1. Introduction
Sensors are the pen and paper of the next wave of medical data acquisition. Although medicine is a very conservative industry, adoption of this new wave of medicine is underway. Early sensor products, that are now coming to market, will change the way we think about diagnosis and outcomes. A combination of broadband internet, micro electrical machine systems (MEMS), and wireless communication standards is facilitating this approach. Several medical sensors have emerged for the purpose of monitoring medical conditions. There are many reasons for this. Uniquely, remote reliable sensing technology allows a new look into old problems. Conditions we previously had difficulty diagnosing and treating can now be more completely explored [
1,
2,
3,
4,
5,
6,
7]. Continuous monitoring offered by sensors can help early detection of emergency medical conditions and improve communication in those situations. Our research group has developed several early sensor concepts in the search for more information on important medical conditions [
8,
9,
10,
11,
12,
13]. One of the goals of this series of studies was to establish a scientific process to better understand acute compartment syndrome (ACS). ACS is a surgical emergency most commonly occurring in the extremities after trauma with resultant swelling within a closed compartment. This closed compartment may result in death of the confined muscle. Release of the muscle with extensive surgery is the treatment. Timely diagnosis with surgery will save the muscle from dying. Unfortunately, many more releases or fasciotomies, are performed than are needed. This results in large costs to the system and significant morbidity to the patient. The concept of compartment pressure and its disease analogue of ACS remained a vexing issue for all trauma surgeons [
14,
15,
16,
17,
18,
19,
20,
21]. There was a knowledge gap regarding the pathophysiology, diagnosis, treatment, and outcome for ACS. For a disease associated with a financial burden to society that represents billions of dollars worldwide, the literature did not have established baseline diagnostic tools to predict treatment and outcomes [
22]. Modeling the disease with scientific process allows a more complete understanding of the disease. Following scientific process (
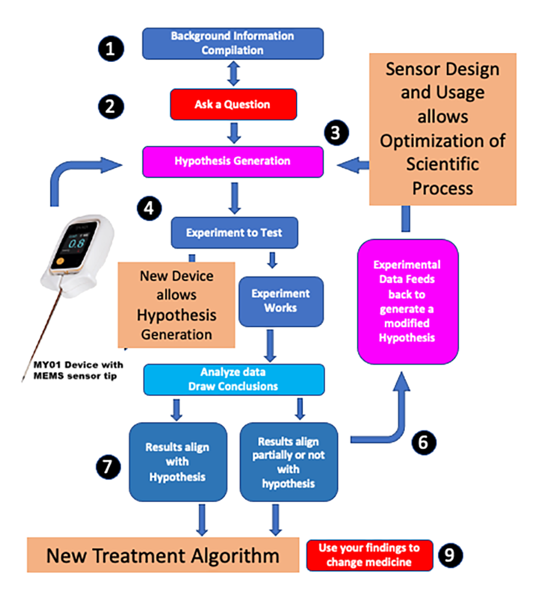
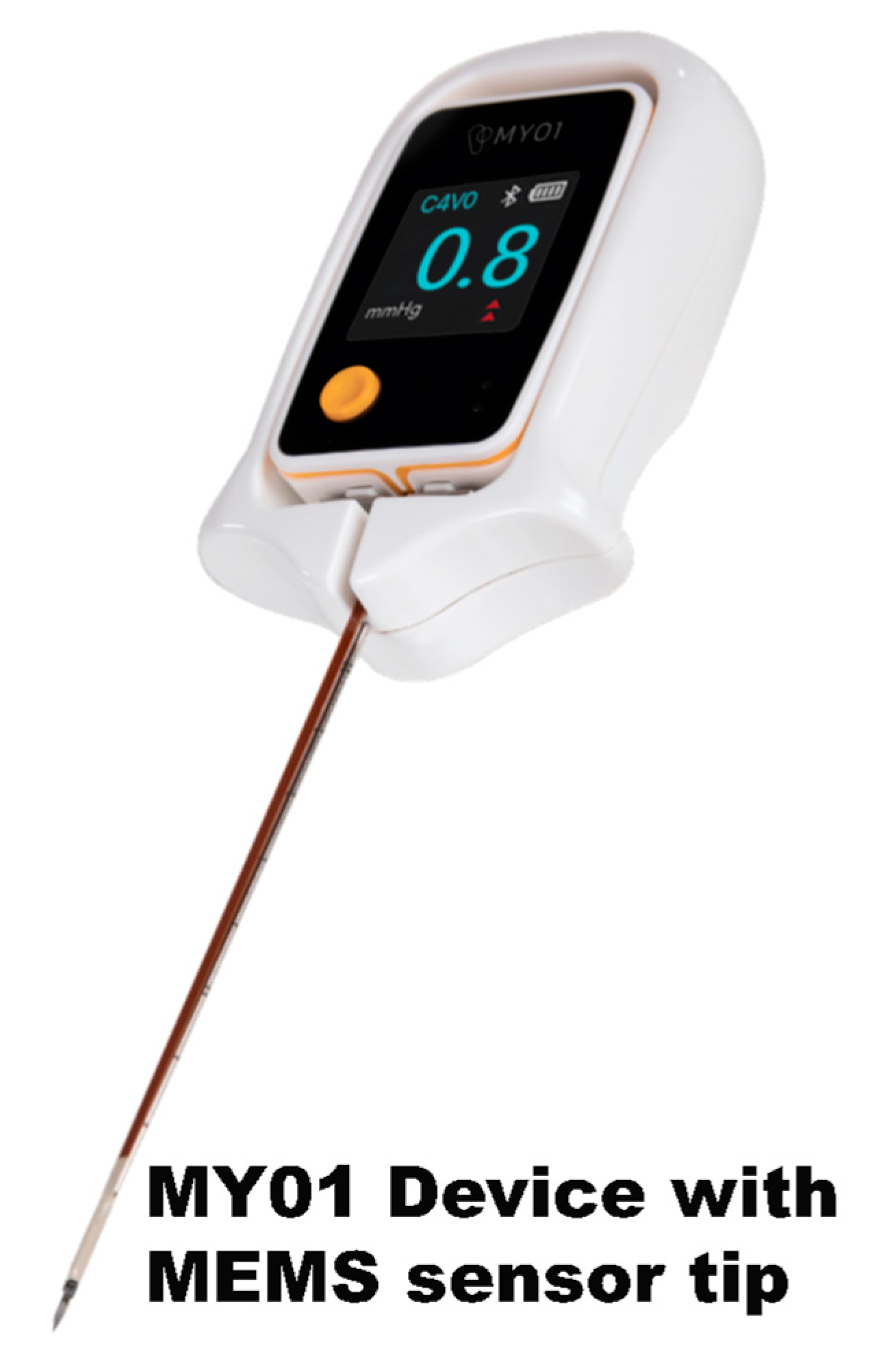
Figure 1), a MEMS sensor (
Figure 2) was developed capable of monitoring tissue trauma in muscle. This was accomplished through a design principle process to allow direct insertion of the sensor assembly into the muscle that is being monitored. Other methods in the literature relied on a standing water column and an external sensor [
19,
23]. The goal of the process was to formulate a hypothesis that could be tested to aid in answering the overall question of making the diagnosis of ACS accurately. The hypothesis tested here was that pressure monitoring, in particular continuous data, was important for diagnosis of ACS.
2. Materials and Methods
Scientific process was applied to the disease process of acute compartment syndrome (
Figure 1). Strict scientific process entails formulation of a question based on current knowledge. Background information was compiled, and the English literature was reviewed for pertinent diagnostic criteria that would allow a question on diagnosis to be formulated (
Figure 1—Areas 1 and 2). Over 350 papers were examined. It was obvious from these readings that two major deficiencies in the literate existed. There was incomplete understanding of the epidemiology of ACS and there was no common classification that allowed review of disparate studies. Studies were then carried out [
9,
10,
13,
19,
21,
22,
23,
24,
25,
26,
27,
28] to fill these deficiencies. This allowed hypothesis generation around the findings—in particular that pressure was relevant to ACS progression and possibly diagnosis (
Figure 1—Area 3). A new device was produced to test the hypothesis (
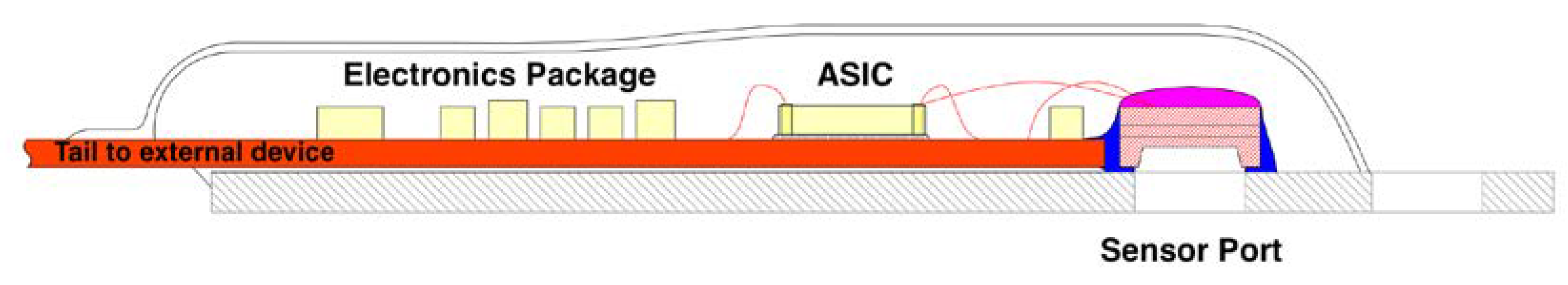
Figure 2 and
Figure 3) Several experiments were then designed to determine if accurate pressure measurement was possible (
Figure 1—Area 4) and then applied to pre-clinical and clinical modeling (
Figure 1—Area 5). These results were examined then used to fine tune the hypothesis (
Figure 1—Area 6 and 7) before being communicated and changing the diagnosis of ACS (
Figure 1—Area 8 and 9).
3. Results
A big data approach (
Figure 1 area 1) was used to better understand the clinical problem [
22,
24]. A common grading system was also validated (in press, 2023) to compare the papers. Several interesting new correlations were found in the big data study but the finding that hypertension was protective for ACS led the author s to believe that pressure measurement in the compartments was an important biomarker. This allowed the formulation of the question of how accurate pressure measurement in diagnosing ACS and the hypothesis was that accurate pressure measurement would be an important tool (
Figure 1- Area 2 and 3). Accurate MEMS sensor-based device was designed and compared to other devices (
Figure 1—Area 4). Results showed that the new device (MY01 Inc) was 600% more accurate than current technology. It was also the only device that allowed continuous pressure sensing. Experiments to test this device were carried out (
Figure 1—Area 5) [
10,
13,
23,
25,
26]. Feedback allowed us to modify the hypothesis to incorporate continuous pressure measurements as a more accurate tool (
Figure 1—Area 6). Eventual results aligned with the hypothesis allowing a change in diagnostic criteria for ACS (
Figure 1- Areas 7,8,9 and
Figure 4).
4. Discussion
Acute Compartment Syndrome (ACS) is a surgical emergency most commonly occurring in the extremities because of trauma and resultant swelling within a closed compartment. It is ultimately the result of increasing pressure leading to circulatory compromise, tissue ischemia, and necrosis [
16,
28,
29,
30,
31]. Monitoring of objective continuous pressure values has been difficult. Micro electrical machine systems (MEMS) techniques can be used to design biomedical sensors to obtain objective data. MEMS are transducers, either sensors or actuators, which convert one type of signal into another type of signal. MEMS devices are advantageous, due to their small size, closely related to characteristics such as ease of integration, light weight, low power consumption. Other advantages include reduced fabrication costs due to high mass production and high accuracy, sensitivity, and throughput. The MY01 device (
Figure 2 and 3) was designed with these advantages in mind. This MEMS sensor product was designed to aid in the diagnosis of acute compartment syndrome [
23]. The device represents a true transition to digital health monitoring as it was combined with a smartphone application for real time data study. This allowed cloud-based storage and integration with electronic health records. The research groups involved [
9,
19,
22,
23,
27,
32,
33] have also used the device for the scientific process (
Figure 1—Area 4) to better understand ACS disease process—an example of how new technology and information can be used to better define medical diseases.
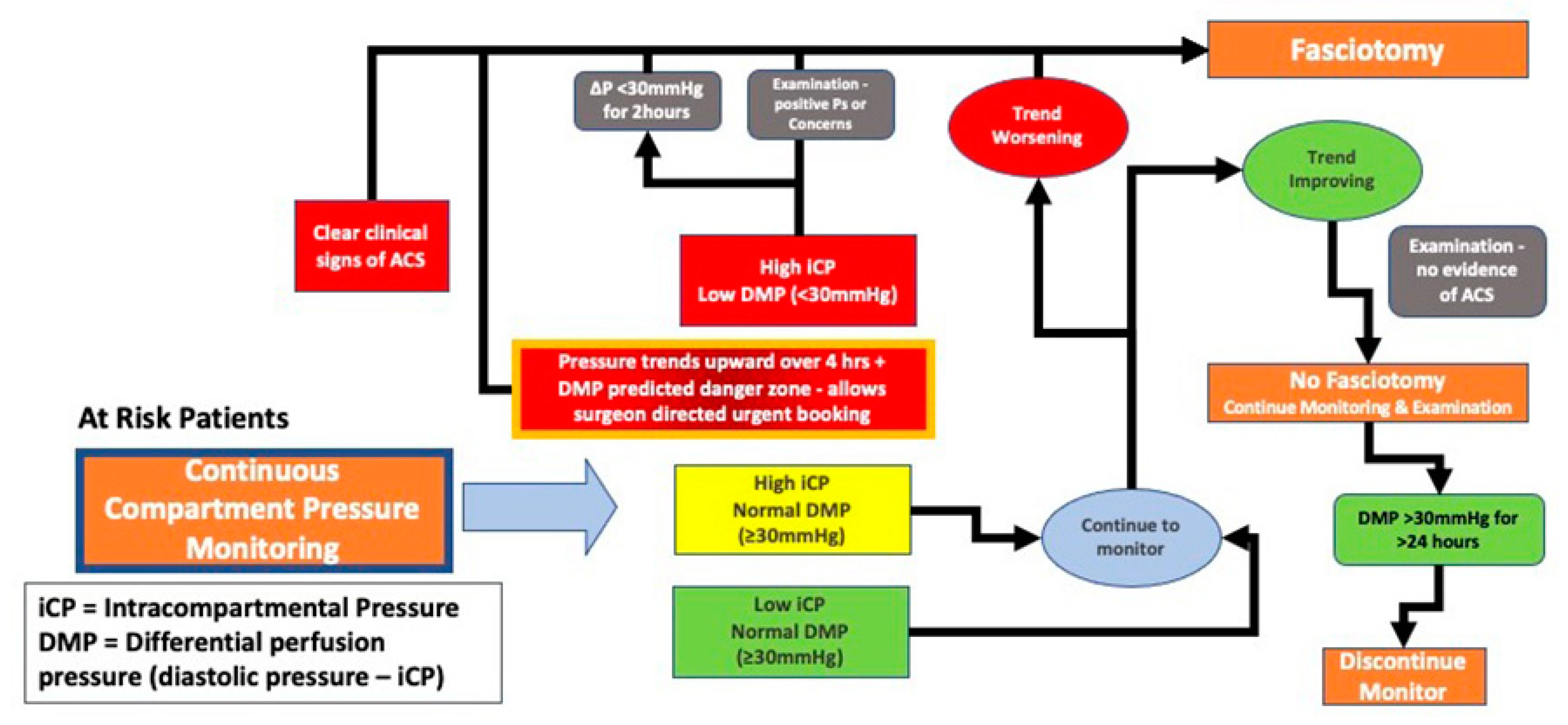
It is now understood that abnormally elevated pressure within a compartment is an early and important marker of ACS [
29,
34]. Continuous pressure monitoring of the affected compartment is a desired marker for the progression or resolution of ACS and this sensor driven information provides the basis for recommendations regarding the need for fasciotomy (
Figure 3). This information now allows the treating physician to deploy a diagnostic sensor proactively with early use aiding care management.
5. Conclusions
What do we get from sensor proliferation? We get better recording of disease progression—whether through medical record amplification or wellness quantification. This example showed how accurate MEMS sensing platforms can permit better disease understanding. MEMS sensor technology has defined a new gold standard of diagnosing acute compartment syndrome. Implantation of a sensor in a muscle compartment allows accurate diagnosis of ACS with continuous trends in pressure.
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “Conceptualization, GM and EJH.; methodology, GM, APH, EJH.; formal analysis, GM and EJH.; investigation, GM, APH, EJH.; resources, GM and EJH.; writing—original draft preparation, EJH.; writing—review and editing, GM, APH, EJH.; project administration, GM EJH.; funding acquisition, GM EJH. All authors have read and agreed to the published version of the manuscript.”
Funding
Portions of this research was funded by the Office of the Assistant Secretary of Defense for Health Affairs through the FY18 Defense Medical Research and Development Program, endorsed by the Department of Defense, through the FY18, DMRDP JPC-6/CCCRP Precision Trauma Care Research Award under Award No. W81XWH1920010. Opinions, interpretations, conclusions, and recommendations are those of the author and are not necessarily endorsed by the Department of Defense.
Institutional Review Board Statement
Not applicable
Informed Consent Statement
Not applicable
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to proprietary sensor technology.
Conflicts of Interest
Dr EJ Harvey is a founder of MY01 Inc, a paid employee and founder of NXTSens Inc, and founder of Stathera. He is a paid consultant at CMAJ Medical publications (Editor in Chief of Canadian Journal of Surgery)
References
- Aldebeyan, S., A. Aoude, and E.J. Harvey, Electronics and orthopaedic surgery. Injury, 2018. 49 Suppl 1: p. S102-S104. [CrossRef]
- Badenes, R., et al., The role of noninvasive brain oximetry in adult critically ill patients without primary non-anoxic brain injury. Minerva Anestesiol, 2021. 87(11): p. 1226-1238. [CrossRef]
- Convertino, V.A., et al., Wearable Sensors Incorporating Compensatory Reserve Measurement for Advancing Physiological Monitoring in Critically Injured Trauma Patients. Sensors (Basel), 2020. 20(22). [CrossRef]
- D, R. Mobile Medicine: The Internet of Things Meets Health. 2016 2016 [cited 2021 December 16]; Web page]. Available from: https://www.goldmansachs.com/insights/pages/iot-meets-health.html.
- Dunn, J., R. Runge, and M. Snyder, Wearables and the medical revolution. Per Med, 2018. 15(5): p. 429-448. [CrossRef]
- Klosterhoff, B.S., et al., Wireless sensor enables longitudinal monitoring of regenerative niche mechanics during rehabilitation that enhance bone repair. Bone, 2020. 135: p. 115311. [CrossRef]
- Sedlmayr, M., H.U. Prokosch, and U. Munch, Towards smart environments using smart objects. Stud Health Technol Inform, 2011. 169: p. 315-9. [CrossRef]
- Vieira, D., et al., Microelectrochemical Smart Needle for Real Time Minimally Invasive Oximetry. Biosensors (Basel), 2020. 10(11). [CrossRef]
- Schupbach, D.E., et al., Percutaneous Forefoot Decompression in a Foot Compartment Syndrome Model. JB JS Open Access, 2021. 6(4). [CrossRef]
- Schupbach, D., et al., Acute Compartment Syndrome Modeling with Sequential Infusion Shows the Deep Posterior Compartment Is Not Functionally Discrete. J Bone Joint Surg Am, 2022. 104(9): p. 813-820. [CrossRef]
- Merle, G., A. Parent-Harvey, and E.J. Harvey, Sensors and digital medicine in orthopaedic surgery. OTA Int, 2022. 5(2 Suppl): p. e189. [CrossRef]
- Merle, G., et al., Sensor technology usage in orthopedic trauma. Injury, 2022. 53 Suppl 3: p. S59-S63. [CrossRef]
- Honjol, Y., et al., Porcine Model of Acute Compartment Syndrome. J Orthop Trauma, 2023. 37(3): p. e122-e127. [CrossRef]
- Blaisdell, F.W., The pathophysiology of skeletal muscle ischemia and the reperfusion syndrome: a review. Cardiovasc Surg, 2002. 10(6): p. 620-30.
- Altay, M.A., et al., Comparison of intracompartmental pressures in a rabbit model of open and closed tibial fractures: an experimental study. Bone Joint J, 2013. 95-B(1): p. 111-4.
- Raza, H. and A. Mahapatra, Acute compartment syndrome in orthopedics: causes, diagnosis, and management. Adv Orthop, 2015. 2015: p. 543412. [CrossRef]
- Weick, J.W., et al., Direct Measurement of Tissue Oxygenation as a Method of Diagnosis of Acute Compartment Syndrome. J Orthop Trauma, 2016. 30(11): p. 585-591. [CrossRef]
- Schmidt, A.H., et al., Predicting Acute Compartment Syndrome (PACS): The Role of Continuous Monitoring. J Orthop Trauma, 2017. 31 Suppl 1: p. S40-S47. [CrossRef]
- Merle, G. and E.J. Harvey, Pathophysiology of Compartment Syndrome, in Compartment Syndrome: A Guide to Diagnosis and Management, C. Mauffrey, D.J. Hak, and I.M. Martin, Editors. 2019: Cham (CH). p. 17-24.
- Phair, J., et al., Malpractice Litigation for Compartment Syndrome. Annals of Vascular Surgery, 2020. 67: p. 143-147. [CrossRef]
- Lorange, J.P., et al., Diagnosis Accuracy for Compartment Syndrome: a Systematic Review and Meta-Analysis. J Orthop Trauma, 2023. [CrossRef]
- Bouklouch, Y., et al., Big data insights into predictors of acute compartment syndrome. Injury, 2022. [CrossRef]
- Merle, G., et al., Comparison of Three Devices to Measure Pressure for Acute Compartment Syndrome. Mil Med, 2020. 185(Suppl 1): p. 77-81. [CrossRef]
- Laverdiere, C., et al., Predictors of Foot Acute Compartment Syndrome: Big Data analysis. J Foot Ankle Surg, 2023. 62(1): p. 27-30. [CrossRef]
- Nasser Eddine, M., et al., Minimal Percutaneous Release for Acute Compartment Syndrome of the Foot: A Case Report. JBJS Case Connect, 2022. 12(3). [CrossRef]
- Montreuil, J., et al., Novel digital continuous sensor for monitoring of compartment pressure: a case report. OTA Int, 2022. 5(3): p. e208. [CrossRef]
- Talbot, M., et al., A pilot study of surgical telementoring for leg fasciotomy. J R Army Med Corps, 2018. 164(2): p. 83-86. [CrossRef]
- Harvey, E.J., et al., What’s new in acute compartment syndrome? J Orthop Trauma, 2012. 26(12): p. 699-702.
- McQueen, M.M. and A.D. Duckworth, The diagnosis of acute compartment syndrome: a review. Eur J Trauma Emerg Surg, 2014. 40(5): p. 521-8. [CrossRef]
- Schmidt, A.H., Acute Compartment Syndrome. Orthop Clin North Am, 2016. 47(3): p. 517-25.
- Merle, G. and E. Harvey. Pathophysiology of Compartment Syndrome. 2019.
- Nooh, A., et al., Acute Thigh Compartment Syndrome due to an Occult Arterial Injury Following a Blunt Trauma: A Case Report. JBJS Case Connect, 2020. 10(1): p. e0506.
- Schupbach, D., et al., Acute Compartment Syndrome Modeling with Sequential Infusion Shows the Deep Posterior Compartment Is Not Functionally Discrete. J Bone Joint Surg Am, 2022.
- McQueen, M.M., P. Gaston, and C.M. Court-Brown, Acute compartment syndrome. Who is at risk? J Bone Joint Surg Br, 2000. 82(2): p. 200-3.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).