1. Introduction
Heart failure (HF) is a progressive cardiovascular disease with significant morbidity and mortality that affects an increasing amount of people worldwide [
1]. Traditionally systolic and/or diastolic left ventricular (LV) dysfunction have been identified as the most important determinants of pathophysiological derangements and clinical manifestations which characterize the disease [
2,
3]. However several recent studies have pointed out that left atrial (LA) function play also a key role in HF [
4]. The development of LA enlargement and dysfunction have been both associated with increased risk of incident atrial fibrillation, poor exercise tolerance, increased morbidity and mortality in HF [
5,
6,
7,
8,
9,
10,
11]. The assessment of LA function by speckle-tracking echocardiography permits to identify LA dysfunction at an early stage, before atrial enlargement occur, and it seems to provide more prognostic informations than LA size itself in HF patients [
6]. In particular LA resevoir function, expressed as peak atrial longitudinal strain (PALS) has proved to be a powerful prognostic marker in HF independently from the left ventricular ejection fraction (LVEF) value [
7]. Il light of these data, LA function is becoming a therapeutical target in HF. While improvements of LA function have recently been observed after some pharmacological therapies [
12] it is not known whether exercise training can have any favorable effect on LA function. Among different exercise modalities, supervised concurrent, aerobic plus resistance, exercise training (SCT) has proved to be superior to aerobic continuous training in improving functional capacity in HF [
13,
14], and it has also showed favorable hemodynamic effects [
15]. The purpose of the present study was to assess the effects of a 12-weeks SCT program on LA function in patients with HF. In particular we focused on patients with HF with mid-range ejection fraction (HFmrEF) and underlying coronary heart disease (CHD). We hypothesized that SCT would improve PALS and other indices of LA function in these patients.
2. Materials and Methods
A total of 70 subjects (57 males and 13 females) in NYHA class I/II, with a previous diagnosis of HFmrEF secondary to CHD, were enrolled for this study. These subjects got referred to the outpatient clinic of the rehabilitation centre of San Raffaele IRCCS in Rome. Inclusion criteria were: stable clinical conditions: patients were required to have no hospitalizations for HF or acute coronary syndrome in the past six months and/or no changes on pharmacological therapy in the past three months; age over 45 years; LV ejection fraction ranging between 40-49%; stable sinus rhythm; normal or mildly enlarged LA volume (<41 ml/m2). Exclusion criteria adopted in this study were: baseline blood pressure levels at rest over 160/100 mmHg; severe heart valve diseases; hypertrophic cardiomyopathy; positive ergometric test showing signs and/or symptoms of myocardial ischemia; uncontrolled arrhythmias; neurological and/or orthopedic contraindications to aerobic and/or resistance exercises; severe chronic obstructive pulmonary disease with FEV1<50%; symptoms of peripheral arterial disease. Also, if patients needed modifications to their pharmacological treatment during the study period, they were excluded from it.
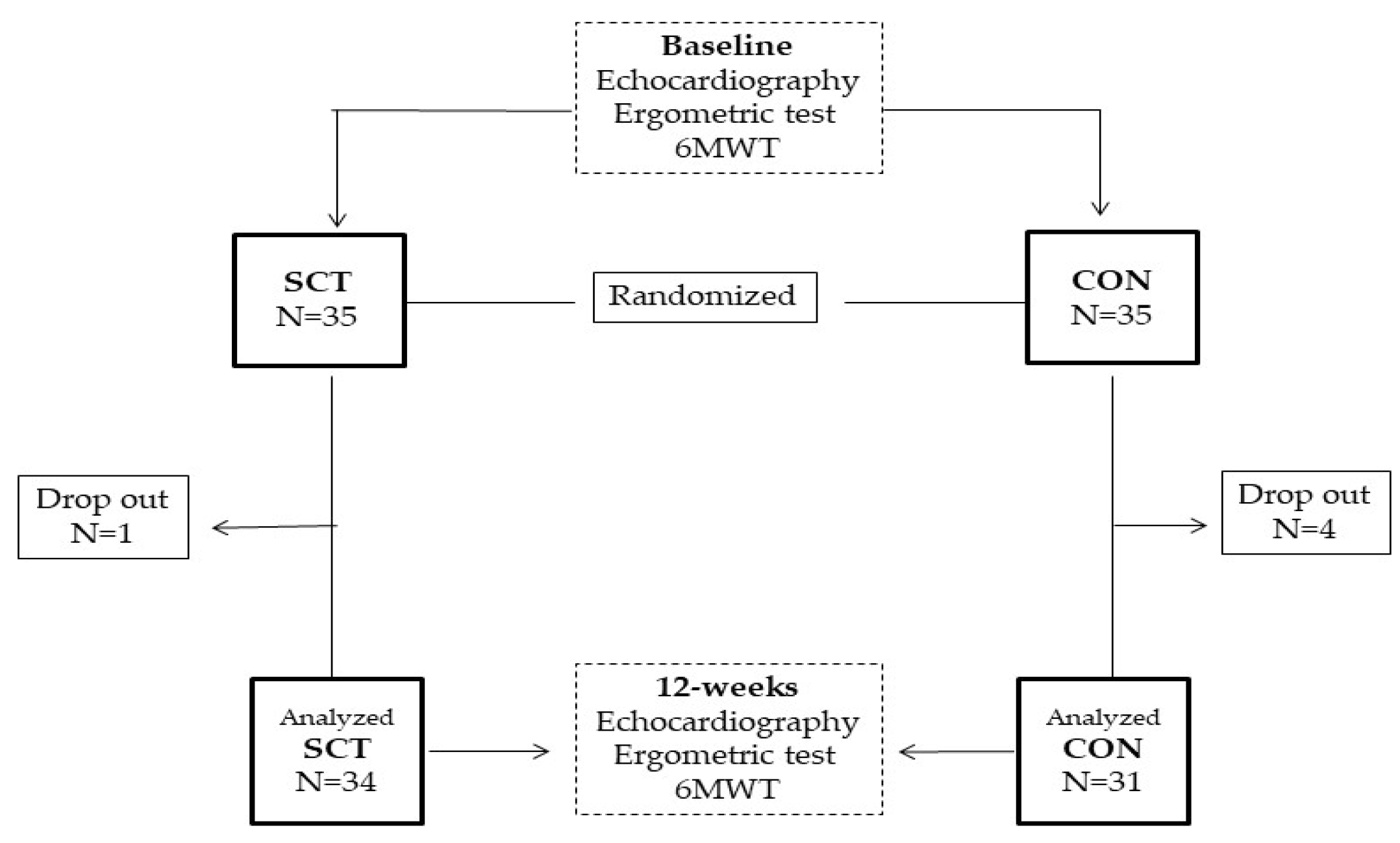
The design of the study is summarized in
Figure 1. This was a prospective, longitudinal, randomized pilot study, with patients randomly assigned on 1:1 basis to either a supervised combined training group (SCT) or a control (CON) group, performing unsupervised home-based exercises. The randomization code was developed with a computed random generator numbers in order to select random permuted blocks. The study was approved by the local Ethics Committee of S. Raffaele IRCCS (protocol number 33/2021) and complied with the Declaration of Helsinki. All patients gave written informed consent before entering the study. At baseline all patients underwent a full clinical evaluation that was divided into two days. The first day included: patient’s medical history; body mass index (BMI), waist circumference, resting heart rate, blood pressure, and a transthoracic echocardiography. The second day included: 6-minute walk test (6MWT), ergometric test; 1-repetition maximum (1-RM) strength test. The 6MWT was supervised by a physical therapist and executed according to current standard procedures [
16]. The ergometric test was performed on a treadmill (Mortara Instr, Bologna, Italy) and the standard Bruce protocol was adopted for all patients. During each stage of the test, the rate of perceived exertion (RPE) was assessed with the 6-20 Borg Scale [
17]. Before starting the assessment of 1-RM patients were allowed to familiarize, for at least 30 minutes, with dynamometers and were supervised by a physiotherapist who had the task of teaching them how to perform properly the different exercises. The 1-RM, for each resistance exercise was assessed according to the following procedure: patients initially performed a warm-up set of 8–10 repetitions at 50-60% of their estimated 1-RM; then, they were asked to perform three attempts of one repetition with maximal effort, with 2 minutes of rest period between each attempt. The highest value achieved was recorded and used as 1-RM [
18]. At 12-week, echocardiography, 6MWT, ergometric test were repeated, within a week from the last exercise session.
Echocardiography
: transthoracic echocardiography was performed in the supine position; a cardiovascular ultrasound Vivid E95
® (GE Healthcare, Chicago, IL) with a 4.0-MHz transducer was used. All echocardiography examinations were performed by one experienced sonographer who was blinded to the type of patient’s allocation. During echocardiography, one-lead electrocardiography monitoring was conducted. All the echocardiographic images were first digitally stored and then reviewed offline. During the review process, an experienced technician performed deformation measures using a proprietary software (version 10.8, EchoPAC; GE Vingmed Ultrasound, Norway). Left ventricular end-diastolic volume (LVEDV) and left ventricular end-systolic volume (LVESV) were calculated from the apical two and four chamber windows; LVEF was assessed by the modified Simpson’s method. LA volume was measured from standard apical four-chamber and three-chamber views before mitral valve opening, at end-systole; the biplane Simpson’s method of disks was adopted. The calculation of LA volume index (LAVI) was made by dividing LA volume by the body surface area of subjects. E/A ratio represented the ratio of peak left ventricle filling velocity (E wave) in early diastole, during atrial relaxation, to peak velocity flow in late diastole (A wave), during atrial contraction. LV E/e’ ratio was calculated as the ratio between E wave velocity and the average between lateral and septal LV e’ wave velocities. Color tissue Doppler tracings were obtained with the range gate placed at the lateral mitral annular segments in the 4-chamber view. LV global longitudinal strain (GLS) was measured through two-, three-, and four-chamber views. The detection of the LV endocardial boundary was automatically provided by the software; however, each time it was deemed appropriate, it was edited, to conform to the visualized LV boundaries. The maximum negative value of strain during systole measured by the software represented the maximum contractility for each segment. The average of the values from each segment was then calculated to determine LV GLS. Using the R wave as a starting point (R-R gating), measures of LA deformation tracking were carried out. During the end-diastolic phase of the long-axis two-chamber and four-chamber cine images. the endocardial and epicardial contours of the LA were traced using automatic contour tracking algorithm and manual adjustments, if necessary. The automatic algorithm places a set of control points on the middle curve of the myocardial wall in the reference phase based on the drawn endocardial and epicardial contours. The software program generated the longitudinal strain curves for each segment and calculated a mean curve of all segments. Longitudinal strain measurements were subdivided into LA reservoir strain, conduit strain and contractile strain [
19]. Peak atrial longitudinal strain (PALS) was measured at the end of atrial diastolic phase (positive peak during LV systole), while peak atrial contraction strain (PACS) was measured right before the start of the atrial systolic phase (positive peak during early diastole). Conduit strain was measured as difference of the strain value at the beginning of atrial contraction minus mitral valve opening [
20].
Exercise training protocol. Patients of the SCT group performed the training sessions in the rehabilitation centre of our hospital. The duration of the sessions was 60 minutes, and each session was preceded by 10 minutes of warm-up and concluded with 10 minutes of cool-down. The frequency of the sessions was set to three time per week. The training sessions were structured as followed: 40 minutes of aerobic exercised with an intensity target of 13-14 RPE on the Borg Scale (patients were free to changer the treadmill settings in order to maintain the effort across the rehabilitation protocol); 20 minutes of resistance training (Technogym Wellness System, Technogym, Cesena, Italy) involving quadriceps, back muscles, deltoids, and biceps muscles consisting of twos sets of 10 repetitions at 60% of the 1-RM for all the exercises with two minutes of rest period between sets. The 1-RM strength tests were repeated every month to update the percentages of the training sessions. The intensity of the aerobic component over the whole training program was established through the RPE method; an intensity target of 13–14 (somewhat hard) during the entire study. In order to maintain the same level of effort during subsequent sessions, patients were free to change the treadmill set-up. The intensity of resistance training was set at 60% of 1-RM for every exercise. The 1-RM test was assessed at baseline and then repeated every month. The following muscle groups were involved: quadriceps, back muscles, deltoids, and biceps. Patients performed two sets for every exercise; each set included 10 repetitions and patients had 2 minutes of rest between sets. The exercise sessions were supervised by a rehabilitation team, including two physiotherapists, that helped the patients setting up the treadmills and the dynamometers during the session, and that checked the executions of the resistance training exercises. A sport medicine physician with experience in the cardiac rehabilitation field and a nurse were also supervising the exercise sessions. Patients’ heart rhythm was monitored by telemetry in the first session. The CON group was advised to follow contemporary preventive guidelines [[
21] but did not receive any supervision nor wearable device for physical activity tracking, throughout the study. In the final assessment at 12-week, patients of the CON group were asked about the number of exercise sessions performed at home weekly.
Statistical analysis
We did not find previous studies investigating the effects of exercise training on LA function in HFmrEF patients; therefore, we conceived this research as a pilot study. Data are presented as mean ± SD or percentage, where appropriate. The assumption of normality was checked using the Shapiro–Wilks hypothesis test. Pre- and post-exercise data were assessed using a repeated measure two-way ANOVA, with Bonferroni corrections for post hoc testing. The Pearson correlation coefficient test was used to measure the strength of a linear association between two variables. The level of significance was set at p < 0.05. All analyses were performed using a commercially available statistical package (SPSS for Windows 20.0, Chicago, IL).
3. Results
Patients of CT and CON groups had similar baseline characteristics, including the number of drugs used for treating CAD and heart failure (
Table 1). Five patients of the 70 initially screened dropped out of the study. Overall, three patients (one in the SCT and three in the CON group) dropped out before completion, for their unwillingness to continue the study. One further patient (of the CON group) was excluded from analyses due to protocol violation in terms of changes in pharmacological therapy. Therefore 65 out of 70 patients completed the study and their data were evaluated in the analysis. The average number of sessions performed by patients of SCT group was 34.6±4.2. Patients of the CON group declared an average number of sessions of 13.9±6.5. Moreover, nine patients of the CON group declared they did not exercise during the study period. At the end of the study, the duration of ergometric test increased significantly in the SCT group compared to CON (SCT from 325.2 ± 46.1 s to 415.3 ± 51.7 s,
P = 0.001; CT from 332.5 ± 54.3 s to 348.3 ± 79 s, between-group p 0.0001); 6MWT also increased significantly in the SCT group compared to CON (SCT from 429.6 ±52.8 m to 512.4 ± 66.3 m; CT from 422.2 ± 47.3 m to 468.3 ±62.4 m, between-group p 0.004). At the end of the study, PALS and conduit strain increased significantly in the SCT group compared to CON (between-groups p 0.008 and 0.001 respectively). PACS increased in the SCT group compared to CON (between-groups p 0.002) (
Table 2). E/e’ was unchanged in the SCT and CON groups. GLS was increased in the SCT compared to CON (between-groups p 0.03). Change occurring on PALS were significantly related to changes in duration of ergometric test (r= 0.42; p 0.006) and to changes in distance walked at 6MWT (r= 0.32; p 0.023). There were not correlations between PACS and duration at exercise training and distance walked at 6MWT. No changes in atral volume and E/e’ ratio occurred in both groups.
4. Discussion
The main finding of the present study is the demonstration that a supervised concurrent exercise program, lasting 12 weeks, was effective in improving LA function of patients with HFmrEF. To our knowledge no previous studies have addressed this topic in HF. Studies assessing the effects of exercise training on LA function are sparse, have been mainly performed in athletes and in healthy subjects, and have showed conflicting results [
22,
23,
24]. LA function progressively deteriorates during the course of HF with both preserved and reduced EF; it represents the first step of a remodelling process triggered by the raising LV pressure and that subsequently involves LA dilation [
25,
26]. Interestingly, in our study including asymptomatic or only mildly symptomatic (NYHA class I or II) HFmrEF patients, PALS and PACS were already reduced compared to normal values [
27] while E/e’ and LA volume were still in their normal range. These findings seem to indicate that LA dysfunction starts at a very early, pre-clinical stage in HFmrEF. Our results are in agreement with the study of Saikhan et al [
28] in which all indices of LA function obtained through speckle-tracking echocardiography were lower in patients with HFmrEF compared to those with preserved EF and healthy subjects. Since the presence of LA remodelling is associated with worsening clinical conditions and poor prognosis [
5,
6,
7,
8,
9,
10,
11], it appears evident that reversing LA dysfunction could be a potential target for both pharmacological and not pharmacological interventions [
4]. A recent study showed that the administration of sacubitril-valsartan in HF with reduced EF promoted LA and LV functional reverse remodelling by improving LA reservoir strain and LV GLS, with these benefits typically observed within 6 months of treatment [
29]. Our preliminary data suggest that SCT elicits LA and LV functional reverse remodelling in patients with HFmrEF. Clearly, larger studies are needed in order to confirm our results and to investigate whether these beneficial effects of SCT can be extended also to patients with HF with more severely reduced EF. In our study, benefits of SCT on LA function appeared already after 12-weeks of SCT, that can be considered a very short period of training. However similar results have been already observed by other authors in different populations. In the study of Wright et al [
22] performed on untrained healthy men, two exercise modalities, high-intensity interval training and continuous moderate-intensity training, produced significant increase of PALS and systolic strain rate already after six training sessions. Taken together these results suggest that benefits of exercise training on LA function appear very early regardless of the type of training used. We observed significant correlations between changes in PALS and increase in the duration of ergometric test and distance walked at 6MWT in the SCT group. These findings suggest the existence of a cause-effect relationship between the improvement of LA resevoir phase and the increase of exercise capacity in HFmrEF and they appear to be in agreement with recent researchers showing that LA function is an independent predictor of exercise capacity in both HFrEF and HFpEF [
30,
31]. In our study distance walked at 6MWT increased in both SCT and CON. However only patients performing exercise in a supervised setting obtained significant effects on LA and LV functions. Our results comply with other researches in which supervised exercise training programs were compared with unsupervised exercise prescribed in routine medical consultations [
32,
33]. In the study of Fabri et al [
33], patients undergoing supervised combined exercise improved LVEF at the end of the training program while no effects on cardiac parameters were observed in the unsupervised group. Our results underline the importance of performing supervised training programs for patients with HFmrEF in order to develop adequate training intensities and to optimize the effects of exercise on cardiac function.
Limitations. This study has been conceived as a pilot study, therefore it has a small sample size and its results should be confirmed in further, adequately powered, studies. In particular, we enrolled a very small proportion of women that should be better represented in further trials. Our results were obtained in patients undergoing concurrent exercise training and cannot be generalized to different exercise modalities. The control group of this study included patients who performed a certain degree of physical activity, and this explains why we observed a rise in fitness in both arms; the lack of a proper control group, without exercise, can have weakened the possibility to observe the neat effect of SCT on LA function parameters. However, the problem of having a control group without physical activity is a common challenge in exercise trials, as it would be unethical not to promote physical activity given its benefits. This study enrolled only patients with HFmrEF with underlying CHD; therefore, its result cannot be extended to patients with severe LVEF or those with preserved LVEF.
Conclusions
The present study indicates that SCT was superior to CON in improving functional capacity, LA and LV function in patients with HFmrEF. Further larger studies are needed in order to generalize the effects of exercise on atrial function in HF and to clarify whether these exercise-induced benefits translate into a better prognosis for HF patients.
Author Contributions
Conceptualization, C.G. and F.I.; Data curation, V.D.A., S.V., A.G.; Formal analysis, G.C., M.A.P. and V.M.; Investigation, G.C. and M.A.P.; Supervision, G.M. and F.I. Writing—review & editing, M.V., G.C., M.A.P. and F.I. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by funding from the Italian Ministry of Health (Ricerca corrente 2021–2022).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of IRCCS San Raffaele Pisana (protocol code 33/2021).
Informed Consent Statement
Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Morris, J.H.; Chen, L. Exercise Training and Heart Failure: A Review of the Literature. Card Fail Rev. 2019, 5, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, P.W. Left ventricular dysfunction: causes, natural history, and hopes for reversal. Heart. 2000, 84 (Suppl 1), i15–7. [Google Scholar] [CrossRef] [PubMed]
- Schwinger, R.H.G. Pathophysiology of heart failure. Cardiovasc Diagn Ther. 2021, 11, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Carpenito, M.; Fanti, D.; Mega, S.; Benfari, G.; Bono, M.C.; Rossi, A.; Ribichini, F.L.; Grigioni, F. The Central Role of Left Atrium in Heart Failure. Front Cardiovasc Med. 2021, 8, 704762. [Google Scholar] [CrossRef] [PubMed]
- Ovchinnikov, A.G.; Potekhina, A.; Belyavskiy, E.; Gvozdeva, A.; Ageev, F. Left atrial dysfunction as the major driver of heart failure with preserved ejection fraction syndrome. J Clin Ultrasound. 2022, 50, 1073–1083. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Lee, A.P.; Yu, C.M. Left atrial function in heart failure with impaired and preserved ejection fraction. Curr Opin Cardiol. 2014, 29, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Maffeis, C.; Rossi, A.; Cannata, L.; Zocco, C.; Belyavskiy, E.; Radhakrishnan, A.K.; Feuerstein, A.; Morris, D.A.; Pieske-Kraigher, E.; Pieske, B.; Edelmann, F.; Temporelli, P.L. Left atrial strain predicts exercise capacity in heart failure independently of left ventricular ejection fraction. ESC Heart Fail. 2022, 9, 842–852. [Google Scholar] [CrossRef]
- Modin, D.; Sengeløv, M.; Jørgensen, P.G.; Olsen, F.J.; Bruun, N.E.; Fritz-Hansen, T.; Andersen, D.M.; Jensen, J.S.; Biering-Sørensen, T. Prognostic Value of Left Atrial Functional Measures in Heart Failure With Reduced Ejection Fraction. J Card Fail. 2019, 25, 87–96. [Google Scholar] [CrossRef]
- Sanchis, L.; Gabrielli, L.; Andrea, R.; Falces, C.; Duchateau, N.; Perez-Villa, F.; Bijnens, B.; Sitges, M. Left atrial dysfunction relates to symptom onset in patients with heart failure and preserved left ventricular ejection fraction. Eur Heart J Cardiovasc Imaging. 2015, 16, 62–67. [Google Scholar] [CrossRef]
- Sargento, L.; Vicente Simões, A.; Longo, S.; Lousada, N.; Palma Dos Reis, R. Left atrial function index predicts long-term survival in stable outpatients with systolic heart failure. Eur Heart J Cardiovasc Imaging. 2017, 18, 119–127. [Google Scholar] [CrossRef]
- Santos, A.B.; Roca, G.Q.; Claggett, B.; Sweitzer, N.K.; Shah, S.J.; Anand, I.S.; Fang, J.C.; Zile, M.R.; Pitt, B.; Solomon, S.D.; Shah, A.M. Prognostic Relevance of Left Atrial Dysfunction in Heart Failure With Preserved Ejection Fraction. Circ Heart Fail. 2016, 9, e002763. [Google Scholar] [CrossRef]
- Castrichini, M.; Manca, P.; Nuzzi, V.; Barbati, G.; De Luca, A.; Korcova, R.; Stolfo, D.; Lenarda, A.D.; Merlo, M.; Sinagra, G. Sacubitril/valsartan induces global cardiac reverse remodeling in long-lasting heart failure with reduced ejection fraction: standard and advanced echocardiographic evidences. J Clin Med. 2020, 9, 906. [Google Scholar] [CrossRef]
- Beckers, P.J.; Denollet, J.; Possemiers, N.M.; Wuyts, F.L.; Vrints, C.J.; Conraads, V.M. Combined endurance-resistance training vs. endurance training in patients with chronic heart failure: a prospective randomized study. Eur Heart J. 2008, 29, 1858–1866. [Google Scholar] [CrossRef]
- Volterrani, M.; Caminiti, G.; Perrone, M.A.; Cerrito, A.; Franchini, A.; Manzi, V.; Iellamo, F. Effects of Concurrent, Within-Session, Aerobic and Resistance Exercise Training on Functional Capacity and Muscle Performance in Elderly Male Patients with Chronic Heart Failure. J Clin Med. 2023, 12, 750. [Google Scholar] [CrossRef]
- Conraads, V.M.; Beckers, P.; Vaes, J.; Martin, M.; Van Hoof, V.; De Maeyer, C.; Possemiers, N.; Wuyts, F.L.; Vrints, C.J. Combined endurance/resistance training reduces NT-proBNP levels in patients with chronic heart failure. Eur Heart J. 2004, 25, 1797–1805. [Google Scholar] [CrossRef]
- Brooks, D.; Solway, S.; Gibbons, W.J. ATS statement on six-minute walk test. Am. J. Respir. Crit. Care Med. 2003, 167, 1287. [Google Scholar] [CrossRef]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Brown, L.E.; Weir, J.P. ASEP Procedures Recommendation I: Accurate Assessment of Muscular Strength and Power. J. Exerc. Physiol. 2001, 4, 1–21. [Google Scholar]
- Gan, G.C.H.; Ferkh, A.; Boyd, A.; Thomas, L. Left atrial function: evaluation by strain analysis. Cardiovasc Diagn Ther. 2018, 8, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Badano, L.P.; Kolias, T.J.; Muraru, D.; Abraham, T.P.; Aurigemma, G.; Edvardsen, T.; D'Hooge, J.; Donal, E.; Fraser, A.G.; Marwick, T.; Mertens, L.; Popescu, B.A.; Sengupta, P.P.; Lancellotti, P.; Thomas, J.D.; Voigt, J.U.; Industry representatives; Reviewers: This document was reviewed by members of the 2016–2018 EACVI Scientific Documents Committee. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. In ; Eur Heart J Cardiovasc Imaging; 2018; Volume 19, pp. 591–600. [Google Scholar]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; Agewall, S.; Dickstein, K.; Edvardsen, T.; Escaned, J.; Gersh, B.J.; Svitil, P.; Gilard, M.; Hasdai, D.; Hatala, R.; Mahfoud, F.; Masip, J.; Muneretto, C.; Valgimigli, M.; Achenbach, S.; Bax, J.J. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.; Esfandiari, S.; Elmayergi, N.; Sasson, Z.; Goodman, J.M. Left atrial functional changes following short-term exercise training. Eur J Appl Physiol. 2014, 114, 2667–2675. [Google Scholar] [CrossRef]
- McNamara, D.A.; Aiad, N.; Howden, E.; Hieda, M.; Link, M.S.; Palmer, D.; Samels, M.; Everding, B.; Ng, J.; Adams-Huet, B.; Opondo, M.; Sarma, S.; Levine, B.D. Left Atrial Electromechanical Remodeling Following 2 Years of High-Intensity Exercise Training in Sedentary Middle-Aged Adults. Circulation. 2019, 139, 1507–1516. [Google Scholar] [CrossRef]
- Sareban, M.; Winkert, K.; Sperlich, B.; Berger, M.M.; Niebauer, J.; Steinacker, J.M.; Treff, G. Speckle tracking-derived bi-atrial strain before and after eleven weeks of training in elite rowers. Sci Rep. 2018, 8, 14300. [Google Scholar] [CrossRef]
- Von Roeder, M.; Rommel, K.P.; Kowallick, J.T.; Blazek, S.; Besler, C.; Fengler, K.; Lotz, J.; Hasenfuß, G.; Lücke, C.; Gutberlet, M.; Schuler, G.; Schuster, A.; Lurz, P. Influence of Left Atrial Function on Exercise Capacity and Left Ventricular Function in Patients With Heart Failure and Preserved Ejection Fraction. Circ Cardiovasc Imaging. 2017, 10, e005467. [Google Scholar] [CrossRef]
- Carluccio, E.; Biagioli, P.; Mengoni, A.; Cerasa, M.F.; Lauciello, R.; Zuchi, C.; Bardelli, G.; Alunni, G.; Coiro, S.; Gronda, E.G.; Ambrosio, G. Left atrial reservoir function and outcome in heart failure with reduced ejection fraction: the importance of atrial strain by speckle tracking echocardiography. Circ Cardiovasc Imaging. 2018, 11, e007696. [Google Scholar] [CrossRef]
- Nielsen, A.B.; Skaarup, K.G.; Hauser, R.; Johansen, N.D.; Lassen, M.C.H.; Jensen, G.B.; Schnohr, P.; Møgelvang, R.; Biering-Sørensen, T. Normal values and reference ranges for left atrial strain by speckle-tracking echocardiography: the Copenhagen City Heart Study. Eur Heart J Cardiovasc Imaging. 2021, 23, 42–51. [Google Scholar] [CrossRef]
- Al Saikhan, L.; Hughes, A.D.; Chung, W.S.; Alsharqi, M.; Nihoyannopoulos, P. Left atrial function in heart failure with mid-range ejection fraction differs from that of heart failure with preserved ejection fraction: a 2D speckle-tracking echocardiographic study. Eur Heart J Cardiovasc Imaging. 2019, 20, 279–290. [Google Scholar] [CrossRef]
- Moon, M.G.; Hwang, I.C.; Lee, H.J.; Kim, S.H.; Yoon, Y.E.; Park, J.B.; Lee, S.P.; Kim, H.K.; Kim, Y.J.; Cho, G.Y. Reverse Remodeling Assessed by Left Atrial and Ventricular Strain Reflects Treatment Response to Sacubitril/Valsartan. JACC Cardiovasc Imaging. 2022, 15, 1525–1541. [Google Scholar] [CrossRef] [PubMed]
- Kusunose, K.; Motoki, H.; Popovic, Z.B.; Thomas, J.D.; Klein, A.L.; Marwick, T.H. Independent association of left atrial function with exercise capacity in patients with preserved ejection fraction. Heart. 2012, 98, 1311–1317. [Google Scholar] [CrossRef] [PubMed]
- D'Andrea, A.; Caso, P.; Romano, S.; Scarafile, R.; Cuomo, S.; Salerno, G.; Riegler, L.; Limongelli, G.; Di Salvo, G.; Romano, M.; Liccardo, B.; Iengo, R.; Ascione, L.; Del Viscovo, L.; Calabrò, P.; Calabrò, R. Association between left atrial myocardial function and exercise capacity in patients with either idiopathic or ischemic dilated cardiomyopathy: a two-dimensional speckle strain study. Int J Cardiol. 2009, 132, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Daskapan, A.; Arikan, H.; Caglar, N.; Tunali, N.; Ataman, S. Comparison of supervised exercise training and home-based exercise training in chronic heart failure. Saudi Med J. 2005, 26, 842–847. [Google Scholar]
- Fabri, T.; Catai, A.M.; Ribeiro, F.H.O.; Junior, J.A.A.; Milan-Mattos, J.; Rossi, D.A.A.; Coneglian, R.C.; Borra, R.C.; Bazan, S.G.Z.; Hueb, J.C.; Matsubara, B.B.; Roscani, M.G. Impact of a Supervised Twelve-Week Combined Physical Training Program in Heart Failure Patients: A Randomized Trial. Cardiol Res Pract 2019, 1718281. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).