Submitted:
05 May 2023
Posted:
06 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Production of Haynes 282 Specimens by Additive Technique
2.2. Ion Nitriding Process
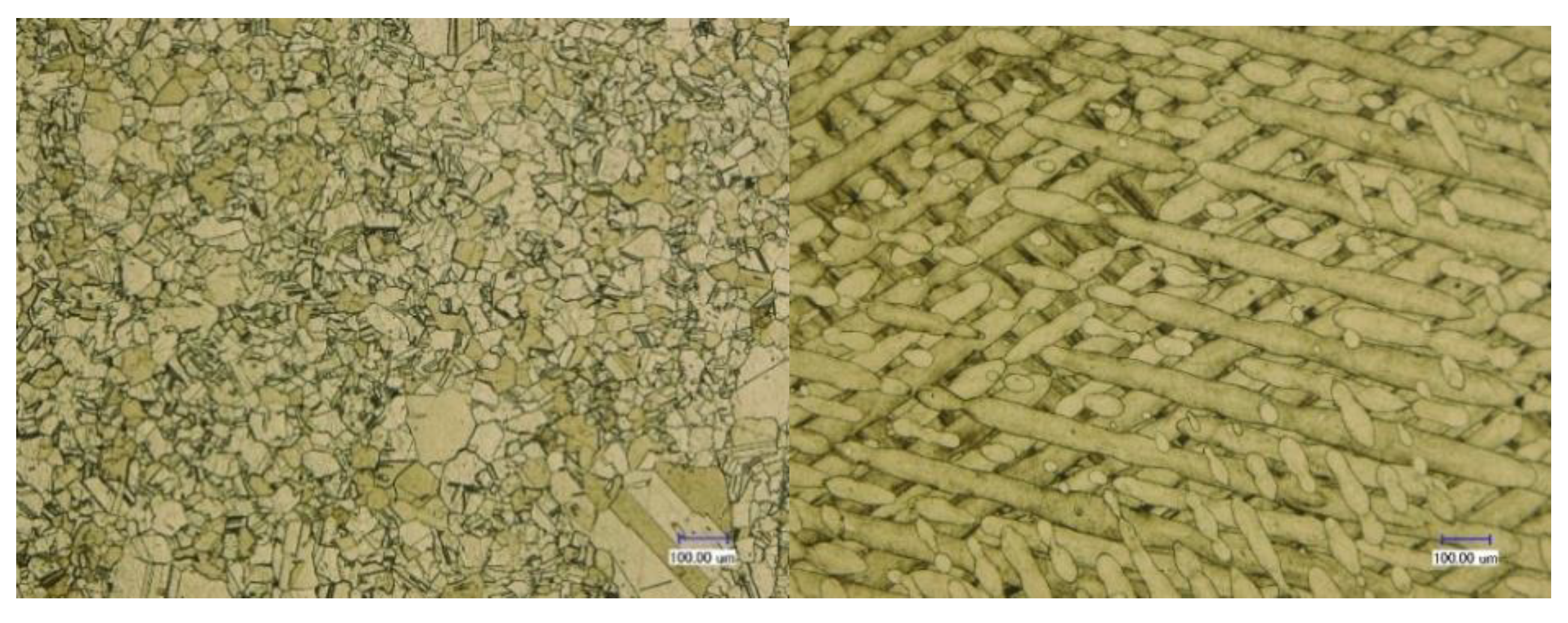
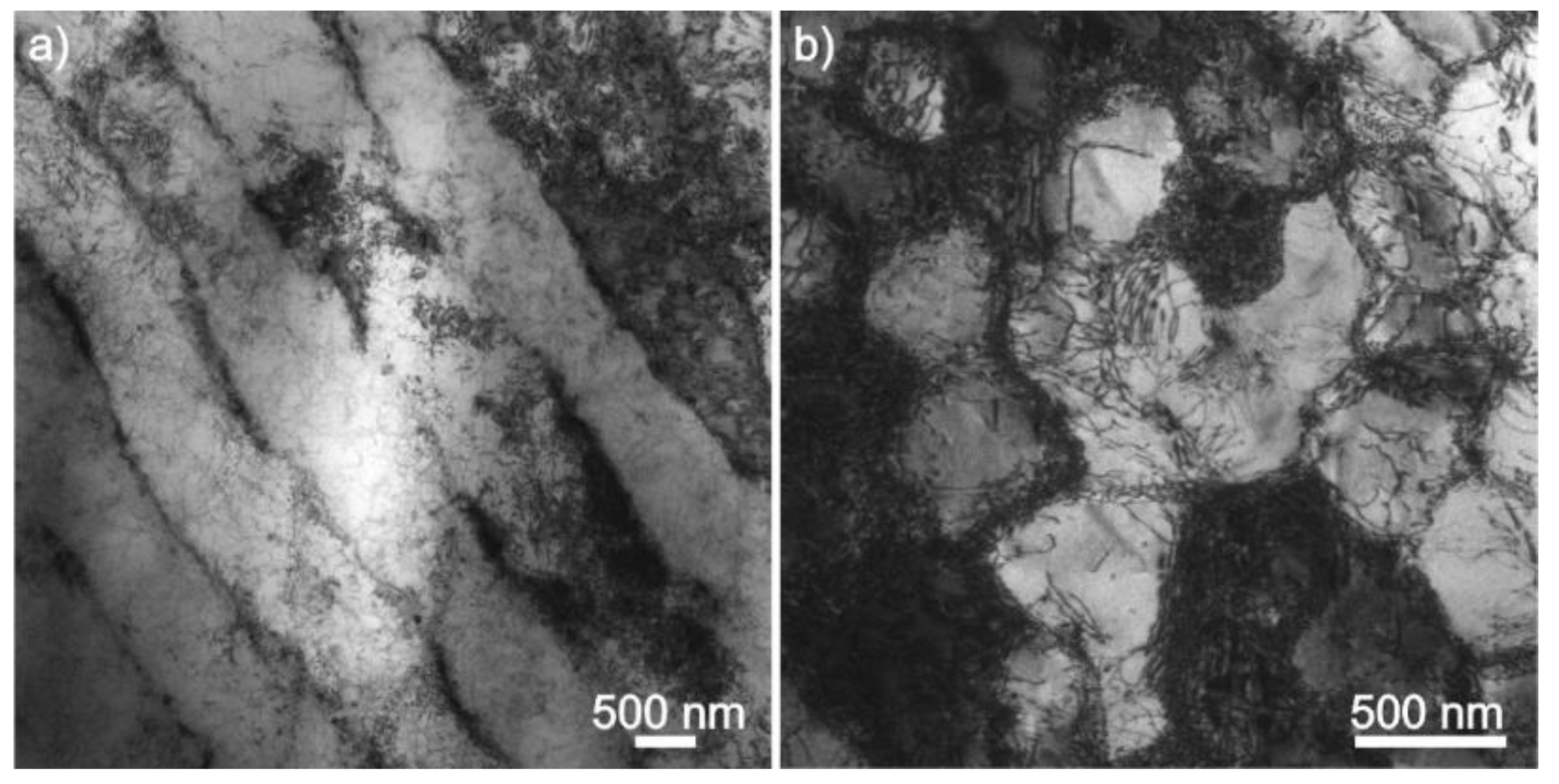
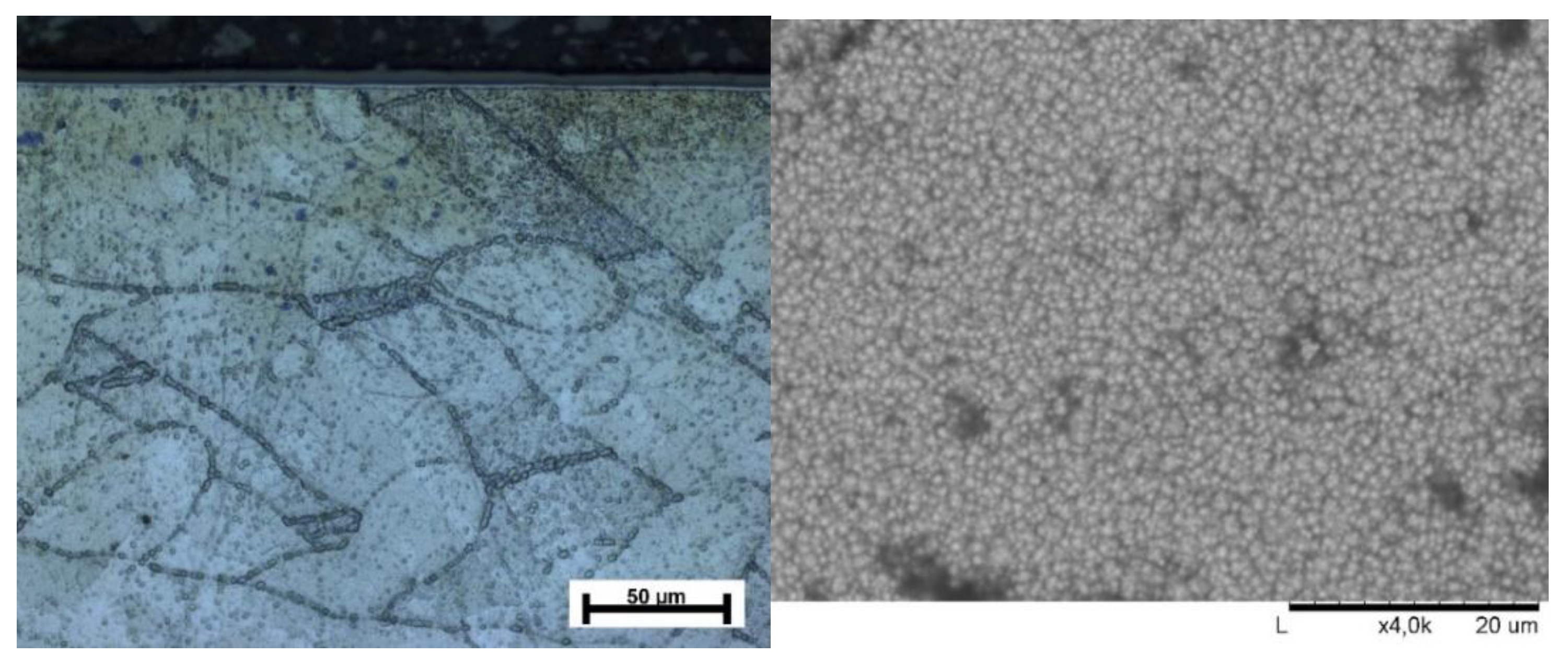
2.3. Microstructure
2.4. Nanohardness of the Manufactured Layers
2.5. Corrosion Resistance Tests
2.6. Analysis of Phase Composition and Residual Stresses
2.7. Static Tensile Test
3. Research Results and Discussion
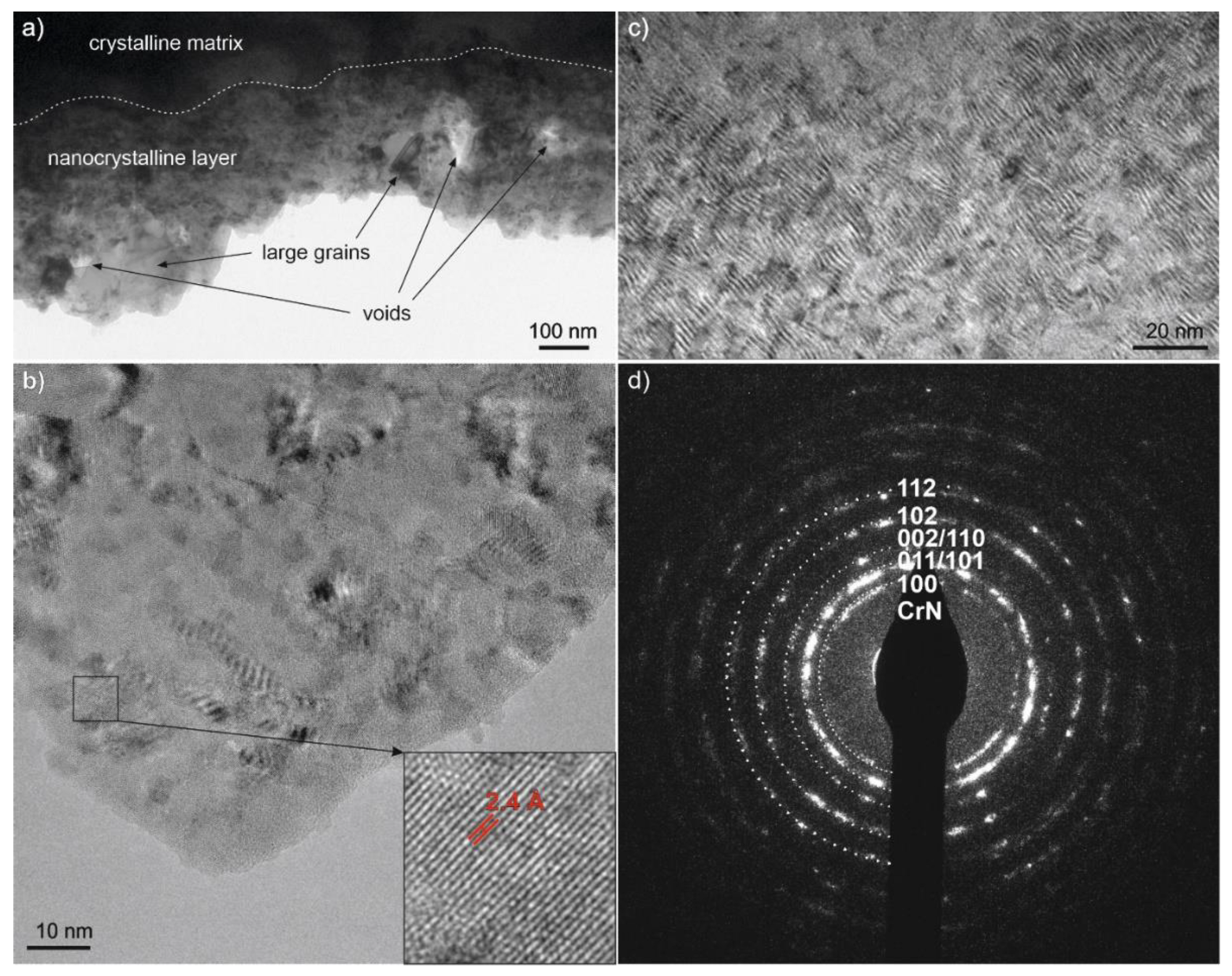
3.1. Microstructure
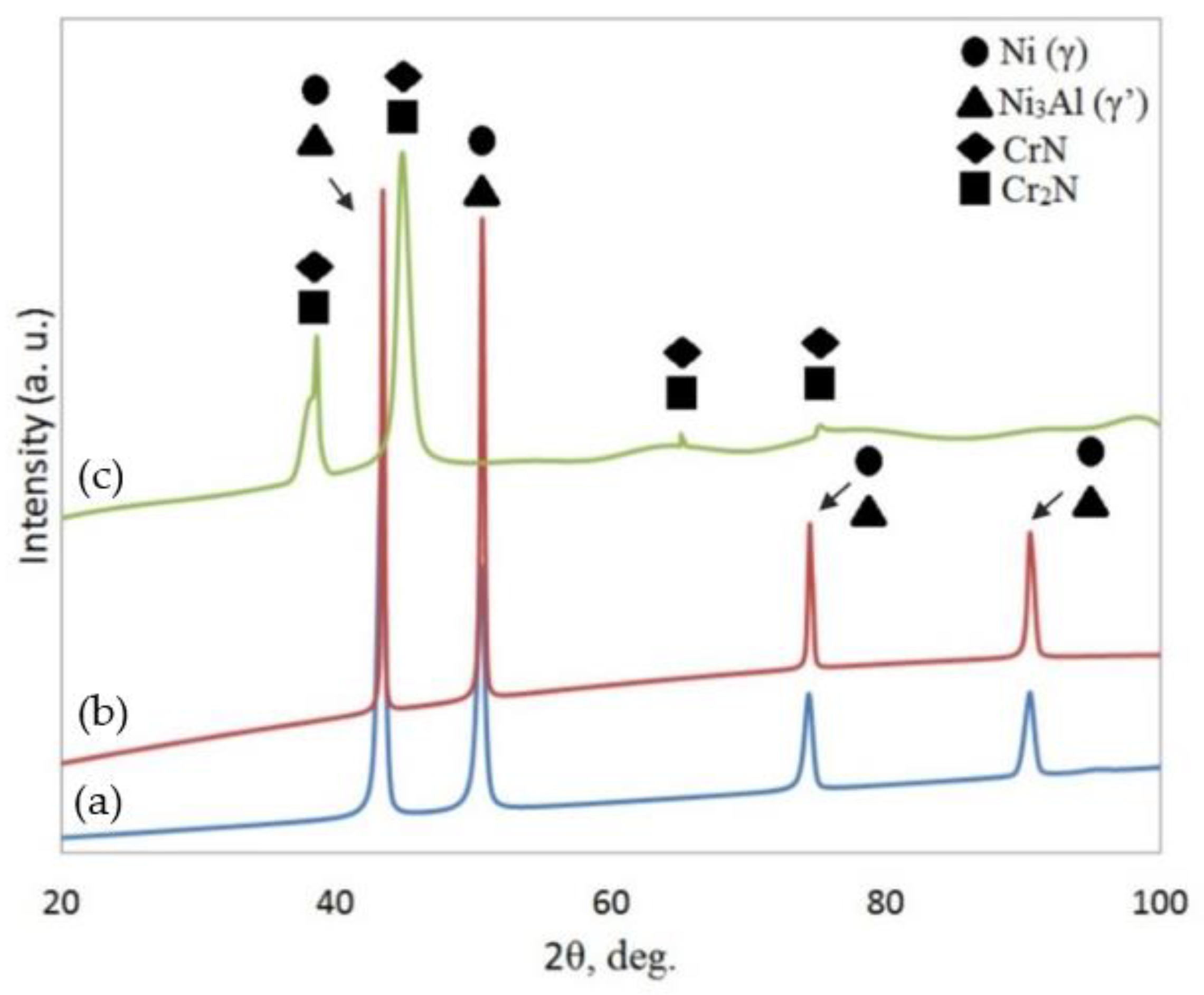
3.2. Phase Analysis and Residual Stress
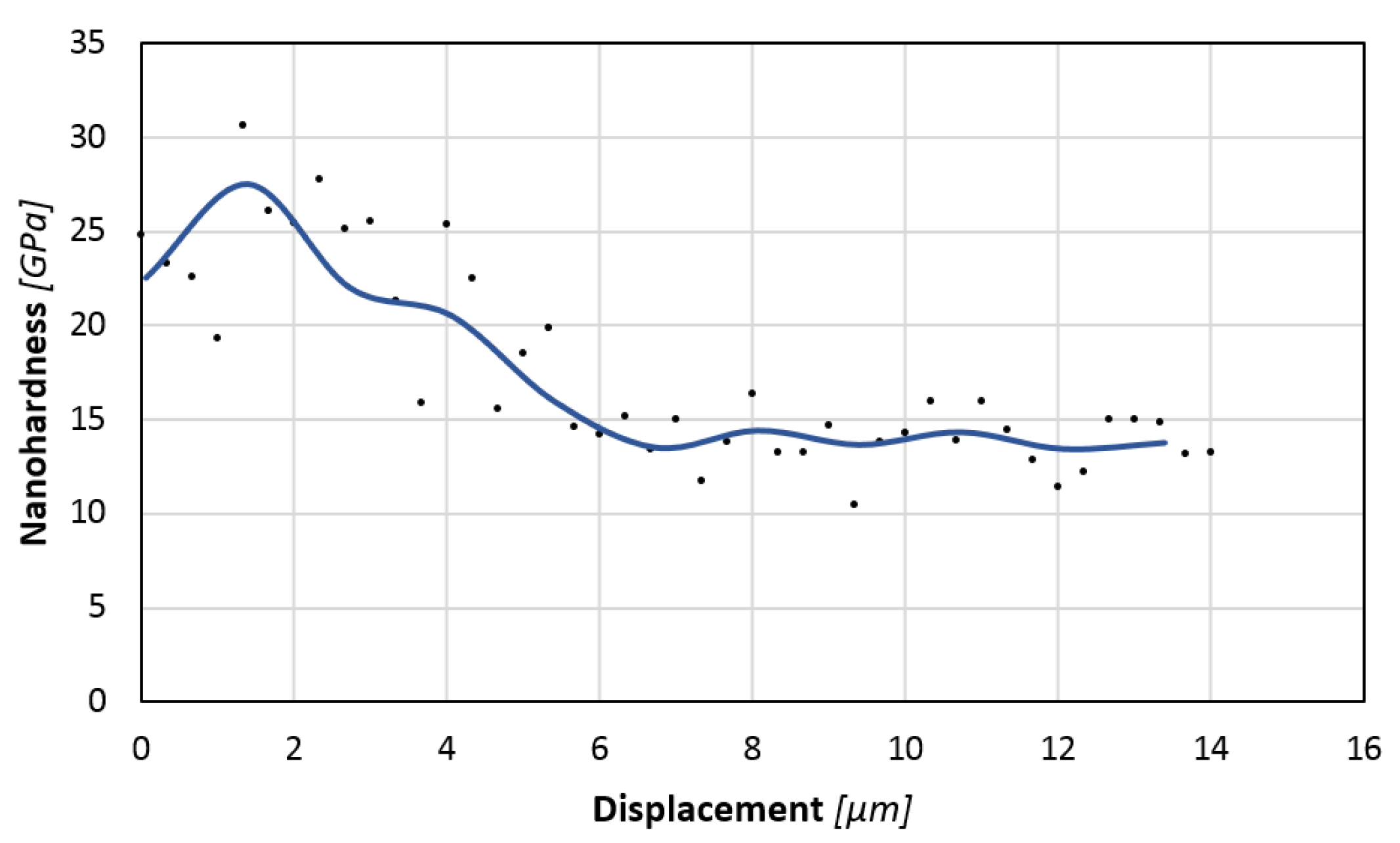
3.3. Nanohardness
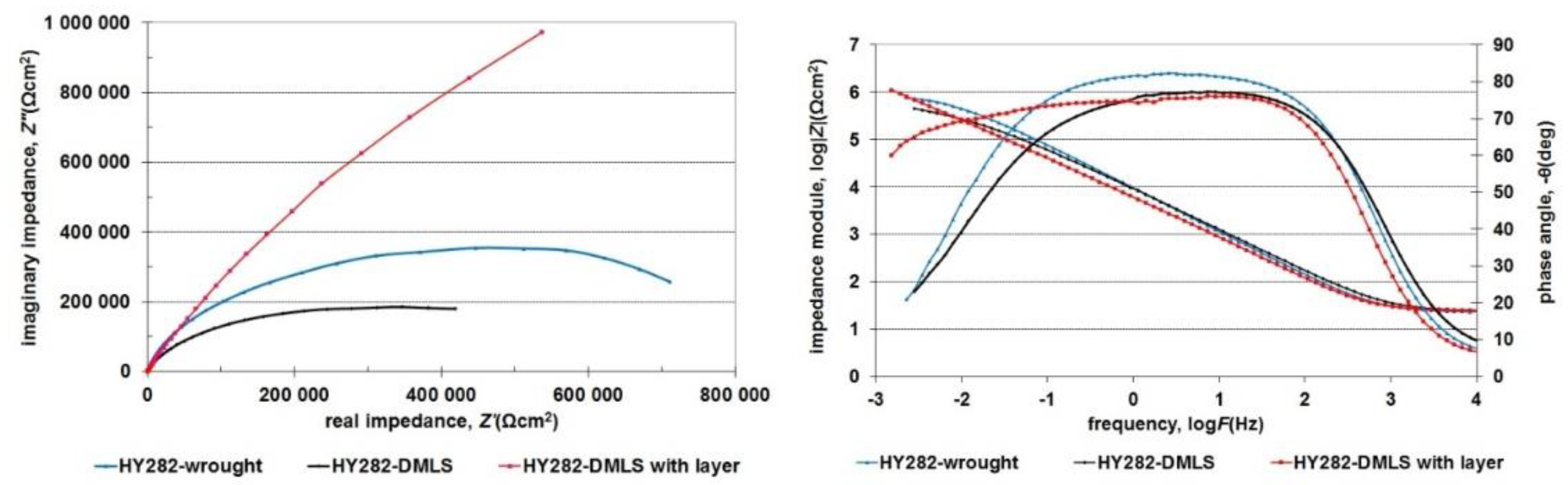
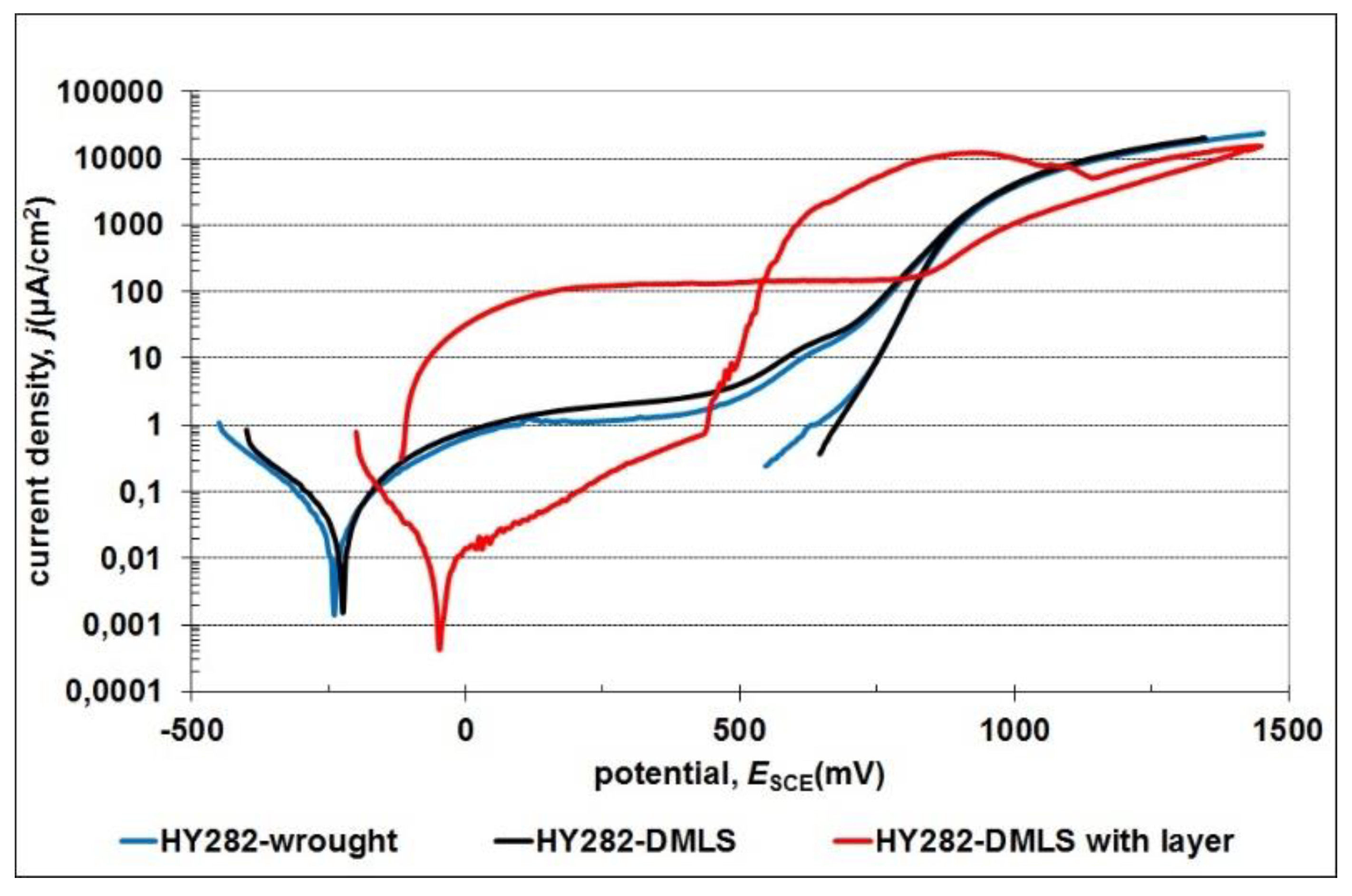
3.4. Corrosion Resistance
| Sa | SD | Ra | SD | Rz | SD | |
|---|---|---|---|---|---|---|
| Haynes 282 wrought | 12.78 ± 0.15 | 7.35 ± 0.62 | 76.14 ± 13.74 | |||
| Haynes 282 DMLS | 16.45 ± 0.24 | 6.39 ± 0.40 | 71.82 ± 10.63 | |||
| Haynes 282 DMLS with CrN+Cr2N layer | 118.93 ± 1.37 | 84.25 ± 5.26 | 1186.65 ± 263.02 | |||
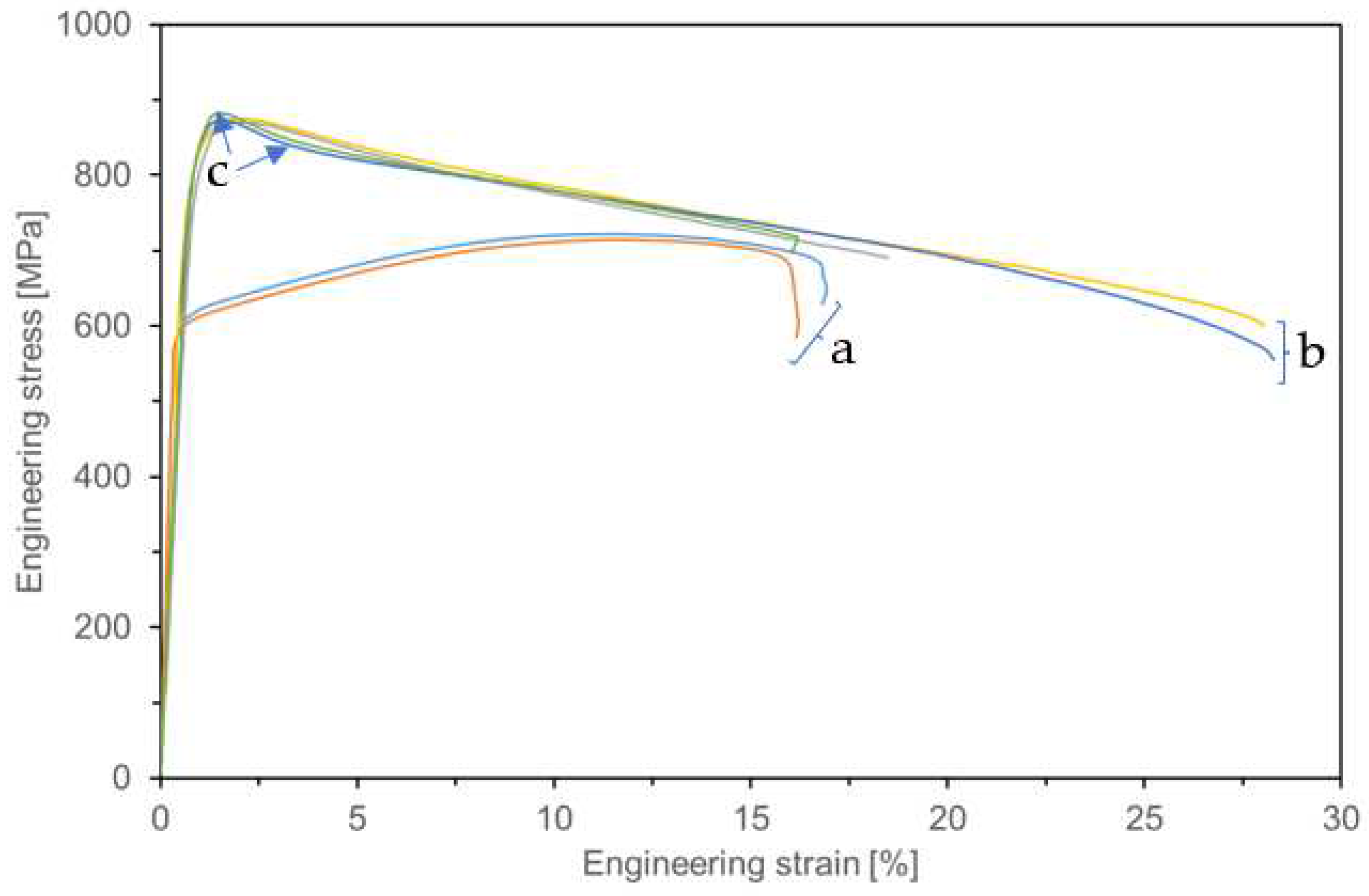
3.7. Static Tensile Test
4. Conclusions
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Di Gianfrancesco, A. Materials for Ultra-Supercritical and Advanced Ultra-Supercritical Power Plants; Elsevier BV: Amsterdam, NX, Netherlands, 2017; Available online: https://app.knovel.com/hotlink/pdf/id:kt0114KRUB/materials-ultra-supercritical/physical-metallurgyISBN 9780081005521.
- Kruger, K. L. , ; Di Gianfrancesco, A., Elsevier: Amsterdam, Netherlands, 2017, 511–545. https://doi.org/10.1016/b978-0-08-100552-1.00015-4.alloy. In Materials for Ultra-Supercritical and Advanced Ultra-Supercritical Power Plants, 1st ed.; Di Gianfrancesco, A., Elsevier: Amsterdam, Netherlands, 2017. [Google Scholar] [CrossRef]
- Tamarin, Y. . 2002. Protective Coatings for Turbine Blades - 2.1 Conditions of Turbine Blade Operation. ASM International. Available online: https://app.knovel.com/hotlink/pdf/id:kt010SPJL2/protective-coatings-turbine/conditions-turbine-blade.
- Ban, S.-D.; Shin, Y.-T.; Lee, S.R.; Lee, H.-W. Corrosion Resistance of Inconel 625 Overlay Welded Inside Pipes as a Function of Heat Treatment Temperature. Int. J. Electrochem. Sci. 2016, 11, 7764–7774. [Google Scholar] [CrossRef]
- Boswell, J.; Jones, J.; Barnard, N.; Clark, D.; Whittaker, M.; Lancaster, R. The effects of energy density and heat treatment on the microstructure and mechanical properties of laser additive manufactured Haynes 282. Mater. Des. 2021, 205. [Google Scholar] [CrossRef]
- Dudziak, T.; Boron, L.; Homa, M.; Nowak, R.; Horton, N.; Sheppard, R.; Purgert, R.M.; Siewiorek, A.; Sobczak, N.; Sobczak, J.J. The Influence of Fabrication Process on the Initial Stages of Steam Oxidation Performed on Haynes® 282® Alloy at 760 °C. J. Mater. Eng. Perform. 2016, 26, 239–249. [Google Scholar] [CrossRef]
- Ban, S.-D.; Shin, Y.-T.; Lee, S.R.; Lee, H.-W. Corrosion Resistance of Inconel 625 Overlay Welded Inside Pipes as a Function of Heat Treatment Temperature. Int. J. Electrochem. Sci. 2016, 11, 7764–7774. [Google Scholar] [CrossRef]
- Sitek, R.; Kwaśniak, P.; Sopicka-Lizer, M.; Borysiuk, J.; Kamiński, J.; Mizera, J.; Kurzydłowski, K. Experimental and ab-initio study of the Zr- and Cr-enriched aluminide layer produced on an IN 713C Inconel substrate by CVD; investigations of the layer morphology, structural stability, mechanical properties, and corrosion resistance. Intermetallics 2016, 74, 15–24. [Google Scholar] [CrossRef]
- Osoba, L.O.; Oladoye, A.M.; Ogbonna, V.E. Corrosion evaluation of superalloys Haynes 282 and Inconel 718 in Hydrochloric acid. J. Alloys Compd. 2019, 804, 376–384. [Google Scholar] [CrossRef]
- Klapper, H.S.; Zadorozne, N.S.; Rebak, R.B. Localized Corrosion Characteristics of Nickel Alloys: A Review. Acta Met. Sin. (English Lett. 2017, 30, 296–305. [Google Scholar] [CrossRef]
- Paulauskas, I.; Brady, M.; Meyer, H.; Buchanan, R.; Walker, L. Corrosion behavior of CrN, Cr2N and π phase surfaces on nitrided Ni–50Cr for proton exchange membrane fuel cell bipolar plates. Corros. Sci. 2006, 48, 3157–3171. [Google Scholar] [CrossRef]
- Bertrand, G.; Mahdjoub, H.; Meunier, C. A study of the corrosion behaviour and protective quality of sputtered chromium nitride coatings. Surf. Coatings Technol. 2000, 126, 199–209. [Google Scholar] [CrossRef]
- Materials Today Communications, 2021, 26, 102038114 Further work on detailed microstructure characterisation and strengthening mechanisms as well as tailored post-AM heat treat-ment is required to reveal the full potential of Haynes 282 by LPBF.
- Wang, L.; Mao, K.; Tortorelli, P.F.; Maziasz, P.J.; Thangirala, M.; Unocic, K.A.; Chen, X.F. Effect of heterogeneous microstructure on the tensile and creep performances of cast Haynes 282 alloy. Mater. Sci. Eng. A 2021, 828, 142099. [Google Scholar] [CrossRef]
- Rozman, K.A.; Kruzic, J.J.; Hawk, J.A. Fatigue Crack Growth Behavior of Nickel-base Superalloy Haynes 282 at 550-750 °C. J. Mater. Eng. Perform. 2015, 24, 2841–2846. [Google Scholar] [CrossRef]
- Pike, L. Development of a Fabricable Gamma Prime (γ ) Strengthened Superalloy. Superalloys. LOCATION OF CONFERENCE, COUNTRYDATE OF CONFERENCE;
- Sitek, R.; Puchlerska, S.; Nejman, I.; Majchrowicz, K.; Pakieła, Z.; Żaba, K.; Mizera, J. The Impact of Plastic Deformation on the Microstructure and Tensile Strength of Haynes 282 Nickel Superalloy Produced by DMLS and Casting. Materials 2022, 15, 7545. [Google Scholar] [CrossRef] [PubMed]
- Boswell, J.; Jones, J.; Barnard, N.; Clark, D.; Whittaker, M.; Lancaster, R. The effects of energy density and heat treatment on the microstructure and mechanical properties of laser additive manufactured Haynes 282. Mater. Des. 2021, 205. [Google Scholar] [CrossRef]
- Fernandez-Zelaia, P.; Kirka, M.M.; Dryepondt, S.N.; Gussev, M.N. Crystallographic texture control in electron beam additive manufacturing via conductive manipulation. Mater. Des. 2020, 195. [Google Scholar] [CrossRef]
- Teng, Y.; Guo, Y.-Y.; Zhang, M.; Yang, Y.-J.; Huang, Z.; Zhou, Y.-W.; Wu, F.-Y.; Liang, Y.-S. Effect of Cr/CrNx transition layer on mechanical properties of CrN coatings deposited on plasma nitrided austenitic stainless steel. Surf. Coatings Technol. 2019, 367, 100–107. [Google Scholar] [CrossRef]
- Zhang, M.; Li, M.-K.; Kim, K.H.; Pan, F. Structural and mechanical properties of compositionally gradient CrNx coatings prepared by arc ion plating. Appl. Surf. Sci. 2009, 255, 9200–9205. [Google Scholar] [CrossRef]
- Yetim, A.; Codur, M.; Yazici, M. Using of artificial neural network for the prediction of tribological properties of plasma nitrided 316L stainless steel. Mater. Lett. 2015, 158, 170–173. [Google Scholar] [CrossRef]
- Bonarski J., T. , „Pomiar i wykorzystanie teksturowo – napreżeniowej charakterystyki mikrostruktury w diagnosyce materiałów”. Kraków: Instytut Metalurgii i Inżynierii Materiałowej PAN; 2013 (in Polish).
- Skrzypek S., J. , „Nowe możliwości pomiaru makronaprężeń własnych materiałów przy zastosowaniu dyfrakcji promieniowania X w geometrii stałego kąta padania”, Kraków: Wydawnictwo AGH; 2002 (in Polish).
- Xu, X.-L.; Yu, Z.-W.; Cui, L.-Y. Microstructure and properties of plasma nitrided layers on Ni-based superalloy Ni-20Cr. Mater. Charact. 2019, 155, 109798. [Google Scholar] [CrossRef]
- Shen, H.; Wang, L.; Sun, J. Characteristics and properties of Cr N compound layer produced by plasma nitriding of Cr-electroplated of AISI 304 stainless steel. Surf. Coatings Technol. 2020, 385, 125450. [Google Scholar] [CrossRef]
- Akhter, R. , Zhou Z., Xie Z., Munroe P. Harmonizing mechanical responses of nanostructured CrN coatings via Ni additions, Appl. Surf. Sci. 2021, 538, 147987. [Google Scholar] [CrossRef]
- Gawroński, Z. , Residual stresses in the surface layer of SW7M steel after conventional and low pressure ’Nitrovac’79’ nitriding processes, Mech. Mech. Eng., 1999, 3, 27–34. [Google Scholar]
- Mendibide, C.; Steyer, P.; Esnouf, C.; Goudeau, P.; Thiaudière, D.; Gailhanou, M.; Fontaine, J. X-ray diffraction analysis of the residual stress state in PVD TiN/CrN multilayer coatings deposited on tool steel. Surf. Coatings Technol. 2005, 200, 165–169. [Google Scholar] [CrossRef]
- Bouaouina, B.; Besnard, A.; Abaidia, S.; Haid, F. Residual stress, mechanical and microstructure properties of multilayer Mo 2 N/CrN coating produced by R.F Magnetron discharge. Appl. Surf. Sci. 2017, 395, 117–121. [Google Scholar] [CrossRef]
- Taguchi, M.; Kurihara, J. Efect of surface nitriding on corrosion resistance of chromium in sulfuric acid solution, Materials Transactions. JIM 1991, 32, 1170–1176. [Google Scholar]
- Huang, L.-F.; Hutchison, M.J.; Santucci, R.J.; Scully, J.R.; Rondinelli, J.M. Improved Electrochemical Phase Diagrams from Theory and Experiment: The Ni–Water System and Its Complex Compounds. J. Phys. Chem. C 2017, 121, 9782–9789. [Google Scholar] [CrossRef]
- Bertsch, K.; de Bellefon, G.M.; Kuehl, B.; Thoma, D. Origin of dislocation structures in an additively manufactured austenitic stainless steel 316L. Acta Mater. 2020, 199, 19–33. [Google Scholar] [CrossRef]
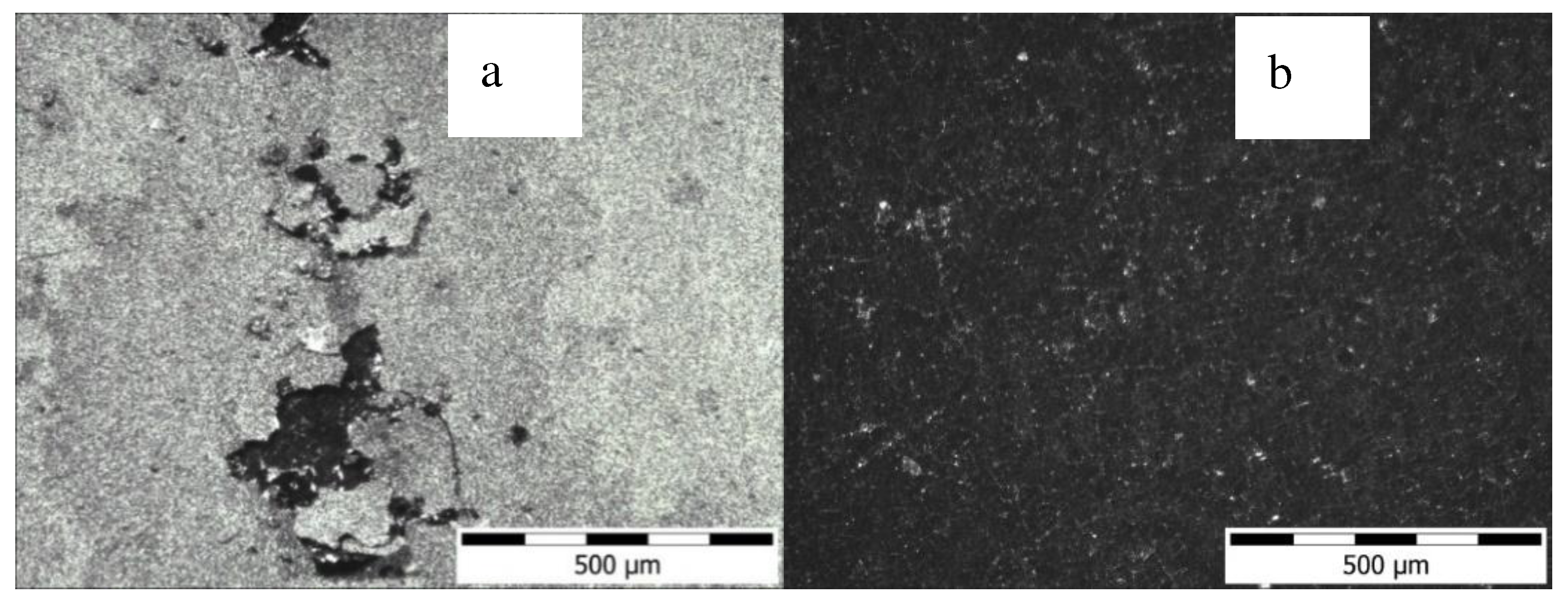
| Substitute arrangement | Dielectric layer | double layer | ||
|---|---|---|---|---|
| Haynes 282-wrought | R(RQ) | R(Ωcm2) | 8.07 × 105 | |
| Y0 CPE(Fcm-2*sn-1) | 1.99 × 10-5 | |||
| n | 0.91 | |||
| Haynes 282-DMLS | R(RQ)(RQ) | R(Ωcm2) | 6.26 × 104 | 4.36 × 105 |
| Y0 CPE(Fcm-2*sn-1) | 4.99 × 10-5 | 3.87 × 10-5 | ||
| n | 0.86 | 0.88 | ||
| Haynes 282 DMLS with layer | R(Q[R(RQ)]) | R(Ωcm2) | 8.61 × 103 | 7.43 × 106 |
| Y0 CPE(Fcm-2*sn-1) | 2.67 × 10-5 | 1.03 × 10-5 | ||
| n | 0.89 | 0.66 |
| Haynes 282 wrought | Haynes 282 DMLS | Haynes 282 DMLS with layer |
|
|---|---|---|---|
| Rp (kΩcm2) | 809 | 637 | 2763 |
| Icorr (µA/cm2) | 3.88 × 10-2 | 4.09 × 10-2 | 0.89 × 10-2 |
| Ecorr (mV) | -240 | -225 | -45 |
| Specimens | YS0.2 [MPa] | UTS [MPa] | A [%] |
|---|---|---|---|
| Haynes 282 alloy (wrought) | 600±4 | 719±4 | 15±1.5 |
| Haynes 282 DMLS | 808±3 | 873±4 | 22±6.4 |
| Haynes 282 DMLS with a CrN+Cr2N layer | 834±5 | 878±6 | 21±8.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





