Introduction
Paint is a term used to describe a number of synthetic substances commonly used to provide texture, protection and aesthetic value to infrastructure, furniture and utensil of everyday life (1). With a brush, roller, or spray gun, paint is applied in a thin coat to various surfaces such as wood, metal, or stone (2). Samples of the first known paintings, made between 20,000 and 25,000 years ago, survive in caves in France and Spain. There are many different types of paints; each with a different set of characters, these include primers, house paints, masonry paints, marine paints and fire retardants (3). They have different basic qualities which include consistency, opacity(hiding power), spreading capacity, adhesion and durability. Primarily, it is composed of pigments, binders, solvents and certain additives. Paints are found either in the form of Emulsion or Oil based formulations (4). Organic chemicals such as volatile organic compounds (VOCs) are used in paints as solvents (5). These solvents may cause short and long-term environmental impacts (6), and may lead to respiratory, allergic, or immunogenic defects in humans (7,21)
Water-based paints are paints formulated on the base of water as the major solvent serving as a vehicle carrying the solid components (binders, pigments and additives). In most cases, water-based coatings contain up to 80% water with small quantities of other solvents, such as glycol ethers. Water-based paints commonly contain up to 15% of Hydrocarbon solvents, which control the paint's viscosity and wettability. Water-based paints are non-toxic and non-combustible, but they are characterized by long drying time due to the slow evaporation rate of water (8).
Paints often lose their aesthetic and protective value as a result of microbial activities whether in-can or on the dry paint film. Owing to the fact that microorganisms have a simple approach to life; they use whatever is available as a food source, attach themselves to practically all surfaces, multiply and build biomass provided that favourable environmental conditions (humidity, temperature, light, and, to a lesser extent pH) are met (9). The various organic materials of paints represent a carbon source for practically all species of microorganisms and act as nutrients to stimulate microbial growth both in-can and on the dry paint film. This seriously compromises the adhesion and durability of the paint as well as its decorative function (10). The types of microbial attack implicated are bacterial, fungal and algal (11). Bacteria, which are small, single-celled organisms, grow readily in unpreserved water-based systems(23,25).
A wide range of aerobic and anaerobic bacterial strains including Bacillus brevis, Bacillus laterosporus, Bacillus polymyxa, Lactobacillus gasseri, Lactobacillus brevis, Proteus mirabilis, Escherichia coli, Bacteroides, Clostridium, Desulphovibrio and Bifidobacterium have been isolated in water-based paints (2,12,). Some other studies have also identified some viruses from paints(26;
This study was therefore saddled with the objective to isolate and Identify the bacteria associated with fresh water-based paint and determining the predominant bacteria implicated in the biodeterioration of water-based paint
Methods
Collection of Samples
A total of 6 fresh paint samples (A, B, C, D, E and F) were collected into different sterile capped containers from a paint manufacturing industry in Lagos, Nigeria. The samples were transported aseptically to the laboratory, stored at room temperature and subjected to microbiological analysis fortnightly.
Isolation Procedures
Having prepared a ten-fold dilution series, 1ml of each sample was added to 9ml of sterile distilled water and serial dilution was carried out to 10-7. An inoculum size of 1µl(0.1ml) from dilution factor 10-7 was plated onto the surface of the entire agar directly using the spread plate method. The dilution factor 10-7 was plated only for weeks zero and two, while 10-5 was used for the subsequent weeks. The inoculated Nutrient agar plates were incubated in an inverted position aerobically at 37°C for 24 hours. After incubation, the number of resulting colonies was counted and characterized based on shape, size, margin, elevation, pigmentation, consistency, optical characteristics and colony surface. Distinct bacterial colonies were subcultured onto other agar plates to get the pure culture of isolates. Pure isolates were stored in slants and For identification of bacteria, the colonies were submitted to the Gram stain, and the following biochemical tests: oxidase and oxidation-fermentation (OF). The organisms were confirmed subsequently using Analytical Profiled Index (API) kit 20NE and 20E.
Automated Biochemical Identification Using Analytical Profile Index (API)
API test strips consists of microtubes (cupules) containing dehydrated substrates to detect the enzymatic activity or the assimilation/fermentation of sugars by the inoculated organisms. During incubation, metabolism produces colour changes that are either spontaneous or revealed by the addition of reagents. When the carbohydrates are fermented, the pH within the cupule changes and is shown by an indicator. Assimilation tests are inoculated with a minimal medium (API AUX medium) and the bacteria grow if they are able to utilize the corresponding substrate: a positive result is indicated by growth. Test results were entered into an online database to determine the bacterial identity.
Procedure:
The API carrier tray was labelled with each isolate code. Then 5ml of sterile distilled water was put into the tray to provide a moist atmosphere which prevent the drying of the strip and the strip was laid in the tray. A suspension of the organisms was made and inoculated into the wells
For 20NE:
A suspension of the test organism was prepared in 2ml saline.
The saline suspension was Inoculated into NO3 to PNPG wells (the first 8 cupules)
API AUX ampoule was opened (provided with the kit) and 4 drops (200µl) of the saline suspension were added to it.
This suspension was inoculated into the remainder of the cupules.
The wells were overlaid with liquid paraffin where indicated.
Incubation was for 48 hours at 37°C.
The purity of the plate was examined to ensure the culture is pure and then the reagents were added.
Having done the above, the results were documented in the form of figures and the organisms were identified using an API application software.
Results
The nature of the samples collected from the paint industry are shown in
Table 1 and the cultural characteristics of the microorganisms isolated from the paint samples are also shown in
Table 2.
Automated Biochemical Identification Using Analytical Profile Index (API)
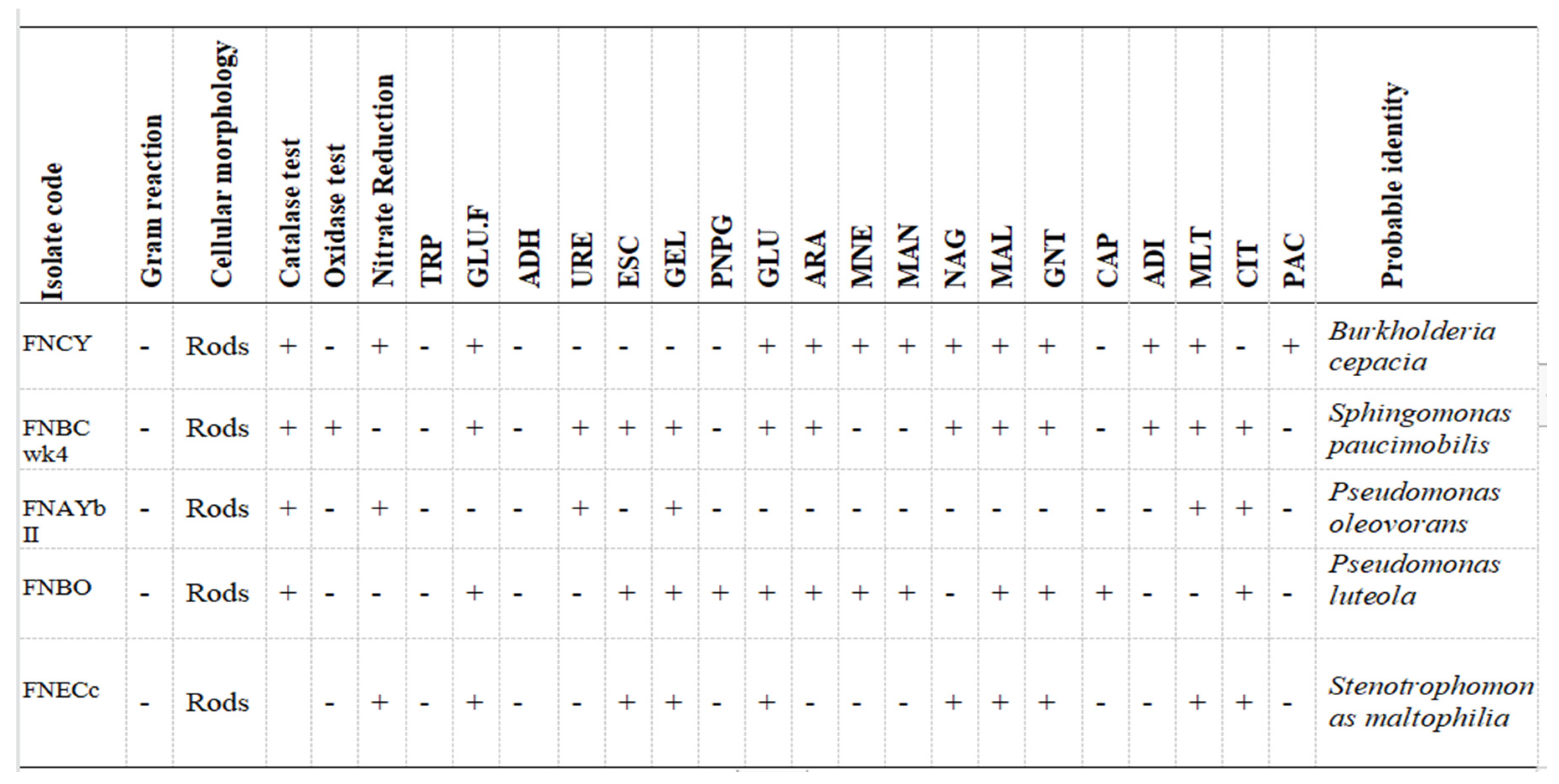
The result of the biochemical identification showed the presence of
Pseudomonas oleovorans, P. luteola, Burkholderia cepacia, Sphingomonas paucimobilis, Stenotrophomonas maltophilia . Table 3 shows the biochemical tests as wells as the confirmed organisms
Total Heterotrophic Count of Samples on Nutrient Agar
Bacterial count was carried out after the paint samples were serially diluted and aliquots of 0.1 ml of the dilution factor 10-5 and 10-7 were plated out on nutrient agar plates in duplicate. The total bacterial population monitored in the paint samples for a period of 3 months ranged from 1×106 CFU/ml to 7.8×109 CFU/ml.
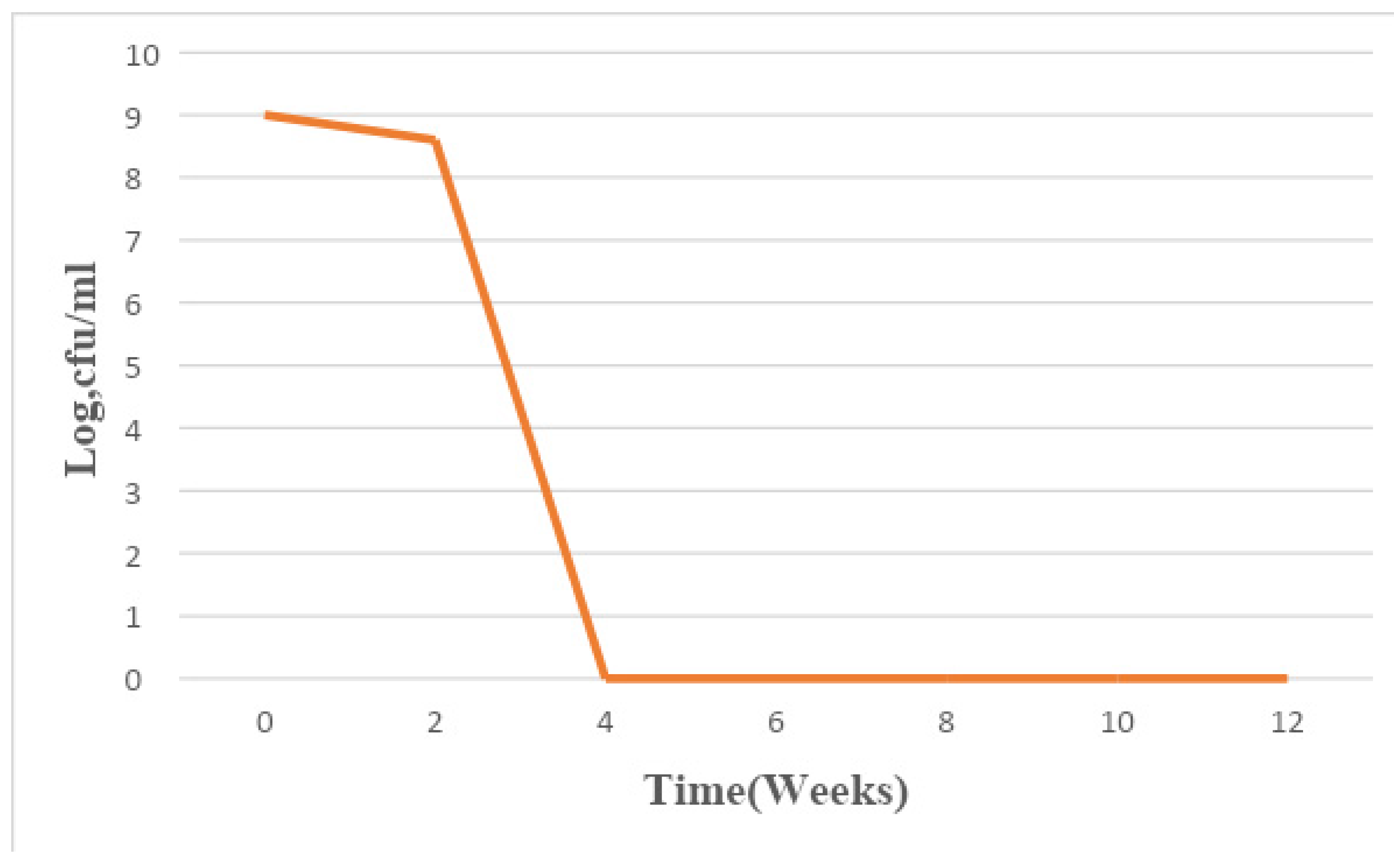
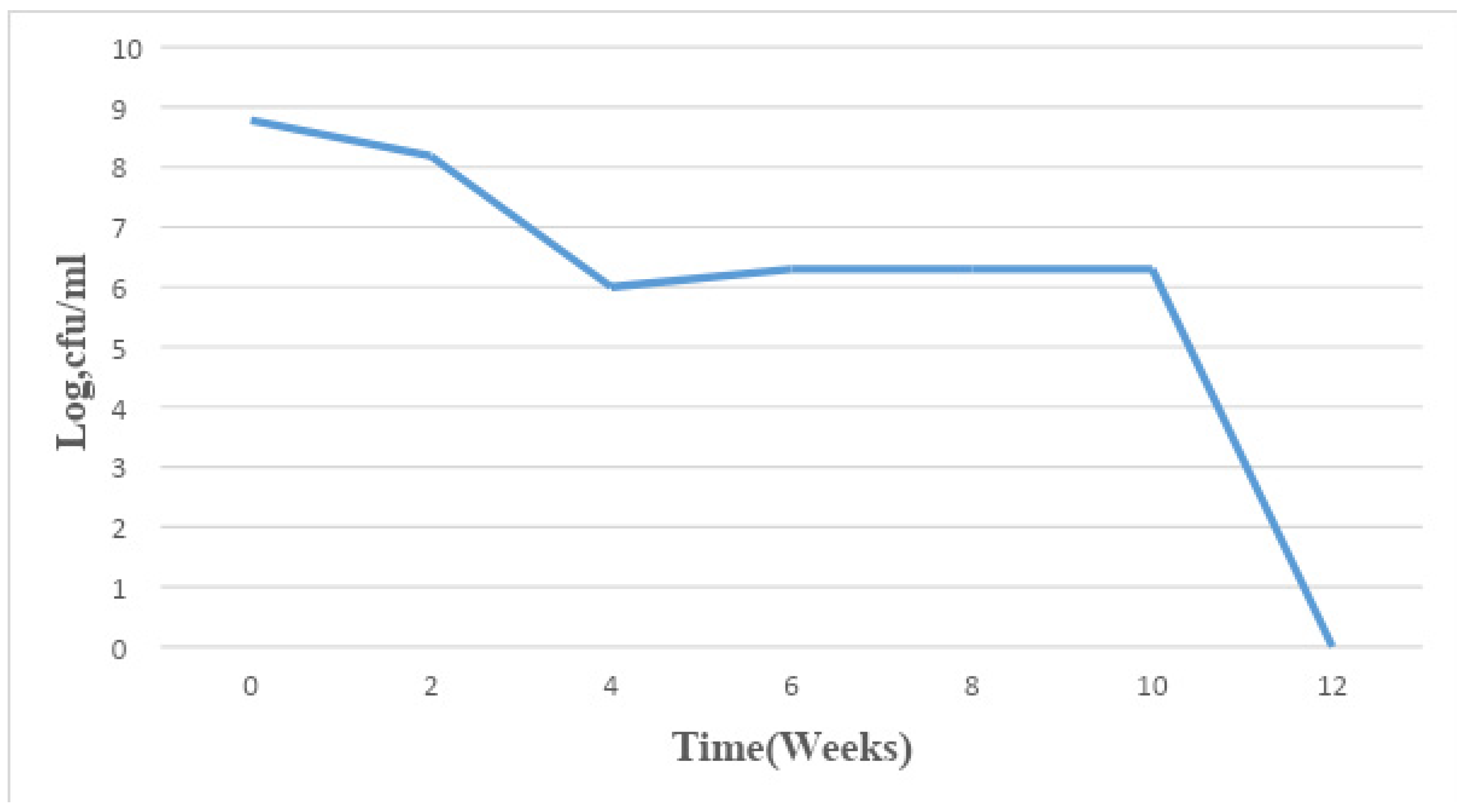
Figure 1.
Mean changes in microbial population density in sample A for a period of 12 weeks.
Figure 1.
Mean changes in microbial population density in sample A for a period of 12 weeks.
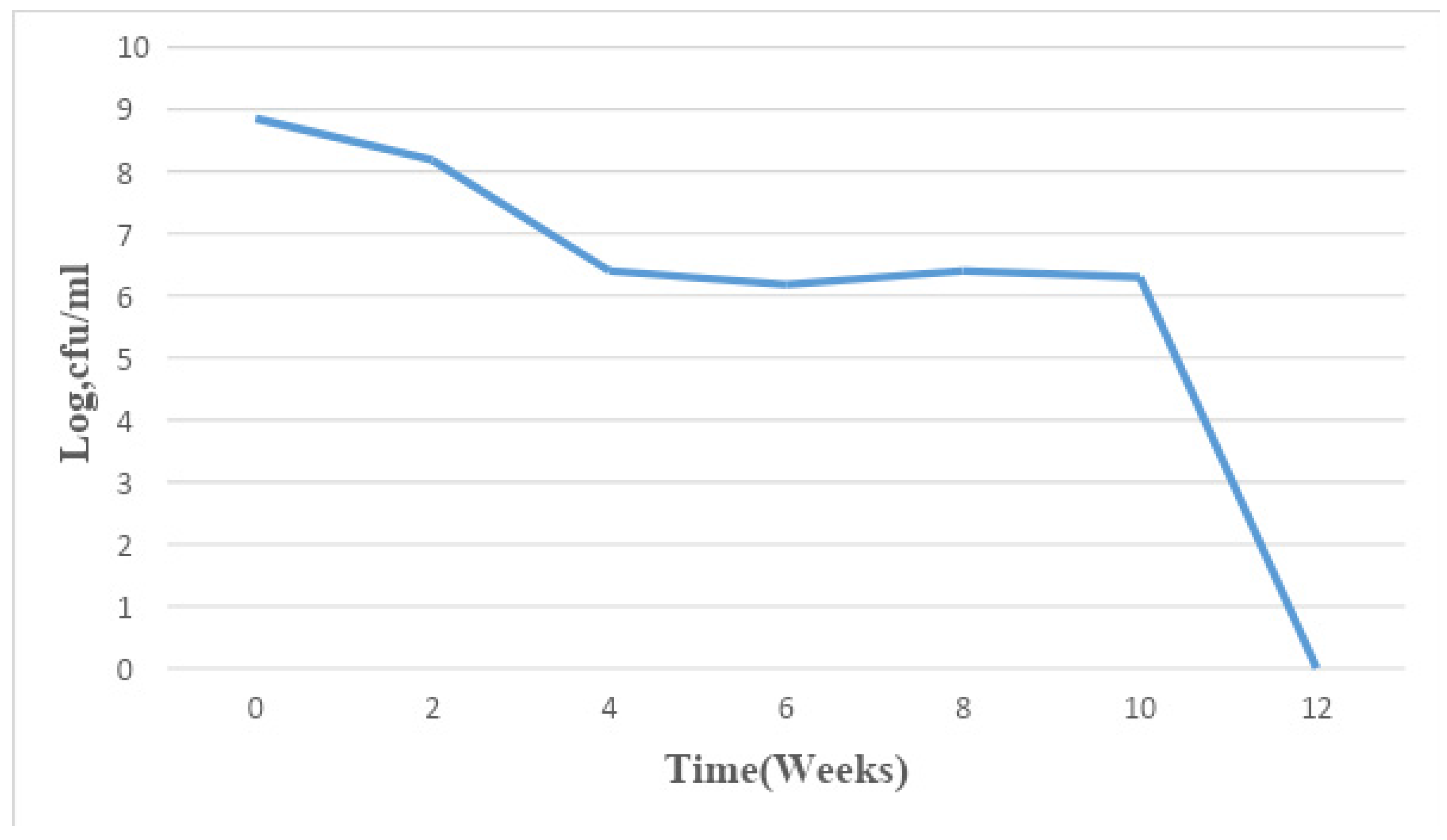
Figure 2.
Mean changes in microbial population density in sample B for a period of 12 weeks.
Figure 2.
Mean changes in microbial population density in sample B for a period of 12 weeks.
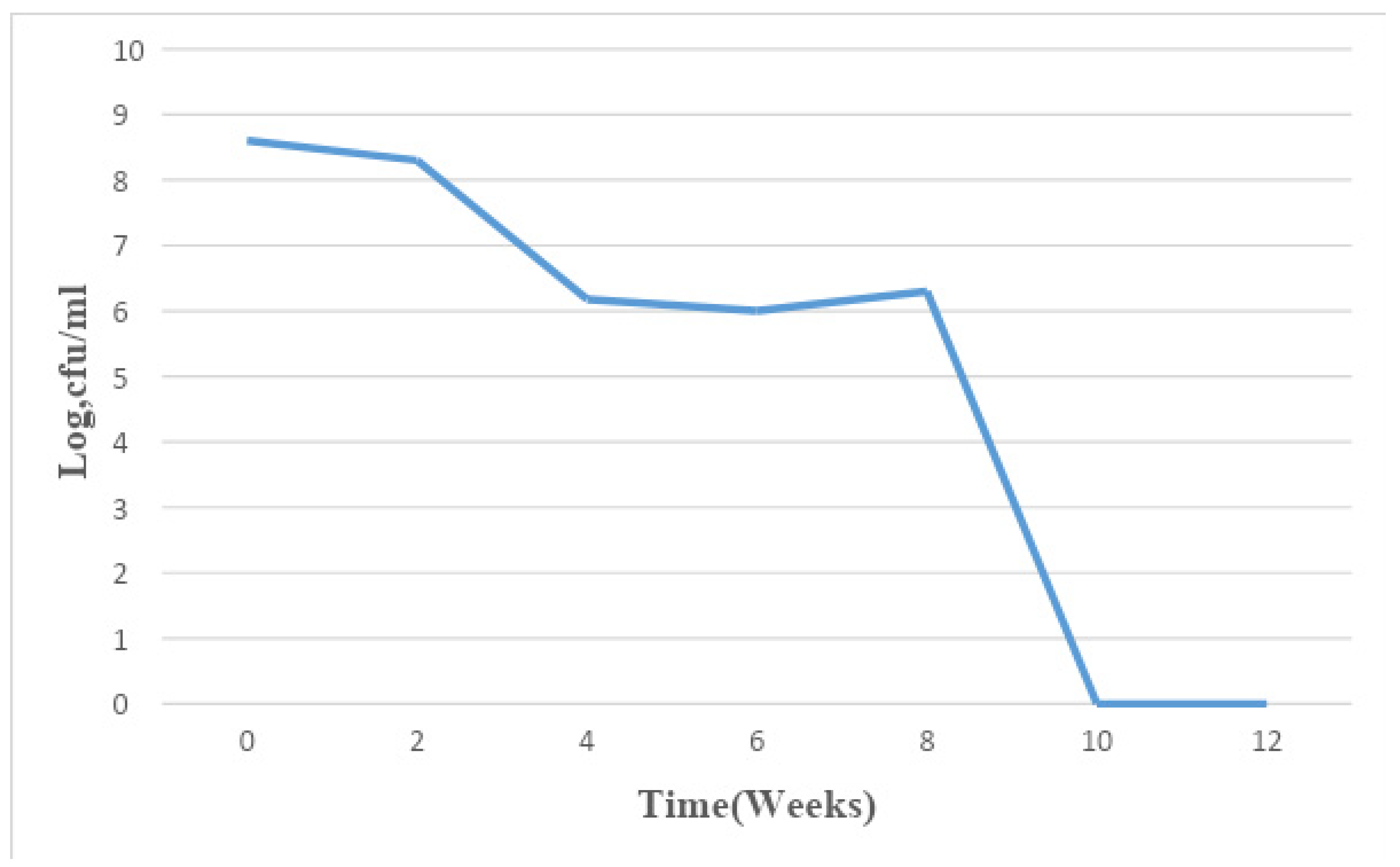
Figure 3.
Mean changes in microbial population density in sample C for a period of 12 weeks.
Figure 3.
Mean changes in microbial population density in sample C for a period of 12 weeks.
Figure 4.
Mean changes in microbial population density in sample D for a period of 12 weeks.
Figure 4.
Mean changes in microbial population density in sample D for a period of 12 weeks.
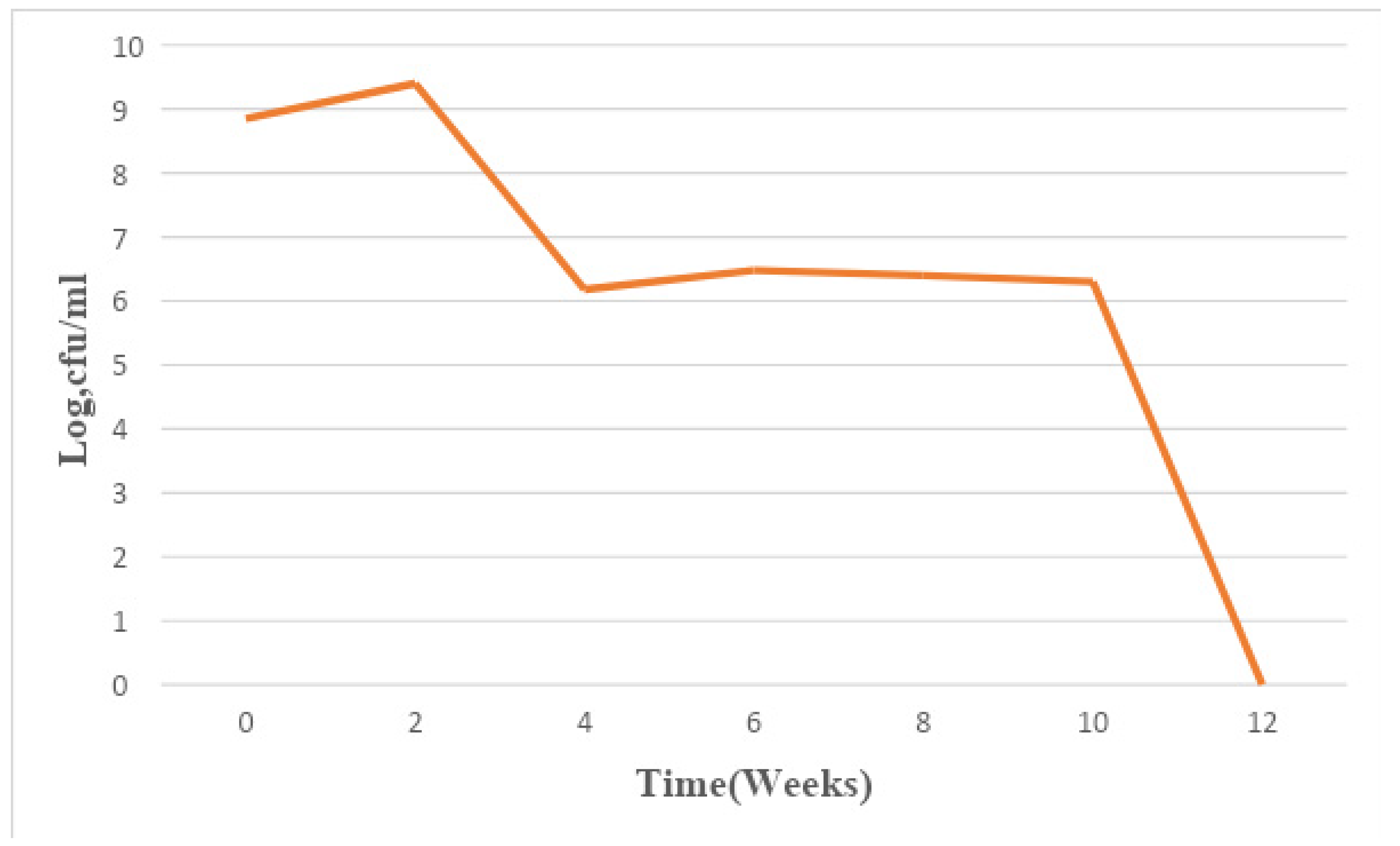
Figure 5.
Mean changes in microbial population density in sample E for a period of 12 weeks.
Figure 5.
Mean changes in microbial population density in sample E for a period of 12 weeks.
Figure 6.
Mean changes in microbial population density in sample F for a period of 12 weeks.
Figure 6.
Mean changes in microbial population density in sample F for a period of 12 weeks.
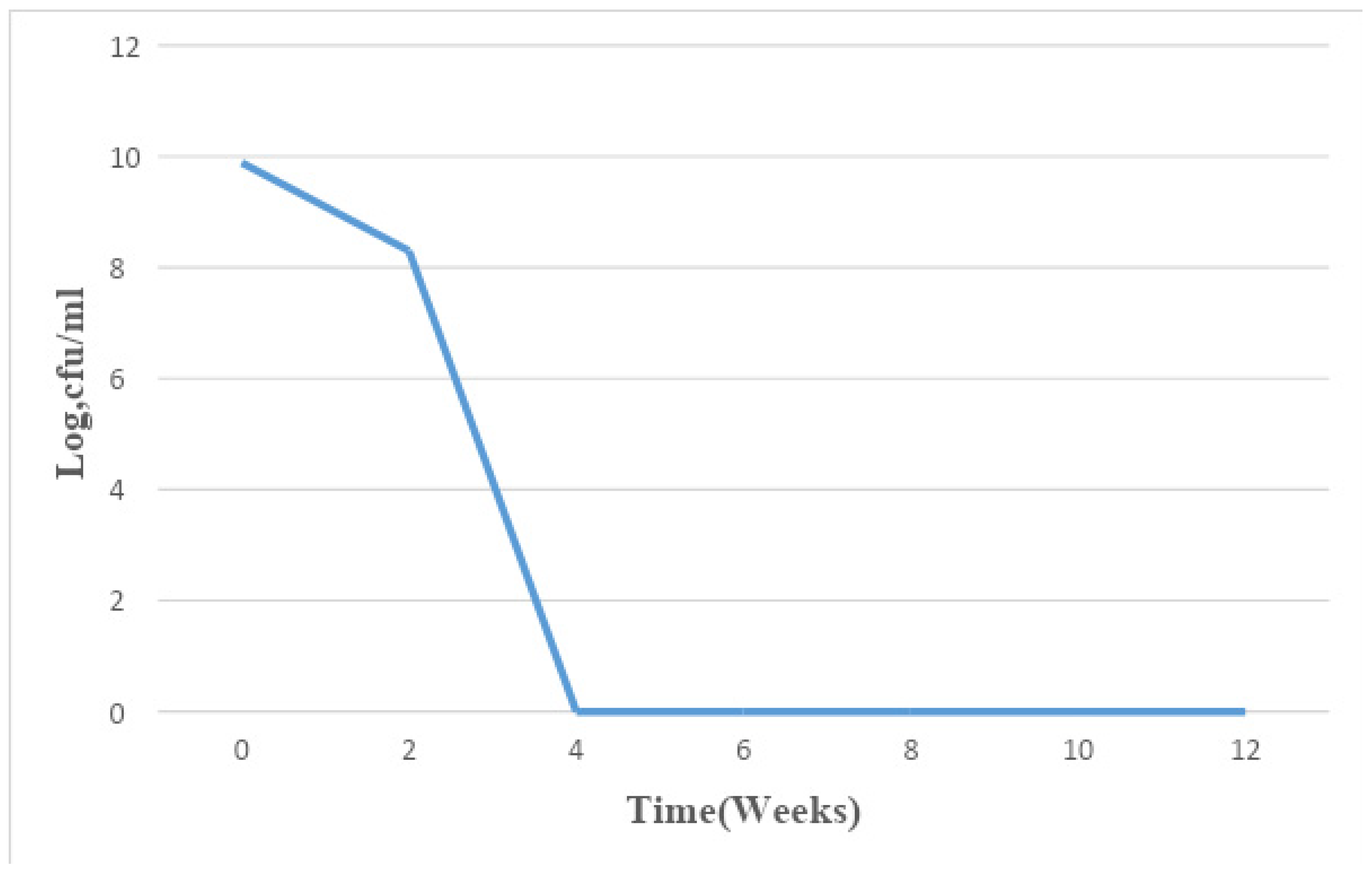
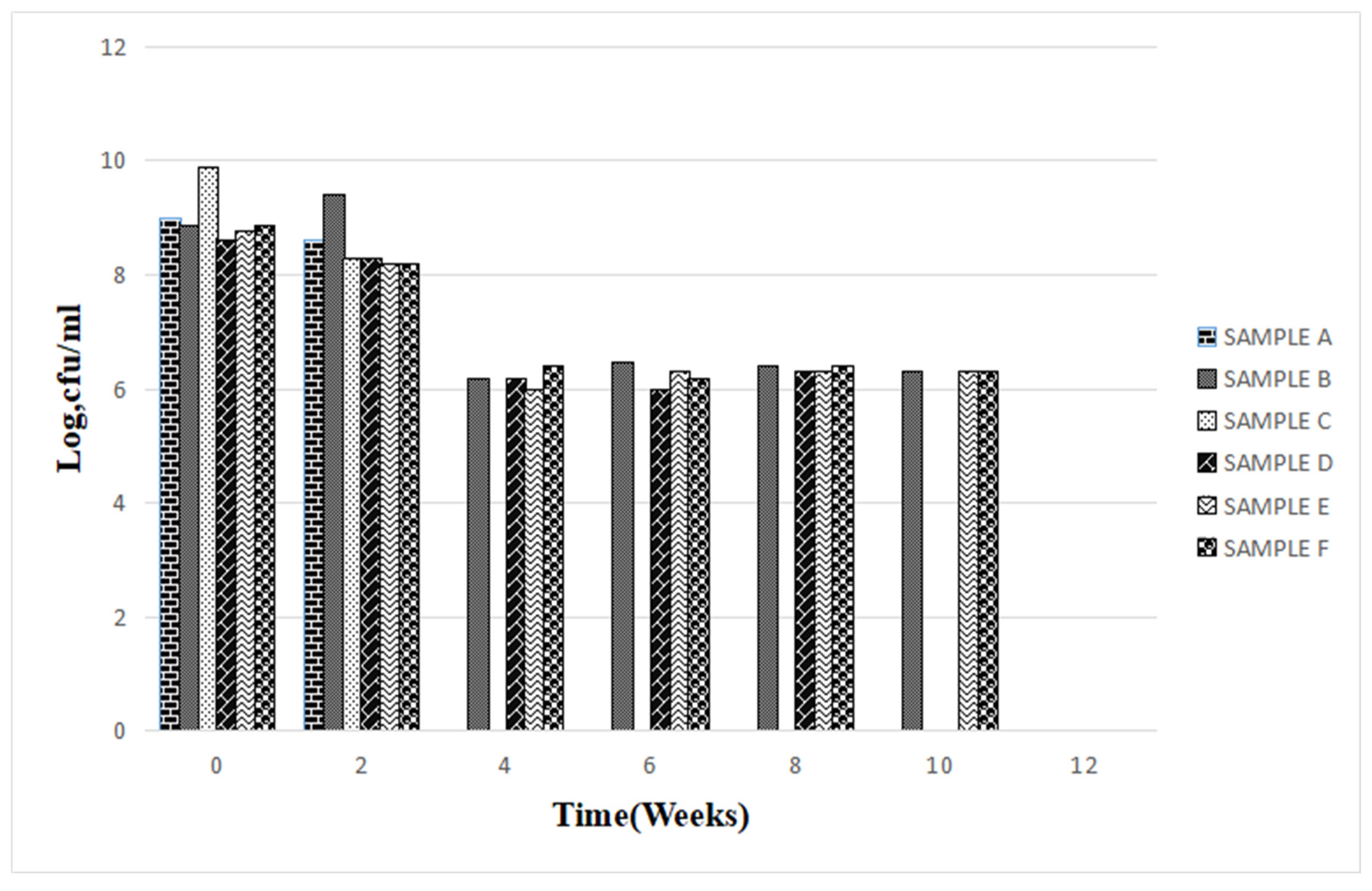
Figure 7.
Summary of mean changes in microbial population density in the six fresh paint samples for a period of 12 weeks.
Figure 7.
Summary of mean changes in microbial population density in the six fresh paint samples for a period of 12 weeks.
Discussion and Conclusion
The ability of bacteria to utilize a wide range of organic matter as a source of carbon and energy resulting in biomass and population build-up does not exempt paints(). The results obtained from this study show that as long as the environmental conditions remain favourable, a can of water-based paint will continue to serve as a substrate for bacterial growth regardless of its freshness. A similar observation was made by Allsopp et al. (9) and Obidi et al. (2). This implies that fresh paint is not equivalent to being sterile, hence the shelf life could be adversely affected and this could lead to an increase in the rate of deterioration of painted surfaces as the organisms are present in the container. Work previously carried out by Gillatt (13) showed that microbial contamination of paints can be from a number of sources such as raw materials, manufacturing plant process units, packaging materials, and final containers.
Study results were obtained by Cappitelli et al., (14), who studied surfaces coated with epoxy resins and isolated Gram-positive bacteria of the Bacillus, Brevibacillus, Micrococcus, Staphylococcus and Kocuria genus, as well as Gram-negative bacteria from the genus of Pseudomonas, Agrobacterium and Ochrobacter(22-24).
The total bacterial population monitored in the 6 paint samples ranged from 1×106 CFU/ml to 7.8×109 CFU/ml. The population density decreased in all the paint samples throughout the period of study, though not uniformly. An early stationary phase was experienced in samples A and C at week 4, and sample D at week 8, while samples B, E, and F experienced a seemingly stationary phase from week 4 to week 10 before an exponential death phase. This is in sharp contrast with the observations made by several researchers, in which there is usually an exponential growth phase after a protracted lag phase indicating the gradual reduction in the impact of the biocides incorporated in paints during production (15,16, 17,18, 19,24,26-27). The observed decrease in population density could be owed to the effectiveness of the biocide present in the paint samples and another environmental factor such as the presence of heavy metals concentration. The paint samples from week 0 to week 10 however supported the growth of Pseudomonas oleovorans, P. luteola, Burkholderia cepacia, Sphingomonas paucimobilis, Stenotrophomonas maltophilia .
Degradation of paints can cause infections due to the microbial impact of degraded materials. These microorganisms can become a source of infection in asymptomatic individuals leading to varying forms of resistance and other public health concerns (20).
It is evident from this study that bacteria are capable of using paints as a substrate and thereby proliferate which leads to undesirable effects such as loss of viscosity, discolouration and malodour on the paint with huge economic loss to the manufacturer. Bacteria like Pseudomonas were prevalent in these paints and their biodeterioration activity is well-documented. Therefore, to increase the shelf-life of these paints, manufacturers are employed to implement the use of biocides that are very active against Pseudomonas sp, as it was present in all the paint samples. Storage conditions that do not favour the growth of microorganisms should also be communicated to retailers.
References
- Bently, J. and Turner, G. P. A. (1997). Introduction to Paint Chemistry and Principles of paint technology. Unk. ISBN 0412723204.
- Obidi, O. F., Aboaba, O. O., Makanjuola, M. S. and Nwachukwu, S. C. U. (2009). Microbial evaluation and deterioration of paints and paint products. Journal of Environmental Biology. 30(5): 835–840.
- Kampman, L. (1972). The Children Handbook of Painting. Journal of Paint Chemistry. 2: 284.
- Spurgeon, A. (2006). Organic Solvent Syndrome in Late-Twentieth-Century Britain. Medical History.50(2): 167-188. [CrossRef]
- Goldstein, A. H. and Galbally, I. E. (2007). Known and Unexplored Organic Constituents in the Earth’s Atmosphere. Environmental Science and Technology 41(9): 1515-1521.
- 6. Spurgeon, A. (2006). Organic Solvent Syndrome in Late-Twentieth-Century Britain. Medical History.50(2): 167-188. [CrossRef]
- Mendell, M. J. (2007). Indoor residential chemical emissions as risk factors for respiratory and allergic effects in children. Indoor Air. 17(4): 259-277. [CrossRef]
- Suma, K. K., Jacob, S. and Joseph, R. (2009). Paint Formulation Using Water-Based Binder and Property Studies. Macromolecular Symposia. 277(1): 144–51. [CrossRef]
- Allsopp, D., Seal, K. J. and Gaylarde, C. C. (2003). Introduction to Biodeterioration. 2nd ed. Cambridge University Press, Cambridge. pp.103-106.
- Da Silva, V. Q. (2003). Microbial deterioration of paints. Microbiologist. 4(1): 43.
- Gaylarde, C. C. and Gaylarde, P. M. (2005). A comparative study of the major microbial biomass of biofilms on exteriors of buildings in Europe and Latin America. International Biodeterioration and Biodegradation 55:131–139. [CrossRef]
- Opperman, A. A. and Gull, M. (1984). Presence and effects of anaerobic bacteria in water-based paints. Journal of Coatings Technology. 56: 51-57.
- Gillatt, A. C. (1992): Bacterial and fungal spoilage of water-borne formulations. Additives 10: 387-393.
- Cappitelli, F., Principi, P., Pedrazzani, R., Toniolo, L. and Sorlini, C. (2007) Bacterial and fungal deterioration of the Milan Cathedral marble treated with protective synthetic resins. Science of the Total Environment 385: 172–181. [CrossRef]
- Russell, A. D. and Mc Donell, G. (2000). Concentration: A major factor in studying biocidal action. Journal of Hospital Infection. 44: 1-3. [CrossRef]
- Gilbert, P. and McBain, A. J. (2001). Biofilms: Their impact upon health and their recalcitrance towards biocides. American Journal of Infection Control. 29: 252-255.
- Stickler, D. J. (2002). Susceptibility of antibiotic-resistant Gram-negative bacteria to biocides: A perspective from the study of catheter biofilms. Journal of Applied Microbiology. 92: 163S-170S.
- Russell, A. D. (2003). Similarities and differences in the response of microorganisms to biocides. Journal of Antimicrobial Chemotherapy. 52:750-763. [CrossRef]
- Petersen, G. D., Dahlllof, J. and Nielsen, L. P. (2004). Effects of zinc pyrithione and copper pyrithione on microbial community function and structure in sediments. Environmental Toxicology and Chemistry. 23: 921-928.
- Favour EN, Isaac AA. Phenotypic characterization of biofilm formation and efflux pump ac.
- Orababa, O. Q., Soriwei, J. D., Akinsuyi, S. O., Essiet, U. U., & Solesi, O. M. (2021). A systematic review and meta-analysis on the prevalence of vancomycin-resistant enterococci (VRE) among Nigerians. Porto biomedical journal, 6(1). [CrossRef]
- Kurowski, G., Vogt, O., & Ogonowski, J. Paint-degrading microorganisms Mikroorganizmy zdolne do degradacji powłok malarskich.
- Ikpe, R. T., Agbendeh, N. L., & Akinsuyi, O. S. (2022). Prevalence study of zoonotic gastrointestinal parasitic infections in goats in Makurdi Metropolis, Nigeria. Journal of Zoonotic Diseases, 6(1), 11-16.
- Oladiran, O. J., Umeadi, C. N., & Onatayo, D. A. (2018). Evaluating change orders and their impacts on construction project performance in Lagos, Nigeria. FUTY Journal of the Environment, 12(2), 81-89.
- Akinsuyi, O., Orababa, O., Juwon, O., Oladunjoye, I., Akande, E., Ekpueke, M., & Emmanuel, H. (2021). One Health approach, a solution to reducing the menace of multidrug-resistant bacteria and zoonoses from domesticated animals in Nigeria–A review. Global Biosecurity, 3(1).
- Mohite, V. S., Darade, M. M., Sharma, R. K., & Pawar, S. H. (2022). Nanoparticle Engineered Photocatalytic Paints: A Roadmap to Self-Sterilizing against the Spread of Communicable Diseases. Catalysts, 12(3), 326. [CrossRef]
- Babalola, B. A., Adetobi, T. E., Akinsuyi, O. S., Adebisi, O. A., & Folajimi, E. O. (2021). Computational study of the therapeutic potential of novel heterocyclic derivatives against SARS-CoV-2. COVID, 1(4), 757-774.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).