Submitted:
05 May 2023
Posted:
05 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Susceptibility profile
2.2. Molecular identification of ESBL and carbapenemases genes
2.3. Biofilm formation assay
2.4. Minimum inhibitory concentration
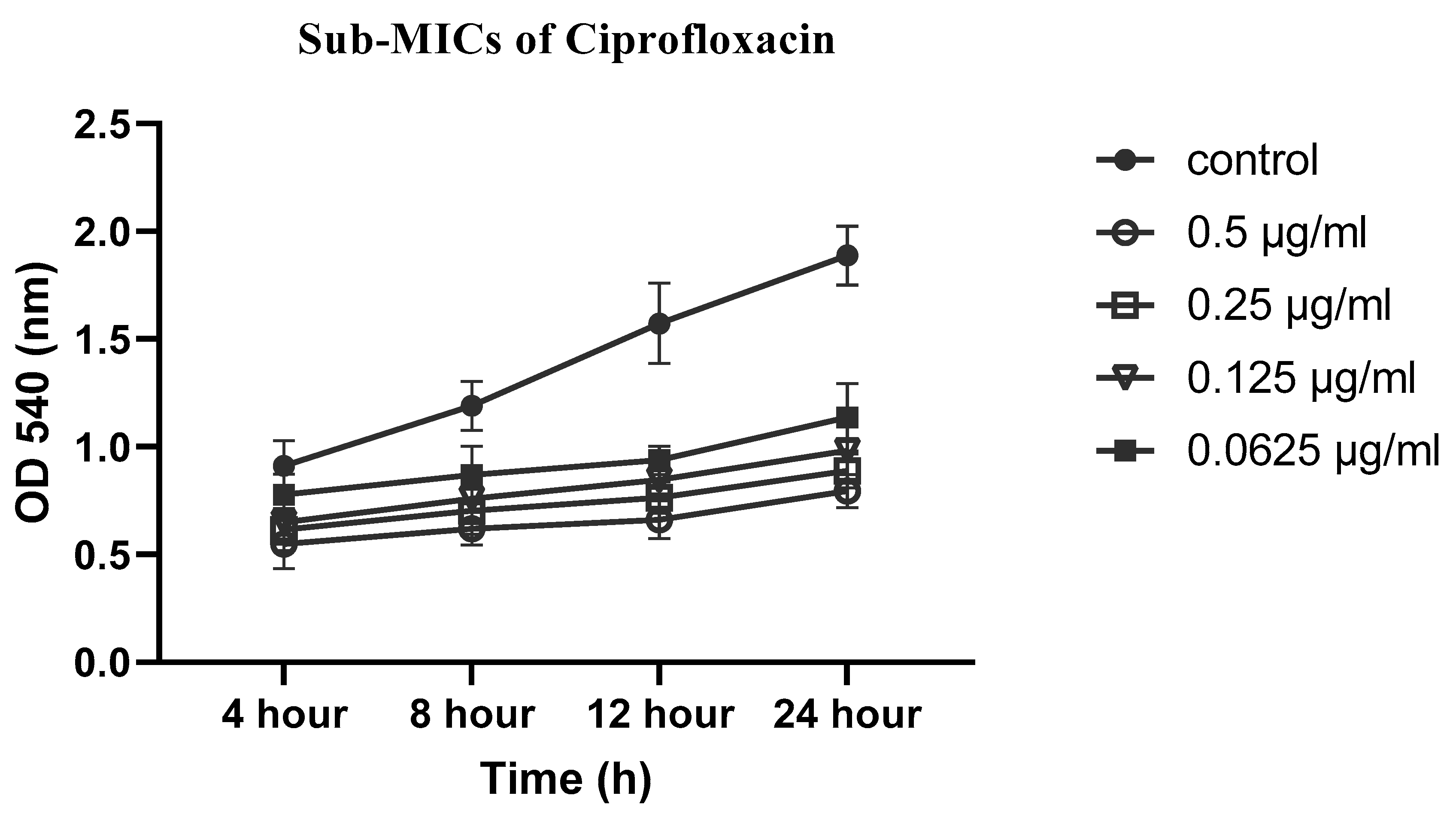
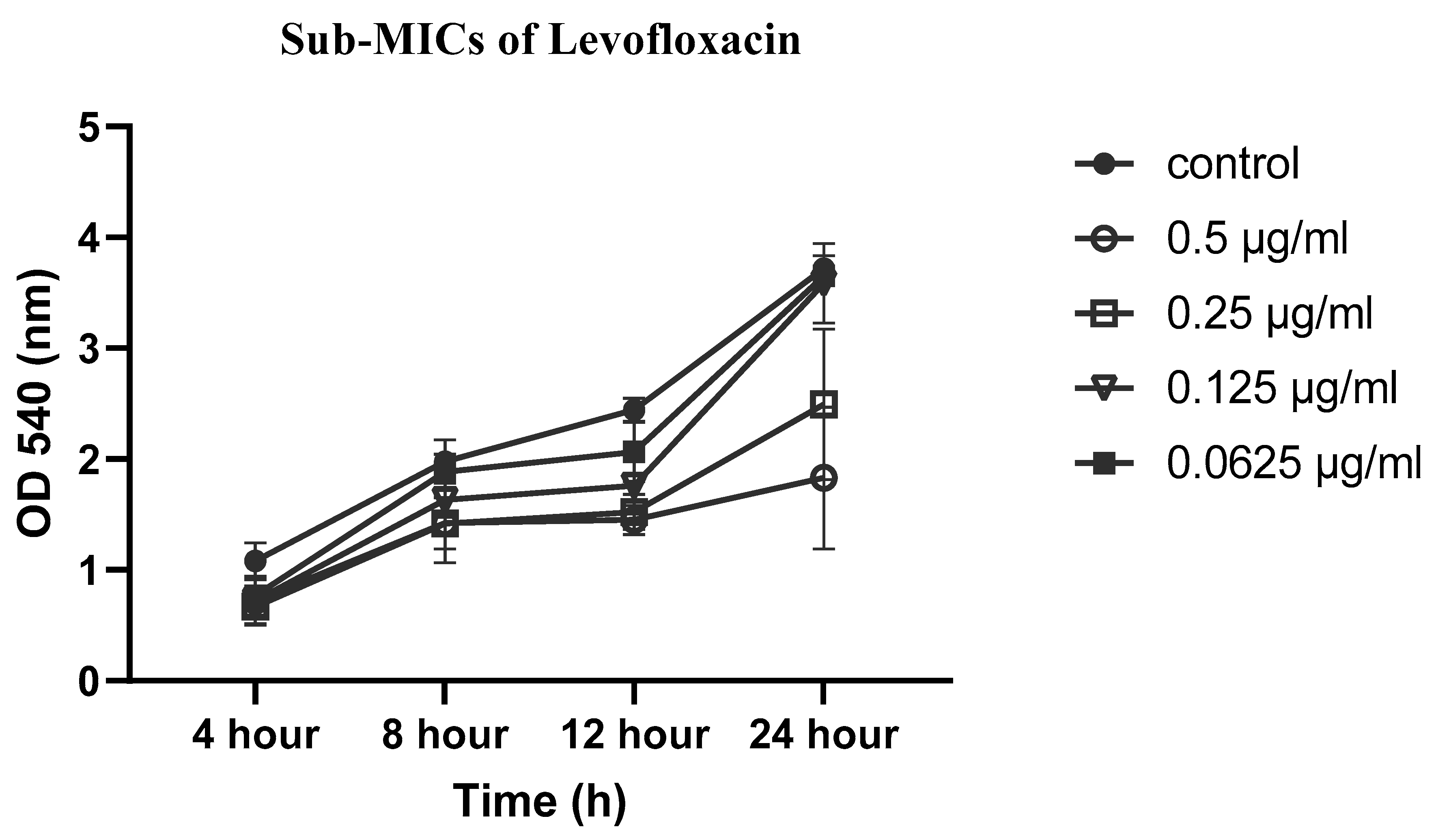
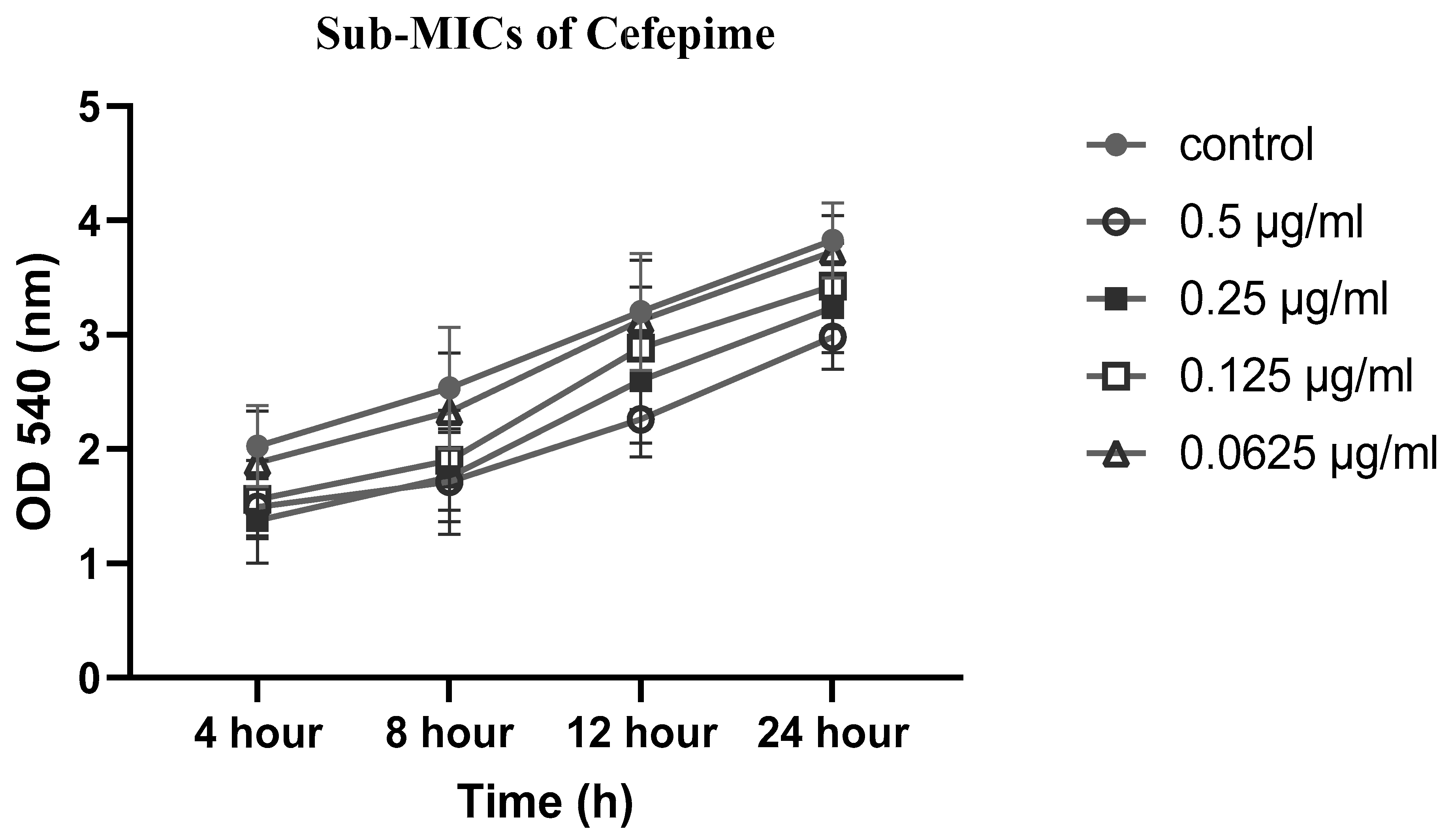
2.5. Effects of sub-MICs of CIP, LEV and CEF on biofilm formation
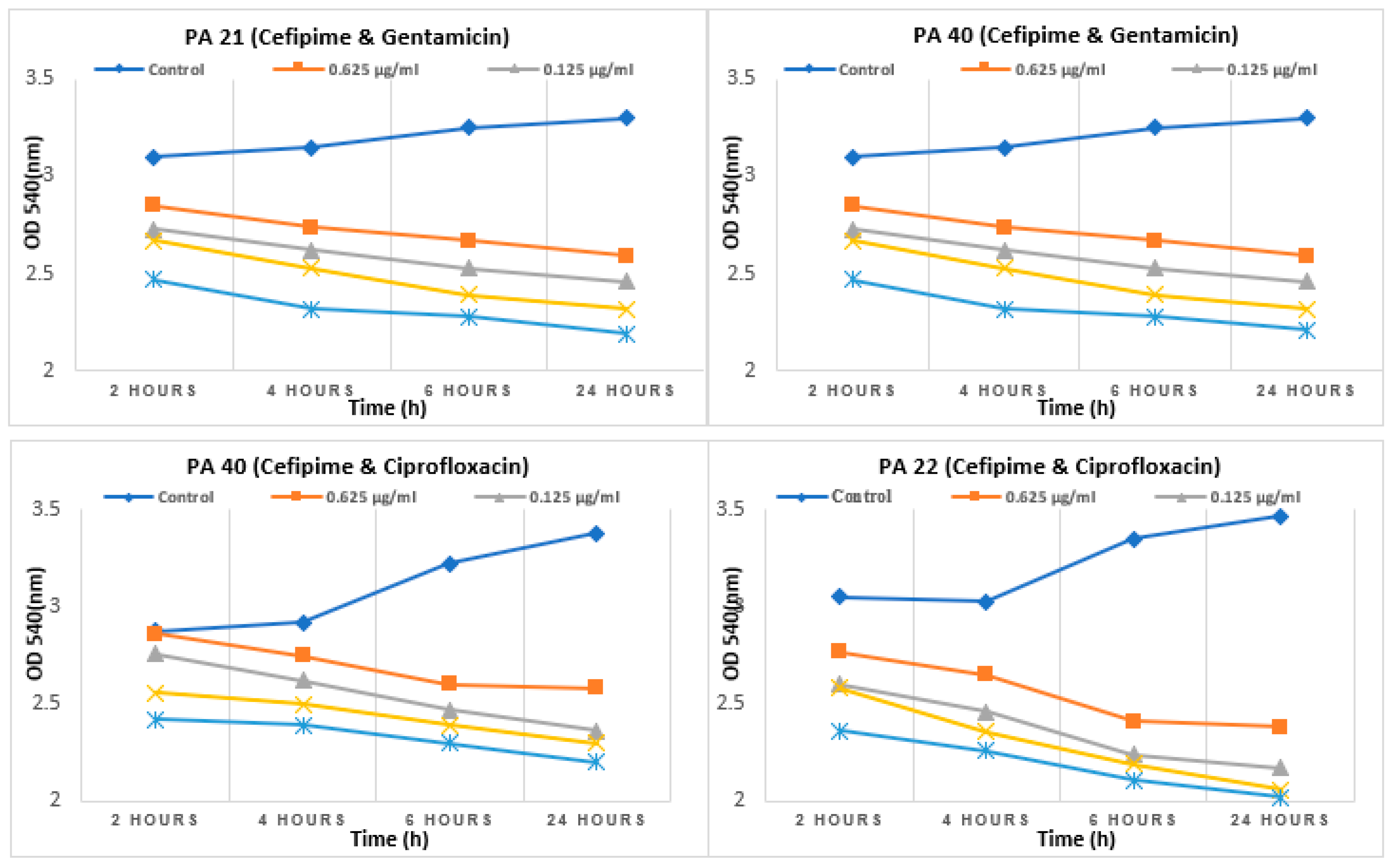
2.6. Evaluation of synergistic effects by checkerboard method
2.7. Effect of sub-MIC on synergism
2.8. Figures and Tables
| Type of Specimen | P. aeruginosa (n=266) | MDRs | XDRs | Non-MDRs | P value |
|---|---|---|---|---|---|
| Pus | 102(38.34%) | 16(15.68%) | 25(24.5%) | 61(50%) | 0.44 |
| Urine | 49 (18.42%) | 7(14.28%) | 13(26.53%) | 29(28.6%) | 0.5670 |
| Endobronchial sec. | 10 (3.75%) | 0 | 1(10.0%) | 9(88.88%) | 0.1815 |
| Sputum | 14 (5.26%) | 1(7.14%) | 1(7.14%) | 12(75%) | 0.2251 |
| NBL | 09 (3.38%) | 4(44.9%) | 2(22.25%) | 3(14.3%) | 0.0257 |
| Tissue | 18 (6.76%) | 1(5.55%) | 1(5.55%) | 16(100%) | 0.0774 |
| Fluid | 11 (4.13%) | 1(9.09%) | 1(9.09%) | 9(50%) | 0.4537 |
| Ear, Throat and Wound swab | 27 (10.15%) | 4(14.81%) | 3(11.11%) | 20(100%) | 0.3778 |
| Blood | 13 (4.88%) | 0 | 7(53.84%) | 6(66.66%) | 0.0105 |
| Pleural fluid | 02 (0.75%) | 1(50%) | 0 | 1(50%) | 0.3174 |
| CVP tip | 05 (1.87%) | 1(20%) | 2(40%) | 2(40%) | 0.4956 |
| Peritoneal fluid | 02 (0.75%) | 1(50%) | 0 | 1(50%) | 0.3174 |
| Tracheal tube tip | 01 (0.37%) | 0 | 1(100%) | 0 | 0.1588 |
| Chest tube tip | 01 (0.37%) | 0 | 0 | 1(100%) | 0.7567 |
| Catheter tip | 02 (0.75%) | 1(50%) | 0 | 1(50%) | 0.3174 |
| Sample ID | MIC-p (µg/ml) | MIC-b (µg/ml) | MBEC (µg/ml) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CIP | LEV | CEF | CIP | LEV | CEF | CIP | LEV | CEF | |
| 20 | <0.25 | 2 | 0.5 | 128 | 64 | 128 | >2048 | >2048 | >2048 |
| 44 | 1 | 0.5 | 2 | 256 | 16 | 128 | >2048 | >2048 | >2048 |
| 92 | 1 | 2 | 1 | 128 | 64 | 256 | >2048 | >2048 | >2048 |
| 147 | 0.25 | 0.5 | 1 | 128 | 64 | 256 | >2048 | >2048 | >2048 |
| 172 | 0.25 | 0.5 | 0.5 | 256 | 64 | 128 | >2048 | >2048 | >2048 |
| 188 | 1 | 0.5 | 1 | 256 | 64 | 256 | >2048 | >2048 | >2048 |
| S. no. | Isolate no. | Gentamicin (GEN) | Cefepime (CEF) | GEN+CEF (Combination) | |||
|---|---|---|---|---|---|---|---|
| MBIC (µg/ml) | MBEC (µg/ml) | MBIC (µg/ml) | MBEC (µg/ml) | MBIC (µg/ml) | MBEC (µg/ml) | ||
| 1. | PA21 | 512 | 1024 | 512 | 1024 | 32CEF+16GEN | 32 CEF + 32GEN |
| 2. | PA40 | 512 | 1024 | 256 | 512 | 32CEF+16GEN | 32CEF+ 32GEN |
| S. no. | Isolate no. | Ciprofloxacin (CIP) | Cefepime (CEF) | CIP+CEF (Combination) | |||
| MBIC (µg/ml) | MBEC (µg/ml) | MBIC (µg/ml) | MBEC (µg/ml) | MBIC (µg/ml) | MBEC (µg/ml) | ||
| 1. | PA21 | 512 | 1024 | 512 | 1024 | 64CIP+ 16CEF | 64 CIP+ 32 CEF |
| 2. | PA40 | 512 | 1024 | 256 | 512 | 128CIP+16CEF | 128 CIP+ 32 CEF |
| S.No./ Isolate no. | Well no. | Drug Concentration Drug A: Cefepime Drug B: Gentamicin |
FIC index value Synergy ≤ 0.5 |
S.No./ Isolate no. | Well no. | Drug Concentration Drug A: Ciprofloxacin Drug B: Cefepime |
FIC index value Synergy ≤ 0.5 |
|---|---|---|---|---|---|---|---|
| 1. | D2 | 128A, 16B | 0.28 | 3. | D2 | 128A, 16B | 0.28 |
| D3 | 128A, 32B | 0.3 | D3 | 128A, 32B | 0.312 | ||
| D4 | 128A, 64B | 0.37 | D4 | 128A, 64B | 0.375 | ||
| E2 | 64A, 16B | 0.15 | D5 | 128A, 128B | 0.5 | ||
| E3 | 64A, 32B | 0.18 | E2 | 64A, 16B | 0.15 | ||
| E4 | 64A, 64B | 0.25 | E3 | 64A, 32B | 0.19 | ||
| E5 | 64A, 128B | 0.37 | E5 | 64A, 128B | 0.375 | ||
| F2 | 32A, 16B | 0.09 | 4. | D2 | 128A, 16B | 0.31 | |
| F3 | 32A, 32B | 0.12 | D3 | 128A, 32B | 0.375 | ||
| F4 | 32A, 64B | 0.18 | D4 | 128A, 64B | 0.5 | ||
| F5 | 32A, 128B | 0.31 | |||||
| 2. | E2 | 64A, 16B | 0.28 | ||||
| E3 | 64A, 32B | 0.31 | |||||
| E4 | 64A, 64B | 0.375 | |||||
| F2 | 32A, 16B | 0.156 | |||||
| F3 | 32A, 32B | 0.186 | |||||
| F4 | 32A, 64B | 0.25 | |||||
| F5 | 32A, 128B | 0.375 |
3. Discussion
4. Materials and Methods
4.1. Sample collection and susceptibility profile
4.2. Phenotypic and genotypic detection of ESBLs and carbapenemases
4.3. Minimum Inhibitory Concentration planktonic (MIC-p)
4.4. Biofilm formation assay
4.5. Effect of sub-MICs of CIP, LEV, and CEF on biofilm formation
4.6. Evaluation of synergistic effect of antibioticsby checkerboard method
4.7. Evaluation of synergistic effect of antibiotics at sub-MIC level by checkerboard method
Conclusion
Author Contributions
Funding
Conflicts of Interest
References
- Barbier, M.; Owings, J.P.; Martínez-Ramos, I.; Damron, F.H.; Gomila, R.; Blázquez, J.; Goldberg, J.B.; Albertí, S. Lysine trimethylation of EF-Tu mimics platelet-activating factor to initiate Pseudomonas aeruginosa pneumonia. MBio 2013, 4, e00207-13. [Google Scholar] [CrossRef] [PubMed]
- Fazeli, H.; Akbari, R.; Moghim, S.; Narimani, T.; Arabestani, M.R.; Ghoddousi, A.R. Pseudomonas aeruginosa infections in patients, hospital means, and personnel's specimens. Journal of research in medical sciences: the official journal of Isfahan University of Medical Sciences 2012, 17, 332. [Google Scholar] [PubMed]
- Thaden, J.T.; Park, L.P.; Maskarinec, S.A.; Ruffin, F.; Fowler, V.G., Jr.; van Duin, D. Results from a 13-year prospective cohort study show increased mortality associated with bloodstream infections caused by Pseudomonas aeruginosa compared to other bacteria. Antimicrobial agents and chemotherapy 2017, 61, e02671-16. [Google Scholar] [CrossRef] [PubMed]
- Micek, S.T.; Wunderink, R.G.; Kollef, M.H.; Chen, C.; Rello, J.; Chastre, J.; Antonelli, M.; Welte, T.; Clair, B.; Ostermann, H. An international multicenter retrospective study of Pseudomonas aeruginosa nosocomial pneumonia: impact of multidrug resistance. Critical Care 2015, 19, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tamma, P.; Aitken, S.; Bonomo, R., IDSA guidance on the treatment of antimicrobial-resistant gram-negative infections: version 2.0. 2022. 2022.
- Kadri, S.S.; Adjemian, J.; Lai, Y.L.; Spaulding, A.B.; Ricotta, E.; Prevots, D.R.; Palmore, T.N.; Rhee, C.; Klompas, M.; Dekker, J.P. Difficult-to-treat resistance in gram-negative bacteremia at 173 US hospitals: retrospective cohort analysis of prevalence, predictors, and outcome of resistance to all first-line agents. Clinical Infectious Diseases 2018, 67, 1803–1814. [Google Scholar] [CrossRef]
- Al Salman, J.; Al Dabal, L.; Bassetti, M.; Alfouzan, W.A.; Al Maslamani, M.; Alraddadi, B.; Elhoufi, A.; Enani, M.; Khamis, F.A.; Mokkadas, E. Management of infections caused by WHO critical priority Gram-negative pathogens in Arab countries of the Middle East: a consensus paper. International journal of antimicrobial agents 2020, 56, 106104. [Google Scholar] [CrossRef]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E. Sobrevivir Sepsis Campaign: Directrices internacionales para el manejo de la sepsis y el shock séptico: 2016. Intensive Care Med 2017, 43, 304–377. [Google Scholar] [CrossRef]
- Bassetti, M.; Vena, A.; Croxatto, A.; Righi, E.; Guery, B. How to manage Pseudomonas aeruginosa infections. Drugs in context 2018, 7. [Google Scholar] [CrossRef]
- Bassetti, M.; Vena, A.; Russo, A.; Croxatto, A.; Calandra, T.; Guery, B. Rational approach in the management of Pseudomonas aeruginosa infections. Current opinion in infectious diseases 2018, 31, 578–586. [Google Scholar] [CrossRef]
- Zakhour, J.; Sharara, S.L.; Hindy, J.-R.; Haddad, S.F.; Kanj, S.S. Antimicrobial Treatment of Pseudomonas aeruginosa Severe Sepsis. Antibiotics 2022, 11, 1432. [Google Scholar] [CrossRef]
- Macia, M.; Rojo-Molinero, E.; Oliver, A. Antimicrobial susceptibility testing in biofilm-growing bacteria. Clinical Microbiology and Infection 2014, 20, 981–990. [Google Scholar] [CrossRef]
- Noreen, A.; Masood, H.; Zaib, J.; Rafaque, Z.; Fatima, A.; Shabbir, H.; Alam, J.; Habib, A.; Noor, S.; Dil, K. Investigating the Role of Antibiotics on Induction, Inhibition and Eradication of Biofilms of Poultry Associated Escherichia coli Isolated from Retail Chicken Meat. Antibiotics 2022, 11, 1663. [Google Scholar] [CrossRef]
- Nucleo, E.; Steffanoni, L.; Fugazza, G.; Migliavacca, R.; Giacobone, E.; Navarra, A.; Pagani, L.; Landini, P. Growth in glucose-based medium and exposure to subinhibitory concentrations of imipenem induce biofilm formation in a multidrug-resistant clinical isolate of Acinetobacter baumannii. BMC microbiology 2009, 9, 1–14. [Google Scholar] [CrossRef]
- Abbey, T.C.; Deak, E. What's New from the CLSI Subcommittee on Antimicrobial Susceptibility Testing M100. Clinical Microbiology Newsletter 2019, 41, 203–209. [Google Scholar] [CrossRef]
- Amjad, A.; Mirza, I.A.; Abbasi, S.; Farwa, U.; Malik, N.; Zia, F. Modified Hodge test: A simple and effective test for detection of carbapenemase production. Iranian journal of microbiology 2011, 3, 189. [Google Scholar]
- Saha, M.R.; Jhora, S.T. Detection of extended spectrum beta-lactamase producing Gram-negative organisms: hospital prevalence and comparison of double disc synergy and E-test methods. IMC Journal of Medical Science 2018, 12, 32–36. [Google Scholar] [CrossRef]
- Ali, I.; Rafaque, Z.; Ahmed, I.; Tariq, F.; Graham, S.E.; Salzman, E.; Foxman, B.; Dasti, J.I. Phylogeny, sequence-typing and virulence profile of uropathogenic Escherichia coli (UPEC) strains from Pakistan. BMC infectious diseases 2019, 19, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rafaque, Z.; Abid, N.; Liaqat, N.; Afridi, P.; Siddique, S.; Masood, S.; Kanwal, S.; Dasti, J.I. In-vitro Investigation of Antibiotics Efficacy Against Uropathogenic Escherichia coli Biofilms and Antibiotic Induced Biofilm Formation at Sub-Minimum Inhibitory Concentration of Ciprofloxacin. Infection and Drug Resistance 2020, 13, 2801. [Google Scholar] [CrossRef] [PubMed]
- Drago, L.; De Vecchi, E.; Nicola, L.; Colombo, A.; Guerra, A.; Gismondo, M.R. Activity of levofloxacin and ciprofloxacin in combination with cefepime, ceftazidime, imipenem, piperacillin-tazobactam and amikacin against different Pseudomonas aeruginosa phenotypes and Acinetobacter spp. Chemotherapy 2004, 50, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Thiyagarajan, D.; Das, G.; Ramesh, A. Amphiphilic Cargo-Loaded Nanocarrier Enhances Antibiotic Uptake and Perturbs Efflux: Effective Synergy for Mitigation of Methicillin-Resistant Staphylococcus aureus. ChemMedChem 2017, 12, 1125–1132. [Google Scholar] [CrossRef]
- Wang, L.; Di Luca, M.; Tkhilaishvili, T.; Trampuz, A.; Gonzalez Moreno, M. Synergistic activity of fosfomycin, ciprofloxacin, and gentamicin against Escherichia coli and Pseudomonas aeruginosa biofilms. Frontiers in microbiology 2019, 10, 2522. [Google Scholar] [CrossRef]
- Dosler, S.; Karaaslan, E.; Alev Gerceker, A. Antibacterial and anti-biofilm activities of melittin and colistin, alone and in combination with antibiotics against Gram-negative bacteria. Journal of Chemotherapy 2016, 28, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Almaaytah, A.; Abualhaijaa, A.; Alqudah, O. The evaluation of the synergistic antimicrobial and antibiofilm activity of AamAP1-Lysine with conventional antibiotics against representative resistant strains of both Gram-positive and Gram-negative bacteria. Infection and drug resistance 2019, 12, 1371. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, H.; Memar, M.Y.; Sefidan, F.Y.; Yekani, M.; Ghotaslou, R. In vitro synergy of antibiotic combinations against planktonic and biofilm Pseudomonas aeruginosa. GMS hygiene and infection control 2017, 12. [Google Scholar]
- Gupta, P.; Chhibber, S.; Harjai, K. Subinhibitory concentration of ciprofloxacin targets quorum sensing system of Pseudomonas aeruginosa causing inhibition of biofilm formation & reduction of virulence. The Indian journal of medical research 2016, 143, 643. [Google Scholar]
- Ellappan, K.; Narasimha, H.B.; Kumar, S. Coexistence of multidrug resistance mechanisms and virulence genes in carbapenem-resistant Pseudomonas aeruginosa strains from a tertiary care hospital in South India. Journal of global antimicrobial resistance 2018, 12, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Ullah, W.; Qasim, M.; Rahman, H.; Bari, F.; Khan, S.; Rehman, Z.U.; Khan, Z.; Dworeck, T.; Muhammad, N. Multi drug resistant Pseudomonas aeruginosa: Pathogen burden and associated antibiogram in a tertiary care hospital of Pakistan. Microbial pathogenesis 2016, 97, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Gimeno, C.; Cantón, R.; García, A.; Gobernado, M. Comparative activity of doripenem, meropenem, and imipenem in recent clinical isolates obtained during the COMPACT-Spain epidemiological surveillance study. Revista Espanola de Quimioterapia: Publicacion Oficial de la Sociedad Espanola de Quimioterapia 2010, 23, 144–152. [Google Scholar]
- Shenkutie, A.M.; Zhang, J.; Yao, M.; Asrat, D.; Chow, F.W.; Leung, P.H. Effects of sub-minimum inhibitory concentrations of imipenem and colistin on expression of biofilm-specific antibiotic resistance and virulence genes in Acinetobacter baumannii sequence type 1894. International Journal of Molecular Sciences 2022, 23, 12705. [Google Scholar] [CrossRef]
- Paul, M.; Carrara, E.; Retamar, P.; Tängdén, T.; Bitterman, R.; Bonomo, R.A.; De Waele, J.; Daikos, G.L.; Akova, M.; Harbarth, S. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gram-negative bacilli (endorsed by European society of intensive care medicine). Clinical Microbiology and Infection 2022, 28, 521–547. [Google Scholar] [CrossRef]
- Anderl, J.N.; Franklin, M.J.; Stewart, P.S. Role of antibiotic penetration limitation in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrobial agents and chemotherapy 2000, 44, 1818–1824. [Google Scholar] [CrossRef] [PubMed]
- Vrany, J.D.; Stewart, P.S.; Suci, P.A. Comparison of recalcitrance to ciprofloxacin and levofloxacin exhibited by Pseudomonas aeruginosa bofilms displaying rapid-transport characteristics. Antimicrobial Agents and Chemotherapy 1997, 41, 1352–1358. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





