Submitted:
03 May 2023
Posted:
04 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Postmortem Biomarkers
3. Biomarkers of Vascular Homeostasis
3.1. Vascular Biomarkers and Omics
3.2. Circulating Markers of the Extracellular Matrix—Biomarkers Related to the Vascular Wall
3.3. Proteins Associated with Vascular Lumen—Inflammation and Thrombosis Biomarkers
3.4. Vascular Cognitive Impairment—Room for Postmortem Biomarkers
4. Options for Traditional Autopsy
Applying Clinical Biomarkers in a Postmortem Setting
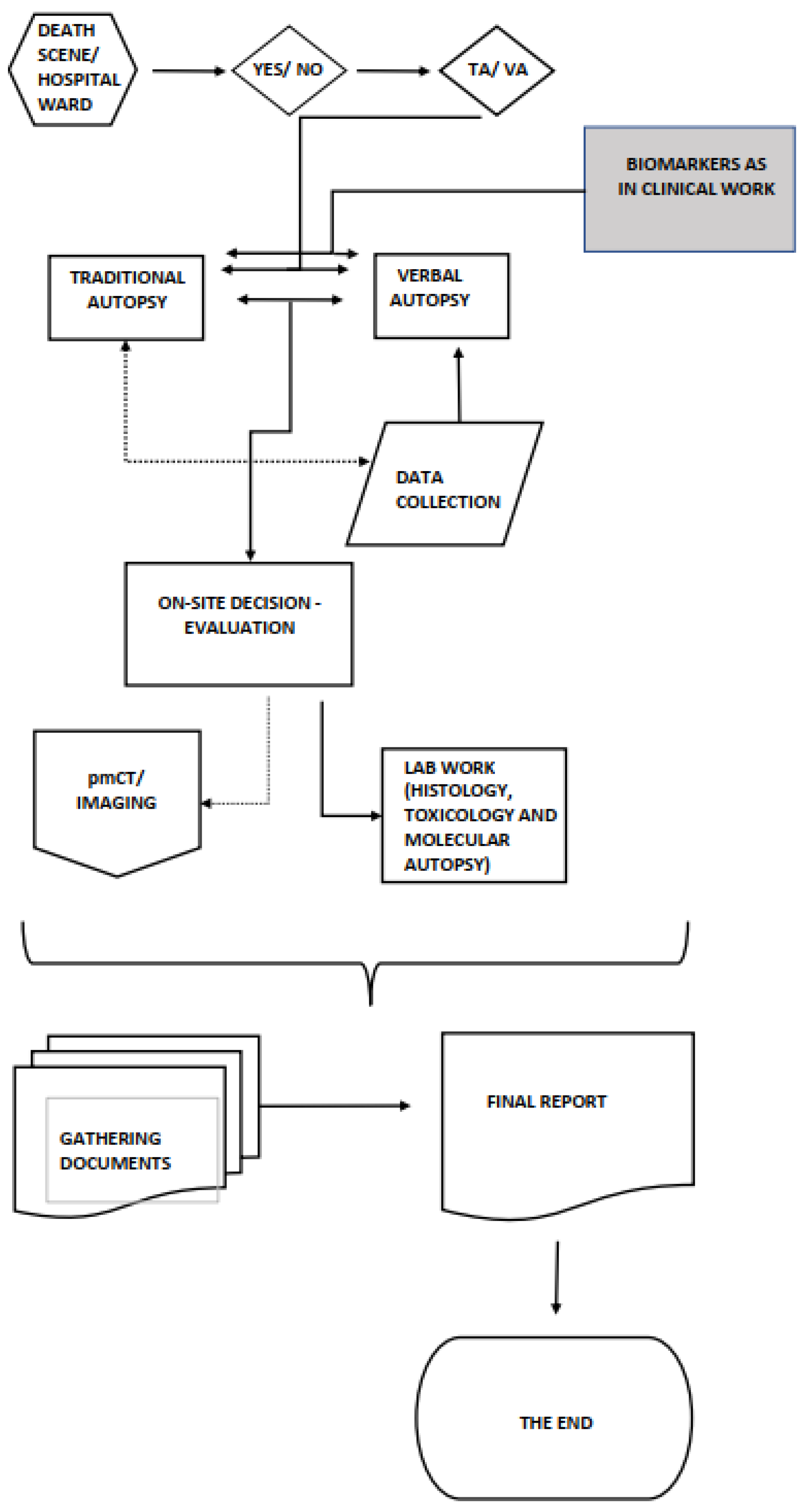
5. Conclusions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thiene, G.; Saffitz, J.E. Autopsy as a Source of Discovery in Cardiovascular Medicine: Then and Now. Circulation 2018, 137, 2683–2685. [Google Scholar] [CrossRef] [PubMed]
- Bombi, J.A.; Llebaria, C.; Rives, A. [Analysis of a series of 500 clinical post mortem studies. II. Basic diagnosis (author’s transl)]. Med Clin (Barc) 1981, 77, 185–189. [Google Scholar] [PubMed]
- Smith, A.M.; Lingard, L.; Heslop, P.; Gray, J.; Walker, D.J. Vascular disease as a cause of death in patients with severe disability due to osteoarthritis and rheumatoid arthritis. Springerplus 2015, 4, 328. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.; Rayner, M.; Leal, J.; Luengo-Fernandez, R.; Gray, A. European cardiovascular disease statistics; British Heart Foundation: 2000.
- EUROSTAT. Causes of death statistics. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Causes_of_death_statistics (accessed on.
- Earle, W.; Misra, S.; Herzig, M.; Abdallah, G.; Ross, C.D.; Secemsky, E.A.; Carroll, B. CAUSE OF DEATH ANALYSIS IN PATIENTS WITH INTERMEDIATE RISK ACUTE PULMONARY EMBOLISM. Journal of the American College of Cardiology 2023, 81, 2088–2088. [Google Scholar] [CrossRef]
- Hamer, H.M.; Stroobants, A.K.; Bavalia, R.; Ponjee, G.A.E.; Klok, F.A.; van der Hulle, T.; Huisman, M.V.; Hendriks, H.A.; Middeldorp, S. Diagnostic accuracy of four different D-dimer assays: A post-hoc analysis of the YEARS study. Thromb Res 2021, 201, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, A.E.; Lamont, I.L. Biochemistry changes that occur after death: potential markers for determining post-mortem interval. PLoS One 2013, 8, e82011. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Cao, J.; Wang, Q.; Shi, Q.; Liu, K.; Luo, Z.; Chen, X.; Chen, S.; Yu, K.; Huang, Z.; et al. D-dimer as a biomarker for disease severity and mortality in COVID-19 patients: a case control study. J Intensive Care 2020, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Aronson, J.K. When I use a word.... Too much healthcare—Biomarkers. 2022, 379.
- Aronson, J.K.; Ferner, R.E. Biomarkers-A General Review. Curr Protoc Pharmacol 2017, 76, 9–23. [Google Scholar] [CrossRef]
- Nordon, I.M.; Hinchliffe, R.J. Biomarkers in Vascular Disease. Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists [Internet] 2011.
- Cui, Z.; Zhao, G.; Liu, X. Blood fibrinogen level as a biomarker of adverse outcomes in patients with coronary artery disease: A systematic review and meta-analysis. Medicine (Baltimore) 2022, 101, e30117. [Google Scholar] [CrossRef]
- Davis, K.D.; Aghaeepour, N.; Ahn, A.H.; Angst, M.S.; Borsook, D.; Brenton, A.; Burczynski, M.E.; Crean, C.; Edwards, R.; Gaudilliere, B.; et al. Discovery and validation of biomarkers to aid the development of safe and effective pain therapeutics: challenges and opportunities. Nat Rev Neurol 2020, 16, 381–400. [Google Scholar] [CrossRef]
- Kutlu, E.; Cil, N.; Avci, E.; Bir, F.; Kilic, I.D.; Dereli, A.K.; Acar, K. Significance of postmortem biomarkers and multimarker strategy in sudden cardiac death. Leg Med (Tokyo) 2023, 61, 102212. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Zhao, M.; Xu, C.; Zhang, T.; Jia, Y.; Wang, T.; Zhu, B. Evaluation of Agonal Cardiac Function for Sudden Cardiac Death in Forensic Medicine with Postmortem Brain Natriuretic Peptide (BNP) and NT-proBNP: A Meta-analysis. J Forensic Sci 2020, 65, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Puchenkova, O.A.; Soldatov, V.O.; Belykh, A.E.; Bushueva, O.; Piavchenko, G.A.; Venediktov, A.A.; Shakhpazyan, N.K.; Deykin, A.V.; Korokin, M.V.; Pokrovskiy, M.V. Cytokines in Abdominal Aortic Aneurysm: Master Regulators With Clinical Application. Biomark Insights 2022, 17, 11772719221095676. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, F.A. Biomarkers and Surrogate Endpoints: Lessons Learned From Glaucoma. Invest Ophthalmol Vis Sci 2017, 58, BIO20–BIO26. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, C.; Xaplanteris, P.; Aboyans, V.; Brodmann, M.; Cifkova, R.; Cosentino, F.; De Carlo, M.; Gallino, A.; Landmesser, U.; Laurent, S.; et al. The role of vascular biomarkers for primary and secondary prevention. A position paper from the European Society of Cardiology Working Group on peripheral circulation: Endorsed by the Association for Research into Arterial Structure and Physiology (ARTERY) Society. Atherosclerosis 2015, 241, 507–532. [Google Scholar] [CrossRef]
- Basso, C.; Stone, J.R. Autopsy in the era of advanced cardiovascular imaging. Eur Heart J 2022, 43, 2461–2468. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, M.; MovaseghiGargari, M.; Chand, M.T. The importance of autopsies despite the declining number amidst the COVID-19 pandemic. Autops Case Rep 2022, 12, e2021371. [Google Scholar] [CrossRef]
- Waidhauser, J.; Martin, B.; Trepel, M.; Markl, B. Can low autopsy rates be increased? Yes, we can! Should postmortem examinations in oncology be performed? Yes, we should! A postmortem analysis of oncological cases. Virchows Arch 2021, 478, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Bunei, M.; Muturi, P.; Otiato, F.; Njuguna, H.N.; Emukule, G.O.; Otieno, N.A.; Dawa, J.; Chaves, S.S. Factors Influencing Acceptance of Post-Mortem Examination of Children at a Tertiary Care Hospital in Nairobi, Kenya. Ann Glob Health 2019, 85. [Google Scholar] [CrossRef]
- Lawrence, S.; Namusanya, D.; Hamuza, A.; Huwa, C.; Chasweka, D.; Kelley, M.; Molyneux, S.; Voskuijl, W.; Denno, D.M.; Desmond, N. Hypothetical acceptability of hospital-based post-mortem pediatric minimally invasive tissue sampling in Malawi: The role of complex social relationships. PLoS One 2021, 16, e0246369. [Google Scholar] [CrossRef]
- Puntmann, V.O. How-to guide on biomarkers: biomarker definitions, validation and applications with examples from cardiovascular disease. Postgrad Med J 2009, 85, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Bondareva, O.; Sheikh, B.N. Vascular Homeostasis and Inflammation in Health and Disease-Lessons from Single Cell Technologies. Int J Mol Sci 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Almulhim, A.M.; Menezes, R.G. Evaluation of postmortem changes. 2020.
- Blokker, B.M.; Wagensveld, I.M.; Weustink, A.C.; Oosterhuis, J.W.; Hunink, M.G. Non-invasive or minimally invasive autopsy compared to conventional autopsy of suspected natural deaths in adults: a systematic review. Eur Radiol 2016, 26, 1159–1179. [Google Scholar] [CrossRef] [PubMed]
- Hyde, G.; Rummery, R.; Whitby, E.H.; Bloor, J.; Raghavan, A.; Cohen, M.C. Benefits and Limitations of the Minimally Invasive Postmortem: A Review of an Innovative Service Development. Pediatr Dev Pathol 2020, 23, 431–437. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, H.; Shenfine, R.; Brown, M.; Beyer, F.; Rankin, J. Are non-invasive or minimally invasive autopsy techniques for detecting cause of death in prenates, neonates and infants accurate? A systematic review of diagnostic test accuracy. BMJ Open 2023, 13, e064774. [Google Scholar] [CrossRef] [PubMed]
- Ricard, N.; Bailly, S.; Guignabert, C.; Simons, M. The quiescent endothelium: signalling pathways regulating organ-specific endothelial normalcy. Nat Rev Cardiol 2021, 18, 565–580. [Google Scholar] [CrossRef]
- Schlereth, K.; Weichenhan, D.; Bauer, T.; Heumann, T.; Giannakouri, E.; Lipka, D.; Jaeger, S.; Schlesner, M.; Aloy, P.; Eils, R.; et al. The transcriptomic and epigenetic map of vascular quiescence in the continuous lung endothelium. Elife 2018, 7. [Google Scholar] [CrossRef]
- Hao, X.; Cheng, S.; Jiang, B.; Xin, S. Applying multi-omics techniques to the discovery of biomarkers for acute aortic dissection. Front Cardiovasc Med 2022, 9, 961991. [Google Scholar] [CrossRef]
- Smejkal, G.B. I’m an -omics, you’re an -omics. Expert Review of Proteomics 2006, 3, 383–385. [Google Scholar] [CrossRef]
- Janiszewska, D.; Szultka-Mlynska, M.; Pomastowski, P.; Buszewski, B. “Omic” Approaches to Bacteria and Antibiotic Resistance Identification. Int J Mol Sci 2022, 23, 9601. [Google Scholar] [CrossRef]
- Mischak, H.; Ioannidis, J.P.; Argiles, A.; Attwood, T.K.; Bongcam-Rudloff, E.; Broenstrup, M.; Charonis, A.; Chrousos, G.P.; Delles, C.; Dominiczak, A.; et al. Implementation of proteomic biomarkers: making it work. Eur J Clin Invest 2012, 42, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Kumarasamy, G.; Ismail, M.N.; Tuan, S.E.; Sharif, C.D.; Mittal, P.; Hoffmann, P.; Kaur, G. Conference Proceedings–6th International Conference on Molecular Diagnostics and Biomarker Discovery (MDBD 2022): Building Resilience in Biomedical Research. In Proceedings of the BMC Proceedings, 2022; p. 1.
- Palstrom, N.B.; Matthiesen, R.; Rasmussen, L.M.; Beck, H.C. Recent Developments in Clinical Plasma Proteomics-Applied to Cardiovascular Research. Biomedicines 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Qian, G.; Adeyanju, O.; Olajuyin, A.; Guo, X. Abdominal Aortic Aneurysm Formation with a Focus on Vascular Smooth Muscle Cells. Life (Basel) 2022, 12, 191. [Google Scholar] [CrossRef] [PubMed]
- Pal, D.; Szilagyi, B.; Berczeli, M.; Szalay, C.I.; Sardy, B.; Olah, Z.; Szekely, T.; Racz, G.; Banga, P.; Czinege, Z.; et al. Ruptured Aortic Aneurysm and Dissection Related Death: an Autopsy Database Analysis. Pathol Oncol Res 2020, 26, 2391–2399. [Google Scholar] [CrossRef] [PubMed]
- Takada, M.; Yamagishi, K.; Tamakoshi, A.; Iso, H. Height and Mortality from Aortic Aneurysm and Dissection. J Atheroscler Thromb 2022, 29, 1166–1175. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.; Goyal, A.; Grigorova, Y.; Farci, F.; Le, J.K. Aortic Dissection. In StatPearls; StatPearls Publishing Copyright © 2023, StatPearls Publishing LLC.: Treasure Island (FL), 2023.
- La Russa, R.; Maiese, A.; Viola, R.V.; De Matteis, A.; Pinchi, E.; Frati, P.; Fineschi, V. Searching for highly sensitive and specific biomarkers for sepsis: State-of-the-art in post-mortem diagnosis of sepsis through immunohistochemical analysis. Int J Immunopathol Pharmacol 2019, 33, 2058738419855226. [Google Scholar] [CrossRef]
- Imanaka-Yoshida, K.; Matsumoto, K.I. Multiple Roles of Tenascins in Homeostasis and Pathophysiology of Aorta. Ann Vasc Dis 2018, 11, 169–180. [Google Scholar] [CrossRef]
- Brady, A.R.; Thompson, S.G.; Fowkes, F.G.; Greenhalgh, R.M.; Powell, J.T.; Participants, U.K.S.A.T. Abdominal aortic aneurysm expansion: risk factors and time intervals for surveillance. Circulation 2004, 110, 16–21. [Google Scholar] [CrossRef]
- Pierrakos, C.; Velissaris, D.; Bisdorff, M.; Marshall, J.C.; Vincent, J.L. Biomarkers of sepsis: time for a reappraisal. Crit Care 2020, 24, 287. [Google Scholar] [CrossRef]
- Bown, M.J.; Sutton, A.J.; Bell, P.R.; Sayers, R.D. A meta-analysis of 50 years of ruptured abdominal aortic aneurysm repair. Br J Surg 2002, 89, 714–730. [Google Scholar] [CrossRef]
- Rastogi, V.; Stefens, S.J.M.; Houwaart, J.; Verhagen, H.J.M.; de Bruin, J.L.; van der Pluijm, I.; Essers, J. Molecular Imaging of Aortic Aneurysm and Its Translational Power for Clinical Risk Assessment. Front Med (Lausanne) 2022, 9, 814123. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Jiang, B.; Li, X.; Sun, H.Y.; Li, X.T.; Jing, J.J.; Yang, J. Serum matrix metalloproteinase-9 is a valuable biomarker for identification of abdominal and thoracic aortic aneurysm: a case-control study. BMC Cardiovasc Disord 2018, 18, 202. [Google Scholar] [CrossRef] [PubMed]
- Bihlet, A.R.; Karsdal, M.A.; Sand, J.M.; Leeming, D.J.; Roberts, M.; White, W.; Bowler, R. Biomarkers of extracellular matrix turnover are associated with emphysema and eosinophilic-bronchitis in COPD. Respir Res 2017, 18, 22. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, J.H.; Karsdal, M.A.; Sand, J.M.; Willumsen, N.; Diefenbach, C.; Svensson, B.; Hagglund, P.; Oersnes-Leeming, D.J. Serological assessment of neutrophil elastase activity on elastin during lung ECM remodeling. BMC Pulm Med 2015, 15, 53. [Google Scholar] [CrossRef]
- Aragon-Vela, J.; Alcala-Bejarano Carrillo, J.; Moreno-Racero, A.; Plaza-Diaz, J. The Role of Molecular and Hormonal Factors in Obesity and the Effects of Physical Activity in Children. Int J Mol Sci 2022, 23, 15413. [Google Scholar] [CrossRef] [PubMed]
- Demestre, M.; Parkin-Smith, G.; Petzold, A.; Pullen, A.H. The pro and the active form of matrix metalloproteinase-9 is increased in serum of patients with amyotrophic lateral sclerosis. J Neuroimmunol 2005, 159, 146–154. [Google Scholar] [CrossRef]
- Silvello, D.; Narvaes, L.B.; Albuquerque, L.C.; Forgiarini, L.F.; Meurer, L.; Martinelli, N.C.; Andrades, M.E.; Clausell, N.; dos Santos, K.G.; Rohde, L.E. Serum levels and polymorphisms of matrix metalloproteinases (MMPs) in carotid artery atherosclerosis: higher MMP-9 levels are associated with plaque vulnerability. Biomarkers 2014, 19, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Beck-Joseph, J.; Lehoux, S. Molecular Interactions Between Vascular Smooth Muscle Cells and Macrophages in Atherosclerosis. Front Cardiovasc Med 2021, 8, 737934. [Google Scholar] [CrossRef]
- Makita, S.; Nakamura, M.; Hiramori, K. The association of C-reactive protein levels with carotid intima-media complex thickness and plaque formation in the general population. Stroke 2005, 36, 2138–2142. [Google Scholar] [CrossRef]
- Andrade, C.; Bosco, A.; Sandrim, V.; Silva, F. MMP-9 Levels and IMT of Carotid Arteries are Elevated in Obese Children and Adolescents Compared to Non-Obese. Arq Bras Cardiol 2017, 108, 198–203. [Google Scholar] [CrossRef]
- Antoniou, G.A.; Georgiadis, G.S.; Antoniou, S.A.; Murray, D.; Smyth, J.V.; Serracino-Inglott, F.; Paraskevas, K.I. Plasma matrix metalloproteinase 9 levels may predict endoleaks after endovascular aortic aneurysm repair. Angiology 2013, 64, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Maguire, E.M.; Pearce, S.W.A.; Xiao, R.; Oo, A.Y.; Xiao, Q. Matrix Metalloproteinase in Abdominal Aortic Aneurysm and Aortic Dissection. Pharmaceuticals (Basel) 2019, 12. [Google Scholar] [CrossRef] [PubMed]
- Nehring, S.; Goyal, A.; Patel, B. C Reactive Protein. 2021 Dec 28. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing 2022.
- Nourkami-Tutdibi, N.; Graf, N.; Beier, R.; Zemlin, M.; Tutdibi, E. Plasma levels of osteopontin from birth to adulthood. Pediatr Blood Cancer 2020, 67, e28272. [Google Scholar] [CrossRef] [PubMed]
- Sikora-Skrabaka, M.; Skrabaka, D.; Ruggeri, P.; Caramori, G.; Skoczynski, S.; Barczyk, A. D-dimer value in the diagnosis of pulmonary embolism-may it exclude only? J Thorac Dis 2019, 11, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, B.; Ruiz, C.; Chacon, P.; Alvarez-Sabin, J.; Matas, M. Serum values of metalloproteinase-2 and metalloproteinase-9 as related to unstable plaque and inflammatory cells in patients with greater than 70% carotid artery stenosis. J Vasc Surg 2004, 40, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.Z.; Xue, Q.; Shao, H. Inflammatory Markers Related to Innate and Adaptive Immunity in Atherosclerosis: Implications for Disease Prediction and Prospective Therapeutics. J Inflamm Res 2021, 14, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, Z.; Amighi, F.; Vakili, Z.; Momen-Heravi, M.; Moravveji, S.A. Diagnostic value of procalcitonin, erythrocyte sedimentation rate (ESR), quantitative C-reactive protein (CRP) and clinical findings associated with osteomyelitis in patients with diabetic foot. Hum Antibodies 2021, 29, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Albu, E.; Filip, C.; Zamosteanu, N.; Jaba, I.M.; Linic, I.S.; Sosa, I. Hyperhomocysteinemia is an indicator of oxidant stress. Med Hypotheses 2012, 78, 554–555. [Google Scholar] [CrossRef]
- Atre, A.S.; CR, W.D.S.; Suresh, V.; Nagaraja, M.; Madhuvan, H. Evaluation of Plasma Total Antioxidant Capacity Levels and Osteocalcin in Prediabetes and Healthy Subjects. RGUHS Journal of Medical Sciences 2020, 10. [Google Scholar] [CrossRef]
- Hirano, T. IL-6 in inflammation, autoimmunity and cancer. Int Immunol 2021, 33, 127–148. [Google Scholar] [CrossRef]
- Ridker, P.M.; MacFadyen, J.G.; Glynn, R.J.; Bradwin, G.; Hasan, A.A.; Rifai, N. Comparison of interleukin-6, C-reactive protein, and low-density lipoprotein cholesterol as biomarkers of residual risk in contemporary practice: secondary analyses from the Cardiovascular Inflammation Reduction Trial. Eur Heart J 2020, 41, 2952–2961. [Google Scholar] [CrossRef]
- Holcomb, D.; Alexaki, A.; Hernandez, N.; Hunt, R.; Laurie, K.; Kames, J.; Hamasaki-Katagiri, N.; Komar, A.A.; DiCuccio, M.; Kimchi-Sarfaty, C. Gene variants of coagulation related proteins that interact with SARS-CoV-2. PLoS Comput Biol 2021, 17, e1008805. [Google Scholar] [CrossRef] [PubMed]
- Petel, D.; Winters, N.; Gore, G.C.; Papenburg, J.; Beltempo, M.; Lacroix, J.; Fontela, P.S. Use of C-reactive protein to tailor antibiotic use: a systematic review and meta-analysis. BMJ Open 2018, 8, e022133. [Google Scholar] [CrossRef] [PubMed]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front Immunol 2018, 9, 754. [Google Scholar] [CrossRef] [PubMed]
- Dawson, J.; Cockerill, G.W.; Choke, E.; Belli, A.M.; Loftus, I.; Thompson, M.M. Aortic aneurysms secrete interleukin-6 into the circulation. J Vasc Surg 2007, 45, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Artemiou, P.; Charokopos, N.; Rouska, E.; Sabol, F.; Chrysogonidis, I.; Tsavdaridou, V.; Paschalidis, G. C-reactive protein/interleukin-6 ratio as marker of the size of the uncomplicated thoracic aortic aneurysms. Interact Cardiovasc Thorac Surg 2012, 15, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Cui, N.; Zhang, H.; Chen, Z.; Yu, Z. Prognostic significance of PCT and CRP evaluation for adult ICU patients with sepsis and septic shock: retrospective analysis of 59 cases. J Int Med Res 2019, 47, 1573–1579. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Lu, Y.; Jiang, H.; Zhang, L. The diagnostic accuracy of procalcitonin and C-reactive protein for sepsis: A systematic review and meta-analysis. J Cell Biochem 2019, 120, 5852–5859. [Google Scholar] [CrossRef]
- Hung, S.K.; Lan, H.M.; Han, S.T.; Wu, C.C.; Chen, K.F. Current Evidence and Limitation of Biomarkers for Detecting Sepsis and Systemic Infection. Biomedicines 2020, 8, 494. [Google Scholar] [CrossRef]
- Al-Barjas, H.S.; Ariens, R.; Grant, P.; Scott, J.A. Raised plasma fibrinogen concentration in patients with abdominal aortic aneurysm. Angiology 2006, 57, 607–614. [Google Scholar] [CrossRef]
- Menekşe, E.; Düz, M.E. Changes in D-dimer, Ferritin, and Fibrinogen in Healthy Smokers and Nonsmokers during the Covid-19 Outbreak. Journal of Surgery and Research 2023, 6, 94–99. [Google Scholar] [CrossRef]
- Ezaki, M.; Wada, H.; Ichikawa, Y.; Ikeda, N.; Shiraki, K.; Yamamoto, A.; Moritani, I.; Shimaoka, M.; Shimpo, H. Plasma Soluble Fibrin Is Useful for the Diagnosis of Thrombotic Diseases. Journal of Clinical Medicine 2023, 12, 2597. [Google Scholar] [CrossRef] [PubMed]
- Di Castelnuovo, A.; de Curtis, A.; Costanzo, S.; Persichillo, M.; Olivieri, M.; Zito, F.; Donati, M.B.; de Gaetano, G.; Iacoviello, L.; Investigators, M.-S.P. Association of D-dimer levels with all-cause mortality in a healthy adult population: findings from the MOLI-SANI study. Haematologica 2013, 98, 1476–1480. [Google Scholar] [CrossRef] [PubMed]
- Takagi, H.; Manabe, H.; Kawai, N.; Goto, S.; Umemoto, T. Plasma fibrinogen and D-dimer concentrations are associated with the presence of abdominal aortic aneurysm: a systematic review and meta-analysis. Eur J Vasc Endovasc Surg 2009, 38, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Crawford, F.; Andras, A.; Welch, K.; Sheares, K.; Keeling, D.; Chappell, F.M. D-dimer test for excluding the diagnosis of pulmonary embolism. Cochrane Database Syst Rev 2016, 2016, CD010864. [Google Scholar] [CrossRef] [PubMed]
- Wang, H. Evaluation of D-dimer in postmortem blood using the SERATEC PMB Test. Boston University, 2019.
- Gevsemezoglu, O.F.; Karadayi, B.; Koca, Y.; Cetin, G. Investigation of the use of seratec pmb test on postmortem peripheral blood samples for forensic purposes. Medicine 2022, 11, 159–165. [Google Scholar] [CrossRef]
- Goncalves, F.A.R.; Besen, B.; Lima, C.A.; Cora, A.P.; Pereira, A.J.R.; Perazzio, S.F.; Gouvea, C.P.; Fonseca, L.A.M.; Trindade, E.M.; Sumita, N.M.; et al. Use and misuse of biomarkers and the role of D-dimer and C-reactive protein in the management of COVID-19: A post-hoc analysis of a prospective cohort study. Clinics (Sao Paulo) 2021, 76, e3547. [Google Scholar] [CrossRef] [PubMed]
- Gorelick, P.B.; Scuteri, A.; Black, S.E.; Decarli, C.; Greenberg, S.M.; Iadecola, C.; Launer, L.J.; Laurent, S.; Lopez, O.L.; Nyenhuis, D.; et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke 2011, 42, 2672–2713. [Google Scholar] [CrossRef]
- Han, X.W.; Wang, W.J.; He, J.J.; Jiang, L.; Li, X. Osteopontin as a biomarker for osteosarcoma therapy and prognosis. Oncology Letters 2019, 17, 2592–2598. [Google Scholar] [CrossRef]
- Chai, Y.L.; Chong, J.R.; Raquib, A.R.; Xu, X.; Hilal, S.; Venketasubramanian, N.; Tan, B.Y.; Kumar, A.P.; Sethi, G.; Chen, C.P.; et al. Plasma osteopontin as a biomarker of Alzheimer’s disease and vascular cognitive impairment. Sci Rep 2021, 11, 4010. [Google Scholar] [CrossRef]
- Wei, R.; Wong, J.P.C.; Kwok, H.F. Osteopontin -- a promising biomarker for cancer therapy. J Cancer 2017, 8, 2173–2183. [Google Scholar] [CrossRef] [PubMed]
- Bruha, R.; Vitek, L.; Smid, V. Osteopontin - A potential biomarker of advanced liver disease. Ann Hepatol 2020, 19, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Farrokhi, V.; Chabot, J.R.; Neubert, H.; Yang, Z.Y. Assessing the Feasibility of Neutralizing Osteopontin with Various Therapeutic Antibody Modalities. Scientific Reports 2018, 8, 7781. [Google Scholar] [CrossRef] [PubMed]
- Roberts, I.S.; Benamore, R.E.; Benbow, E.W.; Lee, S.H.; Harris, J.N.; Jackson, A.; Mallett, S.; Patankar, T.; Peebles, C.; Roobottom, C.; et al. Post-mortem imaging as an alternative to autopsy in the diagnosis of adult deaths: a validation study. Lancet 2012, 379, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, D.; Obbelode, F.; Vogel, H.; Hoepker, W.W.; Nierhaus, A.; Braune, S.; Sauter, G.; Pueschel, K.; Kluge, S. Virtual autopsy as an alternative to traditional medical autopsy in the intensive care unit: a prospective cohort study. Ann Intern Med 2012, 156, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Flaxman, A.D.; Stewart, A.; Joseph, J.C.; Alam, N.; Alam, S.S.; Chowdhury, H.; Mooney, M.D.; Rampatige, R.; Remolador, H.; Sanvictores, D.; et al. Collecting verbal autopsies: improving and streamlining data collection processes using electronic tablets. Popul Health Metr 2018, 16, 3. [Google Scholar] [CrossRef] [PubMed]
- Sinard, J.H. Factors affecting autopsy rates, autopsy request rates, and autopsy findings at a large academic medical center. Exp Mol Pathol 2001, 70, 333–343. [Google Scholar] [CrossRef]
- Paratz, E.D.; Rowe, S.J.; Stub, D.; Pflaumer, A.; La Gerche, A. A systematic review of global autopsy rates in all-cause mortality and young sudden death. Heart Rhythm 2023, 20, 607–613. [Google Scholar] [CrossRef]
- Michaud, K.; Jacobsen, C.; Basso, C.; Banner, J.; Blokker, B.M.; de Boer, H.H.; Dedouit, F.; O’Donnell, C.; Giordano, C.; Magnin, V. Application of postmortem imaging modalities in cases of sudden death due to cardiovascular diseases–current achievements and limitations from a pathology perspective. Virchows Archiv 2023, 482, 385–406. [Google Scholar] [CrossRef]
- Thomas, L.M.; D’Ambruoso, L.; Balabanova, D. Verbal autopsy in health policy and systems: a literature review. BMJ Glob Health 2018, 3, e000639. [Google Scholar] [CrossRef]
- D’Ambruoso, L.; Kahn, K.; Wagner, R.G.; Twine, R.; Spies, B.; van der Merwe, M.; Gomez-Olive, F.X.; Tollman, S.; Byass, P. Moving from medical to health systems classifications of deaths: extending verbal autopsy to collect information on the circumstances of mortality. Glob Health Res Policy 2016, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- Mahesh, B.P.K.; Hart, J.D.; Acharya, A.; Chowdhury, H.R.; Joshi, R.; Adair, T.; Hazard, R.H. Validation studies of verbal autopsy methods: a systematic review. BMC Public Health 2022, 22, 2215. [Google Scholar] [CrossRef] [PubMed]
- Rosen, T.; Safford, M.M.; Sterling, M.R.; Goyal, P.; Patterson, M.; Al Malouf, C.; Ballin, M.; Del Carmen, T.; LoFaso, V.M.; Raik, B.L.; et al. Development of the Verbal Autopsy Instrument for COVID-19 (VAIC). J Gen Intern Med 2021, 36, 3522–3529. [Google Scholar] [CrossRef] [PubMed]
- Nasaruddin, N.H.; Ganapathy, S.S.; Awaluddin, S.M.; Anuar, M.F.M.; Binti Alias, N.; Mang, C.Y.; Wan-Fei, K. Conducting verbal autopsy by telephone interview during the pandemic to support mortality surveillance: a feasibility study in Malaysia. Western Pac Surveill Response J 2022, 13, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Singh, A. Childhood Malnutrition in India. In Perspective of Recent Advances in Acute Diarrhea; IntechOpen: 2020.
- Krehbiel, K.; Pinckard, J.K. The Toolbox Approach to Forensic Pathology. Academic Forensic Pathology 2015, 5, 534–547. [Google Scholar] [CrossRef]
- Yi-Li, G.W.; Lai, P.S.; Noor, M.H.M.; Chinna, K.; Ibrahim, M. Reliability of Post-Mortem Computed Tomography in Measuring Foramen Magnum Dimensions: A Pilot Study. Forensic Anthropology 2023, 1–9-1–9.
- Cartocci, G.; Santurro, A.; Neri, M.; Zaccagna, F.; Catalano, C.; La Russa, R.; Turillazzi, E.; Panebianco, V.; Frati, P.; Fineschi, V. Post-mortem computed tomography (PMCT) radiological findings and assessment in advanced decomposed bodies. Radiol Med 2019, 124, 1018–1027. [Google Scholar] [CrossRef] [PubMed]
- Filograna, L.; Manenti, G.; O’Donnell, C.; Floris, R.; Oliva, A. Potentials of post-mortem CT (PMCT) in paediatric cases related to SARS-CoV-2 infection. Forensic Sci Med Pathol 2023, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Herath, J.C.; Herath, S.O. Is it time for targeted and minimally invasive post-mortem examination using total body computed tomography in a medicolegal autopsy? Forensic Science, Medicine and Pathology 2021, 17, 175–176. [Google Scholar] [CrossRef]
- Mercala, E.; Benbow, E.W. Autopsy by Imaging: The Last 10 Years. Forensic Sciences 2022, 2, 696–714. [Google Scholar] [CrossRef]
- Joshi, R.; Praveen, D.; Jan, S.; Raju, K.; Maulik, P.; Jha, V.; Lopez, A.D. How much does a verbal autopsy based mortality surveillance system cost in rural India? PLoS One 2015, 10, e0126410. [Google Scholar] [CrossRef]
- Zech, W.D.; Jackowski, C.; Schwendener, N.; Brencicova, E.; Schuster, F.; Lombardo, P. Postmortem CT versus forensic autopsy: frequent discrepancies of tracheobronchial content findings. Int J Legal Med 2016, 130, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Mondello, C.; Baldino, G.; Bottari, A.; Sapienza, D.; Perri, F.; Argo, A.; Asmundo, A.; Ventura Spagnolo, E. The role of PMCT for the assessment of the cause of death in natural disaster (landslide and flood): a Sicilian experience. Int J Legal Med 2022, 136, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Chatzaraki, V.; Thali, M.J.; Ampanozi, G. Diagnostic accuracy of postmortem computed tomography for bleeding source determination in cases with hemoperitoneum. Int J Legal Med 2021, 135, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Shelmerdine, S.C.; Sebire, N.J.; Arthurs, O.J. Diagnostic accuracy of postmortem ultrasound vs postmortem 1.5-T MRI for non-invasive perinatal autopsy. Ultrasound Obstet Gynecol 2021, 57, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Thayyil, S.; Chandrasekaran, M.; Chitty, L.S.; Wade, A.; Skordis-Worrall, J.; Bennett-Britton, I.; Cohen, M.; Withby, E.; Sebire, N.J.; Robertson, N.J.; et al. Diagnostic accuracy of post-mortem magnetic resonance imaging in fetuses, children and adults: a systematic review. Eur J Radiol 2010, 75, e142–148. [Google Scholar] [CrossRef]
- Elmsjo, A.; Vikingsson, S.; Soderberg, C.; Kugelberg, F.C.; Green, H. Post-Mortem Metabolomics: A Novel Approach in Clinical Biomarker Discovery and a Potential Tool in Death Investigations. Chem Res Toxicol 2021, 34, 1496–1502. [Google Scholar] [CrossRef]
- Rodrigues, F.S.; Oliveira, I.C.; Cat, M.N.L.; Mattos, M.C.L.; Silva, G.A. Agreement between Clinical and Anatomopathological Diagnoses in Pediatric Intensive Care. Rev Paul Pediatr 2021, 39, e2019263. [Google Scholar] [CrossRef]
- Hudak, L.; Nagy, A.C.; Molnar, S.; Mehes, G.; Nagy, K.E.; Olah, L.; Csiba, L. Discrepancies between clinical and autopsy findings in patients who had an acute stroke. Stroke Vasc Neurol 2022, 7, 215–221. [Google Scholar] [CrossRef]
- Lilla, H. Personal communication. 2023.
- Goldman, L. Autopsy 2018: still necessary, even if occasionally not sufficient. Circulation 2018, 137, 2686–2688. [Google Scholar] [CrossRef]
- Lunetta, P.; Lounamaa, A.; Sihvonen, S. Surveillance of injury-related deaths: medicolegal autopsy rates and trends in Finland. Inj Prev 2007, 13, 282–284. [Google Scholar] [CrossRef]
- Kurz, S.D.; Sido, V.; Herbst, H.; Ulm, B.; Salkic, E.; Ruschinski, T.M.; Buschmann, C.T.; Tsokos, M. Discrepancies between clinical diagnosis and hospital autopsy: A comparative retrospective analysis of 1,112 cases. PLoS One 2021, 16, e0255490. [Google Scholar] [CrossRef] [PubMed]
- Perkins, G.D.; McAuley, D.F.; Davies, S.; Gao, F. Discrepancies between clinical and postmortem diagnoses in critically ill patients: an observational study. Crit Care 2003, 7, R129–132. [Google Scholar] [CrossRef] [PubMed]
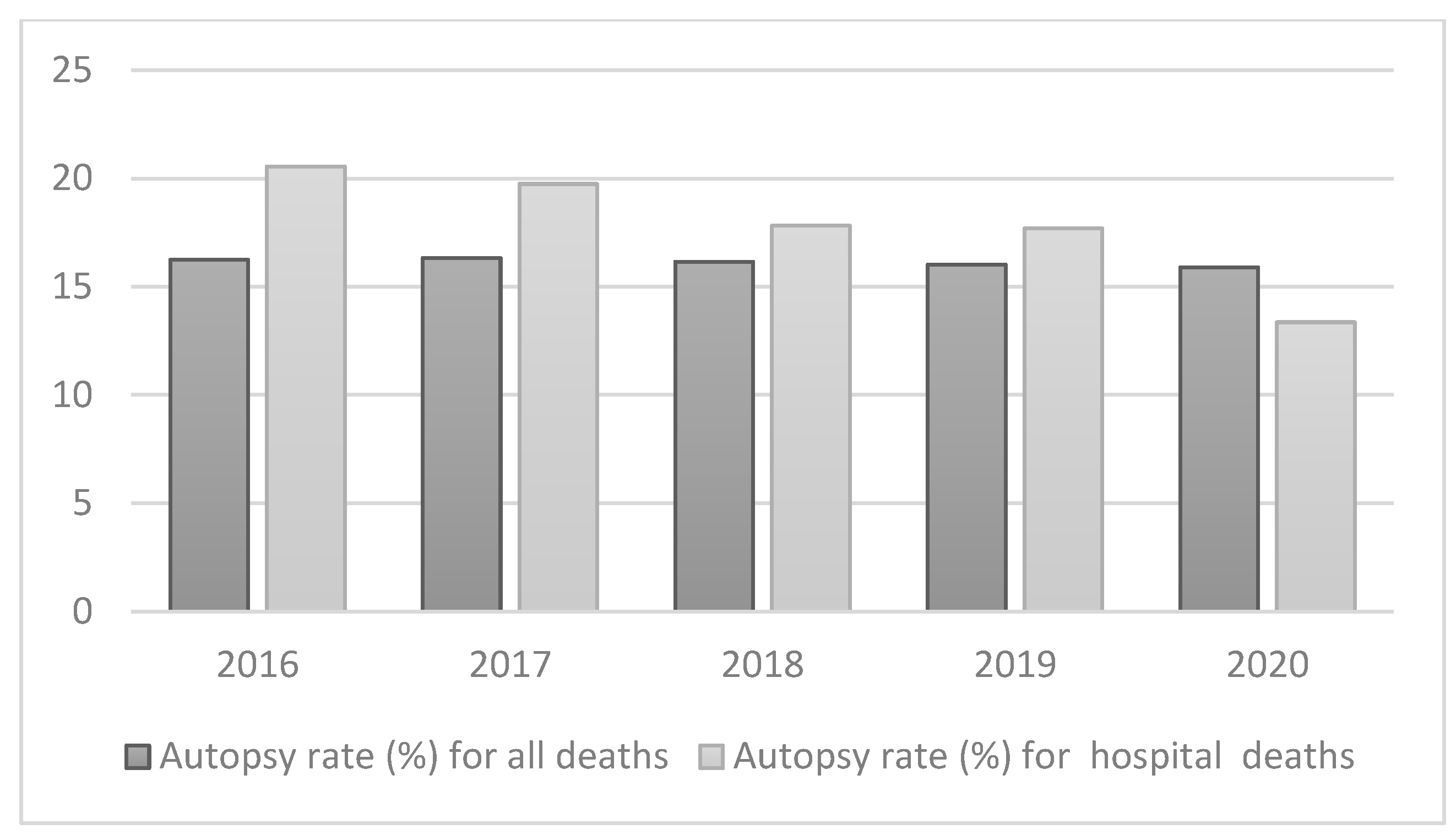
- (WHO), W.H.O. Autopsy rate (%) for all deaths. Available online: https://gateway.euro.who.int/en/indicators/hfa_545-6410-autopsy-rate-for-all-deaths/ (accessed on.
- (WHO), W.H.O. Autopsy rate (%) for hospital deaths. Available online: https://gateway.euro.who.int/en/indicators/hfa_544-6400-autopsy-rate-for-hospital-deaths/ (accessed on.
- Stambouly, J.J.; Kahn, E.; Boxer, R.A. Correlation between clinical diagnoses and autopsy findings in critically ill children. Pediatrics 1993, 92, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Mitrovic, D.; Savic, I.; Jankovic, R. Discrepancies between clinical and autopsy diagnosis of cause of death among psychiatric patients who died due to natural causes. A retrospective autopsy study. Vojnosanitetski Pregled 2019, 76, 278–283. [Google Scholar] [CrossRef]
| Related Process | Biomarker | Medium | Reference Values |
|---|---|---|---|
| Inflammation | C-reactive protein (CRP) | S | < 0.3 mg/dL: Normal (level seen in most healthy adults); 0.3 to 1.0 mg/dL: Normal or minor elevation (can be seen in obesity, pregnancy, diabetes, common cold, gingivitis, periodontitis, sedentary lifestyle, cigarette smoking, and genetic polymorphisms) [60] |
| Osteopontin (OPT) | S | 122.3 ± 39.2 ng/mL | |
| P | 463.7 ng/mL - 587.0 ng/mL [61] | ||
| Related to thrombus | D-Dimer | S | <2,152 ng/mL [62] |
| Matrix-degrading enzymes | MMP-9 | S | mean 436 ng/mL (range, 169-705 ng/mL) [63] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





