Submitted:
02 May 2023
Posted:
03 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Evidence of multi-targeted action of curcuminoids against cancers invivo, historical overview and recent findings
2.1. Historical overview
2.1.1. Chemopreventive properties
2.1.2. Modulation of immune functions and inhibition of signaling pathways
2.1.3. Extension of preclinical studies and first clinical trials
2.2. Recent findings, and new developments
| Major findings | Tumor type | Adm. route | Hist. | Ref |
|---|---|---|---|---|
| sympomatic relief, ↓ lesion size | Patients with external cancers | topical | - | [5] |
| tumor growth inhibition | Head & neck carcinoma (M) | topical | - | [37] |
| ↓ CD31 and HIF-1α expression | Head & neck carcinoma (M) | p.o. | + | [63] |
| ↑ IFN-γ and granzyme expression | Head & neck carcinoma (M) | i.p. | + | [67] |
| ↑ T-cell proliferation, ↓ PD-1 expression | Head & Neck carcinoma (M) | i.p. | + | [85] |
| humoral response (+ immunization) | Melanoma B16-R (M) | i.p. | - | [40] |
| ↓ ATP-synthase activity, ATP/AMP ratio | Melanoma B16-R (M) | i.v. | + | [66] |
| ↓ Foxp3+ Tregs in tumor | Melanoma B16-F10 (M) | i.p. GO-Y030 * | - | [71] |
| ↓tumor formation | Dalton’s lymphoma (M) | i.p. (liposomes) | - | [4] |
| downregulation of DNMT1 | Acute myeloid leukemia (M) | i.p. | - | [65] |
| ↓ MMP2, MMP9, vimentin expression | Monocytic leukaemia (M) | i.p. | + | [75] |
| ↓ EZH2, H3K4me3, H3K27me3 | Myelodysplastic syndrome (M) | - | - | [86] |
| apoptosis induction | Colon adenocarcinoma (R) | p.o. (diet) | + | [24] |
| inhibition of colon tumorigenesis | Colon adenocarcinoma (R) | p.o. (diet) | - | [26] |
| tumor prevention, ↑ CD4+ T-cells, B cells | Genetic colon cancer (M) | p.o. (diet) | + | [29] |
| inhibition of proteasome activity | Colon cancer HCT-116 (M) | p.o. | + | [64] |
| ↓ PCNA, β-catenin, Axin-2 | Colon cancer (M) | ? | + | [77] |
| ↑oxidative stress, mitochondrial Ca2+ | Colorectal cancer (M) | i.p. | + | [82] |
| inhibition of tumor-induced immune cell ↓ |
Ehrlich’s ascites carcinoma (M) | p.o. | + | [30] |
| no peritoneal bulge + survival | Histiocytic tumor AK-5 (R) | i.p. | - | [27] |
| ER stress-associated apoptosis | Liposarcoma (M) | i.p. | + | [44] |
| ↓ MDSCs, lL-6, Il-1β, GM-CSF secretion | Hepatocarcinoma HepG2 (M) | p.o. | + | [70] |
| inactivation of JAK2/STAT3 pathway | Hepatocellular carcinoma (M) | i.p. GL63 * | - | [79] |
| suppression of Wnt/ β-catenin signaling | Gastric carcinoma (M) | p.o. | - | [58] |
| inhibition of β-catenin and STAT3 | Genetic gastric cancer (M) | p.o. (diet) GO-Y031* |
+ | [59] |
| activation of ER stress pathway | Adrenocortical carcinoma (M) | i.p. | + | [83] |
| induction of cleaved caspase-3 and PARP | Prostate cancer PC-3 (M) | i.p. | + | [35] |
| ↑apoptosis, ↓ tumor angiogenesis | Prostate cancer LNCaP (M) | p.o. (diet) | + | [36] |
| ↓lung metastasis | Prostate cancer PC-3 (M) | p.o. (diet) | + | [48] |
| ↑membrane localization of β-catenin | Prostate cancer C4-2 (M) | intratumoral | + | [57] |
| NF-κB and p-STAT3 suppression, ↓ Il-8 | Ovarian cancer (M) | p.o. | + | [47] |
| synergically↑ IFN-β induced apoptosis | Breast cancer (M) | p.o. | - | [54] |
| ↓tumor incidence, DNA adducts | Mammary carcinoma (R) | i.p. | - | [23] |
| inhibition of SERCA2, ER stress | Breast cancer (M) | i.p. (RL71) * | + | [46] |
| inhibition of STAT3 phosphorylation | Breast cancer (M) | i.p. Curcumin-BTP hybrid * | + | [61] |
| N-cadherin, MMP2, MMP9 suppressed | Breast cancer (M) | i.p. WZ35 * | + | [80] |
| ↓ HIF1α/mTOR/VEGF cascade | Lewis lung cancer (M) | ? | - | [49] |
| ↑ expression of FOXO3a, p27, p21 | Lung cancer A549 (M) | i.p. | + | [55] |
| activation of p53-miR-192-5p/215-XIAP | Lung cancer (M) | p.o. | + | [62] |
| ↓circ-PRKCA, ITGB1 expression | Lung cancer A549 (M) | p.o. | - | [78] |
| induction of ferroptosis via autophagy | Lewis Lung carcinoma (M) | i.p. | + | [81] |
| immune response (CD8+ T cells), ↓ Il-6 | Mesothelioma (R) | i.p. | + | [42] |
| ↑p38, MAPK and CARP-1 | Patient mesothelioma (M) | p.o. | + | [43] |
| proteome changes (liver invasion) | Mesothelioma (R) | i.p. | + | [72] |
| proteome changes (residual tumors) | Mesothelioma (R) | i.p. | + | [74] |
| ↓tumor volume, hemorrhage | Glioblastoma C6 (R) | i.p. | + | [51] |
| ↑ PTEN and P53 expression | Glioblastoma U87 (M) | i.p. | + | [76] |
| ↑ HSP70, ER stress, immune cells | Glioma GL261 (M) | i.p. | + | [84] |
3. Curcumin and chemosensitization through metabolic reprogramming
3.3. Curcumin: Glycolysis and Lactate Production
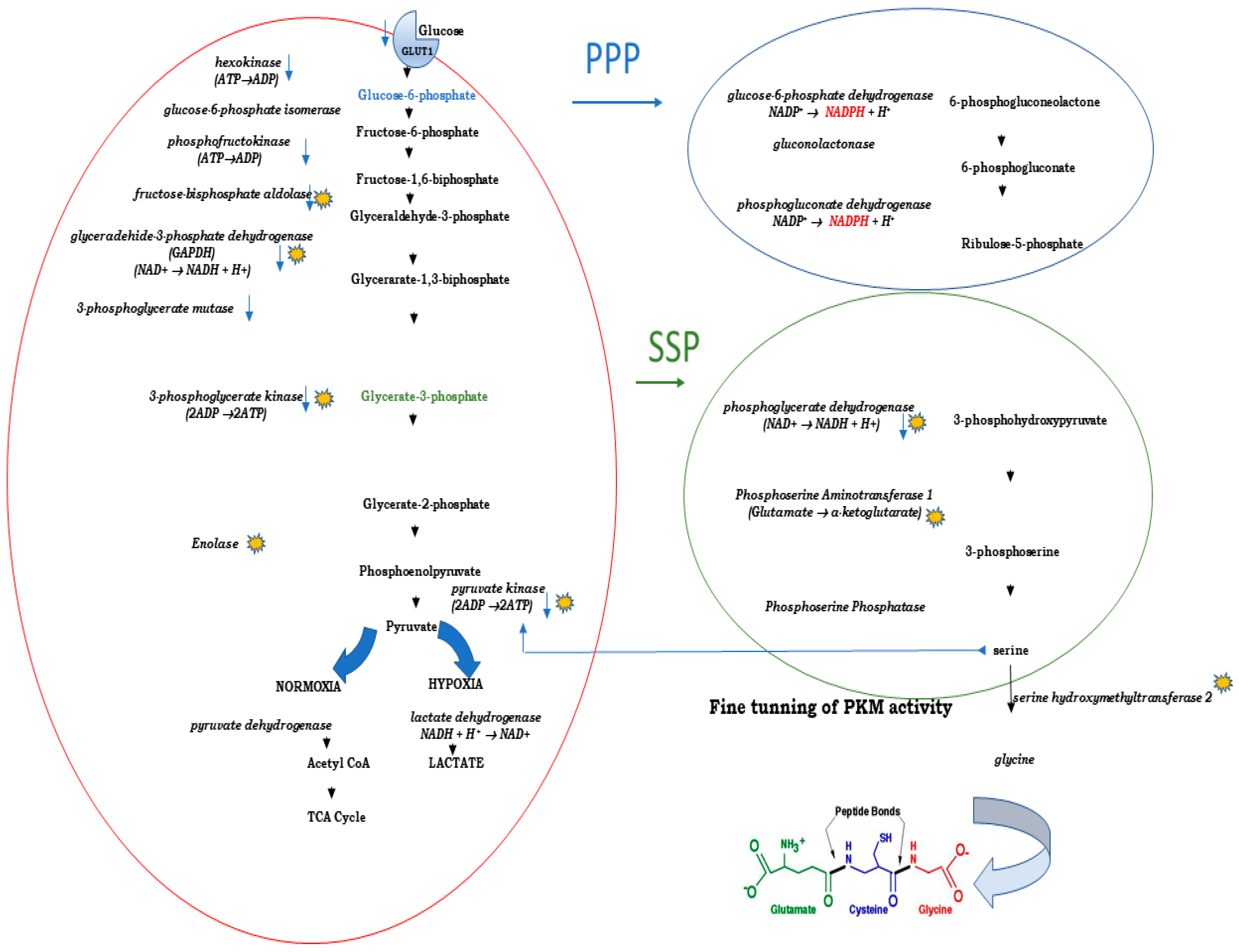
3.2. Curcumin and Lactate Excretion
3.3. Curcumin and Pentose Phosphate Pathway (PPP)
3.4. Curcumin, Pyruvate Kinase and Serine Synthesis Pathway (SSP)
4. Curcumin as a chemosensitizer in conventional chemotherapy
4.1. Chemosensitization: need of the hour.
4.2. Curcumin: the celebrity among nutraceuticals
4.3. Molecular targets of curcumin as a chemosensitizer
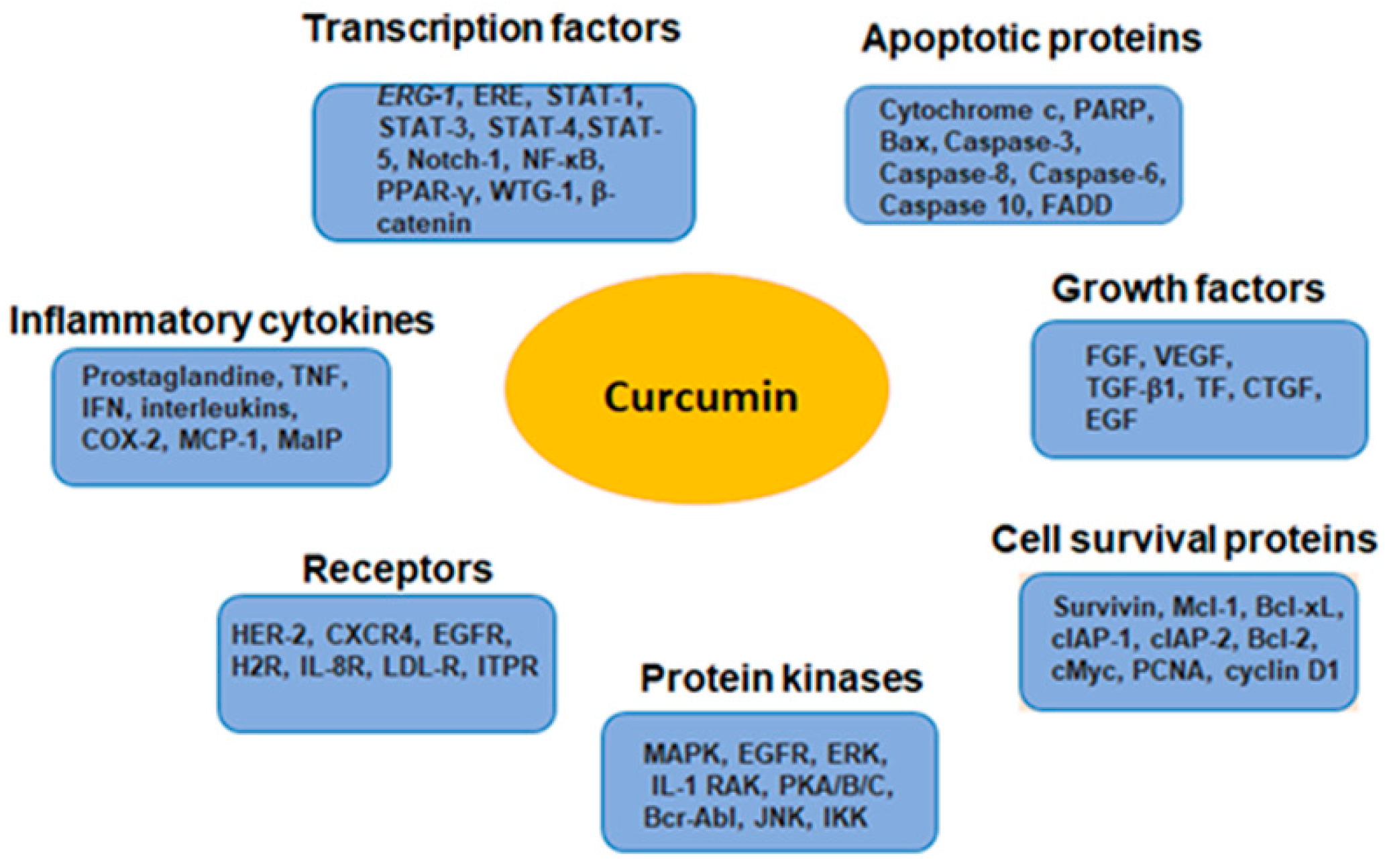
4.4. Curcumin as a chemosensitizer in adjuvant chemotherapy
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gerelene, J. Turmeric, The Golden Spice: From Asia to Africa. Open Access Journal of Archaeology & Anthropology (OAJAA) 2020, 2. [Google Scholar] [CrossRef]
- Schraufstatter, E.; Bernt, H. Antibacterial action of curcumin and related compounds. Nature 1949, 164, 456. [Google Scholar] [CrossRef] [PubMed]
- Ally, B. Turmeric Flower as a Remedy for Gonorrhoea. Ind Med Gaz 1876, 11, 273. [Google Scholar] [PubMed]
- Kuttan, R.; Bhanumathy, P.; Nirmala, K.; George, M.C. Potential anticancer activity of turmeric (Curcuma longa). Cancer Lett 1985, 29, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Kuttan, R.; Sudheeran, P.C.; Josph, C.D. Turmeric and curcumin as topical agents in cancer therapy. Tumori 1987, 73, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Ruby, A.J.; Kuttan, G.; Babu, K.D.; Rajasekharan, K.N.; Kuttan, R. Anti-tumour and antioxidant activity of natural curcuminoids. Cancer Lett 1995, 94, 79–83. [Google Scholar] [CrossRef]
- Anto, R.J.; George, J.; Babu, K.V.; Rajasekharan, K.N.; Kuttan, R. Antimutagenic and anticarcinogenic activity of natural and synthetic curcuminoids. Mutat Res 1996, 370, 127–131. [Google Scholar] [CrossRef]
- Steward, W.P.; Gescher, A.J. Curcumin in cancer management: recent results of analogue design and clinical studies and desirable future research. Mol Nutr Food Res 2008, 52, 1005–1009. [Google Scholar] [CrossRef]
- Rodrigues, F.C.; Kumar, N.A.; Thakur, G. The potency of heterocyclic curcumin analogues: An evidence-based review. Pharmacol Res 2021, 166, 105489. [Google Scholar] [CrossRef]
- Mehta, H.J.; Patel, V.; Sadikot, R.T. Curcumin and lung cancer--a review. Target Oncol 2014, 9, 295–310. [Google Scholar] [CrossRef]
- Pouliquen, D.L.; Boissard, A.; Henry, C.; Coqueret, O.; Guette, C. Curcuminoids as Modulators of EMT in Invasive Cancers: A Review of Molecular Targets With the Contribution of Malignant Mesothelioma Studies. Front Pharmacol 2022, 13, 934534. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, R.A.; Garcia-Smith, R.; Dorsey, J.; Griffith, J.K.; Bisoffi, M.; Trujillo, K.A. Tumor necrosis factor alpha induces Warburg-like metabolism and is reversed by anti-inflammatory curcumin in breast epithelial cells. Int J Cancer 2013, 133, 2504–2510. [Google Scholar] [CrossRef] [PubMed]
- Troselj, K.G.; Samarzija, I.; Tomljanovic, M.; Kujundzic, R.N.; Dakovic, N.; Mojzes, A. Implementing Curcumin in Translational Oncology Research. Molecules 2020, 25. [Google Scholar] [CrossRef] [PubMed]
- Amekyeh, H.; Alkhader, E.; Sabra, R.; Billa, N. Prospects of Curcumin Nanoformulations in Cancer Management. Molecules 2022, 27. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, M.; van Nostrum, C.F.; Kok, R.J.; Storm, G.; Hennink, W.E.; Heger, M. Utility of Intravenous Curcumin Nanodelivery Systems for Improving In Vivo Pharmacokinetics and Anticancer Pharmacodynamics. Mol Pharm 2022, 19, 3057–3074. [Google Scholar] [CrossRef] [PubMed]
- Maleki Dizaj, S.; Alipour, M.; Dalir Abdolahinia, E.; Ahmadian, E.; Eftekhari, A.; Forouhandeh, H.; Rahbar Saadat, Y.; Sharifi, S.; Zununi Vahed, S. Curcumin nanoformulations: Beneficial nanomedicine against cancer. Phytother Res 2022, 36, 1156–1181. [Google Scholar] [CrossRef] [PubMed]
- Obeid, M.A.; Alsaadi, M.; Aljabali, A.A. Recent updates in curcumin delivery. J Liposome Res 2022, 10.1080/08982104.2022.2086567, 1-12. [CrossRef]
- Bava, S.V.; Puliyappadamba, V.T.; Deepti, A.; Nair, A.; Karunagaran, D.; Anto, R.J. Sensitization of taxol-induced apoptosis by curcumin involves down- regulation of nuclear factor-kappaB and the serine/threonine kinase Akt and is independent of tubulin polymerization. J Biol Chem 2005, 280, 6301–6308. [Google Scholar] [CrossRef]
- Horbach, S.; Halffman, W. The ghosts of HeLa: How cell line misidentification contaminates the scientific literature. PLoS One 2017, 12, e0186281. [Google Scholar] [CrossRef]
- Conney, A.H.; Lysz, T.; Ferraro, T.; Abidi, T.F.; Manchand, P.S.; Laskin, J.D.; Huang, M.T. Inhibitory effect of curcumin and some related dietary compounds on tumor promotion and arachidonic acid metabolism in mouse skin. Adv Enzyme Regul 1991, 31, 385–396. [Google Scholar] [CrossRef]
- Polasa, K.; Sesikaran, B.; Krishna, T.P.; Krishnaswamy, K. Turmeric (Curcuma longa)-induced reduction in urinary mutagens. Food Chem Toxicol 1991, 29, 699–706. [Google Scholar] [CrossRef]
- Nagabhushan, M.; Bhide, S.V. Curcumin as an inhibitor of cancer. J Am Coll Nutr 1992, 11, 192–198. [Google Scholar] [CrossRef]
- Singletary, K.; MacDonald, C.; Wallig, M.; Fisher, C. Inhibition of 7,12-dimethylbenz[a]anthracene (DMBA)-induced mammary tumorigenesis and DMBA-DNA adduct formation by curcumin. Cancer Lett 1996, 103, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Samaha, H.S.; Kelloff, G.J.; Steele, V.; Rao, C.V.; Reddy, B.S. Modulation of apoptosis by sulindac, curcumin, phenylethyl-3-methylcaffeate, and 6-phenylhexyl isothiocyanate: apoptotic index as a biomarker in colon cancer chemoprevention and promotion. Cancer Res 1997, 57, 1301–1305. [Google Scholar] [PubMed]
- Kato, K.; Ito, H.; Kamei, K.; Iwamoto, I. Stimulation of the stress-induced expression of stress proteins by curcumin in cultured cells and in rat tissues in vivo. Cell Stress Chaperones 1998, 3, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Kawamori, T.; Lubet, R.; Steele, V.E.; Kelloff, G.J.; Kaskey, R.B.; Rao, C.V.; Reddy, B.S. Chemopreventive effect of curcumin, a naturally occurring anti- inflammatory agent, during the promotion/progression stages of colon cancer. Cancer Res 1999, 59, 597–601. [Google Scholar] [PubMed]
- Khar, A.; Ali, A.M.; Pardhasaradhi, B.V.; Begum, Z.; Anjum, R. Antitumor activity of curcumin is mediated through the induction of apoptosis in AK-5 tumor cells. FEBS Lett 1999, 445, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Bhaumik, S.; Jyothi, M.D.; Khar, A. Differential modulation of nitric oxide production by curcumin in host macrophages and NK cells. FEBS Lett 2000, 483, 78–82. [Google Scholar] [CrossRef]
- Churchill, M.; Chadburn, A.; Bilinski, R.T.; Bertagnolli, M.M. Inhibition of intestinal tumors by curcumin is associated with changes in the intestinal immune cell profile. J Surg Res 2000, 89, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Bhattacharyya, S.; Choudhuri, T.; Datta, G.K.; Das, T.; Sa, G. Amelioration of immune cell number depletion and potentiation of depressed detoxification system of tumor-bearing mice by curcumin. Cancer Detect Prev 2005, 29, 470–478. [Google Scholar] [CrossRef]
- Lin, J.K.; Pan, M.H.; Lin-Shiau, S.Y. Recent studies on the biofunctions and biotransformations of curcumin. Biofactors 2000, 13, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.K. Suppression of protein kinase C and nuclear oncogene expression as possible action mechanisms of cancer chemoprevention by Curcumin. Arch Pharm Res 2004, 27, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.K.; Cha, S.H.; Jeon, H.G. Curcumin-induced histone hypoacetylation enhances caspase-3-dependent glioma cell death and neurogenesis of neural progenitor cells. Stem Cells Dev 2006, 15, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Han, R. Highlight on the studies of anticancer drugs derived from plants in China. Stem Cells 1994, 12, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Khor, T.O.; Keum, Y.S.; Lin, W.; Kim, J.H.; Hu, R.; Shen, G.; Xu, C.; Gopalakrishnan, A.; Reddy, B.; Zheng, X.; et al. Combined inhibitory effects of curcumin and phenethyl isothiocyanate on the growth of human PC-3 prostate xenografts in immunodeficient mice. Cancer Res 2006, 66, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Dorai, T.; Cao, Y.C.; Dorai, B.; Buttyan, R.; Katz, A.E. Therapeutic potential of curcumin in human prostate cancer. III. Curcumin inhibits proliferation, induces apoptosis, and inhibits angiogenesis of LNCaP prostate cancer cells in vivo. Prostate 2001, 47, 293–303. [Google Scholar] [CrossRef] [PubMed]
- LoTempio, M.M.; Veena, M.S.; Steele, H.L.; Ramamurthy, B.; Ramalingam, T.S.; Cohen, A.N.; Chakrabarti, R.; Srivatsan, E.S.; Wang, M.B. Curcumin suppresses growth of head and neck squamous cell carcinoma. Clin Cancer Res 2005, 11, 6994–7002. [Google Scholar] [CrossRef]
- Shehzad, A.; Lee, Y.S. Molecular mechanisms of curcumin action: signal transduction. Biofactors 2013, 39, 27–36. [Google Scholar] [CrossRef]
- Calabrese, V.; Bates, T.E.; Mancuso, C.; Cornelius, C.; Ventimiglia, B.; Cambria, M.T.; Di Renzo, L.; De Lorenzo, A.; Dinkova-Kostova, A.T. Curcumin and the cellular stress response in free radical-related diseases. Mol Nutr Food Res 2008, 52, 1062–1073. [Google Scholar] [CrossRef]
- Odot, J.; Albert, P.; Carlier, A.; Tarpin, M.; Devy, J.; Madoulet, C. In vitro and in vivo anti-tumoral effect of curcumin against melanoma cells. Int J Cancer 2004, 111, 381–387. [Google Scholar] [CrossRef]
- Chang, Y.F.; Chuang, H.Y.; Hsu, C.H.; Liu, R.S.; Gambhir, S.S.; Hwang, J.J. Immunomodulation of curcumin on adoptive therapy with T cell functional imaging in mice. Cancer Prev Res (Phila) 2012, 5, 444–452. [Google Scholar] [CrossRef]
- Pouliquen, D.L.; Nawrocki-Raby, B.; Nader, J.; Blandin, S.; Robard, M.; Birembaut, P.; Grégoire, M. Evaluation of intracavitary administration of curcumin for the treatment of sarcomatoid mesothelioma. Oncotarget 2017, 8, 57552–57573. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Rishi, A.K.; Wu, W.; Polin, L.; Sharma, S.; Levi, E.; Albelda, S.; Pass, H.I.; Wali, A. Curcumin suppresses growth of mesothelioma cells in vitro and in vivo, in part, by stimulating apoptosis. Mol Cell Biochem 2011, 357, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, L.; Song, R.; Shen, Y.; Sun, Y.; Gu, Y.; Shu, Y.; Xu, Q. Targeting sarcoplasmic/endoplasmic reticulum Ca(2)+-ATPase 2 by curcumin induces ER stress-associated apoptosis for treating human liposarcoma. Mol Cancer Ther 2011, 10, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiao, J.; Zhou, H.; Yang, S.; Wu, X.; Jiang, C.; Zhao, Y.; Liang, D.; Li, X.; Liang, G. A novel monocarbonyl analogue of curcumin, (1E,4E)-1,5-bis(2,3-dimethoxyphenyl)penta-1,4-dien-3-one, induced cancer cell H460 apoptosis via activation of endoplasmic reticulum stress signaling pathway. J Med Chem 2011, 54, 3768–3778. [Google Scholar] [CrossRef]
- Gao, J.; Fan, M.; Peng, S.; Zhang, M.; Xiang, G.; Li, X.; Guo, W.; Sun, Y.; Wu, X.; Wu, X.; et al. Small-molecule RL71-triggered excessive autophagic cell death as a potential therapeutic strategy in triple-negative breast cancer. Cell Death Dis 2017, 8, e3049. [Google Scholar] [CrossRef]
- Lin, Y.G.; Kunnumakkara, A.B.; Nair, A.; Merritt, W.M.; Han, L.Y.; Armaiz-Pena, G.N.; Kamat, A.A.; Spannuth, W.A.; Gershenson, D.M.; Lutgendorf, S.K.; et al. Curcumin inhibits tumor growth and angiogenesis in ovarian carcinoma by targeting the nuclear factor-kappaB pathway. Clin Cancer Res 2007, 13, 3423–3430. [Google Scholar] [CrossRef]
- Killian, P.H.; Kronski, E.; Michalik, K.M.; Barbieri, O.; Astigiano, S.; Sommerhoff, C.P.; Pfeffer, U.; Nerlich, A.G.; Bachmeier, B.E. Curcumin inhibits prostate cancer metastasis in vivo by targeting the inflammatory cytokines CXCL1 and -2. Carcinogenesis 2012, 33, 2507–2519. [Google Scholar] [CrossRef]
- Fan, S.; Xu, Y.; Li, X.; Tie, L.; Pan, Y.; Li, X. Opposite angiogenic outcome of curcumin against ischemia and Lewis lung cancer models: in silico, in vitro and in vivo studies. Biochim Biophys Acta 2014, 1842, 1742–1754. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Anand, P.; Aggarwal, B.B. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett 2008, 269, 199–225. [Google Scholar] [CrossRef]
- Zanotto-Filho, A.; Braganhol, E.; Edelweiss, M.I.; Behr, G.A.; Zanin, R.; Schroder, R.; Simoes-Pires, A.; Battastini, A.M.; Moreira, J.C. The curry spice curcumin selectively inhibits cancer cells growth in vitro and in preclinical model of glioblastoma. J Nutr Biochem 2012, 23, 591–601. [Google Scholar] [CrossRef]
- Wilken, R.; Veena, M.S.; Wang, M.B.; Srivatsan, E.S. Curcumin: A review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol Cancer 2011, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Borges, G.A.; Rego, D.F.; Assad, D.X.; Coletta, R.D.; De Luca Canto, G.; Guerra, E.N. In vivo and in vitro effects of curcumin on head and neck carcinoma: a systematic review. J Oral Pathol Med 2017, 46, 3–20. [Google Scholar] [CrossRef]
- Ren, M.; Wang, Y.; Wu, X.; Ge, S.; Wang, B. Curcumin synergistically increases effects of beta-interferon and retinoic acid on breast cancer cells in vitro and in vivo by up- regulation of GRIM-19 through STAT3-dependent and STAT3-independent pathways. J Drug Target 2017, 25, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhou, B.H.; Qiu, X.; Wang, H.S.; Zhang, F.; Fang, R.; Wang, X.F.; Cai, S.H.; Du, J.; Bu, X.Z. T63, a new 4-arylidene curcumin analogue, induces cell cycle arrest and apoptosis through activation of the reactive oxygen species-FOXO3a pathway in lung cancer cells. Free Radic Biol Med 2012, 53, 2204–2217. [Google Scholar] [CrossRef] [PubMed]
- Hackler, L., Jr.; Ozsvari, B.; Gyuris, M.; Sipos, P.; Fabian, G.; Molnar, E.; Marton, A.; Farago, N.; Mihaly, J.; Nagy, L.I.; et al. The Curcumin Analog C-150, Influencing NF-kappaB, UPR and Akt/Notch Pathways Has Potent Anticancer Activity In Vitro and In Vivo. PLoS One 2016, 11, e0149832. [Google Scholar] [CrossRef]
- Sundram, V.; Chauhan, S.C.; Ebeling, M.; Jaggi, M. Curcumin attenuates beta-catenin signaling in prostate cancer cells through activation of protein kinase D1. PLoS One 2012, 7, e35368. [Google Scholar] [CrossRef]
- Zheng, R.; Deng, Q.; Liu, Y.; Zhao, P. Curcumin Inhibits Gastric Carcinoma Cell Growth and Induces Apoptosis by Suppressing the Wnt/beta-Catenin Signaling Pathway. Med Sci Monit 2017, 23, 163–171. [Google Scholar] [CrossRef]
- Uehara, Y.; Inoue, M.; Fukuda, K.; Yamakoshi, H.; Hosoi, Y.; Kanda, H.; Oshima, M.; Iwabuchi, Y.; Shibata, H. Inhibition of beta-catenin and STAT3 with a curcumin analog suppresses gastric carcinogenesis in vivo. Gastric Cancer 2015, 18, 774–783. [Google Scholar] [CrossRef]
- Alexandrow, M.G.; Song, L.J.; Altiok, S.; Gray, J.; Haura, E.B.; Kumar, N.B. Curcumin: a novel Stat3 pathway inhibitor for chemoprevention of lung cancer. Eur J Cancer Prev 2012, 21, 407–412. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, J.; Li, S.; Ma, T.; Xu, D.; Han, C.; Liu, F.; Yu, W.; Kong, L. Discovery of monocarbonyl curcumin-BTP hybrids as STAT3 inhibitors for drug-sensitive and drug-resistant breast cancer therapy. Sci Rep 2017, 7, 46352. [Google Scholar] [CrossRef]
- Ye, M.; Zhang, J.; Zhang, J.; Miao, Q.; Yao, L.; Zhang, J. Curcumin promotes apoptosis by activating the p53-miR-192-5p/215-XIAP pathway in non-small cell lung cancer. Cancer Lett 2015, 357, 196–205. [Google Scholar] [CrossRef]
- Hu, A.; Huang, J.J.; Zhang, J.F.; Dai, W.J.; Li, R.L.; Lu, Z.Y.; Duan, J.L.; Li, J.P.; Chen, X.P.; Fan, J.P.; et al. Curcumin induces G2/M cell cycle arrest and apoptosis of head and neck squamous cell carcinoma in vitro and in vivo through ATM/Chk2/p53-dependent pathway. Oncotarget 2017, 8, 50747–50760. [Google Scholar] [CrossRef]
- Milacic, V.; Banerjee, S.; Landis-Piwowar, K.R.; Sarkar, F.H.; Majumdar, A.P.; Dou, Q.P. Curcumin inhibits the proteasome activity in human colon cancer cells in vitro and in vivo. Cancer Res 2008, 68, 7283–7292. [Google Scholar] [CrossRef]
- Yu, J.; Peng, Y.; Wu, L.C.; Xie, Z.; Deng, Y.; Hughes, T.; He, S.; Mo, X.; Chiu, M.; Wang, Q.E.; et al. Curcumin down-regulates DNA methyltransferase 1 and plays an anti- leukemic role in acute myeloid leukemia. PLoS One 2013, 8, e55934. [Google Scholar] [CrossRef]
- Bianchi, G.; Ravera, S.; Traverso, C.; Amaro, A.; Piaggio, F.; Emionite, L.; Bachetti, T.; Pfeffer, U.; Raffaghello, L. Curcumin induces a fatal energetic impairment in tumor cells in vitro and in vivo by inhibiting ATP-synthase activity. Carcinogenesis 2018, 39, 1141–1150. [Google Scholar] [CrossRef]
- Liu, L.; Lim, M.A.; Jung, S.N.; Oh, C.; Won, H.R.; Jin, Y.L.; Piao, Y.; Kim, H.J.; Chang, J.W.; Koo, B.S. The effect of Curcumin on multi-level immune checkpoint blockade and T cell dysfunction in head and neck cancer. Phytomedicine 2021, 92, 153758. [Google Scholar] [CrossRef]
- Kotting, C.; Hofmann, L.; Lotfi, R.; Engelhardt, D.; Laban, S.; Schuler, P.J.; Hoffmann, T.K.; Brunner, C.; Theodoraki, M.N. Immune-Stimulatory Effects of Curcumin on the Tumor Microenvironment in Head and Neck Squamous Cell Carcinoma. Cancers (Basel) 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Miles, G.J.; Demetriou, C.; Sidat, Z.; Foreman, N.; West, K.; Karmokar, A.; Howells, L.; Pritchard, C.; Thomas, A.L.; et al. Ex vivo explant model of adenoma and colorectal cancer to explore mechanisms of action and patient response to cancer prevention therapies. Mutagenesis 2022, 37, 227–237. [Google Scholar] [CrossRef]
- Tian, S.; Liao, L.; Zhou, Q.; Huang, X.; Zheng, P.; Guo, Y.; Deng, T.; Tian, X. Curcumin inhibits the growth of liver cancer by impairing myeloid-derived suppressor cells in murine tumor tissues. Oncol Lett 2021, 21, 286. [Google Scholar] [CrossRef] [PubMed]
- MaruYama, T.; Kobayashi, S.; Nakatsukasa, H.; Moritoki, Y.; Taguchi, D.; Sunagawa, Y.; Morimoto, T.; Asao, A.; Jin, W.; Owada, Y.; et al. The Curcumin Analog GO-Y030 Controls the Generation and Stability of Regulatory T Cells. Front Immunol 2021, 12, 687669. [Google Scholar] [CrossRef]
- Pouliquen, D.L.; Boissard, A.; Henry, C.; Blandin, S.; Richomme, P.; Coqueret, O.; Guette, C. Curcumin Treatment Identifies Therapeutic Targets within Biomarkers of Liver Colonization by Highly Invasive Mesothelioma Cells-Potential Links with Sarcomas. Cancers (Basel) 2020, 12. [Google Scholar] [CrossRef]
- Pouliquen, D.L.; Boissard, A.; Henry, C.; Blandin, S.; Coqueret, O.; Guette, C. Lymphoid Organ Proteomes Identify Therapeutic Efficacy Biomarkers Following the Intracavitary Administration of Curcumin in a Highly Invasive Rat Model of Peritoneal Mesothelioma. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef]
- Pouliquen, D.L.; Malloci, M.; Boissard, A.; Henry, C.; Guette, C. Proteomes of Residual Tumors in Curcumin-Treated Rats Reveal Changes in Microenvironment/Malignant Cell Crosstalk in a Highly Invasive Model of Mesothelioma. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef]
- Zhu, G.; Shen, Q.; Jiang, H.; Ji, O.; Zhu, L.; Zhang, L. Curcumin inhibited the growth and invasion of human monocytic leukaemia SHI-1 cells in vivo by altering MAPK and MMP signalling. Pharm Biol 2020, 58, 25–34. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, F.; Liao, W.; Yu, L.; Hu, Z.; Li, M.; Xia, H. Curcumin suppresses glioblastoma cell proliferation by p-AKT/mTOR pathway and increases the PTEN expression. Arch Biochem Biophys 2020, 689, 108412. [Google Scholar] [CrossRef]
- Hao, J.; Dai, X.; Gao, J.; Li, Y.; Hou, Z.; Chang, Z.; Wang, Y. Curcumin suppresses colorectal tumorigenesis via the Wnt/beta-catenin signaling pathway by downregulating Axin2. Oncol Lett 2021, 21, 186. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, X.; Zhang, Y.; Wang, Z. Curcumin suppresses the malignancy of non-small cell lung cancer by modulating the circ-PRKCA/miR-384/ITGB1 pathway. Biomed Pharmacother 2021, 138, 111439. [Google Scholar] [CrossRef]
- Zhao, J.A.; Nie, W.; Dong, L.; Liu, W.; Wei, W. A curcumin analog GL63 inhibits the malignant behaviors of hepatocellular carcinoma by inactivating the JAK2/STAT3 signaling pathway via the circular RNA zinc finger protein 83/microRNA-324-5p/cyclin-dependent kinase 16 axis. J Gastroenterol Hepatol 2021, 36, 2967–2977. [Google Scholar] [CrossRef]
- Wang, L.; Wang, C.; Tao, Z.; Zhao, L.; Zhu, Z.; Wu, W.; He, Y.; Chen, H.; Zheng, B.; Huang, X.; et al. Curcumin derivative WZ35 inhibits tumor cell growth via ROS-YAP-JNK signaling pathway in breast cancer. J Exp Clin Cancer Res 2019, 38, 460. [Google Scholar] [CrossRef]
- Tang, X.; Ding, H.; Liang, M.; Chen, X.; Yan, Y.; Wan, N.; Chen, Q.; Zhang, J.; Cao, J. Curcumin induces ferroptosis in non-small-cell lung cancer via activating autophagy. Thorac Cancer 2021, 12, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Gueguinou, M.; Ibrahim, S.; Bourgeais, J.; Robert, A.; Pathak, T.; Zhang, X.; Crottes, D.; Dupuy, J.; Ternant, D.; Monbet, V.; et al. Curcumin and NCLX inhibitors share anti-tumoral mechanisms in microsatellite-instability-driven colorectal cancer. Cell Mol Life Sci 2022, 79, 284. [Google Scholar] [CrossRef]
- Huang, X.; Liang, C.; Yang, H.; Li, X.; Deng, X.; Liang, X.; Li, L.; Huang, Z.; Lu, D.; Ma, Y.; et al. Curcumin induces apoptosis and inhibits the growth of adrenocortical carcinoma: Identification of potential candidate genes and pathways by transcriptome analysis. Oncol Lett 2021, 21, 476. [Google Scholar] [CrossRef]
- Xiu, Z.; Sun, T.; Yang, Y.; He, Y.; Yang, S.; Xue, X.; Yang, W. Curcumin Enhanced Ionizing Radiation-Induced Immunogenic Cell Death in Glioma Cells through Endoplasmic Reticulum Stress Signaling Pathways. Oxid Med Cell Longev 2022, 2022, 5424411. [Google Scholar] [CrossRef]
- Liu, L.; Fu, Y.; Zheng, Y.; Ma, M.; Wang, C. Curcumin inhibits proteasome activity in triple-negative breast cancer cells through regulating p300/miR-142-3p/PSMB5 axis. Phytomedicine 2020, 78, 153312. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, X.; Wang, Z.; Huang, L.; Meng, F.; Hu, L.; Chen, Y.; Wei, J. Anti-cancer Effects of Curcumin on Myelodysplastic Syndrome through the Inhibition of Enhancer of Zeste Homolog-2 (EZH2). Curr Cancer Drug Targets 2019, 19, 729–741. [Google Scholar] [CrossRef]
- Shin, J.W.; Chun, K.S.; Kim, D.H.; Kim, S.J.; Kim, S.H.; Cho, N.C.; Na, H.K.; Surh, Y.J. Curcumin induces stabilization of Nrf2 protein through Keap1 cysteine modification. Biochem Pharmacol 2020, 173, 113820. [Google Scholar] [CrossRef]
- Shaikh, S.B.; Najar, M.A.; Prasad, T.S.K.; Bhandary, Y.P. Comparative protein profiling reveals the inhibitory role of curcumin on IL-17A mediated minichromosome maintenance (MCM) proteins as novel putative markers for acute lung injury in vivo. Biomed Pharmacother 2021, 141, 111715. [Google Scholar] [CrossRef]
- Vishvakarma, N.K.; Kumar, A.; Singh, V.; Singh, S.M. Hyperglycemia of tumor microenvironment modulates stage-dependent tumor progression and multidrug resistance: implication of cell survival regulatory molecules and altered glucose transport. Mol Carcinog 2013, 52, 932–945. [Google Scholar] [CrossRef]
- Soni, V.K.; Mehta, A.; Ratre, Y.K.; Chandra, V.; Shukla, D.; Kumar, A.; Vishvakarma, N.K. Counteracting Action of Curcumin on High Glucose-Induced Chemoresistance in Hepatic Carcinoma Cells. Front Oncol 2021, 11, 738961. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Plas, D.R.; Rathmell, J.C.; Fox, C.J.; Harris, M.H.; Thompson, C.B. Growth factors can influence cell growth and survival through effects on glucose metabolism. Mol Cell Biol 2001, 21, 5899–5912. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.B.; Bielska, A.A. Growth factors stimulate anabolic metabolism by directing nutrient uptake. J Biol Chem 2019, 294, 17883–17888. [Google Scholar] [CrossRef]
- Feng, Y.; Xiong, Y.; Qiao, T.; Li, X.; Jia, L.; Han, Y. Lactate dehydrogenase A: A key player in carcinogenesis and potential target in cancer therapy. Cancer Med 2018, 7, 6124–6136. [Google Scholar] [CrossRef]
- Firth, J.D.; Ebert, B.L.; Ratcliffe, P.J. Hypoxic regulation of lactate dehydrogenase A. Interaction between hypoxia-inducible factor 1 and cAMP response elements. J Biol Chem 1995, 270, 21021–21027. [Google Scholar] [CrossRef]
- Cui, X.G.; Han, Z.T.; He, S.H.; Wu, X.D.; Chen, T.R.; Shao, C.H.; Chen, D.L.; Su, N.; Chen, Y.M.; Wang, T.; et al. HIF1/2alpha mediates hypoxia-induced LDHA expression in human pancreatic cancer cells. Oncotarget 2017, 8, 24840–24852. [Google Scholar] [CrossRef]
- Epstein, T.; Xu, L.; Gillies, R.J.; Gatenby, R.A. Separation of metabolic supply and demand: aerobic glycolysis as a normal physiological response to fluctuating energetic demands in the membrane. Cancer Metab 2014, 2, 7. [Google Scholar] [CrossRef]
- Fendt, S.M.; Frezza, C.; Erez, A. Targeting Metabolic Plasticity and Flexibility Dynamics for Cancer Therapy. Cancer Discov 2020, 10, 1797–1807. [Google Scholar] [CrossRef]
- de la Cruz-Lopez, K.G.; Castro-Munoz, L.J.; Reyes-Hernandez, D.O.; Garcia-Carranca, A.; Manzo-Merino, J. Lactate in the Regulation of Tumor Microenvironment and Therapeutic Approaches. Front Oncol 2019, 9, 1143. [Google Scholar] [CrossRef]
- Wittig, R.; Coy, J.F. The role of glucose metabolism and glucose-associated signalling in cancer. Perspect Medicin Chem 2008, 1, 64–82. [Google Scholar] [CrossRef]
- Troselj, K.G.; Kujundzic, R.N. Curcumin in combined cancer therapy. Curr Pharm Des 2014, 20, 6682–6696. [Google Scholar] [CrossRef] [PubMed]
- Wagner, W.; Kania, K.D.; Blauz, A.; Ciszewski, W.M. The lactate receptor (HCAR1/GPR81) contributes to doxorubicin chemoresistance via ABCB1 transporter up-regulation in human cervical cancer HeLa cells. J Physiol Pharmacol 2017, 68, 555–564. [Google Scholar] [PubMed]
- Gunnink, L.K.; Alabi, O.D.; Kuiper, B.D.; Gunnink, S.M.; Schuiteman, S.J.; Strohbehn, L.E.; Hamilton, K.E.; Wrobel, K.E.; Louters, L.L. Curcumin directly inhibits the transport activity of GLUT1. Biochimie 2016, 125, 179–185. [Google Scholar] [CrossRef]
- Mittal, L.; Aryal, U.K.; Camarillo, I.G.; Raman, V.; Sundararajan, R. Effective electrochemotherapy with curcumin in MDA-MB-231-human, triple negative breast cancer cells: A global proteomics study. Bioelectrochemistry 2020, 131, 107350. [Google Scholar] [CrossRef]
- Wang, K.; Fan, H.; Chen, Q.; Ma, G.; Zhu, M.; Zhang, X.; Zhang, Y.; Yu, J. Curcumin inhibits aerobic glycolysis and induces mitochondrial-mediated apoptosis through hexokinase II in human colorectal cancer cells in vitro. Anticancer Drugs 2015, 26, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Majewski, N.; Nogueira, V.; Bhaskar, P.; Coy, P.E.; Skeen, J.E.; Gottlob, K.; Chandel, N.S.; Thompson, C.B.; Robey, R.B.; Hay, N. Hexokinase-mitochondria interaction mediated by Akt is required to inhibit apoptosis in the presence or absence of Bax and Bak. Mol Cell 2004, 16, 819–830. [Google Scholar] [CrossRef] [PubMed]
- Ciscato, F.; Ferrone, L.; Masgras, I.; Laquatra, C.; Rasola, A. Hexokinase 2 in Cancer: A Prima Donna Playing Multiple Characters. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- Geng, C.; Li, J.; Ding, F.; Wu, G.; Yang, Q.; Sun, Y.; Zhang, Z.; Dong, T.; Tian, X. Curcumin suppresses 4-hydroxytamoxifen resistance in breast cancer cells by targeting SLUG/Hexokinase 2 pathway. Biochem Biophys Res Commun 2016, 473, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Nocito, M.C.; Avena, P.; Zavaglia, L.; De Luca, A.; Chimento, A.; Hamad, T.; La Padula, D.; Stancati, D.; Hantel, C.; Sirianni, R.; et al. Adrenocortical Carcinoma (ACC) Cells Rewire Their Metabolism to Overcome Curcumin Antitumoral Effects Opening a Window of Opportunity to Improve Treatment. Cancers (Basel) 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.Q.; Rajadurai, P.; Abas, F.; Othman, I.; Naidu, R. Proteomic Analysis on Anti-Proliferative and Apoptosis Effects of Curcumin Analog, 1,5-bis(4-Hydroxy-3-Methyoxyphenyl)-1,4-Pentadiene-3-One-Treated Human Glioblastoma and Neuroblastoma Cells. Front Mol Biosci 2021, 8, 645856. [Google Scholar] [CrossRef]
- Monteleone, F.; Taverna, S.; Alessandro, R.; Fontana, S. SWATH-MS based quantitative proteomics analysis reveals that curcumin alters the metabolic enzyme profile of CML cells by affecting the activity of miR-22/IPO7/HIF-1alpha axis. J Exp Clin Cancer Res 2018, 37, 170. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Zhang, C.J.; Wong, Y.K.; Lim, T.K.; Hua, Z.C.; Liu, B.; Tannenbaum, S.R.; Shen, H.M.; Lin, Q. In situ Proteomic Profiling of Curcumin Targets in HCT116 Colon Cancer Cell Line. Sci Rep 2016, 6, 22146. [Google Scholar] [CrossRef]
- He, Y.; Luo, Y.; Zhang, D.; Wang, X.; Zhang, P.; Li, H.; Ejaz, S.; Liang, S. PGK1-mediated cancer progression and drug resistance. Am J Cancer Res 2019, 9, 2280–2302. [Google Scholar] [PubMed]
- Elson, D.A.; Ryan, H.E.; Snow, J.W.; Johnson, R.; Arbeit, J.M. Coordinate up-regulation of hypoxia inducible factor (HIF)-1alpha and HIF-1 target genes during multi-stage epidermal carcinogenesis and wound healing. Cancer Res 2000, 60, 6189–6195. [Google Scholar]
- Li, H.; Ko, H.P.; Whitlock, J.P. Induction of phosphoglycerate kinase 1 gene expression by hypoxia. Roles of Arnt and HIF1alpha. J Biol Chem 1996, 271, 21262–21267. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Yang, F.; Shao, C.; Wei, K.; Xie, M.; Shen, H.; Shu, Y. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol Cancer 2019, 18, 157. [Google Scholar] [CrossRef]
- Yang, H.; Geng, Y.H.; Wang, P.; Yang, H.; Zhou, Y.T.; Zhang, H.Q.; He, H.Y.; Fang, W.G.; Tian, X.X. Extracellular ATP promotes breast cancer invasion and chemoresistance via SOX9 signaling. Oncogene 2020, 39, 5795–5810. [Google Scholar] [CrossRef]
- Yang, H.; Geng, Y.H.; Wang, P.; Zhang, H.Q.; Fang, W.G.; Tian, X.X. Extracellular ATP promotes breast cancer chemoresistance via HIF-1alpha signaling. Cell Death Dis 2022, 13, 199. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, A.; Atkin, S.L.; Majeed, M.; Sahebkar, A. Effects of curcumin on hypoxia-inducible factor as a new therapeutic target. Pharmacol Res 2018, 137, 159–169. [Google Scholar] [CrossRef]
- Hahn, Y.I.; Kim, S.J.; Choi, B.Y.; Cho, K.C.; Bandu, R.; Kim, K.P.; Kim, D.H.; Kim, W.; Park, J.S.; Han, B.W.; et al. Curcumin interacts directly with the Cysteine 259 residue of STAT3 and induces apoptosis in H-Ras transformed human mammary epithelial cells. Sci Rep 2018, 8, 6409. [Google Scholar] [CrossRef]
- Webb, B.A.; Chimenti, M.; Jacobson, M.P.; Barber, D.L. Dysregulated pH: a perfect storm for cancer progression. Nat Rev Cancer 2011, 11, 671–677. [Google Scholar] [CrossRef]
- Gerweck, L.E.; Seetharaman, K. Cellular pH gradient in tumor versus normal tissue: potential exploitation for the treatment of cancer. Cancer Res 1996, 56, 1194–1198. [Google Scholar]
- Koltai, T. Cancer: fundamentals behind pH targeting and the double-edged approach. Onco Targets Ther 2016, 9, 6343–6360. [Google Scholar] [CrossRef] [PubMed]
- Li, F.-F.Z., Y-L.; Guo, D-X.; Zhao, C-J.; Yao, Y-F.; Lin, Y-Q.; Wang, S-Q. Biochemometric approach combined with 1D CSSF-TOCSY for the identification of sensitization agents in Curcuma longa L. and prediction of their action mechanisms. Microchemical Journal 2022, 181, 107727. [CrossRef]
- Dong, S.; Liang, S.; Cheng, Z.; Zhang, X.; Luo, L.; Li, L.; Zhang, W.; Li, S.; Xu, Q.; Zhong, M.; et al. ROS/PI3K/Akt and Wnt/beta-catenin signalings activate HIF-1alpha-induced metabolic reprogramming to impart 5-fluorouracil resistance in colorectal cancer. J Exp Clin Cancer Res 2022, 41, 15. [Google Scholar] [CrossRef]
- Kim, S.W.; Cha, M.J.; Lee, S.K.; Song, B.W.; Jin, X.; Lee, J.M.; Park, J.H.; Lee, J.D. Curcumin Treatment in Combination with Glucose Restriction Inhibits Intracellular Alkalinization and Tumor Growth in Hepatoma Cells. Int J Mol Sci 2019, 20. [Google Scholar] [CrossRef] [PubMed]
- Persi, E.; Duran-Frigola, M.; Damaghi, M.; Roush, W.R.; Aloy, P.; Cleveland, J.L.; Gillies, R.J.; Ruppin, E. Systems analysis of intracellular pH vulnerabilities for cancer therapy. Nat Commun 2018, 9, 2997. [Google Scholar] [CrossRef]
- Patra, K.C.; Hay, N. The pentose phosphate pathway and cancer. Trends Biochem Sci 2014, 39, 347–354. [Google Scholar] [CrossRef]
- Kuehne, A.; Emmert, H.; Soehle, J.; Winnefeld, M.; Fischer, F.; Wenck, H.; Gallinat, S.; Terstegen, L.; Lucius, R.; Hildebrand, J.; et al. Acute Activation of Oxidative Pentose Phosphate Pathway as First-Line Response to Oxidative Stress in Human Skin Cells. Mol Cell 2015, 59, 359–371. [Google Scholar] [CrossRef]
- Giacomini, I.; Ragazzi, E.; Pasut, G.; Montopoli, M. The Pentose Phosphate Pathway and Its Involvement in Cisplatin Resistance. Int J Mol Sci 2020, 21. [Google Scholar] [CrossRef]
- Catanzaro, D.; Gaude, E.; Orso, G.; Giordano, C.; Guzzo, G.; Rasola, A.; Ragazzi, E.; Caparrotta, L.; Frezza, C.; Montopoli, M. Inhibition of glucose-6-phosphate dehydrogenase sensitizes cisplatin- resistant cells to death. Oncotarget 2015, 6, 30102–30114. [Google Scholar] [CrossRef]
- Hong, W.; Cai, P.; Xu, C.; Cao, D.; Yu, W.; Zhao, Z.; Huang, M.; Jin, J. Inhibition of Glucose-6-Phosphate Dehydrogenase Reverses Cisplatin Resistance in Lung Cancer Cells via the Redox System. Front Pharmacol 2018, 9, 43. [Google Scholar] [CrossRef]
- Iqbal, M.; Sharma, S.D.; Okazaki, Y.; Fujisawa, M.; Okada, S. Dietary supplementation of curcumin enhances antioxidant and phase II metabolizing enzymes in ddY male mice: possible role in protection against chemical carcinogenesis and toxicity. Pharmacol Toxicol 2003, 92, 33–38. [Google Scholar] [CrossRef]
- Li, L.; Wei, Y.; To, C.; Zhu, C.Q.; Tong, J.; Pham, N.A.; Taylor, P.; Ignatchenko, V.; Ignatchenko, A.; Zhang, W.; et al. Integrated omic analysis of lung cancer reveals metabolism proteome signatures with prognostic impact. Nat Commun 2014, 5, 5469. [Google Scholar] [CrossRef]
- Chaneton, B.; Hillmann, P.; Zheng, L.; Martin, A.C.L.; Maddocks, O.D.K.; Chokkathukalam, A.; Coyle, J.E.; Jankevics, A.; Holding, F.P.; Vousden, K.H.; et al. Serine is a natural ligand and allosteric activator of pyruvate kinase M2. Nature 2012, 491, 458–462. [Google Scholar] [CrossRef]
- Abegg, D.; Frei, R.; Cerato, L.; Prasad Hari, D.; Wang, C.; Waser, J.; Adibekian, A. Proteome-Wide Profiling of Targets of Cysteine reactive Small Molecules by Using Ethynyl Benziodoxolone Reagents. Angew Chem Int Ed Engl 2015, 54, 10852–10857. [Google Scholar] [CrossRef] [PubMed]
- Kujundžić, R.N.; Stepanić, V.; Milković, L.; Gašparović, A.; Tomljanović, M.; Trošelj, K.G. Curcumin and its Potential for Systemic Targeting of Inflamm-Aging and Metabolic Reprogramming in Cancer. Int J Mol Sci 2019, 20. [Google Scholar] [CrossRef]
- Pranzini, E.; Pardella, E.; Muccillo, L.; Leo, A.; Nesi, I.; Santi, A.; Parri, M.; Zhang, T.; Uribe, A.H.; Lottini, T.; et al. SHMT2-mediated mitochondrial serine metabolism drives 5-FU resistance by fueling nucleotide biosynthesis. Cell Rep 2022, 40, 111233. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, F.; Wu, X.R. Inhibition of Pyruvate Kinase M2 Markedly Reduces Chemoresistance of Advanced Bladder Cancer to Cisplatin. Sci Rep 2017, 7, 45983. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wu, J.; Zhang, W.; Luo, H.; Shen, Z.; Cheng, H.; Zhu, X. PKM2 enhances chemosensitivity to cisplatin through interaction with the mTOR pathway in cervical cancer. Sci Rep 2016, 6, 30788. [Google Scholar] [CrossRef]
- Lin, Y.; Lv, F.; Liu, F.; Guo, X.; Fan, Y.; Gu, F.; Gu, J.; Fu, L. High Expression of Pyruvate Kinase M2 is Associated with Chemosensitivity to Epirubicin and 5-Fluorouracil in Breast Cancer. J Cancer 2015, 6, 1130–1139. [Google Scholar] [CrossRef]
- Su, Q.; Luo, S.; Tan, Q.; Deng, J.; Zhou, S.; Peng, M.; Tao, T.; Yang, X. The role of pyruvate kinase M2 in anticancer therapeutic treatments. Oncol Lett 2019, 18, 5663–5672. [Google Scholar] [CrossRef]
- Siddiqui, F.A.; Prakasam, G.; Chattopadhyay, S.; Rehman, A.U.; Padder, R.A.; Ansari, M.A.; Irshad, R.; Mangalhara, K.; Bamezai, R.N.K.; Husain, M.; et al. Curcumin decreases Warburg effect in cancer cells by down-regulating pyruvate kinase M2 via mTOR-HIF1alpha inhibition. Sci Rep 2018, 8, 8323. [Google Scholar] [CrossRef] [PubMed]
- Mojzeš, A.; Tomljanović, M.; Milković, L.; Kujundžić, R.N.; Gašparović, A.; Trošelj, K.G. Cell-Type Specific Metabolic Response of Cancer Cells to Curcumin. Int J Mol Sci 2020, 21. [Google Scholar] [CrossRef]
- Yadav, S.; Bhagat, S.D.; Gupta, A.; Samaiya, A.; Srivastava, A.; Shukla, S. Dietary-phytochemical mediated reversion of cancer-specific splicing inhibits Warburg effect in head and neck cancer. BMC Cancer 2019, 19, 1031. [Google Scholar] [CrossRef]
- Locasale, J.W.; Grassian, A.R.; Melman, T.; Lyssiotis, C.A.; Mattaini, K.R.; Bass, A.J.; Heffron, G.; Metallo, C.M.; Muranen, T.; Sharfi, H.; et al. Phosphoglycerate dehydrogenase diverts glycolytic flux and contributes to oncogenesis. Nat Genet 2011, 43, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Spillier, Q.; Frederick, R. Phosphoglycerate dehydrogenase (PHGDH) inhibitors: a comprehensive review 2015-2020. Expert Opin Ther Pat 2021, 31, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Ngo, B.; Kim, E.; Osorio-Vasquez, V.; Doll, S.; Bustraan, S.; Liang, R.J.; Luengo, A.; Davidson, S.M.; Ali, A.; Ferraro, G.B.; et al. Limited Environmental Serine and Glycine Confer Brain Metastasis Sensitivity to PHGDH Inhibition. Cancer Discov 2020, 10, 1352–1373. [Google Scholar] [CrossRef] [PubMed]
- Bi, F.; An, Y.; Sun, T.; You, Y.; Yang, Q. PHGDH Is Upregulated at Translational Level and Implicated in Platin- Resistant in Ovarian Cancer Cells. Front Oncol 2021, 11, 643129. [Google Scholar] [CrossRef]
- Zou, S.; Qin, B.; Yang, Z.; Wang, W.; Zhang, J.; Zhang, Y.; Meng, M.; Feng, J.; Xie, Y.; Fang, L.; et al. CSN6 Mediates Nucleotide Metabolism to Promote Tumor Development and Chemoresistance in Colorectal Cancer. Cancer Res 2023, 83, 414–427. [Google Scholar] [CrossRef]
- Wei, L.; Lee, D.; Law, C.T.; Zhang, M.S.; Shen, J.; Chin, D.W.; Zhang, A.; Tsang, F.H.; Wong, C.L.; Ng, I.O.; et al. Genome-wide CRISPR/Cas9 library screening identified PHGDH as a critical driver for Sorafenib resistance in HCC. Nat Commun 2019, 10, 4681. [Google Scholar] [CrossRef]
- Rathore, R.; Schutt, C.R.; Van Tine, B.A. PHGDH as a mechanism for resistance in metabolically-driven cancers. Cancer Drug Resist 2020, 3, 762–774. [Google Scholar] [CrossRef]
- Van Nyen, T.; Planque, M.; van Wagensveld, L.; Duarte, J.A.G.; Zaal, E.A.; Talebi, A.; Rossi, M.; Korner, P.R.; Rizzotto, L.; Moens, S.; et al. Serine metabolism remodeling after platinum-based chemotherapy identifies vulnerabilities in a subgroup of resistant ovarian cancers. Nat Commun 2022, 13, 4578. [Google Scholar] [CrossRef] [PubMed]
- Tajan, M.; Hennequart, M.; Cheung, E.C.; Zani, F.; Hock, A.K.; Legrave, N.; Maddocks, O.D.K.; Ridgway, R.A.; Athineos, D.; Suárez-Bonnet, A.; et al. Serine synthesis pathway inhibition cooperates with dietary serine and glycine limitation for cancer therapy. Nat Commun 2021, 12, 366. [Google Scholar] [CrossRef] [PubMed]
- Angelo, L.S.; Maxwell, D.S.; Wu, J.Y.; Sun, D.; Hawke, D.H.; McCutcheon, I.E.; Slopis, J.M.; Peng, Z.; Bornmann, W.G.; Kurzrock, R. Binding partners for curcumin in human schwannoma cells: biologic implications. Bioorg Med Chem 2013, 21, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Vie, N.; Copois, V.; Bascoul-Mollevi, C.; Denis, V.; Bec, N.; Robert, B.; Fraslon, C.; Conseiller, E.; Molina, F.; Larroque, C.; et al. Overexpression of phosphoserine aminotransferase PSAT1 stimulates cell growth and increases chemoresistance of colon cancer cells. Mol Cancer 2008, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Cui, H.; Tu, W.; Li, L.; Gao, Y.; Chen, L.; Li, D.; Chen, X.; Xu, F.; Zhou, C.; et al. An integrated pan-cancer analysis of PSAT1: A potential biomarker for survival and immunotherapy. Front Genet 2022, 13, 975381. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhang, L.; Chen, H.; Lei, Y.; Zhang, T.; Wang, Y.; Jin, P.; Lan, J.; Zhou, L.; Huang, Z.; et al. Regorafenib induces lethal autophagy arrest by stabilizing PSAT1 in glioblastoma. Autophagy 2020, 16, 106–122. [Google Scholar] [CrossRef]
- De Marchi, T.; Timmermans, M.A.; Sieuwerts, A.M.; Smid, M.; Look, M.P.; Grebenchtchikov, N.; Sweep, F.; Smits, J.G.; Magdolen, V.; van Deurzen, C.H.M.; et al. Phosphoserine aminotransferase 1 is associated to poor outcome on tamoxifen therapy in recurrent breast cancer. Sci Rep 2017, 7, 2099. [Google Scholar] [CrossRef]
- Liao, L.; Yu, H.; Ge, M.; Zhan, Q.; Huang, R.; Ji, X.; Liang, X.; Zhou, X. Upregulation of phosphoserine phosphatase contributes to tumor progression and predicts poor prognosis in non-small cell lung cancer patients. Thorac Cancer 2019, 10, 1203–1212. [Google Scholar] [CrossRef]
- Chiang, I.T.; Wang, W.S.; Liu, H.C.; Yang, S.T.; Tang, N.Y.; Chung, J.G. Curcumin alters gene expression-associated DNA damage, cell cycle, cell survival and cell migration and invasion in NCI-H460 human lung cancer cells in vitro. Oncol Rep 2015, 34, 1853–1874. [Google Scholar] [CrossRef]
- Vinod, B.S.; Maliekal, T.T.; Anto, R.J. Phytochemicals as chemosensitizers: from molecular mechanism to clinical significance. Antioxid Redox Signal 2013, 18, 1307–1348. [Google Scholar] [CrossRef]
- Rayan, A.; Raiyn, J.; Falah, M. Nature is the best source of anticancer drugs: Indexing natural products for their anticancer bioactivity. PLoS One 2017, 12, e0187925. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.R.; Chang, C.H.; Hsu, C.F.; Tsai, M.J.; Cheng, H.; Leong, M.K.; Sung, P.J.; Chen, J.C.; Weng, C.F. Natural compounds as potential adjuvants to cancer therapy: Preclinical evidence. Br J Pharmacol 2020, 177, 1409–1423. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Huang, P.; Law, S.; Tian, H.; Leung, W.; Xu, C. Preventive Effect of Curcumin Against Chemotherapy-Induced Side-Effects. Front Pharmacol 2018, 9, 1374. [Google Scholar] [CrossRef] [PubMed]
- Cermak, R.; Wolffram, S. The potential of flavonoids to influence drug metabolism and pharmacokinetics by local gastrointestinal mechanisms. Curr Drug Metab 2006, 7, 729–744. [Google Scholar] [CrossRef] [PubMed]
- Karaboga Arslan, A.K.; Uzunhisarcıklı, E.; Yerer, M.B.; Bishayee, A. The golden spice curcumin in cancer: A perspective on finalized clinical trials during the last 10 years. J Cancer Res Ther 2022, 18, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, K.; Rasoulpoor, S.; Daneshkhah, A.; Abolfathi, S.; Salari, N.; Mohammadi, M.; Rasoulpoor, S.; Shabani, S. Clinical effects of curcumin in enhancing cancer therapy: A systematic review. BMC Cancer 2020, 20, 791. [Google Scholar] [CrossRef] [PubMed]
- Giordano, A.; Tommonaro, G. Curcumin and Cancer. Nutrients 2019, 11. [Google Scholar] [CrossRef]
- Ojo, O.A.; Adeyemo, T.R.; Rotimi, D.; Batiha, G.E.; Mostafa-Hedeab, G.; Iyobhebhe, M.E.; Elebiyo, T.C.; Atunwa, B.; Ojo, A.B.; Lima, C.M.G.; et al. Anticancer Properties of Curcumin Against Colorectal Cancer: A Review. Front Oncol 2022, 12, 881641. [Google Scholar] [CrossRef]
- Hafez Ghoran, S.; Calcaterra, A.; Abbasi, M.; Taktaz, F.; Nieselt, K.; Babaei, E. Curcumin-Based Nanoformulations: A Promising Adjuvant towards Cancer Treatment. Molecules 2022, 27. [Google Scholar] [CrossRef]
- Hesari, A.; Azizian, M.; Sheikhi, A.; Nesaei, A.; Sanaei, S.; Mahinparvar, N.; Derakhshani, M.; Hedayt, P.; Ghasemi, F.; Mirzaei, H. Chemopreventive and therapeutic potential of curcumin in esophageal cancer: Current and future status. Int J Cancer 2019, 144, 1215–1226. [Google Scholar] [CrossRef]
- Puliyappadamba, V.T.; Thulasidasan, A.K.; Vijayakurup, V.; Antony, J.; Bava, S.V.; Anwar, S.; Sundaram, S.; Anto, R.J. Curcumin inhibits B[a]PDE-induced procarcinogenic signals in lung cancer cells, and curbs B[a]P-induced mutagenesis and lung carcinogenesis. Biofactors 2015, 41, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Parsai, S.; Keck, R.; Skrzypczak-Jankun, E.; Jankun, J. Analysis of the anticancer activity of curcuminoids, thiotryptophan and 4-phenoxyphenol derivatives. Oncol Lett 2014, 7, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Shehzad, A.; Wahid, F.; Lee, Y.S. Curcumin in cancer chemoprevention: molecular targets, pharmacokinetics, bioavailability, and clinical trials. Arch Pharm (Weinheim) 2010, 343, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Bachmeier, B.E.; Killian, P.; Pfeffer, U.; Nerlich, A.G. Novel aspects for the application of Curcumin in chemoprevention of various cancers. Front Biosci (Schol Ed) 2010, 2, 697–717. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Wu, J.; Han, S.; Liu, Y.; Su, Z.; Zhu, H.L.; Liu, H.K.; Qian, Y. Biotinylated curcumin as a novel chemosensitizer enhances naphthalimide-induced autophagic cell death in breast cancer cells. Eur J Med Chem 2022, 228, 114029. [Google Scholar] [CrossRef]
- Farghadani, R.; Naidu, R. Curcumin as an Enhancer of Therapeutic Efficiency of Chemotherapy Drugs in Breast Cancer. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef]
- Haritha, N.H.; Nawab, A.; Vijayakurup, V.; Anto, N.P.; Liju, V.B.; Alex, V.V.; Amrutha, A.N.; Aiswarya, S.U.; Swetha, M.; Vinod, B.S.; et al. Targeting Thymidylate Synthase Enhances the Chemosensitivity of Triple-Negative Breast Cancer Towards 5-FU-Based Combinatorial Therapy. Front Oncol 2021, 11, 656804. [Google Scholar] [CrossRef]
- Vijayakurup, V.; Thulasidasan, A.T.; Shankar, G.M.; Retnakumari, A.P.; Nandan, C.D.; Somaraj, J.; Antony, J.; Alex, V.V.; Vinod, B.S.; Liju, V.B.; et al. Chitosan Encapsulation Enhances the Bioavailability and Tissue Retention of Curcumin and Improves its Efficacy in Preventing B[a]P-induced Lung Carcinogenesis. Cancer Prev Res (Phila) 2019, 12, 225–236. [Google Scholar] [CrossRef]
- Thulasidasan, A.K.T.; Retnakumari, A.P.; Shankar, M.; Vijayakurup, V.; Anwar, S.; Thankachan, S.; Pillai, K.S.; Pillai, J.J.; Nandan, C.D.; Alex, V.V.; et al. Folic acid conjugation improves the bioavailability and chemosensitizing efficacy of curcumin-encapsulated PLGA-PEG nanoparticles towards paclitaxel chemotherapy. Oncotarget 2017, 8, 107374–107389. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, C.; Bao, J.; Jia, X.; Liang, Y.; Wang, X.; Chen, M.; Su, H.; Li, P.; Wan, J.B.; et al. Synergistic chemopreventive effects of curcumin and berberine on human breast cancer cells through induction of apoptosis and autophagic cell death. Sci Rep 2016, 6, 26064. [Google Scholar] [CrossRef]
- Bava, S.V.; Sreekanth, C.N.; Thulasidasan, A.K.; Anto, N.P.; Cheriyan, V.T.; Puliyappadamba, V.T.; Menon, S.G.; Ravichandran, S.D.; Anto, R.J. Akt is upstream and MAPKs are downstream of NF-κB in paclitaxel-induced survival signaling events, which are down-regulated by curcumin contributing to their synergism. Int J Biochem Cell Biol 2011, 43, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Sreekanth, C.N.; Bava, S.V.; Sreekumar, E.; Anto, R.J. Molecular evidences for the chemosensitizing efficacy of liposomal curcumin in paclitaxel chemotherapy in mouse models of cervical cancer. Oncogene 2011, 30, 3139–3152. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.H.; Alsahli, M.A.; Aly, S.M.; Khan, M.A.; Aldebasi, Y.H. Role of Curcumin in Disease Prevention and Treatment. Adv Biomed Res 2018, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Sumathi, C.S.; Rajesh, P.; Kannan, V.R. The Biological Potentialsof Indian Traditional Medicine, Curcumin for Treating Human Diseases. Cardiovasc Hematol Agents Med Chem 2017, 10.2174/1871525715666170830130555. [CrossRef]
- Banik, U.; Parasuraman, S.; Adhikary, A.K.; Othman, N.H. Curcumin: the spicy modulator of breast carcinogenesis. J Exp Clin Cancer Res 2017, 36, 98. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, F.L.; Chen, W.D. Modulation of apoptosis-related cell signalling pathways by curcumin as a strategy to inhibit tumor progression. Mol Biol Rep 2014, 41, 4583–4594. [Google Scholar] [CrossRef]
- Lev-Ari, S.; Vexler, A.; Starr, A.; Ashkenazy-Voghera, M.; Greif, J.; Aderka, D.; Ben-Yosef, R. Curcumin augments gemcitabine cytotoxic effect on pancreatic adenocarcinoma cell lines. Cancer Invest 2007, 25, 411–418. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Guha, S.; Krishnan, S.; Diagaradjane, P.; Gelovani, J.; Aggarwal, B.B. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res 2007, 67, 3853–3861. [Google Scholar] [CrossRef]
- Kanai, M.; Yoshimura, K.; Asada, M.; Imaizumi, A.; Suzuki, C.; Matsumoto, S.; Nishimura, T.; Mori, Y.; Masui, T.; Kawaguchi, Y.; et al. A phase I/II study of gemcitabine-based chemotherapy plus curcumin for patients with gemcitabine-resistant pancreatic cancer. Cancer Chemother Pharmacol 2011, 68, 157–164. [Google Scholar] [CrossRef]
- Vinod, B.S.; Antony, J.; Nair, H.H.; Puliyappadamba, V.T.; Saikia, M.; Narayanan, S.S.; Bevin, A.; Anto, R.J. Mechanistic evaluation of the signaling events regulating curcumin-mediated chemosensitization of breast cancer cells to 5-fluorouracil. Cell Death Dis 2013, 4, e505. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Diagaradjane, P.; Anand, P.; Harikumar, K.B.; Deorukhkar, A.; Gelovani, J.; Guha, S.; Krishnan, S.; Aggarwal, B.B. Curcumin sensitizes human colorectal cancer to capecitabine by modulation of cyclin D1, COX-2, MMP-9, VEGF and CXCR4 expression in an orthotopic mouse model. Int J Cancer 2009, 125, 2187–2197. [Google Scholar] [CrossRef]
- Hour, T.C.; Chen, J.; Huang, C.Y.; Guan, J.Y.; Lu, S.H.; Pu, Y.S. Curcumin enhances cytotoxicity of chemotherapeutic agents in prostate cancer cells by inducing p21(WAF1/CIP1) and C/EBPbeta expressions and suppressing NF-kappaB activation. Prostate 2002, 51, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.B.; Sengupta, R.; Qazi, S.; Vachhani, H.; Yu, Y.; Rishi, A.K.; Majumdar, A.P. Curcumin enhances the effects of 5-fluorouracil and oxaliplatin in mediating growth inhibition of colon cancer cells by modulating EGFR and IGF-1R. Int J Cancer 2008, 122, 267–273. [Google Scholar] [CrossRef]
- Deeb, D.; Jiang, H.; Gao, X.; Hafner, M.S.; Wong, H.; Divine, G.; Chapman, R.A.; Dulchavsky, S.A.; Gautam, S.C. Curcumin sensitizes prostate cancer cells to tumor necrosis factor-related apoptosis-inducing ligand/Apo2L by inhibiting nuclear factor-kappaB through suppression of IkappaBalpha phosphorylation. Mol Cancer Ther 2004, 3, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Ganapathy, S.; Chen, Q.; Srivastava, R.K. Curcumin sensitizes TRAIL-resistant xenografts: molecular mechanisms of apoptosis, metastasis and angiogenesis. Mol Cancer 2008, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Fu, L.; Huang, J.; Dai, Y.; Wang, B.; Xu, G.; Wu, L.; Zhou, H. Curcumin reverses doxorubicin resistance via inhibition the efflux function of ABCB4 in doxorubicin-resistant breast cancer cells. Mol Med Rep 2019, 19, 5162–5168. [Google Scholar] [CrossRef]
- Notarbartolo, M.; Poma, P.; Perri, D.; Dusonchet, L.; Cervello, M.; D’Alessandro, N. Antitumor effects of curcumin, alone or in combination with cisplatin or doxorubicin, on human hepatic cancer cells. Analysis of their possible relationship to changes in NF-kB activation levels and in IAP gene expression. Cancer Lett 2005, 224, 53–65. [Google Scholar] [CrossRef]
- Attia, Y.M.; El-Kersh, D.M.; Ammar, R.A.; Adel, A.; Khalil, A.; Walid, H.; Eskander, K.; Hamdy, M.; Reda, N.; Mohsen, N.E.; et al. Inhibition of aldehyde dehydrogenase-1 and p-glycoprotein-mediated multidrug resistance by curcumin and vitamin D3 increases sensitivity to paclitaxel in breast cancer. Chem Biol Interact 2020, 315, 108865. [Google Scholar] [CrossRef]
- Chirnomas, D.; Taniguchi, T.; de la Vega, M.; Vaidya, A.P.; Vasserman, M.; Hartman, A.R.; Kennedy, R.; Foster, R.; Mahoney, J.; Seiden, M.V.; et al. Chemosensitization to cisplatin by inhibitors of the Fanconi anemia/BRCA pathway. Mol Cancer Ther 2006, 5, 952–961. [Google Scholar] [CrossRef]
- Meiyanto, E.; Putri, D.D.; Susidarti, R.A.; Murwanti, R.; Sardjiman; Fitriasari, A.; Husnaa, U.; Purnomo, H.; Kawaichi, M. Curcumin and its analogues (PGV-0 and PGV-1) enhance sensitivity of resistant MCF-7 cells to doxorubicin through inhibition of HER2 and NF-kB activation. Asian Pac J Cancer Prev 2014, 15, 179–184. [CrossRef]
- Xiao-ai, L.; Bei, W.; Xiao-hong, X.; Lei, P.; Bin, W.; Xiao-xue, D.; Chen-hui, Z.; Qi-wei, D. Curcumin re-sensitizes multidrug resistant (MDR) breast cancer to cisplatin through inducing autophagy by decreasing CCAT1 expression. RSC Advances 2017, 7, 33572–33579. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E. Curcumin Combination Chemotherapy: The Implication and Efficacy in Cancer. Molecules 2019, 24. [Google Scholar] [CrossRef]
- Fang, Z.; Meng, Q.; Xu, J.; Wang, W.; Zhang, B.; Liu, J.; Liang, C.; Hua, J.; Zhao, Y.; Yu, X.; et al. Signaling pathways in cancer-associated fibroblasts: recent advances and future perspectives. Cancer Commun (Lond) 2023, 43, 3–41. [Google Scholar] [CrossRef] [PubMed]
- Ball, P. Water is an active matrix of life for cell and molecular biology. Proc Natl Acad Sci U S A 2017, 114, 13327–13335. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Bagchi, B. From structure and dynamics to biomolecular functions: The ubiquitous role of solvent in biology. Curr Opin Struct Biol 2022, 77, 102462. [Google Scholar] [CrossRef] [PubMed]
- Conti, M.; Bolzan, I.; Dal Zilio, S.; Parisse, P.; Andolfi, L.; Lazzarino, M. Water-Air Interface to Mimic In Vitro Tumoral Cell Migration in Complex Micro-Environments. Biosensors (Basel) 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.L.; Bordallo, H.N.; Mamontov, E. Water Dynamics in Cancer Cells: Lessons from Quasielastic Neutron Scattering. Medicina (Kaunas) 2022, 58. [Google Scholar] [CrossRef] [PubMed]
- Amelio, I.; Lisitsa, A.; Knight, R.A.; Melino, G.; Antonov, A.V. Polypharmacology of Approved Anticancer Drugs. Curr Drug Targets 2017, 18, 534–543. [Google Scholar] [CrossRef]
- Bao, L.; Wang, Z.; Wu, Z.; Luo, H.; Yu, J.; Kang, Y.; Cao, D.; Hou, T. Kinome-wide polypharmacology profiling of small molecules by multi-task graph isomorphism network approach. Acta Pharm Sin B 2023, 13, 54–67. [Google Scholar] [CrossRef]
- Lamens, A.; Bajorath, J. Explaining Accurate Predictions of Multitarget Compounds with Machine Learning Models Derived for Individual Targets. Molecules 2023, 28. [Google Scholar] [CrossRef]
- Mitchell, D.C.; Kuljanin, M.; Li, J.; Van Vranken, J.G.; Bulloch, N.; Schweppe, D.K.; Huttlin, E.L.; Gygi, S.P. A proteome-wide atlas of drug mechanism of action. Nat Biotechnol 2023, 10.1038/s41587-022-01539-0. [CrossRef]
- Drost, J.; Clevers, H. Organoids in cancer research. Nat Rev Cancer 2018, 18, 407–418. [Google Scholar] [CrossRef]
- de Visser, K.E.; Joyce, J.A. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wang, Q.; Li, M.; Guo, H.; Liu, W.; Wang, F.; Tian, X.; Yang, Y. Single-cell RNA-seq reveals dynamic change in tumor microenvironment during pancreatic ductal adenocarcinoma malignant progression. EBioMedicine 2021, 66, 103315. [Google Scholar] [CrossRef] [PubMed]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of dietary polyphenols: The role of metabolites. Crit Rev Food Sci Nutr 2020, 60, 626–659. [Google Scholar] [CrossRef] [PubMed]
- Pluta, R.; Januszewski, S.; Ułamek-Kozioł, M. Mutual Two-Way Interactions of Curcumin and Gut Microbiota. Int J Mol Sci 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Luo, W.; Chen, Q.; Chen, X.; Zhou, G.; Sun, H. Curcumin sensitizes response to cytarabine in acute myeloid leukemia by regulating intestinal microbiota. Cancer Chemother Pharmacol 2022, 89, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.M.; Jennings, L.; Thomas, O.P. Marine Biodiscovery in a Changing World. Prog Chem Org Nat Prod 2021, 116, 1–36. [Google Scholar] [CrossRef]
- Nohara, L.; Ellis, S.; Dada, S.; Saranchova, I.; Munro, L.; Pfeifer, C.; Coyle, K.; Dreier, C.; Morrice, J.R.; Shim, D.J.; et al. A NOVEL cell-based screen identifies entities from sea sponges that reverse the immune-escape phenotype of metastatic tumours. Frontiers in Pharmacology 2023, 14. [Google Scholar] [CrossRef]
- Huo, C.; Han, F.; Xiao, Y.; Kim, H.J.; Lee, I.S. Microbial Transformation of Yakuchinone A and Cytotoxicity Evaluation of Its Metabolites. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef]
- Saeed, M.E.M.; Yücer, R.; Dawood, M.; Hegazy, M.F.; Drif, A.; Ooko, E.; Kadioglu, O.; Seo, E.J.; Kamounah, F.S.; Titinchi, S.J.; et al. In Silico and In Vitro Screening of 50 Curcumin Compounds as EGFR and NF-κB Inhibitors. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef]
- Pandya, N.; Khan, E.; Jain, N.; Satham, L.; Singh, R.; Makde, R.D.; Mishra, A.; Kumar, A. Curcumin analogs exhibit anti-cancer activity by selectively targeting G-quadruplex forming c-myc promoter sequence. Biochimie 2021, 180, 205–221. [Google Scholar] [CrossRef]
- Wang, T.; Wang, J.; Jiang, H.; Ni, M.; Zou, Y.; Chen, Y.; Wu, T.; Ding, D.; Xu, H.; Li, X. Targeted regulation of tumor microenvironment through the inhibition of MDSCs by curcumin loaded self-assembled nano-filaments. Mater Today Bio 2022, 15, 100304. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Feng, Z.; Wang, C.; Su, Q.; Song, H.; Zhang, C.; Huang, P.; Liang, X.J.; Dong, A.; Kong, D.; et al. Co-localized delivery of nanomedicine and nanovaccine augments the postoperative cancer immunotherapy by amplifying T-cell responses. Biomaterials 2020, 230, 119649. [Google Scholar] [CrossRef] [PubMed]
- Das, A.P.; Agarwal, S.M. Recent advances in the area of plant-based anti-cancer drug discovery using computational approaches. Mol Divers 2023, 10.1007/s11030-022-10590-7, 1-25. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





