Submitted:
29 April 2023
Posted:
30 April 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental and Analysis Overview
2.1. Diatomic Molecular Analysis
2.2. Air Wavelength vs. Vacuum Wavenumber
| Parameter | Value (m) |
|---|---|
| 238.0185 | |
| 5,792,105 | |
| 57.362 | |
| 167,917 |
3. Results
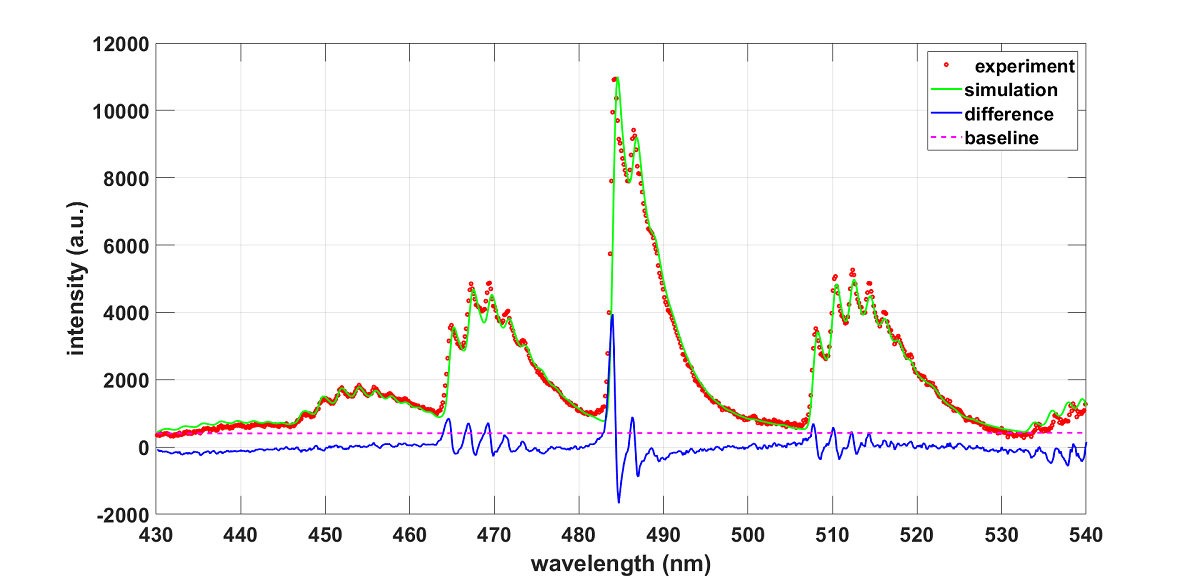
3.1. Analysis with NMT Program and ExoMol Line Strengths
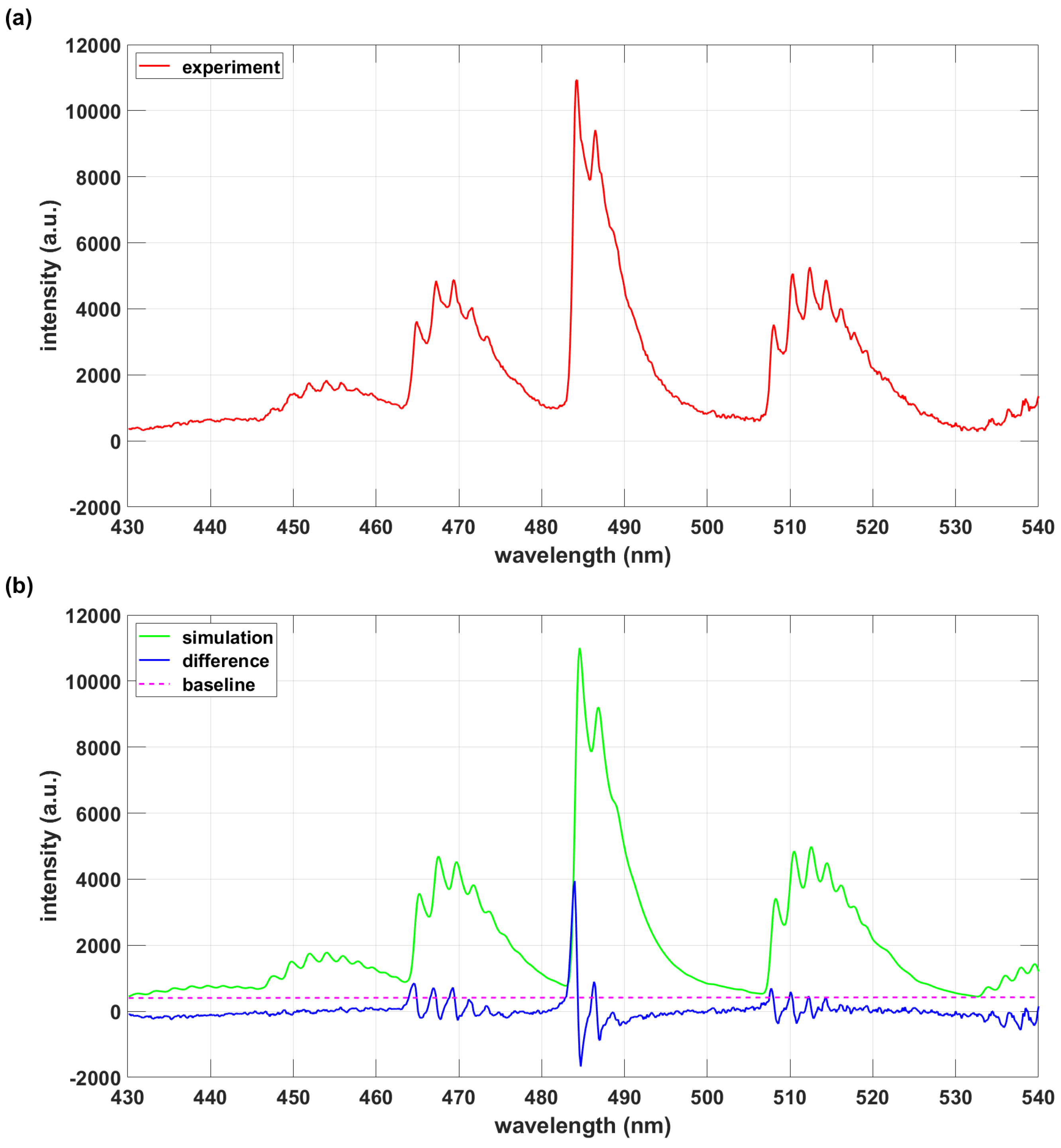
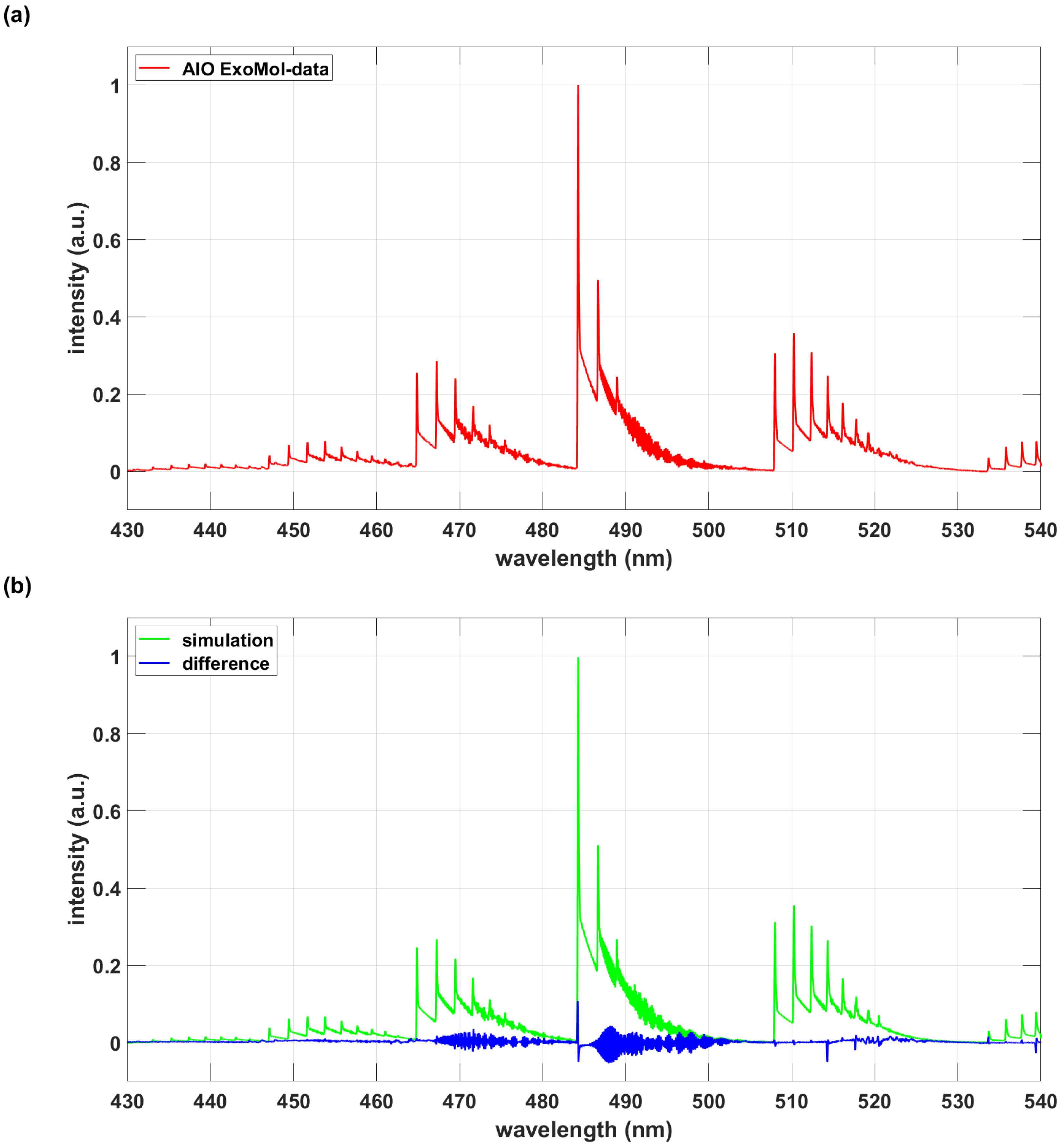
3.2. Exomol AlO and AlO-lsf Data Comparisons
| Database | AlO B-X | AlO B-X 430 nm to 540 nm |
|---|---|---|
| ExoMol | 774,575 | 169,143 |
| AlO-lsf | 33,484 | 29,258 |
| Database | AlO B-X 430 nm to 540 nm A |
|---|---|
| ExoMol | 104,260 |
| AlO-lsf | 29,258 |
| Database | ||||||
|---|---|---|---|---|---|---|
| ExoMol | 747 | 3,146 | 10,843 | 14,425 | 21,036 | 22,609 |
| Database | AlO B-X 483 nm to 493 nm A |
|---|---|
| ExoMol | 10,159 |
| AlO-lsf | 2,818 |
| Database | |||
|---|---|---|---|
| ExoMol | 96 | 506 | 1,517 |
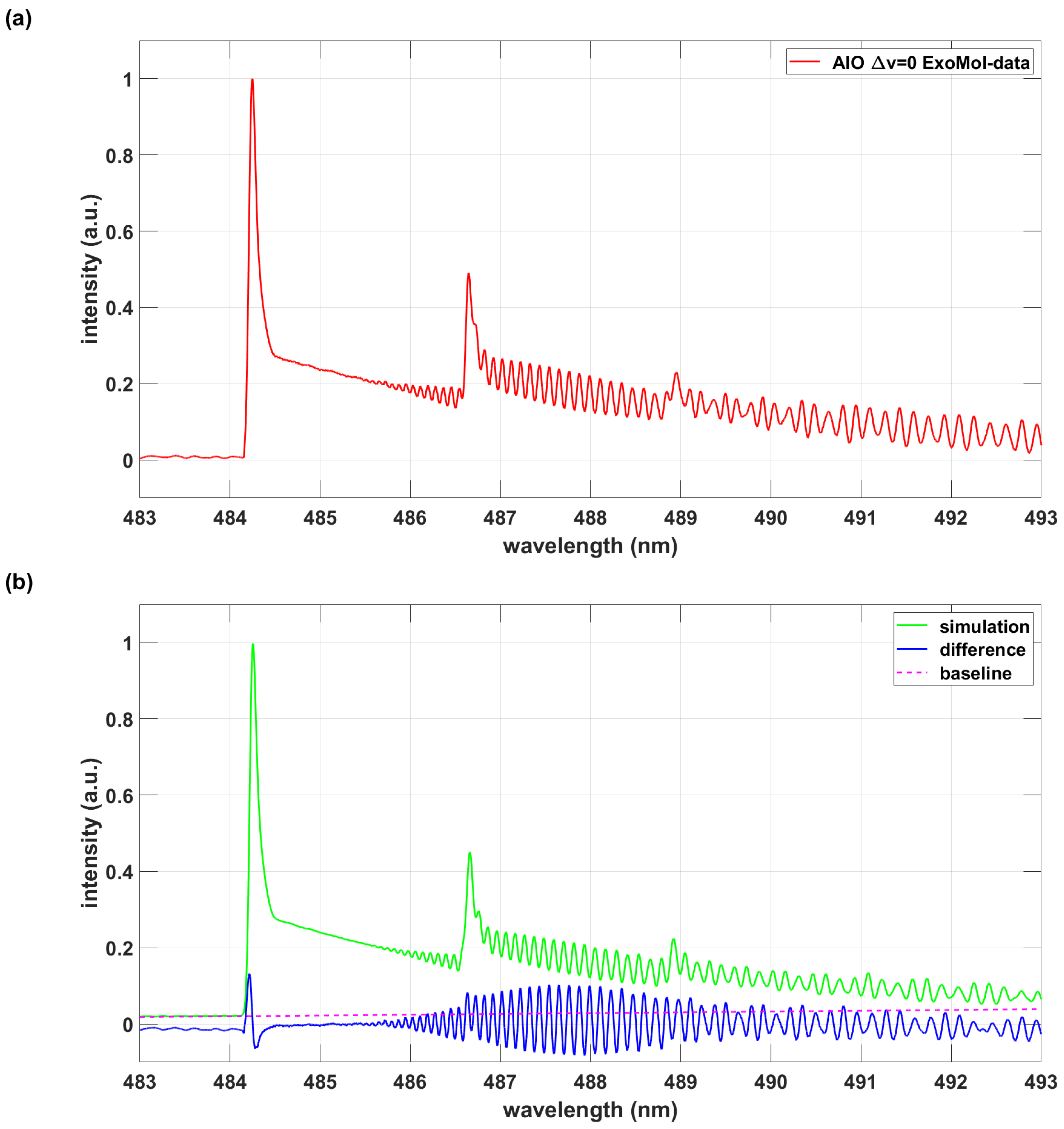
4. Discussion
Acknowledgments
References
- Dors, I.G.; Parigger, C.G.; Lewis, J.W.L. Spectroscopic Temperature Determination of Aluminum Monoxide in Laser Ablation with 266-nm Radiation. Opt. Lett. 23, 1778, (1998). [CrossRef]
- Surmick, D.M.; Parigger, C.G. Aluminum Monoxide Emission Measurements in a Laser-Induced Plasma. Appl. Spectrosc. 68, 992 , (2014). [CrossRef]
- Parigger, C.G.; Woods, A.C.; Surmick, D.M.; Donaldson, A.B.; Height, J.L. Aluminum Flame Temperature Measurements in Solid Propellant Combustion. Appl. Spectrosc. 68, 362, (2014). [CrossRef]
- Parigger, C.G.; Hornkohl, J.O. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 81, 404, (2011). [CrossRef]
- Parigger, C.G. Diatomic Line Strengths for Fitting Selected Molecular Transitions of AlO, C2, CN, OH, , NO, and TiO, Spectra Foundations 3, 1, (2023). [CrossRef]
- Parigger, C.G.; Hornkohl, J.O. Quantum Mechanics of the Diatomic Molecule with Applications; IOP Publishing: Bristol, UK, 2020.
- Tennyson, J.; Yurchenko, S.N.; Al-Refaie, A.F.; Clark, V.H.J.; Chubb, K.L.; Conway, E.K.; Dewan, A.; Gorman, M.N.; Hill, C.; Lynas-Gray, A.E.; Mellor, T.; McKemmish, L.K.; Owens, A.; Polyansky, O.L.; Semenov, M.; Somogyi, W.; Tinetti, G.; Upadhyay, A.; Waldmann, I.; Wang, Y.; Wright, S.; Yurchenko, O.P. The 2020 release of the ExoMol database: Molecular line lists for exoplanet and other hot atmospheres. J. Quant. Spectrosc. Radiat. Transf. 255, 107228, (2020). [CrossRef]
- Parigger, C.G. Laser Plasma Cyanide Emission Spectra Analysis. Submitted to Int. Rev. At. Mol. Phys. (2022).
- Kunze, H.-J. Introduction to Plasma Spectroscopy; Springer: Berlin/Heidelberg, Germany, 2009.
- Fujimoto, T. Plasma Spectroscopy; Clarendon Press; Oxford, UK, 2004.
- Ochkin, V.N. Spectroscopy of Low Temperature Plasma; Wiley-VCH: Weinheim, Germany, 2009.
- Demtröder, W. Laser Spectroscopy 1: Basic Principles, 5th ed.; Springer: Heidelberg, Germany, 2014.
- Demtröder, W. Laser Spectroscopy 2: Experimental Techniques, 5th ed.; Springer: Heidelberg, Germany, 2015.
- Miziolek, A.W., Palleschi, V., Schechter, I. (Eds.) Laser Induced Breakdown Spectroscopy (LIBS): Fundamentals and Applications; Cambridge Univ. Press: New York, NY, USA, 2006.
- Singh, J.P.; Thakur, S.N. (Eds.) Laser-Induced Breakdown Spectroscopy, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2020.
- Western, C.M. A Program for Simulating Rotational, Vibrational and Electronic Spectra. J. Quant. Spectrosc. Radiat. Transf. 186, 221, (2017).
- McKemmish, L.K. Molecular diatomic spectroscopy data. WIREs Comput. Mol. Sci. 11, e1520, (2021). [CrossRef]
- Rothman, L.S.; Gordon, I.E.; Barber, R.J.; Dothe, H.; Gamache, R.R.; Goldman, A.; Perevalov, V.I.; Tashkun, S.A.; Tennyson, J. HITEMP, the high-temperature molecular spectroscopic database. J. Quant. Spectrosc. Radiat. Transf. 2010, 111, 2139. [CrossRef]
- MATLAB Release R2022a Update 5, The MathWorks, Inc.: Natick, Massachusetts, US, 2022.
- Nelder, J.A.; Mead, R. A simplex method for function minimization. Comp. J. 7, 308, (1965). [CrossRef]
- Patrascu, A.T.; Yurchenko, S.N.; Tennyson J. ExoMol molecular line lists: IX The spectrum of AlO. Mon. Notices Royal Astron. Soc. 449, 3613, (2015). [CrossRef]
- Bowesman, C.A.; Shuai, M.; Yurchenko, S.N.; Tennyson, J. A high resolution line list for AlO. Mon. Notices Royal Astron. Soc. 508, 3181, (2021). [CrossRef]
- Condon, E.U.; Shortley, G.H. The Theory of Atomic Spectra; Cambridge Univ Press: Cambridge, UK, 1964.
- Hilborn, R.C. Einstein coefficients, cross sections, f values, dipole moments, and all that. Am. J. Phys. 50, 982, (1982). [CrossRef]
- Thorne, A.P. Spectrophysics, 2nd ed.; Chapman and Hall: New York, NY, US, 1988.
- Wigner, E.; Witmer, E.E. Über die Struktur der zweiatomigen Molekelspektren nach der Quantenmechanik. Z. Phys. 51, 859, (1928). [CrossRef]
- Wigner, E.; Witmer E.E. On the structure of the spectra of two-atomic molecules according to quantum mechanics. In Hettema H. (Ed) Quantum Chemistry: Classic Scientific Papers. World Scientific: Singapore, SG, 2000; 287.
- Ciddor, P.E. Refractive index of air: New equations for the visible and near infrared. Appl. Opt. 35, 1567, (1996). [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





