1. Introduction
The essential component to neonatal adaptation after birth is the initiation of adequate respiratory effort. Approximately 10-15% of newborns require support for respiratory transition at birth, 3% require positive pressure ventilation by mask, 2% intubation and only < 1% cardiopulmonary resuscitation with chest compressions or ephinephrine to establish cardiorespiratory function [
1,
2]. The major cause for delivery room cardiopulmonary resuscitation is birth asphyxia, a condition of insufficient oxygen supply to vital organs that results in hypoxia, hypercarbia and metabolic acidosis and, if prolonged, may progress to multiorgan failure, including the developing brain [
3,
4]. Asphyxia may originate from prenatal, perinatal or postnatal pathology. Prenatal maternal pathologies that increase the risk for birth asphyxia include diabetes mellitus or gestational diabetes, arterial hypertension, placental insufficiency, pregnancy toxemia, eclamptic seizure, infections, or drugs. Perinatal risk factors are e.g., placental abruption, fetomaternal hemorrhage, amniotic fluid embolism, umbilical cord compression (knot or prolapse), insertio velamentosa of the umbilical cord, placenta previa, shoulder dystocia). Postnatal causes of birth asphyxia are fetal anemia due to twin-to-twin transfusion in monochoriotic twins or fetal isoimmunization, airway anomalies, neurologic disorders, severe cardiopulmonary disease, infection, congenital malformations, intrauterine growth retardation, medication effects).
Hypoxic-ischemic encephalopathy is a form of brain dysfunction (i.e. following brain injury) that occurs due to insufficient blood flow to the brain and/or insufficient oxygenation. The pattern of damage in HIE depends on the severity, duration, and reoccurrence of hypoxia-ischemia and may result in involvement of the deep gray matter (basal ganglia and thalami), brainstem, and/or brain white matter in various combinations [
5]. Hypoxic-ischemic encephalopathy is classified into three severity grades according to Sarnat et al. based on clinical symptoms. For the diagnosis of moderate or severe hypoxic-ischemic encephalopathy (grade II, III), at least three of the six categories (e.g., vigilance, activity, reflexes, muscle tone, apnea, seizures) must be met [
6]. Birth asphyxia accounts for 900.000 neonatal deaths worldwide annually and hypoxic-ischemic encephalopathy is estimated to cause up to a quarter of all postnatal deaths [
7,
8,
9]. In developed countries birth asphyxia occurs in 1.5-2.5 per 1000 live births and is one of the mayor causes for the development of cerebral palsy [
10].
Therapeutic hypothermia is the only evidence-based neuroprotective therapeutic intervention currently available and is standard of care in high income countries for moderate and severe hypoxic-ischemic encephalopathy (grade II, III). According to guidelines, therapeutic hypothermia is indicated in neonates ≥ 36+0 weeks of gestation if one of the following criteria is met: Umbilical cord pH or pH < 1h: ≤ 7,0; Apgar Score at 10 min ≤ 5; Base excess ≤ -16 mmol/l, ongoing cardiopulmonary resuscitation, ventilatory support at 10 min plus either a clinical sign of hypoxic-ischemic encephalopathy (see above) or pathological background activity on amplitude-integrated electroencephalogram (aEEG), such as low voltage and burst suppression [
11].
Therapeutic hypothermia improved the prognosis significantly: lower incidences of death, less severe cerebral palsy and epilepsy were reported in major randomized controlled trials on hypothermia [
12,
13,
14,
15]. However, mortality rates and the prevalence of severe disability are still high with 28% and 16-30%, respectively[
16,
17,
18].
In severe hypoxic-ischemic encephalopathy, early prognosis is essential for parental counseling and treatment decisions such as withdrawal of care. Currently available biomarkers and clinical parameters have in common that they require high personnel and technical resources. Furthermore, applicability and validity are limited in the first hours of life [
19]. In a situation of a life-threatening illness, as severe hypoxic-ischemic encephalopathy, accurate and reliable determination of organ dysfunction and mortality risk is urgently needed.
The Sepsis-related Organ Failure Assessment (SOFA) Score was designed to quantify organ dysfunction and mortality risk in adult intensive care patients with sepsis [
20,
21,
22]. In recent years its use is no longer limited to sepsis and the acronym is sometimes translated into
Sequential Organ Failure Assessment, reflecting the broad dissemination of the SOFA score.
The neonatal modification of the SOFA (nSOFA) was proposed to address the need for a consensus definition of neonatal sepsis in 2020 [
23]. nSOFA uses three objective and broadly available clinical parameters to quantify organ dysfunction: respiratory, cardiovascular, and hematological scores (total scores range from 0 to 15).
It has been previously used for predicting mortality and severe morbidity in preterm infants [
24], preterm infants with late onset sepsis [
25], respiratory distress syndrome (RDS) [
26] and neonates with proven sepsis [
23,
27].
The aim of the present study was to evaluate the accuracy of the nSOFA for predicting in-hospital mortality (sensitivity, specificity) following hypoxic-ischemic encephalopathy and therapeutic hypothermia.
2. Materials and Methods
2.1. Participants
For this retrospective study the charts of all neonates with a gestational age of ≥ 36+0 weeks were reviewed, who received therapeutic hypothermia for hypoxic-ischemic encephalopathy following birth asphyxia at the level III NICU of the University Hospital Essen, Germany between December 1st 2007 and January 31st 2023.
Therapeutic hypothermia was initiated based on clinical and laboratory criteria derived from large, randomized trials [12-15]. therapeutic hypothermia after birth asphyxia was officially recommended by the American Heart Association in 2010 followed by the German Guideline in 2013. The eligibility criteria for therapeutic hypothermia at our institution did not change in the evaluated time period and were as follows:
Birth asphyxia defined by at least one of the following criteria:
documented severe acidosis with a pH ≤ 7.00
base excess of ≤ -16 mmol/l (blood from umbilical cord or neonate within the first 60 minutes of life)
Apgar-Score ≤ 5 at 10 minutes of life
prolonged cardiorespiratory support (at least 10 minutes) consisting of chest compressions, intubation, bag and mask ventilation or continuous positive airway pressure (CPAP) and moderate to severe hypoxic-ischemic encephalopathy defined by at least one of the following:
modified Sarnat-Score ≥ 5 (hypoxic-ischemic encephalopathy Grade II and III)
clinical seizures
pathologic amplitude integrates electroencephalogram (aEEG, discontinuous normal voltage, low voltage, burst-suppression, flat trace, electroencephalographic seizures)
Contraindications for therapeutic hypothermia were life-threatening congenital malformation (e.g., diaphragmatic hernia, cerebral malformation), suspected metabolic disease, coagulopathy with active bleeding, cerebral hemorrhage, cerebral venous thrombosis, or hemorrhagic infarction, small for gestational age with birth weight < 1800g, severe pulmonary hypertension.
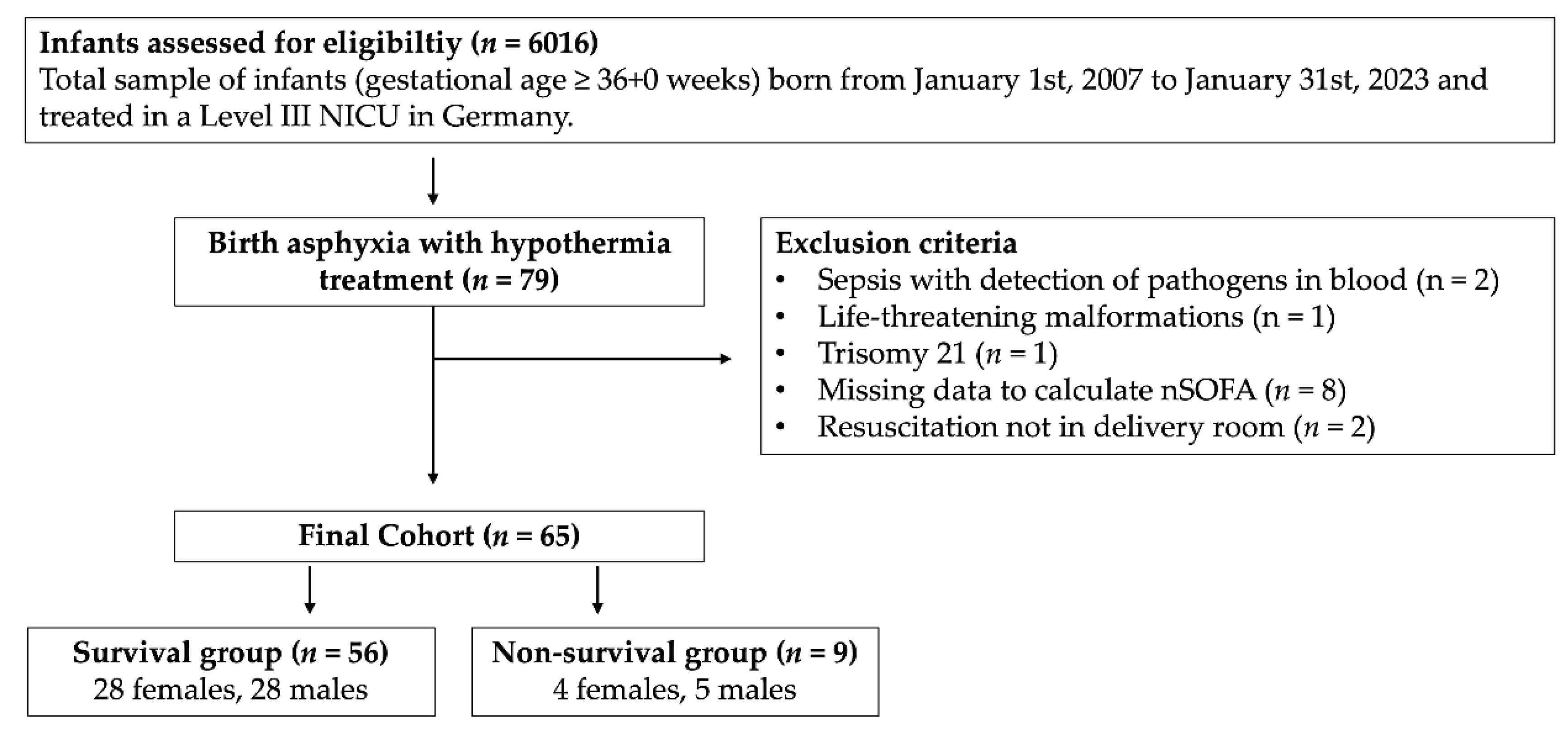
A summary of inclusion and exclusion criteria is presented in
Figure 1.
2.2. Therapeutic Hypothermia
Therapeutic hypothermia was delivered following a standardized protocol. Whole body hypothermia was initiated as early postnatally as possible but within 6 hours after birth. A target temperature of 33.5 to 34.5°C, which was monitored by continuous rectal probe. It was sustained for 72 hours followed by a rewarming phase. Temperature was increased 0.5°C per hour until 37.0°C were reached. Until 2015 this was done manually. After that a servo-controlled cooling mattress was used.
2.3. Calculation of nSOFA
The nSOFA is calculated from 3 subcategories for respiratory, cardiovascular and hematological status (
Table 1). The respiratory category takes the status of mechanical ventilation, oxygen saturation (SpO2) and fraction of inspired oxygen (FiO2) into account (score range 0-8). Cardiovascular status analyses the number of vasoactive drugs necessary to maintain normal blood pressure including the use of corticosteroids (score range 0-4). The hematologic score is based on the presence and severity of thrombocytopenia (score range 0-3). The total score can therefore range from 0 (best) to 15 (worst)[
23]. Data to calculate nSOFA in our cohort were derived from the patient charts, choosing the worst score value from the first 6 hours of life. Monitoring included continuous amplitude-integrated electroencephalogram (aEEG), Thompson-Score and 1-2 hourly vital signs including changes in ventilator settings. A complete blood count is included in the initial blood work on admission.
2.4. Limitation and Withdrawal of Care
Decision to withdraw care was based on a combination of clinical considerations, mainly lack of respiratory effort in ventilated infants, severe CNS injury on Magnetic resonance imaging (MRI) or sonography and severe organ dysfunction. Four of the non-surviving infants did receive MRI. The preferred method was extubation under sufficient analgo-sedation and/or weaning of cardiovascular support while maintaining enteral nutrition and warming for comfort.
2.5. Statistical Analysis
Analyses were performed using SPSS 29 (IBM Corp., New York, NY, USA). Patient demographics, clinical characteristics and nSOFA scores are presented as medians and ranges or interquartile ranges for continuous variables and for categorical variables as counts and category percentages. Mann-Whitney U tests and Fisher exact Test were used to compare continuous and non-continuous data, respectively. Two-sided p-values < 0.05 were considered statistically significant.
Logistic regression was performed to calculate the odds ratio for inhospital-mortality with the nSOFA score as regressor. A receiver operating characteristics curve (ROC) was generated to plot true positive rate (sensitivity) against false positive rate (1—specificity) across varying threshold settings and areas under the receiver operating characteristic curves (AUCs) were calculated. An optimal cut-off value for odds of in hospital-mortality by nSOFA was determined using the Youden index and closest top left methods. Positive and negative predictive value were calculated.
3. Results
Out of 6016 infants screened, 79 infants received therapeutic hypothermia. One infant, 37 +0 weeks of gestation, was included although the birth weight was 1745 g (contrary to recommendations of the American Heart Association) based on an individual treatment decision. We excluded 4 infants from the analysis, because of a baseline condition with influence on organ function or mortality other than hypoxic-ischemic encephalopathy: two infants with blood-culture proven early-onset sepsis, one infant with congenital malformation and one infant with chromosomal abnormality. Two infants had life-threatening congenital malformations, so they were included. Two infants were excluded since they did not suffer from birth asphyxia, but required resuscitation later than in the delivery room. 45 of 79 infants were inborn. Outborn infants were transferred to our institution and therapeutic hypothermia was commenced within the first 6 hours of life. From these, 3 infants were born outside of a hospital, which led to missing data in one case. 7 additional cases of outborn infants had to be excluded, because data to calculate nSOFA were not sufficient. This led to a final cohort of 65 infants with 21 outborn infants (survival cohort n = 16; non-survival cohort n = 5) (
Figure 1).
3.1. Clinical Characteristics
Survivors (
n = 56) and non-survivors (
n = 9) were similar with respect to gestational age, birth weight, small for gestational age, head circumference, child sex, umbilical cord pH and umbilical base excess (BE) (
Table 2).
Non-survivors had lower pH and BE in the first infant blood gas analysis, Apgar-Score at 1, 5, and 10 minutes and Thompson-Score. Non-survivors were more often born via c-section, or emergency c-section (
Table 2). One surviving infant was born as twin, there were no multiple births in the non-survival cohort. All but one infant received cardiopulmonary resuscitation and/or respiratory support during transition after birth. Non-survivors were more likely to have received cardiopulmonary resuscitation during postnatal care. There was the need for respiratory support in both groups. However, there were more intubations in the non-surviving group while temporary mask ventilation via t-piece was sufficient for stabilization in the group of survivors (
Table 2).
3.2. nSOFA Scores in Survivors and Non-Survivors
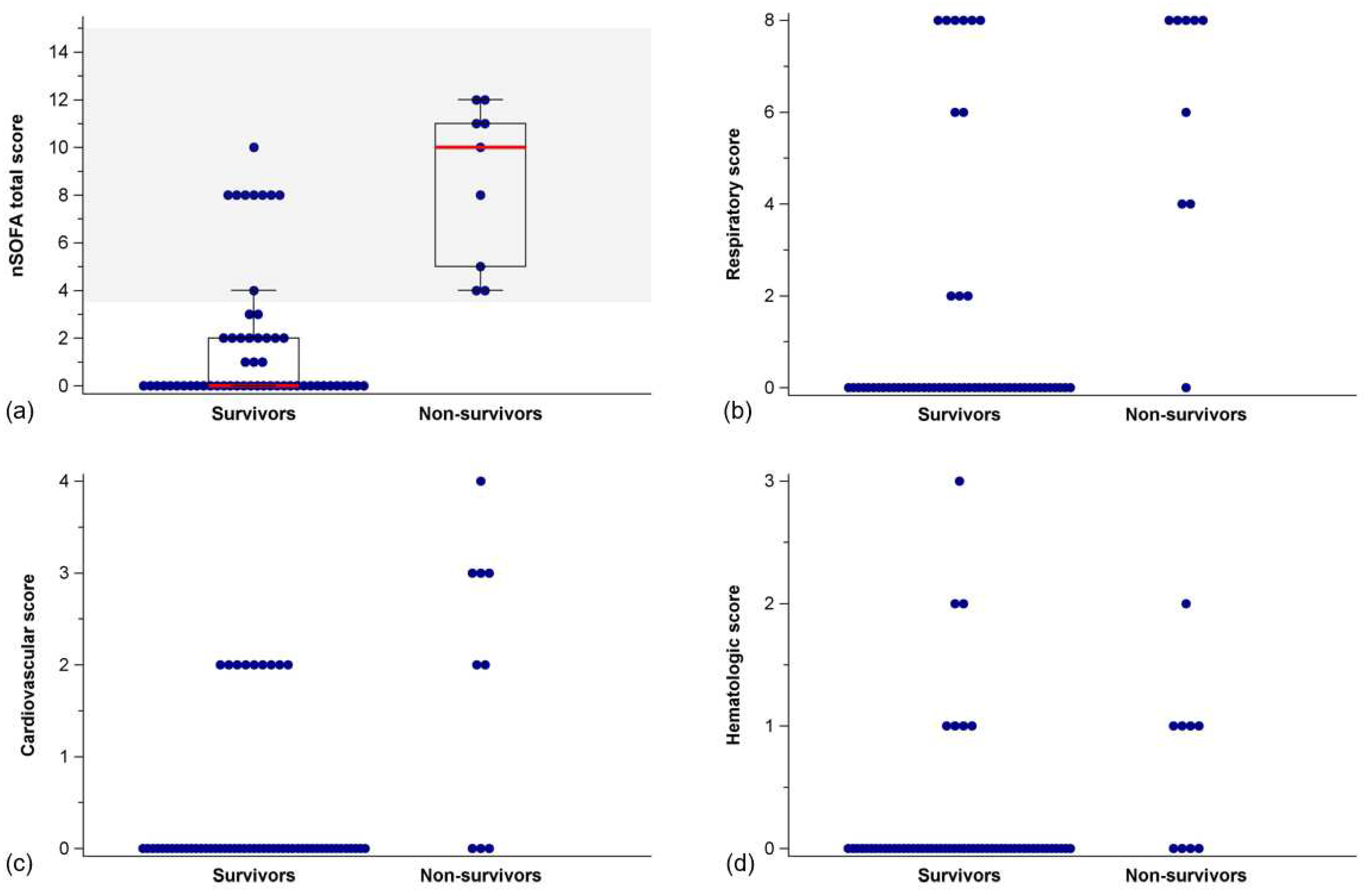
nSOFA sum scores were lower in survivors than in non-survivors (Mann-Whitney U test,
p < 0.001) (
Figure 2,
Table 3). This also held true for respiratory (
p < 0.001), cardiovascular (
p < 0.001), and hematologic sub scores (
p = 0.003), respectively (
Figure 2,
Table 3).
3.3. Prediction of in-Hospital Mortality by nSOFA
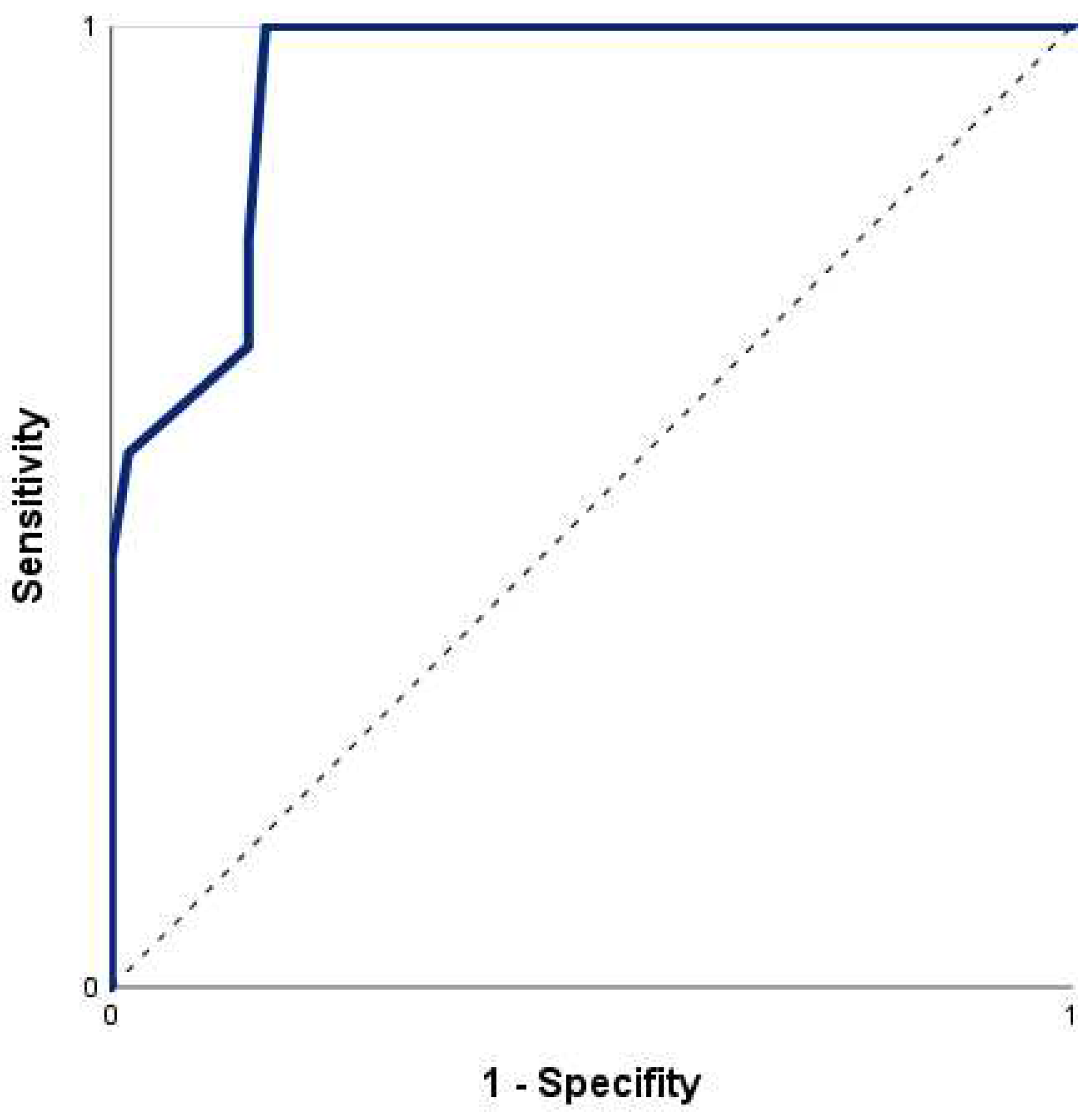
A significant relationship of nSOFA and mortality was confirmed with an odds ratio for mortality of 1.61 [95% CI = 1.24 - 2.08] per one-point increase in nSOFA score (Χ
2 (1) = 25.98,
p < 0.001, Nagelkerkes R
2 = 0.53). The ROC curve for risk of death by nSOFA (
Figure 3) had an area under the curve (AUC) of 0.94 (95% CI = 0.88 - 1.00). The optimal cut-off value of the nSOFA score according to Youden-Index and closest top left method was 3.5 (sensitivity 100.0%, specificity 83.9%). Using a cut-off of 3.5 points on the nSOFA score, the positive and negative predictive values were 50.0% and 100.0%, respectively (cross-tabulation in
Table 4).
4. Discussion
4.1. Prediction of in-Hospital Mortality
The nSOFA proved useful for predicting in-hospital mortality in neonates with hypoxic-ischemic encephalopathy undergoing therapeutic hypothermia. Non-survivors showed significantly higher sum scores, as well as respiratory, cardiovascular, and hematologic sub scores. A one-point increase in nSOFA increased the odds for in-hospital mortality by 1.6. None of the infants with a nSOFA score < 3.5 died in this cohort (negative predictive value: 100%). Thus, the nSOFA serves well as an operational definition of organ dysfunction identifying neonates at risk for death following birth asphyxia.
Despite the excellent negative predictive value of nSOFA scores < 3.5 several survivors had nSOFA scores of 8, limiting the positive predictive value of the cut-off value. This is likely due to the fact that the respiratory sub score has the highest weight in the nSOFA sum score, with mechanical ventilation contributing 8 points of the possible total of 15. All non-survivors and 41% of survivors were intubated and received mechanical ventilation within the first 6 hours of life, which resulted in respiratory sub scores of 2 to 8 points.
4.2. Respiratory and Cardiovascular Sub Scores
Factors contributing to the need for mechanical ventilation in hypoxic-ischemic encephalopathy and therapeutic hypothermia are manifold: Hypoxia at birth may prevent the onset of spontaneous breathing, lead to apnea and bradycardia, and prevent physiologic transition of circulation to extrauterine life, resulting in pulmonary hypertension and persistent fetal circulation [
3]. Therapeutic hypothermia exerts direct effects on respiration by increasing pulmonary vascular resistance and reducing oxygen consumption and release (hemoglobin dissociation) [
28]. Neuroactive medication, injury to the respiratory control center in the brain stem, and status epilepticus affect respiratory drive, potentially exacerbating hypoxia and disturbed CO
2 elimination [
29]. In a previous study it was shown that the need for mechanical ventilation was significantly higher in the group with severe asphyxia and unfavorable outcome (death and severe brain injury on MRI) compared to infants with better short term outcome [
30]. However, in this cohort, the need for mechanical ventilation without another sign of organ dysfunction was not inextricably associated with death, calling for caution when interpreting nSOFA scores in neonates on mechanical ventilation who are otherwise stable.
Because establishment of sufficient oxygenation and ventilation is the most important aspect of delivery room resuscitation, the fact that few neonates require chest compressions and/or administration of epinephrine should not be misinterpreted. A neonate who experiences birth asphyxia may still develop multi-organ failure and become life-threateningly ill due to the redistribution of cardiac output to vital organs such as the brain, myocardium, and adrenal gland. Reduced perfusion to the other organs may cause local hypoxia/ischemia and may result in organ failure [
31]. If birth asphyxia is prolonged, cardiovascular deterioration occurs that eventually causes myocardial dysfunction. The fact that the cardiovascular sub score showed fewer differences between survivors and non-survivors in this study may indicate that this cohort was less affected, or that cardiovascular impairment in asphyxia is less common than in sepsis, for which the nSOFA was originally developed for.
4.3. Hematologic Sub Score
Thombocytopenia may result from both asphyxia and therapeutic hypothermia. There is a reduced release of platelets from the bone marrow and an increased destruction of circulating platelets in birth asphyxia, platelet dysfunction under therapeutic hypothermia. The nadir of platelet count is on the 3
rd day of life following asphyxia and 5
th day of life under therapeutic hypothermia, suggesting an additive effect of therapeutic hypothermia [
32,
33,
34]. An influence of therapeutic hypothermia on the early nSOFA score is therefore unlikely. It is reasonable to assume that cardiovascular and hematological sub scores of the nSOFA increase during the acute phase of post-resuscitation treatment before dropping again.
4.4. Decision Making
However, early determination of organ dysfunction and risk of mortality is desirable. All infants in this study had hypoxic-ischemic encephalopathy as an expression of moderate to severe brain injury. In such a serious situation, objective and easily accessible prognostic markers can help in parental counseling and decision making.
Redirection of care is common on (N)ICUs for critically ill patients with poor prognosis either for survival, severe disabilities and a high burden of treatment or poor quality of life. The window of opportunity for decisions to limit therapy in severe hypoxic-ischemic encephalopathy exists while the infant is critically ill and physiologically unstable and therefore highly likely to die when treatment is withheld or withdrawn. The extend of the time window depends on the severity of the injury, the extent of cerebral edema, and the use of sedatives and anticonvulsants. Sarkar et al. demonstrated that 40% of infants with hypoxic-ischemic encephalopathy were extubated within the first 3 days [
35]. Physicians must consider the competing factors of prognostic certainty and this time window [
36]. In the cooling era, most deaths after elective extubation occurred within the first 72 hours in very unstable infants, whereas withdrawal of nutrition and hydration was a more difficult option in infants who survived the acute phase with severe brain damage and palliative care [
37]. Decisions about life-sustaining treatments or withdrawal of support are among the most sensitive and difficult conversations parents ever must face about their infant. Medico-ethical guidelines recommend shared decision making with parents in these situations [
38] and parents appreciated being fully informed about the medical situation and possible options, and especially appreciated being encouraged to ask questions [
39]. A recent study showed that both parents and physicians share the wish for certainty in end-of-life decisions [
40]. However, prognosis in hypoxic-ischemic encephalopathy is challenging.
4.5. Available Biomarkers
First-hour clinical parameters, such as umbilical cord pH and Apgar scores have limited reliability in predicting individual mortality. In addition, the Apgar includes subjective components with high inter-observer variability. Currently available biomarkers, physical examination, chemical, electrophysiological, and imaging studies, all have specific limitations. Clinical examinations require experience and may be influenced by treatment/medication. Chemical biomarkers, e.g. plasma biomarkers, proteomics and metabolomics, require specific lab resources and may respond delayed to the injury [
19]. Electrophysiology has a good predictive marker for abnormal brain activity, but it necessitates equipment, resources, and expertise [
41]. MRI examinations also involves a large amount of time and effort, the risk of transporting a critically ill infant and limited accuracy of early scans compared to those obtained at the end of the first week of life [
42]. The fact that only 4 of the non-surviving infants in this study received MRI highlights that they presented with such severe symptomatology that the extent of cerebral damage was either not necessary for decision making to discontinue therapy or the infants died despite full therapy. Existing neonatal critical illness scores are either designed for very preterm infants (Clinical risk index for Babies – CRIB I and II; Berlin Score) are too complex and inconvenient to use and /or variables are collected over a longer period of time – up to 24 hours after birth (Score for Neonatal Acute Physiology - (Perinatal Extension) - SNAP I/II and SNAP-PE I/II; (Extended) Sick Neonatal Score – (E)SNS)[
43,
44]. It is therefore desirable to have a very early and at the same time accurate assessment of prognosis, which can easily be performed without technical effort or special expertise.
4.6. Strengths and Limitations
The strength of our study lies in the fact that we were able to show for the first time that the nSOFA offers the potential to identify infants at risk of mortality following birth asphyxia and hypoxic-ischemic encephalopathy within the first 6 hours of life. The nSOFA is easy to apply, does not require a large number of human resources and no technical equipment. It is based on variables that can be objectified and measured even in low-resource settings. The nSOFA may serve as a valuable tool in the discussion of treatment termination after severe birth asphyxia with hypoxic-ischemic encephalopathy and therapeutic hypothermia.
The nSOFA has already proven its suitability as a predictor for unfavorable outcome in neonates diagnosed with sepsis. Therefore, neonates with sepsis, which must be considered a potential confounder, were excluded from our study. From clinical experience, a infants suffering from birth asphyxia may also have sepsis. However, our study had a small sample size, which limits the statistical power to allow for subgroup analysis.
Other limitations of this study need to be recognized. This single-center study was performed retrospectively over a long time period. Although inclusion criteria and major therapeutic regimes did not change, it cannot be excluded that neonatal intensive care of infants has changed slightly over time.
5. Future Aspects and Conclusions
Therefore, the results of this study should be prospectively replicated in multiple centers and larger samples to investigate influencing factors such as maternal diseases, delivery mode, socio-economic status, and fetal factors e.g., child sex, small for gestational age and sepsis. In addition, it may be of interest to explore the extent to which the nSOFA is helpful in future decision making and in counseling with parents. The nSOFA is easy to apply, measurable even in low-resource settings and can be used to identify infants at risk of in-hospital mortality due to asphyxia with hypoxic-ischemic encephalopathy and therapeutic hypothermia. Early accurate prognosis after asphyxia with hypoxic-ischemic encephalopathy and therapeutic hypothermia is essential to guide end-of-life decisions.
Author Contributions
Conceptualization, Anne-Kathrin Dathe, Anja Stein, Nora Bruns and Britta Hüning; Formal analysis, Anne-Kathrin Dathe and Nora Bruns; Investigation, Elena-Diana Craciun, Laura Tuda, Johanna Bialas and Maire Brasseler; Methodology, Anja Stein; Validation, Anne-Kathrin Dathe, Anja Stein, Johanna Bialas and Maire Brasseler; Writing – original draft, Anja Stein and Britta Hüning; Writing – review & editing, Nora Bruns and Ursula Felderhoff-Müser. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the University Duisburg-Essen (protocol code 18-8191-BO and 07.06.2018).
Informed Consent Statement
Patient consent was waived due to the retrospective analysis of charts on clinical routine data.
Data Availability Statement
The dataset used and/or analyzed for the study is available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Perlman, J.M.; Risser, R. Cardiopulmonary resuscitation in the delivery room. Associated clinical events. Arch. Pediatr. Adolesc. Med. 1995, 149, 20–25. [Google Scholar] [CrossRef]
- Madar, J.; Roehr, C.C.; Ainsworth, S.; Ersdal, H.; Morley, C.; Rudiger, M.; Skare, C.; Szczapa, T.; Te Pas, A.; Trevisanuto, D.; et al. European Resuscitation Council Guidelines 2021: Newborn resuscitation and support of transition of infants at birth. Resuscitation 2021, 161, 291–326. [Google Scholar] [CrossRef]
- Rainaldi, M.A.; Perlman, J.M. Pathophysiology of Birth Asphyxia. Clin. Perinatol. 2016, 43, 409–422. [Google Scholar] [CrossRef]
- Aziz, K.; Chadwick, M.; Baker, M.; Andrews, W. Ante- and intra-partum factors that predict increased need for neonatal resuscitation. Resuscitation 2008, 79, 444–452. [Google Scholar] [CrossRef]
- Volpe, J.J. Neonatal encephalopathy: An inadequate term for hypoxic-ischemic encephalopathy. Ann. Neurol. 2012, 72, 156–166. [Google Scholar] [CrossRef]
- Sarnat, H.B.; Sarnat, M.S. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch. Neurol. 1976, 33, 696–705. [Google Scholar] [CrossRef]
- Word Health Organisation, W. Available online:. Available online: https://www.who.int/teams/maternal-newborn-child-adolescent-health-and-ageing/newborn-health/perinatal-asphyxia (accessed on 26.01.2023).
- Liu, L.; Oza, S.; Hogan, D.; Chu, Y.; Perin, J.; Zhu, J.; Lawn, J.E.; Cousens, S.; Mathers, C.; Black, R.E. Global, regional, and national causes of under-5 mortality in 2000–2015: An updated systematic analysis with implications for the Sustainable Development Goals. Lancet 2016, 388, 3027–3035. [Google Scholar] [CrossRef]
- McIntyre, S.; Nelson, K.B.; Mulkey, S.B.; Lechpammer, M.; Molloy, E.; Badawi, N.; Newborn Brain Society, G.; Publications, C. Neonatal encephalopathy: Focus on epidemiology and underexplored aspects of etiology. Semin. Fetal Neonatal Med. 2021, 26, 101265. [Google Scholar] [CrossRef]
- Kurinczuk, J.J.; White-Koning, M.; Badawi, N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum. Dev. 2010, 86, 329–338. [Google Scholar] [CrossRef]
- Arbeitsgemeinschaft der Medizinischen Fachgesellschaften. S2k-Leitlinie/Hypothermiebehandlung des asphyktischen Neugeborenen. 2013, 024-023, 024–023.
- Simbruner, G.; Mittal, R.A.; Rohlmann, F.; Muche, R.; neo. n, E.n.T.P. Systemic hypothermia after neonatal encephalopathy: Outcomes of neo.nEURO.network RCT. Pediatrics 2010, 126, e771–778. [Google Scholar] [CrossRef]
- Azzopardi, D.V.; Strohm, B.; Edwards, A.D.; Dyet, L.; Halliday, H.L.; Juszczak, E.; Kapellou, O.; Levene, M.; Marlow, N.; Porter, E.; et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N. Engl. J. Med. 2009, 361, 1349–1358. [Google Scholar] [CrossRef]
- Shankaran, S.; Laptook, A.R.; Ehrenkranz, R.A.; Tyson, J.E.; McDonald, S.A.; Donovan, E.F.; Fanaroff, A.A.; Poole, W.K.; Wright, L.L.; Higgins, R.D.; et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N. Engl. J. Med. 2005, 353, 1574–1584. [Google Scholar] [CrossRef]
- Jacobs, S.E.; Morley, C.J.; Inder, T.E.; Stewart, M.J.; Smith, K.R.; McNamara, P.J.; Wright, I.M.; Kirpalani, H.M.; Darlow, B.A.; Doyle, L.W.; et al. Whole-body hypothermia for term and near-term newborns with hypoxic-ischemic encephalopathy: A randomized controlled trial. Arch. Pediatr. Adolesc. Med. 2011, 165, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jary, S.; Cowan, F.; Thoresen, M. Reduced infancy and childhood epilepsy following hypothermia-treated neonatal encephalopathy. Epilepsia 2017, 58, 1902–1911. [Google Scholar] [CrossRef]
- Tagin, M.A.; Woolcott, C.G.; Vincer, M.J.; Whyte, R.K.; Stinson, D.A. Hypothermia for neonatal hypoxic ischemic encephalopathy: An updated systematic review and meta-analysis. Arch. Pediatr. Adolesc. Med. 2012, 166, 558–566. [Google Scholar] [CrossRef]
- Perez, A.; Ritter, S.; Brotschi, B.; Werner, H.; Caflisch, J.; Martin, E.; Latal, B. Long-term neurodevelopmental outcome with hypoxic-ischemic encephalopathy. J. Pediatr. 2013, 163, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Ahearne, C.E.; Boylan, G.B.; Murray, D.M. Short and long term prognosis in perinatal asphyxia: An update. World J. Clin. Pediatr. 2016, 5, 67–74. [Google Scholar] [CrossRef]
- Seymour, C.W.; Liu, V.X.; Iwashyna, T.J.; Brunkhorst, F.M.; Rea, T.D.; Scherag, A.; Rubenfeld, G.; Kahn, J.M.; Shankar-Hari, M.; Singer, M.; et al. Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 762–774. [Google Scholar] [CrossRef]
- Shankar-Hari, M.; Phillips, G.S.; Levy, M.L.; Seymour, C.W.; Liu, V.X.; Deutschman, C.S.; Angus, D.C.; Rubenfeld, G.D.; Singer, M.; Sepsis Definitions Task, F. Developing a New Definition and Assessing New Clinical Criteria for Septic Shock: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 775–787. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Wynn, J.L.; Polin, R.A. A neonatal sequential organ failure assessment score predicts mortality to late-onset sepsis in preterm very low birth weight infants. Pediatr. Res. 2020, 88, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Berka, I.; Korcek, P.; Janota, J.; Stranak, Z. Neonatal Sequential Organ Failure Assessment (nSOFA) Score within 72 Hours after Birth Reliably Predicts Mortality and Serious Morbidity in Very Preterm Infants. Diagnostics (Basel) 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Fleiss, N.; Coggins, S.A.; Lewis, A.N.; Zeigler, A.; Cooksey, K.E.; Walker, L.A.; Husain, A.N.; de Jong, B.S.; Wallman-Stokes, A.; Alrifai, M.W.; et al. Evaluation of the Neonatal Sequential Organ Failure Assessment and Mortality Risk in Preterm Infants With Late-Onset Infection. JAMA Netw. Open 2021, 4, e2036518. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Guo, J.; Fu, M.; Liao, L.; Tu, J.; Xiong, J.; Liao, Q.; Chen, W.; Chen, K.; Liao, Y. Evaluation of the neonatal sequential organ failure assessment and mortality risk in neonates with respiratory distress syndrome: A retrospective cohort study. Front. Pediatr. 2022, 10, 911444. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, M.; Kumar, N. Utility of Neonatal Sequential Organ Failure Assessment (nSOFA) Score for Neonatal Mortality prediction. J. Neonatol. 2022, 36, 189–193. [Google Scholar] [CrossRef]
- Polderman, K.H. Mechanisms of action, physiological effects, and complications of hypothermia. Crit. Care Med. 2009, 37, S186–202. [Google Scholar] [CrossRef] [PubMed]
- Szakmar, E.; Jermendy, A.; El-Dib, M. Correction: Respiratory management during therapeutic hypothermia for hypoxic-ischemic encephalopathy. J. Perinatol. 2019, 39, 891. [Google Scholar] [CrossRef]
- Giannakis, S.; Ruhfus, M.; Markus, M.; Stein, A.; Hoehn, T.; Felderhoff-Mueser, U.; Sabir, H. Mechanical Ventilation, Partial Pressure of Carbon Dioxide, Increased Fraction of Inspired Oxygen and the Increased Risk for Adverse Short-Term Outcomes in Cooled Asphyxiated Newborns. Children (Basel) 2021, 8. [Google Scholar] [CrossRef]
- Jensen, A.; Garnier, Y.; Berger, R. Dynamics of fetal circulatory responses to hypoxia and asphyxia. Eur. J. Obstet. Gynecol. Reprod. Biol. 1999, 84, 155–172. [Google Scholar] [CrossRef]
- Christensen, R.D.; Baer, V.L.; Yaish, H.M. Thrombocytopenia in late preterm and term neonates after perinatal asphyxia. Transfusion 2015, 55, 187–196. [Google Scholar] [CrossRef]
- Boutaybi, N.; Razenberg, F.; Smits-Wintjens, V.E.; van Zwet, E.W.; Rijken, M.; Steggerda, S.J.; Lopriore, E. Neonatal thrombocytopenia after perinatal asphyxia treated with hypothermia: A retrospective case control study. Int. J. Pediatr. 2014, 2014, 760654. [Google Scholar] [CrossRef]
- Valeri, C.R.; Feingold, H.; Cassidy, G.; Ragno, G.; Khuri, S.; Altschule, M.D. Hypothermia-induced reversible platelet dysfunction. Ann. Surg. 1987, 205, 175–181. [Google Scholar] [CrossRef]
- Sarkar, S.; Barks, J.D.; Bhagat, I.; Donn, S.M. Effects of therapeutic hypothermia on multiorgan dysfunction in asphyxiated newborns: Whole-body cooling versus selective head cooling. J. Perinatol. 2009, 29, 558–563. [Google Scholar] [CrossRef]
- Wilkinson, D. The window of opportunity for treatment withdrawal. Arch. Pediatr. Adolesc. Med. 2011, 165, 211–215. [Google Scholar] [CrossRef]
- Al Amrani, F.; Racine, E.; Shevell, M.; Wintermark, P. Death after Birth Asphyxia in the Cooling Era. J. Pediatr. 2020, 226, 289–293. [Google Scholar] [CrossRef]
- American Academy of Pediatrics Committee on, F.; Newborn; Bell, E. F. Noninitiation or withdrawal of intensive care for high-risk newborns. Pediatrics 2007, 119, 401–403. [Google Scholar] [CrossRef]
- Beyer, M.F.; Kuehlmeyer, K.; Mang, P.; Flemmer, A.W.; Fuhrer, M.; Marckmann, G.; de Vos, M.; Schouten, E.S. "We Absolutely Had the Impression That It Was Our Decision"-A Qualitative Study with Parents of Critically Ill Infants Who Participated in End-of-Life Decision Making. Children (Basel) 2022, 10. [Google Scholar] [CrossRef]
- Zaal-Schuller, I.H.; Geurtzen, R.; Willems, D.L.; de Vos, M.A.; Hogeveen, M. What hinders and helps in the end-of-life decision-making process for children: Parents' and physicians' views. Acta Paediatr. 2022, 111, 873–887. [Google Scholar] [CrossRef]
- Murray, D.M.; Boylan, G.B.; Ryan, C.A.; Connolly, S. Early EEG findings in hypoxic-ischemic encephalopathy predict outcomes at 2 years. Pediatrics 2009, 124, e459–467. [Google Scholar] [CrossRef]
- Thayyil, S.; Chandrasekaran, M.; Taylor, A.; Bainbridge, A.; Cady, E.B.; Chong, W.K.; Murad, S.; Omar, R.Z.; Robertson, N.J. Cerebral magnetic resonance biomarkers in neonatal encephalopathy: A meta-analysis. Pediatrics 2010, 125, e382–395. [Google Scholar] [CrossRef] [PubMed]
- Dorling, J.S.; Field, D.J.; Manktelow, B. Neonatal disease severity scoring systems. Arch. Dis. Child. Fetal Neonatal. Ed. 2005, 90, F11–16. [Google Scholar] [CrossRef] [PubMed]
- Garg, B.; Sharma, D.; Farahbakhsh, N. Assessment of sickness severity of illness in neonates: Review of various neonatal illness scoring systems. J. Matern. Fetal Neonatal Med. 2018, 31, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).