Submitted:
04 May 2023
Posted:
05 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Chemicals
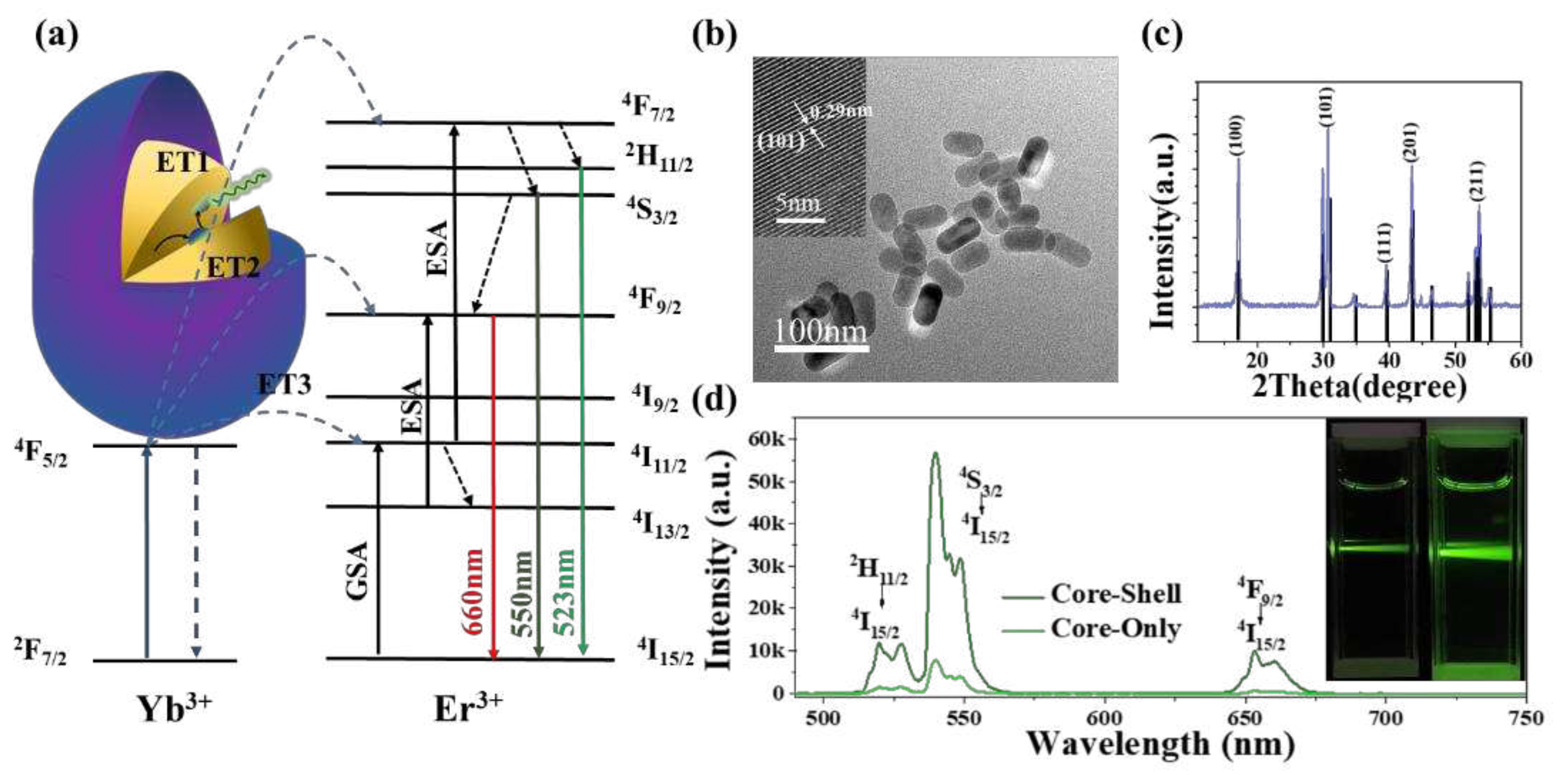
2.2. Synthesis of NaYF4:Er3+/Yb3+@NaYF4 nanoparticles
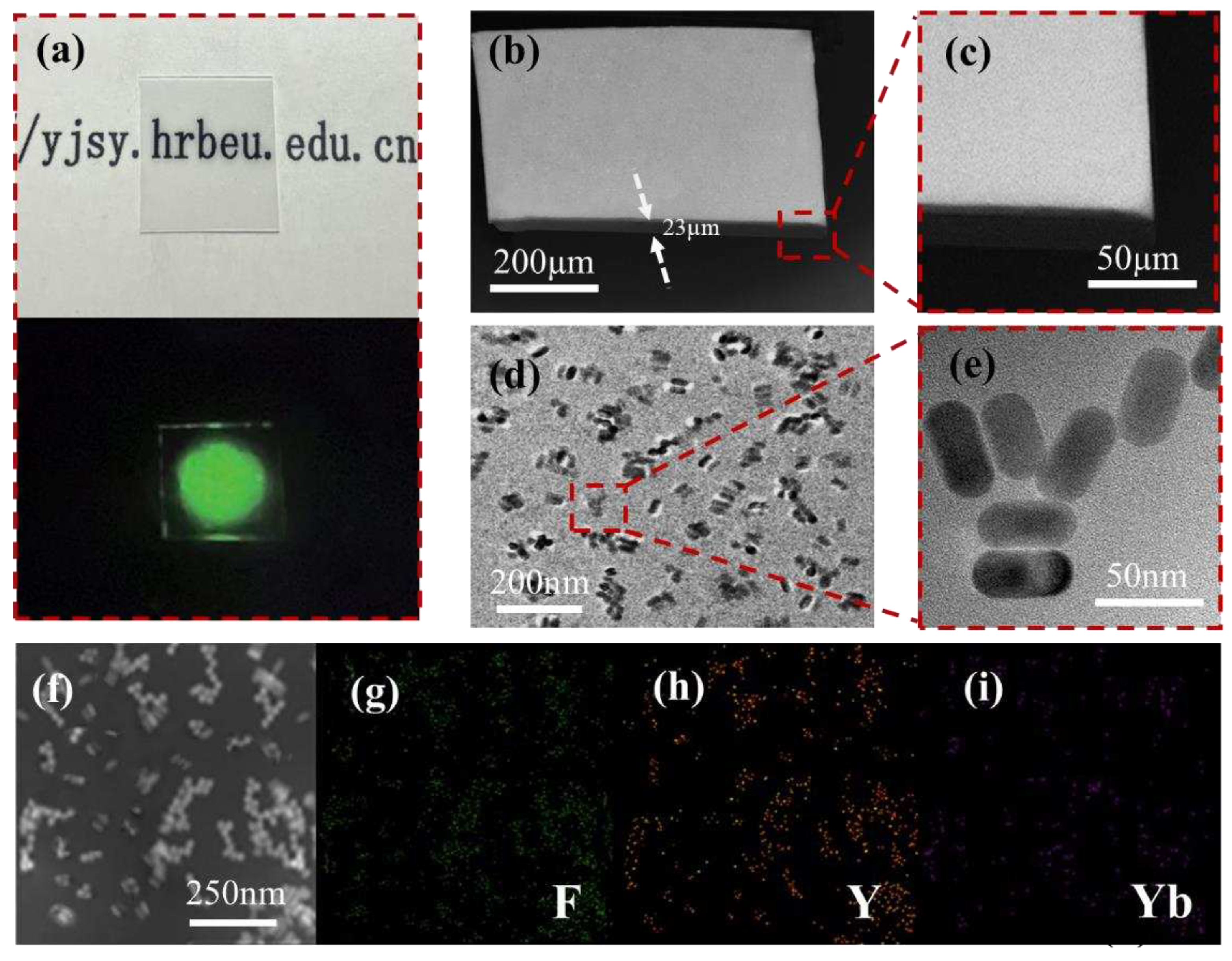
2.3. Fabrication of NaYF4:Er3+/Yb3+@NaYF4-PDMS film
2.4. Characterization
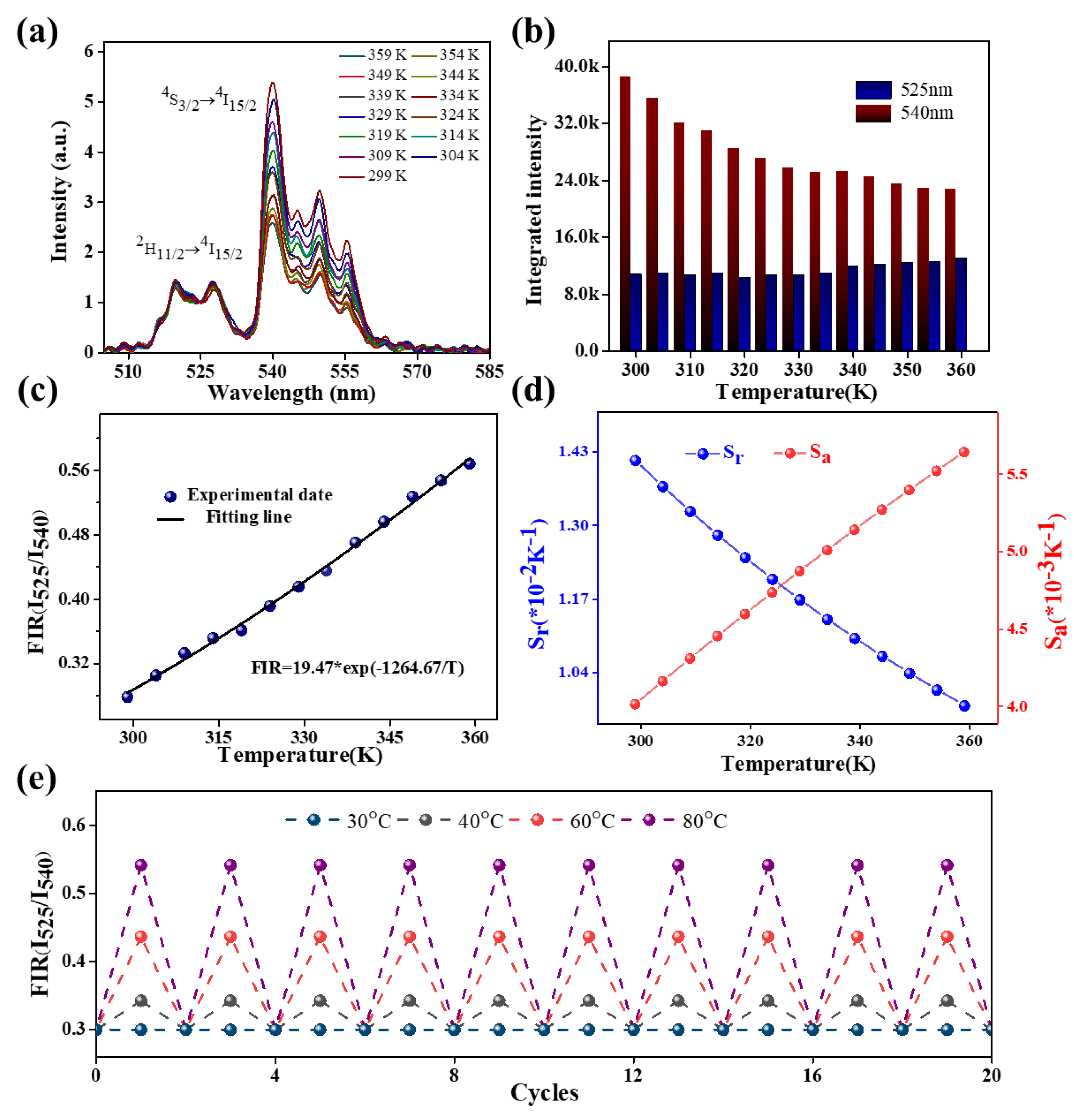
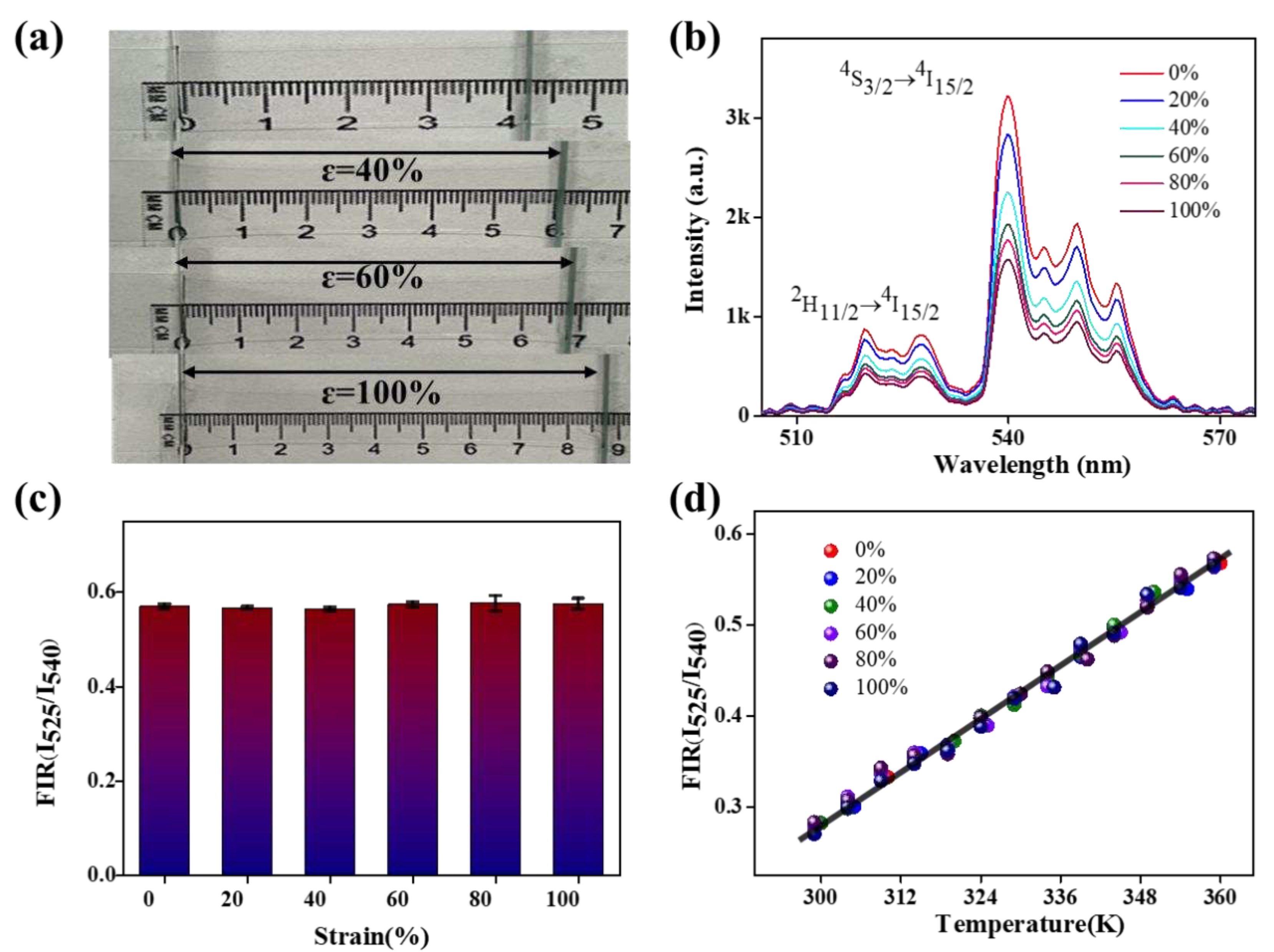
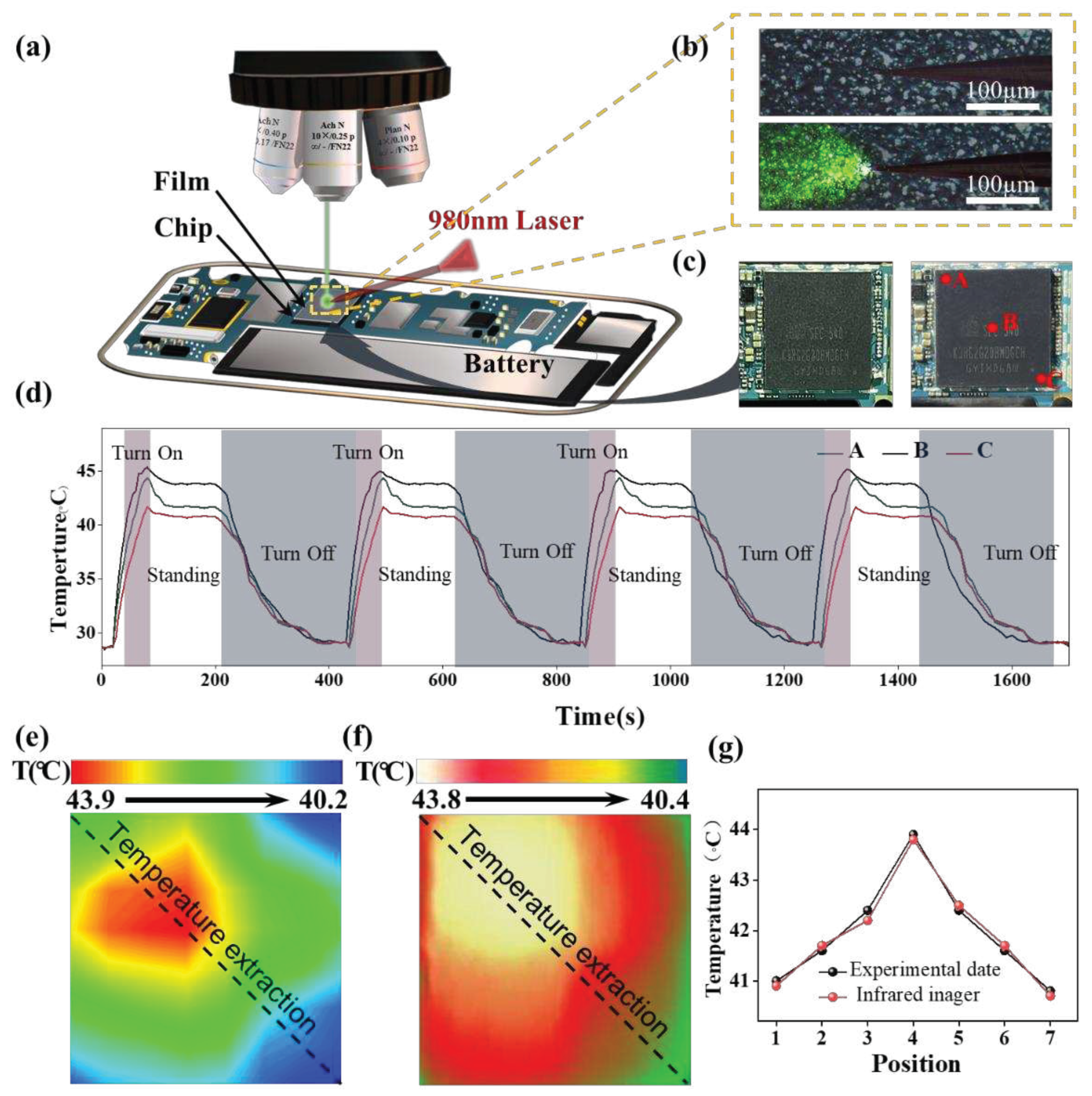
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Brites, C.D.; Xie, X.; Debasu, M.L.; Qin, X.; Chen, R.; Huang, W.; Rocha, J.; Liu, X.; Carlos, L.D. Instantaneous ballistic velocity of suspended Brownian nanocrystals measured by upconversion nanothermometry. Nat. Nanotechnol. 2016, 11, 851. [Google Scholar] [CrossRef]
- Suo, H.; Zhao, X.; Zhang, Z.; Li, T.; Goldys, E.M.; Guo, C. Constructing multiform morphologies of YF: Er3+/Yb3+ up-conversion nano/micro-crystals towards sub-tissue thermometry. Chem. Eng. J. 2017, 313, 65. [Google Scholar] [CrossRef]
- Wang, Z.; Ananias, D.; Carne´-Sa´nchez, A.; Brites, C. D. S.; Imaz, I.; Maspoch, D.; Rocha, J.; Carlos, D. Adv. Funct. Mater. 2015, 25, 2824. [CrossRef]
- Luo, Z.; Cho, H.; Luo, X.; Cho, K. I. System thermal analysis for mobile phone. Applied Thermal Engineering. 2008, 28, 1889–1895. [Google Scholar] [CrossRef]
- Puddu, M.; Stark, W.J.; Grass, R.N. Silica microcapsules for long-term, robust, and reliable room temperature rna preservation. Small. 2016, 12, 452. [Google Scholar] [CrossRef] [PubMed]
- Oleksandr, A.; Savchuk, J.J.; Carvajal, C.; Cascales, M.; Aguilo. Benefits of Silica Core–Shell Structures on the Temperature Sensing Properties of Er, Yb: GdVO4 Up-Conversion Nanoparticles. J. Mater. Chem. C 2016, 4, 6602.
- Borkar, S. Tackling variability and reliability challenges. In IEEE Computer Society Prress. 2006, 23, 520. [Google Scholar] [CrossRef]
- Naouss, M.; Marc, F. Design and implementation of a low cost test bench to assess the reliability of FPGA. Microelectron Reliab 2015, 55, 1341–1345. [Google Scholar] [CrossRef]
- Amrouch, H.; Ebi, T.; Schneider, J.; Parameswaran, S.; Henkel, J. Analyzing the thermal hotspots in FPGA-based embedded systems. International Conference on Field Programmable Logic & Applications 2013, 1–4. [Google Scholar]
- Avenas, Y.; Dupont, L.; Khatir, Z. Temperature measurement of power semiconductor devices by thermo-sensitive electrical parameters-a review. IEEE Trans Power Electron. 2011, 27, 27,3081–3092. [Google Scholar] [CrossRef]
- Grimes, R.; Walsh, E.; Walsh, P. Active Cooling Of A Mobile Phone Handset. Applied Thermal Engineering 2010, 30, 2363–2369. [Google Scholar] [CrossRef]
- Yu, W.J.; Feng, S.W.; Zhang, Y.M.; Shi, B.B. Temperature distribution measurement based on field-programmable gate array embedded ring oscillators. Solid-State Electronics 2019, 158, 16–21. [Google Scholar] [CrossRef]
- Heckmann, T.; Mcevoy, J.P.; Markantonakis, K.; Akram, R.N. Removing epoxy underfill between neighbouring components using acid for component chip-off. Naccache, Digital Investigation 2019, 29, 198–209. [Google Scholar] [CrossRef]
- Suo, H.; Zhao, X.Q.; Zhang, Z.Y.; Shi, R.; Wu, Y.F.; Xiang, J.M.; Guo, C.F. Local symmetric distortion boosted photon up-conversion and thermometric sensitivity in lanthanum oxide nanospheres. Nanoscale. 2018, 10, 9245. [Google Scholar] [CrossRef] [PubMed]
- Sheng, C.; Zheng, J.; Zhao, J.; Yang, Z.; Fang, X. Temperature sensors: robust and stable ratiometric temperature sensor based on Zn-In-S quantum dots with intrinsic dual-dopant ion emissions. Adv. Funct. Mater. 2016, 26, 7224. [Google Scholar]
- Gao, G.J.; Busko, D.; Kauffmann-Weiss, S.; Turshatov, A.; Howard, I. A.; Richards, B. S. Wide-range non-contact fluorescence intensity ratio thermometer based on Yb3+ /Nd3+ co-doped La2O3 microcrystals operating from 290 to 1230 K. J. Mater. Chem. C. 2018, 6, 4163. [Google Scholar] [CrossRef]
- Pan, Y.; Xie, X.J.; Huang, Q.W.; Gao, C.; Wang, Y.B.; Wang, L.X. Inherently Eu2+/Eu3+ codoped Sc2O3 nanoparticles as high-performance nanothermometers. Adv. Mater. 2018, 30, 1705256. [Google Scholar] [CrossRef]
- Back, M.; Trave, E.; Ueda, J. Tanabe S. Ratiometric optical thermometer based on dual near-infrared emission in Cr3+-doped bismuth-based gallate host. Chem. Mater 2016, 28, 8347. [Google Scholar] [CrossRef]
- Jin, Y.; Pang, T. Highly efficient green upconversion luminescence of ZnMoO4:Yb3+/Er3+/Li+ for accurate temperature sensing. Spectrochim. Acta. Part A. 2019, 211, 306. [Google Scholar] [CrossRef]
- Zˇ, Antic´.; Dramicánin, M.D.; Prashanthi, K.; Jovanovic´, D.; Kzman S.; Thundat, T.; Pulsed Laser Deposited Dysprosium-Doped Gadolinium–Vanadate Thin Films for Noncontact, Self-Referencing Luminescence Thermometry. Adv. Mater. 2016, 28, 7745. [CrossRef]
- Debasu, M.L.; Amanias, D.; Pastoriza-Santos, I.; Liz-Matzan, L. M.; Rochaand, J.; Carlos, L.D. All-In-One Optical Heater-Thermometer Nanoplatform Operative From 300 to 2000 K Based on Er3+ Emission and Blackbody Radiation. Adv. Mater. 2013, 25, 4868. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Huang, F.; Lin, H.; Zhou, J.C.; Xu, J.; Wang, Y.S. A Novel Optical Thermometry Strategy Based on Diverse Thermal Response from Two Intervalence Charge Transfer States. Adv. Funct. Mater. 2016, 26, 3139. [Google Scholar] [CrossRef]
- Savchuk, O.A.; Carvajal, J.J.; Cascles, C.; Aguiloánd, M.; Dıáz,F. Benefits of Silica Core–Shell Structures on the Temperature Sensing Properties of Er,Yb:GdVO4 Up-Conversion Nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 7266. [CrossRef] [PubMed]
- Liu, L.X.; Qin, F.; Lv, T.Q.; Zhang, Z.G.; Cao, W.W. Accurate thermometry based on the red and green fluorescence intensity ratio in NaYF4: Yb, Er nanocrystals for bioapplication. Opt. Lett. 2016, 41, 4664. [Google Scholar] [CrossRef] [PubMed]
- Xiang, G.T.; Xia, Q.; Liu, X.T.; Wang, X.J. Optical thermometry based on the thermally coupled energy levels of Er3+ in upconversion materials. Dalton Transactions 2020, 49, 17115–17120. [Google Scholar] [CrossRef] [PubMed]
- Pang, T.; Wan, W.J.; Qian, D.L.; Liu, Z.L. Calibration of optical temperature sensing of Ca1-xNaxMoO4:Yb3+, Er3+ with intense green up-conversion luminescence. Alloys Compd. 2019, 771, 571. [Google Scholar] [CrossRef]
- Tu, Y.Y.; Zhao, S.L.; He, D.Y.; Wu, T.; Zhang, H.; Lei, R.S.; Huang, L.H.; Xu, S.Q. A portable all-fiber thermometer based on the fluorescence intensity ratio (FIR) technique in rare earth doped TeO2–WO3–La2O3–Na2O glass. J. Mater. Chem. C. 2018, 6, 7063. [Google Scholar] [CrossRef]
- Messias, D.N.; Vermelho, M.; Gouveia-Neto, A.S.; Aitchison, J.S. All optical integrated upconversion fluorescence-based point temperature sensing system using Er3+-doped silica-on-silicon waveguides. Rev. Sci. Instrum. 2002, 73, 476–479. [Google Scholar] [CrossRef]
- Mai, H.; Zhang, Y.; Sun, L.; Yan, C. Highly Efficient Multicolor Up-Conversion Emissions and Their Mechanisms of Monodisperse NaYF4:Yb, Er Core and Core/Shell-Structured Nanocrystals. J. Phys. Chem. C. 2007, 111, 13721–13729. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, K.; Song, S.; Fan, D.; Zhang, H.; Liu, X. Remote manipulation of upconversion luminescence. Chem. Soc. Rev. 2018, 47, 6473–6485. [Google Scholar] [CrossRef]
- Gao, H.; Hu, H.; Zhao, Y.; Li, J.; Lei, M.; &, *!!! REPLACE !!!*; Zhang, Y.; Zhang, Y. Highly-sensitive optical fiber temperature sensors based on PDMS/silica hybrid fiber structures. Sensors and Actuators A: Physical 2018, 284, 22–27. [Google Scholar] [CrossRef]
- Hua, Y.b.; Yu, J.S. Synthesis and luminescent properties of near-UV excited NaLa(MoO4)2:Er3+ phosphors for multifunctional applications. Journal of Alloys and Compounds. 2019, 811, 152050. [Google Scholar] [CrossRef]
- Cai, D.; Zhu, D.H.; Yuan, X.; Wei, G.D.; Li, H.B.; Zhao, J.L.; Han, W. Thermally stable luminescence of Mn2+ in Mn doped CsPbCl3 nanocrystals embedded in polydimethylsiloxane films. Journal of Luminescence. 2018, 202, 157–162. [Google Scholar] [CrossRef]
- Tong, L.; Li, X.; Hua, R.; Cheng, L.; Sun, J.; Zhang, J.; Xu, S.; Zheng, H.; Zhang, Y.; Chen, B. Microwave-assisted hydrothermal synthesis, temperature quenching and laser-induced heating effect of hexagonal microplate β-NaYF4: Er3+/Yb3+ microcrystals under 1550 nm laser irradiation. Curr. Appl. Phys. 2017, 17, 999–1004. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, S.L.; Wang, X.L.; Ren, X.T.; Ye, J.T.; Huang, L.H.; Xu, S.Q. The enhanced photoluminescence and temperature sensing performance in rare earth doped SrMoO4 phosphors by aliovalent doping: from material design to device applications. J. Mater. Chem. C. 2020, 7, 15007–15013. [Google Scholar] [CrossRef]
- Peng, Y. P.; Lu, W.; Ren, P.; Ni, Y.; Wang, Y.; Yan, P.; Zeng, Y.-J.; Zhang, W.; Ruan, S. Multi-Band Up-Converted Lasing Behavior in NaYF4:Yb/Er Nanocrystals. Nanomaterials 2018, 8, 497. [Google Scholar] [CrossRef] [PubMed]
- Ju, D.; Song, F.; Khan, A.; Song, F.; Zhou, A.; Gao, X.; Hu, H.; Sang, X.; Zadkov, V. Simultaneous Dual-mode Emission and Tunable Multicolor in the Time Domain from Lanthanide-doped Core-shell Microcrystals. Nanomaterials 2018, 8, 1023. [Google Scholar] [CrossRef] [PubMed]
- Nahorniak, M.; Patsula, V.; Mareková, D.; Matouš, P.; Shapoval, O.; Oleksa, V.; Vosmanská, M.; Machová Urdzíková, L.; Jendelová, P.; Herynek, V.; Horák, D. Chemical and Colloidal Stability of Polymer-Coated NaYF4:Yb,Er Nanoparticles in Aqueous Media and Viability of Cells: The Effect of a Protective Coating. Int. J. Mol. Sci. 2023, 24, 2724. [Google Scholar] [CrossRef]
- Yadhuraj, S.R.; Gandla, S.B.; Sudarshan, B.G.; Prasanna Kumar, S.C. Preparation and Study of PDMS Material. Materials Today: Proceedings. 2018, 5, 21406–21412. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, S.; Liu, Y.; Hou, J.; Xu, X.; Zhao, X.; Wei, B. Influence of NaYF4 Inert and Active Layer on Upconversion Luminescence. Nanomaterials 2022, 12, 3288. [Google Scholar] [CrossRef]
- Guo, J.J.; Zhou, B.Q.; Yang, C.X.; Dai, Q.H.; Kong, L.J. Stretchable and upconversion-luminescent polymeric optical sensor for wearable multifunctional sensing. Optics Letters. 2019, 44, 5747–5750. [Google Scholar] [CrossRef] [PubMed]
- Chockalingam, S.; Roth, C.; Henzel, T.; Cohen, T. Probing local nonlinear viscoelastic properties in soft materials. Journal of the Mechanics and Physics of Solids. 2021, 146, 104172. [Google Scholar] [CrossRef]
- Li, H.Y.; Wei, F.; Li, Y.Z.; Yu, M.; Zhang, Y.; Liu, L.; Liu, Z.H. Optical fiber sensor based on upconversion nanoparticles for internal temperature monitoring of Li-ion batteries. J. Mater. Chem. C. 2021, 9, 14757–14765. [Google Scholar] [CrossRef]
- Seethapathy, S.; Gorecki, T. Applications of polydimethylsiloxane in analytical chemistry: A review. Analytica Chimica Acta. 2012, 750, 48–62. [Google Scholar] [CrossRef]
- Sheng, W.; Wang, X.; Tao, Y.; Yan, X. Detecting Variable Resistance by Fluorescence Intensity Ratio Technology. Sensors 2019, 19, 2400. [Google Scholar] [CrossRef]
- Xu, L.; Wang, M.; Chen, Q.; Yang, J.; Zheng, W.; Lv, G.; Quan, Z.; Li, C. Rare Earth Hydroxide as a Precursor for Controlled Fabrication of Uniform β-NaYF4 Nanoparticles: A Novel, Low Cost, and Facile Method. Molecules 2019, 24, 357. [Google Scholar] [CrossRef]
- Gao, J.; Ren, X.; Yang, K.; Zhao, S.; Huang, L.; Xu, S. Detection of hydrogen concentration based on an all-fiber fluorescence intensity ratio optical thermometer with Er3+/Yb3+ codoped NaBi(WO4)2 phosphors. Optik 2021, 242, 167280. [Google Scholar] [CrossRef]
- Wu, T.; Zhao, S.; Lei, R.; Huang, L.; Xu, S. Optical thermometry based on green upconversion emission in Er3+/Yb3+ codoped BaGdF5 glass ceramics.Mater. Res. Express 2018, 5, 025201. [Google Scholar] [CrossRef]
- Dong, B.; Liu, D.P.; Wang, X.J.; Yang, T.; Miao, S.M.; Li, C.R. Optical thermometry through infrared excited green upconversion emissions in Er3+–Yb3+ codoped Al2O3. Appl. Phys. Lett. 2007, 90, 181117. [Google Scholar] [CrossRef]
- Zhang, H.; Ye, J.T.; Wang, X.L.; Zhao, S.L.; Lei, R.S.; Huanga, L.H.; Xu, S.Q. Highly reliable all-fiber temperature sensor based on the fluorescence intensity ratio (FIR) technique in Er3+/Yb3+ co-doped NaYF4 phosphors. J. Mater. Chem. C. 2019, 7, 15269–15275. [Google Scholar] [CrossRef]
- Giang, L. T. K.; Trejgis, K.; Marciniak, L.; Vu, N.; Minh, L. Q. Fabrication and characterization of up-converting β-NaYF4: Er3+, Yb3+@ NaYF4 core–shell nanoparticles for temperature sensing applications. Scientific Reports 2020, 1, 14672. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, M.; Hu, G. Gain Enhancement of the Optical Waveguide Amplifier Based on NaYF4/NaLuF4: Yb, Er NPs-PMMA Integrated with a Si3N4 Slot. Nanomaterials 2022, 12, 2937. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Wu, J.; Yang, Y.; Liu, X.; Jia, J.; Dong, J.; Zhang, L.; Lin, J.M.; Huang, M.L.; Wei, Y.L.; Huang, Y.F. High performance perovskite solar cells based on β-NaYF4: Yb3+/Er3+/Sc3+@ NaYF4 core-shell upconversion nanoparticles. Journal of Power Sources 2019, 426, 178–187. [Google Scholar] [CrossRef]
- Li, H.Y.; Sun, X.; Shahzad, M.K.; Liu, L. Facile preparation of upconversion microfibers for efficient luminescence and distributed temperature measuremen. J. Mater. Chem. C. 2019, 7, 7984–7992. [Google Scholar] [CrossRef]
- Li, X.; Yang, L.; Zhu, Y.; Zhong, J.; Chen, D. Upconversion of transparent glass ceramics containing β-NaYF4: Yb3+, Er3+ nanocrystals for optical thermometry. RSC advances 2019, 14, 7948–7954. [Google Scholar] [CrossRef]
| Phosphor | Maximum Sensitivity (% K-1) |
Temperature range (K) |
Ref. |
| NaBi(WO4)2:Yb3+/Er3+ | 1.24 | 298–373 | [47] |
| BaGdF5:Yb3+/Er3+ | 1.28 | 298–681 | [48] |
| Al2O3:Yb3+/Er3+ | 0.51 | 295–973 | [49] |
| NaYF4:Yb3+/Er3+ | 1.68 | 258–423 | [50] |
| NaYF4:Yb3+/Er3+@NaYF4 | 1.43 | 299-359 | This work |
| Chip Type | Mobile phone | Maximun Temperature(°С) |
Minimun Temperature(°С) |
Temperature difference(°С) |
| A10 | Iphone 7plus | 34.7 | 32.0 | 2.7 |
| Kirin970 | Huawei p20 | 36.9 | 34.6 | 2.3 |
| MTK6752 | Vivo X5s | 40.4 | 37.4 | 3.0 |
| Exynos7420 | Galaxy S6 edge+ | 43.9 | 43.9 | 3.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





