Submitted:
03 May 2023
Posted:
04 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
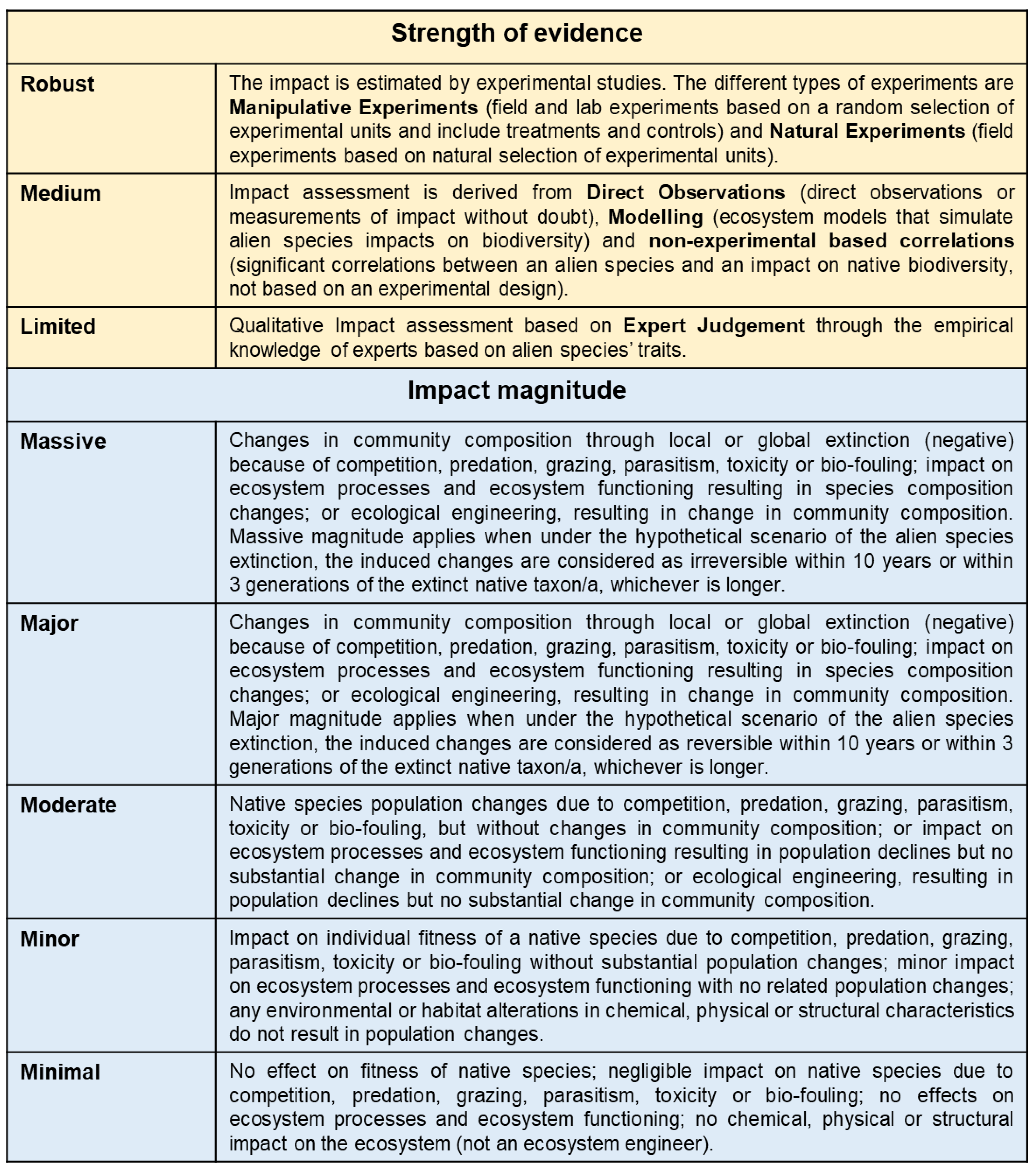
2. Materials and Methods
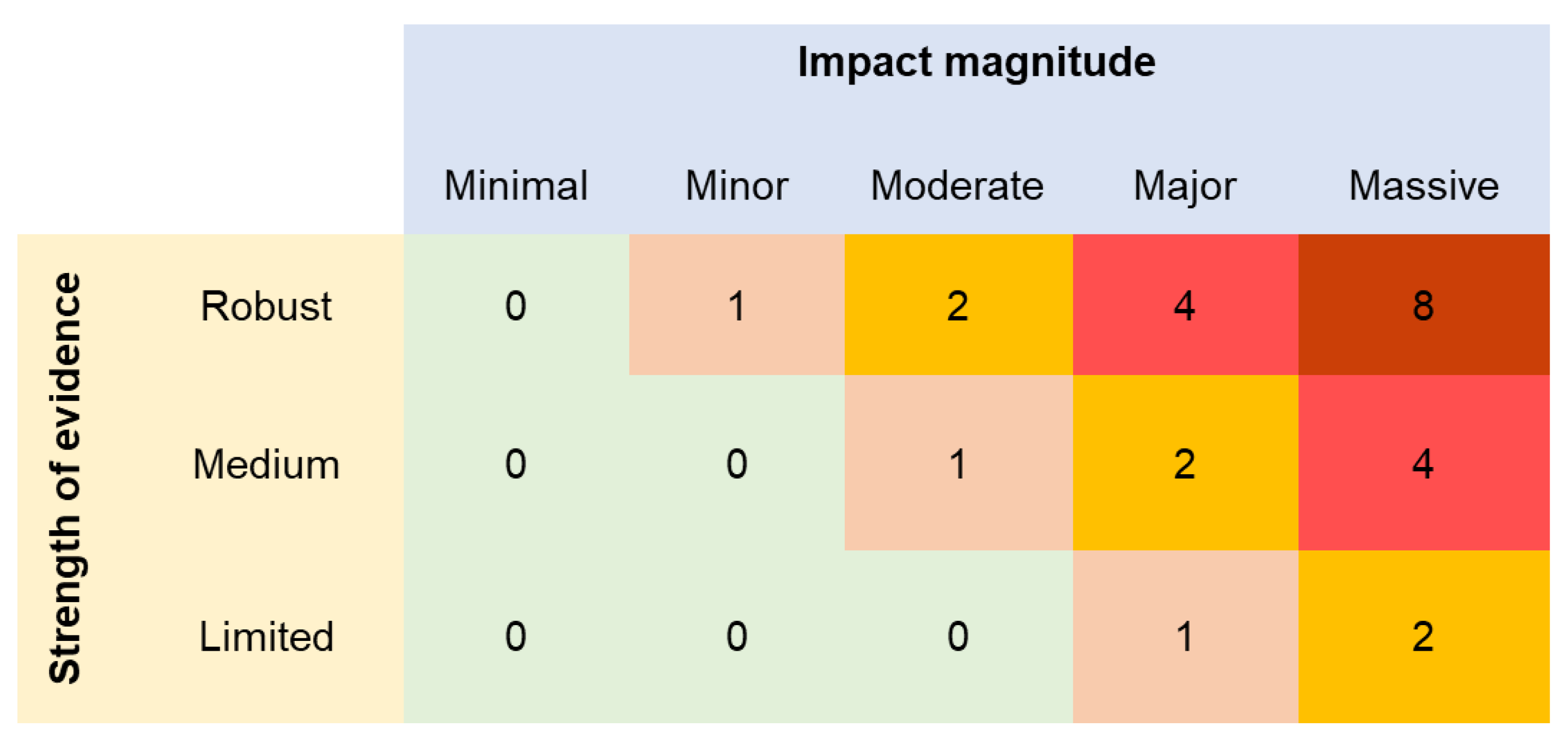
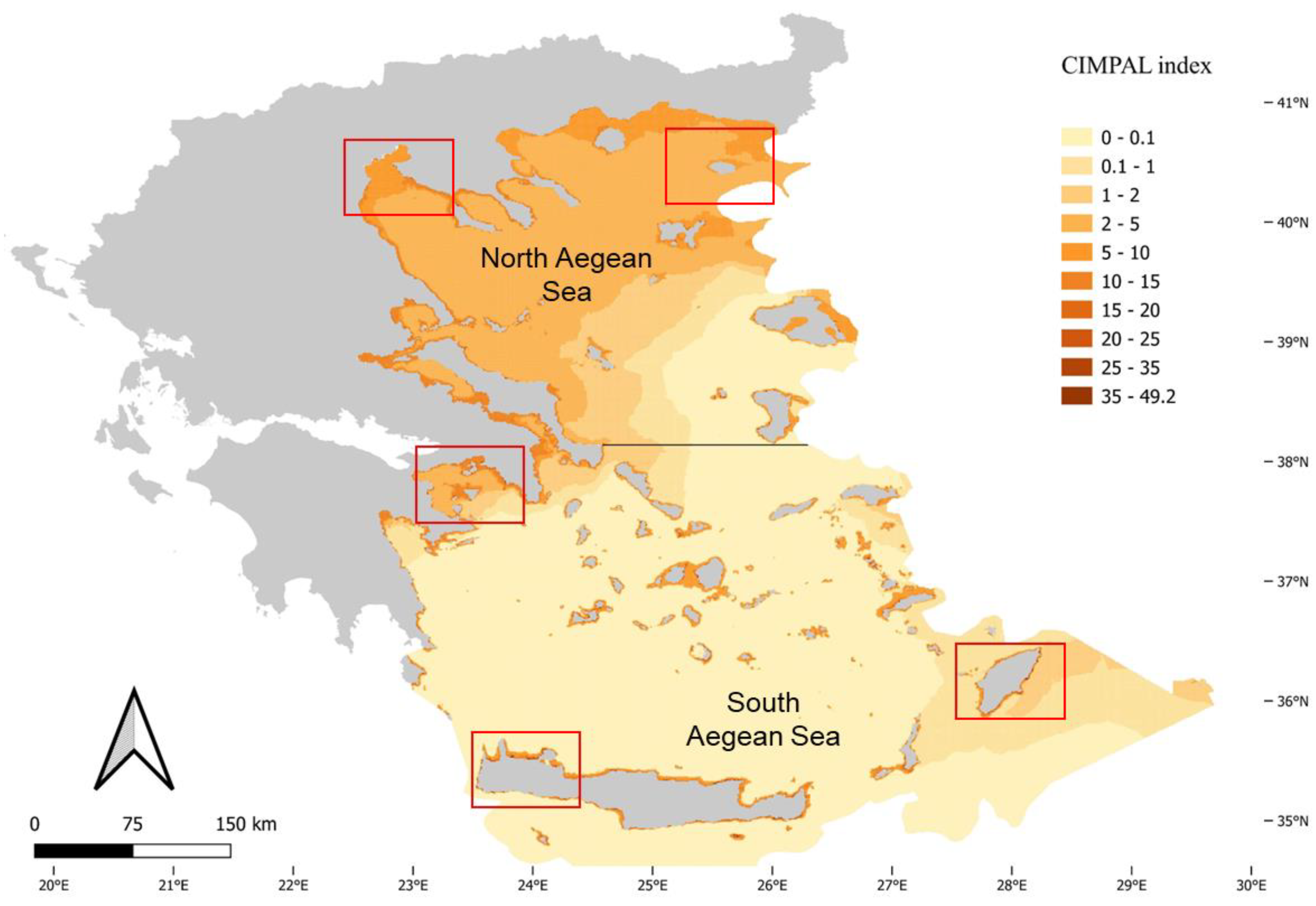
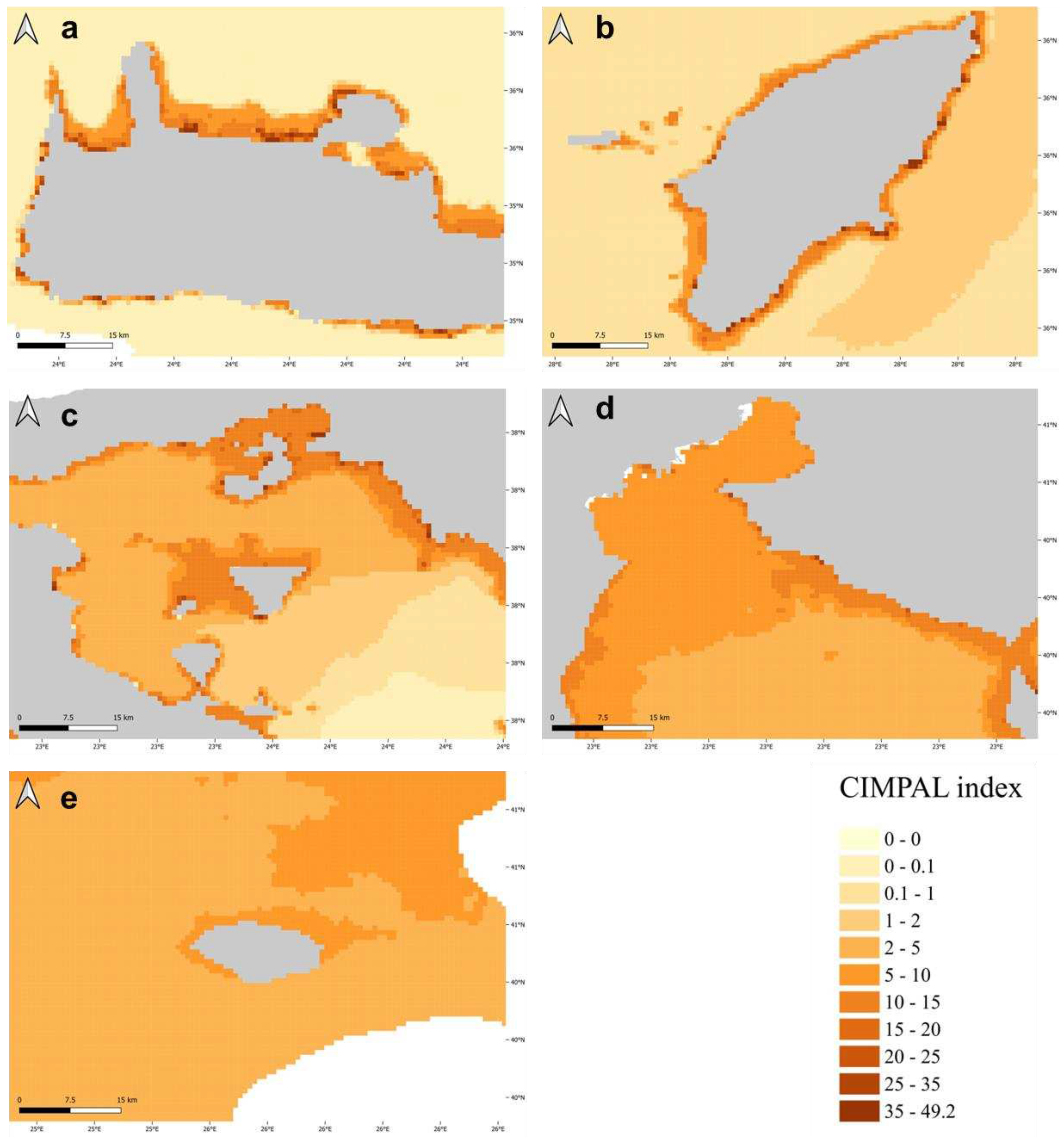
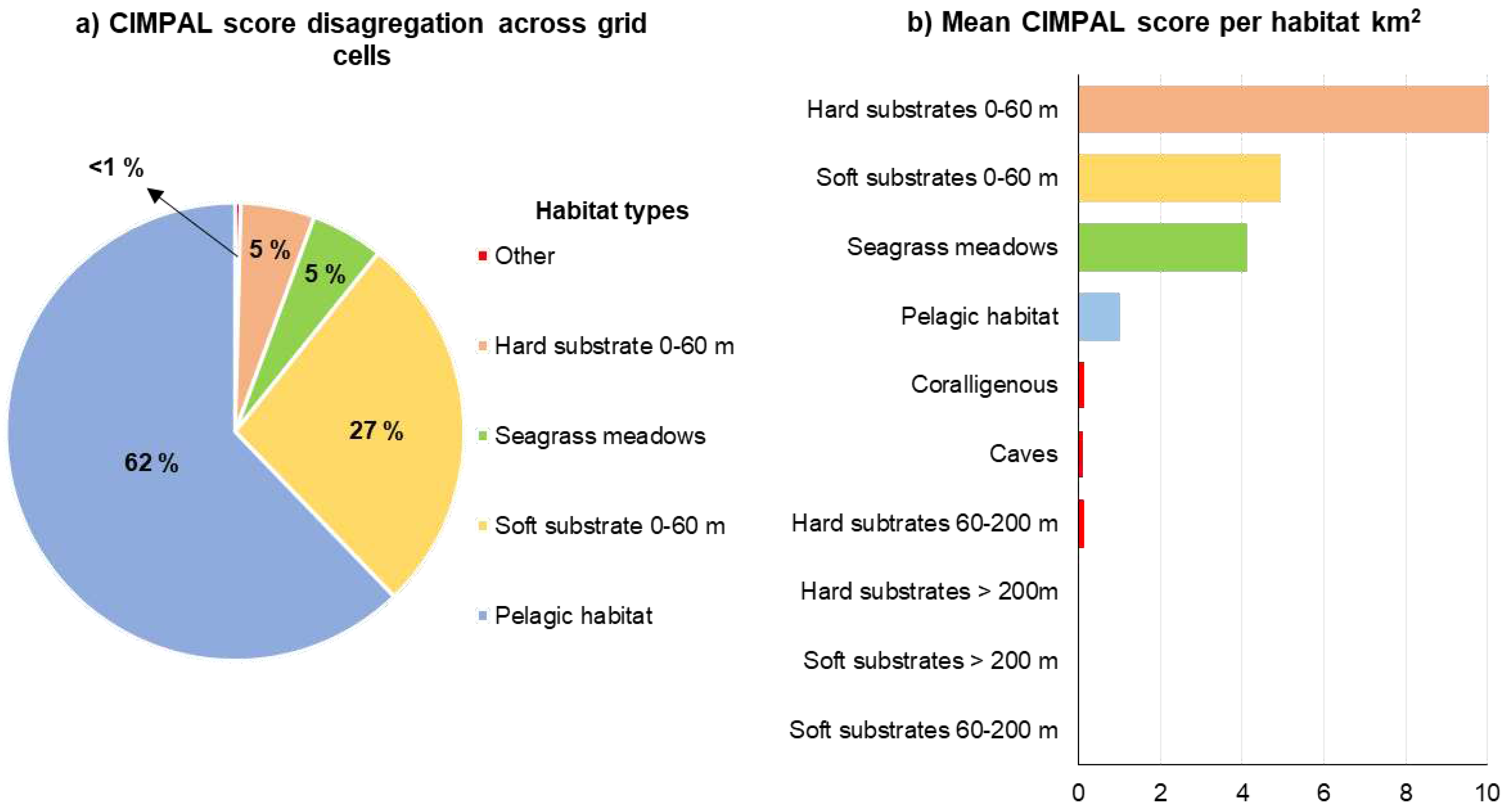
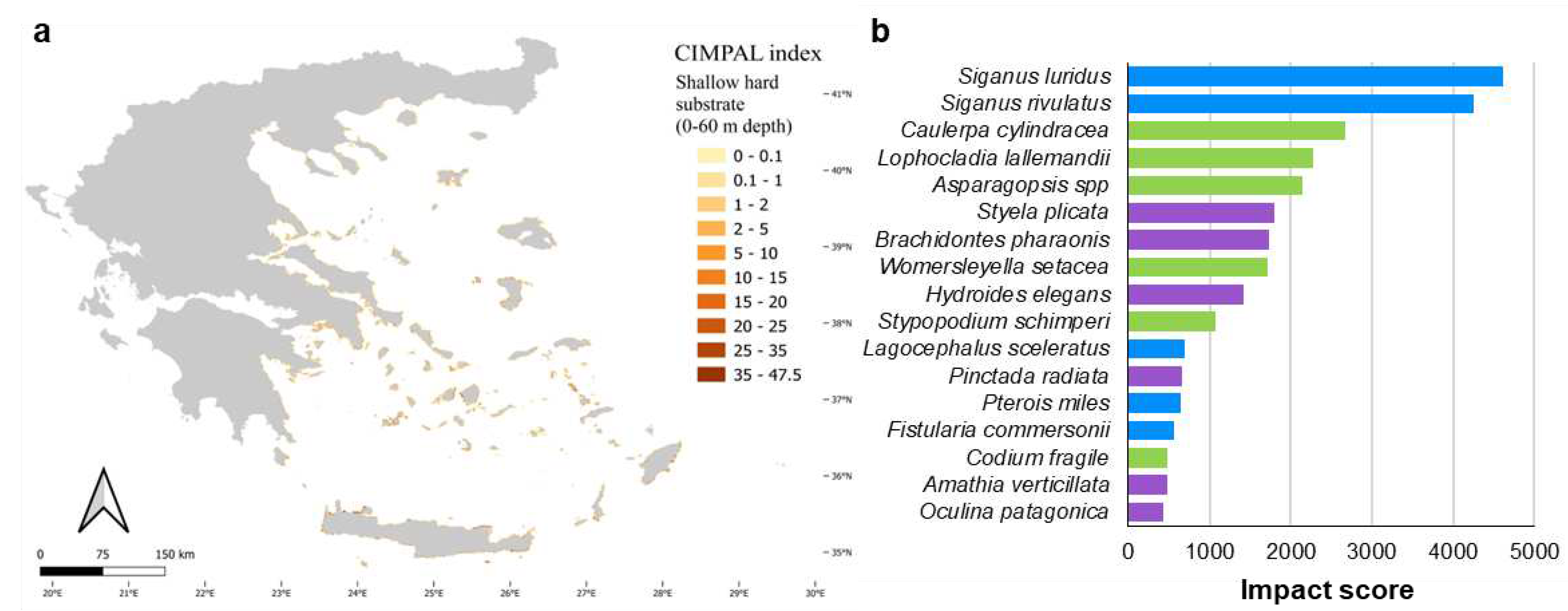
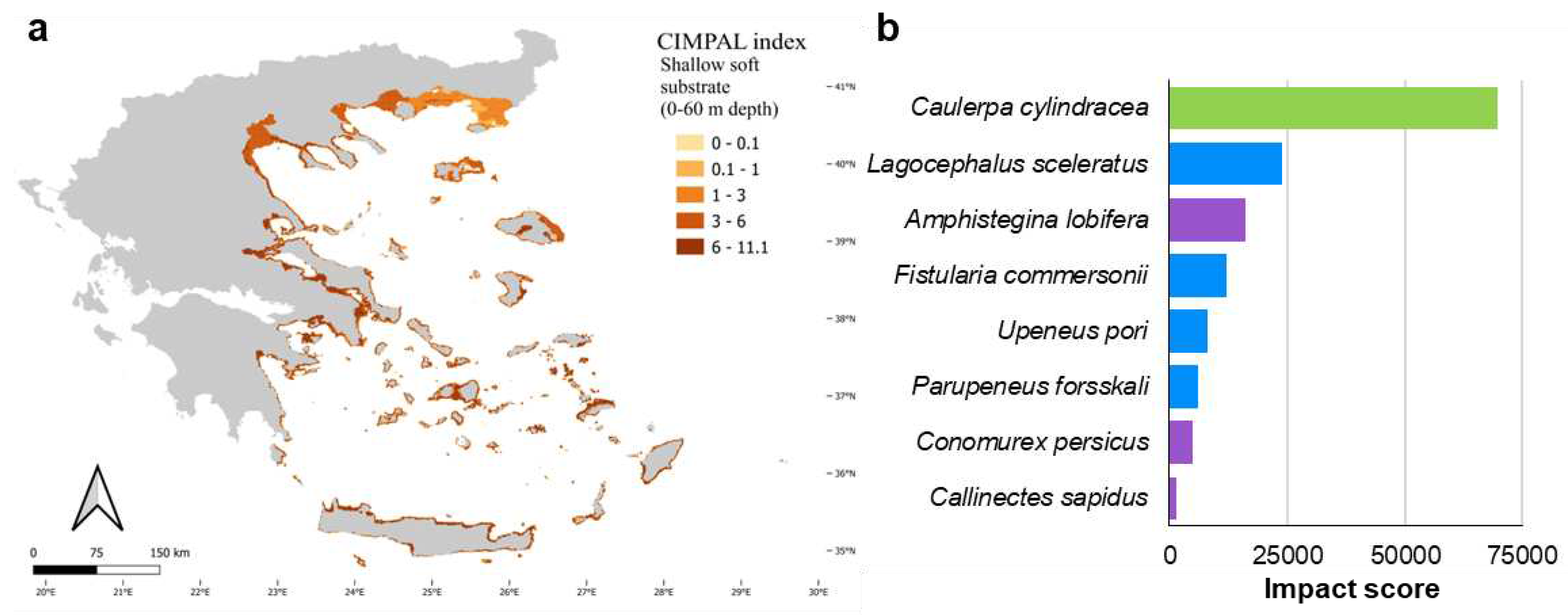
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Solomon, S.; Plattner, G.K.; Knutti, R.; Friedlingstein, P. Irreversible climate change due to carbon dioxide emissions. PNAS 2009, 106, 1704–1709. [Google Scholar] [CrossRef] [PubMed]
- Canfield, D.E.; Glazer, A.N.; Falkowski, P.G. The evolution and future of Earth’s nitrogen cycle. Science 2010, 330, 192–196. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Summary for Policymakers. In: IPCC Special Report on the Ocean and Cryosphere in a Changing Climate; Pörtner, H.O., Roberts, D.C. Eds.; Cambridge University Press, Cambridge, UK and New York, NY, USA, 2019; pp. 3–35. [CrossRef]
- Murphy, G.E.; Romanuk, T.N. A meta-analysis of declines in local species richness from human disturbances. Ecol. Evol. 2014, 4, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Pyšek, P.; Hulme, P.E.; Simberloff, D.; Bacher, S.; Blackburn, T.M.; Carlton, J.T.; Dawson, W.; Essl, F.; Foxcroft, L.C.; Genovesi, P.; et al. Scientists’ warning on invasive alien species. Biol. Rev. 2020, 95, 1511–1534. [Google Scholar] [CrossRef]
- Seebens, H.; Blackburn, T.M.; Dyer, E.E.; Genovesi, P.; Hulme, P.E.; Jeschke, J.M.; Pagad, S.; Pyšek, P.; Winter, M.; Arianoutsou, M.; et al. No saturation in the accumulation of alien species worldwide. Nat. Commun. 2017, 8, 14435. [Google Scholar] [CrossRef]
- Seebens, H.; Bacher, S.; Blackburn, T.M.; Capinha, C.; Dawson, W.; Dullinger, S.; Genovesi, P.; Hulme, P.E.; Kleunen, M.; Kühn, I.; et al. Projecting the continental accumulation of alien species through to 2050. Global Change Biol. 2021, 27, 970–982. [Google Scholar] [CrossRef]
- Essl, F.; Lenzner, B.; Bacher, S.; Bailey, S.; Capinha, C.; Daehler, C.; Dullinger, S.; Genovesi, P.; Hui, C.; Hulme, P.E; et al. Drivers of future alien species impacts: An expert-based assessment. Global Change Biol. 2020, 26, 4880–4893. [Google Scholar] [CrossRef]
- Lewis, S.L.; Maslin, M.A. Defining the Anthropocene. Nature 2015, 519, 171–180. [Google Scholar] [CrossRef]
- Simberloff, D.; Martin, J.L.; Genovesi, P.; Maris, V.; Wardle, D.A.; Aronson, J.; Courchamp, F.; Galil, B.; García-Berthou, E.; Pascal, M.; Pyšek, P.; Sousa, R.; Tabacchi, E.; Vilà, M. Impacts of biological invasions: what’s what and the way forward. Trends Ecol. Evol. 2013, 28, 58–66. [Google Scholar] [CrossRef]
- Blackburn, T.M.; Essl, F.; Evans, T.; Hulme, P.E.; Jeschke, J.M.; Kühn, I.; Kumschick, S.; Marková, Z.; Mrugała, A.; Nentwig, W. et al. A Unified Classification of Alien Species Based on the Magnitude of their Environmental Impacts. PLoS Biol. 2014, 12, e1001850. [Google Scholar] [CrossRef]
- Tsirintanis, K.; Azzurro, E.; Crocetta, F.; Dimiza, M.; Froglia, C.; Gerovasileiou, V.; Langeneck, J.; Mancinelli, G.; Rosso, A.; Stern, N.; et al. Bioinvasion impacts on biodiversity, ecosystem services, and human health in the Mediterranean Sea. Aquat. Invasions 2022, 17, 308–352. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Tempera, F.; Teixeira, H. Mapping the impact of alien species on marine ecosystems: the Mediterranean Sea case study. Divers. Distrib. 2016, 22, 694–707. [Google Scholar] [CrossRef]
- Strayer, D.L.; Eviner, V.T.; Jeschke, J.M.; Pace, M.L. Understanding the long-term effects of species invasions. Trends Ecol. Evol. 2006, 21, 645–651. [Google Scholar] [CrossRef]
- Vergés, A.; Tomas, F.; Cebrian, E.; Ballesteros, E.; Kizilkaya, Z.; Dendrinos, P.; Karamanlidis, A.A.; Spiegel, D.; Sala, E. Tropical rabbitfish and the deforestation of a warming temperate sea. J. Ecol. 2014, 102, 1518–1527. [Google Scholar] [CrossRef]
- Hulme, P.E. Climate change and biological invasions: evidence, expectations, and response options. Biol. Rev. 2017, 92, 1297–1313. [Google Scholar] [CrossRef]
- Gissi, E.; Manea, E.; Mazaris, A.D.; Fraschetti, S.; Almpanidou, V.; Bevilacqua, S.; Coll, M.; Guarnieri, G.; Lloret-Lloret, E.; Pascual, M.; et al. A review of the combined effects of climate change and other human stressors on the marine environment. Sci Total Environ. 2021, 755, 142564. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Moustakas, A. Uncertainty in Marine Invasion Science. Front. Mar. Sci. 2018, 5, 38. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Wallentinus, I.; Zenetos, A.; Leppäkoski, E.; Çinar, M.E.; Oztürk, B.; Grabowski, M.; Golani, D.; Cardoso, A.C. Impacts of invasive alien marine species on ecosystem services and biodiversity: a pan-European review. Aquat. Invasions 2014, 9, 391–423. [Google Scholar] [CrossRef]
- Halpern, B.S.; Walbridge, S.; Selkoe, K.A.; Kappel, C.V.; Micheli, F.; D’Agrosa, C.; Bruno, J.F.; Casey, K.S.; Ebert, C.; Fox, H.E.; et al. A global map of human impact on marine ecosystems. Science 2008, 319, 948–952. [Google Scholar] [CrossRef]
- Halpern, B.; Frazier, M.; Potapenko, J.; Casey, K.S.; Koenig, K.; Longo, C.; Lowndes, J.S.; Rockwood, R.S.; Selig, E.R.; Selkoe, K.A.; et al. Spatial and temporal changes in cumulative human impacts on the world’s ocean. Nat. Commun. 2015, 6, 7615. [Google Scholar] [CrossRef] [PubMed]
- Ban, N.C.; Alidina, H.M.; Ardron, J.A. Cumulative impact mapping: advances, relevance and limitations to marine management and conservation, using Canada's Pacific waters as a case study. Mar. Policy 2010, 34, 876–886. [Google Scholar] [CrossRef]
- Halpern, B.S.; McLeod, K.L.; Rosenberg, A.A.; Crowder, L.B. Managing for cumulative impacts in ecosystem- based management through ocean zoning. Ocean Coast. Manag. 2008, 51, 203–211. [Google Scholar] [CrossRef]
- Halpern, B.S.; Fujita, R. Assumptions, challenges, and future directions in cumulative impact analysis. Ecosphere 2013, 4, 131. [Google Scholar] [CrossRef]
- Micheli, F.; Halpern, B.S.; Walbridge, S.; Ciriaco, S.; Ferretti, F.; Fraschetti, S.; Lewison, R.; Nykjaer, L.; Rosenberg, A.A. Cumulative human impacts on Mediterranean and Black Sea marine ecosystems: assessing current pressures and opportunities. PLoS ONE 2013, 8, e79889. [Google Scholar] [CrossRef]
- Costello, M.J.; Dekeyzer, D.; Galil, B.S.; Hutchings, P.; Katsanevakis, S.; Pagad, S.; Robinson, T.B.; Turon, X.; Vandepitte, L.; Vanhoorne, B.; et al. Introducing the World Register of Introduced Marine Species (WRiMS). Manag. Biol. Invasions 2021, 12, 792–811. [Google Scholar] [CrossRef]
- Zenetos, A.; Albano, P.G.; López Garcia, E.; Stern, N.; Tsiamis, K.; Galanidi, M. Established non-indigenous species increased by 40% in 11 years in the Mediterranean Sea. Mediterr. Mar. Sci. 2022, 23, 196–212. [Google Scholar] [CrossRef]
- Zenetos, A.; Albano, P.G.; López Garcia, E.; Stern, N.; Tsiamis, K.; Galanidi, M. Corrigendum to the Review Article (Medit. Mar. Sci. 23/1 2022, 196-212). Mediterr. Mar. Sci. 2022, 23, 876–878. [Google Scholar] [CrossRef]
- Galanidi, M.; Zenetos, A. Data-Driven Recommendations for Establishing Threshold Values for the NIS Trend Indicator in the Mediterranean Sea. Diversity 2022, 14, 57. [Google Scholar] [CrossRef]
- Gerovasileiou, V.; Bancila, R.I.; Katsanevakis, S.; Zenetos, A. Introduced species in Mediterranean marine caves: an increasing but neglected threat. Mediterr. Mar. Sci. 2022, 23, 995–1005. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Coll, M.; Piroddi, C.; Steenbeek, J.; Ben Rais Lasram, F.; Zenetos, A.; Cardoso, A.C. Invading the Mediterranean Sea: biodiversity patterns shaped by human activities. Front. Mar. Sci. 2014, 1, 32. [Google Scholar] [CrossRef]
- Rilov, G.; Peleg, O.; Yeruham, E.; Garval, T.; Vichik, A.; Raveh, O. Alien turf: Overfishing, overgrazing and invader domination in south-eastern Levant reef ecosystems. Aquat. Conserv. Mar. Freshwater Ecosyst. 2018, 28, 351–369. [Google Scholar] [CrossRef]
- Yeruham, E.; Shpigel, M.; Abelson, A.; Rilov, G. Ocean warming and tropical invaders erode the performance of a key herbivore. Ecology 2020, 101, e02925. [Google Scholar] [CrossRef]
- Spalding, M.D.; Fox, H.E.; Allen, G.R.; Davidson, N.; Ferdaña, Z.A.; Finlayson, M.; Halpern, B.S.; Jorge, M.A.; Lombana, A.; Lourie, S.A.; et al. Marine ecoregions of the world: A bioregionalization of coastal and shelf areas. BioScience 2007, 57, 573–583. [Google Scholar] [CrossRef]
- Sini, M.; Katsanevakis, S.; Koukourouvli, N.; Gerovasileiou, V.; Dailianis, T.; Buhl-Mortensen, L.; Damalas, D.; Dendrinos, P.; Dimas, X.; Frantzis, A.; et al. Assembling Ecological Pieces to Reconstruct the Conservation Puzzle of the Aegean Sea. Front. Mar. Sci. 2017, 4, 347. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Zenetos, A.; Corsini-Foka, M.; Tsiamis, K. Biological Invasions in the Aegean Sea: Temporal Trends, Pathways, and Impacts. In The Handbook of Environmental Chemistry. Anagnostou, C.L., Kostianoy, A.G., Eds.; Publisher: Springer, Berlin, Heidelberg, Germany, 2020; pp. 1–34. [Google Scholar] [CrossRef]
- Ragkousis, M.; Sini, M.; Koukourouvli, N.; Zenetos, A.; Katsanevakis, S. Invading the Greek Seas: Spatiotemporal Patterns of Marine Impactful Alien and Cryptogenic Species. Diversity 2023, 15, 353. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Tsirintanis, K.; Sini, M.; Gerovasileiou, V.; Koukourouvli, N. Aliens in the Aegean - A sea under siege (ALAS). RIO 2020, 6, e53057. [Google Scholar] [CrossRef]
- Pancucci-Papadopoulou, M.; Zenetos, A.; Corsini-Foka, M.; Politou, C. Update of marine alien species in Hellenic waters. Mediterr. Mar. Sci. 2005, 6, 147–158. [Google Scholar] [CrossRef]
- Zenetos, A.; Corsini-Foka, M.; Crocetta, F.; Gerovasileiou, V.; Karachle, V.; Simboura, M.; Tsiamis, K.; Pancucci-Papadopoulou, M. Deep cleaning of alien and cryptogenic species records in the Greek Seas (2018 update). Manag. Biol. Inv. 2018, 9, 209–226. [Google Scholar] [CrossRef]
- Zenetos, A.; Arianoutsou, M.; Bazos, I.; Balopoulou, S.; Corsini-Foka, M.; Dimiza, M.; Drakopoulou, P.; Katsanevakis, S.; Kondylatos, G.; Koutsikos, N.; Kytinou, E. ELNAIS: a collaborative network on aquatic alien species in Hellas (Greece). Manag. Biol. Inv. 2015, 6, 185–196. [Google Scholar] [CrossRef]
- Karachle, P.; Corsini Foka, M.; Crocetta, F.; Dulcic, J.; Dzhembekova, N.; Galanidi, M.; Ivanova, P.; Shenkar, N.; Skolka, M.; Stefanova, E.; et al. Setting-up a billboard of marine invasive species in the ESENIAS area: current situation and future expectancies. Acta Adriat. 2017, 58, 429–458. [Google Scholar] [CrossRef]
- Giovos, I.; Kleitou, P.; Poursanidis, D.; Batjakas, I.; Bernardi, G.; Crocetta, F.; Doumpas, N.; Kalogirou, S.; Kampouris, T.E.; Keramidas, I.; et al. Citizen-science for monitoring marine invasions and stimulating public engagement: a case project from the eastern Mediterranean. Biol. Invasions 2019, 21, 3707–3721. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Poursanidis, D.; Hoffman, R.; Rizgalla, J.; Rothman, S.B.-S.; Levitt-Barmats, Y.; Hadjioannou, L.; Trkov, D.; Garmendia, J.M.; Rizzo, M.; et al. Unpublished Mediterranean records of marine alien and cryptogenic species. Bioinvasions Rec. 2020, 9, 165–182. [Google Scholar] [CrossRef]
- Ragkousis, M.; Zenetos, A.; Ben Souissi, J.; Hoffman, R.; Ghanem, R.; Taşkın, E.; Muresan, M.; Karpova, E.; Slynko, E.; Dağlı, E.; et al. Unpublished Mediterranean and Black Sea records of marine alien, cryptogenic, and neonative species. BioInvasions Rec. 2023, in press. [Google Scholar] [CrossRef]
- Zenetos, A.; Karachle, P.K.; Corsini-Foka, M.; Gerovasileiou, V.; Simboura, N.; Xentidis, N. J.; Tsiamis, K. Is the trend in new introductions of marine non-indigenous species a reliable criterion for assessing good environmental status? Τhe case study of Greece. Mediterr. Mar. Sci. 2020, 21, 775–793. [Google Scholar] [CrossRef]
- Topouzelis, K.; Makri, D.; Stoupas, N.; Papakonstantinou, A.; Katsanevakis, S. Seagrass mapping in Greek territorial waters using Landsat-8 satellite images. Int. J. Appl. Earth Obs. Geoinf. 2018, 67, 98–113. [Google Scholar] [CrossRef]
- Ragkousis, Μ.; Papazekou, M.; Sini, M.; Zenetos, A.; Mazaris, A.D.; Katsanevakis, S. Modelling the distribution of impactful alien and cryptogenic species in the Aegean Sea. Mediterr. Mar. Sci. 2023, to be submitted.
- Yemshanov, D.; Koch, F.H.; Ducey, M.; Koehler, K. Mapping ecological risks with a portfolio-based technique: incorporating uncertainty and decision-making preferences. Divers. Distrib. 2013, 19, 567–579. [Google Scholar] [CrossRef]
- Volery, L.; Blackburn, T.M.; Bertolino, S.; Evans, T.; Genovesi, P.; Kumschick, S.; Roy, H.E.; Smith, K.G.; Bacher, S. Improving the Environmental Impact Classification for Alien Taxa (EICAT): a summary of revisions to the framework and guidelines. In Frameworks used in Invasion Science. Wilson, J.R., Bacher, S., Eds.; NeoBiota 2020, 62, 547–567. [Google Scholar] [CrossRef]
- Coll, M.; Piroddi, C.; Steenbeek, J.; Kaschner, K.; Ben Rias Lasram, F.; Aguzzi, J.; Ballesteros, E.; Bianchi, C.N.; Corbera, J.; Dailianis, T.; et al. The Biodiversity of the Mediterranean Sea: estimates, patterns, and threats. PLoS ONE 2010, 5, e11842. [Google Scholar] [CrossRef]
- Siapatis, A.; Giannoulaki, M.; Valavanis, V.D.; Palialexis, A.; Schismenou, E.; Machias, A.; Somarakis, S. Modelling potential habitat of the invasive ctenophore Mnemiopsis leidyi in Aegean Sea. In Essential Fish Habitat Mapping in the Mediterranean. Developments in Hydrobiology. Valavanis, V.D. Eds.;, Publisher: Springer, Dordrecht, Germany, 2008, 203, pp. 281–295. [CrossRef]
- Ikhtiyar, S.; Durgham, H.; The biochemical composition of two marine gelatin species Marivagia stellata and Mnemiopsis leidyi in coastal waters of Latakia city. Al-Baath University Journal of Medical, Engineering, Basic and Applied Sciences 2020, 42.
- Galil, B.S.; Kress, N.; Shiganova, T.A. First record of Mnemiopsis leidyi A. Agassiz, 1865 (Ctenophora; Lobata; Mnemiidae) off the Mediterranean coast of Israel. Aquat. Invasions 2009, 4, 357–360. [Google Scholar] [CrossRef]
- Gómez, F. Phytoplankton invasions: Comments on the validity of categorizing the nonindigenous dinoflagellates and diatoms in European Seas. Mar. Pollut. Bull. 2008, 56, 620–628. [Google Scholar] [CrossRef]
- Gómez, F. Comments on the non-indigenous microalgae in the European seas. Mar. Pollut. Bull. 2019, 148, 1–2. [Google Scholar] [CrossRef]
- Tsiamis, K.; Azzurro, E.; Bariche, M.; Çinar, M.E.; Crocetta, F.; De Clerck, O.; Galil, B.; Gomez, F.; Hoffman, R.; Jensen, K. Prioritizing marine invasive alien species in the EU through Horizon Scanning. Aquat. Conserv. Mar. Freshwater Ecosyst. 2020, 30, 794–845. [Google Scholar] [CrossRef]
- Karachle, P.; Oikonomou, A.; Pantazi, M.; Stergiou, K.I.; Zenetos, A. Can biological traits serve as predictors for fishes’ introductions, establishment, and interactions? The Mediterranean Sea as a case study. Biology 2022, 11, 1625. [Google Scholar] [CrossRef] [PubMed]
- Androulidakis, Y.S.; Krestenitis, Y.N. Sea Surface Temperature Variability and Marine Heat Waves over the Aegean, Ionian, and Cretan Seas from 2008–2021. J. Mar. Sci. Eng. 2022, 10, 42. [Google Scholar] [CrossRef]
- Nguyen, H.M.; Yadav, N.S.; Barak, S.; Lima, F.P.; Sapir, Y.; Winters, G. Responses of Invasive and Native Populations of the Seagrass Halophila stipulacea to Simulated Climate Change. Front. Mar. Sci. 2020, 6, 812. [Google Scholar] [CrossRef]
- Bernal-Ibáñez, A.; Gestoso, I.; Ramalhosa, P.; Campanati, C.; Cacabelos, E. Interaction of marine heatwaves and grazing on two canopy-forming algae. J. Exp. Mar. Biol. Ecol. 2022, 556, 151795. [Google Scholar] [CrossRef]
- Molnar, J.L.; Gamboa, R.L.; Revenga, C.; Spalding, M.D. Assessing the global threat of invasive species to marine biodiversity. Front. Ecol. Environ 2008, 6, 485–492. [Google Scholar] [CrossRef]
- Costello, K.E.; Lynch, S.A.; McAllen, R.; O'Riordan, R.M.; Culloty, S.C. Assessing the potential for invasive species introductions and secondary spread using vessel movements in maritime ports. Mar. Pollut. Bull. 2022, 177, 113496. [Google Scholar] [CrossRef]
- Tempesti, J.; Mangano, M.C.; Langeneck, J.; Lardicci, C.; Maltagliati, F.; Castelli, A. Nonindigenous species in Mediterranean ports: A knowledge baseline. Mar. Environ. Res. 2020, 161, 105056. [Google Scholar] [CrossRef]
- Zenetos, A.; Gofas, S.; Morri, C.; Rosso, A.; Violanti, D.; García Raso, J.E.; Çinar, M.E.; Almogi Labin, A.; Ates, A.S.; Azzuro, E.; et al. Alien species in the Mediterranean Sea by 2012. A contribution to the application of European Union’s Marine Strategy Framework Directive (MSFD). Part 2. Introduction trends and pathways. Mediterr. Mar. Sci. 2012, 13, 328–352. [Google Scholar] [CrossRef]
- Zenetos, A.; Ovalis, P.; Giakoumi, S.; Kontadakis, C.; Lefkaditou, E.; Mpazios, G.; Simboura, N.; Tsiamis, K. Saronikos Gulf: a hotspot area for alien species in the Mediterranean Sea. BioInvasions Rec. 2020, 9, 873–889. [Google Scholar] [CrossRef]
- Zenetos, A.; Tsiamis, K.; Galanidi, M.; Carvalho, N.; Bartilotti, C.; Canning Clode, J.; Castriota, L.; Chainho, P.; Comas-González, R.; Costa, A.C.; et al. Status and Trends in the Rate of Introduction of Marine Non-Indigenous Species in European Seas. Diversity 2022, 14, 1077. [Google Scholar] [CrossRef]
- Sala, E.; Kizilkaya, Z.; Yildirim, D.; Ballesteros, E. Alien marine fishes deplete algal biomass in the eastern Mediterranean. PLoS ONE 2011, 6, e17356. [Google Scholar] [CrossRef]
- Giakoumi, S. Distribution patterns of the invasive herbivore Siganus luridus (Rüppell, 1829) and its relation to native benthic communities in the central Aegean Sea, Northeastern Mediterranean. Mar. Ecol. 2013, 35, 96–105. [Google Scholar] [CrossRef]
- Vergés, A.; Tomas, F.; Cebrian, E.; Ballesteros, E.; Kizilkaya, Z.; Dendrinos, P.; Karamanlidis, A.A.; Spiegel, D.; Sala, E. Tropical rabbitfish and the deforestation of a warming temperate sea. J. Ecol. 2014, 102, 1518–1527. [Google Scholar] [CrossRef]
- Salomidi, M.; Katsanevakis, S.; Borja, A.; Braeckman, U.; Damalas, D.; Galparsoro, I.; Mifsud, R.; Mirto, S.; Pascual, M.; Pipitone, C.; et al. Assessment of goods and services, vulnerability, and conservation status of European seabed biotopes: a stepping stone towards ecosystem-based marine spatial management. Mediterr. Mar. Sci., 2012, 13, 49–88. [Google Scholar] [CrossRef]
- Kytinou, E.; Issaris, Y.; Sini, M.; Salomidi, M.; Katsanevakis, S. ECOfast - an integrative ecological evaluation index for an ecosystem-based assessment of shallow rocky reefs. J. Environ. Manage. 2023. submitted . [Google Scholar] [CrossRef]
- Savin, A.; Sini, M.; Xynogala, I.; Vougioukalou, V.; Stamatis, K.; Noe, S.; Ragkousis, M.; Gerovasileiou, V.; Dailianis, T.; Katsanevakis, S Assessment of macroalgal communities on shallow rocky reefs in the Aegean Sea indicates an impoverished ecological status. Mediterr. Mar. Sci. 2023, in press.
- Lodola, A. Distribution and abundance of the tropical macroalgae Caulerpa racemosa var. cylindracea (Chlorophyta: Caulerpaceae) and Asparagopsis taxiformis (Rhodophyta: Bonnemaisoniaceae) in the upper infralittoral fringe of Linosa island (Pelagian Islands, Italy). Scientifica Acta 2013, 7, 3–11.
- Cebrian, E.; Tomas, F.; López-Sendino, P.; Vilà, M.; Ballesteros, E. Biodiversity influences invasion success of a facultative epiphytic seaweed in a marine forest. Biol. Invasions 2018, 20, 2839–2848. [Google Scholar] [CrossRef]
- Rueda, J.L.; Gofas, S.; Aguilar, R.; de la Torriente, A.; García Raso, J.E.; Lo Iacono, C.; Luque, Á.A.; Marina, P.; Mateo-Ramírez, Á.; Moya-Urbano, E. et al. Benthic Fauna of Littoral and Deep-Sea Habitats of the Alboran Sea: A Hotspot of Biodiversity. In: Alboran Sea - Ecosystems and Marine Resources. Báez, J.C., Vázquez, J.T., Eds.; Publisher: Springer, Cham, Germany, 2021, pp. 285–358. [CrossRef]
- Bedini, R.; Bedini, M.; Bonechi, L.; Piazzi, L. Effects of non-native turf-forming Rhodophyta on mobile macro-invertebrate assemblages in the north-western Mediterranean Sea. Mar. Biol. Res. 2015, 11, 430–437. [Google Scholar] [CrossRef]
- Mancuso, F.P.; D'Agostaro, R.; Milazzo, M.; Badalamenti, F.; Musco, L.; Mikac, B.; Brutto, S.L.; Chemello, R. The invasive seaweed Asparagopsis taxiformis erodes the habitat structure and biodiversity of native algal forests in the Mediterranean Sea. Mar. Environ. Res. 2022, 173, 105515. [Google Scholar] [CrossRef]
- Pica, D.; Bloecher, N.; Dell’Anno, A.; Bellucci, A.; Pinto, T.; Pola, L.; Puce, S. Dynamics of a biofouling community in finfish aquaculture: a case study from the South Adriatic Sea. Biofouling 2019, 35, 696–709. [Google Scholar] [CrossRef] [PubMed]
- Argyrou, M.; Demetropoulos, A.; Hadjichristophorou, M. Expansion of the macroalga Caulerpa racemosa and changes in softbottom macrofaunal assemblages in Moni Bay, Cyprus. Oceanolog. Acta 1999, 22, 517–528. [Google Scholar] [CrossRef]
- Rizzo, L.; Pusceddu, A.; Stabili, L.; Alifano, P.; Fraschetti, S. Potential effects of an invasive seaweed (Caulerpa cylindracea, Sonder) on sedimentary organic matter and microbial metabolic activities. Sci. Rep. 2017, 7, 12113. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, L.; Pusceddu, A.; Bianchelli, S.; Fraschetti, S. Potentially combined effect of the invasive seaweed Caulerpa cylindracea (Sonder) and sediment deposition rates on organic matter and meiofaunal assemblages. Mar. Environ. Res. 2020, 159, 104966. [Google Scholar] [CrossRef] [PubMed]
- Kalogirou, S. Ecological characteristics of the invasive pufferfish Lagocephalus sceleratus (Gmelin, 1789) in the eastern Mediterranean Sea – a case study from Rhodes. Mediterr. Mar. Sci. 2013, 14, 251–260. [Google Scholar] [CrossRef]
- Kalogirou, S.; Corsini, M.; Kondilatos, G.; Wennhage, H. (2007) Diet of the invasive piscivorous fish Fistularia commersonii in a recently colonized area of the eastern Mediterranean. Biol. Invasions 2007, 9, 887–896. [Google Scholar] [CrossRef]
- Bariche, M.; Alwan, N.; El-Assi, H.; Zurayk, R. Diet composition of the Lessepsian bluespotted cornetfish Fistularia commersonii in the eastern Mediterranean. J. Appl. Ichthyol. 2009, 25, 460–465. [Google Scholar] [CrossRef]
- Pancucci-Papadopoulou, M.A.; Raitsos, D.E.; Corsini-Foka, M. Biological invasions and climatic warming: implications for south-eastern Aegean ecosystem functioning. J. Mar. Biol. Assoc. U. K. 2012, 92, 777–789. [Google Scholar] [CrossRef]
- Saygu, İ.; Heymans, J.J.; Fox, C.J.; Özbilgin, H.; Eryaşar, A.R.; Gökçe, G. The importance of alien species to the food web and bottom trawl fisheries of the Northeastern Mediterranean, a modelling approach. J. Mar. Syst. 2020, 202, 103253. [Google Scholar] [CrossRef]
- Caruso, A.; Cosentino, C. The first colonization of the Genus Amphistegina and other exotic benthic foraminifera of the Pelagian Islands and south-eastern Sicily (central Mediterranean Sea). Mar. Micropaleontol. 2014, 111, 38–52. [Google Scholar] [CrossRef]
- Mouanga, G.H. Impact and Range Extension of Invasive Foraminifera in the NW Mediterranean Sea. PhD Thesis, University of Bonn, Germany, 2018. [Google Scholar]
- Streftaris, N.; Zenetos, A. Alien Marine Species in the Mediterranean - the 100 ‘Worst Invasives’ and their Impact. Mediterr. Mar. Sci. 2006, 7, 87–118. [Google Scholar] [CrossRef]
- Piazzi, L.; Cinelli, F. Distribution and dominance of two introduced turf-forming macroalgae on the coast of Tuscany, Italy, Northwestern Mediterranean sea in relation to different habitats and sedimentation. Bot. Mar. 2001, 44, 509–520. [Google Scholar] [CrossRef]
- Ballesteros, E.; Cebrian, E.; Alcoverro, T. Mortality of shoots of Posidonia oceanica following meadow invasion by the red alga Lophocladia lallemandii. Bot. Mar. 2007, 50, 8–13. [Google Scholar] [CrossRef]
- Sureda, A.; Box, A.; Terrados, J.; Deudero, S.; Pons, A. Antioxidant response of the seagrass Posidonia oceanica when epiphytized by the invasive macroalgae Lophocladia lallemandii. Mar. Environ. Res. 2008, 66, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Piazzi, L.; Cinelli, F. ; Effets de l’expansion des Rhodophyceae introduites Acrothamnion preissii et Womersleyella setacea sur les communautés algales des rhizomes de Posidonia oceanica de Méditerranée occidentale. Cryptogamie Algol. 2000, 21, 291–300. [Google Scholar] [CrossRef]
- Deudero, S.; Blanco, A.; Box, A.; Mateu-Vicens, G.; Cabanellas-Reboredo, M.; Sureda, A. Interaction between the invasive macroalga Lophocladia lallemandii and the bryozoan Reteporella grimaldii at seagrass meadows: density and physiological responses. Biol. Invasions 2010, 12, 41–52. [Google Scholar] [CrossRef]
- Marchessaux, G.; Faure, V.; Chevalier, C.; Thibault, D. Refugia area for the ctenophore Mnemiopsis leidyi A. Agassiz 1865 in the Berre Lagoon (southeast France): The key to its persistence. Reg. Stud. Mar. Sci. 2020, 39, 101409. [Google Scholar] [CrossRef]
- Marchessaux, G.; Belloni, B.; Gadreaud, J.; Thibault, D. Predation assessment of the invasive ctenophore Mnemiopsis leidyi in a French Mediterranean lagoon. J. Plankton Res. 2021, 43, 161–179. [Google Scholar] [CrossRef]
- Fiori, E.; Benzi, M.; Ferrari, C.R.; Mazziotti, C. Zooplankton community structure before and after Mnemiopsis leidyi arrival. J. Plankton Res. 2019, 41, 803–820. [Google Scholar] [CrossRef]
- Korpinen, S.; Klančnik, K.; Peterlin, M.; Nurmi, M.; Laamanen, L.; Zupančič, G.; Popit, A.; Murray, C.; Harvey, T.; Andersen, J.H.; et al. Multiple pressures and their combined effects in Europe’s seas. ETC/ICM Technical Report 4/2019: European Topic Centre on Inland, Coastal and Marine waters, 2019, 164 pp.
- Magliozzi, C.; Tsiamis, K.; Vigiak, O.; Deriu, I.; Gervasini, E.; Cardoso, A.C. Assessing invasive alien species in European catchments: Distribution and impacts. Sci. Total Environ. 2020, 732, 138677. [Google Scholar] [CrossRef]
- Arianoutsou, M.; Adamopoulou, C.; Andriopoulos, P.; Bazos, I.; Christopoulou, A.; Galanidis, A.; Kalogianni, E.; Karachle, P.K.; Kokkoris, Y.; Martinou, A.F.; et al. HELLAS-ALIENS. The Invasive Alien Species of Greece: time trends, origin and pathways. NEOBIOTA, 2023, accepted.
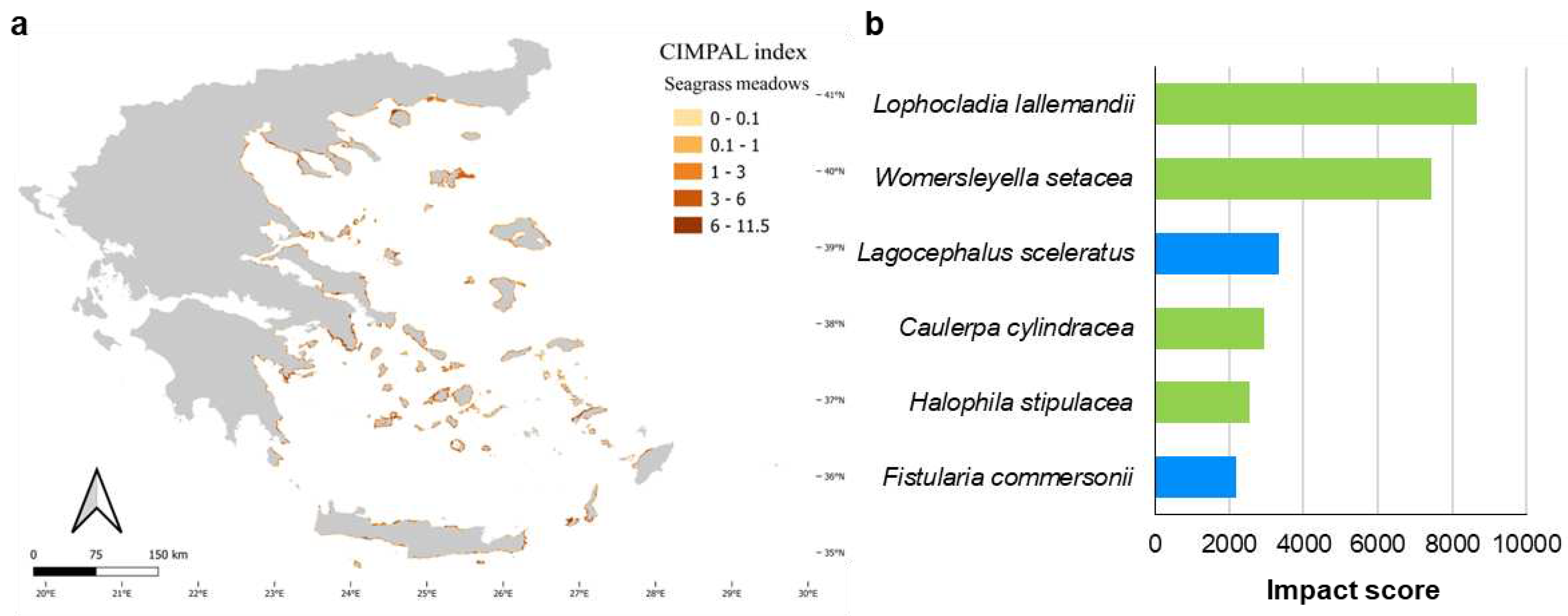
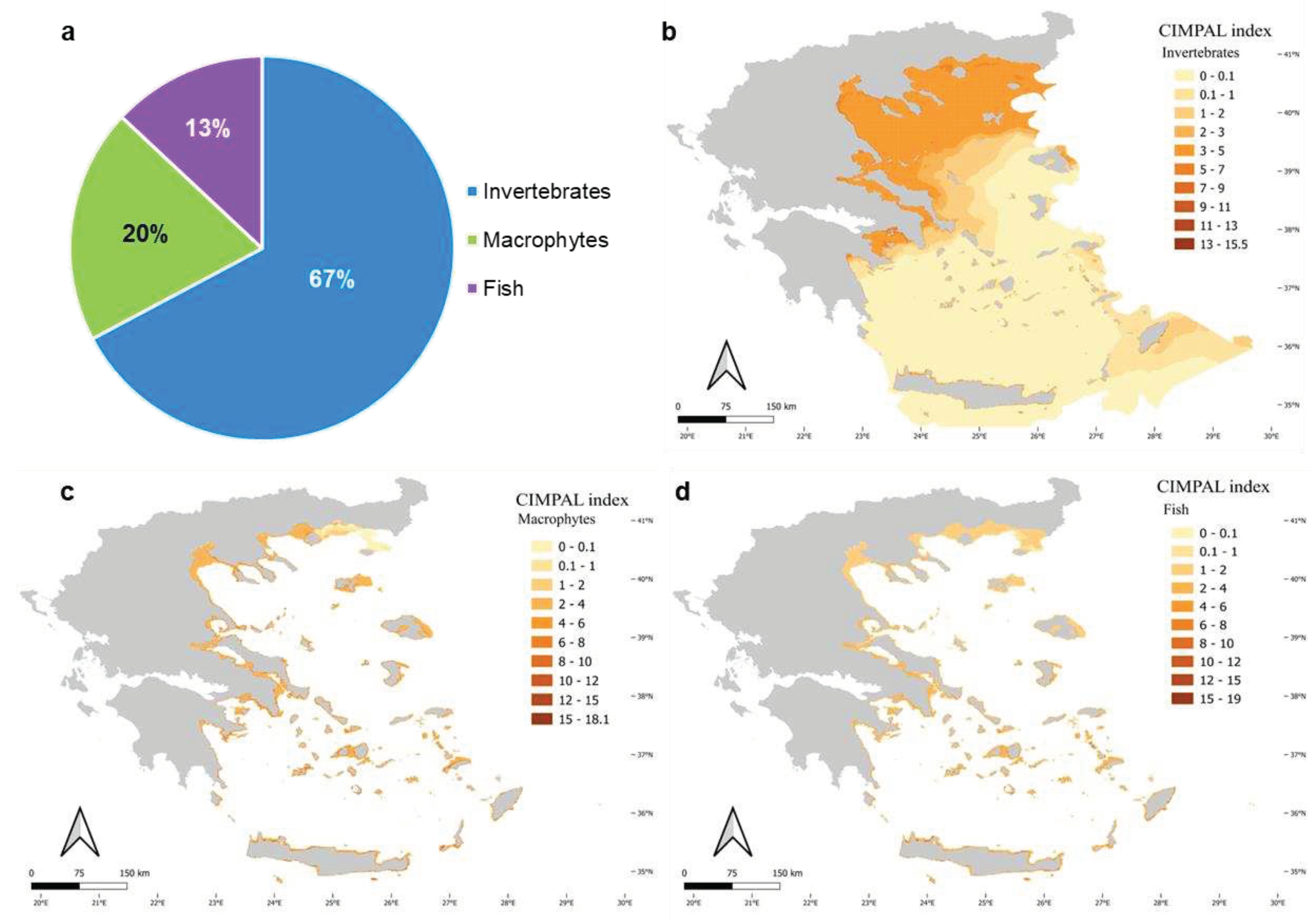
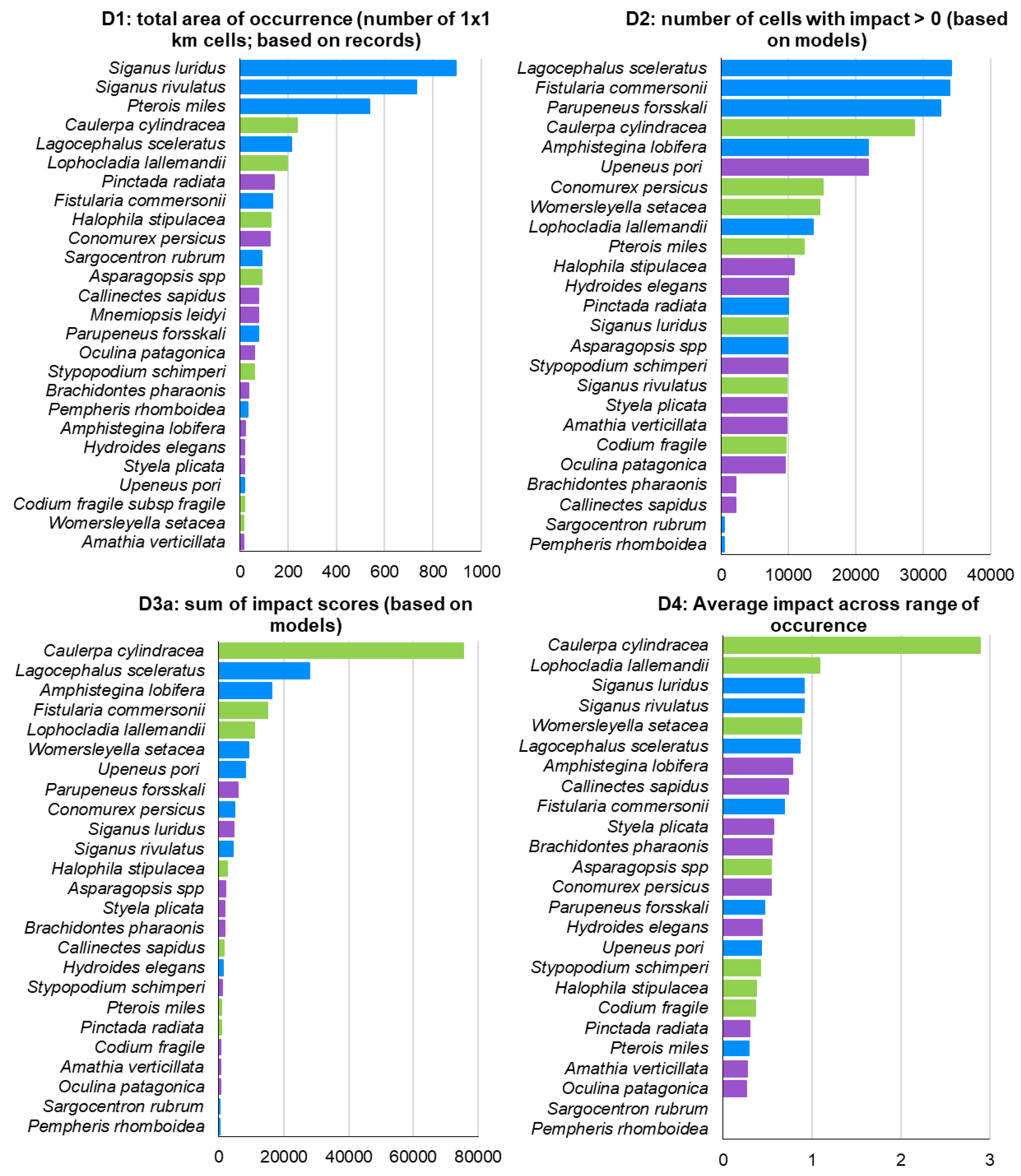
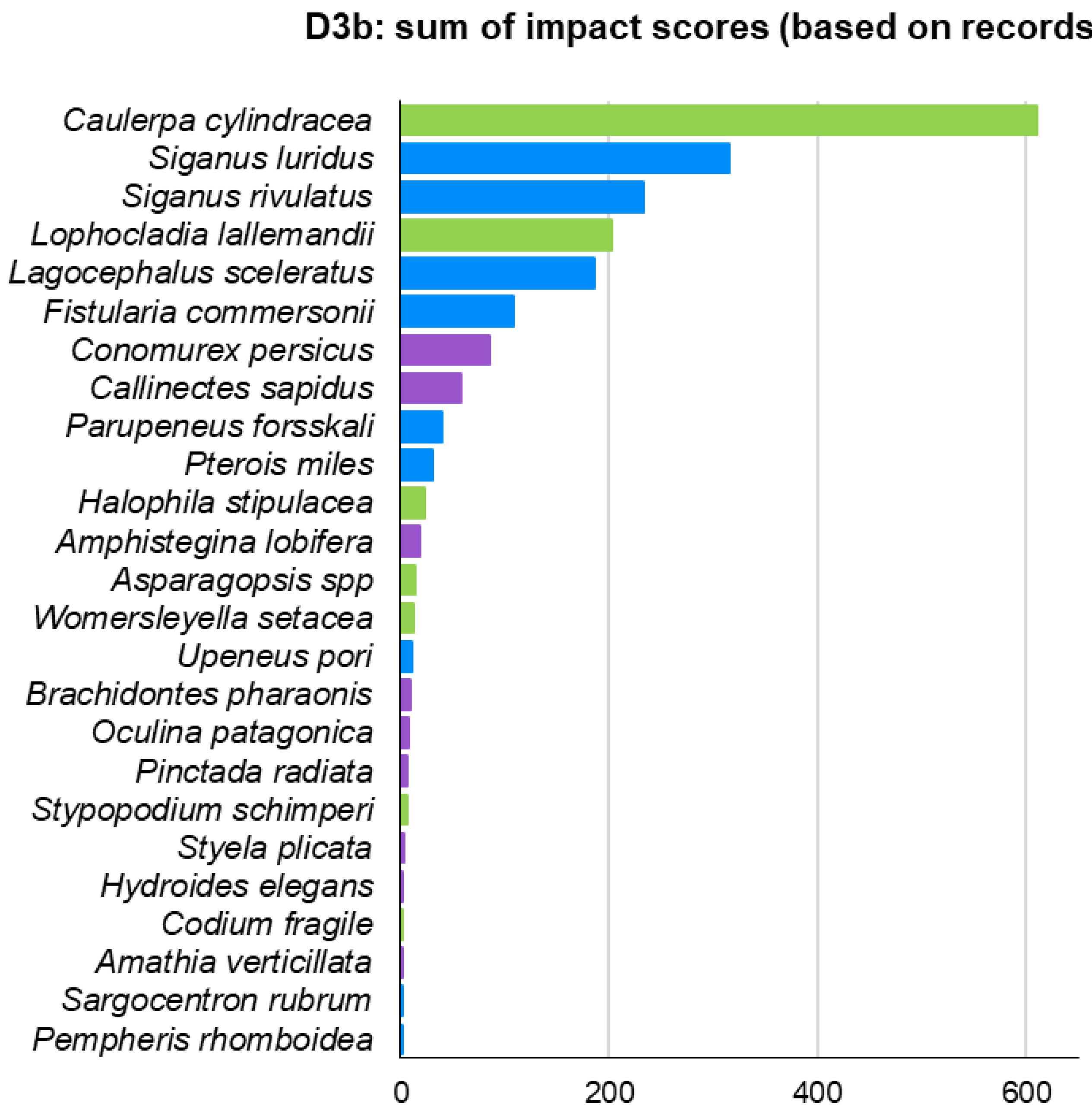
| Phylum, Class | Species | Status | Biotic group | Number of records |
|---|---|---|---|---|
| Arthropoda: Crustacea | Callinectes sapidus | Alien | Invertebrates | 115 |
| Bryozoa: Gymnolaemata | Amathia verticillata | Cryptogenic | Invertebrates | 19 |
| Chlorophyta: Ulvophyceae | Caulerpa cylindracea | Alien | Macrophytes | 283 |
| Chlorophyta: Ulvophyceae | Codium fragile | Alien | Macrophytes | 22 |
| Chordata: Ascidiacea | Styela plicata | Alien | Invertebrates | 21 |
| Chordata: Teleostei | Fistularia commersonii | Alien | Fish | 171 |
| Chordata: Teleostei | Lagocephalus sceleratus | Alien | Fish | 254 |
| Chordata: Teleostei | Parupeneus forsskali | Alien | Fish | 70 |
| Chordata: Teleostei | Pempheris rhomboidea | Alien | Fish | 31 |
| Chordata: Teleostei | Pterois miles | Alien | Fish | 574 |
| Chordata: Teleostei | Sargocentron rubrum | Alien | Fish | 88 |
| Chordata: Teleostei | Siganus luridus | Alien | Fish | 919 |
| Chordata: Teleostei | Siganus rivulatus | Alien | Fish | 748 |
| Chordata: Teleostei | Upeneus pori | Alien | Fish | 23 |
| Cnidaria: Anthozoa | Oculina patagonica | Cryptogenic | Invertebrates | 51 |
| Ctenophora: Tentaculata | Mnemiopsis leidyi | Alien | Invertebrates | 77 |
| Foraminifera: Globothalamea | Amphistegina lobifera | Alien | Invertebrates | 26 |
| Mollusca: Bivalvia | Brachidontes pharaonis | Alien | Invertebrates | 35 |
| Mollusca: Gastropoda | Conomurex persicus | Alien | Invertebrates | 129 |
| Mollusca: Bivalvia | Pinctada radiata | Alien | Invertebrates | 159 |
| Ochrophyta: Phaeophyceae | Stypopodium schimperi | Alien | Macrophytes | 67 |
| Annelida: Polychaeta | Hydroides elegans | Alien | Invertebrates | 24 |
| Rhodophyta: Florideophyceae | Asparagopsis spp. | Alien | Macrophytes | 117 |
| Rhodophyta: Florideophyceae | Lophocladia lallemandii | Alien | Macrophytes | 224 |
| Rhodophyta: Florideophyceae | Womersleyella setacea | Alien | Macrophytes | 24 |
| Tracheophyta: Magnoliopsida | Halophila stipulacea | Alien | Macrophytes | 132 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





