1. Introduction
Nutrition is the critical aspect that influences the productivity and profitability of the aquaculture industry. Commercial diets are formulated with the most available and cost-effective raw materials, considering their nutritional value, nutrient balance and bioavailability. The main challenge in feed formulation is fulfilling the gap between the nutrient requirement of animals and the available nutrient content in the feed. Fortification or enrichment of the diet with specific individual nutrients or blends of nutrients can fulfill the nutrient deficits while improving the growth and health performance of farm animals [
1]. Moreover, when raised in high-density culture conditions, fish are highly susceptible to infectious diseases, which can cause significant economic losses for the farm. Strengthening fish immunity can reduce the risk of emerging and spreading sudden disease outbreaks during immune-deprived conditions. Dietary immunostimulants supplementation is beneficial in improving fish immune responses by enhancing the activity of immune cells such as phagocytes and lymphocytes and the production of cytokines and antibodies [
2]. Naturally originated substances such as plant extracts, prebiotics and probiotics have been shown to convey promising outcomes as immunostimulants [
3,
4]. Numerous studies have shown that specific nutrients including amino acids, fatty acids, nucleotides, vitamins and minerals significantly boost animal immune functions [
5,
6].
Nucleotides are a conditionally essential nutrient in diets for fishes since they are vital in energy metabolism, DNA and RNA synthesis, encoding genetic information, physiological mediators in signal transduction and being a component of coenzymes, cellular agonists and allosteric effectors [
7,
8,
9]. Nucleotides are synthesized in the cell using amino acids, folic acid and CO
2, but this process is metabolically costly [
10]. Previous studies showed that dietary supplementation of nucleotides improved the feed acceptance, growth performance, intestinal structure and immune responses in numerous fish species including olive flounder,
Paralichthys olivaceus [
11], Nile tilapia,
Oreochromis niloticus [
12], Atlantic salmon,
Salmo salar [
13] and common carp,
Cyprinus carpio [
14]. Burrells et al. [
15] showed that the inclusion of nucleotide with β-glucan in the diet for rainbow trout,
Oncorhynchus mykiss, reduced the cumulative mortality by approximately 18% during the bacterial challenge against
Vibrio anguillarum. Therefore, exogenous nucleotide supplementation is essential when aquatic animals are in fast-growing stages or under tissue injuries and stress conditions [
16].
Vitamins and provitamin sources have been studied for fish species since the early twentieth century, and they are required in trace amounts to maintain the growth and health of animals [
17,
18,
19]. Vitamin C (L-ascorbic acid) is an essential micronutrient required for antioxidant activities, immune competence and disease resistance, stress tolerance, steroidogenesis in ovarian follicle cells, connective tissue formation, wound healing and normal physiological functions in fish [
9,
20]. However, external vitamin C sources are essential for teleost fish since they are unable to
de novo synthesize vitamin C owing to the lack of L-gulonolactone oxidase in the vitamin C biosynthesis pathway [
21]. Insufficient levels of dietary vitamin C were reported to cause deficiency symptoms in fish, such as poor growth, reduced immunity, skin hemorrhage, exophthalmia, anorexia, anemia, scoliosis, lordosis, shortened operculum and increased mortality [
22,
23]. In addition, vitamins C and E are hydrophilic and lipophilic antioxidants that scavenge ROS in aqueous and lipidic environments, respectively [
19]. Vitamin E, α-tocopherol, is an important cell membrane component in preventing the oxidation of polyunsaturated membrane phospholipids [
24]. Through the antioxidant activity of vitamin E, vitamin C reduces tocopheroxyl radical back to α-tocopherol, which is known as the synergistic effect between vitamins C and E [
9,
25]. Lee et al. [
26] observed significantly increased weight gain (WG), health improvement and reduced liver mercury deposition with dietary selenomethionine, vitamins C and E. Vitamin E is an essential nutrient for cell membrane formation, maintaining the functional and structural integrity of cells, growth, immunity, reproduction and preventing muscle degeneration [
24,
27]. Vitamin E deficiency in fish can cause white muscle fiber necrosis, anemia, depigmentation, erythropoiesis and ceroid pigmentation in the liver [
9].
Glucans are a group of polysaccharides derived from the cell wall materials of plants, fungi, algae and bacteria [
28]. Furthermore, they are branched or unbranched polymers comprised of β (1,4) and/or β (1,3) linked glucose-monomer units [
29]. Rodrigues et al. [
30] reported that β-glucan administration through different routes, such as dietary supplementation, immersion in baths and injections, improved fish immune responses and stress tolerance. β-glucan triggers host immunity against several pathogen infections since it can act as a pathogen-associated molecular pattern (PAMP) [
31]. The interaction between PAMP and pattern recognition receptors of monomorphonuclear phagocytes and neutrophils of the host can activate phagocytosis, microbial killing, cytokinin production and innate immune memory [
32,
33]. Dietary supplementation of β-glucan is known to significantly alter the gut bacterial populations and reduce the pathogen infections of
Streptococcus iniae,
Aeromonas salmonicida, and
A. hydrophila [
34,
35]. Previous studies have shown that β-glucan improved feed utilization and growth in red seabream (
Pagrus major) and Pacific white shrimp (
Litopenaeus vannamei) [
97,
98]. Especially, β-gluten plays a vital role in improving innate immunity, including lysozyme, ACH50, respiratory burst activity and immune- and cytokine production-related gene expressions in fishes [
35,
36,
37,
38].
Olive flounder is the most important mariculture species in South Korea, representing approximately 50% of the country's production [
39]. However, due to disease outbreaks, a wide range of antibiotics has been used to control infectious diseases, leading to drug-resistant bacteria and remnants of antibiotics in fish products and water contamination [
40]. Dietary immunostimulants supplementation can be a promising strategy to minimize antibiotic usage in the industry. Further, new approaches are required to improve nutritional formulations that aim to simultaneously promote growth and health for the progress of the aquaculture industry. Therefore, the objective of this study was to evaluate the effect of dietary supplementation of a functional immunostimulant mixture (FIM), including nucleotide, β-glucan and vitamins C and E, on growth performance, feed utilization, morphometric indices, intestinal histology, hematological parameters, digestive enzyme activity, innate immunity, antioxidant capacity and inflammation-related gene expressions of olive flounder.
3. Results
During the 12-week feeding trial, fish readily accepted all the experimental diets. Fish fed HB1.5 diet showed significantly higher (
P < 0.05) final body weight (FBW), WG and specific growth rate (SGR) than fish fed the control and HB0.5 diets (
Table 3). Growth-related gene expressions of the fish were significantly upregulated (
P < 0.05) with dietary supplementation of FIM. Compared to the fish fed the control and HB0.5 diets, the relative expression of IGF-1 was significantly upregulated (
P < 0.05) in fish fed HB1.0 and HB1.5 diets. Moreover, significantly upregulated (
P < 0.05) IGF-BP gene expressions were observed in HB1.0 and HB1.5 groups than in the control group. Feed conversion ratio (FCR) was significantly lower (
P < 0.05) in HB1.5 group compared to the control group, but other feed utilization parameters, FI, and protein efficiency ratio (PER) were not significantly affected (
P > 0.05) by FIM. Survival and morphometric parameters including condition factor (CF), hepatosomatic index (HSI) and viscerosomatic index (VSI) showed no significant differences (
P > 0.05) among the dietary treatments.
Blood hemoglobin hematocrit and plasma total protein levels were significantly increased (
P < 0.05) in the fish fed HB1.5 diet than in those fed the control diet (
Table 4). Blood glucose level was significantly reduced (
P < 0.05) in HB1.5 group compared to the control and HB0.5 groups. Plasma triglyceride and cholesterol levels were not affected (
P > 0.05) by FIM. The dietary supplementation of the FIM significantly improved (
P < 0.05) almost all the immune parameters and antioxidant enzyme activities (
Table 5). Fish fed HB1.0 and HB1.5 showed significantly higher (
P < 0.05) lysozyme activity than the control diet. Total immunoglobulin level of the fish was significantly higher (
P < 0.05) in HB1.5 group than in the control and HB0.5 groups. However, antiprotease activity showed no significant differences (
P > 0.05) among dietary groups. HB1.5 group showed a significantly higher (
P < 0.05) NBT activity than the control. MPO activity was significantly improved (
P < 0.05) in the fish of HB groups. SOD and catalase activities were significantly increased (
P < 0.05) with 1.5% FIM inclusion into the control diet. The fish fed HB1.0 and HB1.5 diets showed significantly higher (
P < 0.05) GPx activity than those fed the control and HB0.5 diets and HB0.5 group showed significantly increased GPx activity compared to the control group. Moreover, the dietary supplementation of the FIM at 1.5% in the control diet significantly upregulated (
P < 0.05) TLR-3 and perforin gene expressions.
The anti-inflammatory gene expression of TGF-β was significantly increased (
P < 0.05) with FIM supplementation at 1.5% (
Figure 1). Pro-inflammatory gene expressions of IL-6 and TNF-α were not significantly affected (
P > 0.05) but tended to be down-regulated with increasing dietary FIM supplementation levels.
Digestive enzyme activities are presented in
Table 6. Amylase activity was significantly enhanced (
P < 0.05) at 1.0 or 1.5% of FIM inclusion levels. However, lipase, pepsin, trypsin and chymotrypsin activities were not significantly affected (
P > 0.05). Whole-body compositions of moisture, crude protein, crude lipid and ash were not significantly affected (
P > 0.05) with dietary FIM supplementation (
Table 7). Fish fed HB1.0 and HB1.5 diets showed significantly increased (
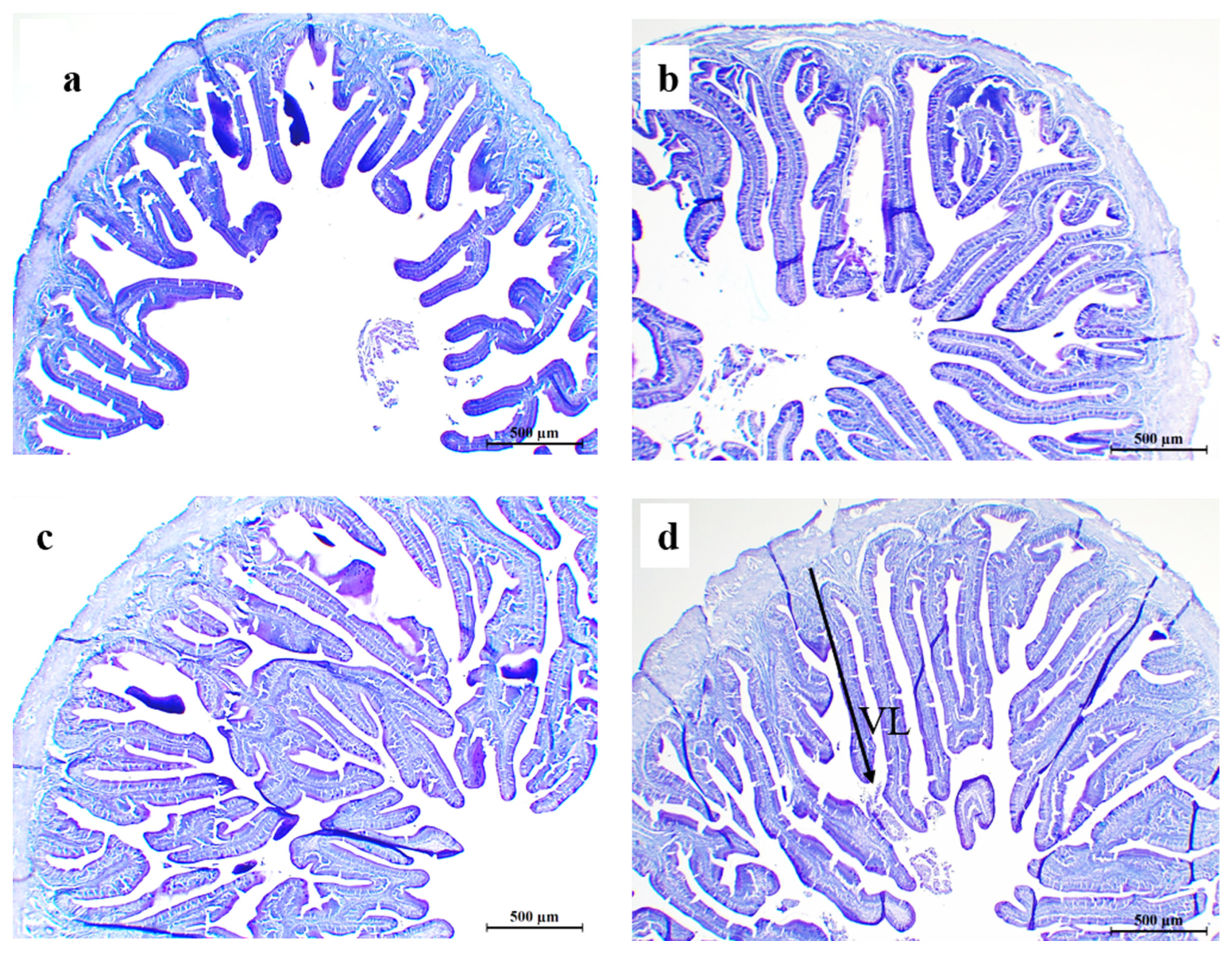
P < 0.05) villus height compared to fish fed the control and HB0.5 diets (
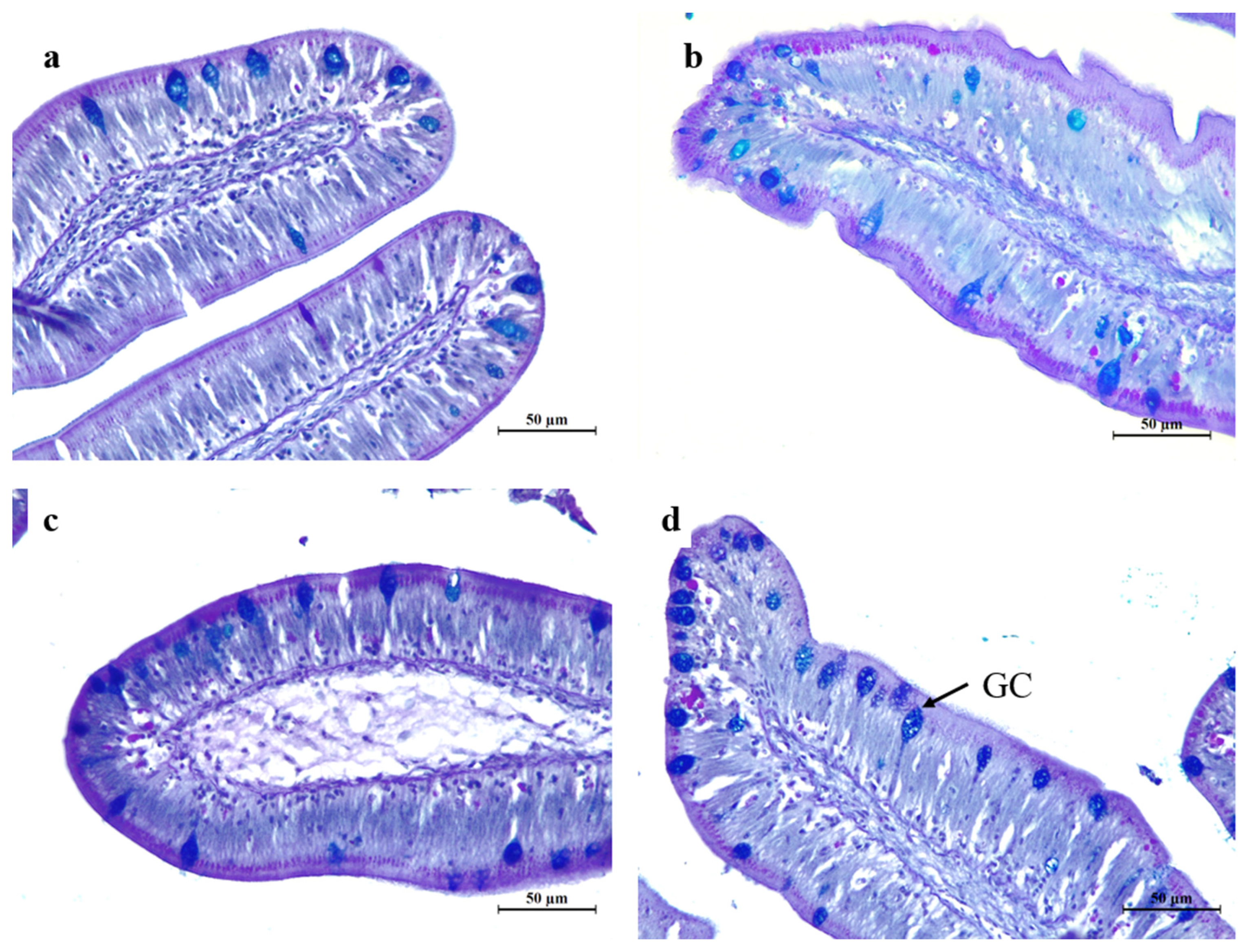
Table 8). The goblet cell counts were significantly higher (
P < 0.05) in fish fed HB1.0 and HB1.5 diets than those fed the control diet. Representative histology images of intestinal villus heights and goblet cell distribution are shown in
Figure 2 and
Figure 3.
4. Discussion
The dietary FIM for juvenile olive flounder showed increased growth, feed utilization and growth-related gene expression. Chuchird et al. [
6] observed increased growth and feed efficiency in Pacific white shrimp fed the same FIM mixture-containing diet. Hossain et al. [
57] reported that exogenous nucleotide supplementation promoted the growth of red seabream and Borda et al. [
58] suggested that increased growth could be attributed to cell proliferation stimulating ability of nucleotides. In our previous study, the inclusion of 0.1-0.4% inosine monophosphate in a fish meal-based diet enhanced the growth and health of olive flounder [
11]. Dietary nucleotide supplementation has shown enhanced growth in turbot (
Scophthalmus maximus), Nile tilapia, and rainbow trout [
59,
60,
61]. Metailler et al. [
59] reported that the growth performances of turbot larvae were significantly increased with 0.77% nucleotide supplementation in diet assuming that this could be attributed to improved FI with increased diet palatability. Moreover, exogenous nucleotide supplementation can save metabolic energy costs, which could beneficially affect fish growth and health [
10]. However, Li et al. [
62] showed that the effect of dietary nucleotide supplementation on the growth of sub-adult or adult fish was limited compared to larval stages. Corresponding to the present study, Asaduzzaman et al. [
12] found that dietary supplementation of 0.2-0.4% inosine monophosphate significantly upregulated the IGF-1 and IGF-2 gene expressions in Nile tilapia. IGF-1 and IGF-BP genes play a vital role in cell growth by enhancing glucose and alanine uptake, myoblast proliferation and DNA, protein, and glycogen synthesis [
63,
64]. Therefore, enhanced growth-related gene expressions with dietary FIM supplementation might have influenced the growth performance in this study. Dietary FIM improved feed conversion, which might correlate with improved intestinal morphology. Previous studies demonstrated that dietary nucleotides and β-glucan increased villus heights and absorptive surface area, suppressed intestinal damage and promoted beneficial bifidobacterial colonization in many fishes including Atlantic salmon [
13], European sea bass (
Dicentrarchus labrax L) [
65], turbot [
66] and red seabream [
58]. Bueno et al. [
67] showed that exogenous nucleotide supplementation improved intestinal ultrastructure compared to natural nucleotide synthesis. Therefore, improved intestinal morphology of olive flounder may have beneficially affected the feed utilization and nutrient uptake.
The FIM supplementation in the diet significantly affected hematological parameters including hemoglobin, hematocrit, total protein and glucose level of the fish. Cao et al. [
68] observed that dietary β-glucan incorporation significantly reduces the blood glucose level by downregulating the gene expression of sodium-glucose transporter-1 in the intestinal mucosa. Hematocrit and hemoglobin concentrations were significantly increased with the increasing FIM level. Tahmasebi-Kohyani et al. [
61] observed that dietary nucleotide supplementation increased hemoglobin, hematocrit and red and white blood cell counts. They suggested this may be due to an increased iron absorption with nucleotide supplementation. Further, they observed increased blood albumin and globulin levels with dietary nucleotide supplementation. Garcia et al. [
69] observed an erythrocyte-protecting effect through dietary vitamins C and E supplementations. Vitamins C and E contained in FIM might have increased the erythrocyte proliferation to obtain the above observations. Thereby it could promote efficient oxygen transportation in the body.
Lysozyme and antiprotease activities are vital in destroying bacteria by cell wall lysis and inhibiting bacterial proteases by trapping the enzymes or attaching to their binding sites. Notably, these enzyme activities were increased with dietary FIM supplementation in this study. Carver et al. [
70] and Gil [
71] showed that dietary nucleotide supplementation influences macrophage and natural killer cell activation, enhancing lysozyme activity. Song et al. [
11] observed that 0.2-0.4% inosine monophosphate supplementation significantly improved the lysozyme activity and disease resistance of olive flounder against
S. iniae. Moreover, dietary nucleotide supplementation significantly affected immunoglobulin production by increasing lymphocyte production in fish [
72,
73]. Supporting the above findings, Leonardi et al. [
74] observed that supplementation of nucleotides in Atlantic salmon diet showed significantly improved antibody production. An oral administration of β-glucan dramatically increased immunoglobulin in fish by binding to the β-glucan receptors of macrophages, leukocytes, neutrophils and NK cells [
75,
76]. Rodrigues et al. [
30] reported that the binding of β-glucan to relevant receptors acting as a PAMP stimulates all the immune activities including phagocytosis and the production of interferon, cytokine and immunoglobulin. Therefore, the increased immunoglobulin content in the present study might be partially attributed to the β-glucan contained in the FIM mixture.
Respiratory burst activity, antioxidative activity and immune parameters were significantly improved with the graded levels of FIM supplementation. Nitroblue tetrazolium activity measures the intercellular superoxide concentration and the MPO enzyme converts hydrogen peroxide into hypochlorous acid in phagocytes during phagocytosis [
77,
78]. Dietary nucleotide supplementation improved phagocytic activities in fishes, including olive flounder and rainbow trout [
11,
71,
79]. Carver et al. [
70] reported that nucleotide supplementation in rats significantly increased the natural killer cell activation and macrophage activity. In a systematic review, Dawood and Kohio [
23] stated that vitamins C and E supplementation in the fish diet has beneficial effects on respiratory burst, phagocytic, lysozyme, and complement activities. However, the underlying mechanism for increasing respiratory burst activity by dietary supplementation of nucleotides, β-glucan and vitamins C and E, or combined effect, remains to be revealed.
In this study, the antioxidant activity in olive flounder was significantly increased. Similarly, Tie et al. [
80] observed upregulated Nrf2 gene expression, which is crucial in initiating the antioxidant enzyme gene expression in grass carp,
Ctenopharyngodon Idella. They suggested that an increased nutrient availability with dietary nucleotide supplementation might have upregulated those gene expressions. Increased serum SOD, GPx and catalase activities were reported in red seabream, turbot and Nile tilapia when fed diets enriched with nucleotide over 0.15% [
57,
67,
81]. Supplementation of β-glucan in the diet also improved antioxidant enzyme activities in immune-suppressed tilapia exposed to atrazine herbicides [
82].
TLR is a PAMP-recognizing protein encoded by the TLR-3 gene, and it mediates cytokine production and activates inflammatory responses [
83]. Several studies have found that dietary supplementation of β-glucan or bacterial cell wall components (peptidoglycan and lipopolysaccharides) increases TLR-3 gene expression, emphasizing the importance of incorporating these compounds in fish feeds as a prophylactic method [
84,
85]. Therefore, dietary β-glucan supplementation might have significantly increased the TLR-3 gene expression in this study. Perforin is a cytosolic protein present in T-lymphocytes and natural killer cells and it involves membrane pore-formation triggering calcium influx and initiating the cell death of targeted infected or damaged cells [
86]. Therefore, upregulation of perforin gene expression may be beneficial to renew the intestinal epithelium and protect olive flounder from pathogenic or parasitic infections.
According to
Table 6, digestibility enzyme activities in the gut were increased with dietary FIM supplementation for olive flounder, even so, only the amylase activity showed a significant improvement. Hunt et al. [
87] observed significantly increased digestive enzyme activities in rainbow trout fed with a yeast-based nucleotide-incorporated diet, including pepsin, trypsin and lipase activities. They suggested that improved intestinal structure and increased surface area and villi density might have increased the digestive enzyme activities through dietary nucleotide supplementations. Bueno et al. [
67] showed that 0.25% dietary nucleotide supplementation significantly developed cellular ultrastructure and intestinal morphological characteristics in weaned rats. A similar mechanism might have led to enhancing the digestive enzyme activities in this study, but the exact influence of nucleotides, β-glucan and vitamins C and E supplementation on the digestive enzyme activity is yet to be revealed. Dietary β-glucan supplementation increases the gut digestive enzyme-secreting bacterial population by regulating the chyme viscosity [
88]. However, information on the digestive enzyme production of bacteria in the intestine and their biological significance is limited.
Previous studies have shown that dietary β-glucan increases short-chain fatty acid assimilation by increasing bifidobacterial populations,
Lactobacilli and
Akkermansia spp and alleviating colonization of detrimental bacterial species in the intestine. Furthermore, these beneficial bacteria species increase acetate, propionate and butyrate fatty acid production by the fermentation of non-digestible oligosaccharides including β-glucans [
89,
90]. However, Medagoda and Lee [
91] showed body fatty acid profile correlate with the dietary fatty acid profile. Thus, the increased whole-body lipid content might have been due to the β-glucan contained in the FIM in the present study.
Nucleotide supplementation was reported to improve the intestinal structure including increased villus height, lateral branching of the villus, mucosal height, gut wall thickness and enterocyte and microvillus height in fishes [
12,
66,
92,
93]. Bueno et al. [
67] revealed that nucleotides develop and recover the intestinal structure after the lactose-induced chronic diarrhea condition in rats suggesting that exogenous nucleotide supplementation spares the cost of metabolic energy in DNA and RNA synthesis in regenerating tissues. Hess and Greenberg [
94] reported that exogenous nucleotide supplementation is essential for intestinal tissue development since nucleotide demand is high in rapidly regenerating tissues. In addition, previous studies have shown that dietary nucleotide and β-glucan supplementation significantly increased the growth of gut health improving beneficial microflora and stimulating mucus secretion from goblet cells [
58,
95,
96]. Accordingly, the enrichment of nucleotide in the diet might have improved intestinal morphology.
In conclusion, the enrichment of nucleotides, Saccharomyces cerevisiae derived β-glucan and vitamins C and E as an FIM in the diet for olive flounder significantly increased the growth by improving feed efficiency, intestinal morphology and growth-related gene expression. Additionally, fish health was promoted by increasing the innate immune parameters, phagocytic activity and antioxidant capacity and upregulating anti-inflammation and immune-related gene expressions. In the present study, the highest two inclusion levels (1-1.5%) showed significantly higher growth and health performance. However, further investigations should design to determine the effect of even higher levels of the FIM (> 1.5%) in the diet of olive flounder and the interactive effect of different nutrient combinations focusing on disease resistance and diet digestibility.
Table 1.
Formulation and proximate compositions of the experimental diets for juvenile olive flounder, Paralichthys olivaceus.
Table 1.
Formulation and proximate compositions of the experimental diets for juvenile olive flounder, Paralichthys olivaceus.
| Ingredients (%, DM basis) |
control |
HB0.5 |
HB1.0 |
HB1.5 |
| Fish meal, Sardine1
|
62.00 |
62.00 |
62.00 |
62.00 |
|
Functional immunostimulant mixture2
|
- |
0.50 |
1.00 |
1.50 |
| Wheat flour3
|
11.90 |
11.40 |
10.90 |
10.40 |
| Starch |
6.00 |
6.00 |
6.00 |
6.00 |
| Tankage meal |
3.50 |
3.50 |
3.50 |
3.50 |
| Soybean meal |
5.00 |
5.00 |
5.00 |
5.00 |
| Corn gluten |
3.00 |
3.00 |
3.00 |
3.00 |
| Wheat gluten |
2.00 |
2.00 |
2.00 |
2.00 |
| Fish oil4
|
4.50 |
4.50 |
4.50 |
4.50 |
| Monocalcium phosphate |
0.50 |
0.50 |
0.50 |
0.50 |
| Lecithin5
|
0.50 |
0.50 |
0.50 |
0.50 |
| Choline chloride |
0.10 |
0.10 |
0.10 |
0.10 |
| Mineral Mix6
|
0.50 |
0.50 |
0.50 |
0.50 |
| Vitamin Mix7
|
0.50 |
0.50 |
0.50 |
0.50 |
| Proximate composition (%) |
|
|
|
|
| Crude protein |
55.79 |
56.20 |
56.65 |
56.90 |
| Crude lipid |
11.36 |
11.86 |
11.61 |
11.99 |
| Ash |
11.76 |
11.78 |
11.92 |
12.04 |
| L-ascorbic acid (g/kg) |
0.24 |
0.40 |
0.80 |
1.27 |
| DL- tocopheryl acetate (g/kg) |
0.11 |
0.20 |
0.30 |
0.41 |
Table 2.
Amino acid compositions (%AA/CP) of the experimental diets formulated to contain graded levels of functional immunostimulant mixture.
Table 2.
Amino acid compositions (%AA/CP) of the experimental diets formulated to contain graded levels of functional immunostimulant mixture.
| %Amino acid/Crude protein |
control |
HB0.5 |
HB1.0 |
HB1.5 |
| Essential amino acids |
|
|
|
|
| Arginine |
5.65 |
5.05 |
5.92 |
5.51 |
| Histidine |
2.15 |
2.11 |
2.35 |
2.39 |
| Isoleucine |
3.46 |
3.36 |
4.02 |
3.54 |
| Leucine |
6.89 |
6.38 |
7.15 |
6.99 |
| Lysine |
5.00 |
5.28 |
5.96 |
6.21 |
| Methionine |
2.48 |
2.26 |
2.54 |
2.48 |
| Phenylalanine |
3.82 |
3.57 |
4.01 |
3.86 |
| Threonine |
3.98 |
3.63 |
3.99 |
3.97 |
| Valine |
4.14 |
3.93 |
4.67 |
4.18 |
| Non-essential amino acids |
|
|
|
|
| Alanine |
5.87 |
5.38 |
6.01 |
5.90 |
| Aspartic acid |
7.70 |
7.39 |
8.23 |
7.99 |
| GABA |
0.02 |
0.02 |
0.02 |
0.02 |
| Glutamic acid |
15.50 |
14.34 |
16.02 |
15.54 |
| Glycine |
5.72 |
5.36 |
5.85 |
5.92 |
| Proline |
5.07 |
4.00 |
4.15 |
2.89 |
| Serine |
3.97 |
3.65 |
3.97 |
4.02 |
| Taurine |
1.06 |
0.97 |
0.92 |
1.09 |
| Tyrosine |
2.62 |
2.38 |
2.69 |
2.59 |
Table 3.
Growth performance, feed utilization, growth-related gene expression and biometric indices of olive flounder, Paralichthys olivaceus, fed graded levels of functional immunostimulant mixture-included diets for 12 weeks.
Table 3.
Growth performance, feed utilization, growth-related gene expression and biometric indices of olive flounder, Paralichthys olivaceus, fed graded levels of functional immunostimulant mixture-included diets for 12 weeks.
| Treatment |
control |
HB0.5 |
HB1.0 |
HB1.5 |
| FBW1
|
177±6b
|
180±1b
|
182±2ab
|
188±2a
|
| WG2
|
571±24b
|
584±5b
|
592±8ab
|
613±7a
|
| SGR3
|
2.12±0.04b
|
2.14±0.01b
|
2.15±0.01ab
|
2.18±0.01a
|
| IGF-14
|
1.00±0.00b
|
1.20±0.48b
|
1.97±0.38a
|
2.09±0.36a
|
| IGF-BP5
|
1.00±0.00b
|
1.71±0.49ab
|
2.38±0.95a
|
2.14±0.72a
|
| FI6
|
137±2 |
137±1 |
138±4 |
139±1 |
| FCR7
|
0.91±0.04a
|
0.89±0.01ab
|
0.88±0.02ab
|
0.86±0.01b
|
| PER8
|
1.99±0.09 |
2.00±0.02 |
2.02±0.05 |
2.04±0.03 |
| Survival |
97.0±3.6 |
99.0±1.5 |
99.0±1.5 |
100±0 |
| CF9
|
1.03±0.02 |
1.03±0.01 |
1.04±0.01 |
1.07±0.01 |
| HSI10
|
1.56±0.12 |
1.57±0.16 |
1.58±0.10 |
1.61±0.08 |
| VSI11
|
4.27±0.10 |
4.28±0.27 |
4.29±0.20 |
4.29±0.23 |
Table 4.
Hematological parameters of olive flounder, Paralichthys olivaceus, fed graded levels of functional immunostimulant mixture-included diets for 12 weeks.
Table 4.
Hematological parameters of olive flounder, Paralichthys olivaceus, fed graded levels of functional immunostimulant mixture-included diets for 12 weeks.
| Treatment |
Hb1
|
Ht2
|
Tg3
|
Chol4
|
TP5
|
Glucose6
|
| control |
4.20±0.24b
|
29.2±2.2b
|
528±62 |
242±29 |
3.61±0.43b
|
75.6±0.6a
|
| HB0.5 |
4.65±0.30ab
|
32.8±1.9ab
|
527±54 |
246±20 |
3.88±0.25ab
|
76.0±3.3a
|
| HB1.0 |
4.58±0.31ab
|
33.2±2.1ab
|
524±54 |
245±25 |
3.98±0.33ab
|
72.9±2.4ab
|
| HB1.5 |
4.96±0.35a
|
34.8±2.1a
|
523±56 |
269±19 |
4.26±0.30a
|
70.5±1.8b
|
Table 5.
Non-specific immune responses, immune-related gene expressions and antioxidant enzyme capacity of olive flounder, Paralichthys olivaceus, fed graded levels of functional immunostimulant mixture-included diets for 12 weeks.
Table 5.
Non-specific immune responses, immune-related gene expressions and antioxidant enzyme capacity of olive flounder, Paralichthys olivaceus, fed graded levels of functional immunostimulant mixture-included diets for 12 weeks.
| Treatment |
Lysozyme1
|
Antiprotease2
|
Ig3
|
NBT4
|
MPO5
|
SOD6
|
Catalase7
|
GPx8
|
TLR-39
|
Perforin10
|
| control |
92.6±1.0b
|
24.4±2.5 |
14.7±3.5c
|
0.845±0.07b
|
0.99±0.12b
|
61.2±3.1b
|
15.2±2.6b
|
51.7±4.8c
|
1.00±0.00b
|
1.00±0.00b
|
| HB0.5 |
95.4±4.2ab
|
25.2±5.0 |
16.0±6.2bc
|
0.943±0.14ab
|
1.25±0.13a
|
68.5±7.4ab
|
17.2±2.2ab
|
66.9±5.9b
|
1.72±0.90ab
|
1.50±0.60ab
|
| HB1.0 |
97.3±2.3a
|
27.2±3.9 |
23.3±4.2ab
|
0.964±0.14ab
|
1.27±0.15a
|
70.2±6.0ab
|
18.9±2.4ab
|
81.9±9.7a
|
1.52±0.75ab
|
1.94±0.94ab
|
| HB1.5 |
98.5±0.8a
|
27.0±1.0 |
25.6±3.5a
|
1.022±0.12a
|
1.48±0.14a
|
77.6±7.0a
|
21.1±1.8a
|
92.1±5.4a
|
2.15±0.67a
|
2.23±0.69a
|
Table 6.
Digestive enzyme activity of olive flounder, Paralichthys olivaceus, fed graded levels of functional immunostimulant mixture-included diets for 12 weeks.
Table 6.
Digestive enzyme activity of olive flounder, Paralichthys olivaceus, fed graded levels of functional immunostimulant mixture-included diets for 12 weeks.
| Treatment |
Lipase1
|
Pepsin2
|
Trypsin3
|
Chymotrypsin4
|
Amylase5
|
| control |
11.0±0.6 |
6.11±0.71 |
0.028±0.004 |
0.0050±0.0009 |
18.6±2.6b
|
| HB0.5 |
11.7±1.2 |
5.59±0.76 |
0.028±0.005 |
0.0052±0.0006 |
21.0±1.9ab
|
| HB1.0 |
12.4±0.8 |
6.73±0.73 |
0.029±0.007 |
0.0053±0.0005 |
23.9±2.0a
|
| HB1.5 |
12.5±1.6 |
6.77±0.51 |
0.029±0.007 |
0.0060±0.0002 |
24.4±1.8a
|
Table 7.
Whole-body composition (% wet basis) of olive flounder, Paralichthys olivaceus, fed graded levels of functional immunostimulant mixture-included diets for 12 weeks.
Table 7.
Whole-body composition (% wet basis) of olive flounder, Paralichthys olivaceus, fed graded levels of functional immunostimulant mixture-included diets for 12 weeks.
| Treatment |
Moisture |
Crude protein |
Crude lipid |
Crude ash |
| control |
71.8±0.8 |
19.4±0.8 |
4.39±0.87 |
3.57±0.47 |
| HB0.5 |
71.8±1.0 |
20.1±1.3 |
4.40±0.77 |
3.31±0.47 |
| HB1.0 |
71.8±0.6 |
19.3±1.0 |
5.03±0.84 |
3.16±0.55 |
| HB1.5 |
71.0±1.0 |
19.9±2.0 |
6.06±1.49 |
3.20±0.57 |
Table 8.
Villus height and goblet cell count of the intestine of olive flounder, Paralichthys olivaceus, fed graded levels of functional immunostimulant mixture-included diets for 12 weeks.
Table 8.
Villus height and goblet cell count of the intestine of olive flounder, Paralichthys olivaceus, fed graded levels of functional immunostimulant mixture-included diets for 12 weeks.
| Treatment |
Villus height (nm) |
Goblet cell count |
| control |
1027±59c
|
833±10c
|
| HB0.5 |
1201±88b
|
926±137ab
|
| HB1.0 |
1362±62a
|
1052±130a
|
| HB1.5 |
1369±72a
|
1068±62a
|
Table 9.
Sequences of primer pairs used for RT-qPCR in this study.
Table 9.
Sequences of primer pairs used for RT-qPCR in this study.
| Target Gene |
Forward primer sequences (5’ to 3’) |
Reverse primer sequences (5’ to 3’) |
Accession number |
| IGF-I1
|
GCCACACCCTCTCACTACTGCT |
GCCTCTCTCTCCACACACAAAC |
AF061278.1 |
| IGF-BP2
|
GGGACCCTGTCATGTTGAACTCC |
CAGAGACGAATCGCACTGCTTGG |
XM_020105928.1 |
| TLR-33
|
AACGCCTGGTTCATCAAGTG |
CGAATGTCGAAGTGCAAGAG |
AB109394 |
| Perforin |
AGCATGTGAGCAAGTTCTGTCT |
GGC ATGACGGGACACATAC |
AB084905.1 |
| IL-64
|
CTCTATCACAGATGCCGACTTGTCCT |
ACCTCCTGCTCCTCACCTGAAA |
DQ884914.3 |
| TNF-α5
|
ACCCTTGCACAATCACACACTCAC |
AAAGTGGTTGGCGGTGCAGA |
AB040449.1 |
| TGF-β6
|
GGGAGTGGATAAGTGGGAGGTTGT |
AGCTCCTCTTTGCTCCCAGTTTCA |
XM_020113775.1 |
| 18S rRNA |
GACTCAACACGGGAAACCTCA |
CAGACAAATCGCTCCACCAA |
EF126037 |
Figure 1.
Inflammatory gene expressions of interleukine-6 (IL-6), tumor necrosis factor-α (TNF-α) and transforming growth factor-β (TGF-β) in the liver of olive flounder (Paralichthys olivaceus) fed graded levels of functional immunostimulant mixture included diets for 12 weeks. Data represent the means of relative gene expression values of five biological replicates in each dietary treatment; error bars indicate the standard deviation. Bars with different letters indicate significant differences (P < 0.05). Abbreviations: control, a basal diet; HB0.5, HB1.0 and HB1.5 are 0.5, 1.0 and 1.5% functional immunostimulant mixture (Rovimax® HB Ultra) supplemented diets.
Figure 1.
Inflammatory gene expressions of interleukine-6 (IL-6), tumor necrosis factor-α (TNF-α) and transforming growth factor-β (TGF-β) in the liver of olive flounder (Paralichthys olivaceus) fed graded levels of functional immunostimulant mixture included diets for 12 weeks. Data represent the means of relative gene expression values of five biological replicates in each dietary treatment; error bars indicate the standard deviation. Bars with different letters indicate significant differences (P < 0.05). Abbreviations: control, a basal diet; HB0.5, HB1.0 and HB1.5 are 0.5, 1.0 and 1.5% functional immunostimulant mixture (Rovimax® HB Ultra) supplemented diets.
Figure 2.
Representative histological images of Alcian blue (pH 2.5), periodic acid, and Schiff reagent stained cross-sections of proximal intestine tissue depicting the micro-morphological characteristics of the intestine (×10) of olive flounder, Paralichthys olivaceus, fed the graded levels of functional immunostimulant mixture included diets for 12 weeks. a - control; b - HB0.5; c - HB1.0, 1; d - HB1.5; and VL - Villus length. Abbreviations: control, a basal diet; HB0.5, HB1.0 and HB1.5 are 0.5, 1.0 and 1.5% functional immunostimulant mixture (Rovimax® HB Ultra) supplemented diets.
Figure 2.
Representative histological images of Alcian blue (pH 2.5), periodic acid, and Schiff reagent stained cross-sections of proximal intestine tissue depicting the micro-morphological characteristics of the intestine (×10) of olive flounder, Paralichthys olivaceus, fed the graded levels of functional immunostimulant mixture included diets for 12 weeks. a - control; b - HB0.5; c - HB1.0, 1; d - HB1.5; and VL - Villus length. Abbreviations: control, a basal diet; HB0.5, HB1.0 and HB1.5 are 0.5, 1.0 and 1.5% functional immunostimulant mixture (Rovimax® HB Ultra) supplemented diets.
Figure 3.
Representative histological images of Alcian blue, periodic acid, and Schiff reagent stained cross-sections of proximal intestine tissue depicting the goblet cell distribution of the intestine cross-sections (×40) of olive flounder, Paralichthys olivaceus, fed the graded levels of functional immunostimulant mixture included diets for 12 weeks. a - control; b - HB0.5; c - HB1.0, 1.0%; d - HB1.5; and GC – Goblet cell. Abbreviations: control, a basal diet; HB0.5, HB1.0 and HB1.5 are 0.5, 1.0 and 1.5% functional immunostimulant mixture (Rovimax® HB Ultra) supplemented diets.
Figure 3.
Representative histological images of Alcian blue, periodic acid, and Schiff reagent stained cross-sections of proximal intestine tissue depicting the goblet cell distribution of the intestine cross-sections (×40) of olive flounder, Paralichthys olivaceus, fed the graded levels of functional immunostimulant mixture included diets for 12 weeks. a - control; b - HB0.5; c - HB1.0, 1.0%; d - HB1.5; and GC – Goblet cell. Abbreviations: control, a basal diet; HB0.5, HB1.0 and HB1.5 are 0.5, 1.0 and 1.5% functional immunostimulant mixture (Rovimax® HB Ultra) supplemented diets.