INTRODUCTION
Anxiety, major depressive disorder and cognitive dysfunction increase substantially following traumatic brain injury [
1] (TBI) contributing to substantial morbidity and mortality in affected persons. Peripheral and central inflammation drive neurodegeneration via activation of innate and adaptive immune mechanisms which may target (in part) neurovascular antigens released during traumatic brain injury. Owing to a global epidemic of obesity and type 2 diabetes mellitus [
2], lifetime TBI-sufferers having metabolic derangements associated with peripheral inflammation may be at increased risk for experiencing certain neurodegenerative complications [
3].
The Zucker diabetic fatty rat (ZDF) is a model of morbid obesity, type 2 diabetes mellitus and hypertension [
4] which exhibits high level of innate immunity, i.e. pro-inflammatory cytokines [
5]. Previously, we reported spontaneously-occurring neurotoxic agonist autoantibodies targeting the 5-hydroxytryptamine (serotonin) 2A receptor in plasma from both ZDF rat and Zucker lean rat (ZLR) sub-strains with the ZDF rats having persistently high level at 5 months of age [
6]. The serotonin 2A receptor is a known treatment target in major depressive disorder and Parkinson’s disease, two neurodegenerative complications following TBI. Here we tested for neurobehavioral differences in emotional/mood disorders as well as recall of spatial learning in substrains of Zucker fatty and lean rats following mild TBI induced by lateral fluid percussion.
In addition, a putative neuroprotective peptide comprised of a 5-hydroxytryptamine 2A receptor second extracellular loop region fragment is effective in preventing in vitro neurotoxicity caused by autoantibodies targeting the serotonin 2A receptor from ZDF rat plasma [
7]. Therefore the efficacy of this peptide in modulating TBI-related anxiety, depressive-like behavior or spatial memory impairments in fatty and lean Zucker rats was tested.
Materials and Methods
Peptides- A linear synthetic peptide, SCLLADDN (SN..8 or P4) having a sequence identical to that of a fragment of the second extracellular loop region of the human 5-hydroxytryptamine 2A receptor was synthesized at Lifetein, Inc (Hillsborough, NJ) and had > 95% purity. Substitutions of SCLLADDN containing a single alanine amino acid replacement, e.g. SALLADDN, SCLLADAN were synthesized at Lifetein, Inc (Hillsborough, NJ) and had purity of > 95%. A scrambled peptide containing the same amino acids as SCLLADDN had a sequence of LASNDCLD (LD.8) and a purity of 96.37%, MW 849.91. The lyophilized peptides were stored (in the presence of dessicant) at −40 degrees C prior to use. On the day of an experiment, an aliquot of lyophilized peptide was reconstituted in sterile saline at the indicated concentration. Reconstituted peptide(s) were prepared fresh before each experiment.
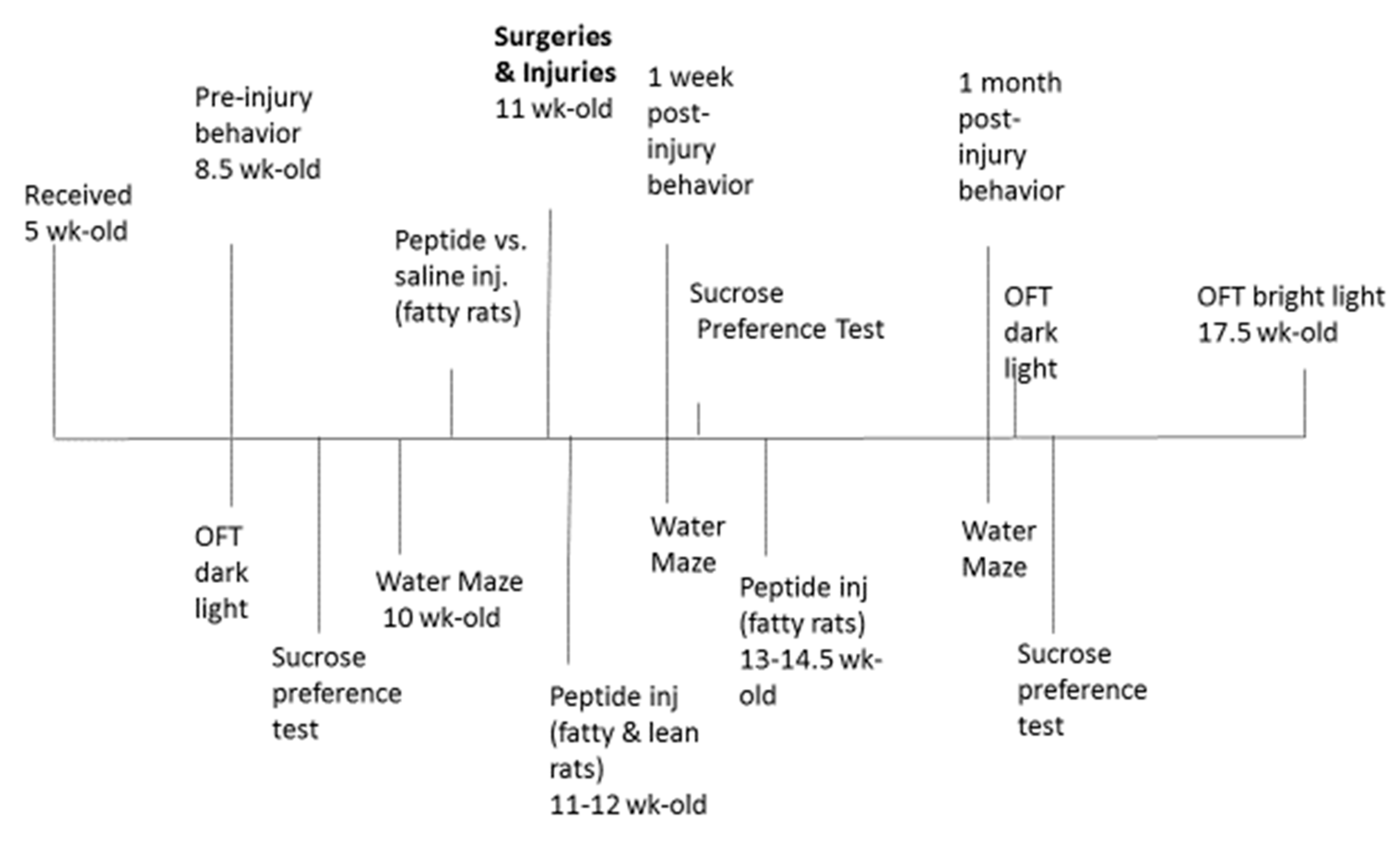
Animals- All procedures were conducted according to the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of the Veterans Affairs Medical Center (East Orange, New Jersey). Male ZDF (fa/fa) and lean (+/?) Zucker rats were obtained from Charles River Laboratories (Kingston, NY) at approximately 6-7 weeks of age. All rats were single housed upon arrival, with modest enrichment (a PVC tube). Rats were provided
ad libitum access to food and water and maintained in a 12 h light/dark cycle with lights on at 0630. All procedures occurred during the light phase of the circadian cycle and a timeline of procedures is shown in
Figure 1.
Injections- Peptide was dissolved in sterile saline (2 mg/kg) and peptide
vs. vehicle (sterile saline) intraperitoneal (IP) injections were administered every other day according to the schedule shown in
Figure 1. Peptide (
vs. saline) injections commenced 1-week prior to surgery/injury in ZDF rats to minimize the risk of excess acute post- injury mortality in ZDF rats thought to be related (in part) to pre-injury moderate hypertension. The peptide SN..8 was previously reported to substantially lower blood pressure in the ZDF rat [
8].
Surgeries/Injury
All animals underwent a surgical procedure to attach a luer-lock connector to a craniectomy, positioned either on the left or right parietal bone plate. Rats were anesthetized with 1-4% isoflurane (5 L/min O2 for 60s) and transferred to a stereotactic instrument with 1-4% isoflurane mixed with 1-1.5 L/min O2 delivered via a nosecone to maintain anesthesia throughout the surgery. A craniectomy (2mm in diameter, centered at 3mm posterior to bregma and 3.5 mm lateral to midline) was made in the skull and super glue was used to secure a luer-lock connector around the edge of the craniectomy. A plastic cylinder (cut from a 10mL syringe) was placed on the skull surrounding the luer-lock connector; this was placed to provide stability and protect the area around the craniectomy. Dental cement was used to fill the space in between the connector and the plastic cylinder. A small amount of sterile saline was deposited into the connector along with a small piece of Kimwipe to keep the dura clean of debris.
One day following implantation of the surgical hub, fluid percussion injury is achieved with a device using a computer-controlled voice-coil to deliver defined pressure waves to the dura through a water piston [
9]. Water is first placed in the surgical cap to prevent any air displacing the pressure waves. Rats were anesthetized with isoflurane in an induction chamber followed by fluid percussion injury or sham. The injury procedure occurs one day after surgery to prevent the dura from drying out and thereby reducing the injury of the brain tissue. Mild to moderate severity TBI will occur at pressure amplitudes of 20 - 30 psi (± 3 psi) and rates of rise between 10-20ms. To reduce the effects of anesthesia as much as possible, rats are connected to the device by the syringe hub and allowed to recover from anesthesia until they respond lightly to a foot pinch before injury occurs. Therefore, the rats were lightly anesthetized at the time of injury. Sham rats are treated the same way as injured rats including isoflurane anesthesia and connection of the device to the rat, but the pressure wave was not delivered. The animal is monitored for startle in response to the pressure wave and then placed on its back in a recovery cage and evaluated for apnea. Latency for the righting reflex to return is recorded and used as an index of traumatic unconsciousness.
Behavioral Tests
Open Field test
Rats were placed in the center of a round, gray, open field apparatus (diameter of 74 cm, height of 51 cm). A light (120 W) was located 135 cm directly above the center of the apparatus which had a measured light intensity, LT300-NIST Light Meter (Extech Instruments, Knoxville, TN) of 400-500 lux. The open field apparatus was placed in a novel environment. The path of the rat in the open field was recorded, digitized, analyzed, and stored on a computer using AnyMaze version 6.21 (64-bit) (Stoelting Co., 620 Wheat Lange, Wood Dale, IL 60191 USA). For analysis, the open field was separated into two zones, the center, and the periphery. The center zone was measured to have ½ the area of the total field (diameter of approximately 52.32 cm). Performance in the open field was scored during a 3-minute time window before returning the animal to its home cage. The apparatus was wiped with 70% ethanol solution between testing of each rat. Increased mobility in the center zone, defined as distance, speed, and time in motion, was used as an indicator of reduced anxiety, whereas staying in the peripheral zone, was used as an indicator of increased anxiety [
10].
Morris Water Maze
The Morris water maze is a test of short-term recall of a spatial learning task. Zucker lean and fatty rats subjected to sham vs traumatic brain injury were tested for the ability to locate a submerged platform in a swimming pool. Sample phase corresponds to acquisition of learning; the choice phase measures recall of newly-acquired spatial learning task. Distance is a measure of how far the rats swam before locating the submerged swim platform. Longer distance (choice phase) is indicative of impairment of recall of short- term spatial learning acquired in the sample phase. Path efficiency (efficiency) is the ratio of the shortest path to the platform vs. the actual swim path taken by the animal. Maximum path efficiency has a value of 1.0 and is a measure of recall of swim platform location.
Delayed Match to Position in Morris Water Maze:
Before injury, the animals underwent extensive training in the Morris water maze apparatus. The delayed match to position (DMTP) test is a short-term recall of a spatial memory task. Rats were tested for the ability to locate a submerged platform in a swimming pool. The swimming pool was made opaque by non-toxic paint (Crayola washable paint, 16 oz, white 54-2016-053). Submerged in the pool was a platform on which the rat could escape from swimming, with the surface of the escape platform approximately 1 cm below the surface of the water. During the first acclimation session, the rat was initially placed on the platform and allowed to remain on the platform for 10 – 20 seconds. In subsequent training trials, the rat was placed further from the escape platform so that the rats learned that there was a place to escape from swimming, learned to climb onto the escape platform, and acclimated to swimming in the pool. During this training phase, the escape platform was in the same location and rats were started twice from 3 distinct locations (6 trials/session). Rats were trained for 4 days with one session per day. Each trial lasted a maximum of 60 seconds and if the rat had not located the platform during that time the rat was led to the platform using a wooden stick. The rat was then allowed to climb onto the platform where they remained for 10- 20 seconds. After this, the rat was dried and moved to a holding cage with a heater if necessary.
One day after the 4th training session, a single probe trial was given in which the platform was removed from the pool and the rat was allowed to swim from one of the start locations for 60 seconds, after which the rat was removed from the pool, dried, and placed back in the home cage. For both training and probe sessions, the swim path of the rat was recorded and analyzed for multiple measures to assess spatial learning using AnyMaze (AnyMaze version 6.21 (64-bit) (Stoelting Co., 620 Wheat Lange, Wood Dale, IL 60191 USA).
Testing of spatial working memory commenced approximately 2-3 days after the probe trial using a DMTP procedure that consisted of 6 trials per rat with each trial having two phases, a sample phase and a choice phase. At the beginning of each trial, an animal entered sample phase by being placed in the water at a novel start position and has 60 seconds maximum swim time to find the platform. After either successfully finding the platform or being led to it after the 60 seconds swim time, the rat was left on the platform for 15 seconds, and then returned to its home cage for 30 seconds. That same animal then began choice phase, was placed back in the water at the same location as in sample phase and had 60 seconds maximum swim time to locate the platform. Each trial has a novel combination of start and platform positions. All rats were tested at the same start-platform positions. The pool was divided by AnyMaze into three equal-area zones, covering 120° of circumference each. At the beginning of the sample phase, each rat was placed in the water facing the perimeter of the tank in a selected zone. The escape platform was in a different zone than that of the start position and remained in that zone for both phases. The rat was allowed a maximum of 60 seconds to find the platform. After either successfully finding the platform or being led to it after 60 seconds, the rat was left on the platform for 10- 20 seconds, and then returned to its home cage for approximately 30 seconds. The same animal then began the choice phase, where it was placed back in the water at the same start location as in sample phase and allowed 60 seconds to locate the platform. On each subsequent trial, the starting location and the platform location were changed across trials, not within trials, within the session. Approximately 1 hour separated each trial.
The swim path was recorded for later analysis. One to two days of working memory testing was required. Swim Distance was used as a measure of how far the rats swam before locating the submerged swim platform.
Sucrose Preference Test:
Sucrose preference was determined over a 48-hour period. Rats were given access to two water bottles in their home cage: one containing 1% sucrose and the other tap water. After 24 hours, the bottle positions were switched to eliminate side biases. The total amount consumed in each bottle is determined by weighing the bottles. A preference score is calculated by the following equation (sucrose consumed)/(sucrose consumed + water consumed) * 100.
Retro orbital sinus bleed (ROB) -was carried out by trained personnel in rats lightly anesthetized with 1-4% isoflurane mixed with 1-1.5 L/min O2 according to a previously reported procedure [
11]. A sterile glass microhematocrit heparinized capillary tube (Fischer, Inc.) was used for blood collection. The blood was centrifuged at 1400 g × 10 minutes at 4 degrees C. The resulting plasma was stored at −4°C for up to 2–3 weeks prior to protein G affinity chromatography.
Zucker rat IgG autoantibodies
EDTA plasma was obtained from 10-week lean, heterozygous (
fa/+) Zucker rats from Charles River Laboratories. The IgG fraction of plasma was isolated using protein-G affinity chromatography as previously reported [
6].
Mouse neuroblastoma N2A neurite retraction assay
The assay was carried out as previously reported [
7]. Both human [
7] and Zucker rat IgG [
6] was previously shown to mediate acute N2A neurite retraction by a mechanism involving sustained Gq11/IP3/Ca2+ and RhoA/Rho kinase signaling pathways activation.
Statistics- Comparisons were made using analysis of variance (ANOVA), repeated measures ANOVA with Tukey’s post-hoc testing, and unpaired Student’s t-test.
Results
Open field test- bright light
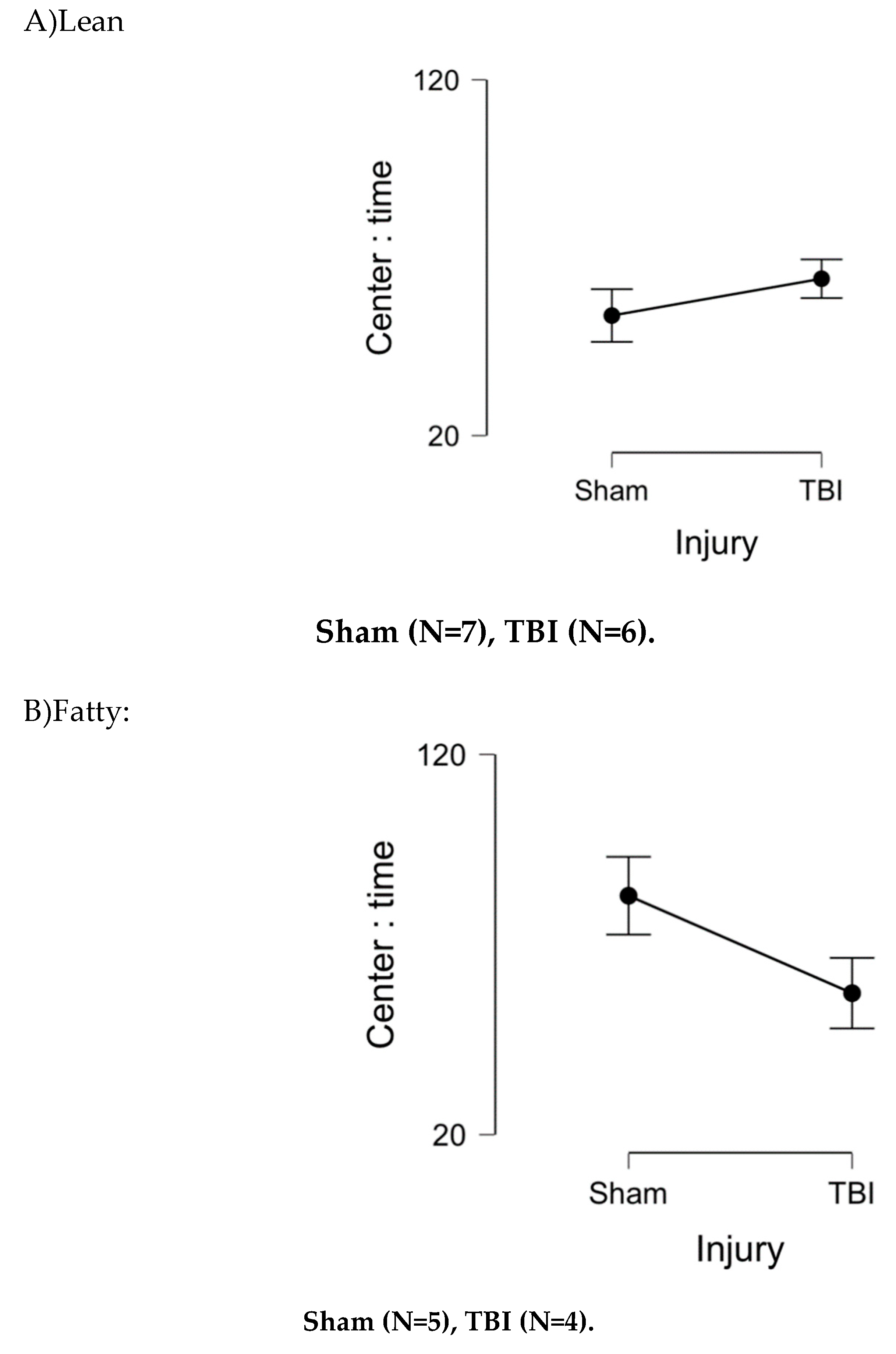
At 1.5 months post-injury, Zucker rats spent more time in center than lean sham Zucker rats (
Figure 2A-B), an ANOVA demonstrated a main effect of strain, P = .037. There was a significant strain x injury interaction (P = 0.025) in favor of TBI Zucker fatty rats spending less time in center than sham-injured, Zucker fatty rats (
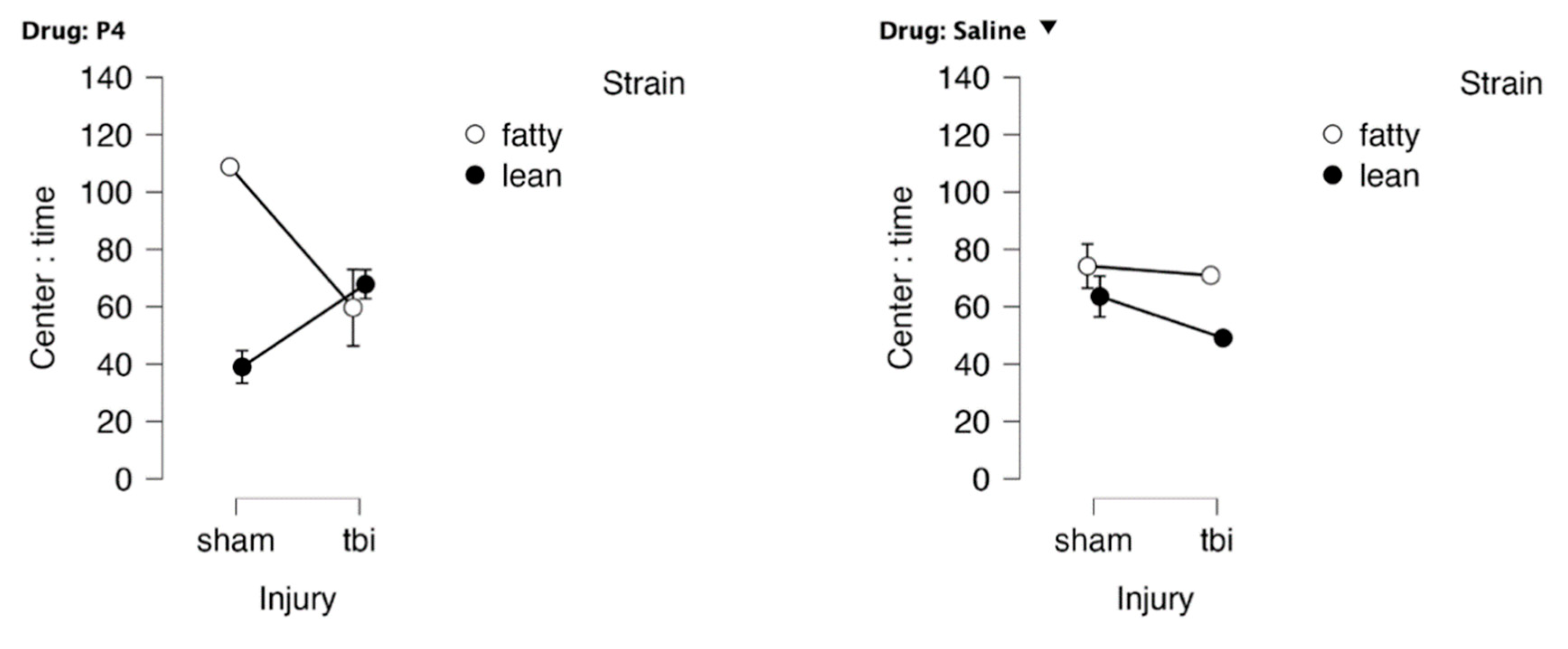
Figure 2). There was also a significant (strain x injury x drug) interaction (P = 0.048) (
Figure 3AB) in which peptide (vs. saline)-treated Zucker lean rats spent more time in center following TBI
vs (sham injury) (
Figure 3AB). Peptide-treated, sham-injured ZDF rats spent significantly more time in center compared to saline-treated, sham-injured ZDF rats (
Figure 3AB) indicative of a possible neuroprotective effect in ZDF rats in the absence of brain injury.
When the rats were retested 3 months post-injury, the pattern was the same as at 1.5 month post-injury. Sham-injured fatty rats spent more time in the center (i.e. reduced anxiety) compared to TBI fatty rats. Time in center did not differ significantly in sham vs TBI lean Zucker rats at the 3-month post-injury evaluations (data not shown).
Figure 3. Drug x strain x injury interaction effect on time in center, bright light OFT.
Drug x injury x strain: Fatty, sham ,P 4 N=2; Fatty, sham, saline n=3; Fatty, tbi, P4 n=3; Fatty tbi saline n=1; Lean, TBI, P4 N= 4; Lean, Sham, P4 N= 2; Lean, TBI, saline N= 2; Lean, Sham, saline N=3 P4 denotes SN..8 peptide.
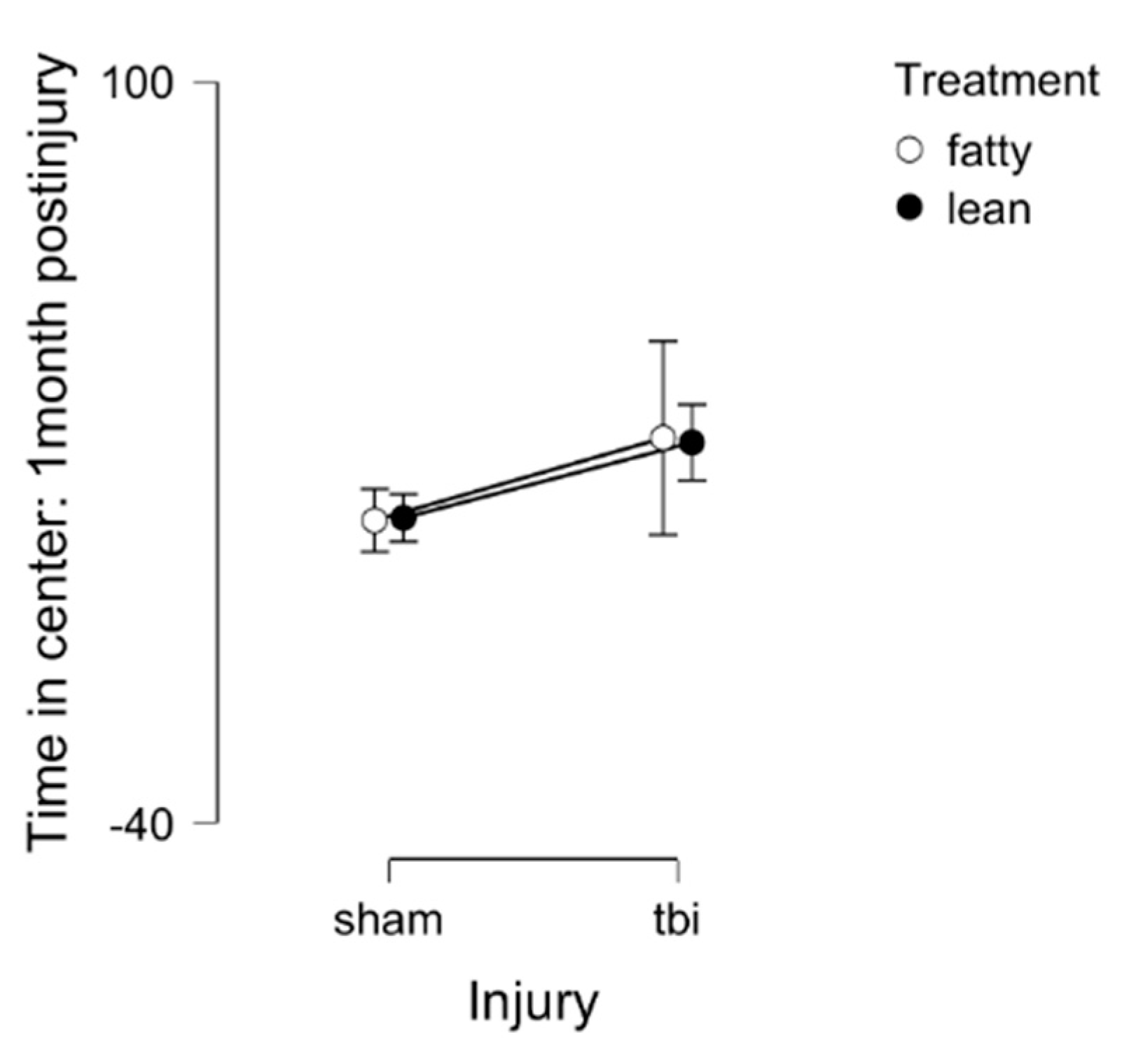
Open Field Test- under dark conditions (mobility test)
General mobility was assessed in the open field test performed under dark conditions. There were no significant differences in time in the center by strain (fatty
vs lean) or injury (sham
vs TBI) (
Figure 4). These results suggest that the differences observed under bright light conditions are likely indicative of anxiety-like behavior.
Lean: Sham (N=7), TBI (N=6)
Fatty: Sham (N=5), TBI (N=4)
Figure 4. Open field test in dark light 1 month post-injury in Zucker lean and fatty rats. Results are mean +/- SEM.
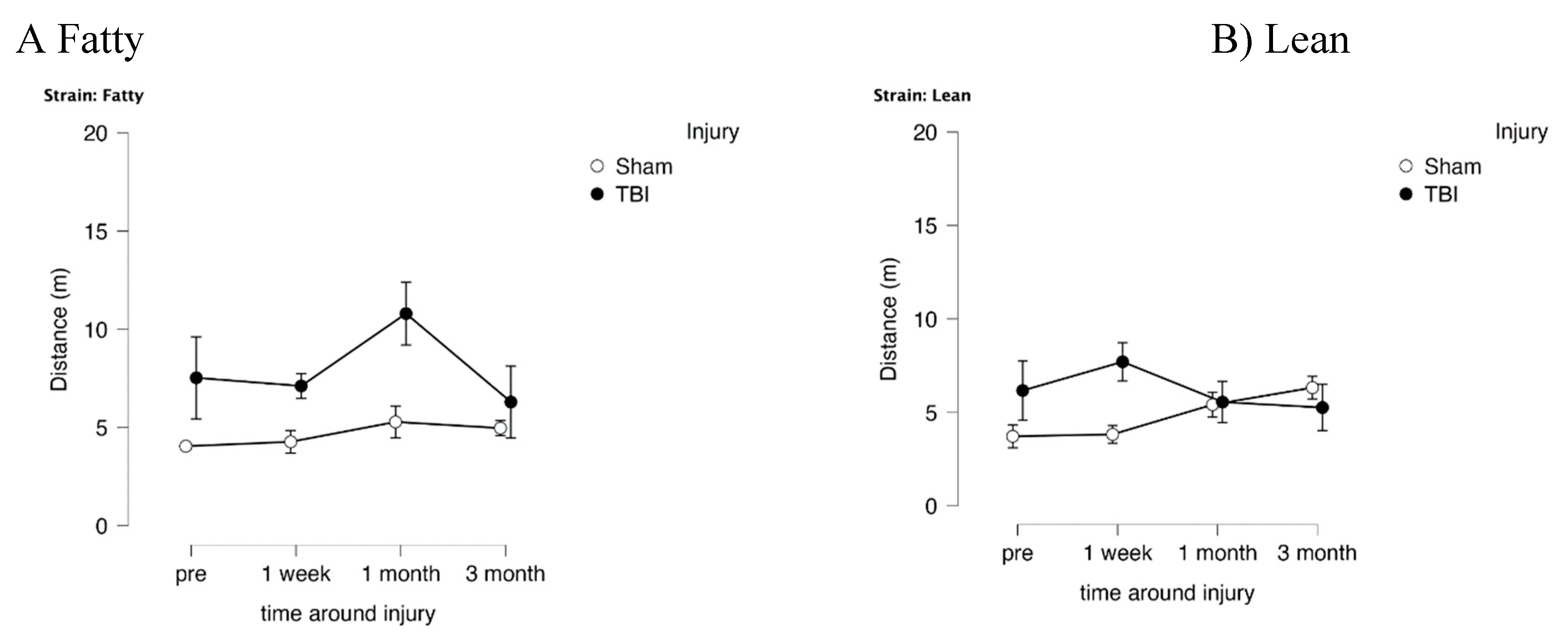
Morris water maze- a test of spatial memory
In fatty and lean Zucker rats exposed to TBI, distance (needed to locate swim platform) in the choice phase (CP) significantly exceeded that of sham-injured Zucker rats (
Figure 5A-B) (F(1,12)=8.314, p=0.014) indicative of TBI-associated spatial memory impairment. In Zucker fatty rats, the mean difference in distance between TBI and sham-injured rats peaked at 1-month post-injury (
Figure 5A) and there was no longer a statistically significant difference 3-months post injury. In lean Zucker rats, the mean difference in distance peaked earlier, at 1-week post injury, and there was no longer a statistically significant difference 1-month or longer post-injury (
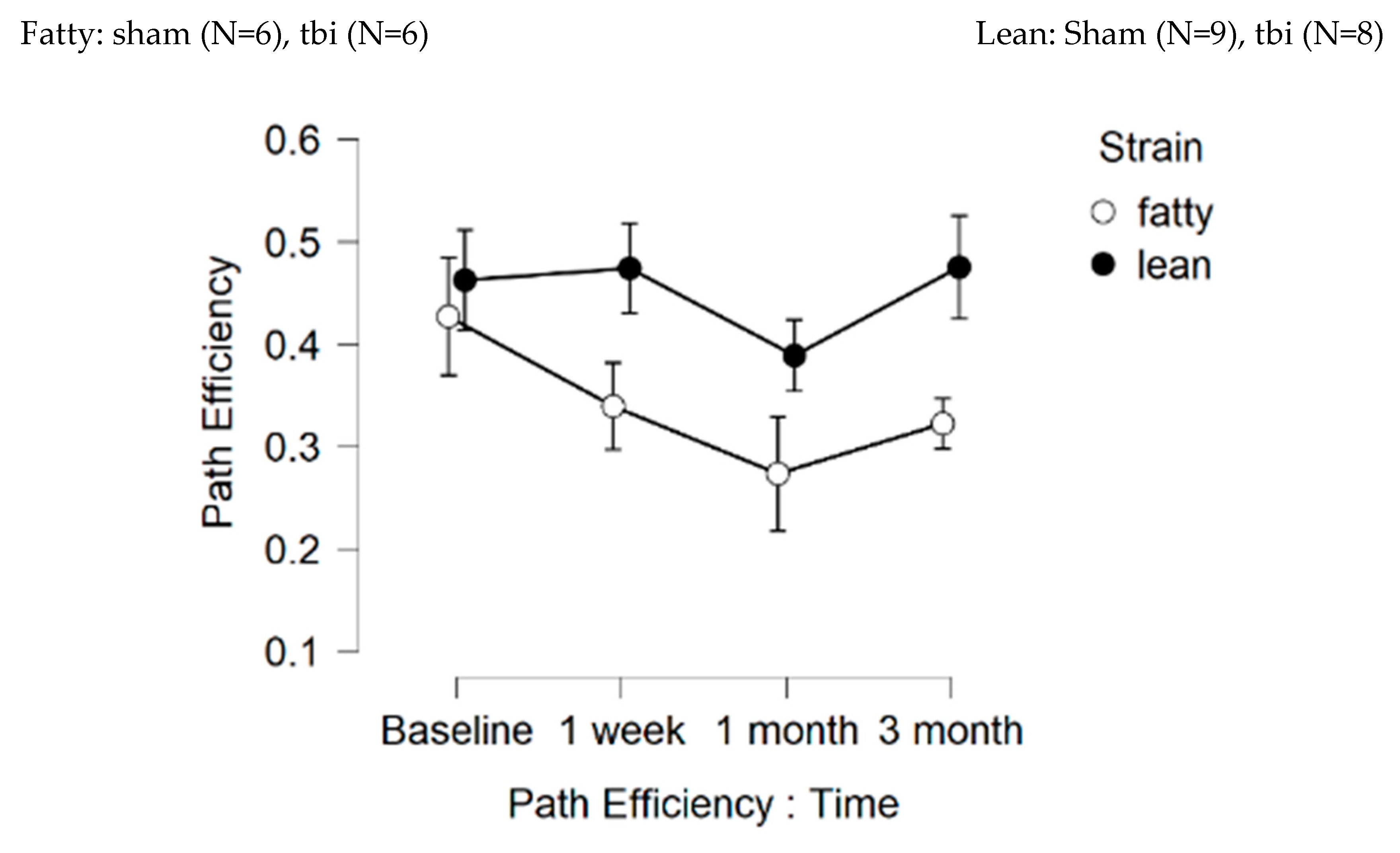
Figure 5B). These data suggest a more prolonged duration of spatial memory impairment in TBI Zucker fatty rats compared to TBI Zucker lean rats. Path efficiency was significantly lower in fatty
vs lean Zucker rats at all timepoints post-baseline (
Figure 6) including TBI and sham-injured fatty rats. These data suggest factors associated with obesity, hypertension and type 2 diabetes contribute to accelerated decline in cognitive function (spatial memory recall) in fatty
vs age-matched lean Zucker rats.
Figure 6. Path efficiency.
Fatty (N=12); Lean (N=17) rats
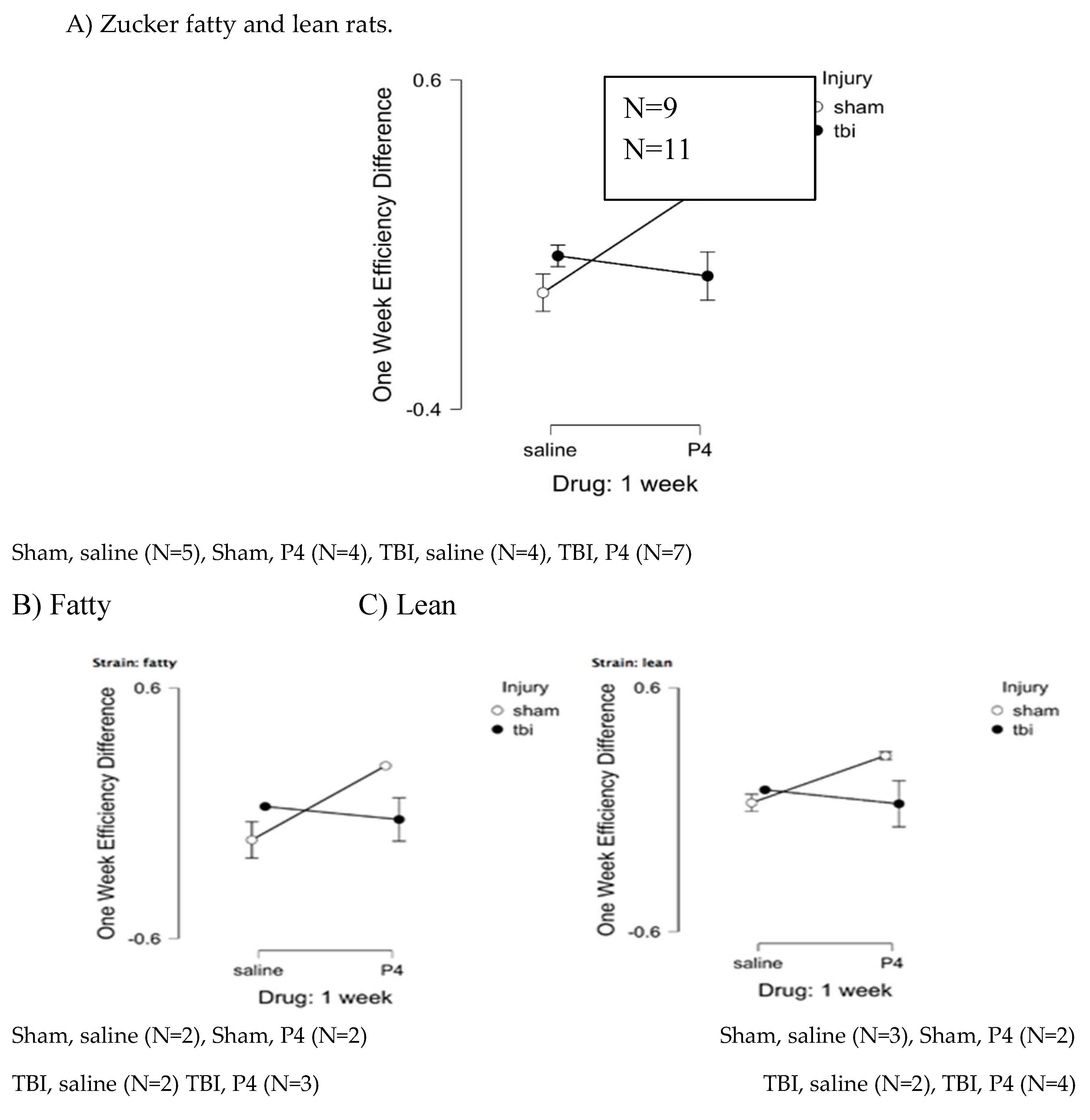
Effect of Short-term Peptide Administration on Spatial Memory
Peptide (SCLLADDN = SN..8) was administered to fatty rats (every other day) for seven days before and after injury and in lean rats for seven days after injury. Path efficiency difference is defined as [choice phase efficiency – sample phase efficiency] and is a measure of the strength of spatial memory recall. One-week post-injury, peptide-treated, sham-injured fatty and lean Zucker rats displayed significantly larger gain in path efficiency compared to saline-treated, sham-injured fatty and lean rats (F(1,10)=5.777, p=0.037) (
Figure 7A-C). Path efficiency difference did not differ significantly in peptide
vs saline-treated, fatty and lean Zucker rats who experienced TBI (filled circles,
Figure 7A-C). These data suggest the neuroprotective peptide (SCLLADDN) may enhance recall of spatial learning in sham-injured lean and fatty Zucker rats, but not in TBI lean or fatty Zucker rats.
Figure 7. Delayed match to position in the Morris water maze: Path efficiency difference at 1-week post injury in Zucker fatty and lean rats. Path efficiency difference was calculated as described in Methods and is a measure of the strength of recall of spatial learning. Results are mean +/- SEM.
There was no significant association between time (baseline, 1 week, 1 month, 3 months post-injury) and learning acquisition, i.e. path efficiency in the sample phase. A repeated measures ANOVA testing for a main effect of time on (sample phase) path efficiency had: F(3,42)=0.718, p=0.547. There were no significant interaction effects of (time x strain) or (time x injury) on learning acquisition, (sample phase, path efficiency). An ANOVA for time x strain had F(3,42)=0.483, p=0.696; and ANOVA for time x injury had (F(3,42)=1.275, p=0.295 (data not shown in Table).
Sucrose preference test
The Zucker fatty rat strain is known to exhibit a low preference for 1% sucrose solutions [
12] compared to other rat strains [
13] including Zucker lean rats [
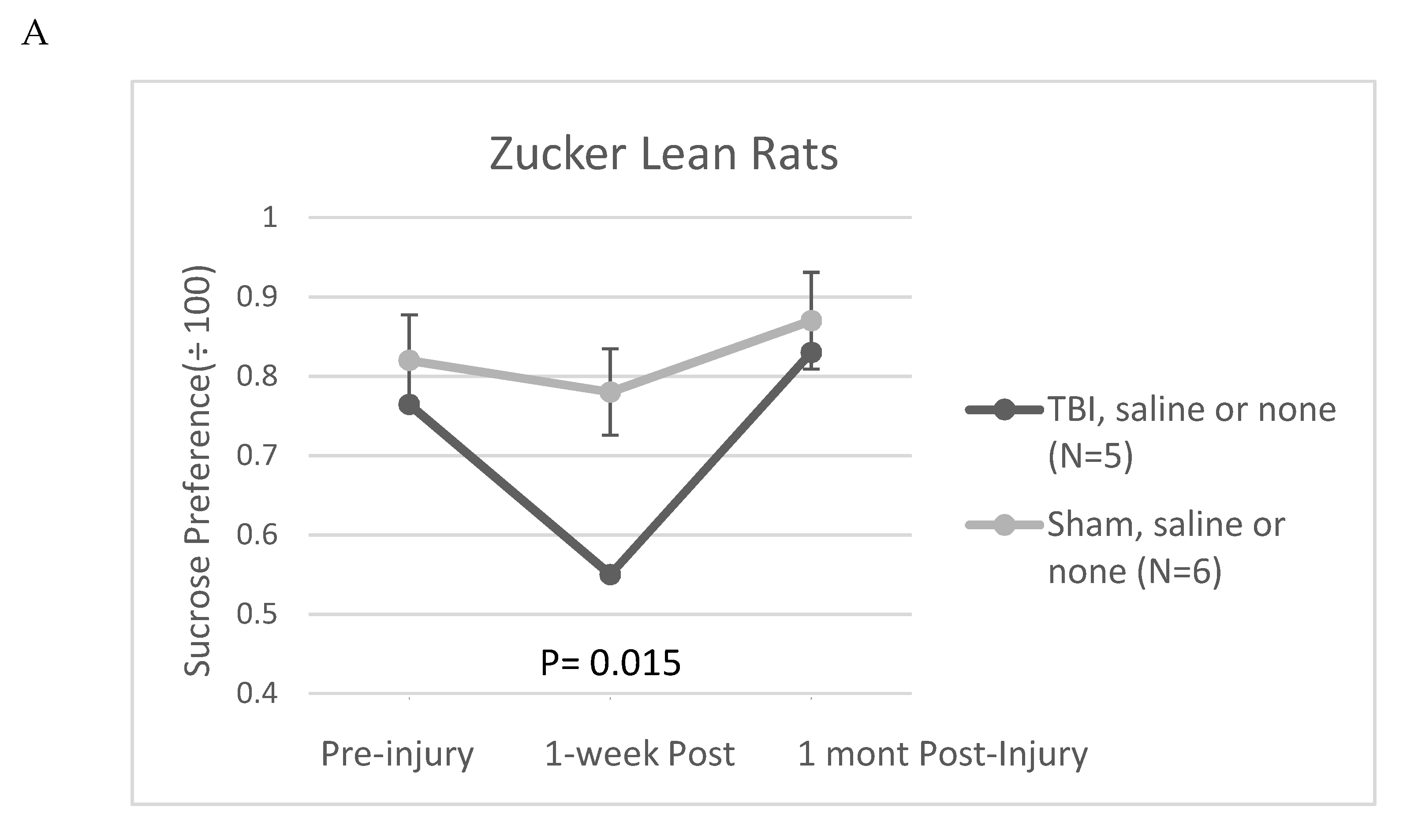
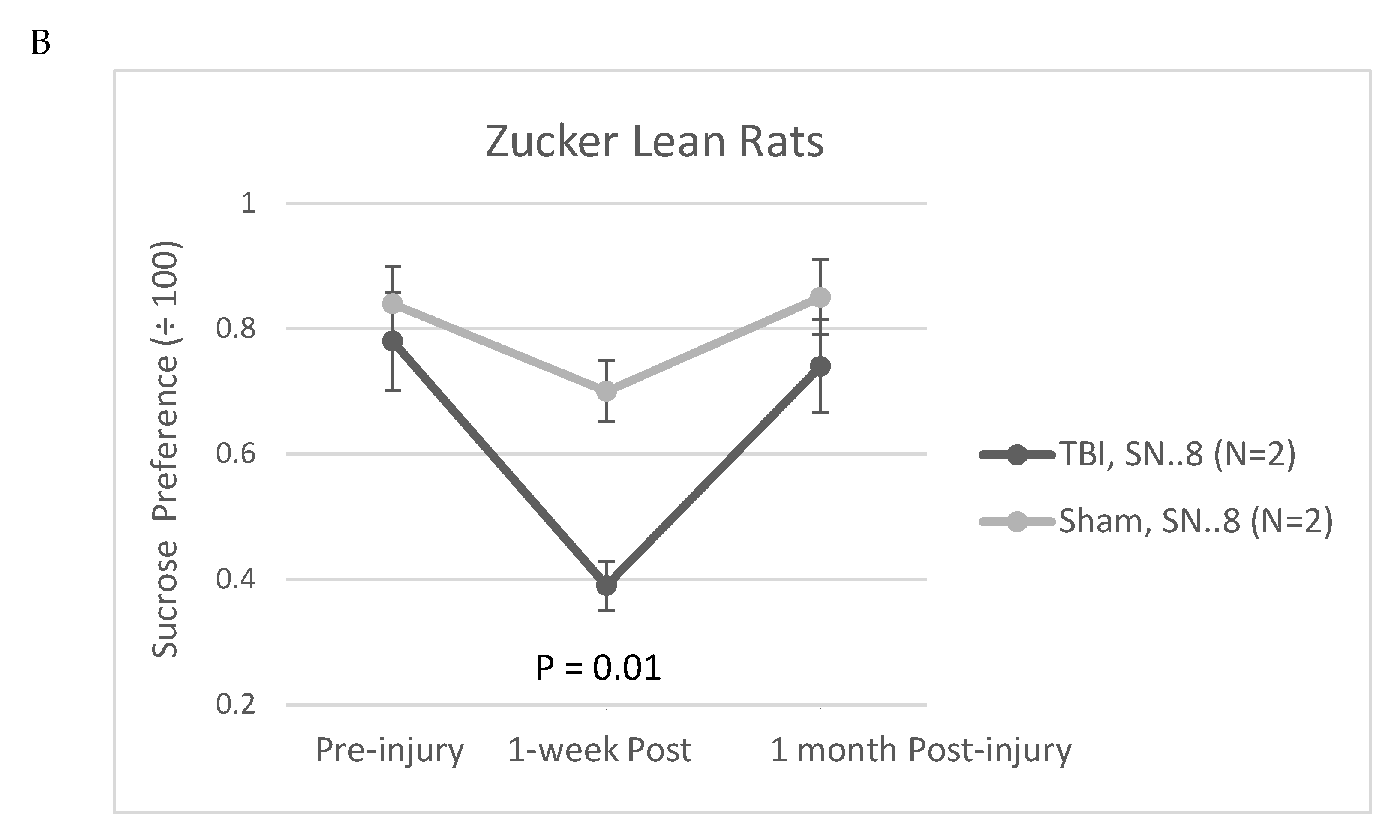
12]. The low baseline sucrose preference in the Zucker strain complicated the use of sucrose preference as a test of post-TBI anhedonia. Still in the Zucker lean rat, TBI (
vs sham injury) was significantly associated with reduced sucrose preference at 1-week post injury (
Figure 8A). In a small exploratory analysis, in a subset of Zucker lean rats, treatment with SN..8 peptide (
vs. saline or no treatment) did not appear to mitigate a TBI-associated decline in sucrose preference 1-week post injury (
Figure 8B).
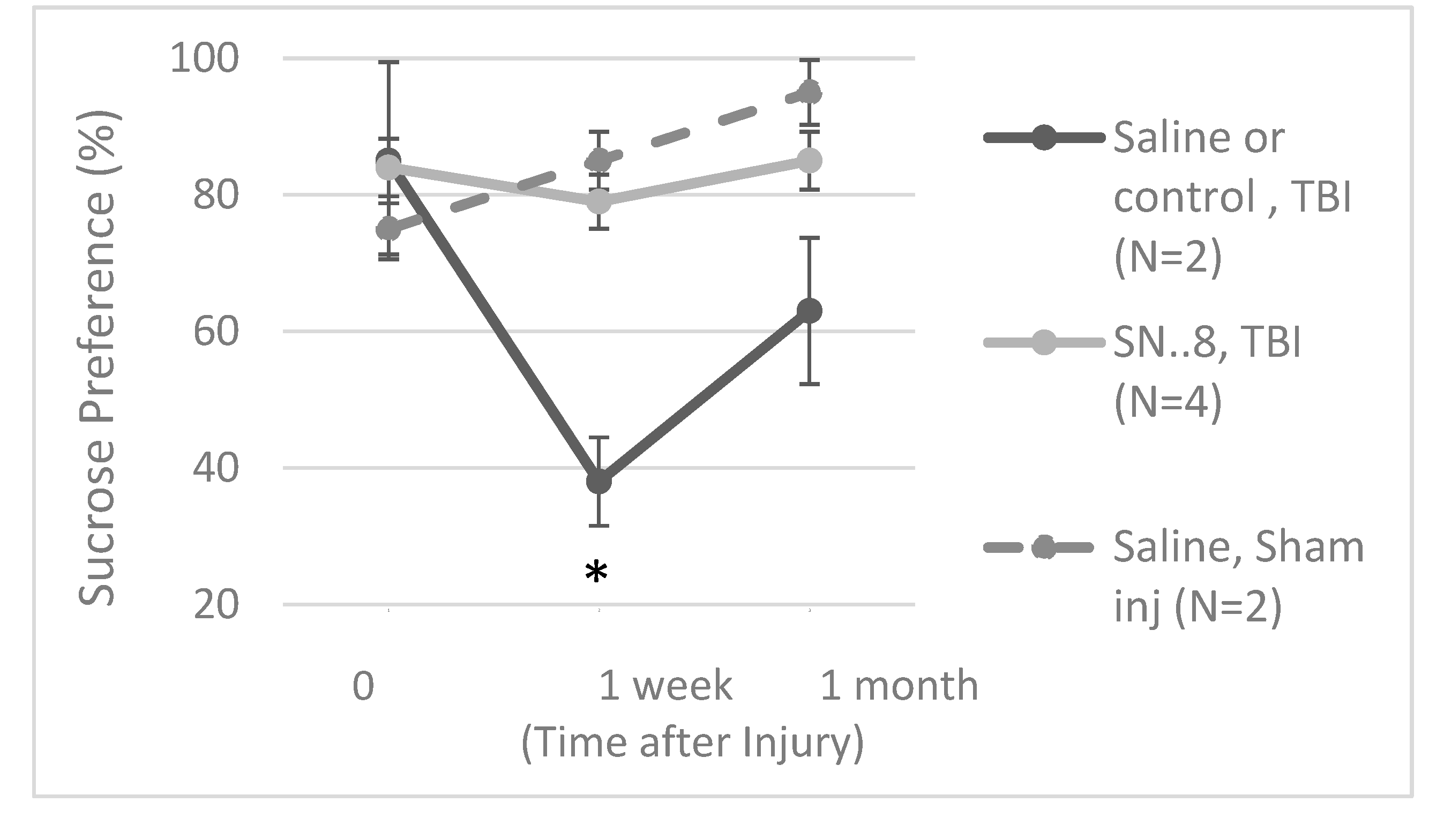
In order to control for the possible effects of stress associated with alternate daily (day 1, 3, 5) injections in the immediate post-injury period, we next tested whether single IP administration of SN..8 (2 mg/kg) vs. saline or identical (2 mg/kg) concentration of a non-protective, scrambled peptide LD..8 at 1-day (N=5 Zucker lean) or 3 days post injury (in N=3 Zucker fatty rats having normal baseline sucrose preference) might modulate sucrose preference post-injury. There was a trend of a significant association between SN…8 peptide (
vs saline or LD..8) treatment and higher sucrose preference 1-week post-injury in TBI Zucker rats (P=0.05;
Figure 9). The data are pooled from both lean and fatty Zucker rats, and only include a small subset of Zucker fatty rats (n=3) who spontaneously expressed high pre-injury sucrose preference (>/
~ 80%). More study in a rat strain expressing higher sucrose preference is needed to test whether SN..8 may protect against early (post-TBI) decreased sucrose preference.
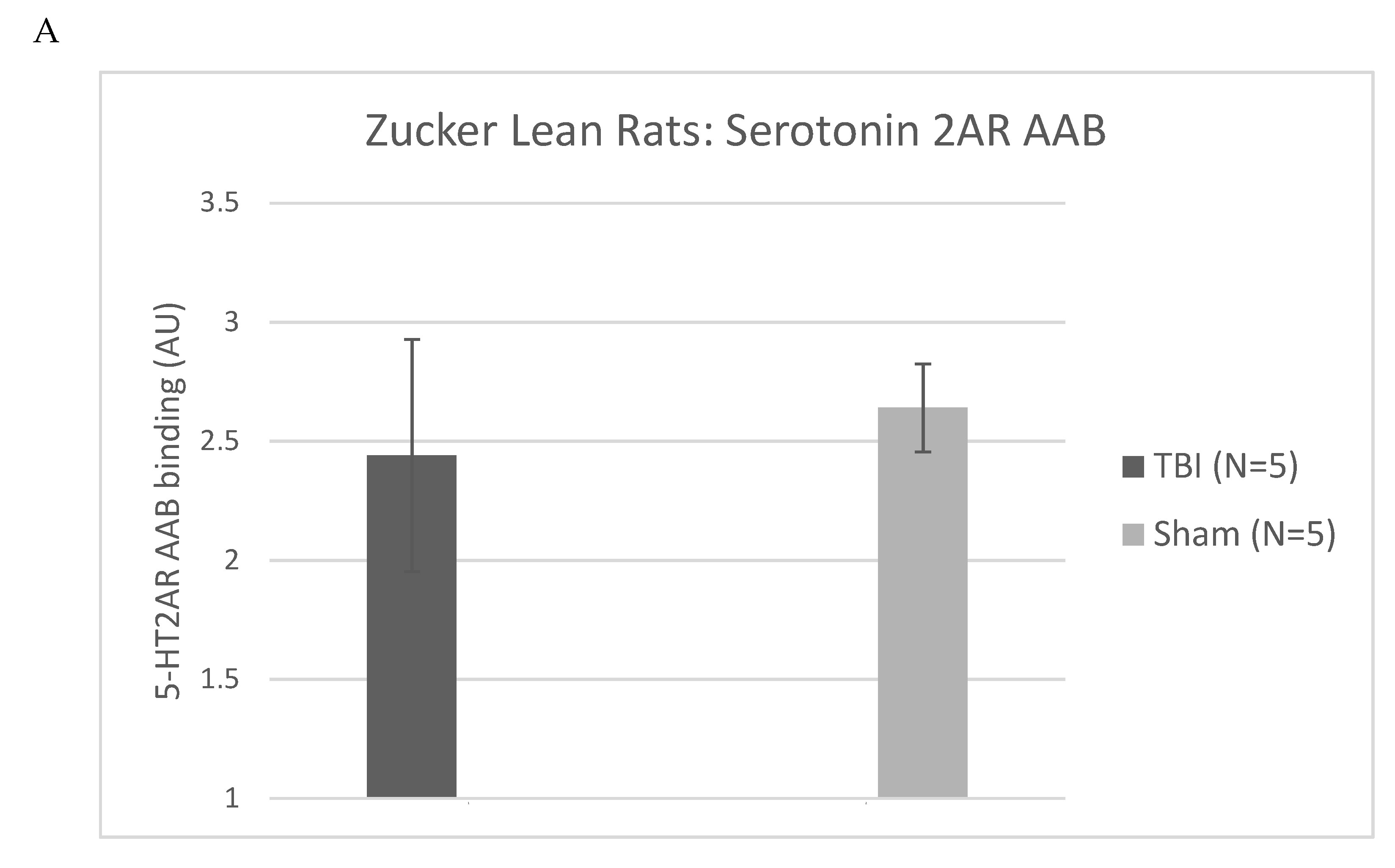
Plasma 5HT2AR agonist autoantibodies: relation to injury (Zucker lean rats)
Zucker fatty and lean rats had blood drawn from the retro-orbital plexus (at week 13) either for determination of plasma triglycerides and general chemistries (fatty) or for determination of IgG (lean) using a previously reported ELISA for epitope-specific 5HT2AR peptide binding [
6,
7]. Plasma 5HT2AR- peptide IgG binding level ranged from undetectable to 4.4-fold above background (N=10 rats), and the mean level (~2.5 times higher than background) did not differ significantly in Zucker lean rats exposed to TBI-
vs sham-injury (
Figure 10A) suggesting that genetic heterogeneity (Fa/?) = Fa/fa or Fa/Fa [6 ] (as opposed to injury
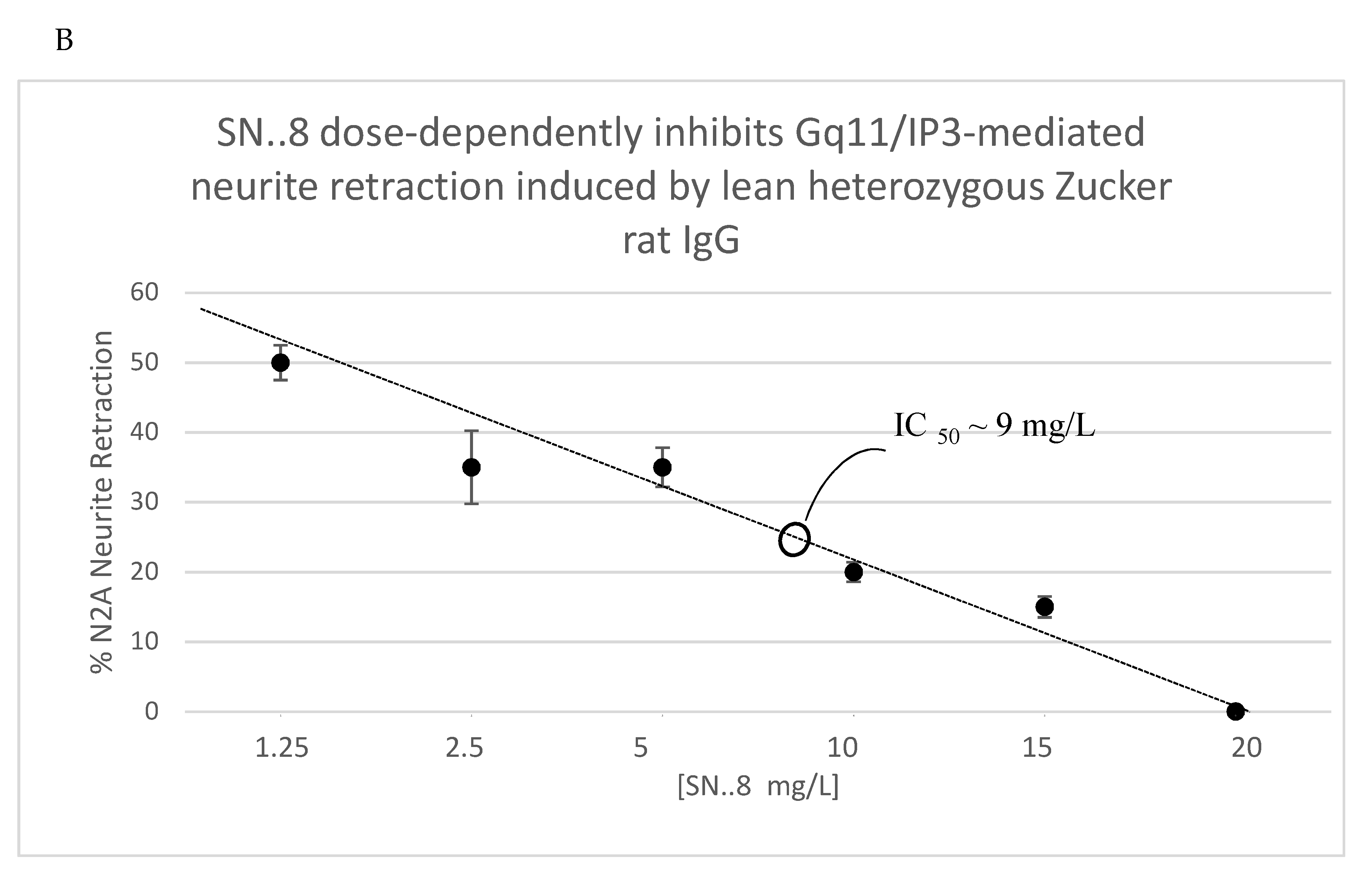
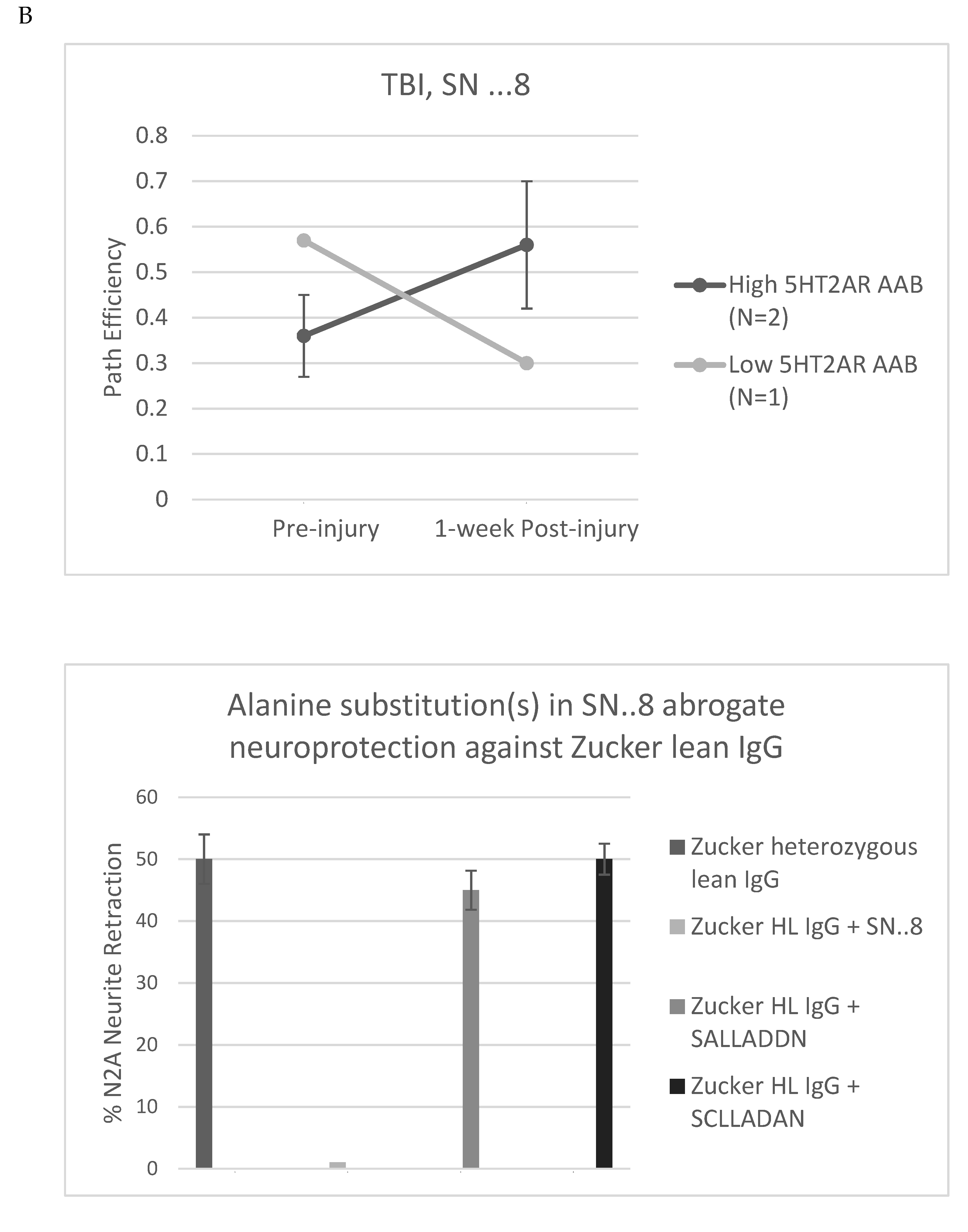
per se) in the lean Zucker rats was a more likely determinant of (13-week-old) plasma Ig level in the lean Zucker rats. The SN..8 peptide dose-dependently inhibited Zucker lean heterozygote rat Ig-induced neurite retraction
in vitro exhibiting an IC
50 of ~ 9 mg/L (
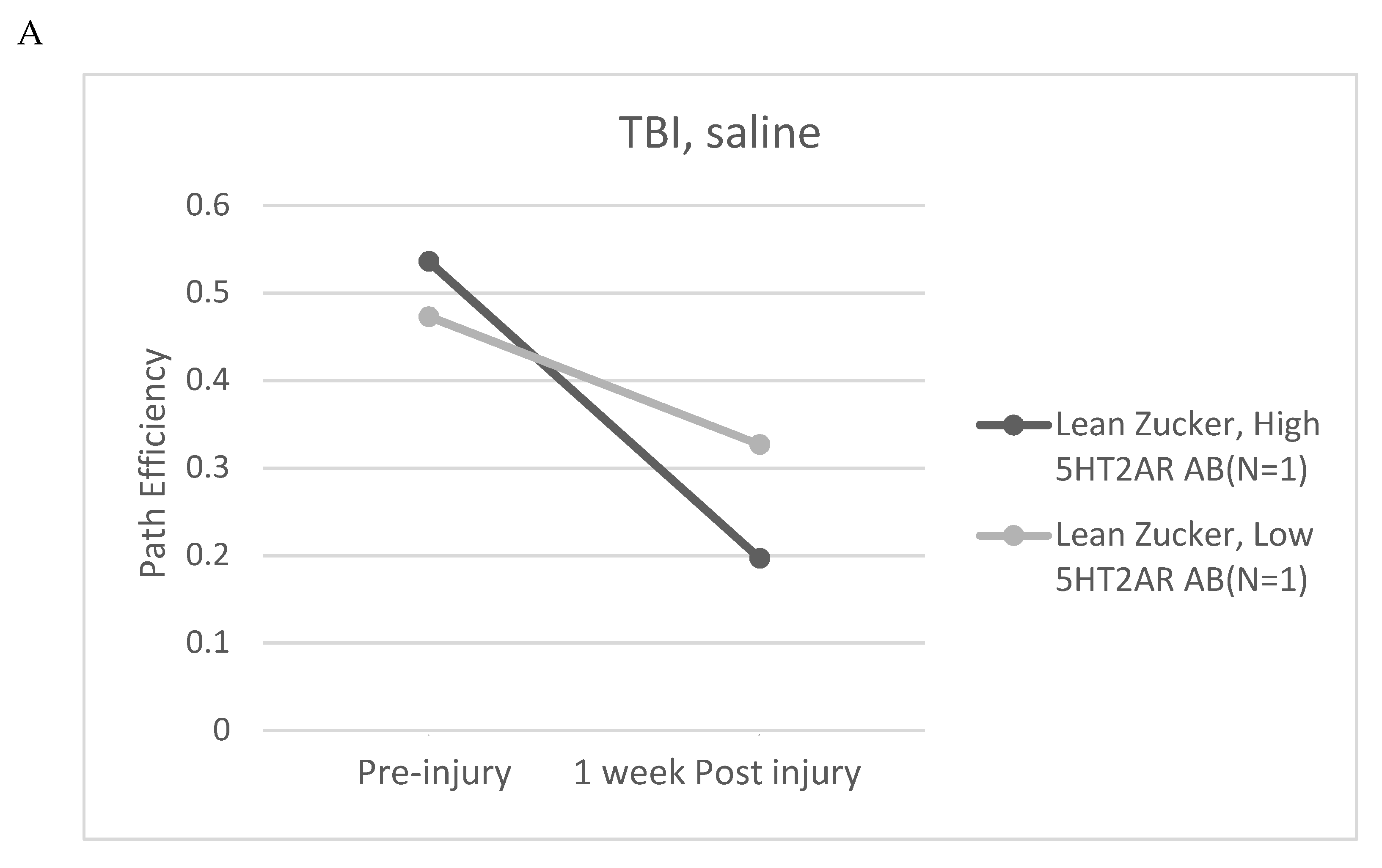
Figure 10B). Zucker lean rats expressing ‘high,’ (i.e. > 2-fold) vs. ‘low’ (i.e. </= 2-fold above background) plasma 5HT2AR-binding AAB were examined for effect of TBI + treatment (SN..8 vs. saline) on change in recall of spatial learning, i.e. 1-week post injury vs. pre-injury. In two lean Zucker rats both treated with saline, high (vs. lower) expression of plasma 5HT2AR AAB was associated with lower path efficiency at 1-week post TBI (
Figure 11A) suggesting that plasma 5-HT2AR agonist AAB may contribute (in part) to the mechanism of TBI-associated impairment of recall of spatial memory. In two lean Zucker rats having high plasma 5HT2AR AAB treatment with SN..8 peptide was associated with higher path efficiency (1-week post TBI) compared to a lean Zucker having low plasma Ig AAB (
Figure 11B). This suggests that SN..8 mechanism of neuroprotection may be selective to rats having high plasma 5HT2AR AAB expression and that other factors (besides AAB) contribute to TBI-associated impairment of spatial memory recall. Determination of plasma 5HT2AR AAB may allow patient selection to optimize benefit from treatment with SN…8. Zucker heterozygous lean (Fa, fa) rats expressed the highest level of plasma 5HT2AR AAB consistent with a role for the leptin receptor mutation in promoting humoral autoimmunity [
14,
15]. More study in Zucker heterozygous (Fa/fa) and homozygous (Fa/Fa) lean Zucker rats, the latter having lower plasma 5HT2AR AAB expression may be useful in isolating the role of different concentration of AAB on behavioral changes after TBI.
Mechanism of action of SCLLADDN (SN….8) peptide
The precise mechanism of SN..8 peptide’s
in vitro and
in vivo neuroprotective action is unknown. We had proposed that the epitope-specific SN..8 peptide may act as a ‘decoy’ which directly binds 5-HT2AR agonist IgG preventing receptor activation. Although we can’t exclude this possibility, we now provide evidence for an additional possible mechanism. Using linear synthetic peptides containing an alanine substitution for a highly conserved cysteine at EL2.50 (Ballesteros-Weinstein residue numbering system [
16]) S
ALLADDN or for an aspartic acid residue at position EL2.55 (SCLLAD
AN) we compared the
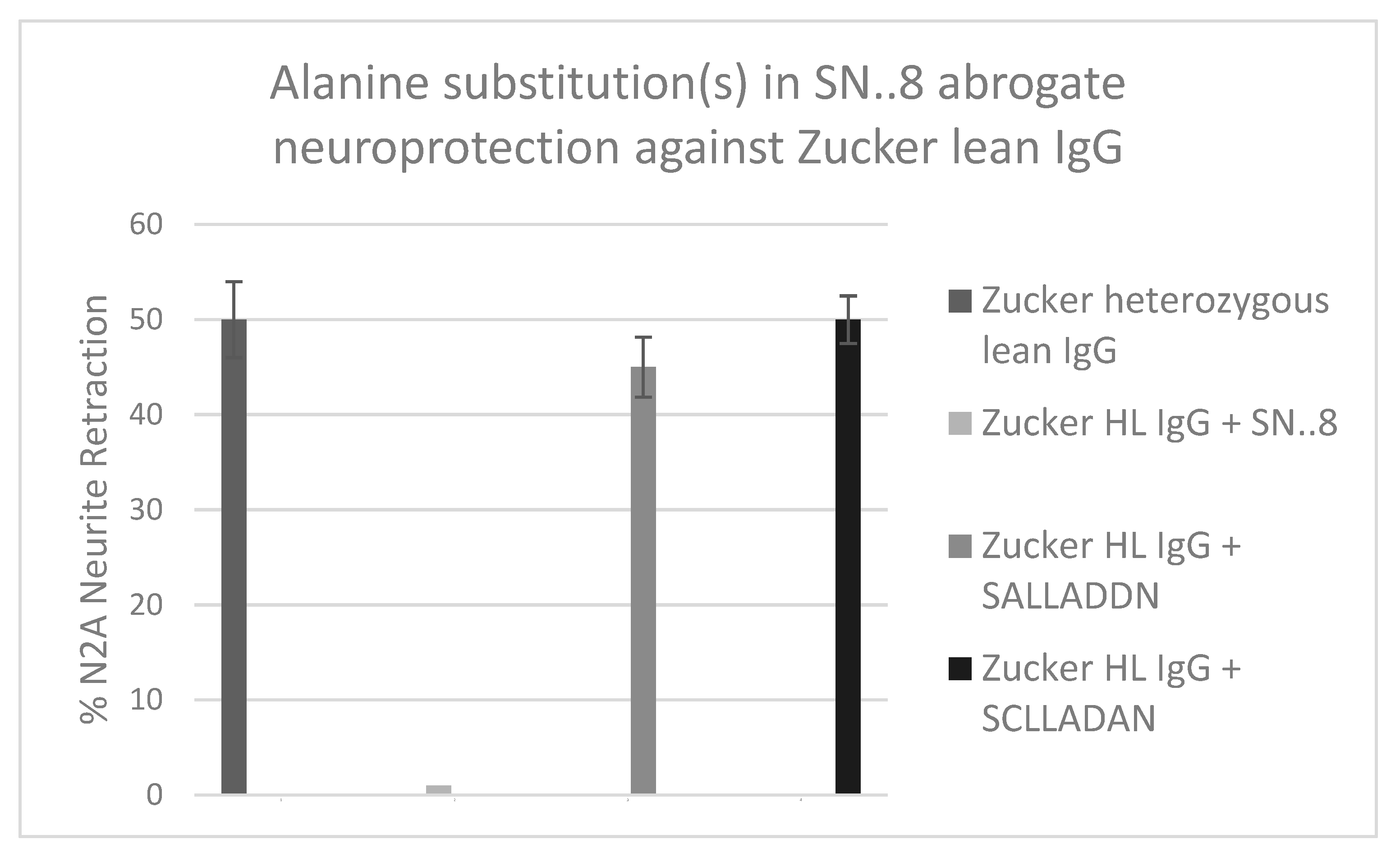
in vitro neuroprotective effects (Gq11/IP-mediated neurite retraction) of mutant vs. ‘wild-type’ SCLLADDN peptide. A 50-100 nanomolar concentration of Zucker heterozygous lean rat IgG caused 50% acute N2A neurite retraction which was completed prevented by co-incubation with twenty μg/mL concentration of wild-type SN..8 (
Figure 12). Pre-incubation of IgG with twenty μg/mL concentration of either ‘C to A’ or ‘D to A’ mutant peptides had no inhibitory effect on Zucker rat IgG-induced neurite retraction. Although (20 ug/mL) wild-type peptide alone had no effect on N2A neurite retraction, we found unexpectedly that incubation of N2A cells with a twenty μg/mL concentration of either ‘C to A’ or ‘D to A’ mutant peptides alone caused transient, reversible (after 5 minutes), Gq11/IP- mediated N2A neurite retraction (not shown in
Figure 12).
The wild-type SCLLADDN (SN..8) is identical to a subregion of the second extracellular loop (ECL) of the human serotonin 2A receptor reported by Wacker
et al [
17] to function as a ‘lid’ modulating the ingress and egress of ligands into and out of the 5HT2(B, or A) receptor’s orthosteric binding pocket (OBP). For example, closure of the lid prevented egress of the hallucinogenic ligand lysergic acid diethylamine (LSD) from the OBP causing an unusually long off-reaction time of LSD at the 5HT2B and 5HT2A receptors [
17]. Mobility of the lid peptide is dependent on its specific amino acid residues which in turn determines the kinetics of the open and closed receptor conformations [
17]. The lid function is conserved among many different aminergic GPCRs [
18] and a highly conserved cysteine residue EL2.50 involved in intrachain disulfide bonding normally prevents constitutive aminergic GPCR activation [
18]. The observation that C to A (S
CLLADDN to S
ALLADDN) or D to A (SCLLADDN to SCLLADAN) single amino acid residue substitution(s) each led to transient constitutive receptor Gq11/IP activation (in the presence of serotonin in the culture medium) is of interest since it suggests a possible direct effect of exogenously administered short lid peptides on stabilizing an active
vs inactive conformation of the receptor. We speculate that cysteine-containing ‘wild-type’ SN..8 (SCLLADDN) may spontaneously form aggregates important in antigen-antibody binding or which interfere with hydrogen bonding between GPCR transmembrane helices [
19] required for receptor activation. The Asp at position EL 2.54, shown in bold SCLLA
DDN in the wild-type peptide, is also highly conserved among family A GPCRs [
19] and it mediates hydrogen bonding between helix 2 and helix 7 underlying receptor activation [
19]. Replacement of the adjacent Asp (EL2.55) with Ala to form SCLLAD
AN may have led to a more highly mobile lid peptide which mediated transient receptor activation by increasing the probability of hydrogen bonding between helices 2 and 7. To our knowledge, these are the first data suggesting that small peptide mimics of an aminergic GPCR receptor ‘lid’ region in which key amino acid residues (Cys EL2.50 or Asp 2.55) have been replaced by alanine not only mediate transient receptor activation, but also abrogate neuroprotection associated with unaltered SN…8 lid peptide.
DISCUSSION
Obese type 2 diabetes mellitus and hypertension are associated with accelerated age-related cognitive decline in older adults [
20], and in a recent study of lifelong TBI- sufferers, (obesity and hypertension) were significant predictors of an increased hazard rate for the occurrence of major depressive disorder, Parkinson’s disease or dementia [
3]. The Zucker fatty rat is a widely-used genetic model of morbid obesity [
4]. Morbid obesity promotes peripheral inflammation associated with increased risk for neuropsychiatric and neurodegenerative disorders in humans [
21], yet there have been relatively few prior neurobehavioral studies in the Zucker fatty rat strain which might shed light on underlying mechanisms.
Here we report that the Zucker diabetic hypertensive fatty rat experienced significantly greater spontaneous decline in spatial memory compared to age-matched lean Zucker rats and longer-lasting spatial memory impairment following mild traumatic brain injury compared to lean Zucker rats.
It is not clear to what extent diabetes or hypertension (in Zucker fatty rats) may have affected the rate of decline in recall of spatial memory. In human studies, older adult type 2 diabetes populations experienced increased rate(s) of decline in executive function and processing speed; and hypertension, hypertriglyceridemia, and diabetes duration, (but not glucose level
per se) was each a significant predictor of accelerated cognitive decline [
3]. Severe hypertriglyceridemia which paralleled the development of morbid obesity in Zucker fatty rat may have contributed in part to observed sub-strain behavioral differences. In a prior report, male albino rats on a high-fat (
vs. normal diet) developed obesity, and elevated lipid profile and had worse performance (on spatial memory task) in the Morris water maze [
22] suggesting a role for metabolic factors in the Zucker fatty rat spatial memory impairment.
Neurogenesis occurs in the dentate gyrus (DG) region of the hippocampus in adult mammals, and it affects not only spatial learning, but also mood regulation [
23]. Hippocampal neural progenitor cells (NPC) develop in a unique vascular niche [
24] exposed to the general circulation. Circulating serotonin 2A receptor modulatory Ig (from morbidly-obese, and adult diabetic patients) had not only anti-endothelial effects [
25] which could adversely impact neurogenesis, but were also reported to adversely affect the maturation, survival and electrical excitability of rat dentate gyrus NPC
in vitro [
26]. The serotonergic system has a well-established role in the regulation of mood and emotional disorders [
27]. Glucocorticoid (GR) and mineralocorticoid receptors (MR) systems are highly expressed in hippocampal brain regions [
28] and regulate the effects of stress on the hypothalamic/pituitary/adrenal axis. Both GR and MR agonists were reported to suppress the rate of adult DG hippocampal neurogenesis and their inhibitory effects (on DG neurogenesis) were reportedly additive [
29].
The Zucker fatty rat has elevated plasma aldosterone [
30] which is predicted to cause increased brain MR activity likely mediating increased TBI- associated suppression of neurogenesis via combined increased MR activity and GR activity (stress associated with injury). It is possible that treatment with the neuroprotective serotonin 2A receptor peptide fragment did not protect against TBI-associated spatial memory impairment (in part) because of a strong inhibitory effect from combined GR and MR effects on DG neurogenesis. In the absence of competing inhibitory effects from GR and/or MR activity (i.e. in sham-injured lean rats) serotonin 2A receptor peptide treatment appeared to have a significant beneficial effect on improving recall of spatial learning- presumably (via enhanced DG neurogenesis) in the absence of mechanical injury and stress associated with TBI. Increased brain MR activity in the Zucker fatty rat may play a role in unexpectedly lower pre-injury, anxiety-like behavior (open field test) compared to Zucker lean rat. Both 5-HT2AR and MR are expressed in cortical brain regions and have a role in anxiety-like behavior. Rozenboom
et al [
31] reported that increased forebrain MR expression in transgenic mice reduced anxiety in both female and male mice in part via a mechanism involving decreased expression of the hippocampal GR and 5-HT 1a receptor genes.
Cortical expression of 5HT2A, -B, -C receptors was identical in ‘high anxiety’ Lewis
vs. ‘low anxiety’ SHR rat strains [
32], however, cortical Gq11/IP accumulation (via 5HT2A, B or C receptors) was substantially higher in the ‘high anxiety’ Lewis rat [
33]. It is possible that transient blood brain barrier disruption following mTBI exposes cortical receptor sites to circulating long-acting 5-HT2AR agonist IgG predicted to enhance anxiety-like behavior. Serotonin 2A receptor expression is normally higher in cortex
vs. hippocampus compared to MR and GR expression which is higher in hippocampus
vs. cortical brain regions. The data suggesting a potential therapeutic benefit of a serotonin 2A receptor peptide on anxiety-like behavior or anhedonia have particular relevance to younger post-deployment military veterans suffering mTBI in whom anxiety and depression may occur in up to 50% of cases and are the most common early neuropsychiatric disturbances [
34].
In reports from different laboratories the systemic administration of a 5-HT2A receptor agonist (psilocin, or TCB-2) significantly impaired spatial memory recall in the Morris water maze (MWM) in rodents [
35]. Our data that a 5HT2AR- specific lid peptide SCLLADDN (SN..8) enhanced spatial memory in the MWM is consistent with the possibility that the receptor peptide may prevent 5HT2A receptor activation by endogenous 5-HT2AR ligand agonists.
Taken together, these data suggest that neuroprotection from a novel serotonin 2A receptor
peptide may be more likely to occur under conditions in which hippocampal neurogenesis is not already strongly suppressed by other hormonal factors principally, increased hippocampal GR and MR activity. A role for DG neurogenesis in mediating the putative neuroprotective effects of a serotonin 2A receptor peptide on recall of spatial learning and/or anhedonia requires more direct study. Traumatic brain injury increases proliferation of immature DG neural progenitor cells, however, several studies have reported either resulting aberrant migration [
36] or incomplete development of such immature NPCs [
37]. Several lines of evidence support a role for 5-HT2AR agonist Ig in mediating anhedonia including our report that intracerebroventricular (ICV) injection of human diabetic depression Ig significantly reduced sucrose preference in c57bl/6 male mice compared to ICV injection of non-depressed diabetic Ig [
25]. Serotonin 2A receptor activation normally depolarizes excitatory and inhibitory neurons [
35] leading to increased synaptic activity which is essential to the normal development of immature DG neural progenitor cells. In our prior studies of cultured rat DG neural progenitor cells, serotonin 2A receptor Ig from human morbidly-obese patients caused long-lasting depolarization in DG NPCs and reduced survival and neurite outgrowth in these immature neurons [38, 26]. These data suggest that the rapid effect (several days) following IP injection of 5-HT2AR peptide on sucrose preference may be due to modulation of synaptic inputs onto hippocampal neurons rather than changes to the DG circuitry from newly-born neurons which is expected to require several weeks [
35].
In summary, systemic administration of a small peptide mimic of the 5HT2A receptor lid peptide appeared to confer neuroprotective effects on anxiety, depression, and recall of spatial memory tasks at early timepoints after sham injury in Zucker rats. TBI was associated with larger post-injury impairments in spatial memory recall, anhedonia and anxiety (compared to sham injury) and in most cases treatment with the neuroprotective peptide was unable to significantly modulate the behavioral impairment(s), especially in the Zucker fatty rat.
Figure 1.
Timeline of treatments, exposures and behavioral assessments in Zucker rats.
Figure 1.
Timeline of treatments, exposures and behavioral assessments in Zucker rats.
Figure 2.
Bright light open field test (OFT) 1.5 months post-injury in Zucker rat substrains. Open field test in bright light 1.5 months post-injury in Zucker lean and fatty rats. Results are mean +/- SEM. Time in center was determined by Any Maze software as described in Methods.
Figure 2.
Bright light open field test (OFT) 1.5 months post-injury in Zucker rat substrains. Open field test in bright light 1.5 months post-injury in Zucker lean and fatty rats. Results are mean +/- SEM. Time in center was determined by Any Maze software as described in Methods.
Figure 3.
Open field test in bright light 1.5 months post-injury in Zucker lean and fatty rats: strain x injury x drug interaction on time in center. Results are mean +/- SEM.
Figure 3.
Open field test in bright light 1.5 months post-injury in Zucker lean and fatty rats: strain x injury x drug interaction on time in center. Results are mean +/- SEM.
Figure 4.
Open field test in dark light (mobility) at 1 month post-injury.
Figure 4.
Open field test in dark light (mobility) at 1 month post-injury.
Figure 5.
Delayed match to position in the Morris water maze: distance as a function of time before and after injury in Zucker lean and fatty rats. Results are mean +/- SEM. Distance was determined by Any Maze software as described in Methods.
Figure 5.
Delayed match to position in the Morris water maze: distance as a function of time before and after injury in Zucker lean and fatty rats. Results are mean +/- SEM. Distance was determined by Any Maze software as described in Methods.
Figure 6.
Delayed match to position in the Morris water maze: path efficiency as a function of time before and after injury in Zucker lean and fatty rats. Results are mean +/- SEM. Path efficiency was determined by Any Maze software as described in Methods.
Figure 6.
Delayed match to position in the Morris water maze: path efficiency as a function of time before and after injury in Zucker lean and fatty rats. Results are mean +/- SEM. Path efficiency was determined by Any Maze software as described in Methods.
Figure 7.
Path efficiency difference: one- week post-injury.
Figure 7.
Path efficiency difference: one- week post-injury.
Figure 8.
Sucrose preference in Zucker lean rats exposed to mild TBI or sham injury and treated with A) saline or nothing or B) SN..8 peptide. Results are mean +/- SEM. Sucrose consumption was calculated as described in Methods.
Figure 8.
Sucrose preference in Zucker lean rats exposed to mild TBI or sham injury and treated with A) saline or nothing or B) SN..8 peptide. Results are mean +/- SEM. Sucrose consumption was calculated as described in Methods.
Figure 9.
Sucrose preference in Zucker lean and fatty rats. Rats were treated with a single intraperitoneal dose of SN..8 (2 mg/kg), saline, or LD..8 (2 mg/kg) (1-3 days) after TBI or sham injury. Data are pooled from two experiments. Results are mean +/- SEM. *P =0.05, Student’s t test comparing results at 1-week in TBI subgroups (saline or LD..8 peptide vs SN.8); N=5 Zucker lean rats: SN..8, TBI (N=2), Saline, TBI (N=1), Saline, sham (N=2). N=3 Zucker fatty rats: SN..8, TBI (N=2); scrambled peptide (LD..8), TBI (N=1). Lean rats were injected 1 day after TBI or sham injury, fatty rats were injected 3 days after TBI
Figure 9.
Sucrose preference in Zucker lean and fatty rats. Rats were treated with a single intraperitoneal dose of SN..8 (2 mg/kg), saline, or LD..8 (2 mg/kg) (1-3 days) after TBI or sham injury. Data are pooled from two experiments. Results are mean +/- SEM. *P =0.05, Student’s t test comparing results at 1-week in TBI subgroups (saline or LD..8 peptide vs SN.8); N=5 Zucker lean rats: SN..8, TBI (N=2), Saline, TBI (N=1), Saline, sham (N=2). N=3 Zucker fatty rats: SN..8, TBI (N=2); scrambled peptide (LD..8), TBI (N=1). Lean rats were injected 1 day after TBI or sham injury, fatty rats were injected 3 days after TBI
Figure 10.
Plasma serotonin 2A receptor peptide binding autoantibodies (AAB) A) in 13-week old Zucker lean rats exposed to TBI vs sham injury. B) Dose-dependent inhibition of N2A neurite retraction from 100 nM concentration of Zucker heterozygous lean by SN..8. Autoantibody ELISA and neurite retraction assay were performed as described in Methods. Results are mean +/- SEM.
Figure 10.
Plasma serotonin 2A receptor peptide binding autoantibodies (AAB) A) in 13-week old Zucker lean rats exposed to TBI vs sham injury. B) Dose-dependent inhibition of N2A neurite retraction from 100 nM concentration of Zucker heterozygous lean by SN..8. Autoantibody ELISA and neurite retraction assay were performed as described in Methods. Results are mean +/- SEM.
Figure 11.
Change in path efficiency in Morris water maze in Zucker lean rats having high vs low plasma serotonin 2A receptor autoantibodies (AAB) and treated either with A) saline or B) SN..8 peptide. Results are mean +/- SEM.
Figure 11.
Change in path efficiency in Morris water maze in Zucker lean rats having high vs low plasma serotonin 2A receptor autoantibodies (AAB) and treated either with A) saline or B) SN..8 peptide. Results are mean +/- SEM.
Figure 12.
Effect of targeted ‘C to A’ or ‘D to A’ amino acid substitutions in ‘wild-type’ SCLLADDN peptide on the resulting mutant peptides’ (SALLADDN) or (SCLLADAN) ability to prevent acute N2A neurite retraction in the presence of 50-100 nM concentration of Zucker heterozygous lean rat IgG. Results are mean +/- SEM. Neurite retraction assay was carried out as described in Methods.
Figure 12.
Effect of targeted ‘C to A’ or ‘D to A’ amino acid substitutions in ‘wild-type’ SCLLADDN peptide on the resulting mutant peptides’ (SALLADDN) or (SCLLADAN) ability to prevent acute N2A neurite retraction in the presence of 50-100 nM concentration of Zucker heterozygous lean rat IgG. Results are mean +/- SEM. Neurite retraction assay was carried out as described in Methods.