1. Introduction
Although infections after anterior cruciate ligament reconstruction (r-ACL) are not as common as other implant-associated infections, the magnitude of this complication is equally important since inappropriate treatment could compromise joint function and return to sports activities [
1,
2].
For this reason, various studies have focused on the study of this pathology in recent years. Most of those studies concluded that aggressive arthroscopic debridement in combination with specific antibiotic therapy should be the treatment of choice for this complication [
1,
3,
4]. Several studies have also focused on the development of infection prophylaxis techniques like the vancomycin bath. It dramatically reduces the incidence of infection [
5,
6,
7,
8,
9,
10].
Other studies have focused on the origin of the infections. Some authors have been able to demonstrate that the infections arise as the result of contamination by coagulase negative staphylococci during the preparation of the graft [
11]. These microorganisms are part of the normal microbiota of the skin and mucous membranes.
There are data published by several authors in which higher rates of infection in r-ACL are observed when quadruplicate hamstring grafts (4xHt grafts) are used when compared to surgeries done with patellar tendon grafts (BPTB grafts) [
6,
12,
13]. Data from a meta-analysis showed an overall estimated infection rate in r-ACL of 0.9% (Confidence interval (CI) 95% 0.8% to 1.0%) [
14]. There was also a higher infection rate in 4xHt autografts surgeries (1.1%, CI 95% 0.9% to 1.2%) than with BPTB autografts (0.7%, CI 95% 0.6% to 0.9%) and allografts of any type (0.5%, CI 95% 0.4% to 0.8%) (Q 5 15.58,
p = 0.001). Therefore, it is thought that 4xHt grafts may be a predisposing factor for infection. However, there are no studies that give a verifiable explanation for this phenomenon. Nevertheless, multiple hypothesis has been proposed.
One of them is that sutures used in 4xHt grafts surgeries may harbor bacteria and that may be a risk factor for the development of an infection [
3]. It has been shown that contamination during surgical procedures is frequent [
15,
16,
17,
18,
19]. However, not all contamination will lead to infection. For an infection to occur, minimal bacterial contamination is required (minimal infective dose MID) [
20]. The probability of an infection to come about is directly proportional to the amount of bacterial inoculation during contamination [
21,
22]. This relation is especially relevant in infections related to foreign bodies [
17,
23,
24]. There is strong evidence that biofilm growth does not grow in the same way on different surfaces [
23,
24,
25,
26]. It has also been shown that as surgical sutures surface are recognized as foreign bodies, it makes for the growth of biofilm [
27,
28].
All this leads one to think that a greater bacterial load is introduced into the body in 4xHt graft surgeries, reaching the MID more frequently. This may be the reason for the higher rate of infections in r-ACL surgeries performed with 4xHt grafts as they require sutures. Those sutures might behave like a foreign body that facilitates the growth of biofilm. With the same contaminating bacterial inoculum, biofilm formation would be more elevated in 4xHt grafts than that produced in BPTB grafts in which sutures are not used. This would allow for the introduction of a greater bacterial load in the subject, raising the risk of infection [
20,
21,
22]. This hypothesis has also been proposed by other authors but never explored [
3].
Something similar was observed in the classic studies of Elek et al. that were performed in 1956. In those studies, they observed that a wound contaminated with the same quantity of bacteria is more likely to develop an infection if there are sutures on it [
29,
30].
The objective of the present study was to evaluate this hypothesis by comparing biofilm formation in BPTB grafts vs. 4xHt grafts, with both contaminated with the same bacterial inoculum in vitro.
The hypothesis is that biofilm growth will be greater in 4xHt grafts than in BPTB grafts due to the greater formation of biofilm around sutures.
2. Materials and Methods
A descriptive in vitro study was carried out. For the production of grafts, a 4xHt graft and a BPTB graft were prepared from sterilized and frozen cadaveric donor samples.
The grafts were provided by Banc de Sang i Teixits (BST) de Barcelona. Subsequently, they were prepared by an orthopedic surgeon with specific training and experience in carrying out this type of grafts. The production of these grafts was done in a manner analogous to the one used during the usual surgical procedures, in a sterile environment. In the case of the 4xHt graft, a high-resistance suture of the same type as those used in normal practice was used (FiberWire, Arthrex, Munich, Germany).
For the BPTB graft, a remodeling of the bony parts to achieve 10mm diameter was performed as in daily clinical practice.
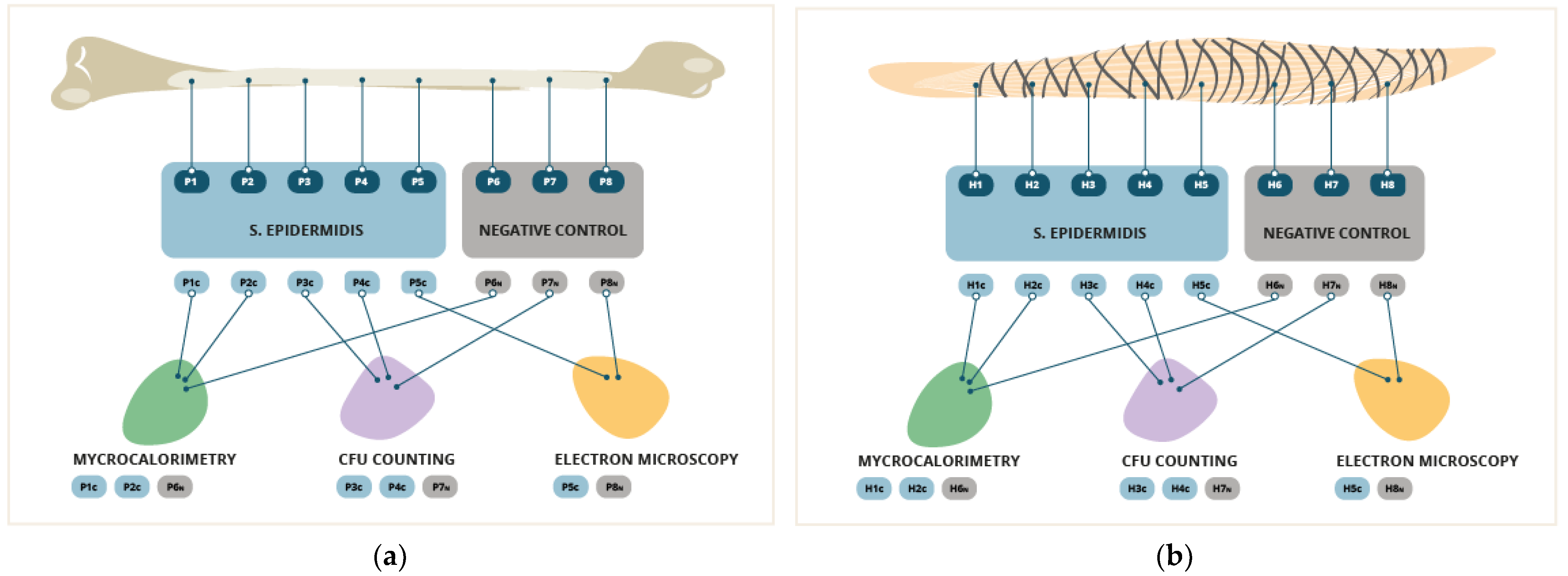
Both of the grafts were divided into 8 representative fragments of each type of graft.
We consider a representative fragment one that contains the representative elements of the complete graft. In the case of the BPTB graft, eight 5mm x 5mm x 1mm fragments of patellar tendon were produced using a surgical scalpel. The decision was taken to use these dimensions because they are the maximum dimensions that can be used in the analysis processes to be carried out later. In the case of the 4xHt graft, a representative fragment was considered to be one that contained a hamstring tendon fragment and a suture fragment. These fragments were made with the same dimensions and followed the technique as those previously described.
This study was approved by Parc de Salut Mar Drug Research Ethics Committee with number (CEIC clinical research project no. 2018/8269/I) on May 29, 2019.
2.1. Contamination and Biofilm Growth Conditions
In 5 fragments from each sample, the formation of artificial biofilm was brought on. To form the bacterial biofilm, the samples were placed in 2ml of heart-brain infusion broth culture medium (BHIb, Sigma-Aldrich, St. Louis, MO, USA) contaminated at 1x10
(5) CFU/ml by Staphylococcus epidermidis (ATCC 35984). They were incubated at 37°C for 24 hours. This bacterium was used because it is one of the most frequent causes of infection in the context of r-ACL [
28]. Moreover, its great capacity for biofilm formation is well known. After the formation of the biofilm, the samples were washed three times with 2ml of 0.9% NaCl to eliminate the bacteria that were in planktonic form. Three fragments from each sample were left uncontaminated as negative controls. These fragments were incubated in a 0.9% NaCl solution at 37°C for 24 hours to simulate the same conditions as the contaminated samples, based on the technique previously described in multiple studies [
31,
32]. (
Figure 1)
2.2. Isothermal Microcalorimetry
In 2 contaminated fragments and 1 negative control of each type of graft, the heat produced by the S. epidermidis bacterial population was monitored with the microcalorimeter using the isothermal microcalorimetry method (TAM III, TA Instruments, Newcastle, DE, USA). It is the same as the one described by Butini et al. [
33].
2.3. Sonication and Plating and CFU Counting
Later, the sonication and seeding analysis was carried out on 2 contaminated fragments and 1 negative control of each type of graft. Each of them was placed in a solution of 1ml 0.9% NaCl, vortexed for 30 seconds and sonicated at an intensity of 40kHza and 0.1 watt/cm2 (BactoSonic, BANDELIN electronic, Berlin, Germany) for 1 minute. Then, they were sonicated again for an additional 30 seconds. Next, 100mcL of the sonication product was seeded in Tryptic Soy Agar (TSA) (Sigma-Aldrich, St. Louis, MO, USA). After 24 hours of incubation at 37ºC, the counting of colony-forming units CFU/mL was carried out in accordance with the previously described technique [
34].
Accepting an alpha risk of 0.05 and a beta risk of less than 0.2 in a bilateral contrast, 2 subjects in the first group and 2 in the second group were needed to detect a difference equal to or greater than 5 units. The common standard deviation was assumed to be 1.5. A loss to follow-up rate was estimated at 0%.
2.4. Scanning Electron Microscopy (SEM)
Lastly, an analysis was performed by means of scanning electron microscopy (GeminiSEM 300, Carl Zeiss, OberkochenDSM 982 GEMINI, Zeiss Oberkochen, Germany) to determine the biofilm growth areas in the samples. This was done in accordance with the previously described technique [
34] with proven validity in biofilm evaluation [
35,
36]. For this analysis, 1 contaminated fragment and 1 negative control fragment of each type of sample were used.
2.5. Statistic Analysis.
The statistical analyses were performed using the SigmaPlot software package (version 13.0; Systat Software, Chicago, IL, USA) and Prism for the graphics (version 8; GraphPad, La Jolla, CA, USA). Continuous variables are presented as means (with standard deviation in parenthesis, SD) and ranges. The unpaired T-test was used to assess CFU counting. A p value under 0.05 was considered statistically significant.
3. Results
3.1. Isothermal Microcalorimetry
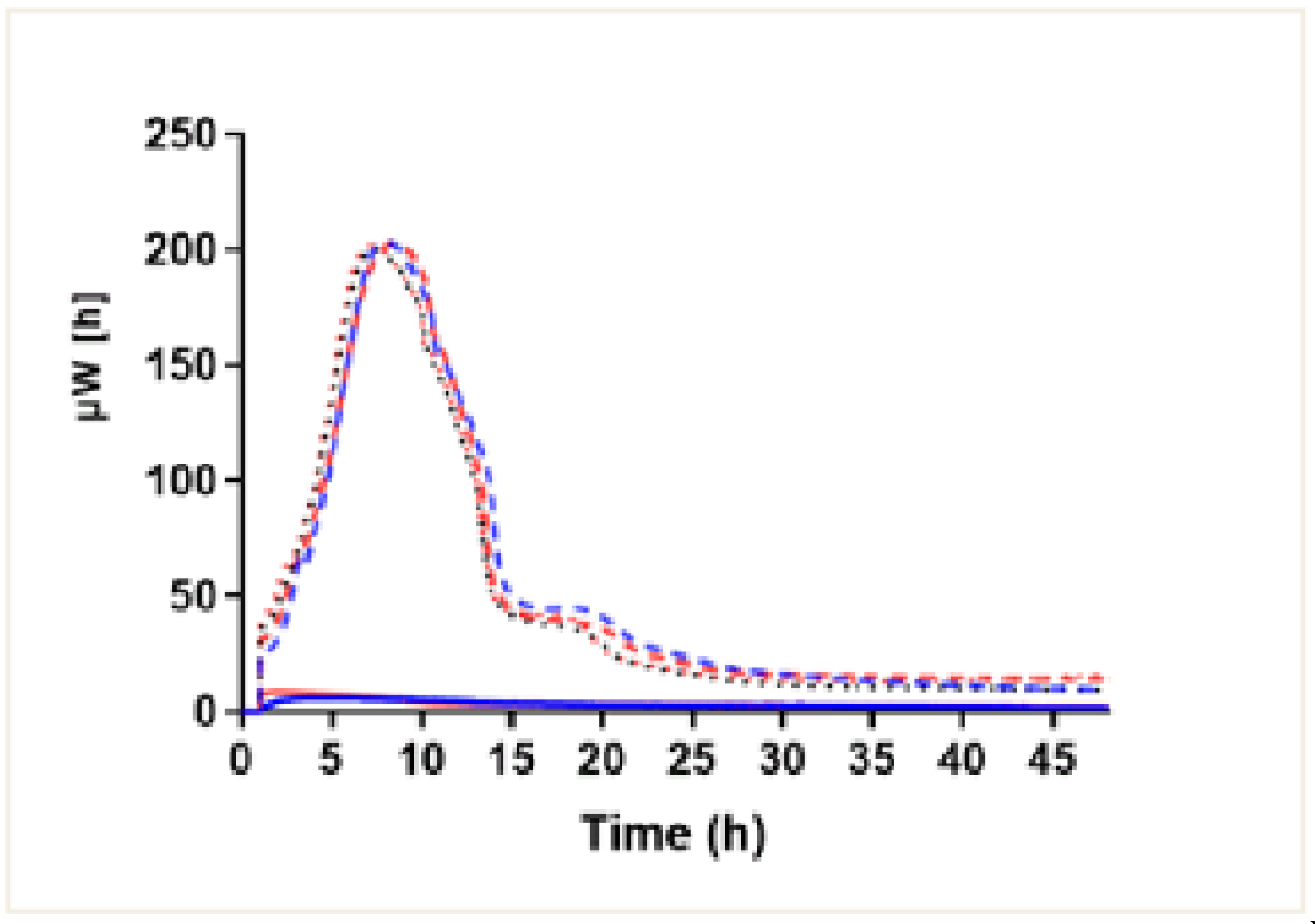
Using the isothermal microcalorimetry method, we observed bacterial growth in the contaminated fragments from both the 4xHt graft and BPTB graft. They showed the same growth dynamics, the one typical of this bacterial population. In the beginning, the microcalorimetry curves showed exponential growth rates until they reached a population peak (200uW) at approximately 8h after contamination. Then, after a short or non-existent stationary phase, a rapid decline in the bacterial population was detected. Later, there was a phase of senescence or death characteristic of this type of population (
Figure 2), with no significant differences between them. In the BPTB and 4xHt graft fragments that were not contaminated (negative controls), no growth profile was observed. Therefore, no significant differences between the bacterial growth profiles of any of the 4xHt and BPTB grafts were found.
3.2. CFU Counting Method
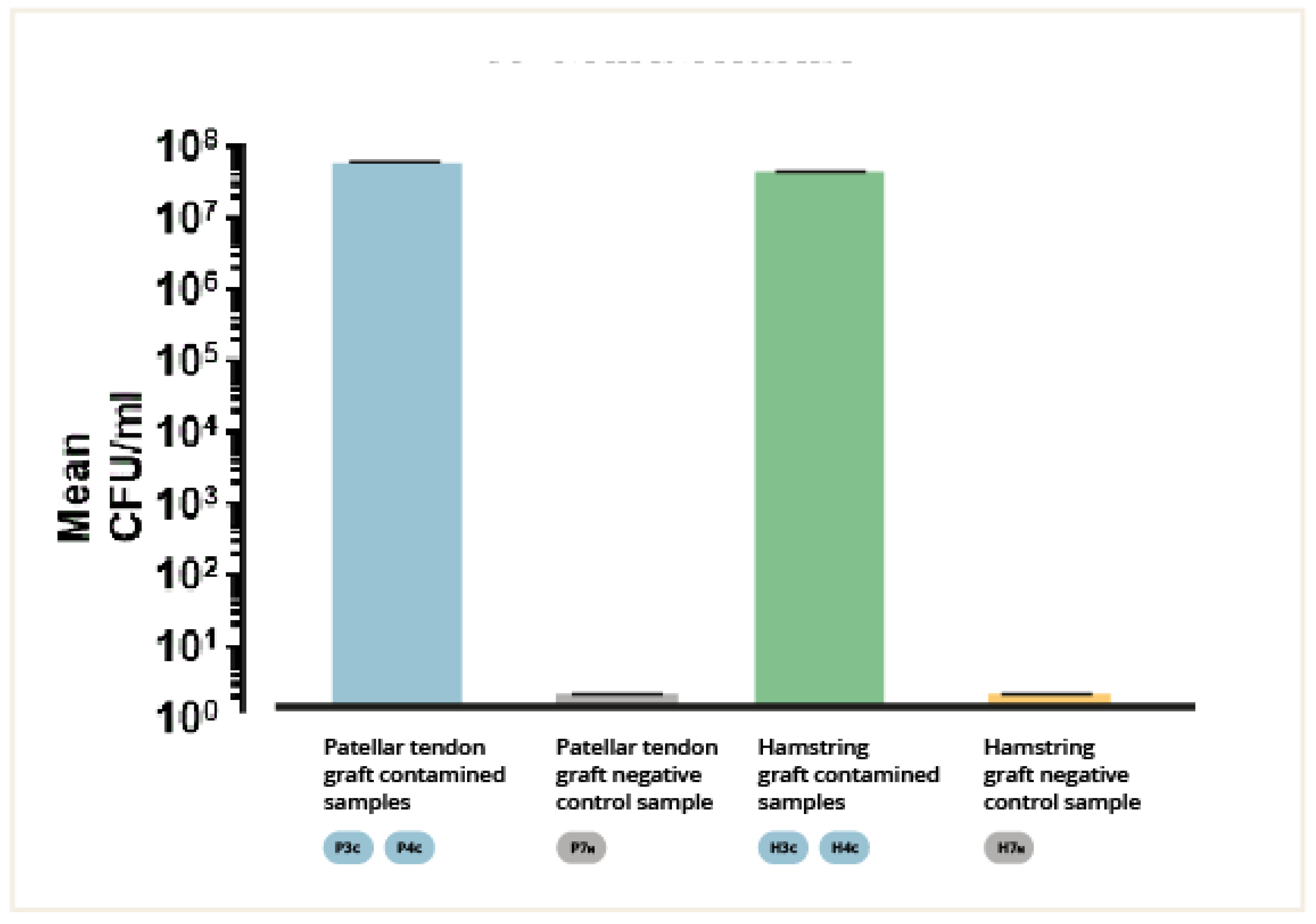
The number of colony-forming units per milliliter (CFU/ml) of the fluid extracted by sonication of the contaminated BPTB and 4xHt grafts fragments was determined. The means of the CFU/ml of the different groups were calculated and analyzed and compared (mean ± SEM, 3.5x107 ± 3450000 CFU/ml and 4.6 x107 ± 1.455e + 7 CFU/ml, respectively, p = 0.6667). (
Figure 3). No significant differences were detected between the 4xHt graft group and the BPTB graft groups (p = n.s.). Seeding of the fluid extracted by sonication of the uncontaminated BPTB and 4xHt grafts fragments (negative controls) did not produce any colony growth.
3.3. Electron Microscopy
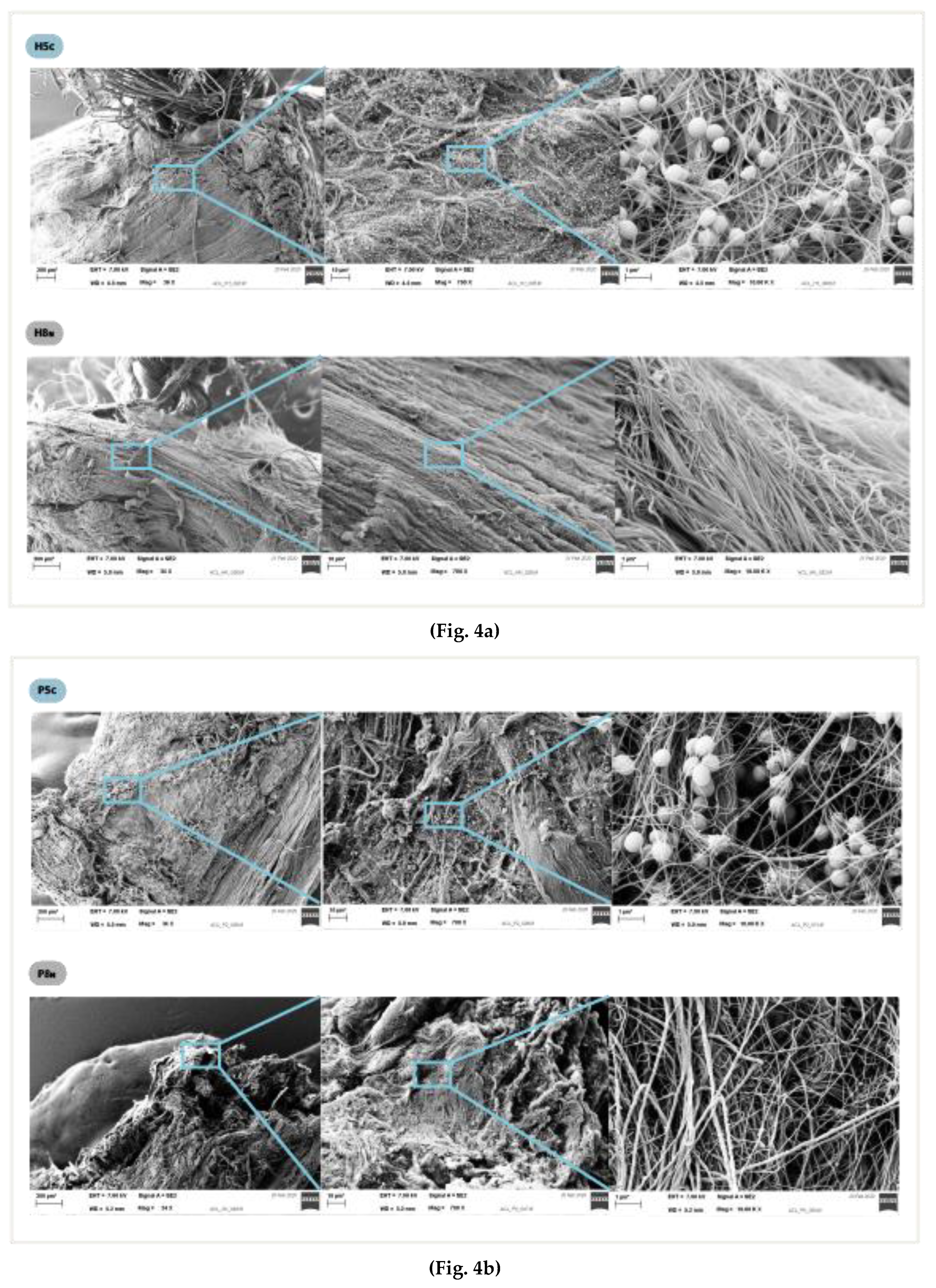
In the electron microscopy analysis, no specific or differential biofilm growth pattern was detected upon comparing the contaminated BPTB graft fragment to the corresponding 4xHt graft fragment. There was a homogeneous growth pattern regardless of the surface on which the biofilm grew. Specifically, an increase in colonization was not observed in the suture areas. (
Figure 3a,b) No bacteria was found in the negative control samples.
Figure 4.
Electron microscopy at magnification of 34x, 700x and10.00 Kx: (a) Hamstring graft samples, contaminated and negative control. (b) Patellar tendon graft samples, contaminated and negative controls.
Figure 4.
Electron microscopy at magnification of 34x, 700x and10.00 Kx: (a) Hamstring graft samples, contaminated and negative control. (b) Patellar tendon graft samples, contaminated and negative controls.
4. Discussion
The initial hypothesis of this study equating the BPTB and 4xHt grafts in their potential for biofilm growth under the contamination of a same bacterial inoculum in vitro has been refuted.
The structural differences between the two grafts types do not have any effect on the production of biofilm during r-ACL surgery, at least outside of the body. Therefore, differences in infection rates in r-ACL surgeries with BPTB and 4xHt grafts cannot be justified in this way.
Electron microscopy analysis showed a homogeneous biofilm growth pattern. These findings rule out the sutures present in 4xHt grafts being a better surface for biofilm growth than the tendon itself. This suggests that both tissues act as foreign bodies that equally make for biofilm growth. Another study showed no significant differences in infection rates between allografts and autografts, which supports our finding that autografts and allografts behave like foreign bodies [
14]. Furthermore, 4xHt autografts are associated with a higher infection rate when compared to both allografts and BPTBp autografts. This study was performed only with frozen cadaveric donor grafts. We are aware of the bias that it may produce. Working with fresh donor grafts would have been more difficult to do for ethical and practical reasons. However, as has been previously stated, we expected no big differences between the use of fresh and cadaveric grafts.
The fact that grafts, by their very nature, are not a predisposing factor for biofilm growth leads us to think that there must be some differences surrounding their processing that changes and conditions the differences in infection rates. One possible reason is that there are differences in the contamination of grafts. It is well known that contamination during a surgical procedures is time-dependent [
37]. The production of the 4xHt graft is more time-consuming due to its greater complexity [
5,
9] . We also know that contamination of grafts occurs during the process of making them [
11]. This further leads us to consider that contamination by considerably more bacterial inoculum might occur during the preparation of 4xHt grafts, more frequently reaching the minimal infectious doses (MID). In this way, we come by the higher infection rates seen in r-ACL surgeries done with 4xHt grafts. Another hypothesis is that slightly more extensive tissue dissection and morbidity is done at the 4xHt autograft harvest site compared to the BPTB autograft harvest site [
38,
39], justifying the difference in infection rates.
This study has some limitations that should be mentioned. Only S. epidermidis was used for contamination and biofilm formation. It would be interesting to perform the same analysis with other types of gram-positive staphylococci that are frequently associated with this pathology. However, it is unlikely that differences would be found due to their similarity to S. epidermidis. On the other hand, we believe that repeating the analysis with pathogens of other strains like anaerobes would be of little relevance due to their low prevalence in r-ACL infections.
Another limitation is the concern around this study being an in vitro study. This does not make for exploring the host-pathogen interaction. However, for the moment, this is the best evidence we can provide relative to this topic for bioethical reasons as the factors mentioned above cannot be explored experimentally in vivo.
Another factor that was beyond our control in this study was the host characteristics (immune status, comorbidities, etc.). Then again, we know that the infection rate differs according to the type of graft used in studies with a large cohort in which the patients are heterogeneous [
6,
12,
13]. This makes us think that the differences in infection rates must be due to differences that are independent of the individuals themselves. That means, that regardless of the individual, their circumstances and their characteristics, infections are more frequent in surgeries performed with hamstring grafts. Therefore, in terms of the host factors, host-pathogen interaction, and the pathogen, the differential factor should not be looked to as the host factor. We believe this is an argument that favors a positive evaluation of our study.
5. Conclusions
We have found that the amount of biofilm formed on BPTB and 4xHt grafts by the same bacterial inoculum is comparable in vitro. Therefore, the 4xHt graft is not intrinsically a predisposing factor for biofilm growth due to its structure and composition when contaminated outside of the body.
Author Contributions
F.C. wrote the manuscript; F.C. and A.T. contributed in methodology design; O.F. and D.P.-P. contributed to sample acquisition; F.C. and S.K. carried out the experiments and prepared the figures; F.C., D.P.-P. and S.K. contributed in investigation, data curation and formal analysis ; F.C., D.P.-P., S.K., A.T., and J.C.M. revised and edited the manuscript; D.P.-P, A.T., and J.C.M. supervised the study; F.C. conceptualized, wrote, and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declartion of Helsinki, and approved by the Ethics Committee of Parc de Salut Mar Drug Research Ethics Committee with number (CEIC clinical research project no. 2018/8269/I) on May 29, 2019.
Acknowledgments
We thank the Proimplant Foundation for their support. We are also grateful to the Core Facility for Electron Microscopy of the Charité-Universitätsmedizin Berlin for their support in image acquisition. Additionally: we would like to thank Mr. Eric L. Goode for his help with English correction and style.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Boström Windhamre, H.; Mikkelsen, C.; Forssblad, M.; Willberg, L. Postoperative Septic Arthritis after Anterior Cruciate Ligament Reconstruction: Does It Affect the Outcome? A Retrospective Controlled Study. Arthrosc. J. Arthrosc. Relat. Surg. Off. Publ. Arthrosc. Assoc. N. Am. Int. Arthrosc. Assoc. 2014, 30, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Zimmerli, W.; Trampuz, A.; Ochsner, P.E. Prosthetic-Joint Infections. N. Engl. J. Med. 2004, 351, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Maletis, G.B.; Inacio, M.C.S.; Reynolds, S.; Desmond, J.L.; Maletis, M.M.; Funahashi, T.T. Incidence of Postoperative Anterior Cruciate Ligament Reconstruction Infections: Graft Choice Makes a Difference. Am. J. Sports Med. 2013, 41, 1780–1785. [Google Scholar] [CrossRef] [PubMed]
- Torres-Claramunt, R.; Pelfort, X.; Erquicia, J.; Gil-González, S.; Gelber, P.E.; Puig, L.; Monllau, J.C. Knee Joint Infection after ACL Reconstruction: Prevalence, Management and Functional Outcomes. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2013, 21, 2844–2849. [Google Scholar] [CrossRef] [PubMed]
- Baron, J.E.; Shamrock, A.G.; Cates, W.T.; Cates, R.A.; An, Q.; Wolf, B.R.; Bollier, M.J.; Duchman, K.R.; Westermann, R.W. Graft Preparation with Intraoperative Vancomycin Decreases Infection After ACL Reconstruction: A Review of 1,640 Cases. J. Bone Joint Surg. Am. 2019, 101, 2187–2193. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, D.; Figueroa, F.; Calvo, R.; Lopez, M.; Goñi, I. Presoaking of Hamstring Autografts in Vancomycin Decreases the Occurrence of Infection Following Primary Anterior Cruciate Ligament Reconstruction. Orthop. J. Sports Med. 2019, 7, 2325967119871038. [Google Scholar] [CrossRef] [PubMed]
- Offerhaus, C.; Balke, M.; Hente, J.; Gehling, M.; Blendl, S.; Höher, J. Vancomycin Pre-Soaking of the Graft Reduces Postoperative Infection Rate without Increasing Risk of Graft Failure and Arthrofibrosis in ACL Reconstruction. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2019, 27, 3014–3021. [Google Scholar] [CrossRef]
- Pérez-Prieto, D.; Perelli, S.; Corcoll, F.; Rojas, G.; Montiel, V.; Monllau, J.C. The Vancomycin Soaking Technique: No Differences in Autograft Re-Rupture Rate. A Comparative Study. Int. Orthop. 2021, 45, 1407–1411. [Google Scholar] [CrossRef]
- Pérez-Prieto, D.; Torres-Claramunt, R.; Gelber, P.E.; Shehata, T.M.A.; Pelfort, X.; Monllau, J.C. Autograft Soaking in Vancomycin Reduces the Risk of Infection after Anterior Cruciate Ligament Reconstruction. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2016, 24, 2724–2728. [Google Scholar] [CrossRef]
- Phegan, M.; Grayson, J.E.; Vertullo, C.J. No Infections in 1300 Anterior Cruciate Ligament Reconstructions with Vancomycin Pre-Soaking of Hamstring Grafts. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2016, 24, 2729–2735. [Google Scholar] [CrossRef]
- Pérez-Prieto, D.; Portillo, M.E.; Torres-Claramunt, R.; Pelfort, X.; Hinarejos, P.; Monllau, J.C. Contamination Occurs during ACL Graft Harvesting and Manipulation, but It Can Be Easily Eradicated. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2018, 26, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Jefferies, J.G.; Aithie, J.M.S.; Spencer, S.J. Vancomycin-Soaked Wrapping of Harvested Hamstring Tendons during Anterior Cruciate Ligament Reconstruction. A Review of the “Vancomycin Wrap.” The Knee 2019, 26, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Naendrup, J.-H.; Marche, B.; de Sa, D.; Koenen, P.; Otchwemah, R.; Wafaisade, A.; Pfeiffer, T.R. Vancomycin-Soaking of the Graft Reduces the Incidence of Septic Arthritis Following ACL Reconstruction: Results of a Systematic Review and Meta-Analysis. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2020, 28, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Kuršumović, K.; Charalambous, C.P. Relationship of Graft Type and Vancomycin Presoaking to Rate of Infection in Anterior Cruciate Ligament Reconstruction: A Meta-Analysis of 198 Studies with 68,453 Grafts. JBJS Rev. 2020, 8, e1900156. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, H.; Zahar, A.; Lausmann, C.; Gehrke, T.; Fickenscher, H.; Suero, E.M.; Gebauer, M.; Citak, M. High Bacterial Contamination Rate of Electrocautery Tips during Total Hip and Knee Arthroplasty. Int. Orthop. 2018, 42, 755–760. [Google Scholar] [CrossRef]
- Beldame, J.; Lagrave, B.; Lievain, L.; Lefebvre, B.; Frebourg, N.; Dujardin, F. Surgical Glove Bacterial Contamination and Perforation during Total Hip Arthroplasty Implantation: When Gloves Should Be Changed. Orthop. Traumatol. Surg. Res. OTSR 2012, 98, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Davis, N.; Curry, A.; Gambhir, A.K.; Panigrahi, H.; Walker, C.R.; Wilkins, E.G.; Worsley, M.A.; Kay, P.R. Intraoperative Bacterial Contamination in Operations for Joint Replacement. J. Bone Joint Surg. Br. 1999, 81, 886–889. [Google Scholar] [CrossRef]
- Robinson, A.H.; Drew, S.; Anderson, J.; Bentley, G.; Ridgway, G.L. Suction Tip Contamination in the Ultraclean-Air Operating Theatre. Ann. R. Coll. Surg. Engl. 1993, 75, 254–256. [Google Scholar]
- Schweitzer, D.; Klaber, I.; Fischman, D.; Wozniak, A.; Botello, E.; Amenábar, P.P. Surgical Light Handles: A Source of Contamination in the Surgical Field. Acta Orthop. Traumatol. Turc. 2015, 49, 421–425. [Google Scholar] [CrossRef]
- Southwood, R.T.; Rice, J.L.; McDonald, P.J.; Hakendorf, P.H.; Rozenbilds, M.A. Infection in Experimental Hip Arthroplasties. J. Bone Joint Surg. Br. 1985, 67, 229–231. [Google Scholar] [CrossRef]
- Johnson, E.N.; Burns, T.C.; Hayda, R.A.; Hospenthal, D.R.; Murray, C.K. Infectious Complications of Open Type III Tibial Fractures among Combat Casualties. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2007, 45, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Lambert, E.W.; Simpson, R.B.; Marzouk, A.; Unger, D.V. Orthopaedic Injuries among Survivors of USS COLE Attack. J. Orthop. Trauma 2003, 17, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.L.; Costerton, J.W. Using Biofilms as Initial Inocula in Animal Models of Biofilm-Related Infections. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Wimpenny, J.; Manz, W.; Szewzyk, U. Heterogeneity in Biofilms. FEMS Microbiol. Rev. 2000, 24, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W. Cystic Fibrosis Pathogenesis and the Role of Biofilms in Persistent Infection. Trends Microbiol. 2001, 9, 50–52. [Google Scholar] [CrossRef]
- Veerachamy, S.; Yarlagadda, T.; Manivasagam, G.; Yarlagadda, P.K. Bacterial Adherence and Biofilm Formation on Medical Implants: A Review. Proc. Inst. Mech. Eng. [H] 2014, 228, 1083–1099. [Google Scholar] [CrossRef]
- Franco, A.R.; Fernandes, E.M.; Rodrigues, M.T.; Rodrigues, F.J.; Gomes, M.E.; Leonor, I.B.; Kaplan, D.L.; Reis, R.L. Antimicrobial Coating of Spider Silk to Prevent Bacterial Attachment on Silk Surgical Sutures. Acta Biomater. 2019, 99, 236–246. [Google Scholar] [CrossRef]
- Henry-Stanley, M.J.; Hess, D.J.; Barnes, A.M.T.; Dunny, G.M.; Wells, C.L. Bacterial Contamination of Surgical Suture Resembles a Biofilm. Surg. Infect. 2010, 11, 433–439. [Google Scholar] [CrossRef]
- Elek, S.D. Experimental Staphylococcal Infections in the Skin of Man. Ann. N. Y. Acad. Sci. 1956, 65, 85–90. [Google Scholar] [CrossRef]
- Elek, S.D.; Conen, P.E. The Virulence of Staphylococcus Pyogenes for Man; a Study of the Problems of Wound Infection. Br. J. Exp. Pathol. 1957, 38, 573–586. [Google Scholar]
- Drago, L.; Romanò, C.L.; Mattina, R.; Signori, V.; De Vecchi, E. Does Dithiothreitol Improve Bacterial Detection from Infected Prostheses? A Pilot Study. Clin. Orthop. 2012, 470, 2915–2925. [Google Scholar] [CrossRef] [PubMed]
- Karbysheva, S.; Di Luca, M.; Butini, M.E.; Winkler, T.; Schütz, M.; Trampuz, A. Comparison of Sonication with Chemical Biofilm Dislodgement Methods Using Chelating and Reducing Agents: Implications for the Microbiological Diagnosis of Implant Associated Infection. PloS One 2020, 15, e0231389. [Google Scholar] [CrossRef] [PubMed]
- Butini, M.E.; Gonzalez Moreno, M.; Czuban, M.; Koliszak, A.; Tkhilaishvili, T.; Trampuz, A.; Di Luca, M. Real-Time Antimicrobial Susceptibility Assay of Planktonic and Biofilm Bacteria by Isothermal Microcalorimetry. Adv. Exp. Med. Biol. 2019, 1214, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Trampuz, A.; Piper, K.E.; Jacobson, M.J.; Hanssen, A.D.; Unni, K.K.; Osmon, D.R.; Mandrekar, J.N.; Cockerill, F.R.; Steckelberg, J.M.; Greenleaf, J.F.; et al. Sonication of Removed Hip and Knee Prostheses for Diagnosis of Infection. N. Engl. J. Med. 2007, 357, 654–663. [Google Scholar] [CrossRef]
- Alhede, M.; Qvortrup, K.; Liebrechts, R.; Høiby, N.; Givskov, M.; Bjarnsholt, T. Combination of Microscopic Techniques Reveals a Comprehensive Visual Impression of Biofilm Structure and Composition. FEMS Immunol. Med. Microbiol. 2012, 65, 335–342. [Google Scholar] [CrossRef]
- Pantanella, F.; Valenti, P.; Natalizi, T.; Passeri, D.; Berlutti, F. Analytical Techniques to Study Microbial Biofilm on Abiotic Surfaces: Pros and Cons of the Main Techniques Currently in Use. Ann. Ig. Med. Prev. E Comunita 2013, 25, 31–42. [Google Scholar] [CrossRef]
- Byrne, A.M.; Morris, S.; McCarthy, T.; Quinlan, W.; O’byrne, J.M. Outcome Following Deep Wound Contamination in Cemented Arthroplasty. Int. Orthop. 2007, 31, 27–31. [Google Scholar] [CrossRef]
- Barker, J.U.; Drakos, M.C.; Maak, T.G.; Warren, R.F.; Williams, R.J.; Allen, A.A. Effect of Graft Selection on the Incidence of Postoperative Infection in Anterior Cruciate Ligament Reconstruction. Am. J. Sports Med. 2010, 38, 281–286. [Google Scholar] [CrossRef]
- Katz, L.M.; Battaglia, T.C.; Patino, P.; Reichmann, W.; Hunter, D.J.; Richmond, J.C. A Retrospective Comparison of the Incidence of Bacterial Infection Following Anterior Cruciate Ligament Reconstruction with Autograft versus Allograft. Arthrosc. J. Arthrosc. Relat. Surg. Off. Publ. Arthrosc. Assoc. N. Am. Int. Arthrosc. Assoc. 2008, 24, 1330–1335. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).