Submitted:
02 May 2023
Posted:
03 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
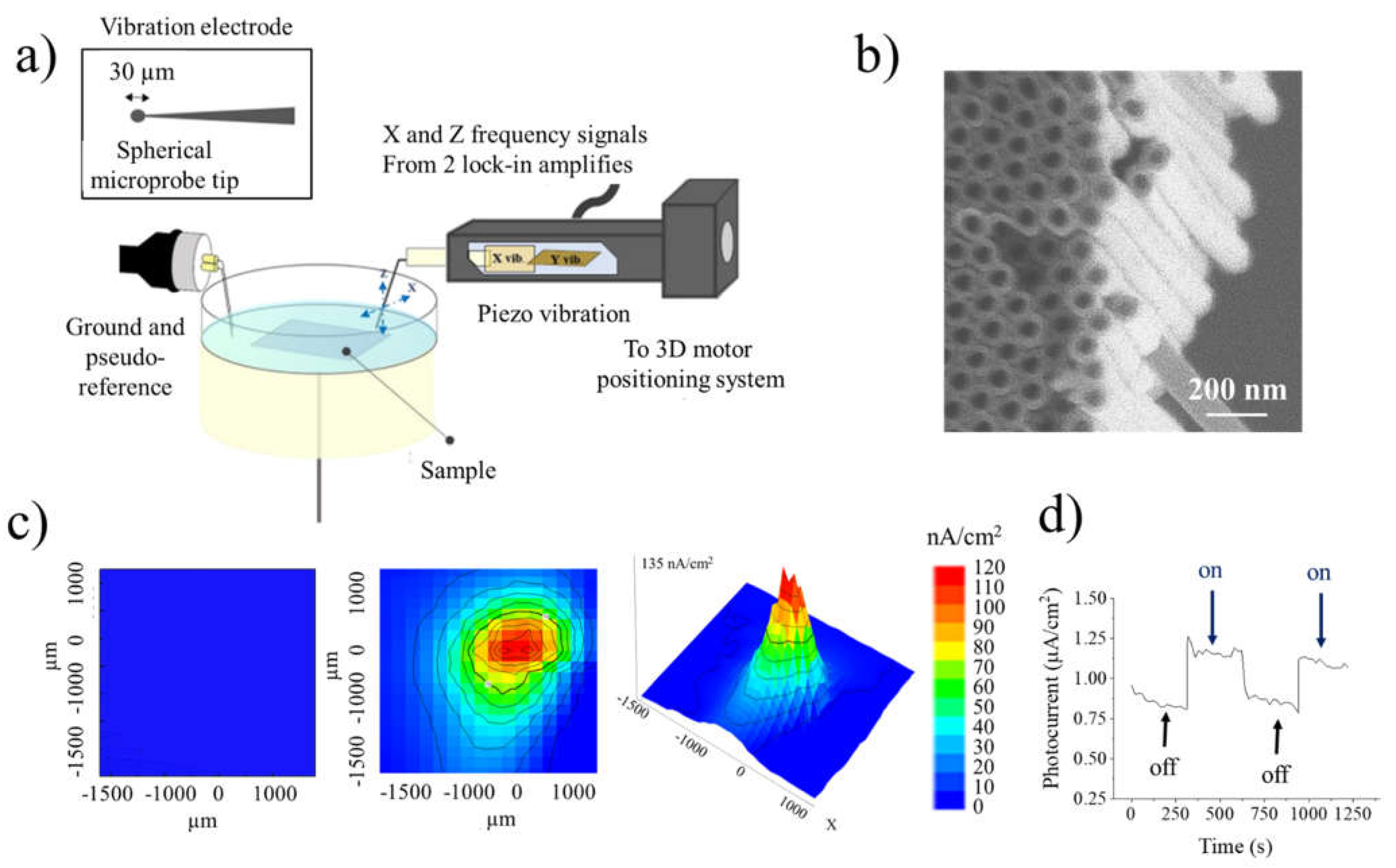
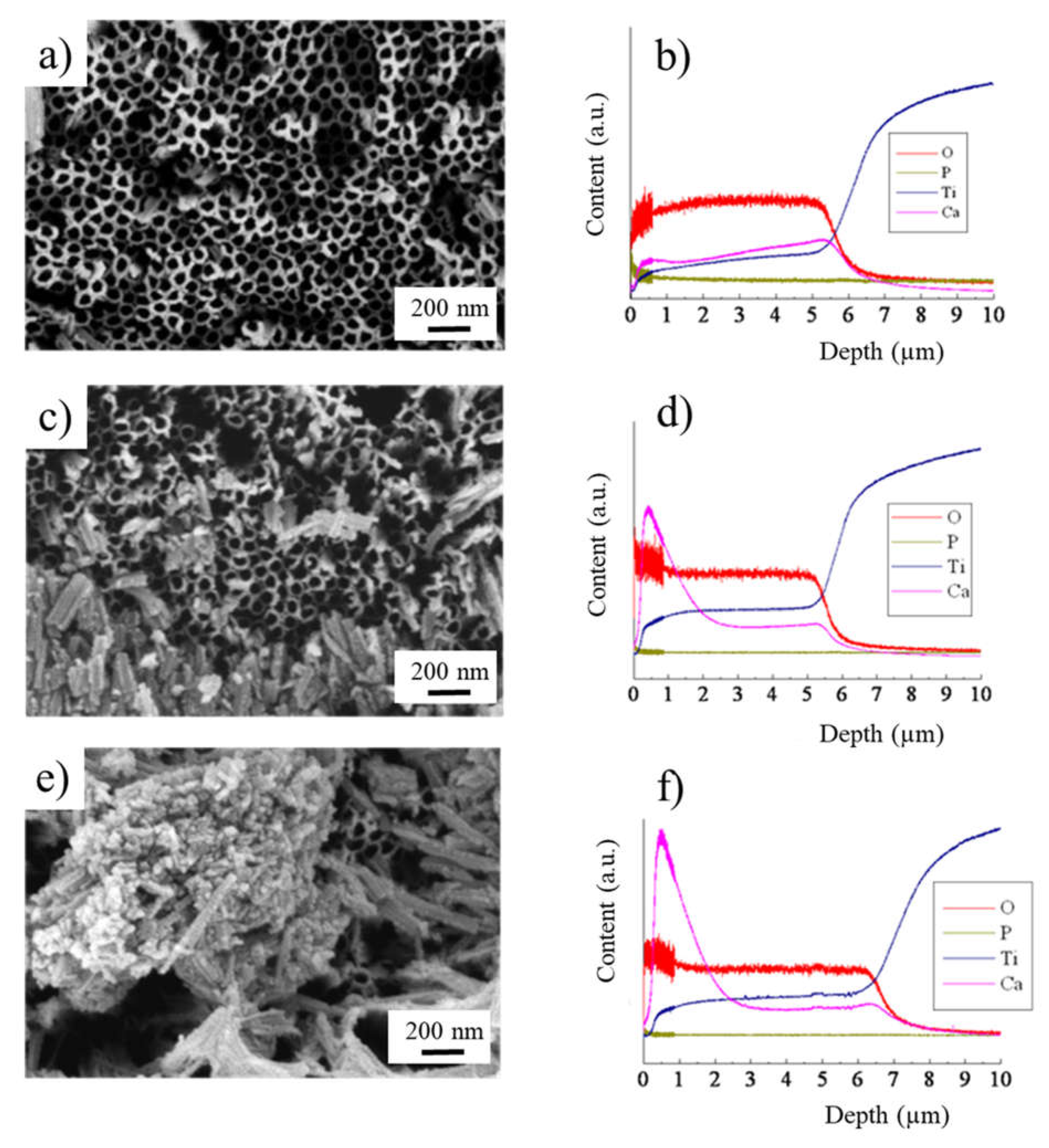
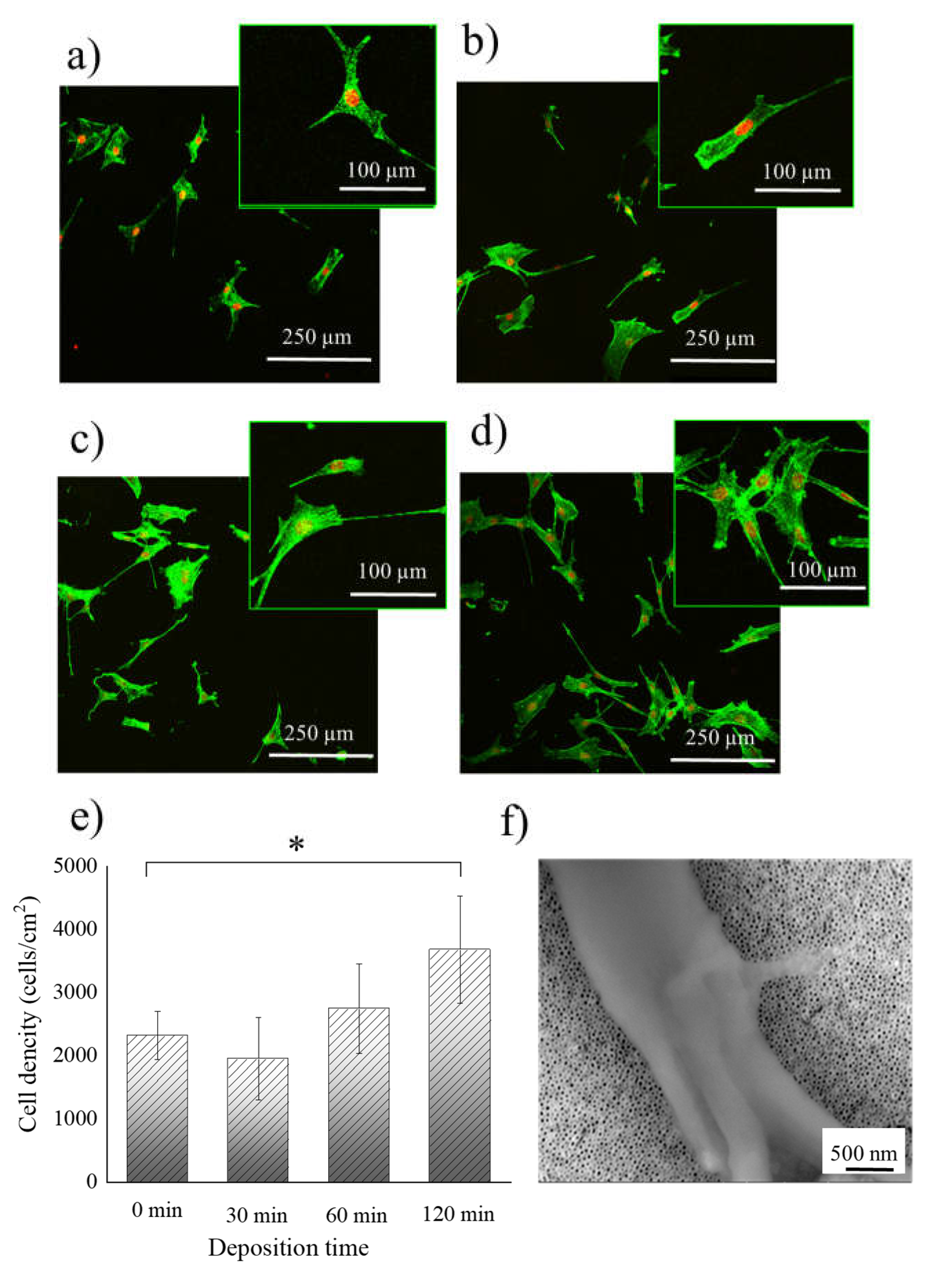
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Preparation of TNT
3.3. Photodegradation of EDTA and Ca(EDTA) complexes study
3.4. Photocatalytic hydroxyapatite deposition
3.5. Sample characterization
3.5. Cell Culture and Immunofluorescent Staining
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siddiqui, H.A.; Pickering, K.L.; Mucalo, M.R. A Review on the Use of Hydroxyapatite- Carbonaceous Structure Composites in Bone Replacement Materials for Strengthening Purposes. Materials (Basel) 2018, 11, 1–32. [Google Scholar] [CrossRef]
- Vdoviaková, K.; Jenca, A.; Jenca, A.; Danko, J.; Kresáková, L.; Simaiová, V.; Reichel, P.; Rusnák, P.; Pribula, J.; Vrzgula, M.; et al. Regenerative Potential of Hydroxyapatite-Based Ceramic Biomaterial on Mandibular Cortical Bone: An In Vivo Study. Biomedicines 2023, 11, 877. [Google Scholar] [CrossRef] [PubMed]
- Tithito, T.; Sillapaprayoon, S.; Pimtong, W.; Thongbunchoo, J.; Charoenphandhu, N.; Krishnamra, N.; Lert-itthiporn, A.; Maneeprakorn, W.; Pon-On, W. Development of Biomaterials Based on Biomimetic Trace Elements Co-Doped Hydroxyapatite: Physical, In Vitro Osteoblast-like Cell Growth and In Vivo Cytotoxicity in Zebrafish Studies. Nanomaterials 2023, 13, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Groth, T.; Barbeck, M.; Sun, P.C.Z. Bioceramics, Biomimetic and Other Compatible Materials Features for Medical Applications; ISBN 9783031172687.
- Francisco, I.; Basílio, Â.; Ribeiro, M.P.; Nunes, C.; Travassos, R.; Marques, F.; Pereira, F.; Paula, A.B.; Carrilho, E.; Marto, C.M.; et al. Three-Dimensional Impression of Biomaterials for Alveolar Graft: Scoping Review. J. Funct. Biomater. 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, S.; Agarwal, A.K.; Rai, K.N.; Garg, A. Development of High Strength Hydroxyapatite by Solid-State-Sintering Process. Ceram. Int. 2007, 33, 419–426. [Google Scholar] [CrossRef]
- Baldassarre, F.; Altomare, A.; Mesto, E.; Lacalamita, M.; Dida, B.; Mele, A.; Bauer, E.M.; Puzone, M.; Tempesta, E.; Capelli, D.; et al. Structural Characterization of Low-Sr-Doped Hydroxyapatite Obtained by Solid-State Synthesis. Crystals 2023, 13. [Google Scholar] [CrossRef]
- Hossain, M.S.; Hasan, M.M.; Mahmud, M.; Mobarak, M. Bin; Ahmed, S. Assessment of Crystallite Size of UV-Synthesized Hydroxyapatite Using Different Model Equations. Chem. Pap. 2023, 77, 463–471. [Google Scholar] [CrossRef]
- Bin Mobarak, M.; Hossain, M.S.; Yeasmin, Z.; Mahmud, M.; Rahman, M.M.; Sultana, S.; Masum, S.M.; Ahmed, S. Probing the Photocatalytic Competency of Hydroxyapatite Synthesized by Solid State and Wet Chemical Precipitation Method. J. Mol. Struct. 2022, 1252, 132142. [Google Scholar] [CrossRef]
- Sadat-Shojai, M.; Khorasani, M.T.; Dinpanah-Khoshdargi, E.; Jamshidi, A. Synthesis Methods for Nanosized Hydroxyapatite with Diverse Structures. Acta Biomater. 2013, 9, 7591–7621. [Google Scholar] [CrossRef]
- Karalkeviciene, R.; Raudonyte-Svirbutaviciene, E.; Zarkov, A.; Yang, J.C.; Popov, A.I.; Kareiva, A. Solvothermal Synthesis of Calcium Hydroxyapatite via Hydrolysis of Alpha-Tricalcium Phosphate in the Presence of Different Organic Additives. Crystals 2023, 13. [Google Scholar] [CrossRef]
- Arce, H.; Montero, M.L.; Sáenz, A.; Castaño, V.M. Effect of PH and Temperature on the Formation of Hydroxyapatite at Low Temperatures by Decomposition of a Ca(EDTA) Complex. Polyhedron 2004, 23, 1897–1901. [Google Scholar] [CrossRef]
- Joshi, K.J.; Shah, N.M. Structural, Morphological & Optical Studies of Hydroxyapatite Microplates Synthesized Using Hydrothermal Technique. Mater. Today Proc. 2023, 1–6. [Google Scholar] [CrossRef]
- Bartkowiak, A.; Zabila, Y.; Menaszek, E.; Zarzycki, A.; Perzanowski, M.; Marszalek, M. Effect of Tantalum Interlayer on Hydroxyapatite Biointerface for Orthopedic Applications. Surf. Coatings Technol. 2022, 447, 128882. [Google Scholar] [CrossRef]
- Muthu, D.; Suresh Kumar, G.; Gowri, M.; Prasath, M.; Viswabaskaran, V.; Kattimani, V.S.; Girija, E.K. Rapid Synthesis of Eggshell Derived Hydroxyapatite with Nanoscale Characteristics for Biomedical Applications. Ceram. Int. 2022, 48, 1326–1339. [Google Scholar] [CrossRef]
- Yokoi, T.; Kawashita, M.; Ohtsuki, C. Biomimetic Mineralization of Calcium Phosphates in Polymeric Hydrogels Containing Carboxyl Groups. J. Asian Ceram. Soc. 2013, 1, 155–162. [Google Scholar] [CrossRef]
- Zhu, Q.; Jiao, H.; Zhao, X.; Tang, Y.; Zhao, K.; Gou, X. Hydroxyapatite Formation in Biomimetic Synthesis with the Interface of a PDA@SIS Membrane. RSC Adv. 2022, 12, 13209–13219. [Google Scholar] [CrossRef] [PubMed]
- Paramasivan, M.; Kumar, T.S.S.; Chandra, T.S. Microbial Synthesis of Hydroxyapatite-Nanocellulose Nanocomposites from Symbiotic Culture of Bacteria and Yeast Pellicle of Fermented Kombucha Tea. Sustain. 2022, 14. [Google Scholar] [CrossRef]
- Orekhov, E.V.; Arbenin, A.Y.; Zemtsova, E.G.; Sokolova, D.N.; Ponomareva, A.N.; Shevtsov, M.A.; Yudintceva, N.M.; Smirnov, V.M. Template Electrochemical Synthesis of Hydroxyapatite on a Titania–Silver Composite Surface for Potential Use in Implantology. Coatings 2022, 12. [Google Scholar] [CrossRef]
- Eliaz, N.; Sridhar, T.M.; Mudali, U.K.; Raj, B. Electrochemical and Electrophoretic Deposition of Hydroxyapatite for Orthopaedic Applications. Surf. Eng. 2005, 21, 238–242. [Google Scholar] [CrossRef]
- Gorejová, R.; Oriňaková, R.; Králová, Z.O.; Sopčák, T.; Šišoláková, I.; Schnitzer, M.; Kohan, M.; Hudák, R. Electrochemical Deposition of a Hydroxyapatite Layer onto the Surface of Porous Additively Manufactured Ti6Al4V Scaffolds. Surf. Coatings Technol. 2023, 455. [Google Scholar] [CrossRef]
- Jaafar, A.; Schimpf, C.; Mandel, M.; Hecker, C.; Rafaja, D.; Krüger, L.; Arki, P.; Joseph, Y. Sol–Gel Derived Hydroxyapatite Coating on Titanium Implants: Optimization of Sol–Gel Process and Engineering the Interface. J. Mater. Res. 2022, 37, 2558–2570. [Google Scholar] [CrossRef]
- Osuchukwu, O.A.; Salihi, A.; Abdullahi, I.; Obada, D.O. Synthesis and Characterization of Sol–Gel Derived Hydroxyapatite from a Novel Mix of Two Natural Biowastes and Their Potentials for Biomedical Applications. Mater. Today Proc. 2022, 62, 4182–4187. [Google Scholar] [CrossRef]
- Farazin, A.; Ghasemi, A.H. Design, Synthesis, and Fabrication of Chitosan/Hydroxyapatite Composite Scaffold for Use as Bone Replacement Tissue by Sol–Gel Method. J. Inorg. Organomet. Polym. Mater. 2022, 32, 3067–3082. [Google Scholar] [CrossRef]
- Fujishiro, Y.; Fujimoto, A.; Sato, T.; Okuwaki, A. Coating of Hydroxyapatite on Titanium Plates Using Thermal Dissociation of Calcium-EDTA Chelate Complex in Phosphate Solutions under Hydrothermal Conditions. J. Colloid Interface Sci. 1995, 173, 119–127. [Google Scholar] [CrossRef]
- Kandori, K.; Horigami, N.; Yasukawa, A.; Ishikawa, T. Texture and Formation Mechanism of Fibrous Calcium Hydroxyapatite Particles Prepared by Decomposition of Calcium-EDTA Chelates. J. Am. Ceram. Soc. 1997, 80, 1157–1164. [Google Scholar] [CrossRef]
- Fujishiro, Y. , Sato, T. , & Okuwaki, A. Coating of hydroxyapatite on metal plates using thermal dissociation of calcium-EDTA chelate in phosphate solutions under hydrothermal conditions. J Mater Sci Mater Med, 1995, 6, 172–176. [Google Scholar] [CrossRef]
- Muthu, D. , Kumar, G.S., Gowri, M., Prasath, M., Viswabaskaran, V., Kattimani, V.S., & Girija, E.K. Rapid synthesis of eggshell derived hydroxyapatite with nanoscale characteristics for biomedical applications. Ceram. Int. 2022, 48, 1326–1339. [Google Scholar] [CrossRef]
- Arokiasamy, P.; Al Bakri Abdullah, M.M.; Abd Rahim, S.Z.; Luhar, S.; Sandu, A.V.; Jamil, N.H.; Nabiałek, M. Synthesis Methods of Hydroxyapatite from Natural Sources: A Review. Ceram. Int. 2022, 48, 14959–14979. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Shen, W.; Bai, Y.; Kong, L. Hydroxyapatite-Based Catalysis in Environmental Decontamination. J. Clean. Prod. 2022, 380, 134961. [Google Scholar] [CrossRef]
- Merzougui, M.; Mezahi, F.Z.; Dakhouche, A.; Kherifi, D.; Sahnoune, F. Improvement of the Reactivity of Triethyl Phosphate and Structural Behavior of Hydroxyapatite versus the Synthesis Conditions by Sol–Gel Route. Chem. Pap. 2022, 76, 1045–1061. [Google Scholar] [CrossRef]
- DileepKumar, V.G.; Sridhar, M.S.; Aramwit, P.; Krut’ko, V.K.; Musskaya, O.N.; Glazov, I.E.; Reddy, N. A Review on the Synthesis and Properties of Hydroxyapatite for Biomedical Applications. J. Biomater. Sci. Polym. Ed. 2022, 33, 229–261. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.M.; Yang, Q.; Troczynski, T.; Tseng, W.J. Structural Evolution of Sol-Gel-Derived Hydroxyapatite. Biomaterials 2002, 23, 1679–1687. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Q.; Vincent, P.; Sayer, M. Bone Growth on Sol-Gel Calcium Phosphate Thin Films In Vitro. Cells Mater. 1993, 3, 351. [Google Scholar]
- Cotrut, C.M.; Vladescu, A.; Dinu, M.; Vranceanu, D.M. Influence of Deposition Temperature on the Properties of Hydroxyapatite Obtained by Electrochemical Assisted Deposition. Ceram. Int. 2018, 44, 669–677. [Google Scholar] [CrossRef]
- Li, T.T.; Ling, L.; Lin, M.C.; Peng, H.K.; Ren, H.T.; Lou, C.W.; Lin, J.H. Recent Advances in Multifunctional Hydroxyapatite Coating by Electrochemical Deposition. J. Mater. Sci. 2020, 55, 6352–6374. [Google Scholar] [CrossRef]
- Gorejová, R. , Oriňaková, R., Králová, Z.O., Sopčák, T., Šišoláková, I., Schnitzer, M.,... & Hudák, R. Electrochemical deposition of a hydroxyapatite layer onto the surface of porous additively manufactured Ti6Al4V scaffolds. 2023, 129207. [Google Scholar] [CrossRef]
- Ulasevich, S.A.; Kulak, A.I.; Poznyak, S.K.; Karpushenkov, S.A.; Lisenkov, A.D.; Skorb, E.V. Deposition of Hydroxyapatite-Incorporated TiO2 Coating on Titanium Using Plasma Electrolytic Oxidation Coupled with Electrophoretic Deposition. RSC Adv. 2016, 6, 62540–62544. [Google Scholar] [CrossRef]
- Sultana, S.; Hossain, M.S.; Mahmud, M.; Mobarak, M. Bin; Kabir, M.H.; Sharmin, N.; Ahmed, S. UV-Assisted Synthesis of Hydroxyapatite from Eggshells at Ambient Temperature: Cytotoxicity, Drug Delivery and Bioactivity. RSC Adv. 2021, 11, 3686–3694. [Google Scholar] [CrossRef]
- Ulasevich, S.A.; Poznyak, S.K.; Kulak, A.I.; Lisenkov, A.D.; Starykevich, M.; Skorb, E.V. Photocatalytic Deposition of Hydroxyapatite onto a Titanium Dioxide Nanotubular Layer with Fine Tuning of Layer Nanoarchitecture. Langmuir 2016, 32, 4016–4021. [Google Scholar] [CrossRef]
- Peters, F. , & Epple, M. Simulating arterial wall calcification in vitro: biomimetic crystallization of calcium phosphates under controlled conditions. Z. Kardiol. 2001, 90, 81–85. [Google Scholar] [CrossRef]
- Aaseth, J.; Crisponi, G.; Anderson, O. Chelation therapy in the treatment of metal intoxication; Chapter 2; Academic Press, 2016; pp. 35–61. [Google Scholar]
- Ryzhkov, N.V. , Nesterov, P., Mamchik, N.A., Yurchenko, S.O., & Skorb, E.V. Localization of ion concentration gradients for logic operation. Front. Chem. 2019, 7, 419. [Google Scholar] [CrossRef] [PubMed]
- Snihirova, D. , Lamaka, S.V., Gonzalez-Garcia, Y., Yilmaz, A., Scharnagl, N., Montemor, M.F., & Zheludkevich, M.L. Influence of inhibitor adsorption on readings of microelectrode during SVET measurements. Electrochim. Acta 2019, 322, 134761. [Google Scholar] [CrossRef]
- Taryba, M. , Lamaka, S.V., Snihirova, D., Ferreira, M.G.S., Montemor, M.F., Wijting, W.K.,... & Grundmeier, G. The combined use of scanning vibrating electrode technique and micro-potentiometry to assess the self-repair processes in defects on “smart” coatings applied to galvanized steel. Electrochim. Acta 2011, 56, 4475–4488. [Google Scholar] [CrossRef]
- Skorb, E.V. , Fix, D., Andreeva, D.V., Möhwald, H., & Shchukin, D.G. Surface-modified mesoporous SiO2 containers for corrosion protection. Adv. Funct. Mater. 2009, 19, 2373–2379. [Google Scholar] [CrossRef]
- Ulasevich, S.A. , Brezhneva, N., Zhukova, Y., Möhwald, H., Fratzl, P., Schacher, F.H.,... & Skorb, E.V. Switching the stiffness of polyelectrolyte assembly by light to control behavior of supported cells. Macromol. Biosci. 2016, 16, 1422–1431. [Google Scholar] [CrossRef]
- Ulasevich, S.A. , Brezesinski, G., Möhwald, H., Fratzl, P., Schacher, F.H., Poznyak, S.K.,... & Skorb, E.V. Light-Induced Water Splitting Causes High-Amplitude Oscillation of pH-Sensitive Layer-by-Layer Assemblies on TiO2. Angew. Chem. 2016, 128, 13195–13198. [Google Scholar] [CrossRef]
- Espenson, J.H. Chemical kinetics and reaction mechanisms; McGraw-Hill: New York, 1995; Volume 102, p. 296. [Google Scholar]
- Van Boekel, M.A.J.S. Kinetic aspects of the Maillard reaction: a critical review. Food/Nahrung 2001, 45, 150–159. [Google Scholar] [CrossRef]
- Chemist's Handbook 21. Available online: https://chem21.info/info/321324/ (accessed on 21 April 2023).
- Mezni, A. , Saber, N.B., Ibrahim, M.M., El-Kemary, M., Aldalbahi, A., Feng, P.,... & Altalhi, T. Facile synthesis of highly thermally stable TiO2 photocatalysts. New J Chem. 2017, 41, 5021–5027. [Google Scholar] [CrossRef]
- Atkins, P.; Atkins, P.W.; de Paula, J. Atkins' physical chemistry; Oxford university press, 2014. [Google Scholar]

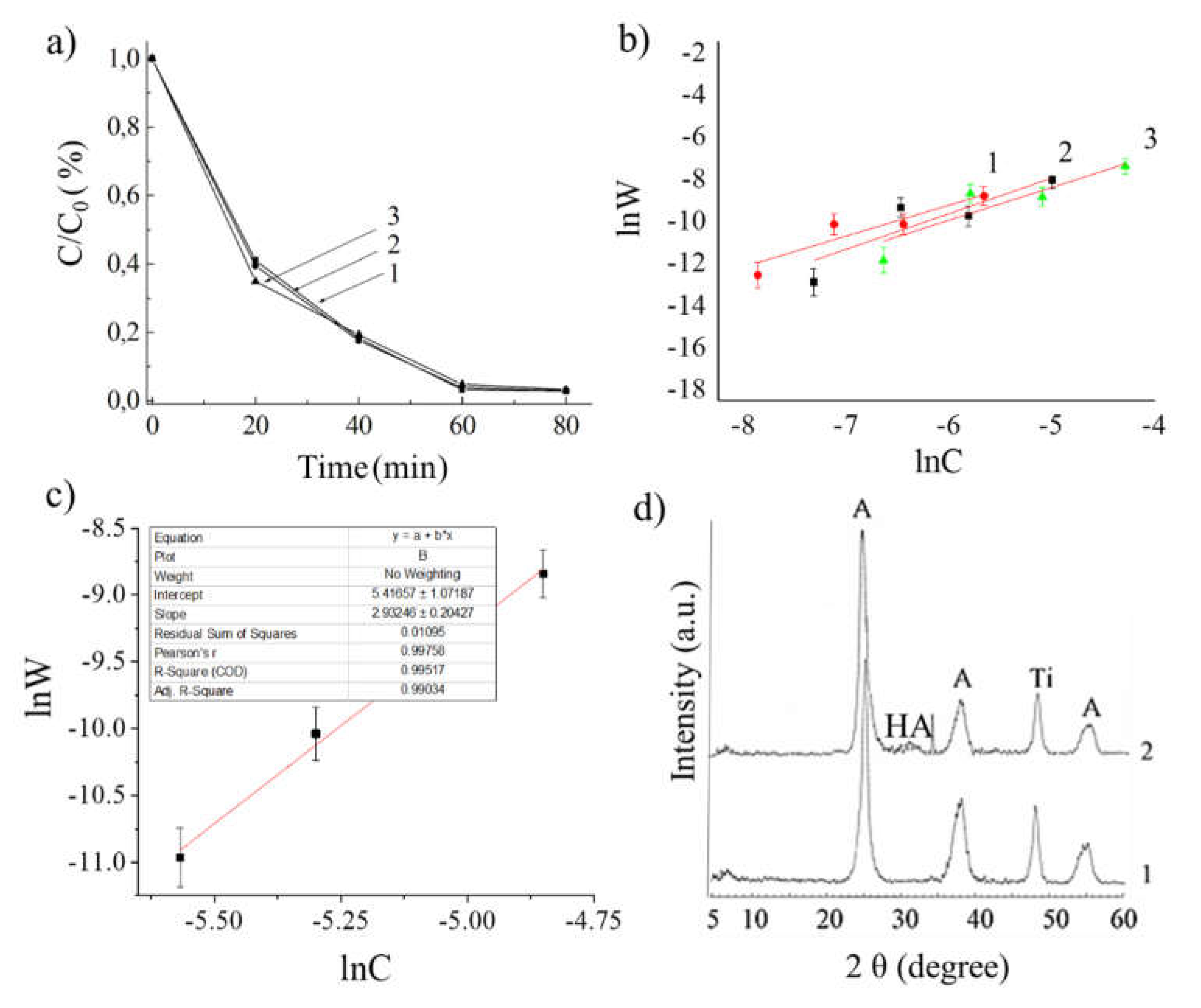
| C(Ca2+)/C(EDTA) | C0(Ca2+),mol/L | C0(EDTA),mol/L | Irradiation time, min | Ct(Ca2+), mol/L |
Ct(EDTA), mol/L |
Ct/C0, % |
|---|---|---|---|---|---|---|
| 1 : 1 | 0.01 | 0.01 | 20 | 0.00353 | 0.00642 | 64.7 |
| 0.01 | 0.01 | 40 | 0.00225 | 0.00769 | 77.5 | |
| 0.01 | 0.01 | 60 | 0.00059 | 0.00931 | 94.1 | |
| 0.01 | 0.01 | 80 | 0.00055 | 0.00936 | 94.5 | |
| 1 : 2 | 0.005 | 0.01 | 20 | 0.00391 | 0,00302 | 60.9 |
| 0.005 | 0.01 | 40 | 0.00237 | 0.00380 | 76.3 | |
| 0.005 | 0.01 | 60 | 0.00067 | 0.00458 | 93.3 | |
| 0.005 | 0.01 | 80 | 0.00060 | 0.00465 | 94.0 | |
| 2 : 1 | 0.02 | 0.01 | 20 | 0.00377 | 0.01241 | 62.3 |
| 0.02 | 0.01 | 40 | 0.00202 | 0.01528 | 79.8 | |
| 0.02 | 0.01 | 60 | 0.00059 | 0.01862 | 94.1 | |
| 0.02 | 0.01 | 80 | 0.00057 | 0.01876 | 94.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





