1. Introduction
Hydrogen is an efficient and renewable energy resource, which is regarded to be highly promising alternative to fossil fuel. It can help mitigate environmental pollution resulting from the combustion of fossil fuel and meet the energy demand of our rapidly developing society. Among the many methods of hydrogen production, water electrolysis has attracted widespread attention as a high-efficiency and environmentally friendly technology to produce hydrogen. Electrolysis of water is composed of hydrogen evolution reaction (HER) in a two-electron process and oxygen evolution reaction (OER) in a four-electron process [
1]. Since the anodic four-electron reaction process of OER is much slower than that of the two-electron reaction process of HER, high overpotential and the sluggish kinetics inevitably occur in the electrolysis of water. Therefore, it is needed to develop an effective electrocatalyst to improve the energy conversion efficiency [
2,
3]. However, the slow kinetics of the OER presents a significant challenge for the widespread implementation of water electrolysis as a large-scale hydrogen production technology. Therefore, it is crucial to develop an efficient OER catalyst.
LDHs are two-dimensional layered functional materials composed of positively charged hydroxide layers with equilibrium charges of anions between the layers [
4]. The general chemical formula of LDHs is [M
1-x2+M
x3+(OH)
2]
x+[A
n-]
x/
n·zH
2O, in which z is the number of interlayer water molecules [
5]. LDHs, as common catalysts, have a unique two-dimensional layered structure and could be exfoliated into ultrathin nanosheets, which are characterized with their large specific surface area and are conductive to the improvement in the catalytic performance of LDHs. On the other hand, the metal cations in LDHs materials have variable valence states, which is beneficial for the electron transfer in the catalytic reactions. To date, many LDHs including NiFe-LDHs, FeCo-LDHs, NiCo-LDHs, NiCr-LDHs and NiMn-LDHs have been prepared and applied in the fields of catalysts, adsorption materials, energy storage materials and sensor materials [
6,
7,
8,
9,
10,
11], etc. Recently, NiMn-LDH was reported to be of quite good catalytic activity in the OER reaction when applied as catalyst in the electrolysis of water [
12]. Wang et al. synthesized cobalt-doped NiMn-LDH by hydrothermal method, which exhibited an overpotential of 310 mV and a Tafel slope of 59 mV dec
-1 at a current density of 10 mA cm
-2 in alkaline media [
13]. Nonetheless, the electrocatalytic performance of LDHs materials is still limited for their poor electrical conductivity and easy agglomeration leaded low surface area (ECSA) [
14,
15,
16,
17].
Because of its unique ultrathin sheet structure and excellent chemical stability and electrical conductivity, graphene is often integrated with some catalysts to improve their catalytic performance [
18]. The composite of cobalt-based metal-organic framework (MOF) and reduced graphene oxide (rGO) synthesized by Yaqoob et al displayed good electrochemical activity with an overpotential of 290 mV at 10 mA cm
-2 [
19]. The composite of graphene and Co
3O
4 prepared by Mao et al demonstrated an improved OER performance owing to the heterojunction created at the interface of graphene sheet and Co
3O
4 nanoparticles [
20]. The composite of copper benzodicar boxylate and rGO prepared by Jahan et al was a promising composite catalyst for OER, because of its quite good stability and much low resistance [
21]. Han et al. prepared the composite of FeNi-LDH and GO by GO guiding the constructing of FeNi-LDH arrays. When applied as a desirable bifunctional electrocatalyst for the splitting of water, it demonstrated better OER performance than conventional commercial catalyst because of its enhanced conductivity and electronic interactions [
22]. Mooni, et al. prepared the bimetal oxides (MnO
2-NiO) and graphene oxide mixed composite electrodes (GO/MnO
2-NiO), which displayed an overpotential of 379 mV and maintained stable for 8 h at 10 mA cm
-2 when applied in the study of anodic water oxidation activities in an aqueous alkaline solution [
23]. Ma et al. firstly synthesized NiMn-LDH nanoplatelets by a hydrothermal treatment of a mixed Ni
2+/Mn
2+ salt solution in the presence of H
2O
2 and hexamethylenetetramine, and then prepared the hetero-assembly of NiMn-LDH nanosheets and GO/rGO through molecular hybridization of the LDH nanosheets with rGO. When applied as electrocatalyst for OER, the face-to-face hetero-assembly of NiMn LDH nanosheets with conductive rGO at an alternating sequence resulted in a small overpotential of 0.26 V and a Tafel slope of 46 mV per decade [
24]. Apart from graphene, other carbon materials [
25,
26], as well as copper and nickel meshes [
4,
5], are also optical candidates to composite with LDHs to improve their OER performance.
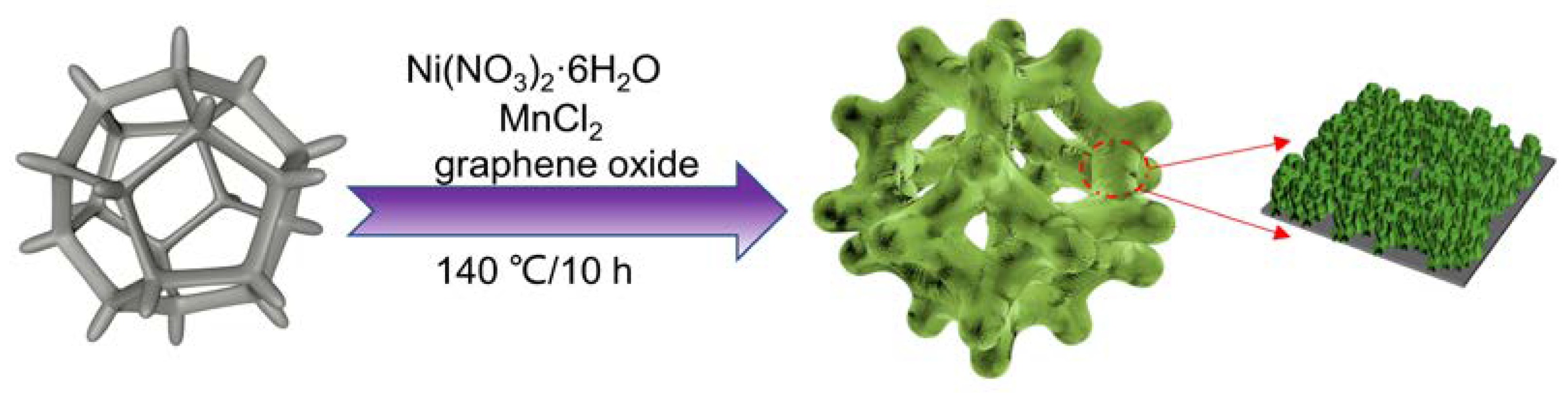
In this study, the composite of NiMn-LDHs and rGO loaded on NF was facilely prepared by a one-pot hydrothermal treatment of a mixed Ni2+/Mn2+ salt solution in the presence of urea and NF. When subjected to the test for OER, the as-prepared NiMn-LDHs/rGO/NF displayed extremely low overpotential at 10 mA cm-2 and much small Tafel slope, which might be owing to the synergistic effect established between NiMn-LDHs and rGO. rGO prevents the NiMn-LDHs nanosheets from agglomeration, whereas the NiMn-LDHs nanosheets alleviate the graphitization of rGO during hydrothermal process.
2. Results
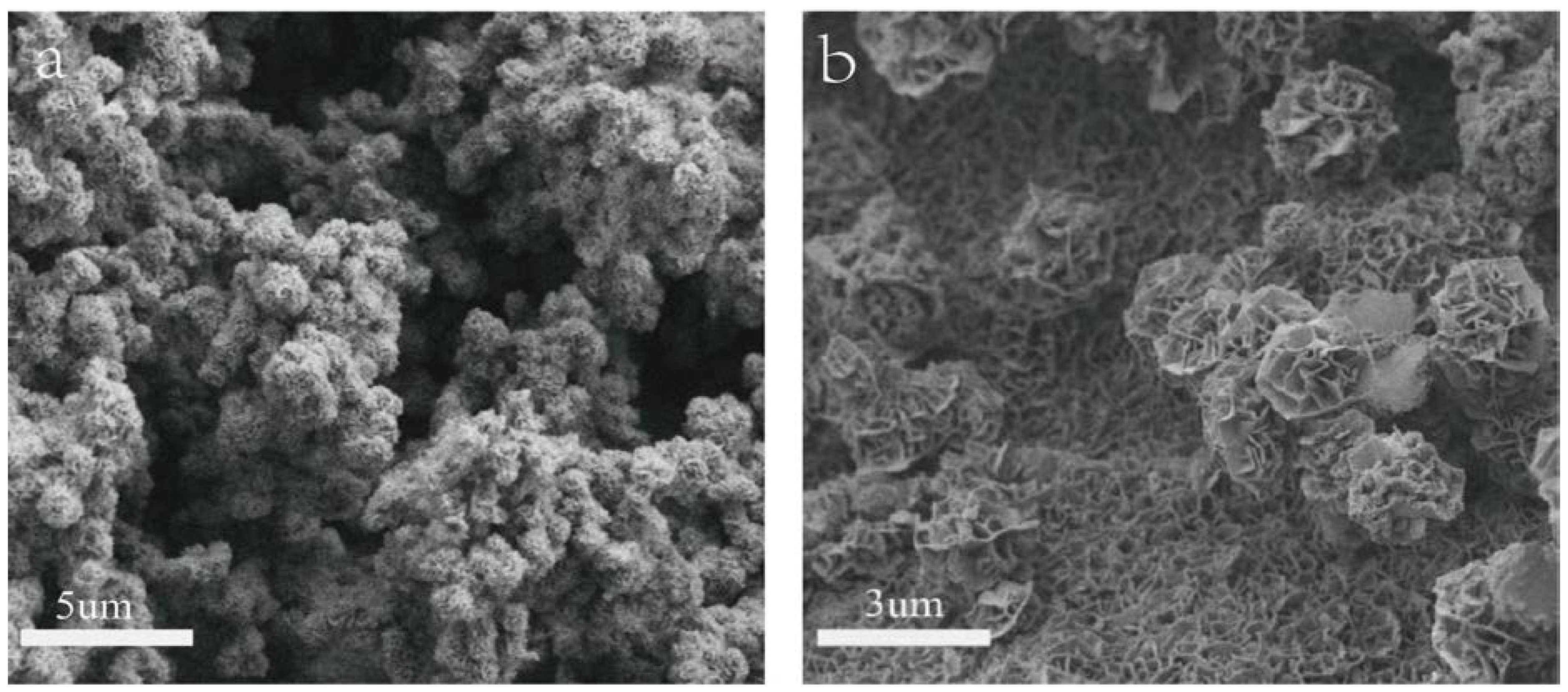
Shown in
Figure 1a & 1b are the SEM images of NiMn-LDHs/NF and NiMn-LDHs/rGO/NF synthesized by hydrothermal method. It can be seen from
Figure 1a that the synthesized NiMn-LDHs agglomerate on the surface of NF to form particles as large as 1
μm. In contrast, as displayed in
Figure 1b the ultra-thin sheets of NiMn-LDHs are cross-linked and assembled vertically and uniformly on the surface of rGO to form nano-walled networks, which is certainly beneficial for the enlargement of the specific area of the synthesized composite electrode. Therefore, rGO plays definitely a role to prevent NiMn-LDHs nanosheets from aggregating together into large particles.
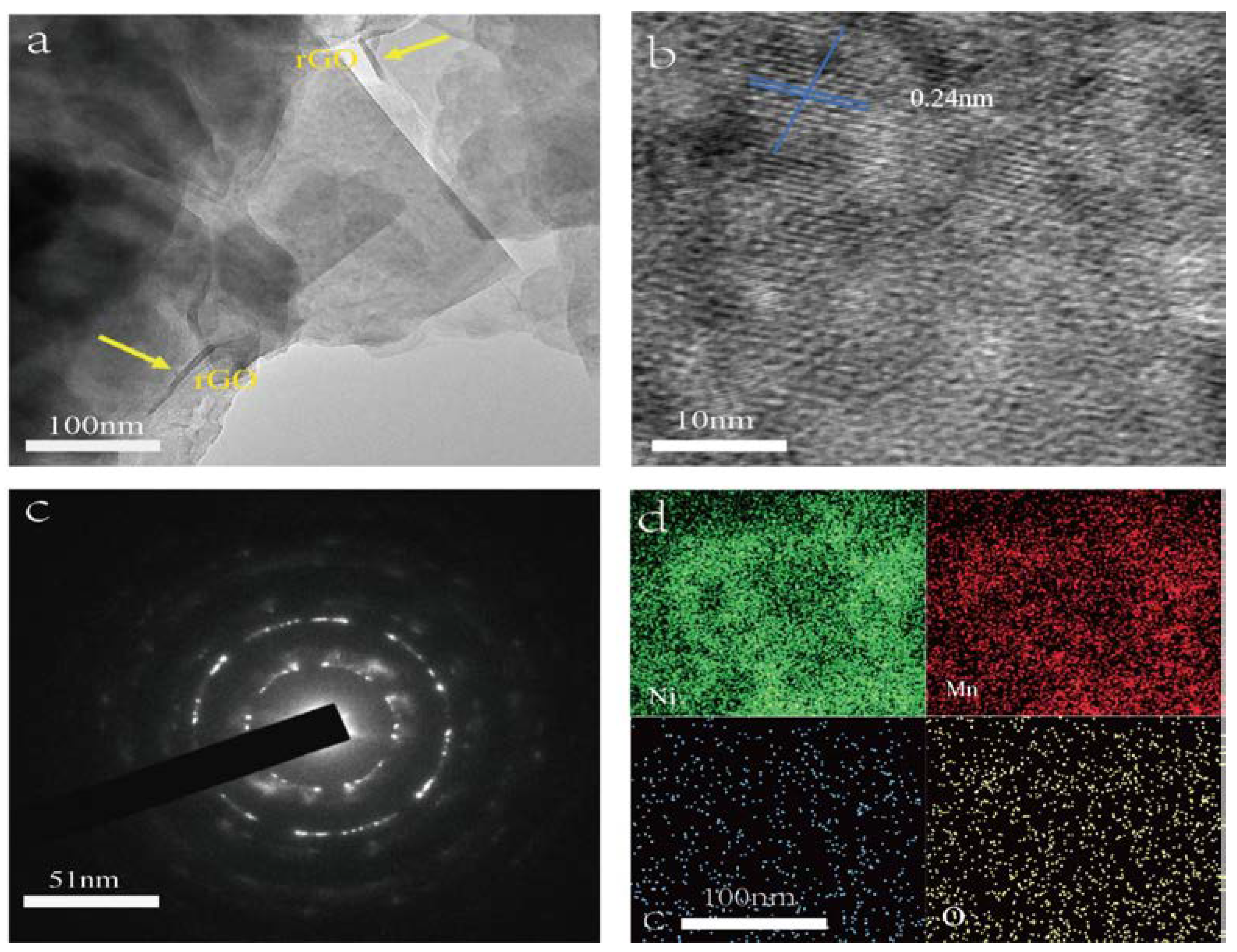
TEM observations were applied to investigate the microstructure of the NiMn-LDHs/rGO composite deposited on the inner surface of NF. In the TEM image shown in
Figure 2a, thin rGO sheets are observed with curved brims, whereas the ultra-thin NiMn-LDHs nanosheets are flat and transparent with straight and sharp edges. In the HR-TEM image displayed in
Figure 2b, the lattice fringes of a NiMn-LDHs nanosheet are arranged regularly with a lattice spacing of 0.24 nm, which corresponds perfectly to the (110) crystallographic plane NiMn-LDHs [
23]. Moreover, these highly oriented lattice fringes indicate that the synthesized NiMn-LDHs in the NiMn-LDHs/rGO composite are of a long range ordered crystal structure, which is strongly supported by the SAED image shown in
Figure 2c. From the energy dispersive spectroscopy (EDS) elemental mapping image demonstrated in
Figure 2d, it can be seen that the Mn, Ni, C and O elements are homogeneous distributed in the NiMn-LDHs/rGO composite, confirming the successful combination of rGO and NiMn-LDHs sheets.
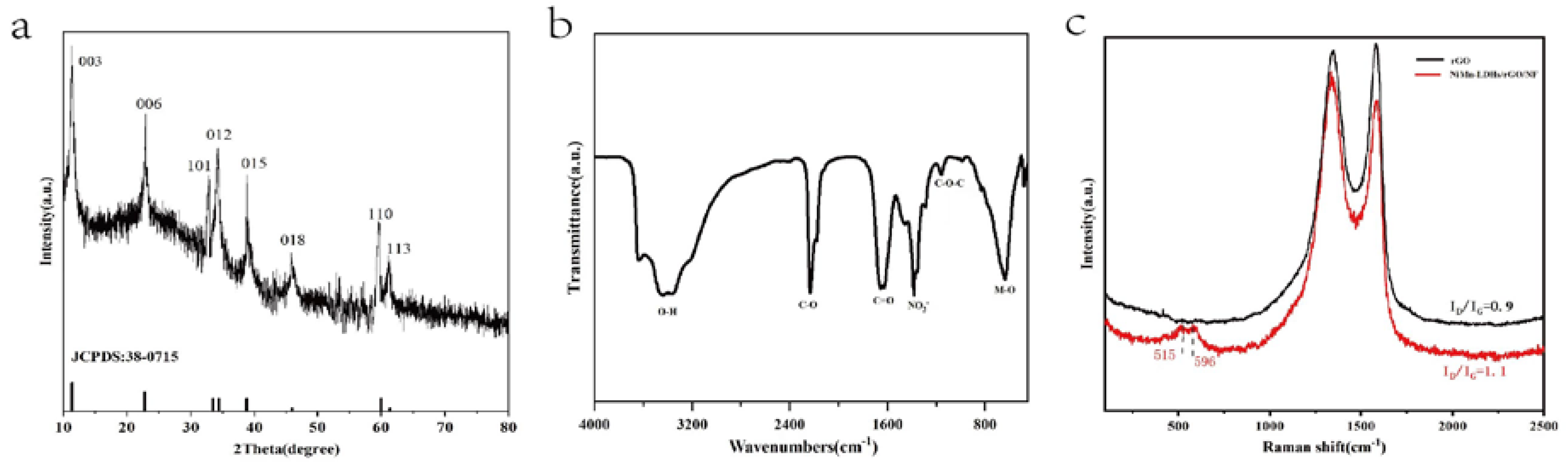
In the X-ray diffraction (XRD) pattern of NiMn-LDHs/rGO shown in
Figure 3a, the indexed crystal planes of all diffraction peaks correspond to the typically layered structure of hydrotalcite-like materials (NiMn-LDHs: PDF 38-0715), and the broad peak centered around 23° is assigned to rGO. Thus, it is concluded that the NiMn-LDHs sheets integrate with rGO with high purity and crystallinity, and that rGO has little effects on the crystalline structure of NiMn-LDHs. In the Fourier transform infrared (FT-IR) spectrum displayed in
Figure 3b, the broad band centered around 3432 cm
-1 is ascribed to the superimposed stretching vibration of hydroxyl groups out of hydrogen-bonded H
2O molecules intercalated between LDHs layers and metal hydroxyl groups in NiMn-LDHs. The strong bands at 640 cm
-1 could be attributed to the vibrational stretching mode of M-O-M and M-O in hydrotalcite-like materials, and the intense band appeared at 1380 cm
-1 is recognized to the asymmetric stretching mode of nitrate ions intercalating in the interlayer [
22,
23]. Whereas, the features located at 2229, 1657 and 1144 cm
-1 correspond to the C=O stretching, C=O ring stretching and C-O-C bending vibrations of rGO, respectively. In the Raman spectrum of NiMn-LDHs/rGO demonstrated in
Figure 3c, the most prominent scattering bands are assigned to the D and G bands of rGO, and the features appeared at 515 and 596 cm
−1 are ascribed to the symmetric stretching vibrations of disordered (or defected) Ni(OH)
2 and MnOOH [
27], respectively. In comparison with that of rGO, the intensity ratio of D band to G band (I
D/I
G) is obviously increased. Therefore, upon the formation of the NiMn-LDHs/rGO composite the rGO is well exfoliated and enriched in structural defects, which contributes a lot both to the enlargement of specific surface and to the increase of active sites. In summary, the data shown in
Figure 3 confirm strongly that the NiMn-LDHs/ rGO composite was successfully synthesized in the pores of NF [
23].
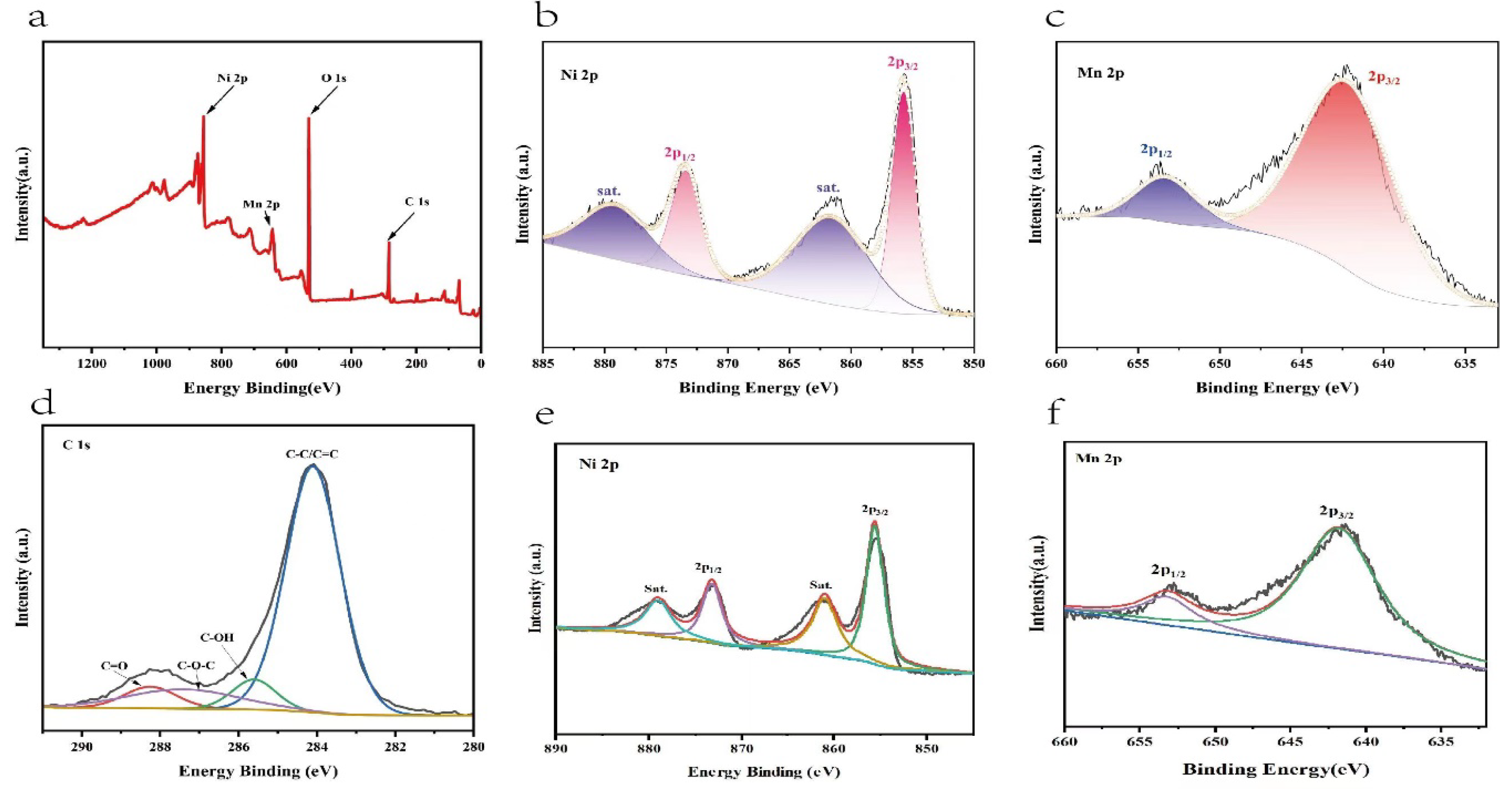
The surface chemical state and elemental composition of the NiMn-LDHs/rGO composite was investigated by XPS. Illustrated in
Figure 4 a-d are the survey and deconvoluted Ni 2p, Mn 2p, C 1s and O 2p XPS spectra of the NiMn-LDHs/rGO composite, respectively, along with the deconvoluted Ni 2p and Mn 2p ones of NiMn-LDHs for comparison. From the survey XPS spectrum displayed in
Figure 4a, it can be seen that elements including Ni, Mn, C and O are present in the NiMn-LDHs/rGO composite, which is consistent with the results of TEM-EDS elemental mapping. In the deconvoluted Ni 2p XPS spectrum (
Figure 4b), the peaks located at 873.5 and 855.8 eV accompanied by satellites are owing to the 2p
1/2 and 2p
3/2 characteristic peaks of Ni
2+ [
13], respectively, confirming the existence of Ni
2+ ions. In the deconvoluted Mn 2p XPS spectrum (
Figure 4c), the peaks located at 653.7 and 642.2 eV are ascribed to the 2p
1/2 and 2p
3/2 characteristic peaks of Mn
3+, respectively, indicative of the presence of Mn
3+ ions. In the deconvoluted XPS C 1s spectrum (
Figure 4d), the peaks located at 284.1 eV, 285.6 eV, 287.5 eV and 288.3 eV could be attributed to the characteristic features of C-C/C=C, C-OH, C-O-C and C=O bonds, respectively, implying that GO has been partly reduced to rGO. The residual oxygen atoms in rGO play an important role for the charge transfer from the metal ions in NiMn-LDHs to rGO, i.e., the interactions between NiMn-LDHs and rGO contributes a lot to the enhancement of the OER catalytic activity of the NiMn-LDHs/rGO composite. Moreover, in comparison with the features of NiMn-LDHs displayed in
Figure 4e and
Figure 4f, the binding energy of Ni
2+ and Mn
3+ of NiMn-LDHs/rGO is positively shifted about 0.4 eV, confirming the charge transfer from the metal ions of NiMn-LDHs to rGO.
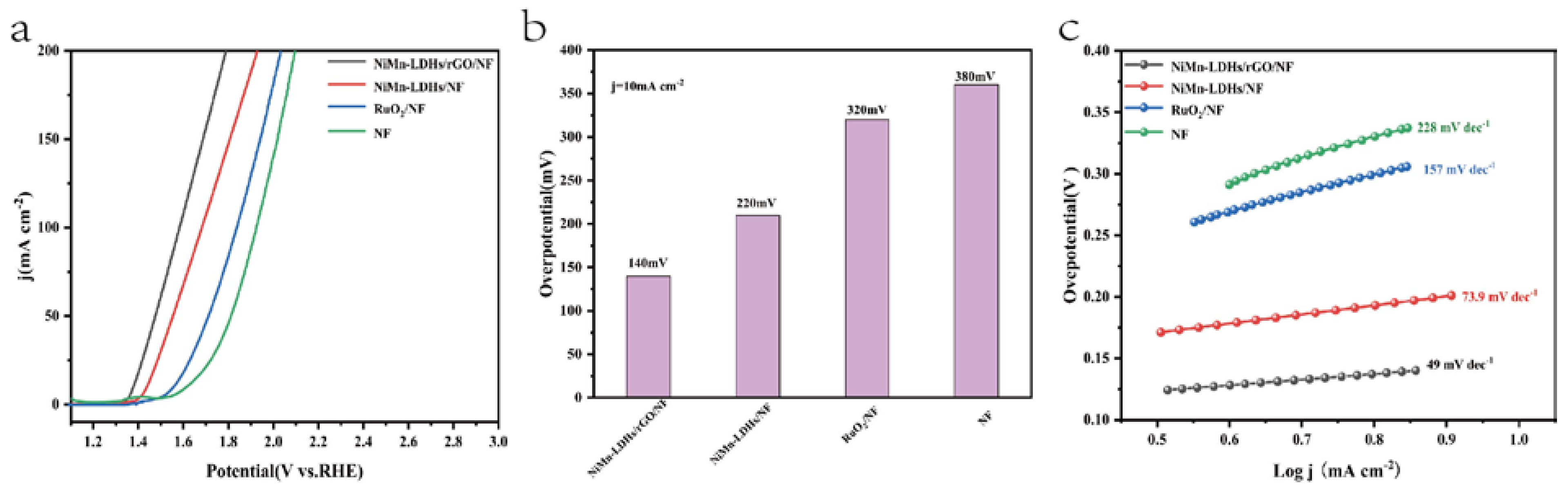
The electrochemical performance of the NiMn-LDHs/rGO/NF composite electrode along with those of NiMn-LDHs/NF, RuO
2/NF and NF for OER was evaluated in a typical three-electrode system using Hg/HgO and Pt wire as the reference and counter electrodes, respectively, in 1 M aqueous KOH solution. Shown in
Figure 5a are the iR-corrected linear sweep voltammetry (LSV) curves at a scan rate of 5 mV s
−1 in the potential range of 1.2−3.0 V (vs. RHE). It is notable that the NiMn-LDHs/rGO/NF electrode exhibits the highest polarization current, which is 1.37 V vs. RHE for achieving a current density of 10 mA cm
−2. Moreover, it can be seen from
Figure 5b that an overpotential as low as 0.14 V is enough for the NiMn-LDHs/rGO/NF electrode to operate at 10 mAcm
-2 current density, which is lower than those of NiMn-LDHs/NF (0.22 V), RuO
2/NF (0.32 V) and NF (0.38 V) electrodes, evidencing the quite good catalytic efficiency of the NiMn-LDHs/rGO/NF electrode owing to the synergistic effect induced by the direct and interfacial contact between ultrathin NiMn-LDH nanosheets and partly reduced rGO [
24].
The Tafel slope represents the reaction kinetics, which is another critical factor for evaluating the performance of a catalyst. As presented in
Figure 5c, the Tafel slopes of the NiMn-LDHs/rGO/NF, NiMn-LDHs/NF, RuO
2/NF and NF electrodes are 49, 73.9, 157 and 228 mV dec
−1, respectively, implying the improved catalytic activity, i.e., the fast reaction kinetics, through the hetero-assembly of NiMn-LDH nanosheets and rGO. On the one hand, rGO can prevent NiMn-LDHs nanoflakes from agglomeration, offer more active sites and accelerate electron migration/transfer for the catalysis. On the other hand, the ultrathin NiMn-LDH sheets also could reduce the graphitization degree of rGO, leading to the increase in the unsaturated sites and in the catalytic activity of the NiMn-LDHs/rGO composite catalyst. More importantly, the charge transfer from Mn and Ni ions of NiMn-LDHs to rGO leads to the increase in the valance state of Mn and Ni ions and the enlargement of charge distribution in rGO, rendering the NiMn-LDHs/rGO composite catalyst to be of the elevated conductivity, the synergistic coupling effect and accordingly the improved electrochemical performance. It should be pointed out that of NiMn-LDHs/rGO/NF composite electrocatalyst outperforms not only RuO
2/NF but also most of the Mn-based and the Ni–Fe containing bimetallic OER catalysts reported in the literature [
8,
9].
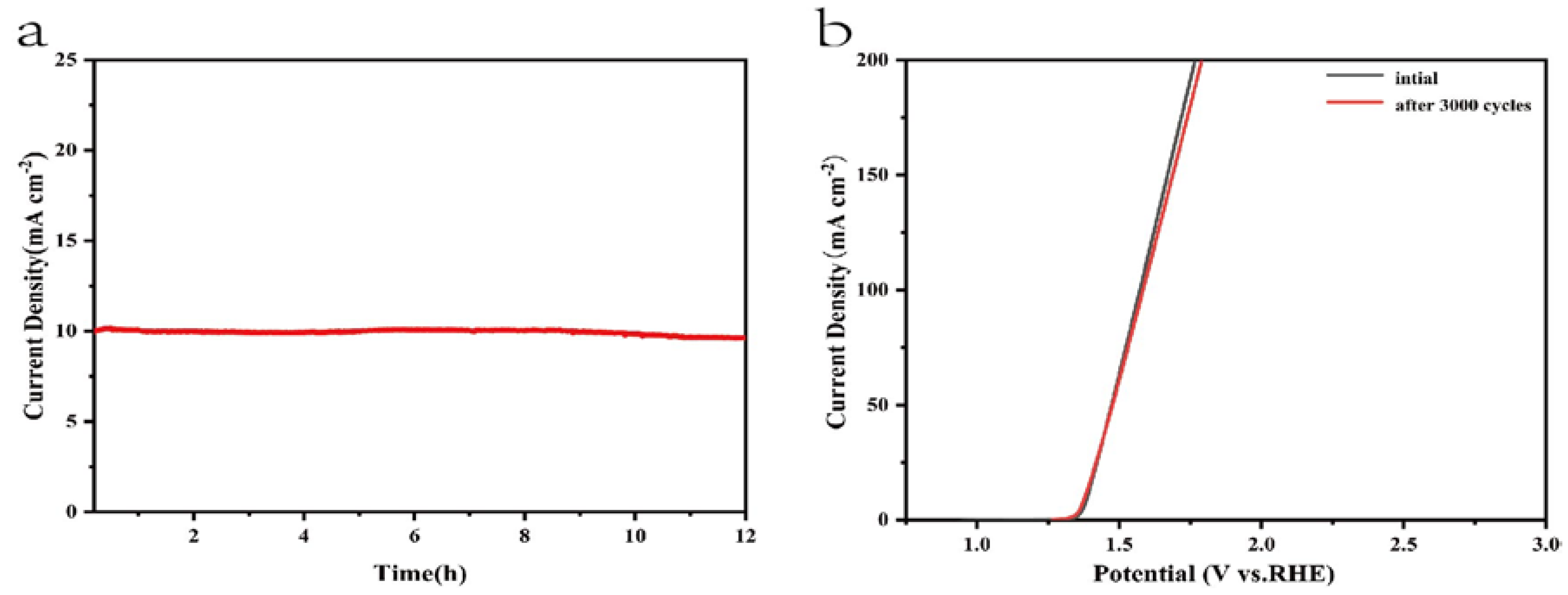
Moreover, the electrochemical stability is another criterion to evaluate the OER catalysts. A chronopotentiometry test under the current density of 10 mA cm
-2 was conducted to estimate the stability and durability of the composite electrocatalysts. As shown in
Figure 6a, the current density of the NiMn-LDHs/rGO/NF electrode was kept nearly constant for 12 h. From
Figure 6b it can be seen that no obvious change is recorded for the original NiMn-LDHs/rGO/NF electrode and that after 3000 CV cycles under the current density of 10 mA cm
−2, indicating that the NiMn-LDHs/rGO/NF composite catalyst has excellent stability and durability in alkaline solution. Such improvement may be a result of the high mechanical strength and electrochemical stability of the NiMn-LDHs/rGO/NF electrode.
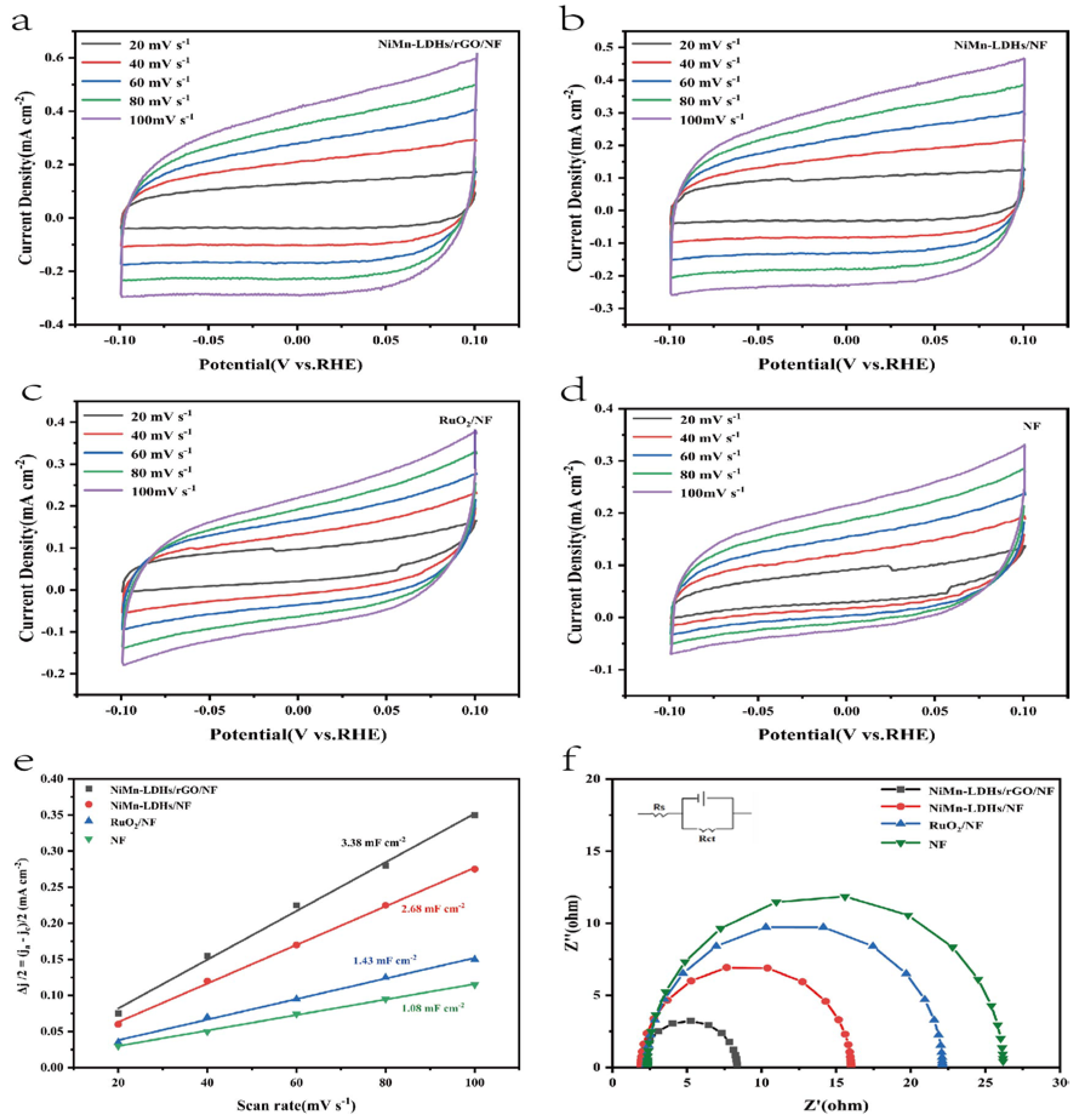
The electrochemical active surface area (ECSA) of the investigated catalysts were estimated via a simple CV scanning (see
Figure 7a-d) for better evaluating the number of catalytic active sites. The slope of Δj/2 vs. Scan rate is known to be equal to the value of Cdl (Cdl is the double layer capacity), which has a linear relationship with ECSA [
14,
15]. From the fitted lines shown in
Figure 7e, it is clear that the Cdl of the investigated catalysts increases gradually in the following order NF <RuO
2 (1.43 mF cm
-2)/NF<NiMn-LDHs/NF (2.68 mF cm
-2) < NiMn-LDHs/rGO/NF (3.38 mF cm
-2). Because of its largest value of ECSA, the NiMn-LDHs/rGO/NF composite catalyst should be of the largest number of active sites, which is one of the crucial factors that determines its excellent OER performance.
Since the Tafel slope obtained directly from the LSV curve is affected by the solution impedance and the material internal resistance, it reflects the overall OER kinetics of the catalyst, but cannot be used to analyze the OER mechanism of the catalyst. Thus, EIS was applied to obtain information on charge transfer between the electrolyte and the electrode surface to elucidate the kinetic differences of the catalysts investigated in this work. From the EIS spectra shown in
Figure 7f, it can be seen that the NiMn-LDHs/rGO/NF composite electrode exhibits the smallest diameter among the Nyquist semicircles, which indicates that the electrode has the lowest charge transfer resistance (Rct). Since Rct influences the conductivity of the electrode and thus electron transfer in the electrochemical reaction process, it is expected that the NiMn-LDHs/rGO/NF electrode also has the quickest charge-transfer rate during reaction process and hence the highest electrochemical activity among the investigated electrodes. Moreover, the catalysts which possessed a layered atomic structure demonstrate a greatly decreased charge transfer resistance relative to RuO
2/NF, suggesting that the atomic structure greatly influences the electronic conductivity of the catalysts.