020, 32, 6318–6325. [Google Scholar] [CrossRef]
- Stoumpos C C, Malliakas C D, Kanatzidis M G. Semiconducting tin and lead iodide perovskites with organic cations: phase transitions, high mobilities, and near-infrared photoluminescent properties[J]. Inorganic chemistry 2013, 52, 9019–9038. [Google Scholar] [CrossRef]
- Leijtens T, Prasanna R, Gold-Parker A, et al. Mechanism of tin oxidation and stabilization by lead substitution in tin halide perovskites[J]. ACS Energy Letters 2017, 2, 2159–2165. [Google Scholar] [CrossRef]
- Han X, Liang J, Yang J H, et al. Lead-free double perovskite Cs2SnX6: facile solution synthesis and excellent stability[J]. Small 2019, 15, 1901650. [Google Scholar] [CrossRef]
- Ullah S, Wang J, Yang P, et al. Lead-Free Cs2SnI6 Perovskites for Optoelectronic Applications: Recent Developments and Perspectives[J]. Solar RRL 2021, 5, 2000830. [Google Scholar] [CrossRef]
- Krishnaiah M, Khan M M I, Kumar A, et al. Impact of CsI concentration, relative humidity, and annealing temperature on lead-free Cs2SnI6 perovskites: Toward visible light photodetectors application[J]. Materials Letters 2020, 269, 127675. [Google Scholar] [CrossRef]
- Huang J, Dong C, Mei Y, et al. The precursor-compensation strategy boosts the photoresponse performance of air-stable, self-powered Cs2SnI6 photodetectors[J]. Journal of Materials Chemistry C 2021, 9, 14217–14225. [Google Scholar] [CrossRef]
- Shen J, Zhu W, Lian Z, et al. Metal Ion-Incorporated Lead-Free Perovskites toward Broadband Photodetectors[J]. ACS Applied Electronic Materials 2023. [Google Scholar]
- Ye X, Liu A, Gao L, et al. Computational screening of Cs based vacancy-ordered double perovskites for solar cell and photocatalysis applications[J]. EcoMat 2022, e12295. [Google Scholar]
- Nouri Y, Hartiti B, Batan A, et al. The structural, mechanical, thermal, electronic and optical properties of halide perovskites Cs2TiX6 (X= Cl, Br, I): First-principles investigations[J]. Solid State Communications 2023, 115087. [Google Scholar]
- Chen M, Ju M G, Carl A D, et al. Cesium titanium (IV) bromide thin films based stable lead-free perovskite solar cells[J]. Joule 2018, 2, 558–570. [Google Scholar] [CrossRef]
- Ju D, Zheng X, Yin J, et al. Tellurium-based double perovskites A2TeX6 with tunable band gap and long carrier diffusion length for optoelectronic applications[J]. ACS Energy Letters 2018, 4, 228–234. [Google Scholar]
- Yoshihiro F, Hideko K, Ryuichi I. Molecular motion in methylammonium hexahalotellurates (IV) as studied by means of the pulsed nuclear magnetic resonance[J]. Bulletin of the Chemical Society of Japan 1981, 54, 103–108. [Google Scholar] [CrossRef]
- Guo J, Xu Y, Yang W, et al. High-stability flexible X-ray detectors based on lead-free halide perovskite Cs2TeI6 films[J]. ACS Applied Materials & Interfaces 2021, 13, 23928–23935. [Google Scholar]
- Zhao P, Su J, Guo Y, et al. A new all-inorganic vacancy-ordered double perovskite Cs2CrI6 for high-performance photovoltaic cells and alpha-particle detection in space environment[J]. Materials Today Physics 2021, 20, 100446. [Google Scholar] [CrossRef]
- Dong Q, Fang Y, Shao Y, et al. Electron-hole diffusion lengths> 175 μm in solution-grown CH3NH3PbI3 single crystals[J]. Science 2015, 347, 967–970. [Google Scholar] [CrossRef]
- Ye F, Lin H, Wu H, et al. High-quality cuboid CH3NH3PbI3 single crystals for high performance X-ray and photon detectors[J]. Advanced Functional Materials 2019, 29, 1806984. [Google Scholar] [CrossRef]
- Hooijer R, Weis A, Biewald A, et al. Silver-Bismuth Based 2D Double Perovskites (4FPEA)4AgBiX8 (X= Cl, Br, I): Highly Oriented Thin Films with Large Domain Sizes and Ultrafast Charge-Carrier Localization[J]. Advanced Optical Materials 2022, 10, 2200354. [Google Scholar] [CrossRef]
- Wu C, Du B, Luo W, et al. Highly efficient and stable self-powered ultraviolet and deep-blue photodetector based on Cs2AgBiBr6/SnO2 heterojunction[J]. Advanced Optical Materials 2018, 6, 1800811. [Google Scholar] [CrossRef]
- Yang J, Bao C, Ning W, et al. Stable, high-sensitivity and fast-response photodetectors based on lead-free Cs2AgBiBr6 double perovskite films[J]. Advanced Optical Materials 2019, 7, 1801732. [Google Scholar] [CrossRef]
- Li Y, Shi Z, Liang W, et al. Recent advances toward environment-friendly photodetectors based on lead-free metal halide perovskites and perovskite derivatives[J]. Materials Horizons 2021, 8, 1367–1389. [Google Scholar] [CrossRef]
- Shao D, Zhu W, Xin G, et al. Inorganic vacancy-ordered perovskite Cs2SnCl6: Bi/GaN heterojunction photodiode for narrowband, visible-blind UV detection[J]. Applied Physics Letters 2019, 115, 121106. [Google Scholar] [CrossRef]
- Li Y, Shi Z, Lei L, et al. Ultrastable lead-free double perovskite photodetectors with imaging capability[J]. Advanced Materials Interfaces 2019, 6, 1900188. [Google Scholar] [CrossRef]
- Lai Z, Wang F, Meng Y, et al. Solution-processed lead-free double perovskite microplatelets with enhanced photoresponse and thermal stability[J]. Sci. China Mater 2022, 65, 1313–1319. [Google Scholar] [CrossRef]
- Sakai N, Haghighirad A A, Filip M R, et al. Solution-processed cesium hexabromopalladate (IV), Cs2PdBr6, for optoelectronic applications[J]. Journal of the American Chemical Society 2017, 139, 6030–6033. [Google Scholar] [CrossRef]
- Shuang Z, Zhou H, Wu D, et al. Low-temperature process for self-powered lead-free Cs2AgBiBr6 perovskite photodetector with high detectivity[J]. Chemical Engineering Journal 2022, 433, 134544. [Google Scholar] [CrossRef]
- Zhang X, Liu X, Sun B, et al. Ultrafast, self-powered, and charge-transport-layer-free ultraviolet photodetectors based on sequentially vacuum-evaporated lead-free Cs2AgBiBr6 thin films[J]. ACS Applied Materials & Interfaces 2021, 13, 35949–35960. [Google Scholar]
- Lee J W, Bae S H, Hsieh Y T, et al. A bifunctional lewis base additive for microscopic homogeneity in perovskite solar cells[J]. Chem 2017, 3, 290–302. [Google Scholar] [CrossRef]
- Ji F, Boschloo G, Wang F, et al. Challenges and Progress in Lead-Free Halide Double Perovskite Solar Cells[J]. Solar RRL 2023, 2201112. [Google Scholar]
- Lei H, Hardy D, Gao F. Lead-free double perovskite Cs2AgBiBr6: fundamentals, applications, and perspectives[J]. Advanced Functional Materials 2021, 31, 2105898. [Google Scholar] [CrossRef]
- Fang Y, Zhang L, Wu L, et al. Pressure-induced emission (PIE) and phase transition of a two-dimensional halide double perovskite (BA)4AgBiBr8 (BA=CH3(CH2)3NH3+)[J]. Angewandte Chemie International Edition 2019, 58, 15249–15253. [Google Scholar] [CrossRef] [PubMed]
- Yao Y, Kou B, Peng Y, et al. (C3H9NI)4AgBiI8: a direct-bandgap layered double perovskite based on a short-chain spacer cation for light absorption[J]. Chemical Communications 2020, 56, 3206–3209. [Google Scholar] [CrossRef] [PubMed]
- Luo J, Wang X, Li S, et al. Efficient and stable emission of warm-white light from lead-free halide double perovskites[J]. Nature 2018, 563, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Euvrard J, Wang X, Li T, et al. Is Cs2TiBr6 a promising Pb-free perovskite for solar energy applications?[J]. Journal of Materials Chemistry A 2020, 8, 4049–4054. [Google Scholar] [CrossRef]
- Keshavarz M, Debroye E, Ottesen M, et al. Tuning the Structural and Optoelectronic Properties of Cs2AgBiBr6 Double-Perovskite Single Crystals through Alkali-Metal Substitution[J]. Advanced Materials 2020, 32, 2001878. [Google Scholar] [CrossRef]
- Lin Q, Wang Z, Young M, et al. Near-Infrared and Short-Wavelength Infrared Photodiodes Based on Dye–Perovskite Composites[J]. Advanced Functional Materials 2017, 27, 1702485. [Google Scholar] [CrossRef]
- Xu W, Guo Y, Zhang X, et al. Room-temperature-operated ultrasensitive broadband photodetectors by perovskite incorporated with conjugated polymer and single-wall carbon nanotubes[J]. Advanced Functional Materials 2018, 28, 1705541. [Google Scholar] [CrossRef]
- Wu G, Fu R, Chen J, et al. Perovskite/Organic Bulk-Heterojunction Integrated Ultrasensitive Broadband Photodetectors with High Near-Infrared External Quantum Efficiency over 70%[J]. Small 2018, 14, 1802349. [Google Scholar] [CrossRef]
- Shen L, Lin Y, Bao C, et al. Integration of perovskite and polymer photoactive layers to produce ultrafast response, ultraviolet-to-near-infrared, sensitive photodetectors[J]. Materials Horizons 2017, 4, 242–248. [Google Scholar] [CrossRef]
- Mak C H, Huang X, Liu R, et al. Recent progress in surface modification and interfacial engineering for high-performance perovskite light-emitting diodes[J]. Nano Energy 2020, 73, 104752. [Google Scholar] [CrossRef]
- Wu Y, Wang D, Liu J, et al. Review of interface passivation of perovskite layer[J]. Nanomaterials 2021, 11, 775. [Google Scholar] [CrossRef] [PubMed]
- Yang J, Wang Y, Huang L, et al. High-Efficiency and Stable Perovskite Photodetectors with an F4-TCNQ-Modified Interface of NiOx and Perovskite Layers[J]. The Journal of Physical Chemistry Letters 2022, 13, 3904–3914. [Google Scholar] [CrossRef]
- Gkini K, Orfanoudakis S, Tsipas P, et al. ZrCl4 for energy level alignment at the perovskite/TiO2 interface[J]. Electrochimica Acta 2022, 433, 141214. [Google Scholar] [CrossRef]
- Li Y, Xie H, Lim E L, et al. Recent progress of critical interface engineering for highly efficient and stable perovskite solar cells[J]. Advanced Energy Materials 2022, 12, 2102730. [Google Scholar] [CrossRef]
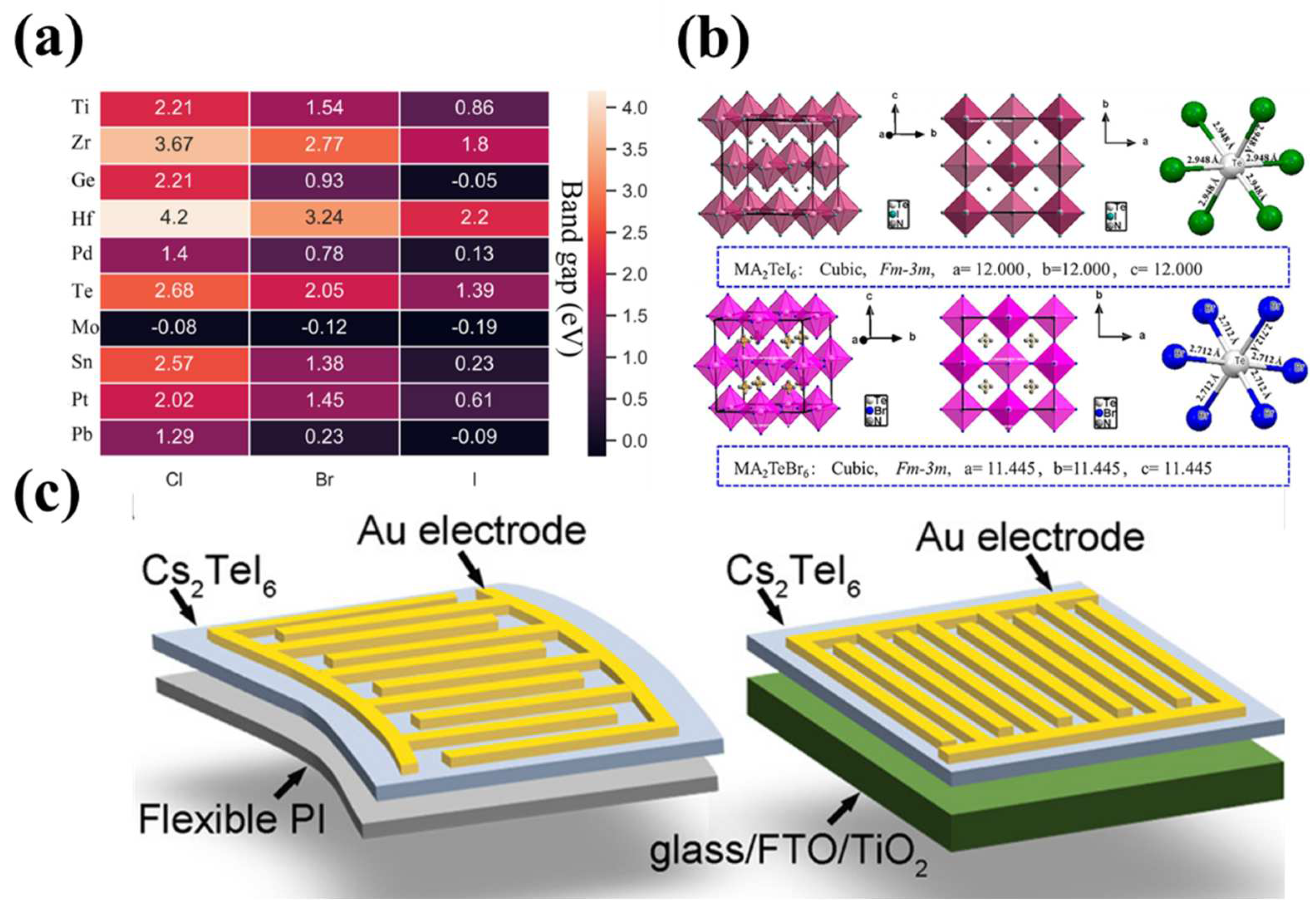
| Device configuration | Spectral range (nm) | R(A W-1) | D* (jones) | Response time (ms) | On/off ratio | Ref. |
|---|---|---|---|---|---|---|
| Au/Cs2AgBiBr6/Au | 300-800 | 7.01 | 5.66×1011 | 0.956/0.955 | 2.2×104 | 8 |
| ALD-NiOx modified FTO/Cs2AgBiBr6/TiO2/Au | 350-550 | - | 1.2×1013 | - | - | 9 |
| FTO/TiO2/Cs2AgBiBr6/CuSCN/Au | 300-600 | 0.34 | 1.03×1013 | 28.75/32.95 | - | 10 |
| FTO/SnO2/ZnO/Cs2AgBiBr6/Au | - | 0.608 | 2.97×1010 | 124/61 | - | 14 |
| FTO/Cs2AgBiBr6/Au | 350-500 | 9.8 | - | 1.2×10-3/0.5×10-3 | - | 21 |
| Au/Cs2AgInCl6/Au | 340–400 | 0.97 | ~1012 | 0.8/1.0 | ~500 | 46 |
| FTO/Cs2SnI6/FTO | - | 0.006 | 2×109 | - | - | 56 |
| FTO/c-TiO2/Cs2SnI6/Spiro OMeTAD/Au | 300-1000 | 0.001 | 6.03×1010 | 590/190 | 151 | 59 |
| FTO/TiO2/Cs2SnI6-Ni3+/TiO2/FTO | 350-950 | 160 | 4.52×1012 | - | - | 60 |
| FTO/TiO2/Cs2SnI6-Zn2+/TiO2/FTO | 350−900 | 710 | 1.56×1013 | 190/530 | - | 60 |
| Au/(4FPEA)4AgBiI8/Au | 400 | 0.002 | 5×108 | - | - | 70 |
| Au/(4FPEA)4AgBiI8/Au | 400 | 0.01 | 6×109 | - | - | 70 |
| ITO/Cs2AgBiBr6/SnO2/Au | 350 | 0.11 | 2.1×1010 | 2 | - | 71 |
| SnO2/Cs2AgBiBr6/TFB/Au | 300-550 | 0.14 | 3.3×1012 | 1.7×10-5 | - | 72 |
| FTO/Cs2SnI6/FTO | 500–900 | - | - | 100/100 | - | 73 |
| MWCNT/Cs2SnCl6:Bi/GaN | 350–400 | 0.208 | 1.2×1012 | 7.5×10-4/9.1×10-4 | - | 74 |
| In/GaN/Cs2AgBiBr6/Ag | 200–550 | 1.46 | 9.4×1012 | 3.463/8.442 | - | 75 |
| Au/Cs2AgBiBr6 microplatelets/Au | 450 | 0.245 | 1.3×1011 | 145×10-3/136×10-3 | 2.8×103 | 76 |
| Au/MA2AgBiBr6 microplatelets/Au | 450 | 0.058 | 2.9×1010 | - | 281 | 76 |
| ITO/Cs2PdBr6/Ag | - | - | - | - | - | 77 |
| Flexible ITO/SnO2/Cs2AgBiBr6/Carbon | - | 0.031 | 8.04×1011 | - | 0.5×104 | 78 |
| ITO/Cs2AgBiBr6/Ag | 375 | - | - | 6.13×10-3/28.02×10-3 | 6.6×103 | 79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





