Submitted:
04 May 2023
Posted:
05 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
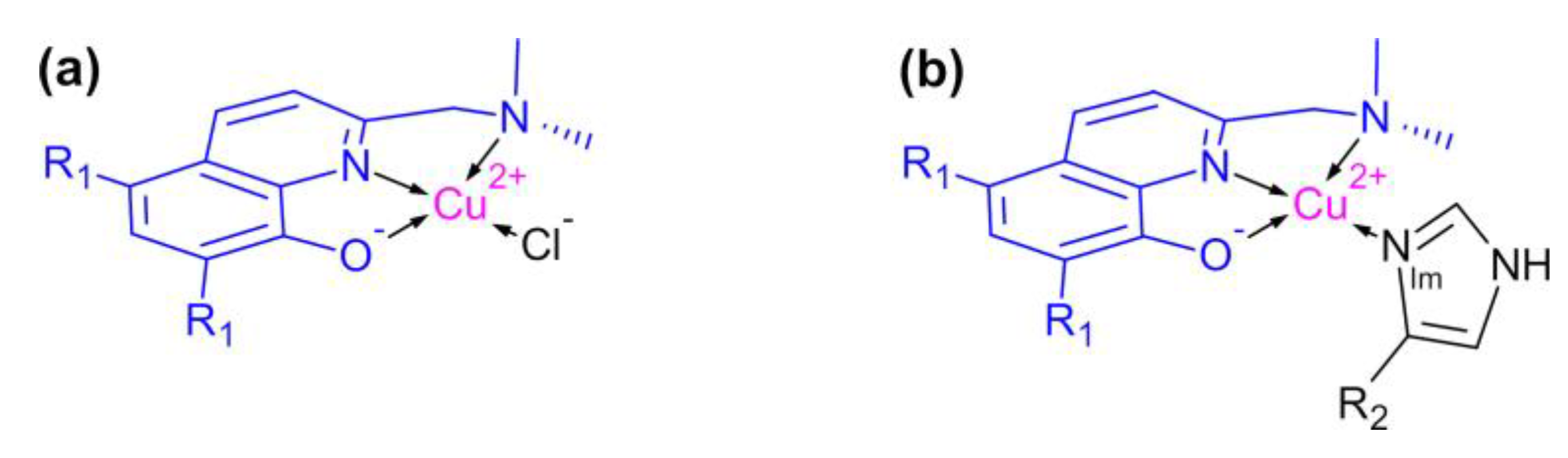
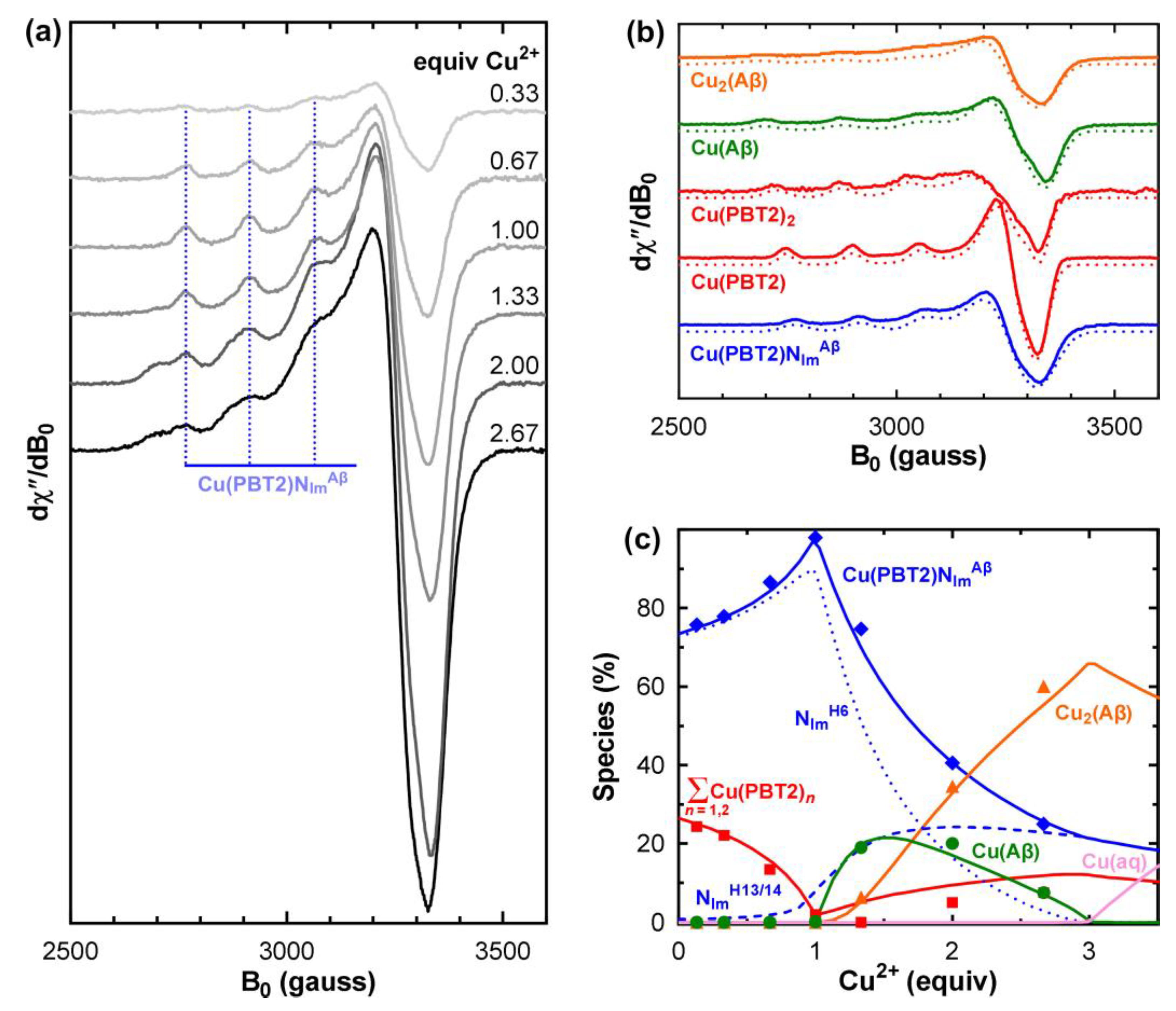
2. Results
3. Discussion
4. Materials and Methods
4.1. Sample preparation
4.2. EPR spectroscopy
4.3. Determination of the ternary formation constants for Cu/PBT2/Aβ1–40
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bush, A.I. Metals and neuroscience. Curr. Opin. Chem. Biol. 2000, 4, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Bush, A.I. Drug development based on the metals hypothesis of Alzheimer’s disease. J. Alzheimers Dis. 2008, 15, 223–240. [Google Scholar] [CrossRef]
- Adlard, P.A.; Bica, L.; White, A.R.; Nurjono, M.; Filiz, G.; Crouch, P.J.; Donnelly, P.S.; Cappai, R.; Finkelstein, D.I.; Bush, A.I. Metal ionophore treatment restores dendritic spine density and synaptic protein levels in a mouse model of Alzheimer’s disease. PLoS ONE 2011, 6, e17669. [Google Scholar] [CrossRef] [PubMed]
- Sampson, E.L.; Jenagaratnam, L.; McShane, R. Metal protein attenuating compounds for the treatment of Alzheimer’s dementia. Cochrane Database Syst. Rev. 2014, CD005380. [Google Scholar] [CrossRef] [PubMed]
- www.alzforum.org/news/research-news/pbt2-takes-dive-phase-2-alzheimers-trial . 1 April 2014.
- Kenche, V.B.; Zawisza, I.; Masters, C.L.; Bal, W.; Barnham, K.J.; Drew, S.C. Mixed ligand Cu2+ complexes of a model therapeutic with Alzheimer's amyloid-β peptide and monoamine neurotransmitters. Inorg. Chem. 2013, 52, 4303–4318. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.; Vendier, L. ; Stigliani, J-L., Meunier, B.; Robert, A. Structures of the Copper and Zinc Complexes of PBT2, a Chelating Agent Evaluated as Potential Drug for Neurodegenerative Diseases. Eur. J. Inorg. Chem. 2017; 600–608. [Google Scholar] [CrossRef]
- Sgarlata, C.; Arena, G.; Bonomo, R.P.; Giuffrida, A.; Tabbì, G. Simple and mixed complexes of copper(II) with 8-hydroxyquinoline derivatives and amino acids: Characterization in solution and potential biological implications. J. Inorg. Biochem. 2018, 180, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Summers, K.L.; Roseman, G.P.; Sopasis, G.J.; Millhauser, G.L.; Harris, H.H.; Pickering, I.J.; George, G.N. Copper(II) Binding to PBT2 Differs from That of Other 8-Hydroxyquinoline Chelators: Implications for the Treatment of Neurodegenerative Protein Misfolding Diseases. Inorg. Chem. 2020, 59, 17519–17534. [Google Scholar] [CrossRef]
- Summers, K.L.; Roseman, G.; Schilling, K.M.; Dolgova, N.V.; Pushie, M.J.; Sokaras, D.; Kroll, T.; Harris, H.H.; Millhauser, G.L.; Pickering, I.J.; George, G.N. Alzheimer’s Drug PBT2 Interacts with the Amyloid β 1–42 Peptide Differently than Other 8-Hydroxyquinoline Chelating Drugs. Inorg. Chem. 2022, 61, 14626–14640. [Google Scholar] [CrossRef]
- Mital, M.; Zawisza, I.A.; Wiloch, M.Z.; Wawrzyniak, U.E.; Kenche, V.; Wróblewski, W.; Bal, W.; Drew, S.C. Copper Exchange and Redox Activity of a Prototypical 8-Hydroxyquinoline: Implications for Therapeutic Chelation. Inorg Chem. 2016, 55, 7317–7319. [Google Scholar] [CrossRef]
- Drew, S. C.; Barnham, K. J. The Heterogeneous Nature of Cu2+ Interactions with Alzheimer’s Amyloid-β Peptide. Acc. Chem. Res. 2011, 44, 1146–1155. [Google Scholar] [CrossRef]
- Faller, P.; Hureau, C.; La Penna, G. Metal ions and intrinsically disordered proteins and peptides: from Cu/Zn amyloid-β to general principles. Acc. Chem. Res. 47, 2252–2259. [CrossRef]
- Drew, S.C.; Noble, C.J.; Masters, C.L.; Hanson, G.R.; Barnham, K.J. Pleomorphic copper coordination by Alzheimer’s amyloid-β peptide. J. Am. Chem. Soc. 2009, 131, 1195–1207. [Google Scholar] [CrossRef] [PubMed]
- Alies, B.; Renaglia, E.; Rózga, M.; Bal, W.; Faller, P.; Hureau, C. Cu(II) affinity for the Alzheimer's peptide: tyrosine fluorescence studies revisited. Anal. Chem. 2013, 85, 1501–1508. [Google Scholar] [CrossRef] [PubMed]
- Haigh, C.L.; Tumpach, C.; Collins, S.J.; Drew, S.C. A 2-substituted 8-hydroxyquinoline stimulates neural stem cell proliferation by modulating ROS signalling. Cell Biochem. Biophys. 2016, 74, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Drew, S.C. The case for abandoning therapeutic chelation of copper ions in Alzheimer’s disease. Front. Neurosci. 2017, 11, 317. [Google Scholar] [CrossRef] [PubMed]
- Bohlmann, L.; De Oliveira, D.M.P.; El-Deeb, I.M.; Brazel, E.B.; Harbison-Price, N.; Ong, C.Y.; Rivera-Hernandez, T.; Ferguson, S.A.; Cork, A.J.; Phan, M.D.; Soderholm, A.T.; Davies, M.R.; Nimmo, G.R.; Dougan, G.; Schembri, M.A.; Cook, G.M.; McEwan, A.G.; von Itzstein, M.; McDevitt, C.A.; Walker, M.J. Chemical Synergy between Ionophore PBT2 and Zinc Reverses Antibiotic Resistance. mBio. 2018, 9, e02391–18. [Google Scholar] [CrossRef] [PubMed]
- Brazel, E.B.; Tan, A.; Neville, S.L.; Iverson, A.R.; Udagedara, S.R.; Cunningham, B.A.; Sikanyika, M.; De Oliveira, D.M.P.; Keller, B.; Bohlmann, L.; El-Deeb, I.M.; Ganio, K.; Eijkelkamp, B.A.; McEwan, A.G.; von Itzstein, M.; Maher, M.J.; Walker, M.J.; Rosch, J.W.; McDevitt, C.A. Dysregulation of Streptococcus pneumoniae zinc homeostasis breaks ampicillin resistance in a pneumonia infection model. Cell Rep. 2022, 38, 110202. [Google Scholar] [CrossRef] [PubMed]
- Harbison-Price, N.; Ferguson, S.A.; Heikal, A.; Taiaroa, G.; Hards, K.; Nakatani, Y.; Rennison, D.; Brimble, M.A.; El-Deeb, I.M.; Bohlmann, L.; McDevitt, C.A.; von Itzstein, M.; Walker, M.J.; Cook, G.M. Multiple Bactericidal Mechanisms of the Zinc Ionophore PBT2. mSphere. 2020, 5, e00157–20. [Google Scholar] [CrossRef]
- Irving, H.; Williams, R.J.P. The stability of transition-metal complexes. J. Chem. Soc. 1953, 3192–3210. [Google Scholar] [CrossRef]
- .Kuipers, B.J.H.; Gruppen, H. Prediction of Molar Extinction Coefficients of Proteins and Peptides Using UV Absorption of the Constituent Amino Acids at 214 nm To Enable Quantitative Reverse Phase High-Performance Liquid Chromatography-Mass Spectrometry Analysis. J. Agric. Food Chem. 2007, 55, 5445–5451. [Google Scholar] [CrossRef]
- Barnham, K.J.; Gautier, E.C.L.; Kok, G.B.; Krippner, G. Preparation of 8-hydroxyquinolines for treatment of neurological conditions. 2008. U.S. Pat. Appl. Publ. Patent version number 20080161353 A1; United States,
- Wertz, J.E.; Orton, J.W.; Auzins, P. Electron spin resonance studies of radiation effects in inorganic solids. Discuss. Faraday Soc. 1961, 31, 140–150. [Google Scholar] [CrossRef]
- Stoll, S.; Britt, R.D. General and efficient simulation of pulse EPR spectra. Phys. Chem. Chem. Phys. 2009, 11, 6614–6625. [Google Scholar] [CrossRef] [PubMed]
- Stoll, S.; Schweiger, A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson. 2006, 178, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Bossak-Ahmad, K.; Wiśniewska, M.; Bal, W.; Drew, S.C.; Frączyk, T. Ternary Cu(II) complex with GHK peptide and cis-urocanic acid as a potential physiologically functional copper chelate. Int. J. Mol. Sci. 2020, 21, 6190. [Google Scholar] [CrossRef] [PubMed]
- Bossak-Ahmad, K.; Bal, W.; Frączyk, T.; Drew, S.C. Ternary Cu2+ Complexes of Human Serum Albumin and Glycyl-L-histidyl-L-lysine. Inorg. Chem. 2021, 60, 16927–16931. [Google Scholar] [CrossRef]
- Drew, S.C. α-synuclein and β-amyloid form a bridged copper complex. Appl. Magn. Reson. 2015, 46, 1041–1052. [Google Scholar] [CrossRef]
| Complex | gz | Az (63Cu) a | Reference | ||
|---|---|---|---|---|---|
| L = PBT2 | |||||
| CuL | 2.259 ± 0.002 | 151 ± 1 | This work | ||
| CuL2 | 2.283 ± 0.002 | 148 ± 3 | This work | ||
| CuLNImX | |||||
| X = Aβ1–40 | 2.249 ± 0.002 | 147 ± 2 | This work | ||
| X = imidazole | 2.248 ± 0.001 | 143 ± 1 | This work | ||
| X = histamine | 2.248 ± 0.001 | 143 ± 1 | This work | ||
| X = Aβ1–42 | 2.242 ± 0.002 | 142 ± 3 | 10b | ||
| L = non-chlorinated PBT2 homologue | |||||
| CuL | 2.255 ± 0.001 | 153 ± 1 | 6 | ||
| CuL2 | 2.267 ± 0.001 | 149 ± 1 | 6 | ||
| CuLNImX | |||||
| X = imidazole | 2.245 ± 0.001 | 144 ± 1 | This work, 6, 11 | ||
| X = histamine | 2.245 ± 0.001 | 145 ± 1 | This work, 6, 11 | ||
| Aβ | |||||
| Cu(Aβ1–40) | 2.268 ± 0.002 | 174 ± 2 | This workc | ||
| Cu2(Aβ1–40) | |||||
| first site | 2.268 ± 0.002 | 174 ± 2 | This workd | ||
| second site | 2.309 ± 0.005 | 168 ± 5 | This workd | ||
| Complex | Formation constanta | c/ (1 M−1)] at pH 7.4 | Reference |
|---|---|---|---|
| CuL | c | 13.61 ± 0.05 | 8 |
| CuL2 | c | 5.95 ± 0.07 | 8 |
| CuLNImAβ (His6) | c | 6.4 ± 0.1 | This work |
| CuLNImAβ (His13/14) | c | 4.4 ± 0.1 | This work |
| CuLNImimidazole | c | 4.22 ± 0.09 | This work |
| CuLNImhistamine | c | 4.00 ± 0.05 | This work |
| Cu(Aβ1–40) | c | 10.0 ± 0.1 | This work |
| Cu2(Aβ1–40) | c | 8.0 ± 0.1 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





