Submitted:
05 May 2023
Posted:
06 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Chemicals and reagents
2.2. Collection of raw materials
2.3. Preparation of sample extract
2.3.1. Solvent extraction (SE)
2.3.2. Microwave-assisted extraction (MAE)
2.3.3. Ultrasound-assisted extraction (UAE)
2.4. Phytochemical screening
2.4.1. Test for tannins and phenol
2.4.2. Test for alkaloids
2.4.3. Test for saponins
2.4.4. Test for terpenoids
2.4.5. Test for glycosides (Keller–Kiliani test)
2.4.6. Test for amino acids (ninhydrin test)
2.4.7. Test for proteins (Biuret test)
2.5. Determination of phenolic compounds
2.5.1. Total phenolic content (TPC)
2.5.2. Total flavonoid content (TPC)
2.6. Antioxidant activity
2.6.1. DPPH (2,2-diphenyl-1-picryl-hydrazyl-hydrate) radical scavenging activity
2.6.2. Determination of ferric reducing antioxidant power activity (FRAP assay)
2.7. Cell culture and treatment
2.7.1. Cell viability by MTT assay
2.8. High-performance liquid chromatography-mass spectrometry (HPLC-MS) analysis and identification of compounds
2.9. Thin layer chromatography
3. Results and Discussion
3.1. Phytochemical screening
3.2. Percent yield
3.3. Effect of solvent on the extraction of phytochemicals
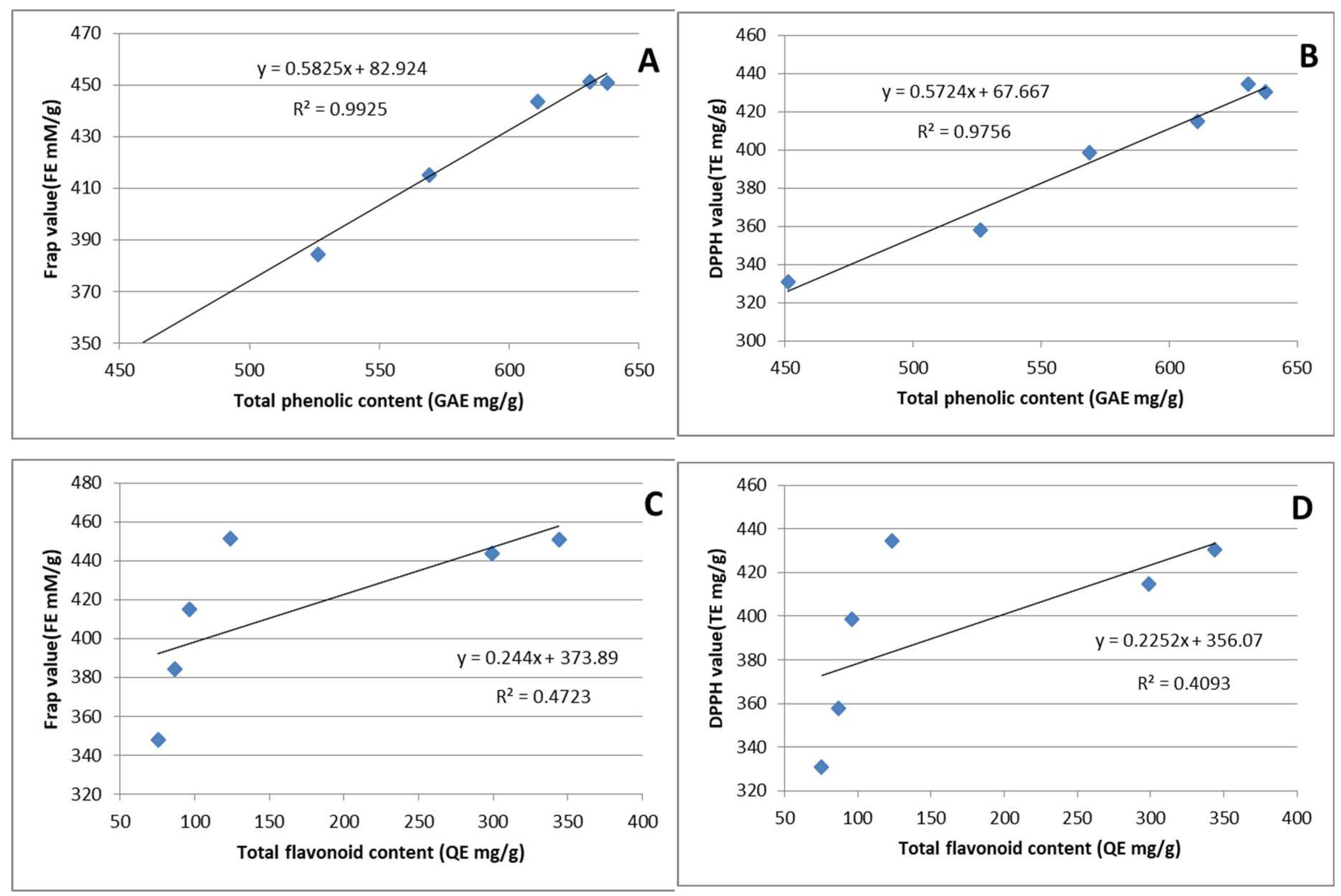
3.3. Phenolic compounds
3.4. Antioxidant activity
| Extraction method |
Solvent | % Yield | TPC (GAE mg/g) |
TFC (QE mg/g) |
DPPH (mg TE/g) |
FRAP (mM Fe(II)/mg) |
|---|---|---|---|---|---|---|
| MAE | Distilled water | 6.85±0.06e | 46.70±3.75d | 75.35±3.12e | 33.08±1.13d | 34.79±1.56d |
| 60% ethanol | 7.11±0.08d | 65.29±1.62a | 123.69±11.77ab | 43.44±0.61a | 45.14±1.24a | |
| UAE | Distilled water | 6.00±0.06f | 54.47±2.21c | 86.54±7.81d | 35.79±0.12c | 38.45±0.87c |
| 60% ethanol | 10.37±0.06a | 63.23±2.91ab | 125.13±4.95a | 41.50±1.21ab | 44.37±1.06a | |
| SE | Distilled water | 7.95±0.04c | 58.89±3.07b | 96.18±1.17c | 39.85±0.58b | 41.50±0.92b |
| 60% ethanol | 9.45±0.13b | 66.00±1.21a | 129.01±9.52a | 43.06±1.04a | 45.10±0.32a |
3.5. The correlation analysis
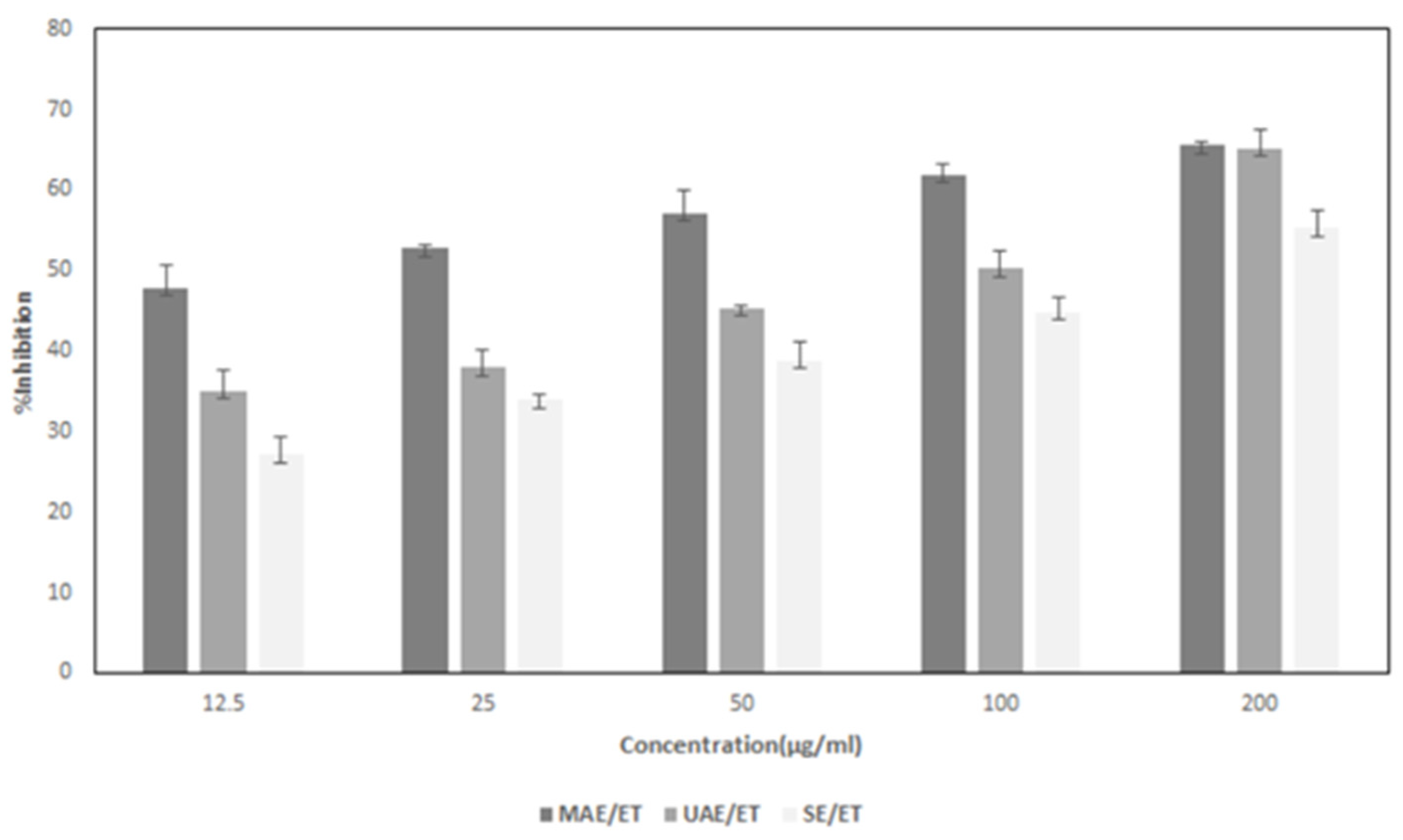
3.6. Anticancer activity
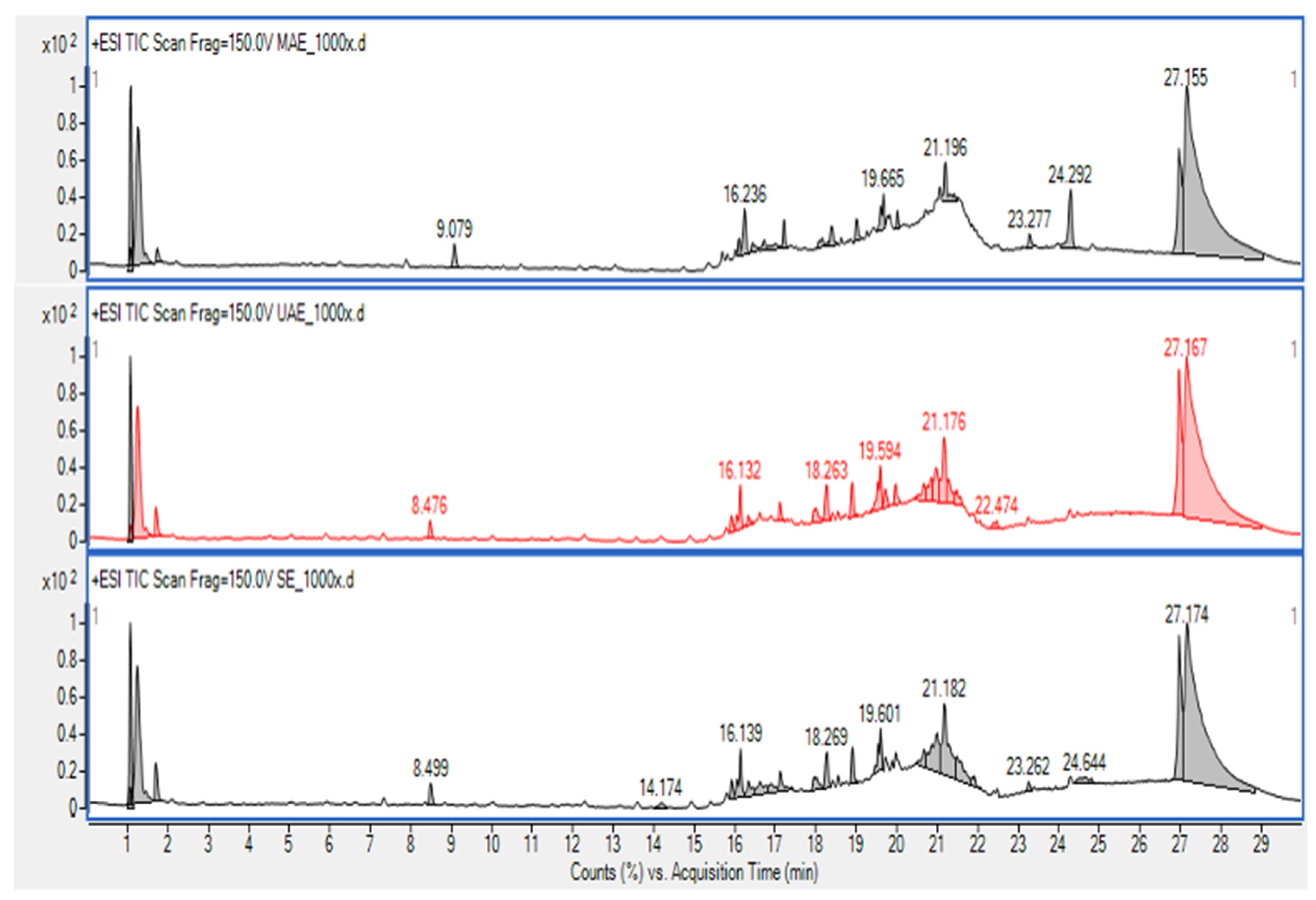
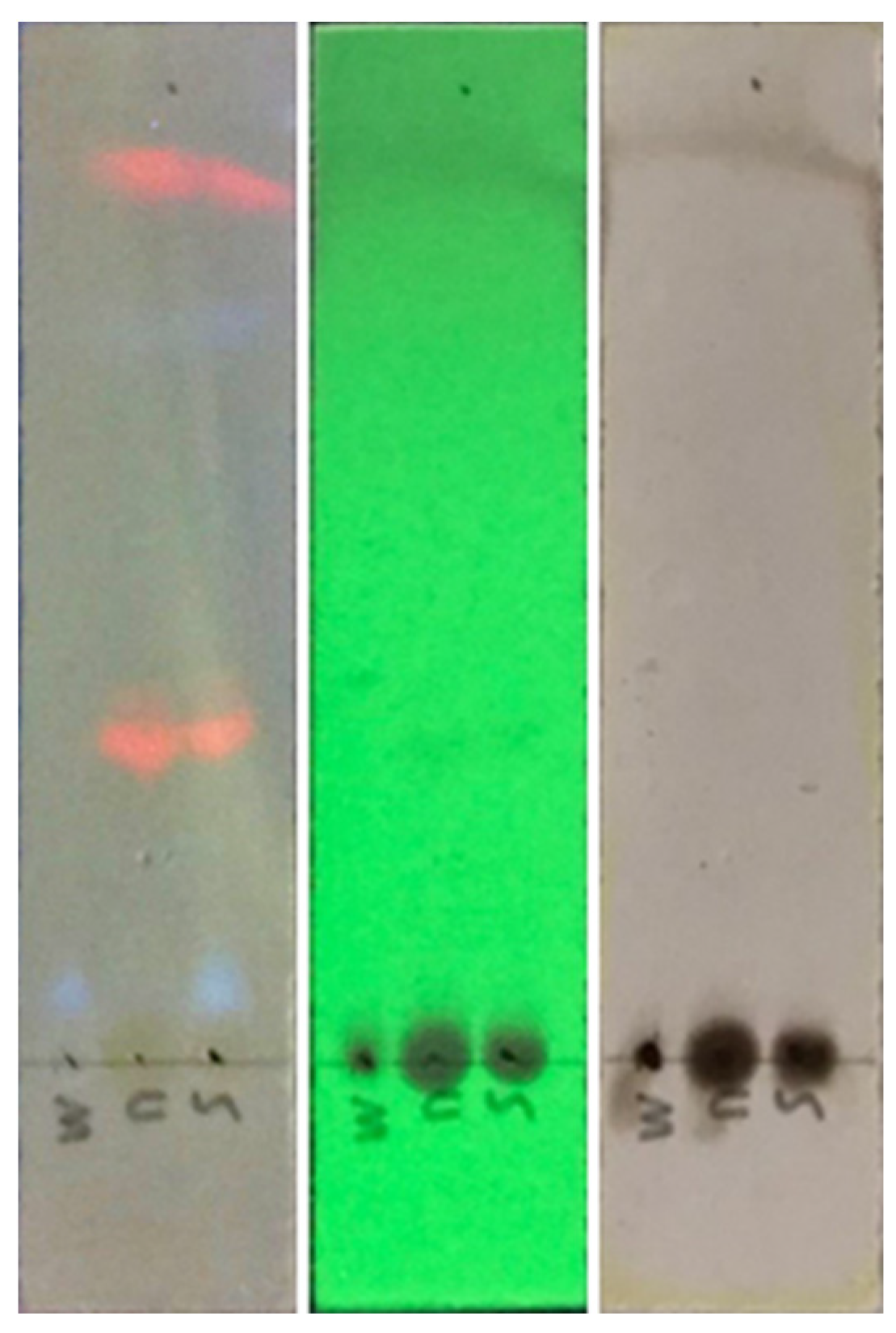
3.7. Extract analysis
3.7.1. HPLC-MS
3.7.2. Thin layer chromatography
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Worldwide Cancer Data. Available online: https://www.wcrf.org/cancer-trends/worldwide-cancer-data/ (accessed on 19 April 2023).
- Spanos, C.P.; Mamopoulos, A.; Tsapas, A.; Syrakos, T.; Kiskinis, D. Female fertility and colorectal cancer. Int. J. Colorectal Dis. 2008, 23, 735–743. [Google Scholar] [CrossRef] [PubMed]
- World Cancer Research Fund and American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective; World Cancer Research Fund and American Institute for Cancer Research: Washington DC, USA, 2007. [Google Scholar]
- Parkin, D.; Whelan, S.; Ferlay, J.; Teppo, L.; Thomas, D. Cancer Incidence in Five Continents, 8th ed.; The World Health Organization and The International Agency for Research on Cancer: Lyon, France, 2002; pp. 1–771. [Google Scholar]
- Center, M.; Siegel, R.; Jemal, A. Global Cancer Facts and Figures, 2nd ed.; American Cancer Society: Atlanta, USA, 2008; pp. 1–52. [Google Scholar]
- Stewart, B.; Wild, C.P. World Cancer Report 2014; International Agency for Research on Cancer: Lyon, France, 2014. [Google Scholar]
- The Global Cancer Observatory, Cancer Observe in Thailand 2021. Available online: http://gco.iarc.fr/today/data/factsheets/populations/764-thailand-fact-sheets.pdf (accessed on 20 April 2023).
- Bishehsari, F.; Mahdavinia, M.; Vacca, M.; Malekzadeh, R.; Mariani Costantini, R. Epidemiological transition of colorectal cancer in developing countries: environmental factors, molecular pathways, and opportunities for prevention. World J. Gastroenterol. 2014, 20, 6055–6072. [Google Scholar] [CrossRef] [PubMed]
- Doll, R.; Peto, R. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J. Natl. Cancer Inst. 1981, 66, 1191–1308. [Google Scholar] [CrossRef]
- Willett, W.C. Diet, nutrition, and avoidable cancer. Environ. Health Perspect. 1995, 103, 165–170. [Google Scholar]
- Surh, Y.J. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer 2003, 3, 768–780. [Google Scholar] [CrossRef]
- Karikas, G.A. Anticancer and chemopreventing natural products: some biochemical and therapeutic aspects. J. Buon. 2010, 15, 627–638. [Google Scholar]
- Saunders, F.R.; Wallace, H.M. On the natural chemoprevention of cancer. Plant Physiol. Biochem. 2010, 48, 621–626. [Google Scholar] [CrossRef]
- Russo, G.L. Ins and outs of dietary phytochemicals in cancer chemoprevention. Biochem. Pharmacol. 2007, 74, 533–544. [Google Scholar] [CrossRef]
- Tarapore, R.S.; Siddiqui, I.A.; Mukhtar, H. Modulation of Wnt/betacatenin signaling pathway by bioactive food components. Carcinogenesis 2012, 33, 483–491. [Google Scholar] [CrossRef]
- Sithara, T.; Arun, K.B.; Syama, H.P.; Reshmitha, T.R.; Nisha, P. Morin inhibits proliferation of SW480 colorectal cancer cells by inducing apoptosis mediated by reactive oxygen species formation and uncoupling of Warburg effect. Front. Pharmacol. 2017, 8, 640. [Google Scholar] [CrossRef] [PubMed]
- Arima, H.; Danno, G. Isolation of antimicrobial compounds from guava (Psidium guajava L.) and their structural elucidation. Biosci. Biotechnol. Biochem. 2002, 66, 1727–1730. [Google Scholar] [CrossRef] [PubMed]
- Chah, K.F.; Eze, C.A.; Emuelosi, C.E.; Esimone, C.O. Antibacterial and wound healing properties of methanolic extracts of some Nigerian medicinal plants. J. Ethnopharmacol. 2006, 140, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Prabu, G.R.; Gnanamani, A.; Sadulla, S. Guaijaverin a plants flavonoid as potential antiplaque agent against Streptococcus mutans. J. Appl. Microbiol. 2006, 101, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Ojewole, J.A. Antiinflamatory and analgesic effects of Psidium guajava Linn (Myrtaceae) leaf aqueous extract in rat and mice. Methods Findings Exp. Clin. Pharmacol. 2006, 28, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Tona, L.; Kambu, K.; Ngimbi, N.; Cimanga, K.; Vlietinck, A.J. Antiamoebic and phytochemical screening of some Congolese medicinal plants. J. Ethnopharmacol. 1998, 61, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Manosroi, J.; Dhumtanom, P.; Manosroi, A. Anti-proliferative activity of essential oil extracted from Thai medicinal plants on KB and P388 cell lines. Cancer Lett. 2006, 235, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.C.; Hsieh, C.L.; Peng, C.C.; Hsieh-Li, H.M.; Chiang, K.D.; Peng, R.Y. Brain derived prostate cancer DU-145 cells are effectively inhibited in vitro by guava leaf extracts. Nutr. Cancer 2007, 58, 93–106. [Google Scholar] [CrossRef]
- Grover, I.S.; Bala, S. Studies on antimutagenic effect of guava (Psidium guajava) in Salmonella typhimurium. Mut. Res. 1993, 300, 1–3. [Google Scholar] [CrossRef]
- Lakhanpal, P.; Rai, D.K. Quercetin: a versatile flavonoid. Int. J. Med. Update 2007, 2, 22–37. [Google Scholar] [CrossRef]
- Hashemzaei, M.; Delarami, Far. ; A., Yari, A.; Heravi, R.E.; Tabrizian, K.; Taghdisi, S.M.; Sadegh, S.E.; Tsarouhas, K.; Kouretas, D.; Tzanakakis, G.; Nikitovic, D.; Anisimov, N.Y.; Spandidos, D.A.; Tsatsakis, A.M.; Rezaee, R. Anticancer and apoptosis-inducing effects of quercetin in vitro and in vivo. Oncolog. Reports 2017, 38, 819–828. [Google Scholar]
- Ahmed, K.; Zaidi, S.F.; Cui, Z.G.; Zhou, D.; Saeed, S.A.; Inadera, H. Potential proapoptotic phytochemical agents for the treatment and prevention of colorectal cancer. Oncolog. Letters 2019, 18, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Rattanachaikunsopon, P.; Phumkhachorn, P. Contents and antibacterial activity of flavonoids extracted from leaves of Psidium guajava. J. Med. Plants Res. 2010, 4, 393–396. [Google Scholar]
- Tiwari, B.K. Ultrasound: A clean, green extraction technology. TrAC Trends Anal. Chem. 2015, 71, 100–109. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, C.; Quirantes-Piné, R.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Optimization of extraction method to obtain a phenolic compounds-rich extract from Moringa oleifera Lam leaves. Ind. Crops Prod. 2015, 66, 246–254. [Google Scholar] [CrossRef]
- Moreno, M.I.N.; Isla, M.I.; Sampietro, A.R.; Vattuone, M.A. Comparison of the free radical scavenging activity of propolis from several regions of Argentina. J. Ethnopharmacol. 2000, 71, 109–114. [Google Scholar] [CrossRef]
- Qian, H.; Nihorimbere, V. Antioxidant power of phytochemicals from Psidium guajava. J. Zhejiang Univ. Sci. 2004, 5, 676–683. [Google Scholar] [CrossRef]
- Bushra, S.; Zaib, H.; Muhammad, A.; Adil, M. Investigation on the antioxidant activity of leaves, peels, stem bark, and kernel of mango (Mangifera indica L. ) J. Food Sci. 2012, 77, 849–852. [Google Scholar]
- Laily, N.; Kusumaningtyas, R.W.; Sukarti, I.; Rini, M.R.D.K. The potency of guava Psidium guajava (L.) leaves as a functional immunostimulatory ingredient. Procedia Chem. 2015, 14, 301–307. [Google Scholar] [CrossRef]
- Patel, P.; Joshi, C.; Birdi, T.; Kothari, V. Anti-infective efficacy of Psidium guajava L. leaves against certain pathogenic bacteria. F1000Res. 2019, 8, 12. [Google Scholar] [CrossRef]
- Luo, Y.; Peng, B.; Liu, Y.; Wu, Y.; Wu, Z. Ultrasound extraction of polysaccharides from guava leaves and their antioxidant and antiglycation activity. Process Biochem. 2018, 73, 228–234. [Google Scholar] [CrossRef]
- Nyström, L.; Lampi, A.-M.; Rita, H.; Aura, A.-M.; Oksman-Caldentey, K.-M.; Piironen, V. Effects of processing on availability of total plant sterols, steryl ferulates and steryl glycosides from wheat and rye bran. J. Agric. Food Chem. 2007, 55, 9059–9065. [Google Scholar] [CrossRef] [PubMed]
- Ajayi, I.A.; Ajibade, O.; Oderinde, R.A. Preliminary phytochemical analysis of some plant seeds. Res. J. Chem. Sci. 2011, 1, 58–62. [Google Scholar]
- Samejo, M.Q.; Sumbul, A.; Shah, S.; Memon, S.B.; Chundrigar, S. Phytochemical screening of Tamarix dioica Roxb. ex Roch. J. Pharm. Res. 2013, 7, 181–183. [Google Scholar] [CrossRef]
- Shaik, S.; Nisha, S.; Ashley, N. Comparison of the selected secondary metabolite content present in the cancer-bush Lessertia frutescens (Sutherlandia) L. extracts. African J. Trad. Comple. Alt. Med. 2011, 8, 429–34. [Google Scholar]
- Biswas, B.; Rogers, K.; Mclaughlin, F.; Daniels, D.; Yadav, A. Antimicrobial activities of leaf extracts of guava (Psidium guajava L.) on two Gram-Negative and Gram-Positive bacteria. Int. J. Microbiol. 2013, 1–7. [Google Scholar] [CrossRef]
- Malik, A.; Ahmad, A.R. Determination of phenolic and flavonoid contents of ethanolic extract of kanunang leaves (Cordia myxa L.). Int. J. Pharm. Tech. 2015, 7, 243–246. [Google Scholar]
- Ashraf, A.; Sarfraz, R.A.; Rashid, M.A.; Mahmood, A.; Shahid, M.; Noor, N. Chemical composition, antioxidant, antitumor, anticancer and cytotoxic effects of Psidium guajava leaf extracts. Pharm. Biol. 2016, 54, 1971–1981. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Food Sci. Tech. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Anal. Biochem. 1996, 239, 70–6. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- López-Cobo, A.; Gómez-Caravaca, A.M.; Cerretani, L.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Distribution of phenolic compounds and other polar compounds in the tuber of Solanum tuberosum L. by HPLC-DAD-q-TOF and study of their antioxidant activity. J. Food Compos. Anal. 2014, 36, 1–11. [Google Scholar] [CrossRef]
- Díaz-de-Cerio, E.; Gómez-Caravaca, A.M.; Verardo, V.; Fernández-Gutiérrez, A.; Segura-Carreter, A. Determination of guava (Psidium guajava L.) leaf phenolic compounds using HPLC-DAD-QTOF-MS. J. Funct. Foods 2016, 22, 376–388. [Google Scholar] [CrossRef]
- Mouraa, P.M.; Prado, G.H.C.; Meireles, M.A.A.; Pereiraa, C.G. Supercritical fluid extraction from guava (Psidium guajava) leaves: Global yield, composition and kinetic data. J. Supercritic. Fluids 2012, 62, 116–122. [Google Scholar] [CrossRef]
- Rahman, Z.; Siddiqui, M.N.; Khatun, M.A.; Kamruzzaman, M. Effect of guava (Psidium guajava) leaf meal on production performances and antimicrobial sensitivity in commercial broiler. J. Nat. Prod. 2013, 6, 177–187. [Google Scholar]
- Thomas, L.A.T.; Anitha, T.; Lasyaja, A.B.; Suganya, M.; Gayathri, P.; Chithra, S. Biochemical and mineral analysis of the undervalued leaves—Psidium guajava L. Int. J. Adv. Sci. Res. 2017, 2, 16–21. [Google Scholar]
- Jassal, K.; Kaushal, S. Phytochemical and antioxidant screening of guava (Psidium guajava) leaf essential oil. Agric. Res. J. 2019, 56, 528. [Google Scholar] [CrossRef]
- Gayathri, V.; Kiruba, D. Preliminary phytochemical analysis of leaf powder extracts of Psidium guajava L. Int J. Pharmacogn. Phytochem. Res. 2014, 6, 332–334. [Google Scholar]
- Geoffrey, K.K.; John, K.M.; Naomi, M.; Simon, K.M. Qualitative phytochemical screening of Camellia sinensis and Psidium guajava leave extracts from Kericho and Baringo Counties. Int J. Adv. Biotechnol. Res. 2014, 5, 506–514. [Google Scholar]
- Taura, D.W.; Yusháu, M.; Bello, U.A; Hassan, A.; Saidu, J.P.T. Antibacterial activity of Psidium guajava in clinical isolates. Acad. J. Microbiol. Res. 2014, 2, 79–83. [Google Scholar]
- Thenmozhi, S.; Rajan, S. GC-MS analysis of bioactive compounds in Psidium guajava leaves. J. Pharmacogn. Phytochem. 2015, 3, 162–166. [Google Scholar]
- Philip, D.; Kaleena, P.K.; Valivittan, K. Phytochemical screening and antimicrobial activity of Sansevieria roxburghiana Schult. & Schult. F. Middle-East J. Sci. Res. 2011, 10, 512–518. [Google Scholar]
- Najafi, S.; Sanadgol, N.; Nejad, B.S.; Beiragi, M.A.; Sanadgo, E. Phytochemical screening and antibacterial activity of Citrullus colocynthis (Linn.) Schrad against Staphylococcus aureus. J. Med. Plants Res. 2010, 4, 2321–2325. [Google Scholar]
- Krishnaiah, D.; Devi, T.; Bono, A.; Sarbatly, R. Studies on phytochemical constituents of six Malaysian medicinal plants. J. Med. Plants Res. 2009, 3, 67–72. [Google Scholar]
- Yadav, R.N.S.; Agarwala, M. Phytochemical analysis of some medicinal plants. J. Phytology. 2011, 3, 10–14. [Google Scholar]
- Markom, M.; Hasan, M.; Wan Daud, W.R.; Singh, H.; Jahim, J.M. Extraction of hydrolysable tannins from Phyllanthus niruri linn.: Effects of solvents and extraction methods. Sep. Purif. Technol. 2007, 52, 487–496. [Google Scholar] [CrossRef]
- Tiwari, P.; Kumar, B.; Kaur, M.; Kaur, G. Kaur, H. Phytochemical screening and extraction: A review. Int. Pharm. Sciencia 2011, 1, 98–106. [Google Scholar]
- Ncube, N.S.; Afolayan, A.J.; Okoh, A.I. Assessment techniques of antimicrobial properties of natural compounds of plant origin: current methods and future trends. African J. Biotechnol. 2008, 7, 1797–1806. [Google Scholar] [CrossRef]
- Eloff, J.N. Which extractant should be used for the screening and isolation of antimicrobial components from plants. J. Ethnopharmacol. 1998, 60, 1–8. [Google Scholar] [CrossRef]
- Chuyen, H.V.; Nguyen, M.H.; Roach, P.D.; Golding, J.B.; Parks, S.E. Microwave-assisted extraction and ultrasound-assisted extraction for recovering carotenoids from Gac peel and their effects on antioxidant capacity of the extracts. Food Sci. Nutr. 2018, 6, 189–196. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Galanakis, C.M.; Brnčić, M.; Orlien, V.; Trujillo, F.J.; Mawson, R.; Barba, F.J. Clean recovery of antioxidant compounds from plant foods, by-products and algae assisted by ultrasounds processing. Modeling approaches to optimize processing conditions. Trends Food Sci. Technol. 2015, 2, 134–149. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, F.; Ma, X. , Sun, S.; Leng, F.; Zhang, W.; Wang, X. Extraction, purification, characterization and antioxidant activity of polysaccharides from Piteguo fruit. Ind. Crops and Prod. 2015, 77, 467–475. [Google Scholar] [CrossRef]
- Corbin, C.; Fidel, T.; Leclerc, E.A.; Barakzoy, E.; Sagot, N.; Falguiéres, A.; Renouard, S.; Blondeau, J.P.; Ferroud, C.; Doussot, J.; Lainé, E.; Hano, C. Development and validation of an efficient ultrasound assisted extraction of phenolic compounds from flax (Linum usitatissimum L.) seeds. Ultrason. Sonochem. 2015, 26, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Moure, A.; Cruz, J.M.; Franco, D.; Domínguez, J.M.; Sineiro, J.; Domínguez, H.; Parajó, J.C. Natural antioxidants from residual sources. Food Chem. 2001, 72, 145–171. [Google Scholar] [CrossRef]
- Mazumder, M.A.R.; Ranganathan, T.V. Encapsulation of isoflavone with milk, maltodextrin and gum acacia improves its stability. Curr. Res. Food Sci. 2020, 2, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, J.; Zu, Y.; Fu, Y.; Luo, M.; Wu, N. , Liu, X.-L. Optimisation of microwave assisted enzymatic extraction of corilagin and geraniin from Geranium sibiricum Linne and evaluation of antioxidant activity. Food Chem. 2010, 122, 373–380. [Google Scholar] [CrossRef]
- Zhang, Z.-S.; Li, D.; Wang, L.-J.; Ozkan, N.; Chen, X.D.; Mao, Z.-H.; Yang, H.-Z. Optimization of ethanol–water extraction of lignans from flaxseed. Sep. Purif. Technol. 2007, 57, 17–24. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, R.; Liu, C. Microwave-assisted extraction of chlorogenic acid from flower buds of Lonicera japonica Thunb. Sep. Purif. Technol. 2008, 62, 480–483. [Google Scholar] [CrossRef]
- Wu, J.-W.; Hsieh, C.-L.; Wang, H.-Y.; Chen, H.-Y. Inhibitory effects of guava (Psidium guajava L.) leaf extracts and its active compounds on the glycation process of protein. Food Chem. 2009, 113, 78–84. [Google Scholar] [CrossRef]
- Ito, T.; Kakino, M.; Tazawa, S.; Watarai, T.; Oyama, M.; Maruyama, H.; Araki, Y.; Hara, H.; Iinuma, M. Quantification of polyphenols and pharmacological analysis of water and ethanol-based extracts of cultivated agarwood leaves. J. Nutr. Sci. Vitaminol. 2012, 58, 136–142. [Google Scholar] [CrossRef]
- Taha, F.S.; Mohamed, G.F.; Mohamed, S.H.; Mohamed, S.S.; Kamil, M.M. Optimization of the extraction of total phenolic compounds from sunflower meal and evaluation of the bioactivities of chosen extracts. Am. J. Food Tech. 2011, 6, 1002–1020. [Google Scholar] [CrossRef]
- Fatiha, B.; Khodir, M.; Farid, D.; Tiziri, R.; Karima, B.; Sonia, O.; Mohamed, C. Optimisation of solvent extraction of antioxidants (phenolic compounds) from Algerian Mint (Mentha spicata L.). Pharmacog. Commun. 2012, 2, 72–86. [Google Scholar]
- Seo, J.; Lee, S.; Elam, M.L.; Johnson, S.A.; Kang, J.; Arjmandi, B.H. Study to find the best extraction solvent for use with guava leaves (Psidium guajava L.) for high antioxidant efficacy. Food Sci. Nutr. 2014, 2, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Nyirenda, K. K.; Saka, J.D.K.; Naidoo, D.; Maharaj, V.J.; Muller, C.J.F. Antidiabetic, anti-oxidant and antimicrobial activities of Fadogia ancylantha extracts from Malawi. J. Ethnopharmacol. 2012, 143, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Kua, S.F.; Ibrahim, J.; Ooi, C K. W.; Nan, K.I.; Hashim, N.; Mohd Yusof, H. Optimisation of phenolic extraction and quantification of phenolics in palm kernel cake. Renew. Bioresour. 2015, 3, 1–7. [Google Scholar] [CrossRef]
- Kıran, T.R.; Otlu, Ö.; Karabulut, E.; Pakdemirli, A.; Özcan, N. Antioxidant effect of grape molasses in rat heart tissues. Medicine Sci. 2019, 8, 814–819. [Google Scholar] [CrossRef]
- Nantitanon, W.; Yotsawimonwat, S.; Okonogi, S. Factors influencing antioxidant activities and total phenolic content of guava leaf extract. LWT - Food Sci. Tech. 2010, 43, 1095–1103. [Google Scholar] [CrossRef]
- Russo, D.; Faraone, I.; Labanca, F.; Sinisgalli, C.; Bartolo, M.; Andrade, P.B.; Valentao, P.; Milella, L. Comparison of different green-extraction techniques and determination of the phytochemical profile and antioxidant activity of Echinacea angustifolia L. extracts. Phytochem. Anal. 2019, 30, 547–555. [Google Scholar] [CrossRef]
- Chan, C.; Yusoff, R.; Ngoh, G.; Kung, F.W. Microwave-assisted extractions of active ingredients from plants. J. Chromatogr. A. 2011, 1218, 6213–6225. [Google Scholar] [CrossRef]
- Saha, M.R.; Hasan, S.M.R.; Akter, R.; Hossain, M.M.; Alam, M.S.; Alam, M.A.; Mazumder, M.E.H. In vitro free radical scavenging activity of methanol extract of leaves of Mimusops elengi Linn. Bangladesh J. Vet. Med. 2008, 6, 197–202. [Google Scholar] [CrossRef]
- Jagadish, L.K.; Krishnan, V.V.; Shenbhagaraman, R.; Kaviyarasan, V. Comparative study on the antioxidant, anticancer and antimicrobial property of Agaricus bisporus Imbach before and after boiling. Afr. J. Biotechnol. 2009, 8, 654–661. [Google Scholar]
- Kosińska, A.; Magdalena, K.; Estrella, I.; Hernandez, I.; Bartolome, B.; Dykes. G.A. Phenolic compound profiles and antioxidant capacity of Persea americana Mill. Peels and seeds of two varieties. J. Agric. Food Chem. 2012, 60, 4613–4619. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Seguin, P.; Ahn, J.K.; Kim, J.J.; Chun, S.C.; Kim, E.H. Seo, S.H.; Kang, E.Y.; Kim, S.L.; Park, Y.J.; Ro, H.M.; Chung, III.M. Phenolic compound concentration and antioxidant activities of edible and medicinal mushrooms from Korea. J. Agric. Food Chem. 2008, 56, 7265–7270. [Google Scholar] [CrossRef] [PubMed]
- Tayade, A.B.; Dhar, P.; Sharma, M.; Chauhan, R.S.; Chaurasia, O.P.; Srivastava, R.B. Antioxidant capacities, phenolic contents, and GC/MS analysis of Rhodiola imbricate edgew. root extracts from trans-himalaya. J. Food Sci. 2013, 78, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.-J.; Chun-Der, L.; Hsien-Jung, C.; Yaw-Huei, L. Antioxidant and antiproliferative activities of sweet potato (Ipomoea baatas [L.] LamTainong 57’) constituents. Bot. Bull. Acad. Sinica 2004, 45, 179–186. [Google Scholar]
- Dahmoune, F.; Boulekbache, L.; Moussi, K.; Aoun, O.; Spigno, G.; Madani, K. Valorization of citrus limon residues for the recovery of antioxidants: evaluation and optimization of microwave and ultrasound application to solvent extraction. Ind. Crops Prod. 2013, 50, 77–87. [Google Scholar] [CrossRef]
- Amarowicz, R.; Pegg, R.; Rahimi-Moghaddam, P.; Barl, B.; Weil, J. Free-radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chem. 2004, 84, 551–562. [Google Scholar] [CrossRef]
- Cedric, Y.; Payne, V.K.; Nadia, N.A.C.; Kodjio, N.; Kollins, E.; Megwi,L. ; Kuiate, J.-R.; Mbida, M. In vitro anticoccidial, antioxidant activities and cytotoxity of Psidium guajava extracts. Res. J. Parasitol. 2018, 13, 1–13. [Google Scholar]
- Kumar, A.; Agarwal, D.K.; Kumar, S.; Reddy, Y.M.; Chintagunta, A.D.; Saritha, K.V.; Pal, G.; Jeevan Kumar, S.P. Nutraceuticals derived from seed storage proteins: implications for health wellness. Biocatal. Agric. Biotechnol. 2019, 17, 710–719. [Google Scholar] [CrossRef]
- Gutiérrez-Grijalva, E.; Picos, S.M.; Leyva, L.N.; Criollo, M.M.; Vazquez, O.G.; Heredia, J. Flavonoids and phenolic acids from Oregano: occurrence, biological activity and health benefits. Plants 2018, 7, 2. [Google Scholar] [CrossRef]
- Shaheena, S.; Chintagunta, A.D.; Dirisala, V.R.; Kumar, N.S.S. Extraction of bioactive compounds from Psidium guajava and their application in dentistry. AMB Express 2019, 9, 208. [Google Scholar] [CrossRef] [PubMed]
- Fidrianny, I.; Sefiany, E.; Ruslan, K. In vitro antioxidant activities from three organs of white ambon banana (musa AAA group) and flavonoid, phenolic carotenoid content. Int. J. Pharmacog. Phytochem. Res. 2015, 7, 590–596. [Google Scholar]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef] [PubMed]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.-T.; Ko, S.-C.; Oh, G.-W.; Heo, S.-Y.; Jung, W.-K. Effects of Psidium guajava leaf extract on apoptosis induction through mitochondrial dysfunction in HepG2 cells. Microbiol. Biotechnol. Lett. 2019, 47, 43–53. [Google Scholar] [CrossRef]
- Peng, C.-C.; Peng, C.-H.; Chen, K.-C.; Hsieh, C.-L.; Peng, R.Y. The aqueous soluble polyphenolic fraction of Psidium guajava leaves exhibits potent anti-angiogenesis and anti-migration actions on DU145 cells. Evid.-Based Complement. Altern. Med. 2011, 2011, 2190069. [Google Scholar] [CrossRef] [PubMed]
- Leadbeater, N.E. Organic synthesis using microwave heating. In Comprehensive Organic Synthesis, 2nd ed.; Knochel, P., Ed.; Elsevier: Amsterdam, Netherlands, 2014; Volume 9, pp. 234–286. [Google Scholar]
- Spigno, G.; De Faveri, D. Microwave-assisted extraction of tea phenols: A phenomenological study. J. Food Eng. 2009, 93, 210–217. [Google Scholar] [CrossRef]
- Sithara, T.; Arun, K.B.; Syama, H.P.; Reshmitha, T.R.; Nisha, P. Morin inhibits proliferation of sw480 colorectal cancer cells by inducing apoptosis mediated by reactive oxygen species formation and uncoupling of Warburg effect. Front. Pharmacol. 2017, 8, 640. [Google Scholar] [CrossRef]
- Díaz-de-Cerio, E.; Verardo, V.; Gómez-Caravaca, A.M.; FernándezGutiérrez, A.; Segura-Carretero, A. Determination of polar compounds in guava leaves infusions and ultrasound aqueous extract by HPLC-ESI-MS. J. Chem. 2015, 1–9. [Google Scholar] [CrossRef]
- Caselli, A.; Cirri, P.; Santi, A.; Paoli, P. Morin: a promising natural drug. Curr. Med. Chem. 2016, 23, 774–791. [Google Scholar] [CrossRef]
- Rajput, S.A.; Wang, X.-q.; Yan, H.-C. Morin hydrate: A comprehensive review on novel natural dietary bioactive compound with versatile biological and pharmacological potential. Biomed. Pharmacother. 2021, 138, 111511. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.N.; Strasser, A. The role of Bcl-2 and its pro-survival relatives in tumourigenesis and cancer therapy. Cell Death Differ. 2011, 18, 1414–1424. [Google Scholar] [CrossRef] [PubMed]
- Strasser, A.; Cory, S.; Adams, J.M. Deciphering the rules of programmed cell death to improve therapy of cancer and other diseases. EMBO J. 2011, 30, 3667–3683. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.H.; Kumar, J.S.; Chellappan, D.R.; Nagarajan, S. Molecular chemoprevention by morin–a plant flavonoid that targets nuclear factor kappa B in experimental colon cancer. Biomed. Pharmacother. 2018, 100, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Vennila, S.; Nalini, N. Modifying effects of morin on the development of aberrant crypt foci and bacterial enzymes in experimental colon cancer. Food Chem. Toxicol. 2009, 47, 309–315. [Google Scholar]
- Chen, R.; Zhang, L. Morin inhibits colorectal tumor growth through inhibition of NF-κB signaling pathway, Immunopharmacol. Immunotoxicol. 2019, 41, 622–629. [Google Scholar] [CrossRef]
- Sreedharan, V.; Venkatachalam, K.K.; Namasivayam, N. Effect of morin on tissue lipid peroxidation and antioxidant status in 1, 2-dimethylhydrazine induced experimental colon carcinogenesis. Invest. N. Drugs 2009, 27, 21–30. [Google Scholar] [CrossRef]
- Lori, G.; Paoli, P.; Femia, A.P.; Pranzini, E.; Caselli, A.; Tortora, K.; Romagnoli, A.; Raugei, G.; Caderni, G. Morin-dependent inhibition of low molecular weight protein tyrosine phosphatase (LMW-PTP) restores sensitivity to apoptosis during colon carcinogenesis: studies in vitro and in vivo, in an Apc-driven model of colon cancer. Mol. Carcinog. 2019, 58, 686–698. [Google Scholar] [CrossRef]
| Class of compounds | MAE | UAE | SE | |||
|---|---|---|---|---|---|---|
| Solvent | Distilled water | 60% ethanol | Distilled water | 60% ethanol | Distilled water | 60% ethanol |
| Total flavonoid | + | ++ | ++ | +++ | +++ | ++++ |
| Total phenolic | + | +++ | +++ | ++ | ++++ | ++++ |
| Tannins and Phenols | ++++ | +++ | +++ | ++ | +++ | + |
| Alkaloids | + | ++ | ++ | ++++ | +++ | ++++ |
| Saponins | + | ++ | + | +++ | + | +++ |
| Terpenoids | + | ++++ | ++ | ++++ | +++ | ++++ |
| Glycosides | - | - | - | - | - | - |
| Protein | + | ++ | + | ++ | + | ++ |
| Amino acids | + | ++ | + | ++ | + | ++ |
| Extraction method | Concentration (µg/ml) | Growth inhibition (%) | IC50 (µg/ml) |
|---|---|---|---|
| MAE | 12.5 | 47.83±2.94 | 16.67±3.65c |
| 25 | 52.73±0.47 | ||
| 50 | 57.12±2.74 | ||
| 100 | 61.59±1.24 | ||
| 200 | 65.51±0.35 | ||
| UAE | 12.5 | 35.05±2.65 | 98.68±0.8b |
| 25 | 37.91±2.13 | ||
| 50 | 45.31±0.33 | ||
| 100 | 50.20±2.11 | ||
| 200 | 65.10±2.41 | ||
| SE | 12.5 | 27.11±2.33 | 144.59±22.30a |
| 25 | 33.89±0.72 | ||
| 50 | 38.78±2.26 | ||
| 100 | 44.83±1.89 | ||
| 200 | 55.19±2.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





