Submitted:
05 May 2023
Posted:
06 May 2023
You are already at the latest version
Abstract
Keywords:
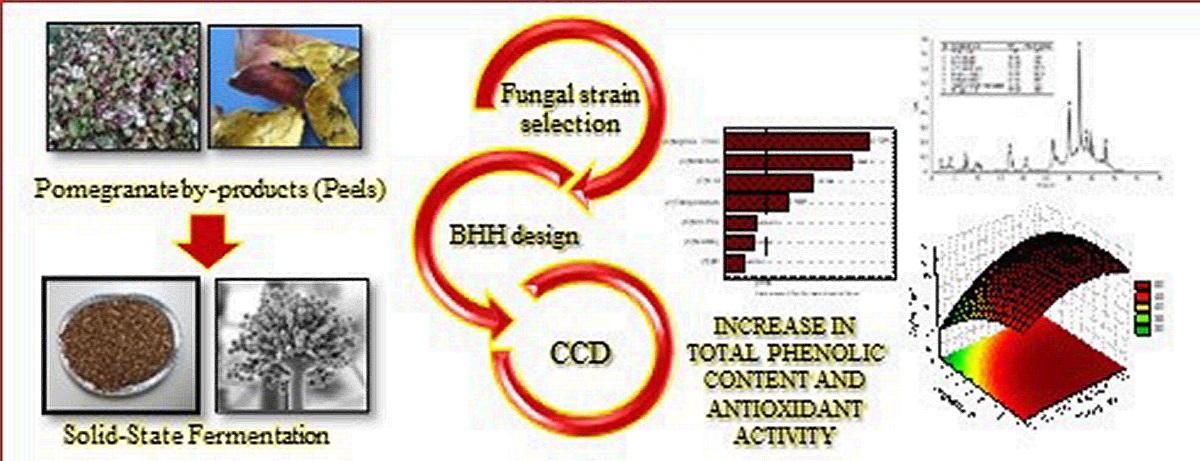
1. Introduction
2. Materials and Methods
2.1. Raw material
2.2. Physicochemical characterization of pomegranate by-products
2.3. Microorganism
2.4. Solid-state fermentation (SSF)
2.5. Analytical analysis.
2.6. Phenolic profile
2.7. Statistical analysis
2.8. Validation of the model
3. Results
3.1. Physicochemical characterization of pomegranate peels
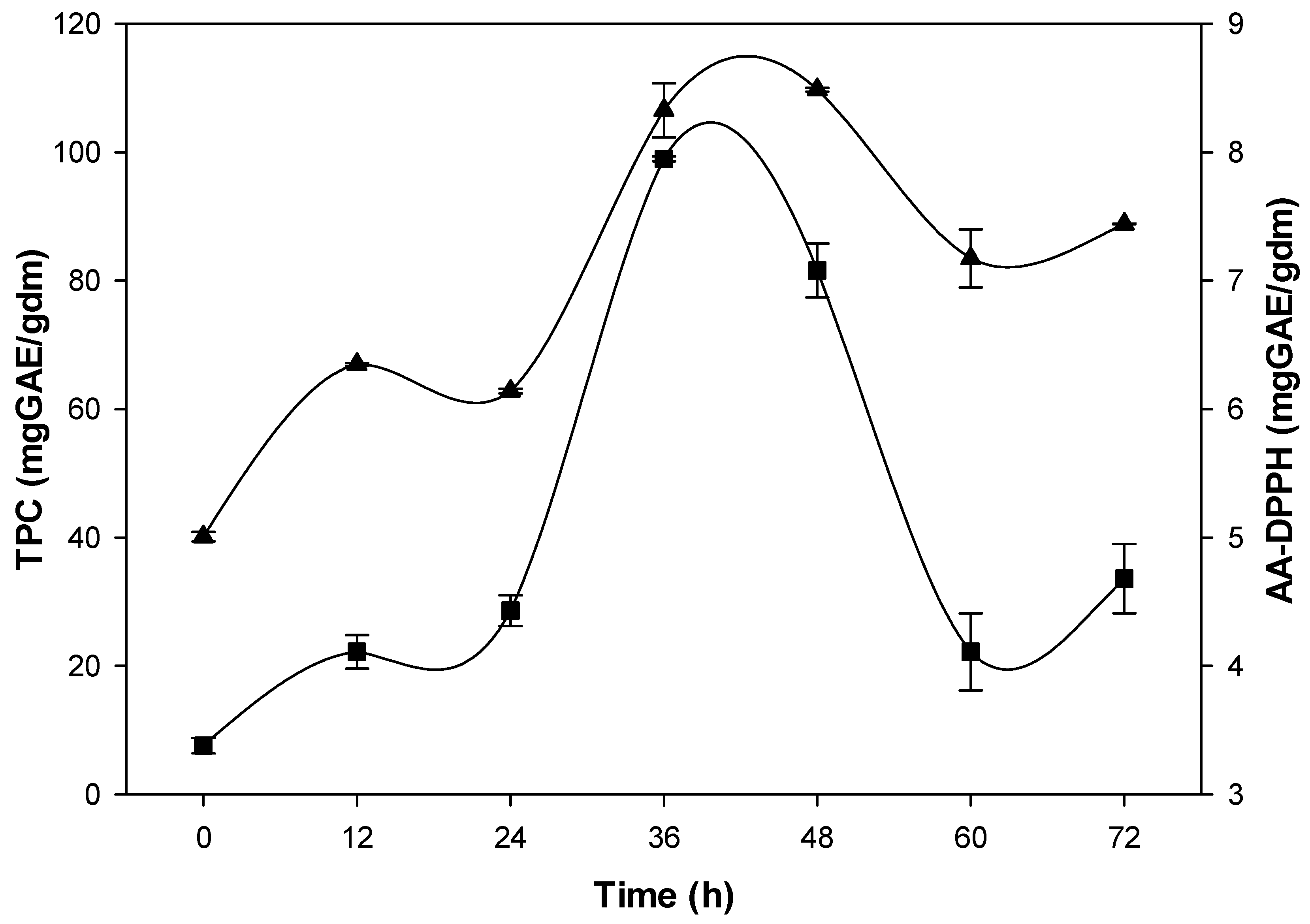
3.2. Kinetics of TPC extraction and AA
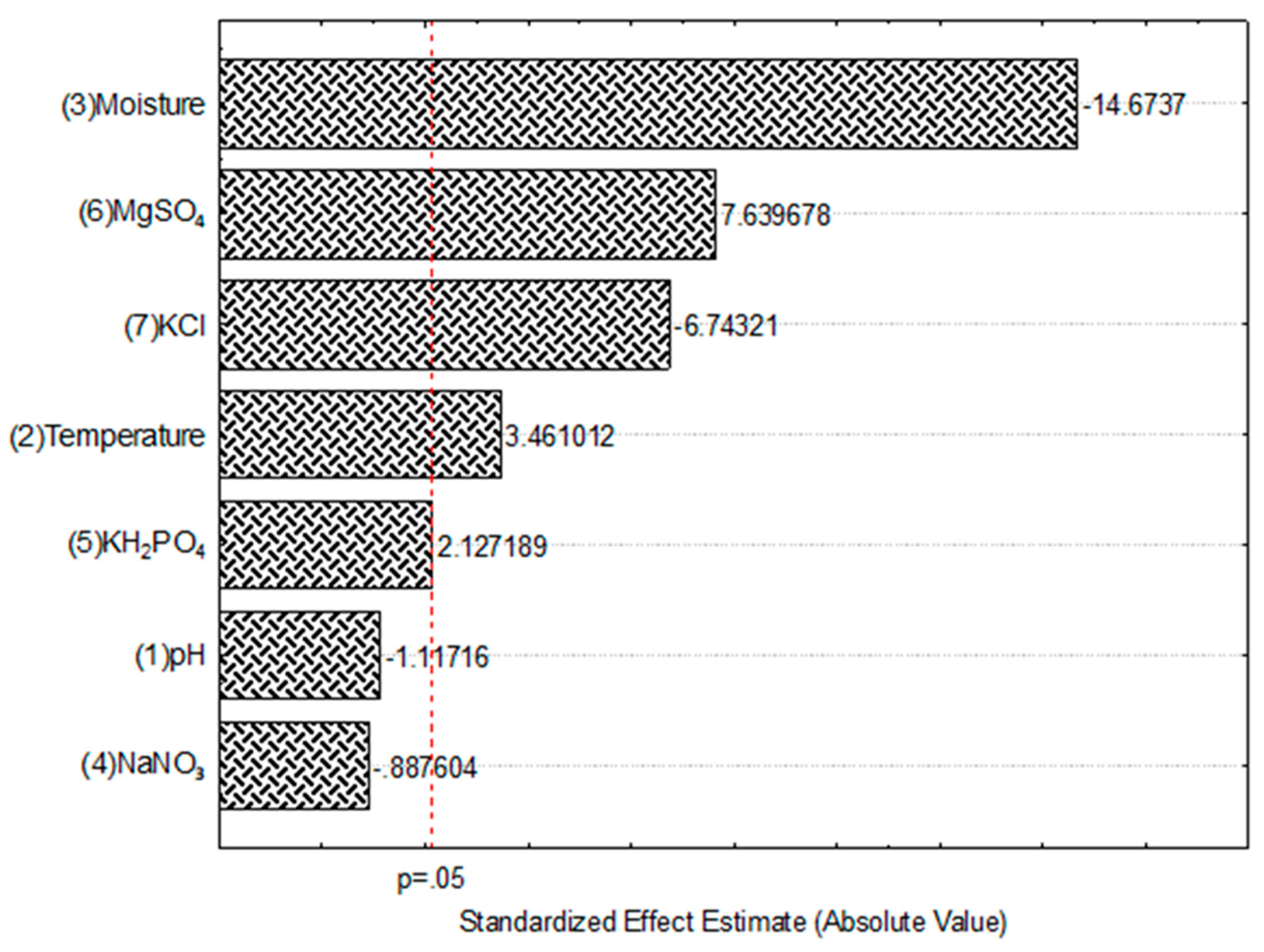
3.3. Significant factors for TPC recovery by SSF
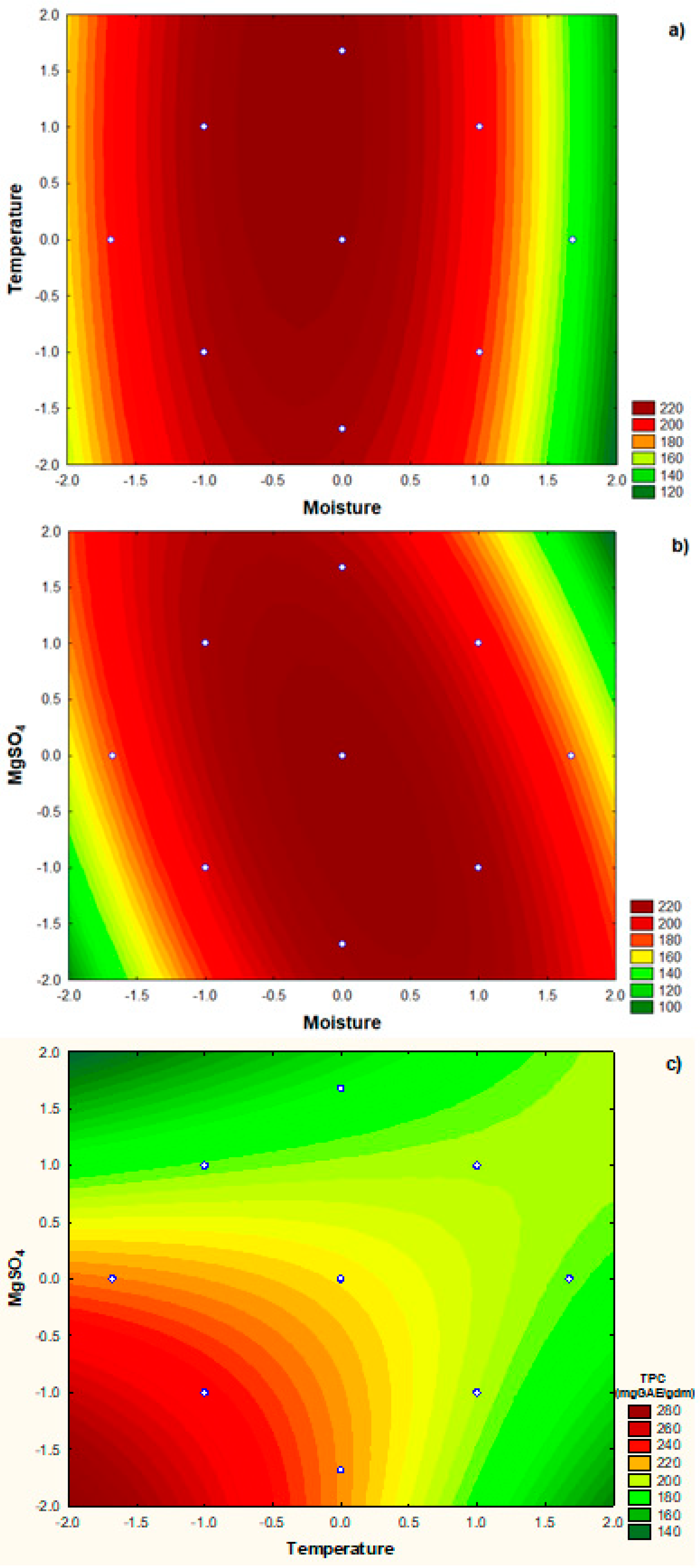
3.4. Optimization of the culture conditions for release of TPC
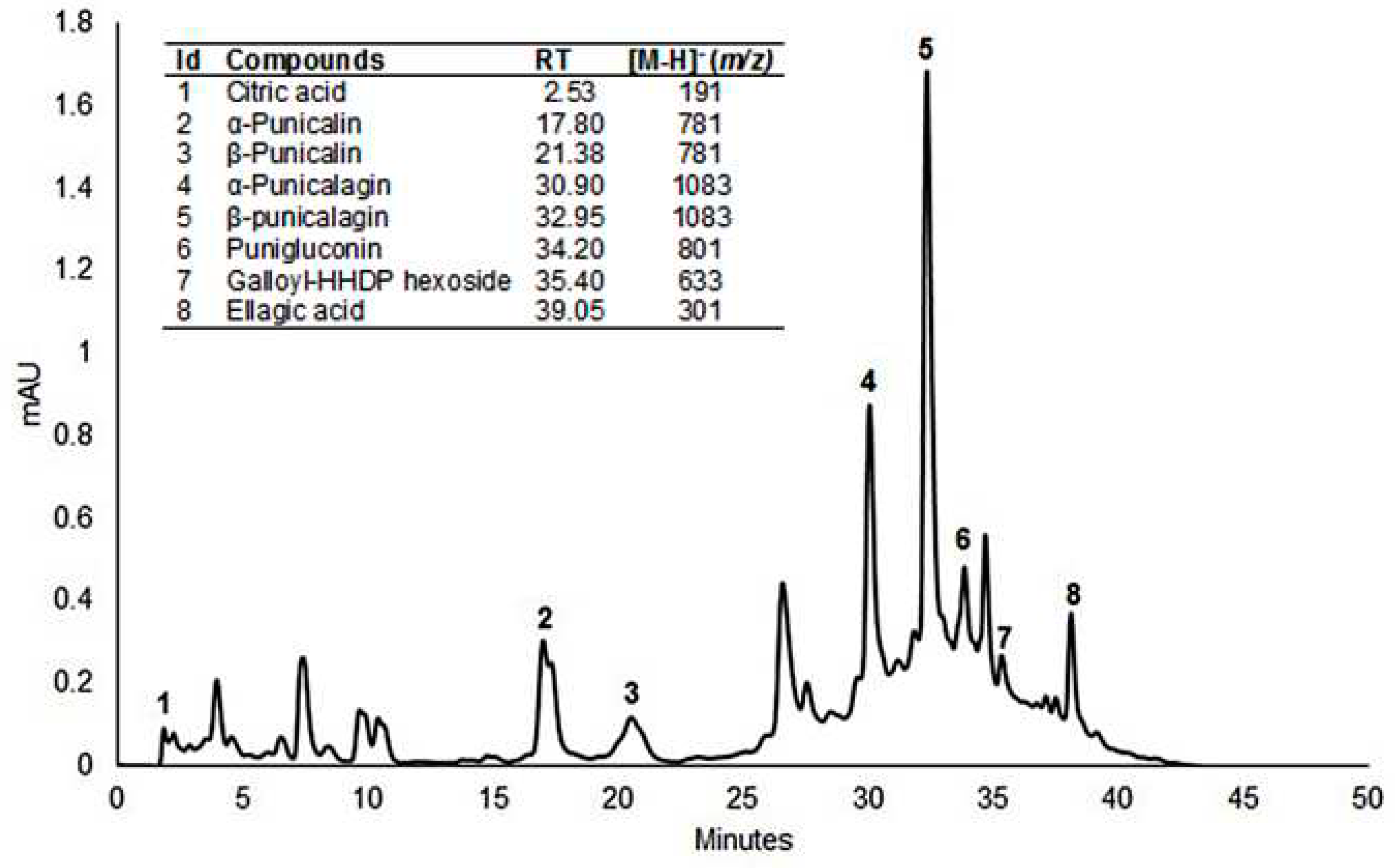
3.5. Identification of phenolic
4. Discussion
Author Contributions
Data Availability Statement
Ethical approval
References
- Aguilera-Carbo A, Augur C, Prado-Barragan LA, Favela-Torres E, Aguilar CN. (2008) Microbial production of ellagic acid and biodegradation of ellagitannins. Appl Microbiol Biotechnol 78: 189–199. [CrossRef]
- Ambigaipalan P, de Camargo AC, Shahidi F (2016) Phenolic Compounds of Pomegranate Byproducts (Outer Skin, Mesocarp, Divider Membrane) and Their Antioxidant Activities. J Agric Food Chem 64(34): 6584-6604. [CrossRef]
- Amyrgialaki E, Makris DP, Mauromoustakos A, Kefalas P (2014) Optimisation of the extraction of pomegranate (Punica granatum) husk phenolics using water/ethanol solvent systems and response surface methodology. Ind Crops Prod 59: 216-222. [CrossRef]
- AOAC International, Latimer GW (2012) Official methods of analysis of AOAC. 19th ed. AOAC International. Gaithersburg, Md. Accessed on 25 October 2022.
- Ascacio-Valdés J, Aguilera-Carbó A, Martínez-Hernández J, Rodríguez-Herrera R, Aguilar, C (2010) Euphorbia antisyphilitica residues as a new source of ellagic acid. Chem Pap 64(4): 528-532. [CrossRef]
- Ascacio-Valdés JA, Buenrostro-Figueroa JJ, Aguilera-Carbó FA, Prado-Barragán LA, Rodríguez-Herrera R, Aguilar CN (2011) Ellagitannins: Biosynthesis, biodegradation and biological properties. J. Med Plants Res 5(19): 4696-4703. http://www.academicjournals.org/JMPR.
- Ascacio-Valdés J, Buenrostro JJ, De la Cruz R, Sepúlveda L, Aguilera AF, Prado A, Contreras JC, Rodríguez R, Aguilar CN (2014) Fungal biodegradation of pomegranate ellagitannins. J Basic Microbiol 54: 28-34. [CrossRef]
- Ascacio-Valdés JA, Aguilera-Carbó AF, Buenrostro JJ, Prado-Barragán A, Rodríguez-Herrera R, Aguilar CN (2016) The complete biodegradation pathway of ellagitannins by Aspergillus niger in solid-state fermentation. J Basic Microbiol 56(4): 329-336. [CrossRef]
- Ben-Ali, S. , Jaouali, I., Souissi-Najar, S., Ouederni, A. 2017. Characterization and adsorption capacity of raw pomegranate peel biosorbent for copper removal. J. Cleaner Production, 142, Part 4, 3809-3821. [CrossRef]
- Beniwal V, Rajesh Goel G, Kumar A, Chhokar V (2013) Production of tannase through solid state fermentation using Indian Rosewood (Dalbergia sissoo) sawdust - A timber industry waste. Ann Microbiol 63(2): 583-590. [CrossRef]
- Bhol S, Lanka D, Bosco SJD (2016) Quality characteristics and antioxidant properties of breads incorporated with pomegranate whole fruit bagasse. Int J Food Sci Technol 53(3): 1717-1721. [CrossRef]
- Buenrostro-Figueroa J, Ascacio-Valdés A, Sepúlveda L, De la Cruz R, Prado-Barragán A, Aguilar-González MA, Rodríguez R, Aguilar CN (2014) Potential use of different agroindustrial by-products as supports for fungal ellagitannase production under solid-state fermentation. Food Bioprod Process 92(4): 376-382. [CrossRef]
- Buenrostro-Figueroa JJ, Velázquez M, Flores-Ortega O, Ascacio-Valdés JA, Huerta-Ochoa S, Aguilar CN, Prado-Barragán LA (2017) Solid state fermentation of fig (Ficus carica L.) by-products using fungi to obtain phenolic compounds with antioxidant activity and qualitative evaluation of phenolics obtained. Process Biochem 62: 16-23. [CrossRef]
- Cano-Lamadrid M, Martínez-Zamora, Castillejo N, Artés-Hernández F (2022) From Pomegranate byproducts Waste to Worth: A Review of Extraction Techniques and Potential Applications for Their Revalorization. Foods, 11, 2596. [CrossRef]
- Coetzee G, Joubert E, van Zyl WH, Viljoen-Bloom M (2014) Improved extraction of phytochemicals from rooibos with enzyme treatment. Food Bioprod Process 92: 393–401. [CrossRef]
- De la Cruz R, Ascacio JA, Buenrostro J, Sepúlveda L, Rodríguez R, Prado-Barragán A, Contreras JC, Aguilera A, Aguilar CN (2014) Optimization of Ellagitannase Production by Aspergillus niger GH1 by Solid-State Fermentation. Prep Biochem Biotechnol 45(7): 617-631. [CrossRef]
- De la Torre I, Martin-Dominguez, MG, Acedos J, Esteban VE, Santos M, Ladero M. (2019). Utilisation/upgrading of orange peel waste from a biological biorefinery perspective. Appl Microbiol Biotechnol 103:5975–5991. [CrossRef]
- Fischer UA, Carle R, Kammerer DR (2011) Identification and quantification of phenolic compounds from pomegranate (Punica granatum L.) peel, mesocarp, aril and differently produced juices by HPLC-DAD–ESI/MSn. Food Chem 127(2): 807-821. [CrossRef]
- Gómez-Caravaca AM, Verardo V, Toselli M, Segura-Carretero A, Fernández-Gutiérrez A, Caboni MF (2013) Determination of the Major Phenolic Compounds in Pomegranate Juices by HPLC–DAD–ESI-MS. J Agric Food Chem 61(22): 5328-5337. [CrossRef]
- Gumienna M, Szwengiel A, Górna B (2016) Bioactive components of pomegranate fruit and their transformation by fermentation processes. Eur Food Res Technol 242(5): 631-640. [CrossRef]
- Jamal P, Idris ZM, Alam MZ (2011) Effects of physicochemical parameters on the production of phenolic acids from palm oil mill effluent under liquid-state fermentation by Aspergillus niger IBS-103ZA. Food Chem 124(4): 1595-1602. [CrossRef]
- Kalaycıoğlu Z, Erim FB (2017) Total phenolic contents, antioxidant activities, and bioactive ingredients of juices from pomegranate cultivars worldwide. Food Chem 221: 496-507. [CrossRef]
- Holic R, Chen G, Xu Y, Caldo KMP, Singer SD, Field CF, Weselake RJ. (2018). Bioactivity and biotechnological production of punicic acid App Microbiol Biotechnol 102:3537–3549. [CrossRef]
- Kazemi M, Karim R, Mirhosseini H, Abdul Hamid A (2016) Optimization of pulsed ultrasound-assisted technique for extraction of phenolics from pomegranate peel of Malas variety: Punicalagin and hydroxybenzoic acids. Food Chem 206: 156-166. [CrossRef]
- Li J, He X, Li M, Zhao W, Liu L, Kong X (2015) Chemical fingerprint and quantitative analysis for quality control of polyphenols extracted from pomegranate peel by HPLC. Food Chem 176:7-11. [CrossRef]
- Lopez-Flores AR, Luna-Urban C, Buenrostro-Figueroa JJ, Hernández-Martínez R, Huerta-Ochoa S, Escalona-Buendía H, Aguilar-González CN, Prado-Barragán LA (2016) Effect of pH, temperature and protein and carbohydrates source in protease production by Yarrowia lipolytica in solid culture. Rev Mex Ing Quim 15(1): 57-67. ISSN 1665-2738. http://www.rmiq.org/ojs311/index.php/rmiq/article/view/1027.
- Lopez-Trujillo J, Medina-Morales MA, Sanchez-Flores A, Arevalo C, Ascacio-Valdes JA, Mellado M, Aguilar CN, Aguilera-Carbo AF (2017) Solid bioprocess of tarbush (Flourensia cernua) leaves for β-glucosidase production by Aspergillus niger: initial approach to fiber–glycoside interaction for enzyme induction. 3 Biotech 7(4): 271. [CrossRef]
- Makris DP, Boskou G, Andrikopoulos NK (2007) Recovery of antioxidant phenolics from white vinification solid by-products employing water/ethanol mixtures. Bioresour Technol 98(15): 2963-2967. [CrossRef]
- Martínez-Ávila GC, Aguilera-Carbó AF, Rodríguez-Herrera R, Aguilar CN (2012) Fungal enhancement of the antioxidant properties of grape waste. Ann Microbiol 62(3): 923-930. [CrossRef]
- Martins S, Mussatto SI, Martínez-Avila G, Montañez-Saenz J, Aguilar CN, Teixeira JA (2011) Bioactive phenolic compounds: Production and extraction by solid-state fermentation. A review. Biotechnol Adv 29(3): 365-373. [CrossRef]
- Martins ZE, Pinho O, Ferreira IMPLVO, Jekle M, Becker T (2017) Development of fibre-enriched wheat breads: impact of recovered agroindustrial by-products on physicochemical properties of dough and bread characteristics. Eur Food Res Technol 243(11): 1973-1988. [CrossRef]
- Meléndez NP, Nevárez-Moorillón GV, Rodriguez-Herrera R, Espinoza JC, Aguilar CN (2014) A microassay for quantification of 2,2-diphenyl-1-picrylhydracyl (DPPH) free radical scavenging. African J Biochem Res 8(1): 14-18. [CrossRef]
- Natarajan K, Rajendran A (2012) Evaluation and optimization of food-grade tannin acyl hydrolase production by a probiotic Lactobacillus plantarum strain in submerged and solid-state fermentation. Food Bioprod Process 90: 780-792. [CrossRef]
- Orzua MC, Mussatto SI, Contreras-Esquivel JC, Rodriguez R, de la Garza H, Teixeira JA, Aguilar CN (2009) Exploitation of agroindustrial wastes as immobilization carrier for solid-state fermentation. Ind Crops Prod 30(1): 24-27. [CrossRef]
- Pande G, Akoh CC (2009) Antioxidant Capacity and Lipid Characterization of Six Georgia-Grown Pomegranate Cultivars. J Agric Food Chem 57(20): 9427-9436. [CrossRef]
- Rajarathnam S, Bano Z, Steinkraus KH (1989) Pleurotus mushrooms. Part III. Biotransformations of natural lignocellulosic wastes: Commercial applications and implications. Crit Rev Food Sci Nutr 28(1): 31-113. [CrossRef]
- Robledo A, Aguilera-Carbó A, Rodriguez R, Martinez J, Garza Y, Aguilar C (2008) Ellagic acid production by Aspergillus niger in solid state fermentation of pomegranate residues. J Ind Microbiol Biotechnol 35(6): 507-513. [CrossRef]
- Saffarzadeh-Matin S, Khosrowshahi FM (2017) Phenolic compounds extraction from Iranian pomegranate (Punica granatum) industrial waste applicable to pilot plant scale. Ind Crops Prod 108: 583-597. [CrossRef]
- Santos da Silveira J, Durand N, Lacour S, Belleville MP, Perez A, Loiseau G, Dornier M (2019) Solid-state fermentation as a sustainable method for coffee pulp treatment and production of an extract rich in chlorogenic acids. Food Bioprod Process 115: 175-184. [CrossRef]
- Sepúlveda L, Aguilera-Carbó A, Ascacio-Valdés JA, Rodríguez-Herrera R, Martínez-Hernández JL, Aguilar CN (2012) Optimization of ellagic acid accumulation by Aspergillus niger GH1 in solid state culture using pomegranate shell powder as a support. Process Biochem 47(12): 2199-2203. [CrossRef]
- Servicio de Información Agroalimentaria y de Pesca. 2021. (https://nube.siap.gob.
- Tehranifar A, Selahvarzi Y, Kharrazi M, Bakhsh VJ (2011) High potential of agro-industrial by-products of pomegranate (Punica granatum L.) as the powerful antifungal and antioxidant substances. Ind Crops Prod 34(3): 1523-1527. [CrossRef]
- Vašák M, Schnabl J (2016) Sodium and Potassium Ions in Proteins and Enzyme Catalysis. In: Sigel A, Sigel H, Sigel RKO (eds) The Alkali Metal Ions: Their Role for Life, 1st edn. Springer International Publishing, pp 259-290.
| Treatment | A | B | C | D | E | F | G | TPC (mg/g) |
|---|---|---|---|---|---|---|---|---|
| 1 | -1 | -1 | -1 | 1 | 1 | 1 | -1 | 189.93±4.40a |
| 2 | 1 | -1 | -1 | -1 | -1 | 1 | 1 | 171.76±0.66bc |
| 3 | -1 | 1 | -1 | -1 | 1 | -1 | 1 | 169.90±5.14bc |
| 4 | 1 | 1 | -1 | 1 | -1 | -1 | -1 | 175.11±5.55b |
| 5 | -1 | -1 | 1 | 1 | -1 | -1 | 1 | 127.67±2.64e |
| 6 | 1 | -1 | 1 | -1 | 1 | -1 | -1 | 144.92±8.96d |
| 7 | -1 | 1 | 1 | -1 | -1 | 1 | -1 | 165.07±4.21c |
| 8 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 151.85±3.04d |
| Code | Factor | Levels | ||||||
| -1 | +1 | |||||||
| A | pH | 5 | 6 | |||||
| B | Temperature (°C) | 30 | 40 | |||||
| C | Moisture (%) | 50 | 60 | |||||
| D | NaNO3 (g/L) | 3.83 | 7.65 | |||||
| E | KH2PO4 (g/L) | 1.52 | 3.04 | |||||
| F | MgSO4 (g/L) | 1.52 | 3.04 | |||||
| G | KCl (g/L) | 1.52 | 3.04 | |||||
| *Different letters mean no significant differences among treatments (Tukey α=0.05). | ||||||||
| Treatment | X1 | X2 | X3 | TPC (mgGAE/gdm) | ||
|---|---|---|---|---|---|---|
| 1 | -1 | -1 | -1 | 236.84±3.89bc | ||
| 2 | -1 | -1 | 1 | 223.01±4.73c | ||
| 3 | -1 | 1 | -1 | 174.52±5.41f | ||
| 4 | -1 | 1 | 1 | 250.78±4.67a | ||
| 5 | 1 | -1 | -1 | 222.57±8.38c | ||
| 6 | 1 | -1 | 1 | 203.02±4.84d | ||
| 7 | 1 | 1 | -1 | 206.66±3.84d | ||
| 8 | 1 | 1 | 1 | 201.14±8.97de | ||
| 9 | -1.68 | 0 | 0 | 148.18±4.54g | ||
| 10 | -1.68 | 0 | 0 | 175.82±6.45f | ||
| 11 | 0 | -1.68 | 0 | 243.17±2.83ab | ||
| 12 | 0 | 1.68 | 0 | 184.67±2.72f | ||
| 13 | 0 | 0 | -1.68 | 226.49±1.72c | ||
| 14 | 0 | 0 | 1.68 | 186.48±6.94ef | ||
| 15 | 0 | 0 | 0 | 233.22±4.46bc | ||
| 16 | 0 | 0 | 0 | 235.39±2.49bc | ||
| Code | Factor | Levels | ||||
| -1.68 | -1 | 0 | 1 | +1.68 | ||
| X1 | Moisture (%) | 42 | 45 | 50 | 55 | 58 |
| X2 | Temperature (°C) | 31 | 35 | 40 | 45 | 48 |
| X3 | MgSO4 (g/L) | 0.48 | 1.52 | 3.04 | 4.56 | 5.59 |
| Component (%) | Value |
|---|---|
| Moisture | 11.86±0.05 |
| Fat | 2.64±0.08 |
| Fibre | 8.81±0.07 |
| Protein | 8.66±0.01 |
| Ash | 4.51±0.01 |
| Carbohydrates | 75.38±0.18 |
| C/N | 41.51 |
| WAI* | 4.38±0.48 |
| CHP* | 10.13±2.13 |
| WAI: Water absorption index CHP: Critical humidity point *gram per gram of dry sample | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





