Submitted:
30 April 2023
Posted:
01 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
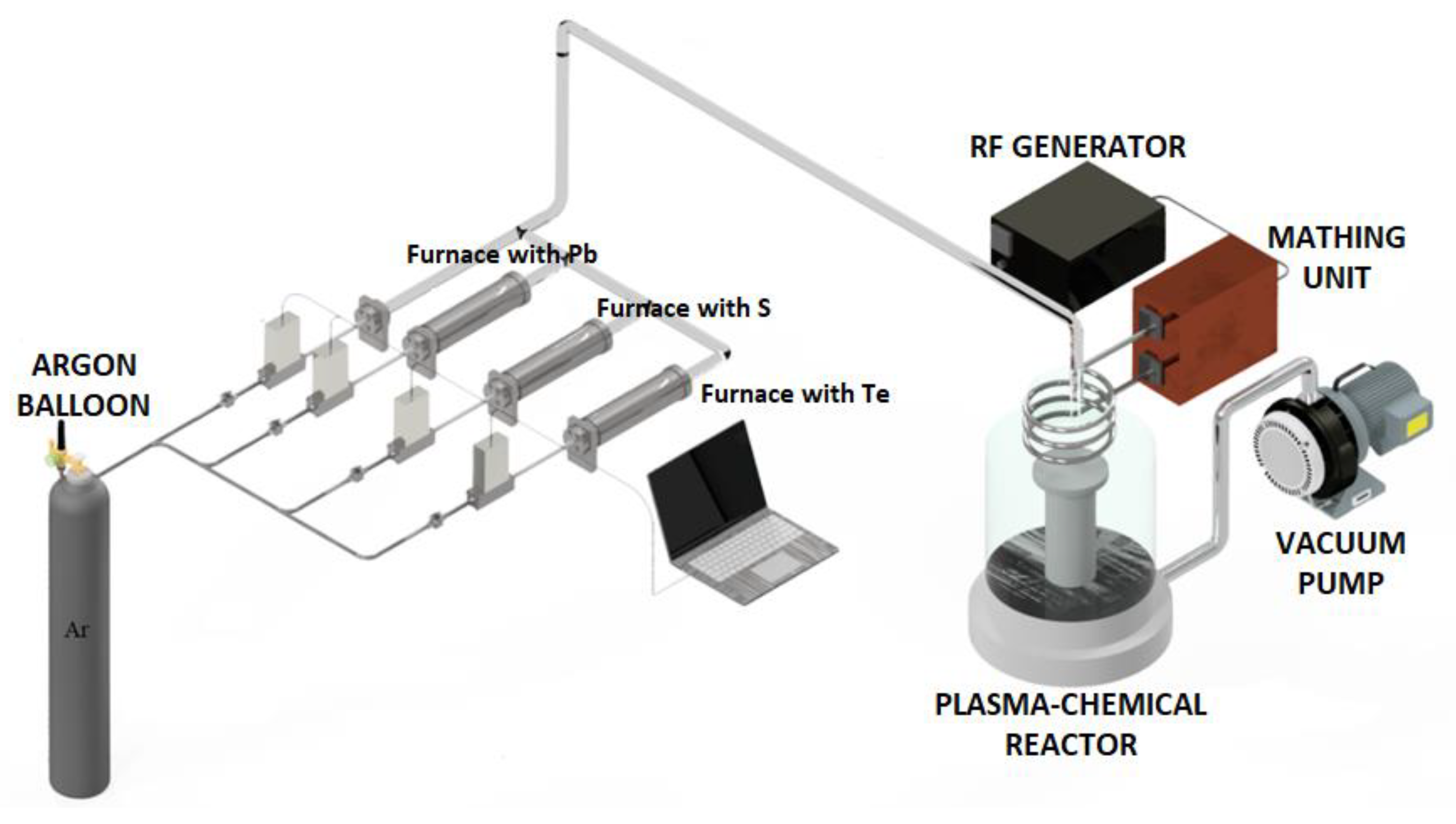
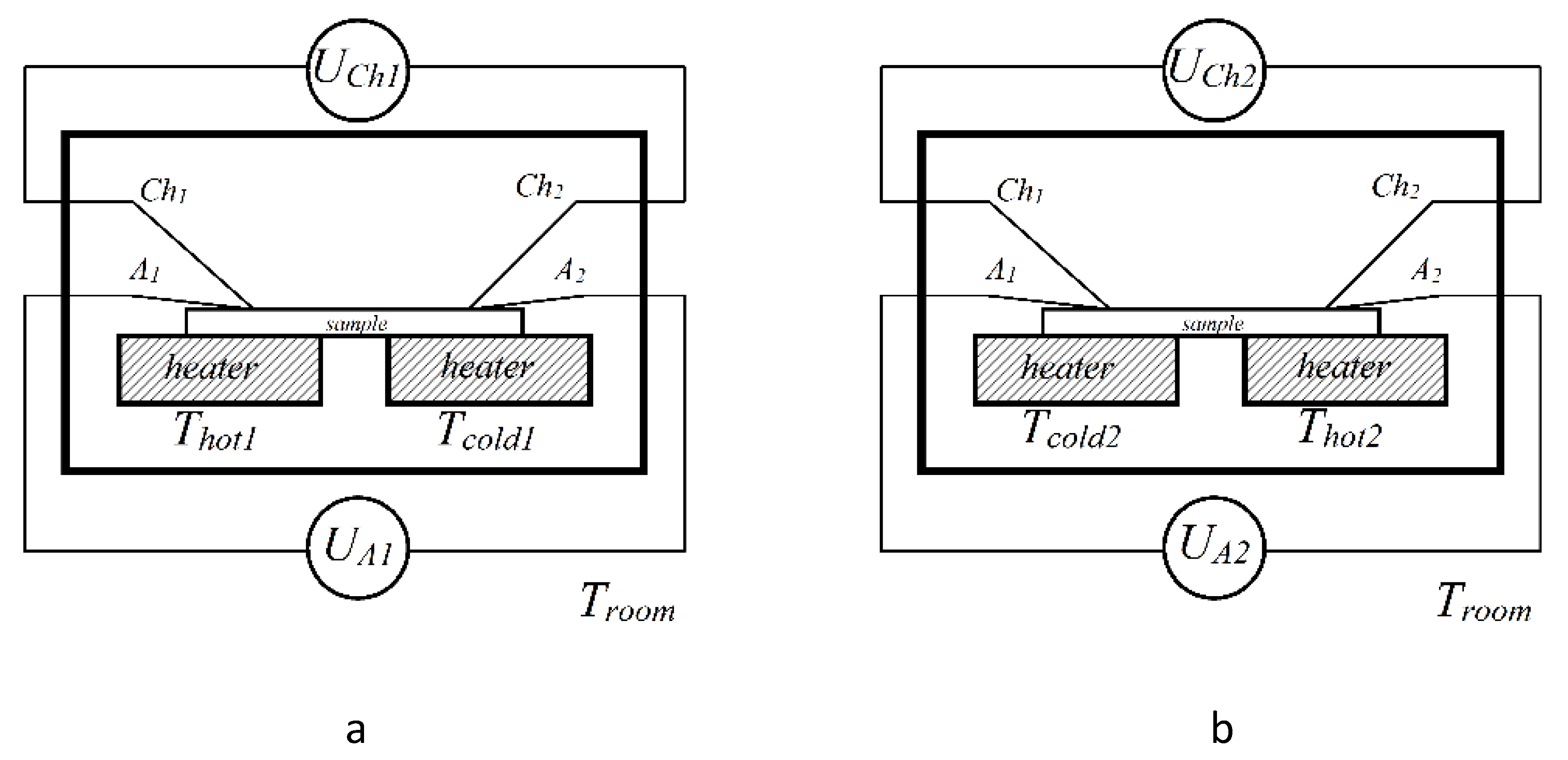
2. Materials and methods
3. Results and discussion
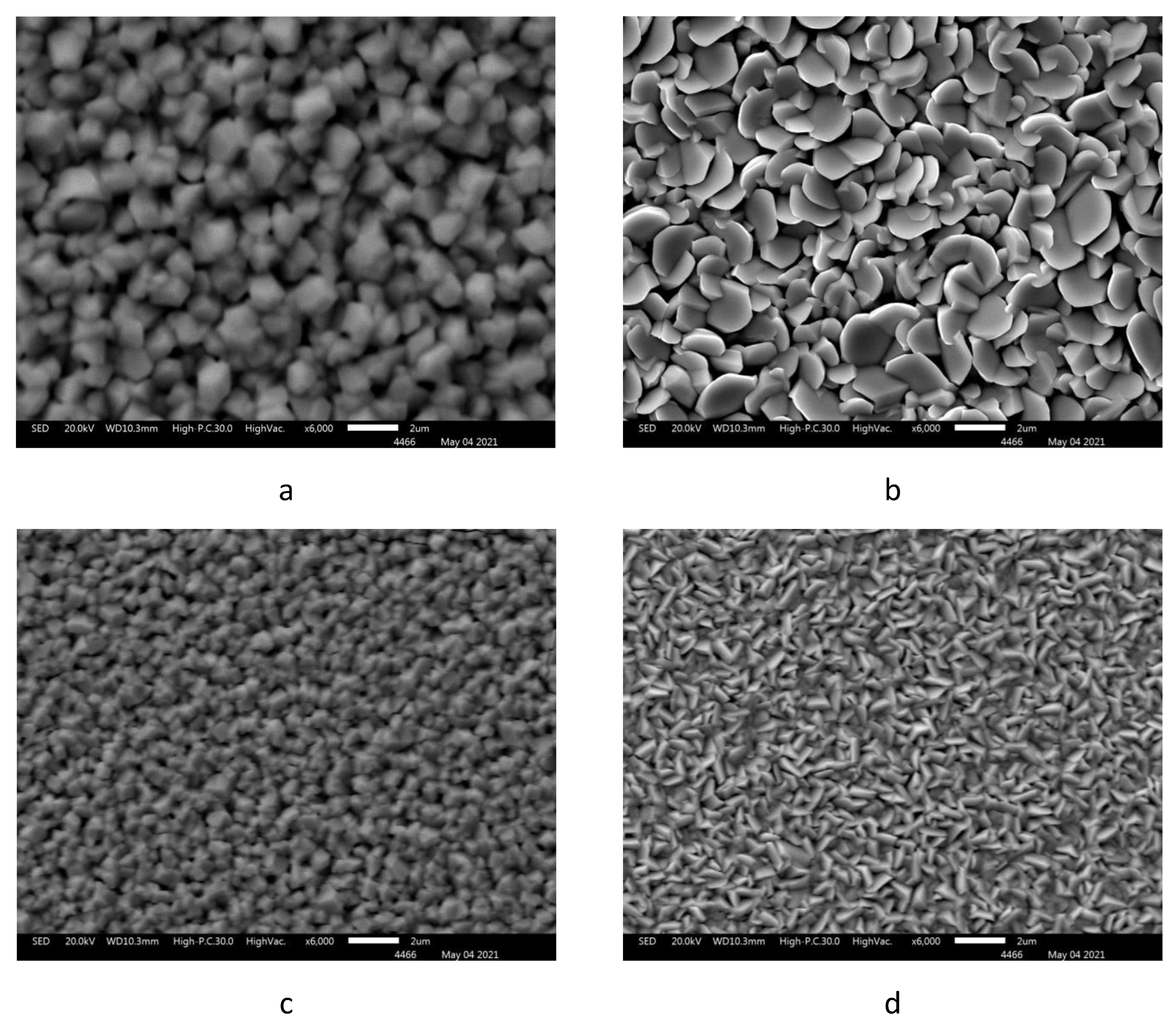
3.1. Elemental analysis and scanning electron microscopy
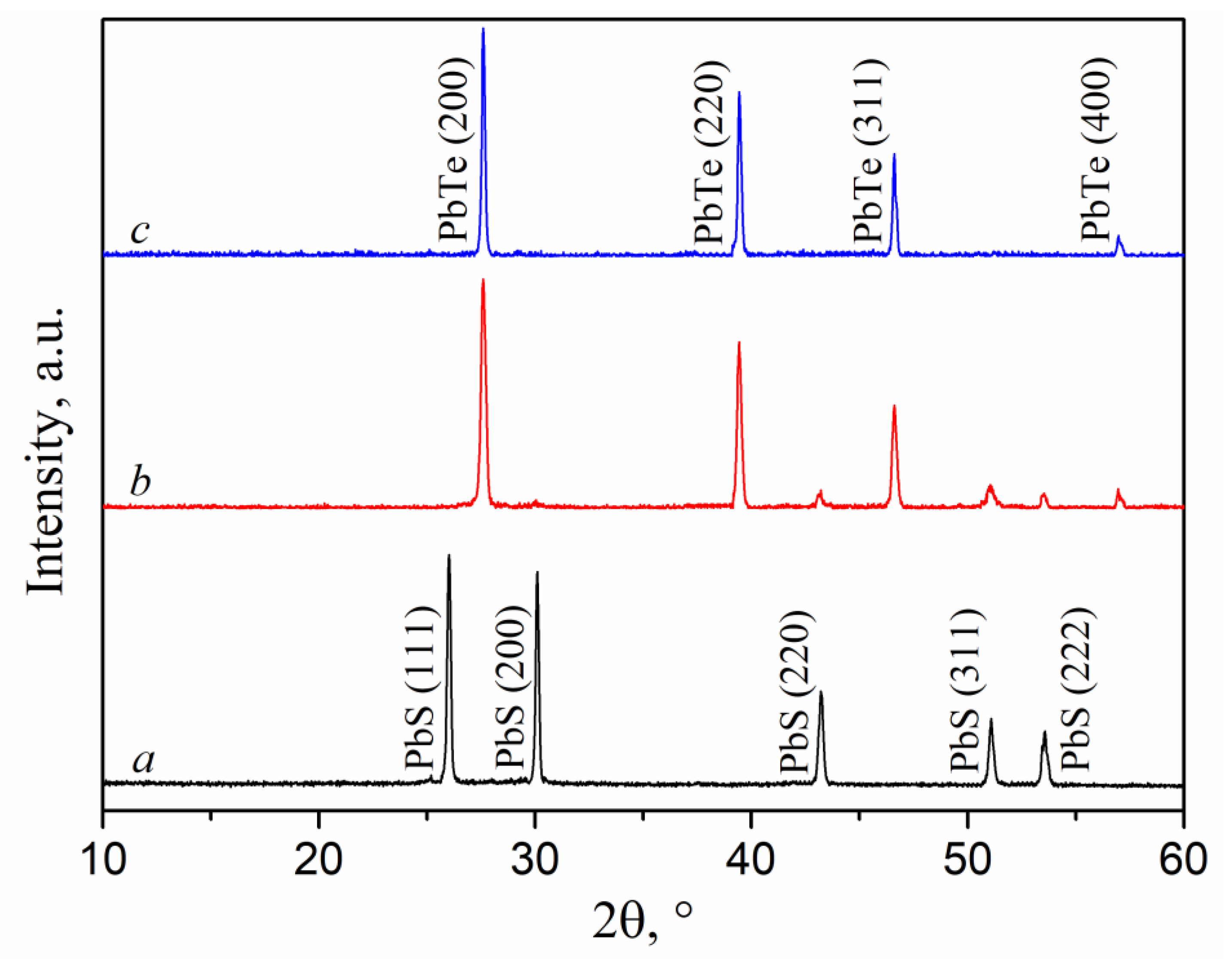
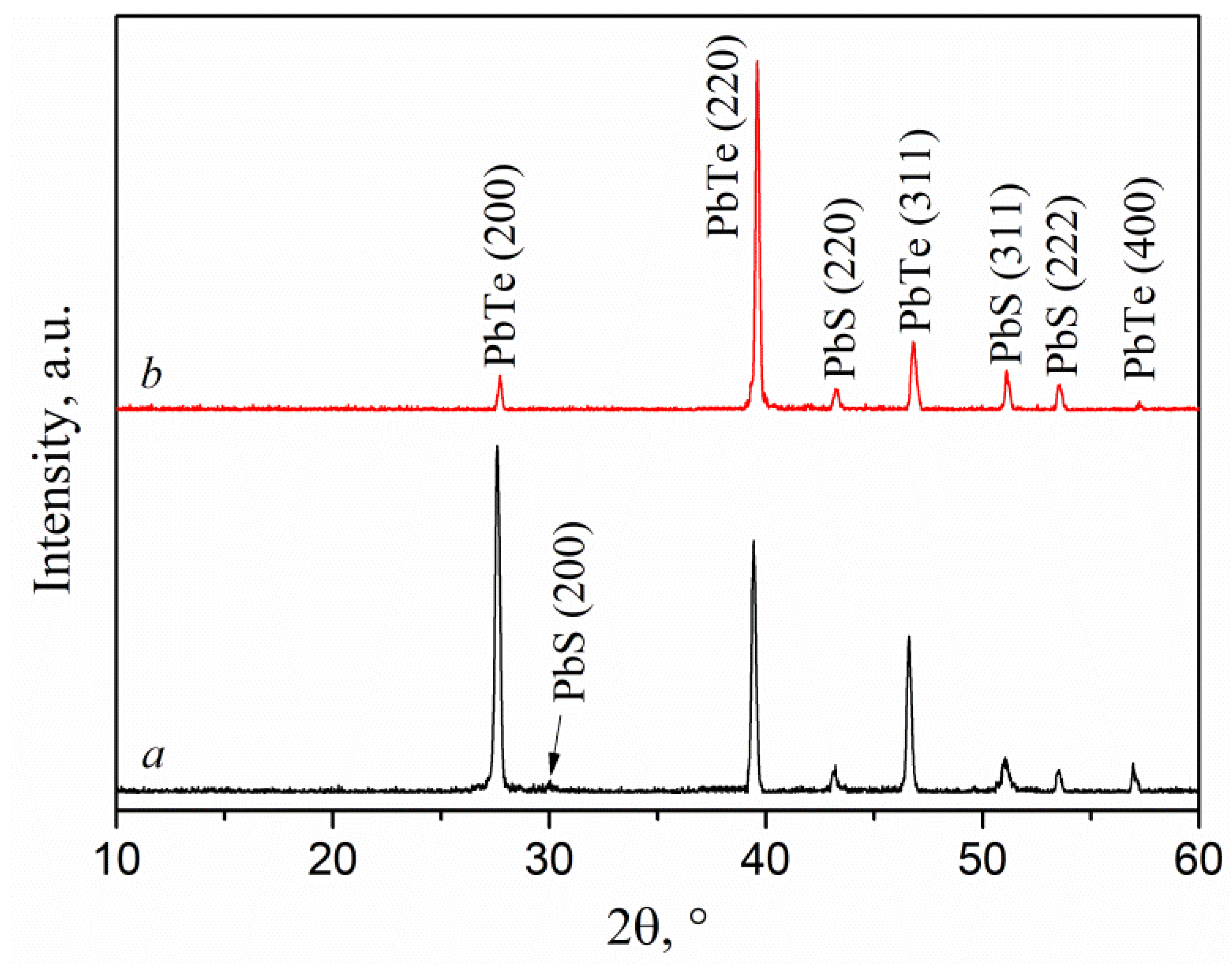
3.2. Results of XRD phase analysis
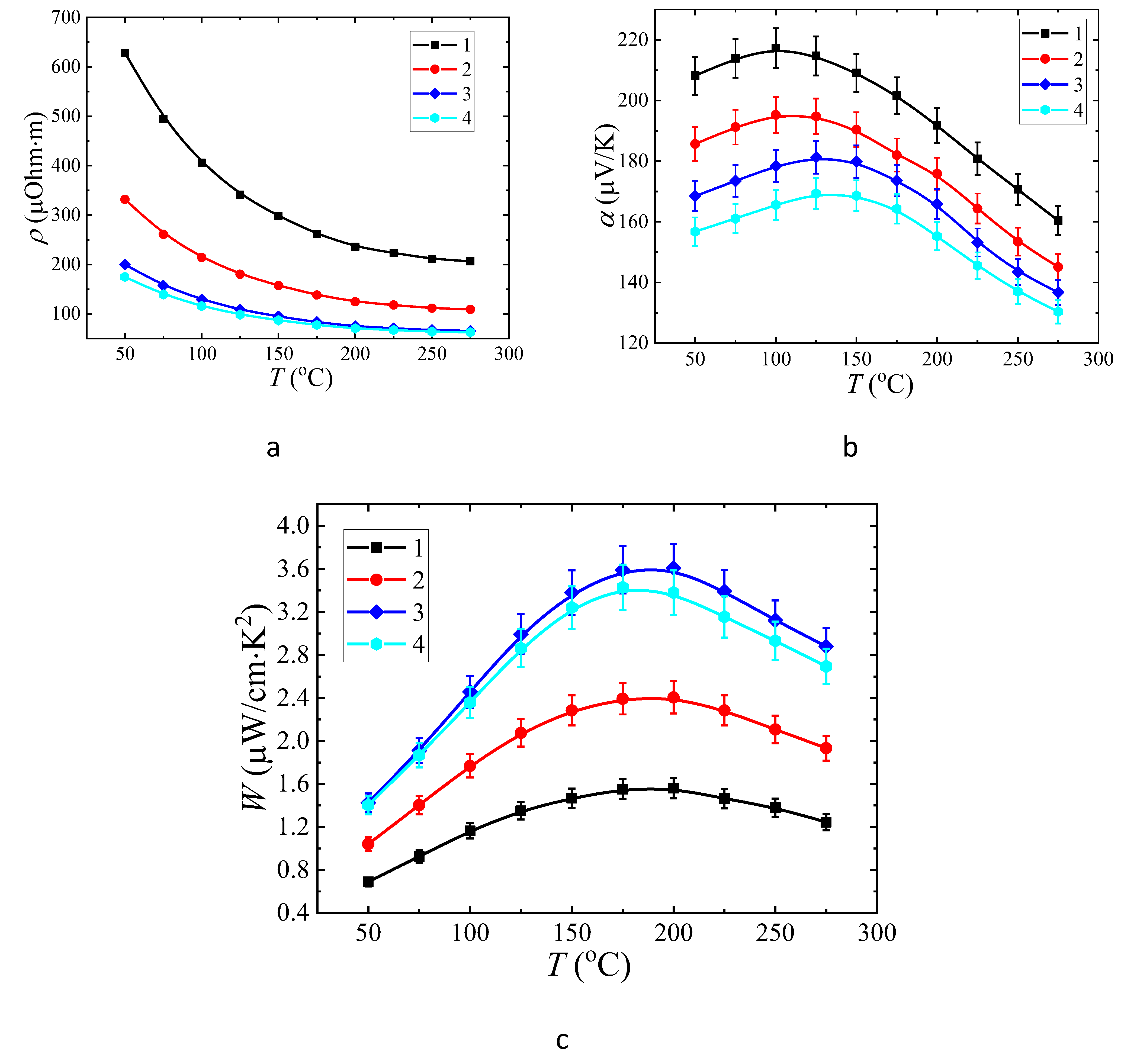
3.3. Thermoelectric properties of the films
4. Conclusions
Acknowledgments
References
- Wang, B.; Zhong, S.P.; Zhang, Z.B.; Zheng, Z.Q.; Zhang, Y.P.; Zhang, H. Broadband photodetectors based on 2D group IVA metal chalcogenides semiconductors. Appl. Mater. Today. 2019, 15, 115–138. [Google Scholar] [CrossRef]
- Xu, Y.; Li, R.; Bai, S.; Li, Y.; Jia, Z.; Yang, Y.; Cui, E.; Yao, F.; Wang, D.; Lei, C.; Lin, Q. Chalcogenide-based narrowband photodetectors for imaging and light communication. Adv. Funct. Mater. 2022, 33, 2212523. [Google Scholar] [CrossRef]
- Tayyab, M.; Liu, Y.; Liu, Z.; Xu, Z.; Yue, W.; Zhou, L.; Lei, J.; Zhang, J. A new breakthrough in photocatalytic hydrogen evolution by amorphous and chalcogenide enriched cocatalysts. Chem. Eng. J. 2023, 455, 140601. [Google Scholar] [CrossRef]
- Tshimangadzo, S.M.; Philiswa, N.N. Review on metal chalcogenides and metal chalcogenide-based nanocomposites in photocatalytic applications. Chem. Afr. 2023, 19, e01509. [Google Scholar]
- Ali, S.A.; Ahmad, T. Chemical strategies in molybdenum based chalcogenides nanostructures for photocatalysis. Int. J. Hydrog. Energy 2022, 47, 29255–29283. [Google Scholar] [CrossRef]
- Li, J.; Jimenez-Calvo, P.; Paineau, E.; Ghazzal, M.N. Metal chalcogenides based heterojunctions and novel nanostructures for photocatalytic hydrogen evolution. Catalysts 2020, 10, 89. [Google Scholar] [CrossRef]
- Yamini, S.A.; Wang, H.; Gibbs, Z.M.; Pei, Y.; Dou, S.X.; Snyder, G.J. Chemical composition tuning in quaternary p-type Pb-chalcogenides – a promising strategy for enhanced thermoelectric performance. Phys. Chem. Chem. Phys. 2014, 16, 1835–1840. [Google Scholar] [CrossRef] [PubMed]
- Yamini, S.A.; Wang, H.; Gibbs, Z.M.; Pei, Y.; Mitchell, D.R.G.; Dou, S.X.; Snyder, G.J. Thermoelectric performance of tellurium-reduced quaternary p-type lead–chalcogenide composites. Acta Mater. 2014, 80, 365–372. [Google Scholar] [CrossRef]
- Yamini, S.A.; Patterson, V.; Santos, R. Band-gap nonlinearity in lead chalcogenide (PbQ, Q = Te, Se, S) alloys. ACS Omega. 2017, 2, 3417–3423. [Google Scholar] [CrossRef]
- Manettas, A.; Santos, R.; Ferreres, X.R.; Yamini, S.A. Thermoelectric performance of single phase p-type quaternary (PbTe)0.65-x(PbSe)0.35(PbS)x alloys. ACS Appl. Energy Mater. 2018, 1, 1898–1903. [Google Scholar] [CrossRef]
- Hone, F.G.; Ampong, F.K.; Nkum, R.K.; Boakye, F. Band gap engineering in lead sulphur selenide (PbS1−xSex) thin films synthesized by chemical bath deposition method. J Mater Sci: Mater Electron. 2017, 28, 2893–2900. [Google Scholar]
- Kudryashov, M.; Mochalov, L.; Nezdanov, A.; Kornev, R.; Logunov, A.; Usanova, D.; Mashin, A.; De Filpo, G.; Gogova, D. A novel plasma-based method for synthesis of As-Se-Te films: impact of plasma parameters on the structure, composition, and optical properties. Superlattices Microstruct. 2019, 128, 334–341. [Google Scholar] [CrossRef]
- Mochalov, L.; Logunov, A.; Prokhorov, I.; Sazanova, T.; Kudrin, A.; Yunin, P.; Zelentsov, S.; Letnianchik, A.; Starostin, N.; Boreman, G.; Vorotyntsev, V. Plasma-chemical synthesis of lead sulphide thin films for near-IR photodetectors. Plasma Chem. Plasma Process. 2020, 41, 493. [Google Scholar] [CrossRef]
- Mochalov, L.; Logunov, A.; Markin, A.; Kitnis, A.; Vorotyntsev, V. Characteristics of the Te-based chalcogenide films dependently on the parameters of the PECVD process. Opt. Quantum Electron. 2020, 52, 197. [Google Scholar] [CrossRef]
- Kumanek, B.; Janas, D. Thermal conductivity of carbon nanotube networks: a review. J. Mater. Sci. 2019, 54, 7397–7427. [Google Scholar] [CrossRef]
- Tambasov, I.A.; Voronin, A.S.; Evsevskaya, N.P.; Kuznetsov, Yu.M.; Luk'yanenko, A.V.; Tambasova, E.V.; Gornakov, M.O.; Dorokhin, M.V.; Loginov, Yu.Yu. Experimental study of the thermal conductivity of single-walled carbon nanotube-based thin films. Phys. Solid State. 2020, 6, 1090–1094. [Google Scholar] [CrossRef]
- Dorokhin, M.V.; Kuznetsov, Yu.M.; Lesnikov, V.P.; Zdoroveishchev, A.V.; Demina, P.B.; Erofeeva, I.V. Studies of thermoelectric properties of superlattices based on manganese silicide and germanium. Phys. Solid State. 2019, 12, 2348–2352. [Google Scholar] [CrossRef]
- Erofeeva, I.V.; Dorokhin, M.V.; Lesnikov, V.P.; Kuznetsov, Yu.M.; Zdoroveyshchev, A.V.; Pitirimova, E.S. Thermoelectric effects in nanoscale layers of manganese silicide. Semiconductors. 2017, 11, 1403–1408. [Google Scholar] [CrossRef]
- Orlova, D.S.; Rogacheva, E.I. Galvanomagnetic properties of thin films of bismuth, doped with tellurium. Nanosyst. Nanomater. Nanotechnologies. 2009, 2, 487–493. (In Russian) [Google Scholar]
- Park, N-W. ; Lee, W-Y.; Yoon, Y-S.; Kim, G-S.; Yoon, Y-G.; Lee, S-K. Achieving out-of-plane thermoelectric figure of merit ZT = 1.44 in a p-type Bi2Te3/Bi0.5Sb1.5Te3 superlattice film with low interfacial resistance. ACS Appl. mater. interfaces. 2019, 11, 38247–38254. [Google Scholar] [CrossRef]
- Zheng, Z-H. ; Shi, X-L.; Ao, D-W.; Liu, W-D.; Li, M.; Kou, L-Z.; Chen, Y-X.; Li, F.; Wei, M.; Liang, G-X.; Fan, P.; Lu, G.Q.M.; Chen, Z-G. Harvesting waste heat with flexible Bi2Te3 thermoelectric thin film. Nat. Sustain. 2022, 6, 180–191. [Google Scholar]
- Zheng, D.; Jin, H.; Liao, Y.; Ji, P. Bi2Te3 nanowires tuning PEDOT:PSS structure for significant enhancing electrical transport property. Mater. Lett. 2023, 338, 134019. [Google Scholar] [CrossRef]
- Lach-hab, M.; Papaconstantopoulos, D.A.; Mehl, M.J. Electronic structure calculations of lead chalcogenides PbS, PbSe, PbTe. J. phys. solids. 2002, 63, 833–841. [Google Scholar] [CrossRef]
- Pei, Ya.; LaLonde, A.; Iwanaga, S.; Snyder, G.J. High thermoelectric figure of merit in heavy hole dominated PbTe. Energy Environ. Sci. 2011, 4, 2085–2089. [Google Scholar] [CrossRef]
- Heremans, J.P.; Jovovic, V.; Toberer, E.S.; Saramat, A.; Kurosaki, K.; Charoenphakdee, A.; Yamanaka, S.; Snyder, G.J. Enhancement of thermoelectric efficiency in PbTe by distortion of the electronic density of states. Science 2008, 321, 554–557. [Google Scholar] [CrossRef] [PubMed]
- Rogacheva, E.I.; Krivulkin, I.M.; Nashchekina, O.N.; Sipatov, A.Yu.; Volobuev, V.V. Effect of oxidation on the thermoelectric properties of PbTe and PbS epitaxial films. Appl. Phys. Lett. 2001, 78, 1661–1663. [Google Scholar] [CrossRef]
- Yang, D.; Lu, C.; Yin, H.; Herman, I.P. Thermoelectric performance of PbSe quantum dot films. Nanoscale. 2013, 5, 7290–7296. [Google Scholar] [CrossRef]
- Yan, Q.; Chen, H.; Zhou, W.; Hng, H.H. Boey, F.Y.C.; Ma, J. A simple chemical approach for PbTe nanowires with enhanced thermoelectric properties. Chem. Mater. 2008, 20, 6298–6300. [Google Scholar] [CrossRef]
- Rogacheva, E.I.; Nashchekina, O.N.; Vekhov, Y.O.; Dresselhaus, M.S.; Cronin, S.B. Effect of thickness on the thermoelectric properties of PbS thin films. Thin Solid Films. 2003, 423, 115–118. [Google Scholar] [CrossRef]
- Geethu, R.; Jacob, R.; Shripathi, T.; Okram, G.S.; Ganesan, V.; Tripathi, S.; Fatima, A.; Sreenivasan, P.V.; Urmila, K.S.; Pradeep, B.; Philip, R.R. Optoelectronic and thermoelectric properties in Ga doped β-PbS2 nanostructured thin films. Appl. Surf. Sci. 2012, 258, 6257–6260. [Google Scholar] [CrossRef]
- Sun, J.; Yeh, M-L. ; Jung, B.J.; Zhang, B.; Feser, J.; Majumdar, A.; Katz, H.E. Simultaneous increase in Seebeck coefficient and conductivity in a doped poly(alkylthiophene) blend with defined density of states. Macromolecules. 2010, 43, 2897–2903. [Google Scholar] [CrossRef]
- Mochalov, L.A.; Kuznetsov, Yu.M.; Dorokhin, M.V.; Fukina, D.G.; Knyazev, A.V.; Kudryashov, M.A.; Kudryashova, Yu.P.; Logunov, A.A.; Mukhina, O.V.; Zdoroveyshchev, A.V.; Zdoroveyshchev, D.A. Thermoelectrical properties of ternary lead chalcogenide plumbum-selenium-tellurium thin films with excess of tellurium prepared by plasma-chemical vapor deposition. Thin Solid Films. 2020, 752, 139244. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, Z.; Lin, Y.-H.; Nan, C. Thermoelectric thin films: Promising strategies and related mechanism on boosting energy conversion performance. J. Mater. 2020, 6, 494–512. [Google Scholar]
- Ibanez, M.; Zamani, R.; Gorsse, S.; Fan, J.; Ortega, S.; Cadavid, D.; Morante, J.R.; Arbiol, J.; Cabot, A. Core-shell nanoparticles as building blocks for the bottom-up production of functional nanocomposites: PbTe-PbS thermoelectric properties. ACS Nano. 2013, 7, 2573–2586. [Google Scholar] [CrossRef]
- Othman, M.S. Mechanical response of PbSSe, PbSTe ternary and PbSnSTe quaternary alloys at high pressure. ARO-sci. j. Koya univ. 2020, 8, 29–33. [Google Scholar] [CrossRef]
- Dorokhin, M.V.; Kuznetsov, Yu.M.; Demina, P.B.; Erofeeva, I.V.; Zavrazhnov, A.Yu.; Boldin, M.S.; Lantsev, E.A.; Popov, A.A.; Boryakov, A.V.; Zdoroveyshchev, A.V.; Ved, M.V.; Zdoroveyshchev, D.A.; Korotkova, M.G. High-efficiency spark plasma sintered Ge0.3Si0.7:P thermoelectric energy converters with silicone phosphide as a source of phosphorus doping. Nanoscale microscale thermophys. eng. 2023, 27, 125–134. [Google Scholar] [CrossRef]
| № | P (W) | Substrate | Compound, at. % | |||
|---|---|---|---|---|---|---|
| Pb | S | Te | ||||
| 1 | 60 | Al2O3 | 50±1 | 3±1 | 47±1 | (PbTe)0.94(PbS)0.06 |
| 2 | 60 | Si | 50±1 | 5±1 | 45±1 | (PbTe)0.90(PbS)0.10 |
| 3 | 100 | Al2O3 | 50±1 | 10±1 | 40±1 | (PbTe)0.80(PbS)0.20 |
| 4 | 100 | Si | 50±1 | 12±1 | 38±1 | (PbTe)0.76(PbS)0.24 |
| № | Сoстав | ρ, мкОм·м | μ, см2/В∙с | p, 1018 см-3 |
|---|---|---|---|---|
| 1 | PbTe | 627±6 | 150±5 | 0.67±0.02 |
| 2 | (PbTe)0.9(PbS)0.1 | 332±3 | 72.6±2.2 | 2.59±0.08 |
| 3 | (PbTe)0.8(PbS)0.2 | 200±2 | 50.3±1.5 | 6.21±0.19 |
| 4 | (PbTe)0.7(PbS)0.3 | 176±2 | 49.2±1.5 | 7.22±0.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





