Submitted:
05 May 2023
Posted:
06 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
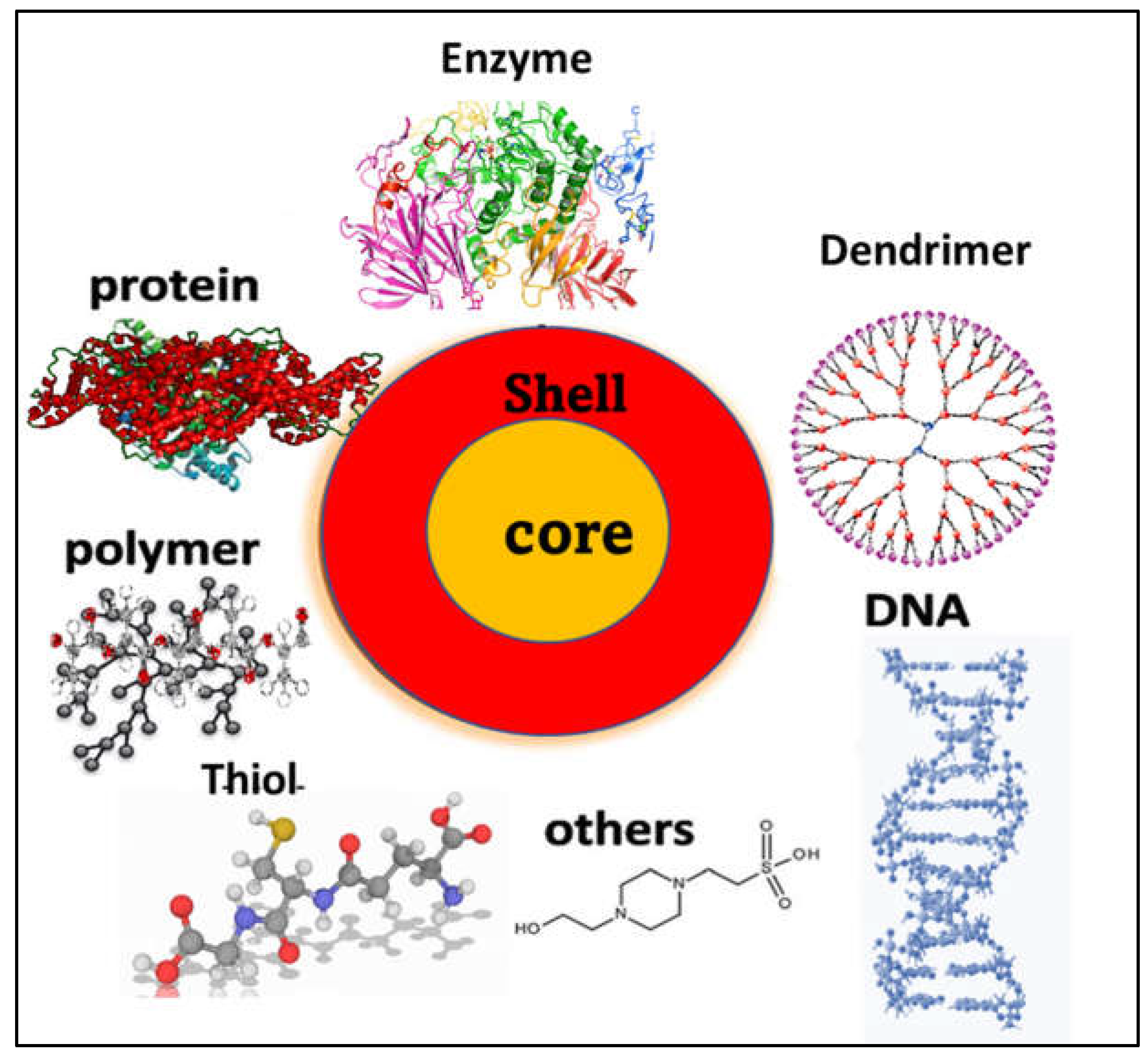
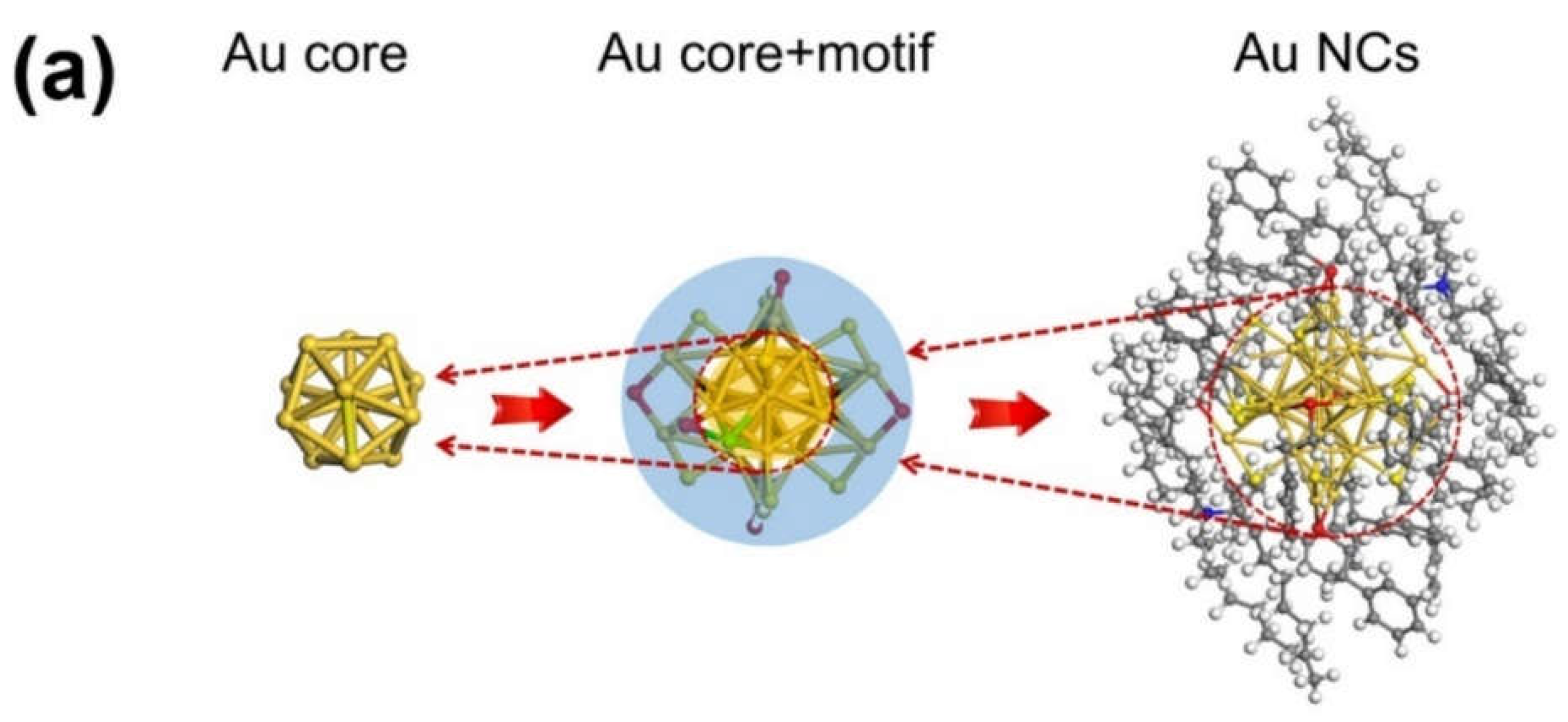
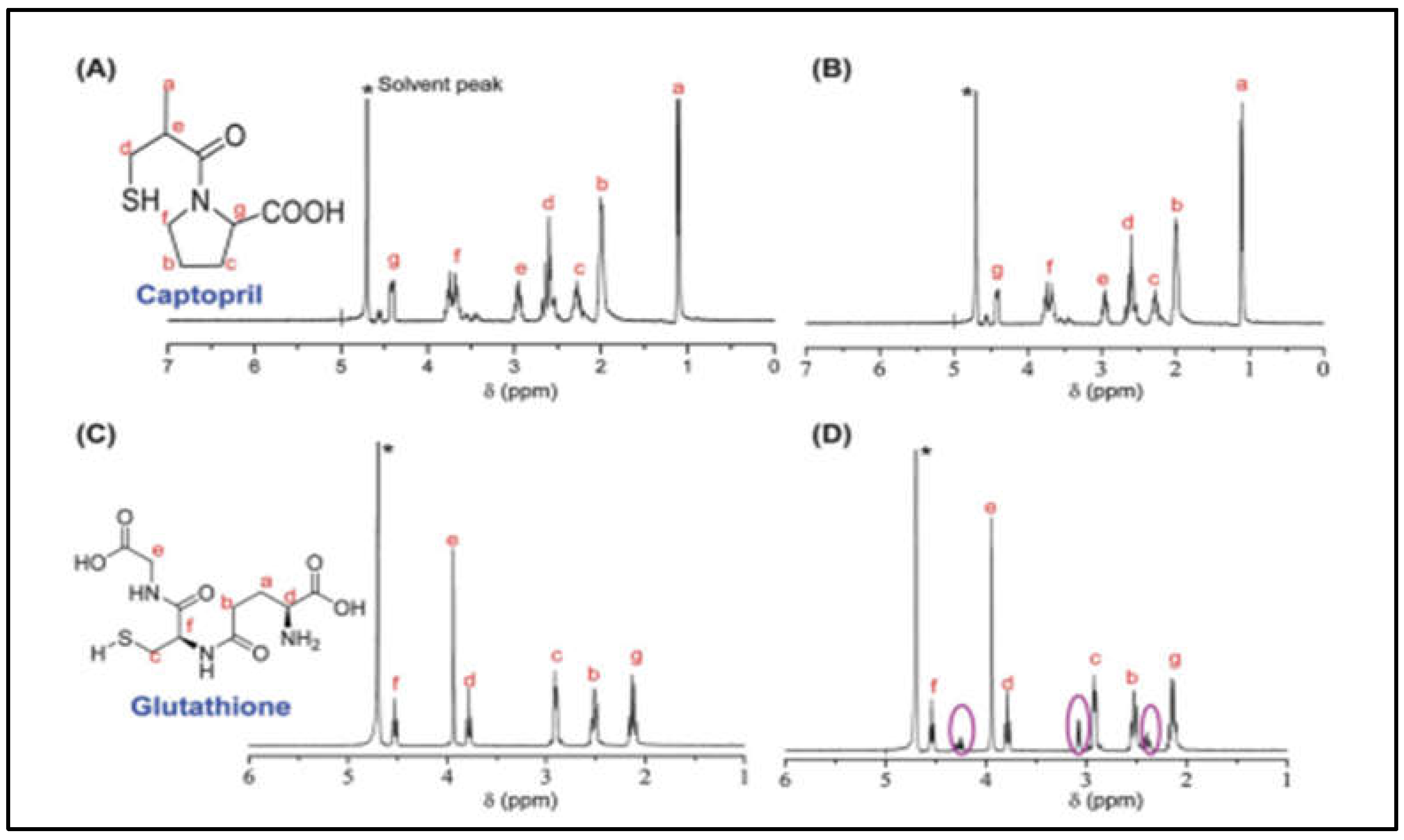
1.1. Chemical Composition and structural properties
Synthesis of Metal Nanocluster
1.2. Key physico-chemical properties of MNCs in photocatalysis
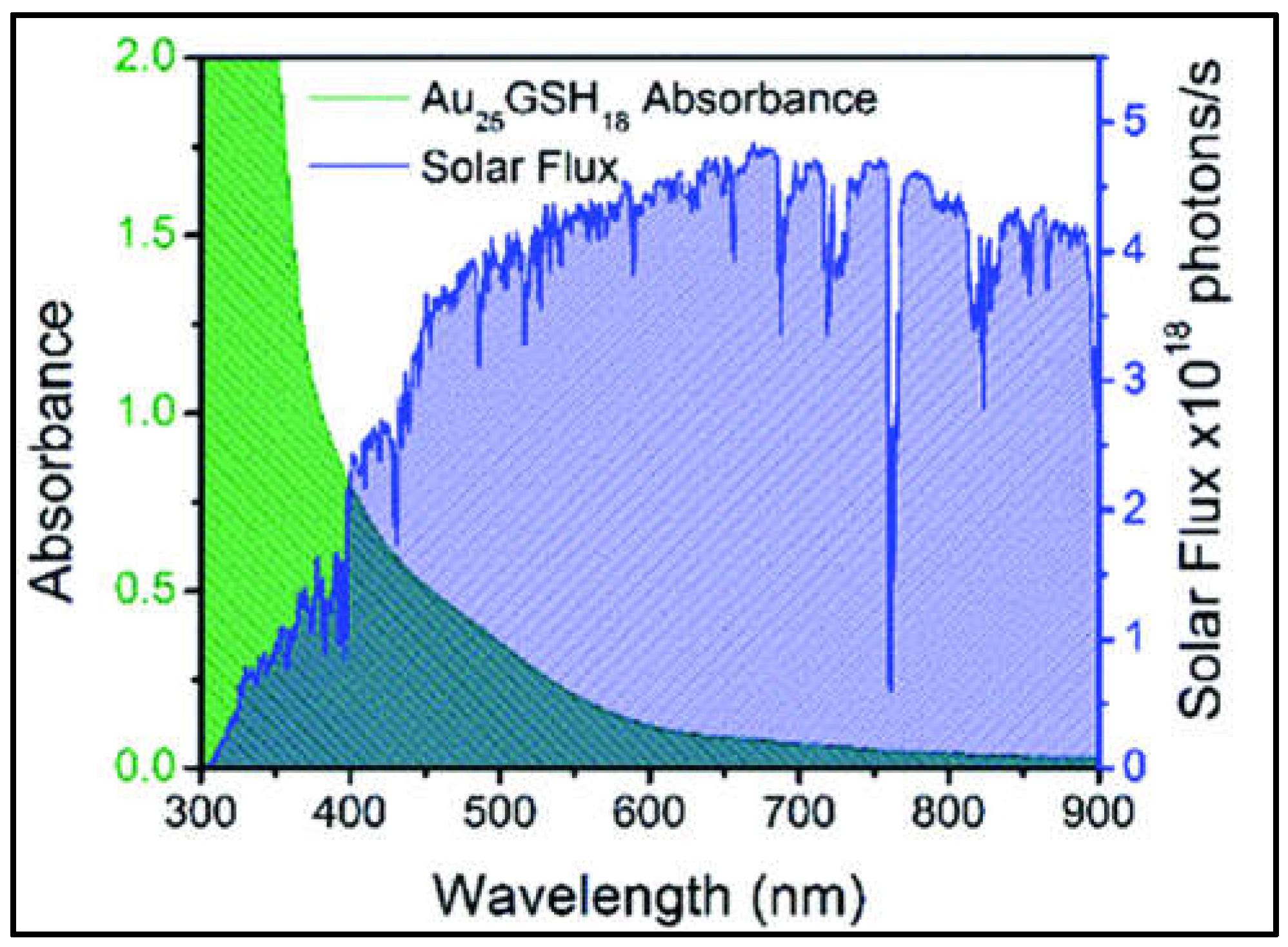
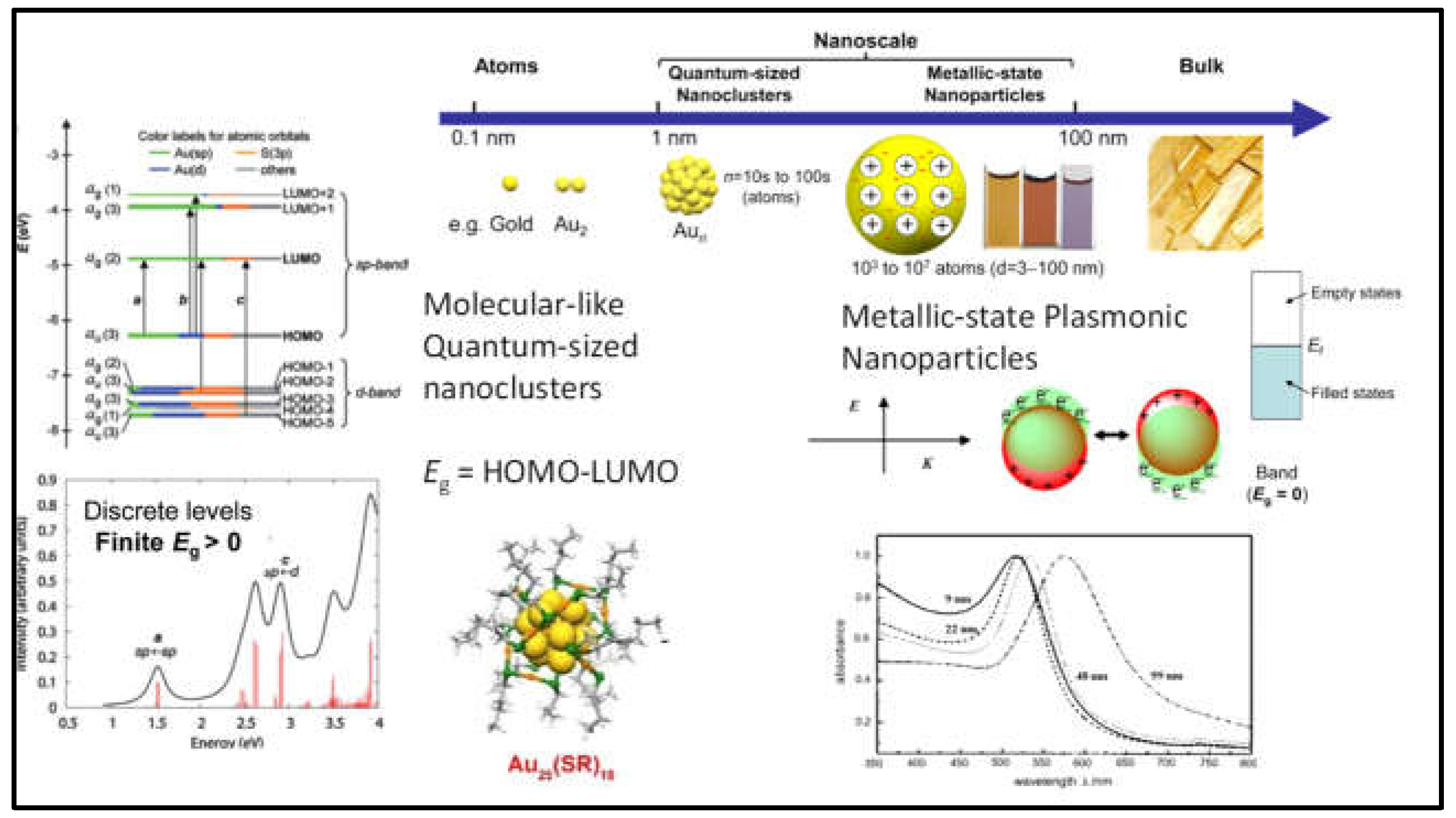
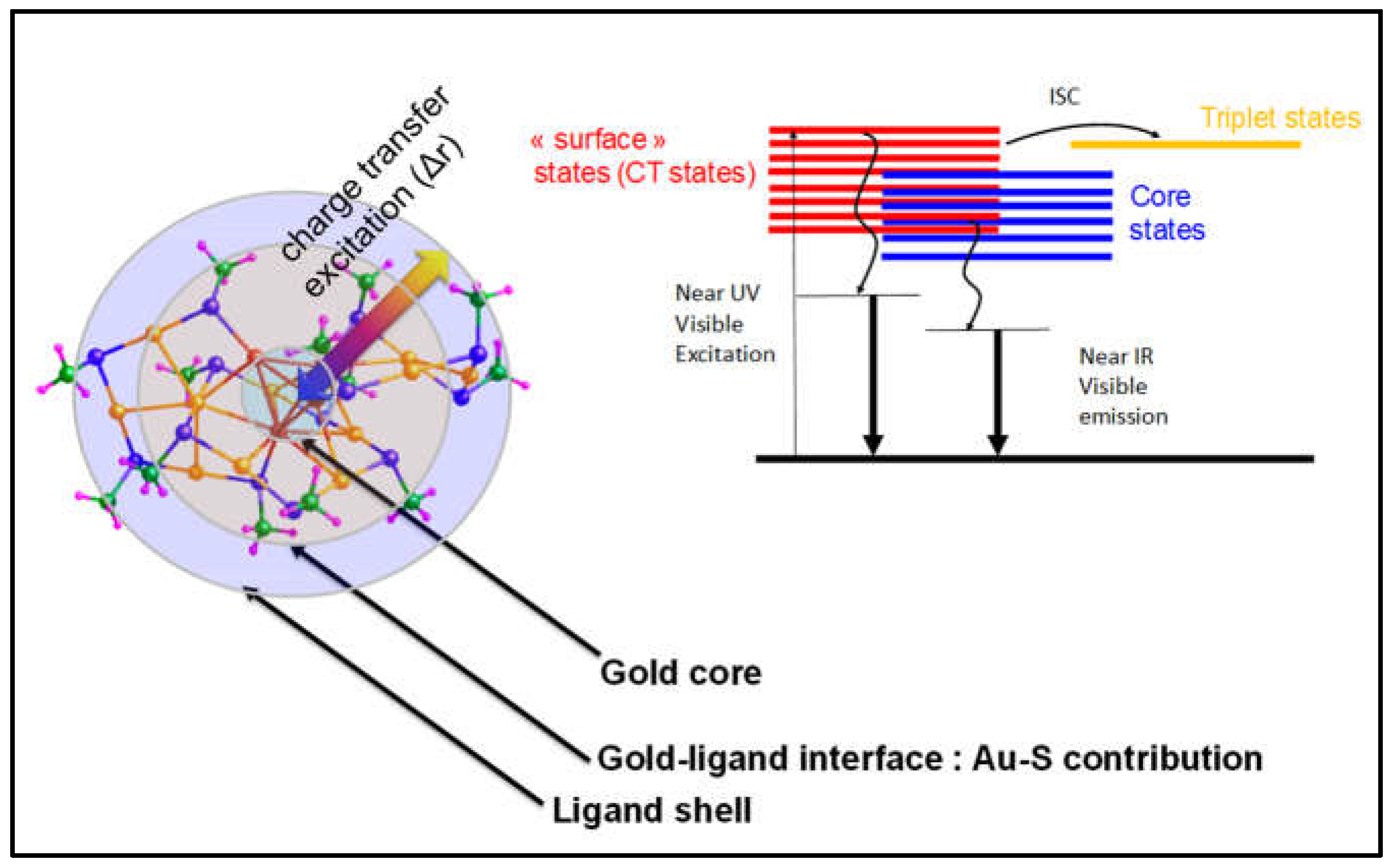
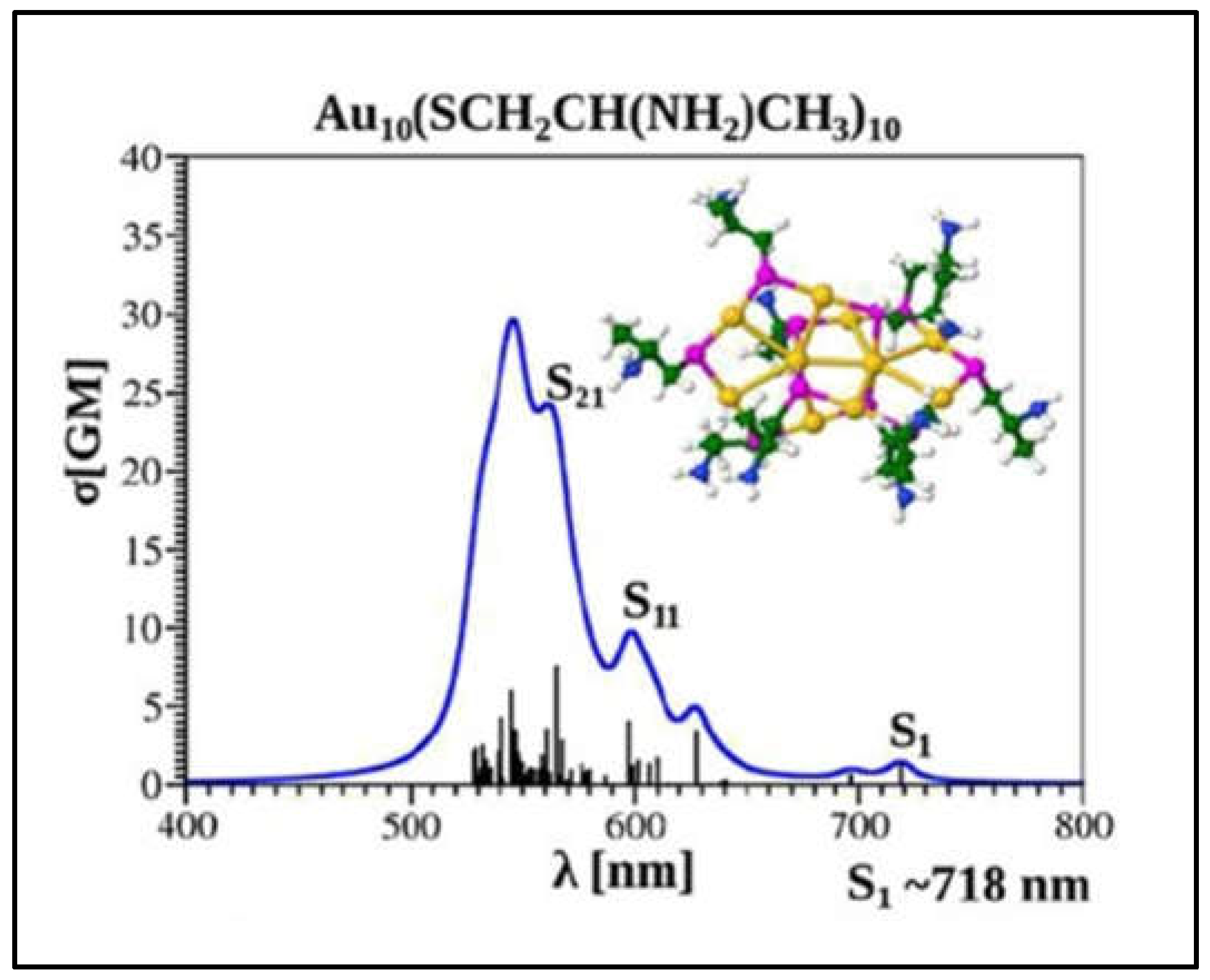
1.3. Optical Properties
1.4. Stability
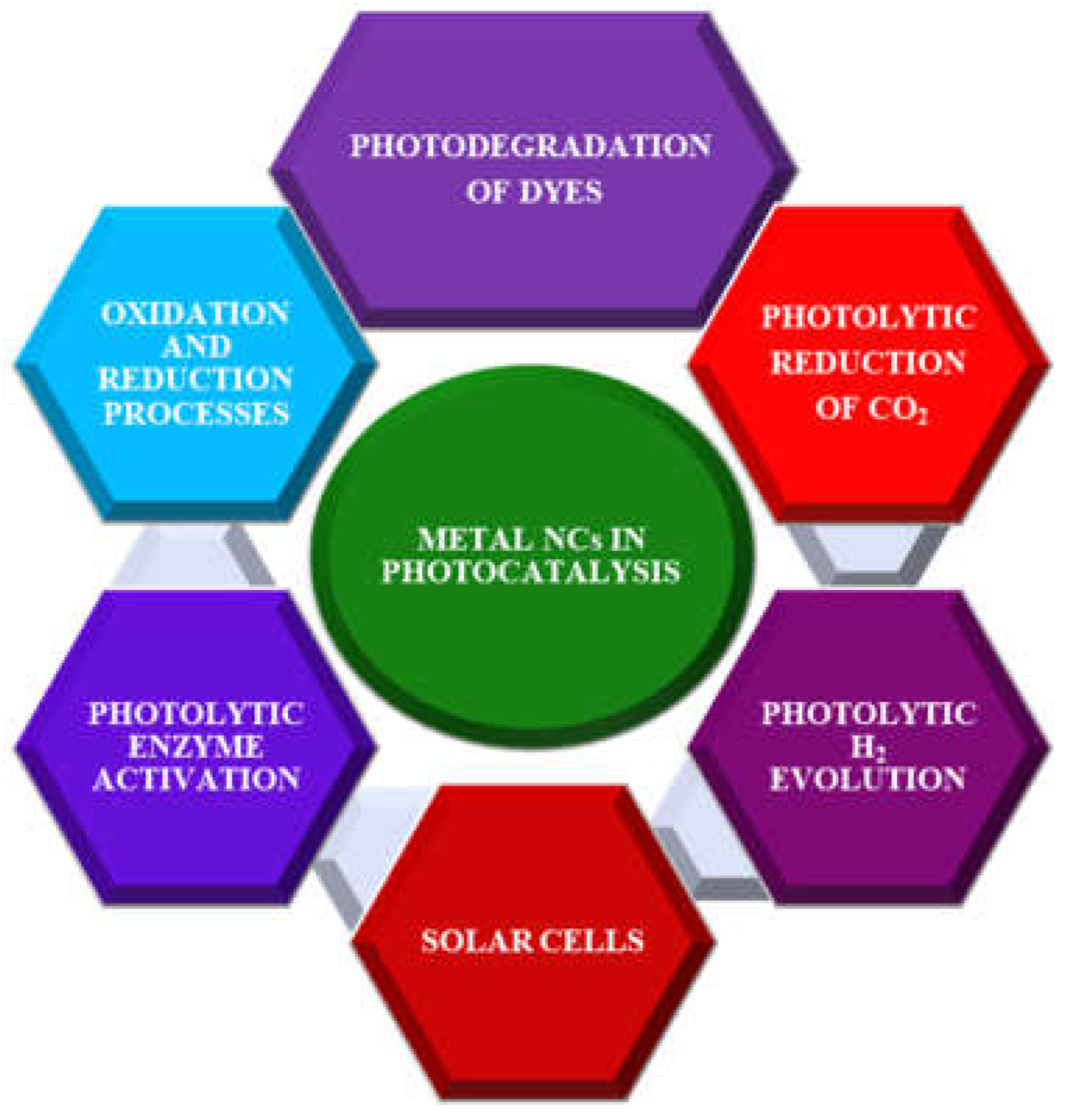
2. Application of Nanoclusters in Photocatalysis
2.1. Photodegradation of organic pollutants
2.2. Oxidation and hydrogenation processes
2.3. Photocatalytic H2 production
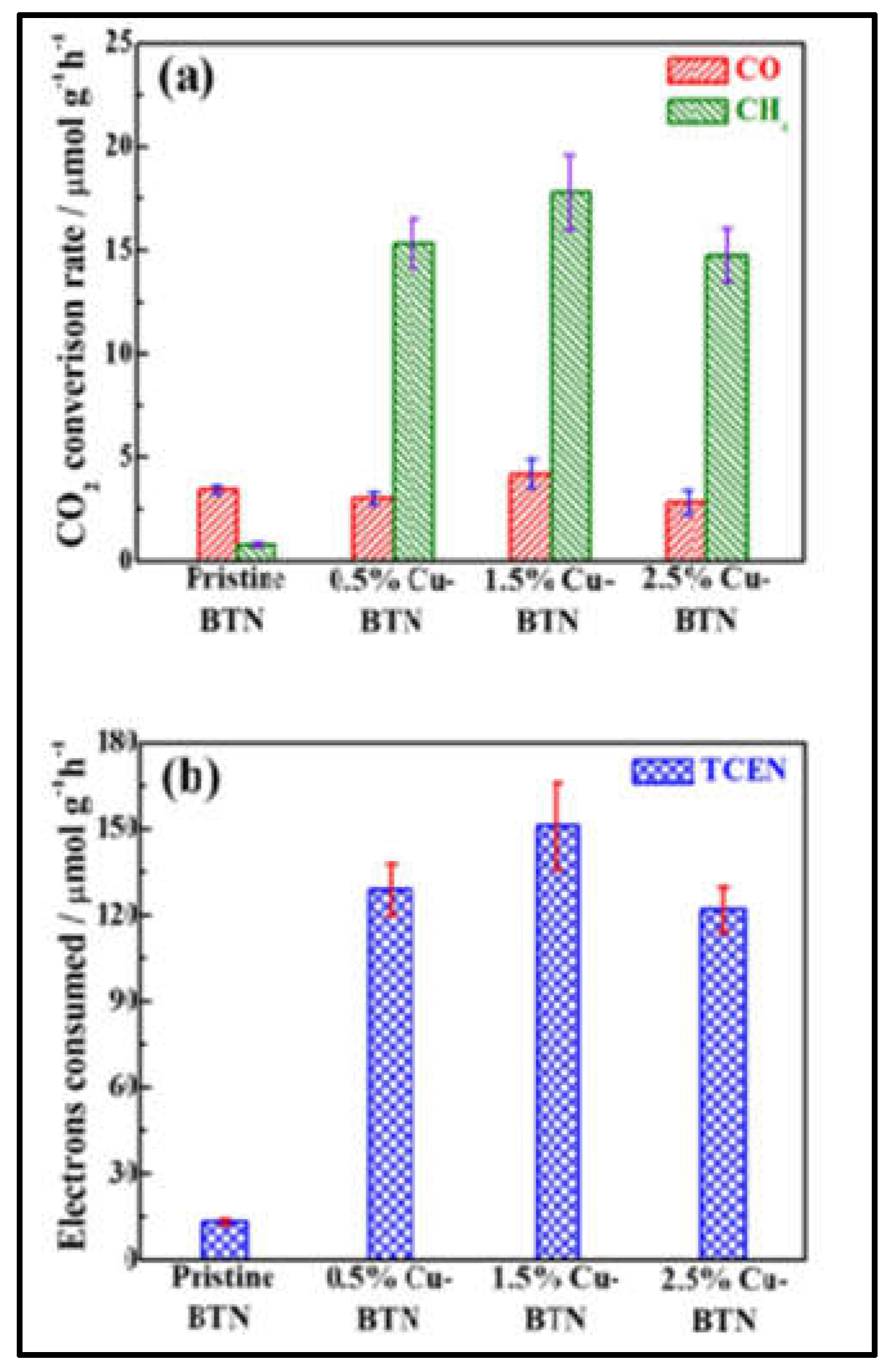
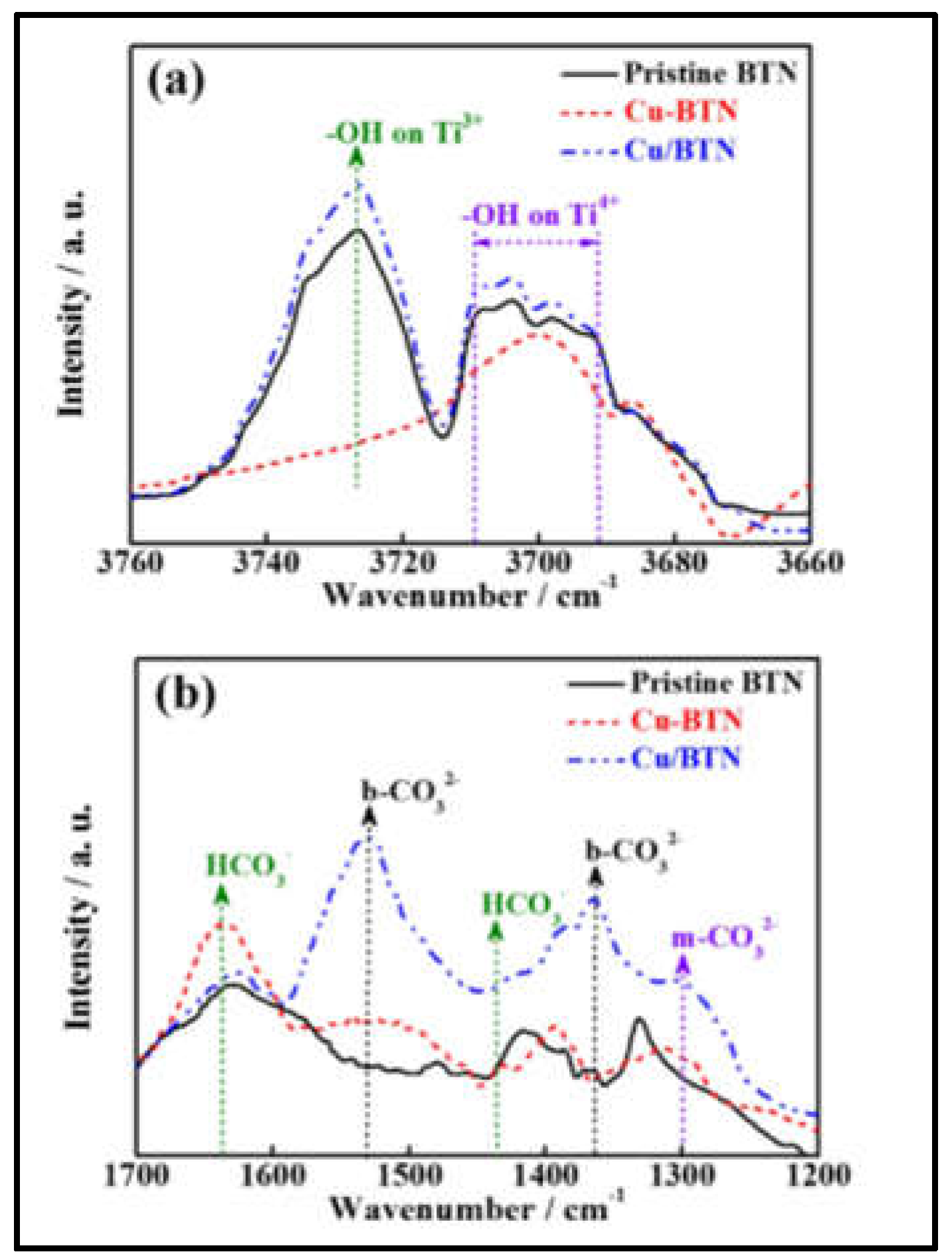
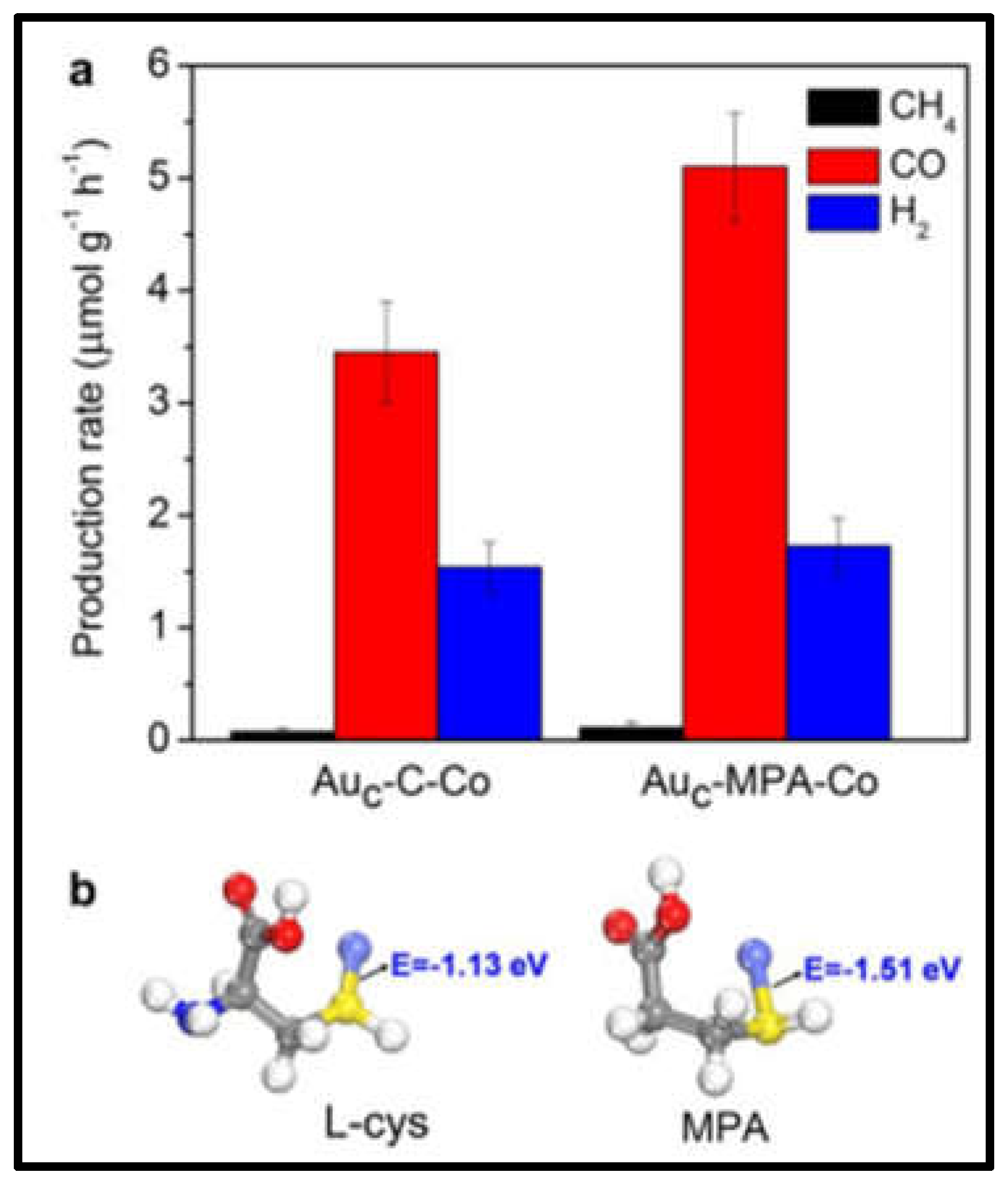
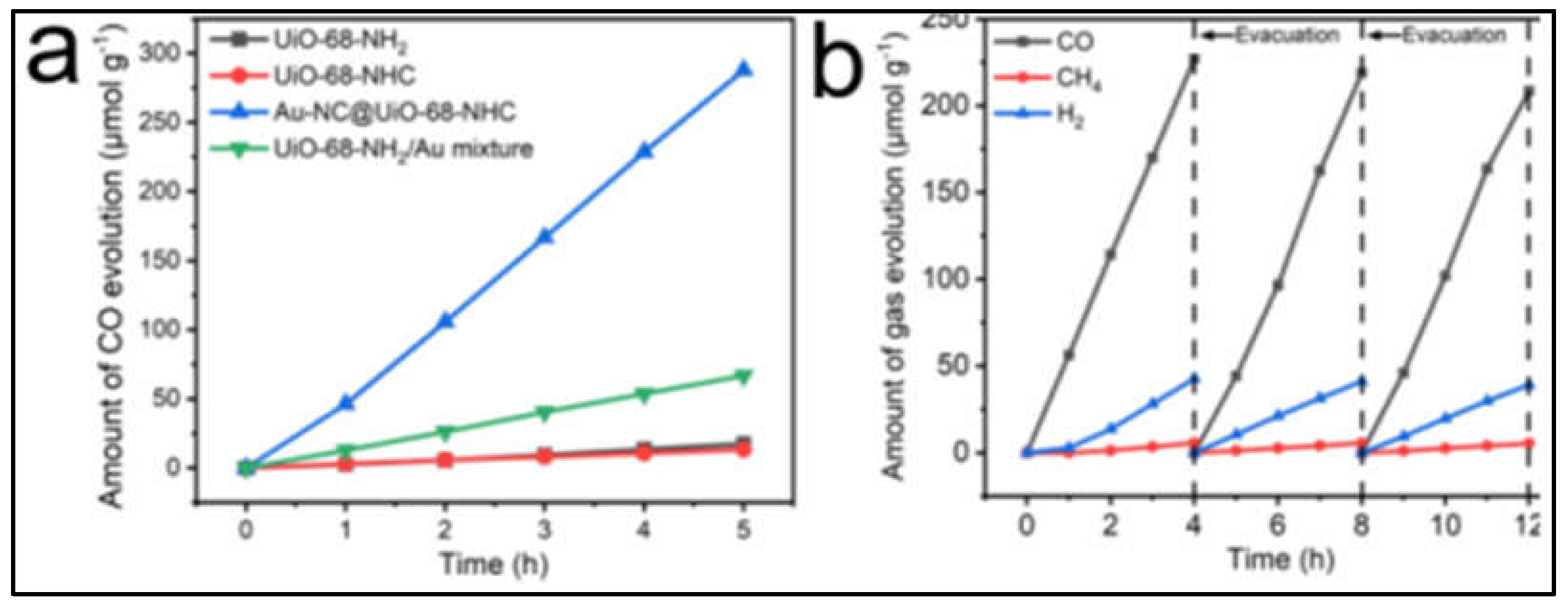
2.4. Photocatalytic CO2 reduction
| Sl No | Photocatalyst | Co catalyst | Application | Efficiency | Reference |
|---|---|---|---|---|---|
| 1 | TiO2 | AuNC | Photodegradation of dyes | 98% degradation in 10 minutes exposure. | [90] |
| 2 | TiO2/Nb2O5 | AgNC | Photodegradation of dyes. | 100% degradation | [92] |
| 3 | CeO2 | AgNC | Photodegradation of dyes. | 80% degradation in 2 hours | [93] |
| 4 | ZnO nanoparticle | AgNC | Photodegradation of dyes. | 100% degradation in 1 hr | [94] |
| 5 | CuNC | No Cocatalyst | Photodegradation of dyes. | 100% degradation in 1 69hr | [95] |
| 6 | AuNC | No cocatalyst | Photodegradation of dyes. | 100% degradation in 1 hr | [96] |
| 7 | AgNC-assembled materials | No cocatalyst | Photodegradation of dyes. | 98% degradation | [98] |
| 8 | TiO2 core- shell | SiO2-Au GSH clusters | Photodegradation of organic dyes. | 99.1% degradation in 0.5 hr | [99] |
| 9 | TiO2 | Au25 NC | Oxidation of phenol derivatives and ferrocyanide and reduction of Ag+, Cu2+ and oxygen | [107] | |
| 10 | Au25NC | No Catalyst | oxidation of styrene and hydrogenation of α,β-unsaturated ketone | 27 ± 1.0% | [108] |
| 11 | TiO2 | AuNC | Oxidation of benzylamines to imines | 73-99% | [101] |
| 12 | Zeolite (ZX-Bi zeolite) | Ag NC | Photooxidation of methanol | 49.60 mmol·g–1·cm–2 after 12 h of reaction | [110] |
| 13 | TiO2 | AuNC | Production of H2 | 0.3 mmol of hydrogen/h/g | [148] |
| 14 | TiO2 nanotube arrays (NP-TNTAs). | AuNC | Photodegradation of organic dyes, photocatalytic reduction of aromatic nitro compounds and photoelectrochemical water splitting. | [114] | |
| 15 | BaLa4Ti4O15 | AuNCs | Photocatalytic water splitting | 190 µmol/h | [115] |
| 16 | SrTiO3 | AuNC | Hydrogen evolution reaction | 41.2 µmol/h of H | [116] |
| 17 | AlSrTiO3 and rGO | AuNCs | photocatalytic production of H2, photocatalytic water splitting | 385 ± 22 nmol h−1 | [117] |
| 18 | Gold nanorods (GNRs) | AgNCs | Hydrogen evolution reaction | 10% | [118] |
| 19 | Monolayer niobate (HTi2NbO7) | Pt NC | Higher H2 production | 10 μmol h−1 | [120] |
| 20 | Modified cadmium sulfide (CdS) nanorod | Pt NC | Photocatalytic water splitting | 1.5‰ h−1 | [121] |
| 21 | BaLa4Ti4O15 | Au24Pd NCs and Au24Pt NCs | photocatalytic H2 evolution | 100-150 µmolh-1 | [124] |
| 22 | Graphitic carbon nitride (g-C3N4) | PtAg NC | photocatalytic H2 production | 39.7 µmolh-1 | [125] |
| 23 | Brookite TiO2 quasi nanocubes | CuNCS | Photocatalytic CO2 reduction | 150.9 μmol g−1 h−1 | [144] |
| 24 | Metal cations -Fe2+, Co2+, Ni2+ and Cu2+ | Au NCs | Photocatalytic CO2 reduction | 3.54 µmol⋅gcat−1⋅h−1 | [145] |
| 25 | carbon monoxide dehydrogenase (CODH) and TiO2 nanoparticles | Ag NC | Photocatalytic CO2 reduction | turnover frequency of 20 s−1 | [149] |
| 26 | Au-NCs | Photocatalytic CO2 reduction | 57.6 μmol g-1h-1 | [146] | |
| 27 | TiO2 | Ni-NCs | photocatalytic CO2 reduction | 10 µmol g-cat−1 | [147] |
| 28 | AgNC | No Cocatalyst | reforming of formic acid to H2 and CO2 |
99% selectivity | [150] |
3. Conclusion and Future Perspective
Acknowledgements
References
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [CrossRef]
- Bakbolat, B.; Daulbayev, C.; Sultanov, F.; Beissenov, R.; Umirzakov, A.; Mereke, A.; Bekbaev, A.; Chuprakov, I. Recent Developments of TiO2-Based Photocatalysis in the Hydrogen Evolution and Photodegradation: A Review. Nanomaterials 2020, 10, 1790. [CrossRef]
- Cheng, L.; Xiang, Q.; Liao, Y.; Zhang, H. CdS-Based Photocatalysts. Energy Environ. Sci. 2018, 11, 1362–1391. [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A Review of ZnO Nanoparticles as Solar Photocatalysts: Synthesis, Mechanisms and Applications. Renewable and Sustainable Energy Reviews 2018, 81, 536–551. [CrossRef]
- Hitam, C.N.C.; Jalil, A.A. A Review on Exploration of Fe2O3 Photocatalyst towards Degradation of Dyes and Organic Contaminants. Journal of Environmental Management 2020, 258, 110050. [CrossRef]
- Lee, G.-J.; Wu, J.J. Recent Developments in ZnS Photocatalysts from Synthesis to Photocatalytic Applications — A Review. Powder Technology 2017, 318, 8–22. [CrossRef]
- Li, J.; Lou, Z.; Li, B. Nanostructured Materials with Localized Surface Plasmon Resonance for Photocatalysis. Chinese Chemical Letters 2022, 33, 1154–1168. [CrossRef]
- Song, X.-R.; Goswami, N.; Yang, H.-H.; Xie, J. Functionalization of Metal Nanoclusters for Biomedical Applications. Analyst 2016, 141, 3126–3140. [CrossRef]
- Goswami, N.; Zheng, K.; Xie, J. Bio-NCs – the Marriage of Ultrasmall Metal Nanoclusters with Biomolecules. Nanoscale 2014, 6, 13328–13347. [CrossRef]
- Basu, S.; Paul, A.; Antoine, R. Controlling the Chemistry of Nanoclusters: From Atomic Precision to Controlled Assembly. Nanomaterials 2021, 12, 62. [CrossRef]
- Wu, Z.; Jin, R. On the Ligand’s Role in the Fluorescence of Gold Nanoclusters. Nano Lett. 2010, 10, 2568–2573. [CrossRef]
- Liu, L.; Corma, A. Metal Catalysts for Heterogeneous Catalysis: From Single Atoms to Nanoclusters and Nanoparticles. Chem. Rev. 2018, 118, 4981–5079. [CrossRef]
- Du, X.; Jin, R. Atomically Precise Metal Nanoclusters for Catalysis. ACS Nano 2019, 13, 7383–7387. [CrossRef]
- Khandelwal, P.; Poddar, P. Fluorescent Metal Quantum Clusters: An Updated Overview of the Synthesis, Properties, and Biological Applications. J. Mater. Chem. B 2017, 5, 9055–9084. [CrossRef]
- Templeton, A.C.; Wuelfing, W.P.; Murray, R.W. Monolayer-Protected Cluster Molecules. Acc. Chem. Res. 2000, 33, 27–36. [CrossRef]
- Qu, X.; Li, Y.; Li, L.; Wang, Y.; Liang, J.; Liang, J. Fluorescent Gold Nanoclusters: Synthesis and Recent Biological Application. Journal of Nanomaterials 2015, 2015, 1–23. [CrossRef]
- Yang, T.-Q.; Peng, B.; Shan, B.-Q.; Zong, Y.-X.; Jiang, J.-G.; Wu, P.; Zhang, K. Origin of the Photoluminescence of Metal Nanoclusters: From Metal-Centered Emission to Ligand-Centered Emission. Nanomaterials 2020, 10, 261. [CrossRef]
- Antoine, R. Supramolecular Gold Chemistry: From Atomically Precise Thiolate-Protected Gold Nanoclusters to Gold-Thiolate Nanostructures. Nanomaterials 2020, 10, 377. [CrossRef]
- Kolay, S.; Bain, D.; Maity, S.; Devi, A.; Patra, A.; Antoine, R. Self-Assembled Metal Nanoclusters: Driving Forces and Structural Correlation with Optical Properties. Nanomaterials 2022, 12, 544. [CrossRef]
- Su, Y.; Xue, T.; Liu, Y.; Qi, J.; Jin, R.; Lin, Z. Luminescent Metal Nanoclusters for Biomedical Applications. Nano Res. 2019, 12, 1251–1265. [CrossRef]
- Bonačić-Koutecký, V.; Antoine, R. Enhanced Two-Photon Absorption of Ligated Silver and Gold Nanoclusters: Theoretical and Experimental Assessments. Nanoscale 2019, 11, 12436–12448. [CrossRef]
- Genji Srinivasulu, Y.; Yao, Q.; Goswami, N.; Xie, J. Interfacial Engineering of Gold Nanoclusters for Biomedical Applications. Mater. Horiz. 2020, 7, 2596–2618. [CrossRef]
- Antoine, R.; Maysinger, D.; Sancey, L.; Bonačić-Koutecký, V. Open Questions on Proteins Interacting with Nanoclusters. Commun Chem 2022, 5, 47. [CrossRef]
- Combes, G.F.; Vučković, A.-M.; Perić Bakulić, M.; Antoine, R.; Bonačić-Koutecky, V.; Trajković, K. Nanotechnology in Tumor Biomarker Detection: The Potential of Liganded Nanoclusters as Nonlinear Optical Contrast Agents for Molecular Diagnostics of Cancer. Cancers 2021, 13, 4206. [CrossRef]
- Rudzińska, M.; Daglioglu, C.; Savvateeva, L.V.; Kaci, F.N.; Antoine, R.; Zamyatnin Jr, A.A. Current Status and Perspectives of Protease Inhibitors and Their Combination with Nanosized Drug Delivery Systems for Targeted Cancer Therapy. DDDT 2021, Volume 15, 9–20. [CrossRef]
- Borghei, Y.; Hosseinkhani, S.; Ganjali, M.R. Bridging from Metallic Nanoclusters to Biomedical in Understanding Physicochemical Interactions at the Nano–Bio Interface. Part & Part Syst Charact 2022, 39, 2100202. [CrossRef]
- Chai, O.J.H.; Liu, Z.; Chen, T.; Xie, J. Engineering Ultrasmall Metal Nanoclusters for Photocatalytic and Electrocatalytic Applications. Nanoscale 2019, 11, 20437–20448. [CrossRef]
- Liang, H.; Liu, B.-J.; Tang, B.; Zhu, S.-C.; Li, S.; Ge, X.-Z.; Li, J.-L.; Zhu, J.-R.; Xiao, F.-X. Atomically Precise Metal Nanocluster-Mediated Photocatalysis. ACS Catal. 2022, 12, 4216–4226. [CrossRef]
- Munir, A.; Joya, K.S.; Ul haq, T.; Babar, N.; Hussain, S.Z.; Qurashi, A.; Ullah, N.; Hussain, I. Metal Nanoclusters: New Paradigm in Catalysis for Water Splitting, Solar and Chemical Energy Conversion. ChemSusChem 2019, 12, 1517–1548. [CrossRef]
- Kauffman, D.R.; Thakkar, J.; Siva, R.; Matranga, C.; Ohodnicki, P.R.; Zeng, C.; Jin, R. Efficient Electrochemical CO 2 Conversion Powered by Renewable Energy. ACS Appl. Mater. Interfaces 2015, 7, 15626–15632. [CrossRef]
- Al Dosari, H.M.; Ayesh, A.I. Nanocluster Production for Solar Cell Applications. Journal of Applied Physics 2013, 114, 054305. [CrossRef]
- Fang, J.; Zhang, B.; Yao, Q.; Yang, Y.; Xie, J.; Yan, N. Recent Advances in the Synthesis and Catalytic Applications of Ligand-Protected, Atomically Precise Metal Nanoclusters. Coordination Chemistry Reviews 2016, 322, 1–29. [CrossRef]
- Serhan, M.; Jackemeyer, D.; Long, M.; Sprowls, M.; Diez Perez, I.; Maret, W.; Chen, F.; Tao, N.; Forzani, E. Total Iron Measurement in Human Serum With a Novel Smartphone-Based Assay. IEEE J. Transl. Eng. Health Med. 2020, 8, 1–9. [CrossRef]
- Yao, Q.; Yuan, X.; Chen, T.; Leong, D.T.; Xie, J. Engineering Functional Metal Materials at the Atomic Level. Adv. Mater. 2018, 30, 1802751. [CrossRef]
- Mathew, A.; Pradeep, T. Noble Metal Clusters: Applications in Energy, Environment, and Biology. Part. Part. Syst. Charact. 2014, 31, 1017–1053. [CrossRef]
- Jadzinsky, P.D.; Calero, G.; Ackerson, C.J.; Bushnell, D.A.; Kornberg, R.D. Structure of a Thiol Monolayer-Protected Gold Nanoparticle at 1.1 Å Resolution. Science 2007, 318, 430–433. [CrossRef]
- Qian, H.; Eckenhoff, W.T.; Zhu, Y.; Pintauer, T.; Jin, R. Total Structure Determination of Thiolate-Protected Au 38 Nanoparticles. J. Am. Chem. Soc. 2010, 132, 8280–8281. [CrossRef]
- Zeng, C.; Qian, H.; Li, T.; Li, G.; Rosi, N.L.; Yoon, B.; Barnett, R.N.; Whetten, R.L.; Landman, U.; Jin, R. Total Structure and Electronic Properties of the Gold Nanocrystal Au 36 (SR) 24. Angew. Chem. Int. Ed. 2012, 51, 13114–13118. [CrossRef]
- Das, A.; Li, T.; Nobusada, K.; Zeng, Q.; Rosi, N.L.; Jin, R. Total Structure and Optical Properties of a Phosphine/Thiolate-Protected Au 24 Nanocluster. J. Am. Chem. Soc. 2012, 134, 20286–20289. [CrossRef]
- Zeng, C.; Li, T.; Das, A.; Rosi, N.L.; Jin, R. Chiral Structure of Thiolate-Protected 28-Gold-Atom Nanocluster Determined by X-Ray Crystallography. J. Am. Chem. Soc. 2013, 135, 10011–10013. [CrossRef]
- Jin, R.; Zeng, C.; Zhou, M.; Chen, Y. Atomically Precise Colloidal Metal Nanoclusters and Nanoparticles: Fundamentals and Opportunities. Chem. Rev. 2016, 116, 10346–10413. [CrossRef]
- Lu, Y.; Chen, W. Application of Mass Spectrometry in the Synthesis and Characterization of Metal Nanoclusters. Anal. Chem. 2015, 87, 10659–10667. [CrossRef]
- Chakraborty, P.; Pradeep, T. The Emerging Interface of Mass Spectrometry with Materials. NPG Asia Mater 2019, 11, 48. [CrossRef]
- Comby-Zerbino, C.; Dagany, X.; Chirot, F.; Dugourd, P.; Antoine, R. The Emergence of Mass Spectrometry for Characterizing Nanomaterials. Atomically Precise Nanoclusters and Beyond. Mater. Adv. 2021, 2, 4896–4913. [CrossRef]
- Halawa, M.I.; Lai, J.; Xu, G. Gold Nanoclusters: Synthetic Strategies and Recent Advances in Fluorescent Sensing. Materials Today Nano 2018, 3, 9–27. [CrossRef]
- Chen, L.-Y.; Wang, C.-W.; Yuan, Z.; Chang, H.-T. Fluorescent Gold Nanoclusters: Recent Advances in Sensing and Imaging. Anal. Chem. 2015, 87, 216–229. [CrossRef]
- Hayyan, M.; Hashim, M.A.; AlNashef, I.M. Superoxide Ion: Generation and Chemical Implications. Chem. Rev. 2016, 116, 3029–3085. [CrossRef]
- Xie, J.; Zheng, Y.; Ying, J.Y. Protein-Directed Synthesis of Highly Fluorescent Gold Nanoclusters. J. Am. Chem. Soc. 2009, 131, 888–889. [CrossRef]
- Le Guével, X.; Hötzer, B.; Jung, G.; Hollemeyer, K.; Trouillet, V.; Schneider, M. Formation of Fluorescent Metal (Au, Ag) Nanoclusters Capped in Bovine Serum Albumin Followed by Fluorescence and Spectroscopy. J. Phys. Chem. C 2011, 115, 10955–10963. [CrossRef]
- Ling, S.; Cui, X.; Zhang, X.; Liu, B.; He, C.; Wang, J.; Qin, W.; Zhang, Y.; Gao, Y.; Bai, G. Glutathione-Protected Gold Nanocluster Decorated Cadmium Sulfide with Enhanced Photostability and Photocatalytic Activity. Journal of Colloid and Interface Science 2018, 530, 120–126. [CrossRef]
- Liu, Z.; Wu, Z.; Yao, Q.; Cao, Y.; Chai, O.J.H.; Xie, J. Correlations between the Fundamentals and Applications of Ultrasmall Metal Nanoclusters: Recent Advances in Catalysis and Biomedical Applications. Nano Today 2021, 36, 101053. [CrossRef]
- Li, Y.; Zaluzhna, O.; Xu, B.; Gao, Y.; Modest, J.M.; Tong, Y.J. Mechanistic Insights into the Brust−Schiffrin Two-Phase Synthesis of Organo-Chalcogenate-Protected Metal Nanoparticles. J. Am. Chem. Soc. 2011, 133, 2092–2095. [CrossRef]
- Meng, X.; Xu, Q.; Wang, S.; Zhu, M. Ligand-Exchange Synthesis of Selenophenolate-Capped Au25 Nanoclusters. Nanoscale 2012, 4, 4161. [CrossRef]
- Wang, Y.; Bürgi, T. Ligand Exchange Reactions on Thiolate-Protected Gold Nanoclusters. Nanoscale Adv. 2021, 3, 2710–2727. [CrossRef]
- Antonello, S.; Dainese, T.; Pan, F.; Rissanen, K.; Maran, F. Electrocrystallization of Monolayer-Protected Gold Clusters: Opening the Door to Quality, Quantity, and New Structures. J. Am. Chem. Soc. 2017, 139, 4168–4174. [CrossRef]
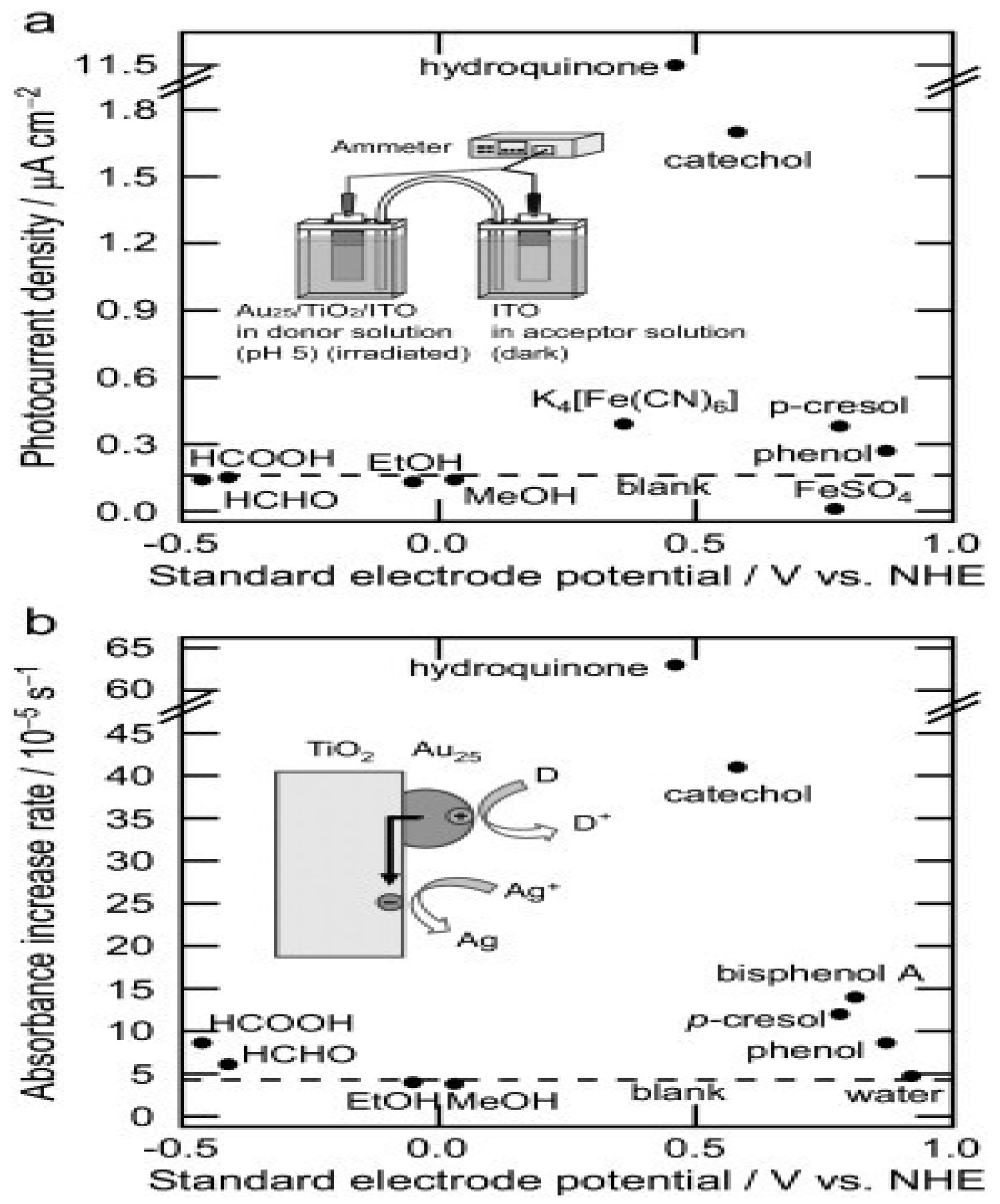
- Stamplecoskie, K.G.; Swint, A. Optimizing Molecule-like Gold Clusters for Light Energy Conversion. J. Mater. Chem. A 2016, 4, 2075–2081. [CrossRef]
- Robinson, D. Synthesis and Characterization of Metal Nanoclusters Stabilized by Dithiolates. [CrossRef]
- Kreibig, U.; Vollmer, M. Optical Properties of Metal Clusters; Springer Series in Materials Science; Springer Berlin Heidelberg: Berlin, Heidelberg, 1995; Vol. 25; ISBN 978-3-642-08191-0.
- Antoine, R.; Bonačić-Koutecký, V. Liganded Silver and Gold Quantum Clusters. Towards a New Class of Nonlinear Optical Nanomaterials; SpringerBriefs in Materials; Springer International Publishing: Cham, 2018; ISBN 978-3-319-64742-5.
- Jin, R.; Higaki, T. Open Questions on the Transition between Nanoscale and Bulk Properties of Metals. Commun Chem 2021, 4, 28. [CrossRef]
- Huang, X.; Li, Z.; Yu, Z.; Deng, X.; Xin, Y. Recent Advances in the Synthesis, Properties, and Biological Applications of Platinum Nanoclusters. Journal of Nanomaterials 2019, 2019, 1–31. [CrossRef]
- Muhammed, M.A.H.; Verma, P.K.; Pal, S.K.; Kumar, R.C.A.; Paul, S.; Omkumar, R.V.; Pradeep, T. Bright, NIR-Emitting Au 23 from Au 25 : Characterization and Applications Including Biolabeling. Chem. Eur. J. 2009, 15, 10110–10120. [CrossRef]
- Zhu, M.; Aikens, C.M.; Hollander, F.J.; Schatz, G.C.; Jin, R. Correlating the Crystal Structure of A Thiol-Protected Au 25 Cluster and Optical Properties. J. Am. Chem. Soc. 2008, 130, 5883–5885. [CrossRef]
- Shang, L.; Dong, S.; Nienhaus, G.U. Ultra-Small Fluorescent Metal Nanoclusters: Synthesis and Biological Applications. Nano Today 2011, 6, 401–418. [CrossRef]
- Mathew, M.S.; Joseph, K. Green Synthesis of Gluten-Stabilized Fluorescent Gold Quantum Clusters: Application As Turn-On Sensing of Human Blood Creatinine. ACS Sustainable Chem. Eng. 2017, 5, 4837–4845. [CrossRef]
- Bertorelle, F.; Wegner, D.; Bakulić, M.P.; Fakhouri, H.; Comby-Zerbino, C.; Sagar, A.; Bernadó, P.; Resch-Genger, U.; Koutecky, V.B.; Guével, X.L.; et al. Tailoring NIR-II Photoluminescence of Single Thiolated Au25 Nanoclusters by Selective Binding to Proteins; In Review, 2021;
- Tang, J.-H.; Han, G.; Li, G.; Yan, K.; Sun, Y. Near-Infrared Light Photocatalysis Enabled by a Ruthenium Complex-Integrated Metal–Organic Framework via Two-Photon Absorption. iScience 2022, 25, 104064. [CrossRef]
- Russier-Antoine, I.; Bertorelle, F.; Calin, N.; Sanader, Ž.; Krstić, M.; Comby-Zerbino, C.; Dugourd, P.; Brevet, P.-F.; Bonačić-Koutecký, V.; Antoine, R. Ligand-Core NLO-Phores: A Combined Experimental and Theoretical Approach to the Two-Photon Absorption and Two-Photon Excited Emission Properties of Small-Ligated Silver Nanoclusters. Nanoscale 2017, 9, 1221–1228. [CrossRef]
- Antoine, R. Ligand-Core NLO-Phores: Two-Photon Absorption and Two-Photon Excited Emission Properties of Atomically Precise Clusters of Gold and Silver. In Molecular Spectroscopy—Experiment and Theory; Koleżyński, A., Król, M., Eds.; Challenges and Advances in Computational Chemistry and Physics; Springer International Publishing: Cham, 2019; Vol. 26, pp. 139–160 ISBN 978-3-030-01354-7.
- Sanader, Ž.; Krstić, M.; Russier-Antoine, I.; Bertorelle, F.; Dugourd, P.; Brevet, P.-F.; Antoine, R.; Bonačić-Koutecký, V. Two-Photon Absorption of Ligand-Protected Ag 15 Nanoclusters. Towards a New Class of Nonlinear Optics Nanomaterials. Phys. Chem. Chem. Phys. 2016, 18, 12404–12408. [CrossRef]
- Hao, C.; Xu, L.; Ma, W.; Wu, X.; Wang, L.; Kuang, H.; Xu, C. Unusual Circularly Polarized Photocatalytic Activity in Nanogapped Gold-Silver Chiroplasmonic Nanostructures. Adv. Funct. Mater. 2015, 25, 5816–5822. [CrossRef]
- Schaaff, T.G.; Knight, G.; Shafigullin, M.N.; Borkman, R.F.; Whetten, R.L. Isolation and Selected Properties of a 10.4 KDa Gold:Glutathione Cluster Compound. J. Phys. Chem. B 1998, 102, 10643–10646. [CrossRef]
- Bertorelle, F.; Russier-Antoine, I.; Calin, N.; Comby-Zerbino, C.; Bensalah-Ledoux, A.; Guy, S.; Dugourd, P.; Brevet, P.-F.; Sanader, Ž.; Krstić, M.; et al. Au 10 (SG) 10 : A Chiral Gold Catenane Nanocluster with Zero Confined Electrons. Optical Properties and First-Principles Theoretical Analysis. J. Phys. Chem. Lett. 2017, 8, 1979–1985. [CrossRef]
- Tsukuda, T.; Häkkinen, H. Introduction. In Frontiers of Nanoscience; Elsevier, 2015; Vol. 9, pp. 1–7 ISBN 978-0-08-100086-1.
- Shichibu, Y.; Negishi, Y.; Tsunoyama, H.; Kanehara, M.; Teranishi, T.; Tsukuda, T. Extremely High Stability of Glutathionate-Protected Au25 Clusters Against Core Etching. Small 2007, 3, 835–839. [CrossRef]
- Boyen, H.-G.; Kästle, G.; Weigl, F.; Koslowski, B.; Dietrich, C.; Ziemann, P.; Spatz, J.P.; Riethmüller, S.; Hartmann, C.; Möller, M.; et al. Oxidation-Resistant Gold-55 Clusters. Science 2002, 297, 1533–1536. [CrossRef]
- Tracy, J.B.; Kalyuzhny, G.; Crowe, M.C.; Balasubramanian, R.; Choi, J.-P.; Murray, R.W. Poly(Ethylene Glycol) Ligands for High-Resolution Nanoparticle Mass Spectrometry. J. Am. Chem. Soc. 2007, 129, 6706–6707. [CrossRef]
- Kumar, S.; Jin, R. Water-Soluble Au25(Capt)18 Nanoclusters: Synthesis, Thermal Stability, and Optical Properties. Nanoscale 2012, 4, 4222. [CrossRef]
- Negishi, Y.; Kurashige, W.; Niihori, Y.; Iwasa, T.; Nobusada, K. Isolation, Structure, and Stability of a Dodecanethiolate-Protected Pd1Au24 Cluster. Phys. Chem. Chem. Phys. 2010, 12, 6219. [CrossRef]
- Jiang, D.; Dai, S. From Superatomic Au 25 (SR) 18 − to Superatomic M@Au 24 (SR) 18 q Core−Shell Clusters. Inorg. Chem. 2009, 48, 2720–2722. [CrossRef]
- Negishi, Y.; Munakata, K.; Ohgake, W.; Nobusada, K. Effect of Copper Doping on Electronic Structure, Geometric Structure, and Stability of Thiolate-Protected Au 25 Nanoclusters. J. Phys. Chem. Lett. 2012, 3, 2209–2214. [CrossRef]
- Akola, J.; Walter, M.; Whetten, R.L.; Häkkinen, H.; Grönbeck, H. On the Structure of Thiolate-Protected Au 25. J. Am. Chem. Soc. 2008, 130, 3756–3757. [CrossRef]
- Tlahuice-Flores, A.; Muñoz-Castro, A. Bonding and Properties of Superatoms. Analogs to Atoms and Molecules and Related Concepts from Superatomic Clusters. Int J Quantum Chem 2019, 119, e25756. [CrossRef]
- De Heer, W.A.; Knight, W.D.; Chou, M.Y.; Cohen, M.L. Electronic Shell Structure and Metal Clusters. In Solid State Physics; Elsevier, 1987; Vol. 40, pp. 93–181 ISBN 978-0-12-607740-7.
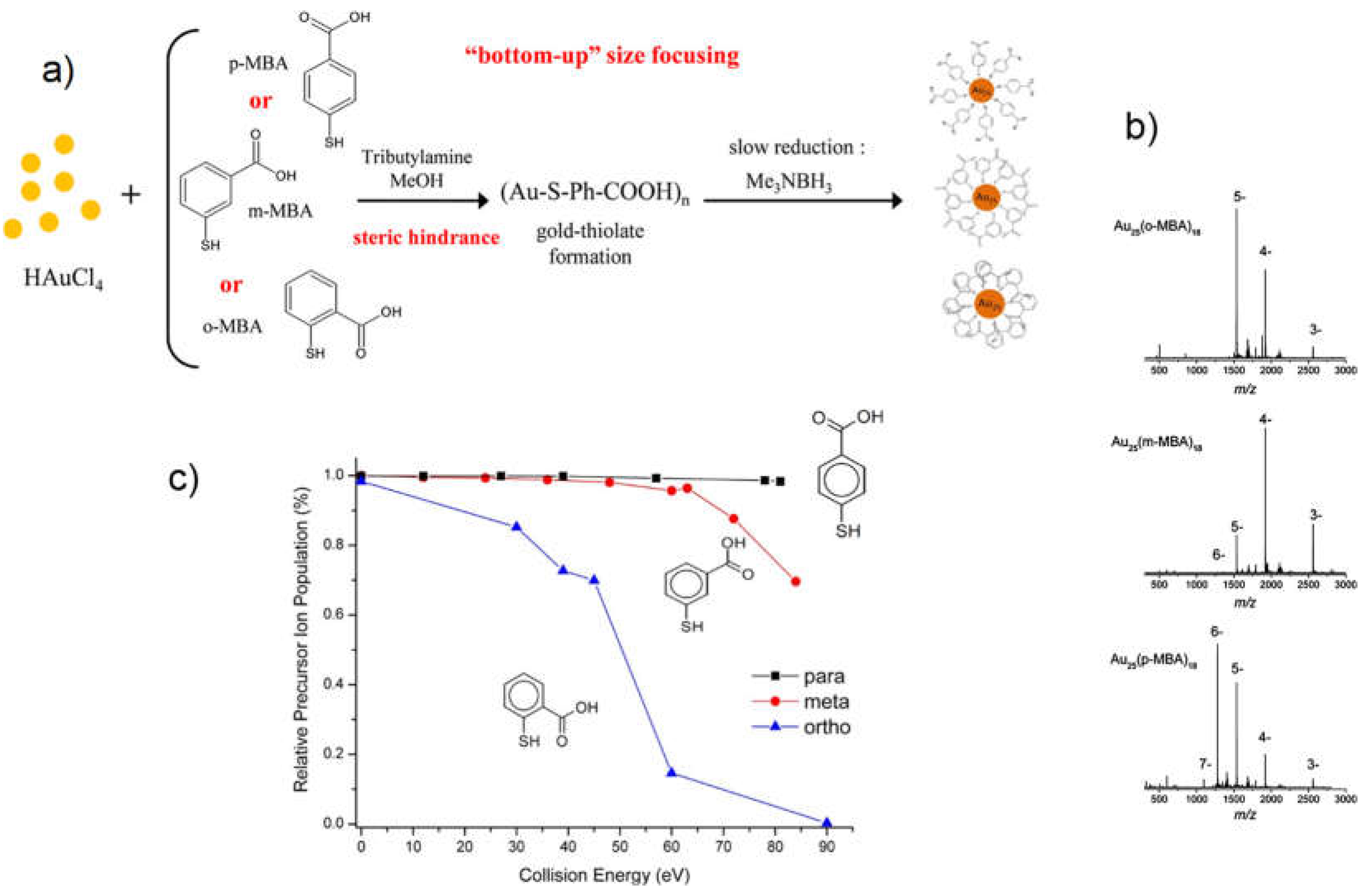
- Bertorelle, F.; Russier-Antoine, I.; Comby-Zerbino, C.; Chirot, F.; Dugourd, P.; Brevet, P.-F.; Antoine, R. Isomeric Effect of Mercaptobenzoic Acids on the Synthesis, Stability, and Optical Properties of Au 25 (MBA) 18 Nanoclusters. ACS Omega 2018, 3, 15635–15642. [CrossRef]
- Das, K.; Roychoudhury, A. Reactive Oxygen Species (ROS) and Response of Antioxidants as ROS-Scavengers during Environmental Stress in Plants. Front. Environ. Sci. 2014, 2. [CrossRef]
- Sacco, O.; Stoller, M.; Vaiano, V.; Ciambelli, P.; Chianese, A.; Sannino, D. Photocatalytic Degradation of Organic Dyes under Visible Light on N-Doped TiO 2 Photocatalysts. International Journal of Photoenergy 2012, 2012, 1–8. [CrossRef]
- Guo, W.; Yang, L.; Lu, J.; Gao, P.; Li, W.; Feng, Z. An Accurate Growth Mechanism and Photocatalytic Degradation Rhodamine B of Crystalline Nb2O5 Nanotube Arrays. Catalysts 2020, 10, 1480. [CrossRef]
- Goswami, T.; Singh, M.; Reddy, K.M.; Mishra, A.K. Facile Synthesis of Ag-TiO 2 Hybrid Nanocluster:A Comprehensive Experimental and Computational Insight into the Role of Surface Ligands on Enhanced Visible Light Photo-Catalysis. ChemistrySelect 2018, 3, 10892–10899. [CrossRef]
- Zhu, H.; Goswami, N.; Yao, Q.; Chen, T.; Liu, Y.; Xu, Q.; Chen, D.; Lu, J.; Xie, J. Cyclodextrin–Gold Nanocluster Decorated TiO 2 Enhances Photocatalytic Decomposition of Organic Pollutants. J. Mater. Chem. A 2018, 6, 1102–1108. [CrossRef]
- Sharma, V.; Kumar, S.; Krishnan, V. Clustered Au on TiO 2 Snowman-Like Nanoassemblies for Photocatalytic Applications. ChemistrySelect 2016, 1, 2963–2970. [CrossRef]
- Goswami, T.; Reddy, K.M.; Bheemaraju, A. Silver Nanocluster Anchored TiO 2 /Nb 2 O 5 Hybrid Nanocomposite as Highly Efficient and Selective Visible-Light Sensitive Photocatalyst. ChemistrySelect 2019, 4, 6790–6799. [CrossRef]
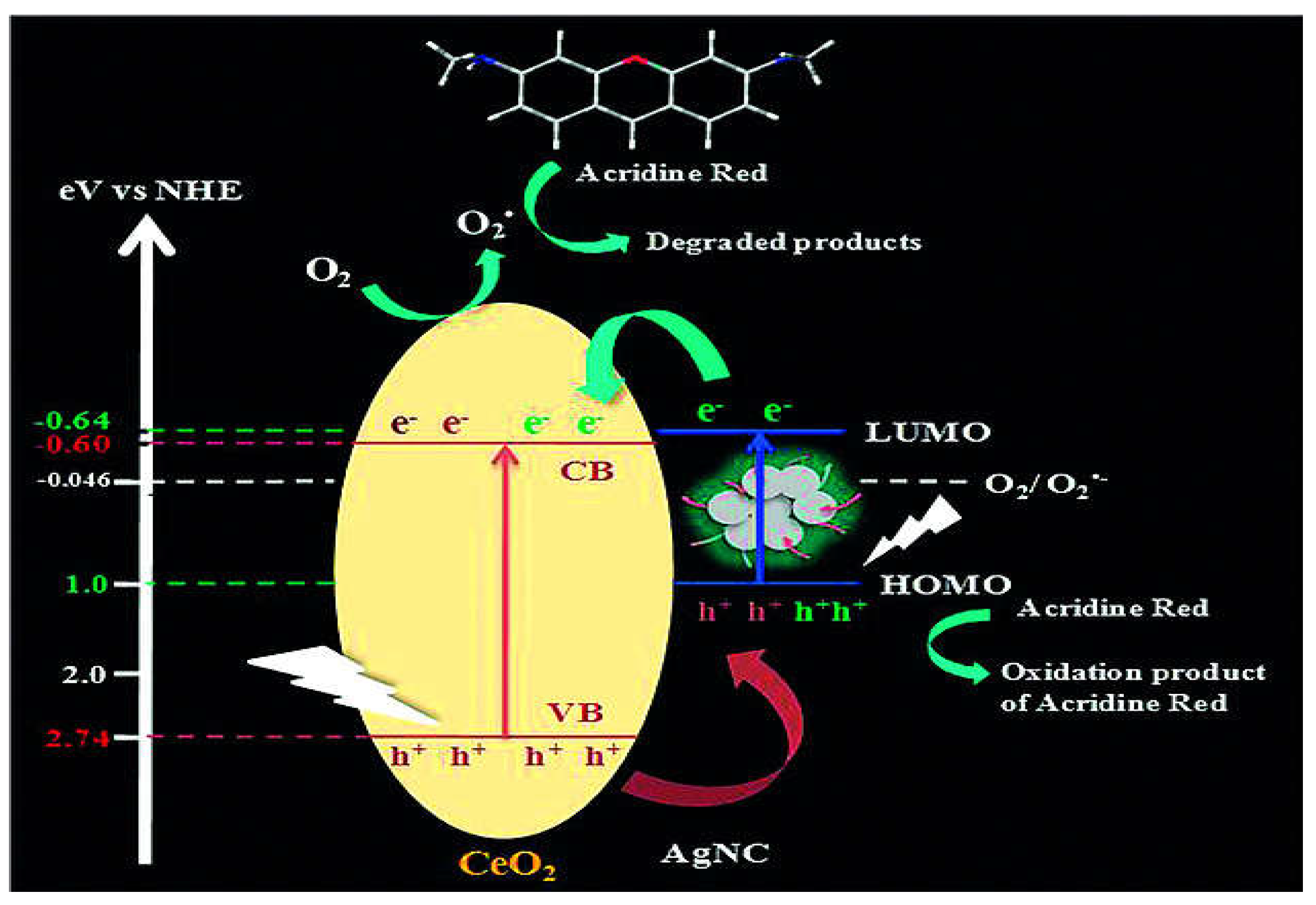
- Samai, B.; Chall, S.; Mati, S.S.; Bhattacharya, S.C. Role of Silver Nanoclusters in the Enhanced Photocatalytic Activity of Cerium Oxide Nanoparticles. Eur. J. Inorg. Chem. 2018, 2018, 3224–3231. [CrossRef]
- González-Rodríguez, J.; Fernández, L.; Bava, Y.B.; Buceta, D.; Vázquez-Vázquez, C.; López-Quintela, M.A.; Feijoo, G.; Moreira, M.T. Enhanced Photocatalytic Activity of Semiconductor Nanocomposites Doped with Ag Nanoclusters Under UV and Visible Light. Catalysts 2019, 10, 31. [CrossRef]
- Vilar-Vidal, N.; Rey, J.R.; López Quintela, M.A. Green Emitter Copper Clusters as Highly Efficient and Reusable Visible Degradation Photocatalysts. Small 2014, 10, 3632–3636. [CrossRef]
- Zhou, S.; Duan, Y.; Wang, F.; Wang, C. Fluorescent Au Nanoclusters Stabilized by Silane: Facile Synthesis, Color-Tunability and Photocatalytic Properties. Nanoscale 2017, 9, 4981–4988. [CrossRef]
- Veziroglu, S.; Obermann, A.-L.; Ullrich, M.; Hussain, M.; Kamp, M.; Kienle, L.; Leißner, T.; Rubahn, H.-G.; Polonskyi, O.; Strunskus, T.; et al. Photodeposition of Au Nanoclusters for Enhanced Photocatalytic Dye Degradation over TiO 2 Thin Film. ACS Appl. Mater. Interfaces 2020, 12, 14983–14992. [CrossRef]
- Cao, M.; Pang, R.; Wang, Q.-Y.; Han, Z.; Wang, Z.-Y.; Dong, X.-Y.; Li, S.-F.; Zang, S.-Q.; Mak, T.C.W. Porphyrinic Silver Cluster Assembled Material for Simultaneous Capture and Photocatalysis of Mustard-Gas Simulant. J. Am. Chem. Soc. 2019, 141, 14505–14509. [CrossRef]
- Weng, B.; Lu, K.-Q.; Tang, Z.; Chen, H.M.; Xu, Y.-J. Stabilizing Ultrasmall Au Clusters for Enhanced Photoredox Catalysis. Nat Commun 2018, 9, 1543. [CrossRef]
- Al-Shankiti, B.; Al-Maksoud, W.; Habeeb Muhammed, M.A.; Anjum, D.H.; Moosa, B.; Basset, J.-M.; Khashab, N.M. Ligand-Free Gold Nanoclusters Confined in Mesoporous Silica Nanoparticles for Styrene Epoxidation. Nanoscale Adv. 2020, 2, 1437–1442. [CrossRef]
- Chen, H.; Liu, C.; Wang, M.; Zhang, C.; Luo, N.; Wang, Y.; Abroshan, H.; Li, G.; Wang, F. Visible Light Gold Nanocluster Photocatalyst: Selective Aerobic Oxidation of Amines to Imines. ACS Catal. 2017, 7, 3632–3638. [CrossRef]
- Liu, L.; Li, H.; Tan, Y.; Chen, X.; Lin, R.; Yang, W.; Huang, C.; Wang, S.; Wang, X.; Liu, X.Y.; et al. Metal-Support Synergy of Supported Gold Nanoclusters in Selective Oxidation of Alcohols. Catalysts 2020, 10, 107. [CrossRef]
- Kawawaki, T.; Kataoka, Y.; Ozaki, S.; Kawachi, M.; Hirata, M.; Negishi, Y. Creation of Active Water-Splitting Photocatalysts by Controlling Cocatalysts Using Atomically Precise Metal Nanoclusters. Chem. Commun. 2021, 57, 417–440. [CrossRef]
- Li, G.; Zeng, C.; Jin, R. Thermally Robust Au 99 (SPh) 42 Nanoclusters for Chemoselective Hydrogenation of Nitrobenzaldehyde Derivatives in Water. J. Am. Chem. Soc. 2014, 136, 3673–3679. [CrossRef]
- Mu, J.; Yang, J.-L.; Zhang, D.-W.; Jia, Q. Progress in Preparation of Metal Nanoclusters and Their Application in Detection of Environmental Pollutants. Chinese Journal of Analytical Chemistry 2021, 49, 319–329. [CrossRef]
- Haruta, M.; Kobayashi, T.; Sano, H.; Yamada, N. Novel Gold Catalysts for the Oxidation of Carbon Monoxide at a Temperature Far Below 0 °C. Chem. Lett. 1987, 16, 405–408. [CrossRef]
- Kogo, A.; Sakai, N.; Tatsuma, T. Photocatalysis of Au25-Modified TiO2 under Visible and near Infrared Light. Electrochemistry Communications 2010, 12, 996–999. [CrossRef]
- Zhu, Y.; Qian, H.; Zhu, M.; Jin, R. Thiolate-Protected Aun Nanoclusters as Catalysts for Selective Oxidation and Hydrogenation Processes. Adv. Mater. 2010, 22, 1915–1920. [CrossRef]
- Tsunoyama, H.; Ichikuni, N.; Sakurai, H.; Tsukuda, T. Effect of Electronic Structures of Au Clusters Stabilized by Poly( N -Vinyl-2-Pyrrolidone) on Aerobic Oxidation Catalysis. J. Am. Chem. Soc. 2009, 131, 7086–7093. [CrossRef]
- Hamoud, H.I.; Douma, F.; Lafjah, M.; Djafri, F.; Lebedev, O.; Valtchev, V.; El-Roz, M. Size-Dependent Photocatalytic Activity of Silver Nanoparticles Embedded in ZX-Bi Zeolite Supports. ACS Appl. Nano Mater. 2022, 5, 3866–3877. [CrossRef]
- Jain, I.P. Hydrogen the Fuel for 21st Century. International Journal of Hydrogen Energy 2009, 34, 7368–7378. [CrossRef]
- Ahluwalia, R.K.; Wang, X.; Rousseau, A.; Kumar, R. Fuel Economy of Hydrogen Fuel Cell Vehicles. Journal of Power Sources 2004, 130, 192–201. [CrossRef]
- Thoi, V.S.; Sun, Y.; Long, J.R.; Chang, C.J. Complexes of Earth-Abundant Metals for Catalytic Electrochemical Hydrogen Generation under Aqueous Conditions. Chem. Soc. Rev. 2013, 42, 2388–2400. [CrossRef]
- Xiao, F.-X.; Hung, S.-F.; Miao, J.; Wang, H.-Y.; Yang, H.; Liu, B. Metal-Cluster-Decorated TiO 2 Nanotube Arrays: A Composite Heterostructure toward Versatile Photocatalytic and Photoelectrochemical Applications. Small 2015, 11, 554–567. [CrossRef]
- Negishi, Y.; Mizuno, M.; Hirayama, M.; Omatoi, M.; Takayama, T.; Iwase, A.; Kudo, A. Enhanced Photocatalytic Water Splitting by BaLa4Ti4O15 Loaded with ∼1 Nm Gold Nanoclusters Using Glutathione-Protected Au25 Clusters. Nanoscale 2013, 5, 7188. [CrossRef]
- Kurashige, W.; Kumazawa, R.; Mori, Y. Au25 Cluster-Loaded SrTiO3 Water-Splitting Photocatalyst; Preparation and Elucidation of the Effect of Cocatalyst Refinement on Photocatalytic Activity. J. Mater. Appl.
- Mousavi, H.; Small, T.D.; Sharma, S.K.; Golovko, V.B.; Shearer, C.J.; Metha, G.F. Graphene Bridge for Photocatalytic Hydrogen Evolution with Gold Nanocluster Co-Catalysts. Nanomaterials 2022, 12, 3638. [CrossRef]
- Attia, Y.A.; Buceta, D.; Blanco-Varela, C.; Mohamed, M.B.; Barone, G.; López-Quintela, M.A. Structure-Directing and High-Efficiency Photocatalytic Hydrogen Production by Ag Clusters. J. Am. Chem. Soc. 2014, 136, 1182–1185. [CrossRef]
- Shen, P.; Zhao, S.; Su, D.; Li, Y.; Orlov, A. Outstanding Activity of Sub-Nm Au Clusters for Photocatalytic Hydrogen Production. Applied Catalysis B: Environmental 2012, 126, 153–160. [CrossRef]
- Wang, H.; Luo, S.; Song, Y.; Shi, Y.; Wang, Z.; Guo, B.; Wu, L. Enhanced Photocatalytic Hydrogen Evolution over Monolayer HTi2NbO7 Nanosheets with Highly Dispersed Pt Nanoclusters. Applied Surface Science 2020, 511, 145501. [CrossRef]
- Schweinberger, F.F.; Berr, M.J.; Döblinger, M.; Wolff, C.; Sanwald, K.E.; Crampton, A.S.; Ridge, C.J.; Jäckel, F.; Feldmann, J.; Tschurl, M.; et al. Cluster Size Effects in the Photocatalytic Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2013, 135, 13262–13265. [CrossRef]
- Li, S.; Du, X.; Liu, Z.; Li, Y.; Shao, Y.; Jin, R. Size Effects of Atomically Precise Gold Nanoclusters in Catalysis. Precision Chemistry 2023, 1, 14–28. [CrossRef]
- Naveen, M.H.; Khan, R.; Bang, J.H. Gold Nanoclusters as Electrocatalysts: Atomic Level Understanding from Fundamentals to Applications. Chem. Mater. 2021, 33, 7595–7612. [CrossRef]
- Kurashige, W.; Hayashi, R.; Wakamatsu, K.; Kataoka, Y.; Hossain, S.; Iwase, A.; Kudo, A.; Yamazoe, S.; Negishi, Y. Atomic-Level Understanding of the Effect of Heteroatom Doping of the Cocatalyst on Water-Splitting Activity in AuPd or AuPt Alloy Cluster-Loaded BaLa 4 Ti 4 O 15. ACS Appl. Energy Mater. 2019, 2, 4175–4187. [CrossRef]
- Du, X.L.; Wang, X.L.; Li, Y.H.; Wang, Y.L.; Zhao, J.J.; Fang, L.J.; Zheng, L.R.; Tong, H.; Yang, H.G. Isolation of Single Pt Atoms in a Silver Cluster: Forming Highly Efficient Silver-Based Cocatalysts for Photocatalytic Hydrogen Evolution. Chem. Commun. 2017, 53, 9402–9405. [CrossRef]
- Méndez-Medrano, M.G.; Kowalska, E.; Lehoux, A.; Herissan, A.; Ohtani, B.; Rau, S.; Colbeau-Justin, C.; Rodríguez-López, J.L.; Remita, H. Surface Modification of TiO 2 with Au Nanoclusters for Efficient Water Treatment and Hydrogen Generation under Visible Light. J. Phys. Chem. C 2016, 120, 25010–25022. [CrossRef]
- Tawalbeh, M.; Al-Othman, A.; Kafiah, F.; Abdelsalam, E.; Almomani, F.; Alkasrawi, M. Environmental Impacts of Solar Photovoltaic Systems: A Critical Review of Recent Progress and Future Outlook. Science of The Total Environment 2021, 759, 143528. [CrossRef]
- Kondratenko, E.V.; Mul, G.; Baltrusaitis, J.; Larrazábal, G.O.; Pérez-Ramírez, J. Status and Perspectives of CO2 Conversion into Fuels and Chemicals by Catalytic, Photocatalytic and Electrocatalytic Processes. Energy Environ. Sci. 2013, 6, 3112. [CrossRef]
- Roy, S.C.; Varghese, O.K.; Paulose, M.; Grimes, C.A. Toward Solar Fuels: Photocatalytic Conversion of Carbon Dioxide to Hydrocarbons. ACS Nano 2010, 4, 1259–1278. [CrossRef]
- Alper, E.; Yuksel Orhan, O. CO2 Utilization: Developments in Conversion Processes. Petroleum 2017, 3, 109–126. [CrossRef]
- Du, C.; Wang, X.; Chen, W.; Feng, S.; Wen, J.; Wu, Y.A. CO2 Transformation to Multicarbon Products by Photocatalysis and Electrocatalysis. Materials Today Advances 2020, 6, 100071. [CrossRef]
- Halmann, M. Photoelectrochemical Reduction of Aqueous Carbon Dioxide on P-Type Gallium Phosphide in Liquid Junction Solar Cells. Nature 1978, 275, 115–116. [CrossRef]
- Olah, G.A. Beyond Oil and Gas: The Methanol Economy. Angew. Chem. Int. Ed. 2005, 44, 2636–2639. [CrossRef]
- Lu, Q.; Jiao, F. Electrochemical CO2 Reduction: Electrocatalyst, Reaction Mechanism, and Process Engineering. Nano Energy 2016, 29, 439–456. [CrossRef]
- Philip Colombo, D.; Roussel, K.A.; Saeh, J.; Skinner, D.E.; Cavaleri, J.J.; Bowman, R.M. Femtosecond Study of the Intensity Dependence of Electron-Hole Dynamics in TiO2 Nanoclusters. Chemical Physics Letters 1995, 232, 207–214. [CrossRef]
- Kauffman, D.R.; Alfonso, D.; Matranga, C.; Qian, H.; Jin, R. Experimental and Computational Investigation of Au 25 Clusters and CO 2 : A Unique Interaction and Enhanced Electrocatalytic Activity. J. Am. Chem. Soc. 2012, 134, 10237–10243. [CrossRef]
- Guo, S.-X.; MacFarlane, D.R.; Zhang, J. Bioinspired Electrocatalytic CO 2 Reduction by Bovine Serum Albumin-Capped Silver Nanoclusters Mediated by [ α -SiW 12 O 40 ] 4−. ChemSusChem 2016, 9, 80–87. [CrossRef]
- Corma, A.; Garcia, H. Photocatalytic Reduction of CO2 for Fuel Production: Possibilities and Challenges. Journal of Catalysis 2013, 308, 168–175. [CrossRef]
- Qin, L.; Ma, G.; Wang, L.; Tang, Z. Atomically Precise Metal Nanoclusters for (Photo)Electroreduction of CO2: Recent Advances, Challenges and Opportunities. Journal of Energy Chemistry 2021, 57, 359–370. [CrossRef]
- Linsebigler, A.L.; Lu, G.; Yates, J.T. Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results. Chem. Rev. 1995, 95, 735–758. [CrossRef]
- Liu, M.; Qiu, X.; Miyauchi, M.; Hashimoto, K. Cu(II) Oxide Amorphous Nanoclusters Grafted Ti 3+ Self-Doped TiO 2 : An Efficient Visible Light Photocatalyst. Chem. Mater. 2011, 23, 5282–5286. [CrossRef]
- Shoji, S.; Yin, G.; Nishikawa, M.; Atarashi, D.; Sakai, E.; Miyauchi, M. Photocatalytic Reduction of CO2 by Cu O Nanocluster Loaded SrTiO3 Nanorod Thin Film. Chemical Physics Letters 2016, 658, 309–314. [CrossRef]
- Yin, G.; Nishikawa, M.; Nosaka, Y.; Srinivasan, N.; Atarashi, D.; Sakai, E.; Miyauchi, M. Photocatalytic Carbon Dioxide Reduction by Copper Oxide Nanocluster-Grafted Niobate Nanosheets. ACS Nano 2015, 9, 2111–2119. [CrossRef]
- Jin, J.; Luo, J.; Zan, L.; Peng, T. One-Pot Synthesis of Cu-Nanocluster-Decorated Brookite TiO 2 Quasi -Nanocubes for Enhanced Activity and Selectivity of CO 2 Photoreduction to CH 4. ChemPhysChem 2017, 18, 3230–3239. [CrossRef]
- Cui, X.; Wang, J.; Liu, B.; Ling, S.; Long, R.; Xiong, Y. Turning Au Nanoclusters Catalytically Active for Visible-Light-Driven CO 2 Reduction through Bridging Ligands. J. Am. Chem. Soc. 2018, 140, 16514–16520. [CrossRef]
- Jiang, Y.; Yu, Y.; Zhang, X.; Weinert, M.; Song, X.; Ai, J.; Han, L.; Fei, H. N-Heterocyclic Carbene-Stabilized Ultrasmall Gold Nanoclusters in a Metal-Organic Framework for Photocatalytic CO 2 Reduction. Angew. Chem. Int. Ed. 2021, 60, 17388–17393. [CrossRef]
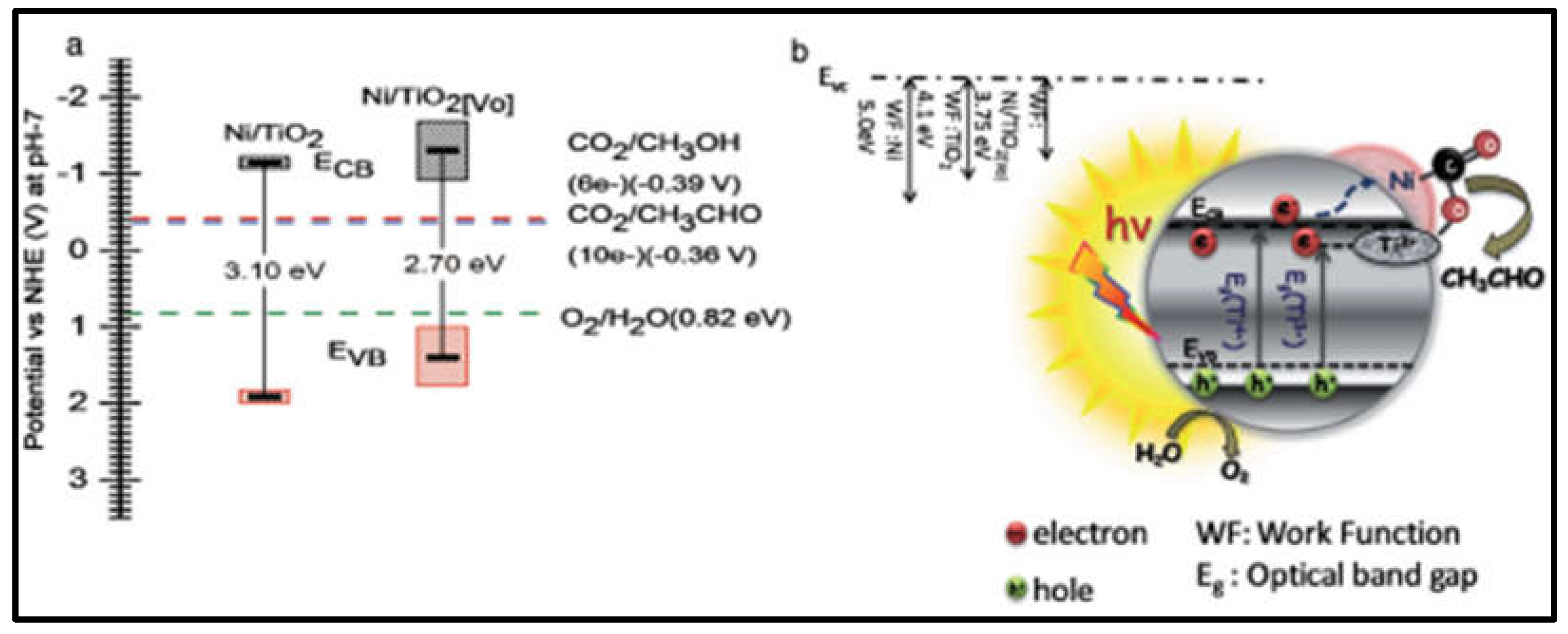
- Billo, T.; Fu, F.-Y.; Raghunath, P.; Shown, I.; Chen, W.-F.; Lien, H.-T.; Shen, T.-H.; Lee, J.-F.; Chan, T.-S.; Huang, K.-Y.; et al. Ni-Nanocluster Modified Black TiO 2 with Dual Active Sites for Selective Photocatalytic CO 2 Reduction. Small 2018, 14, 1702928. [CrossRef]
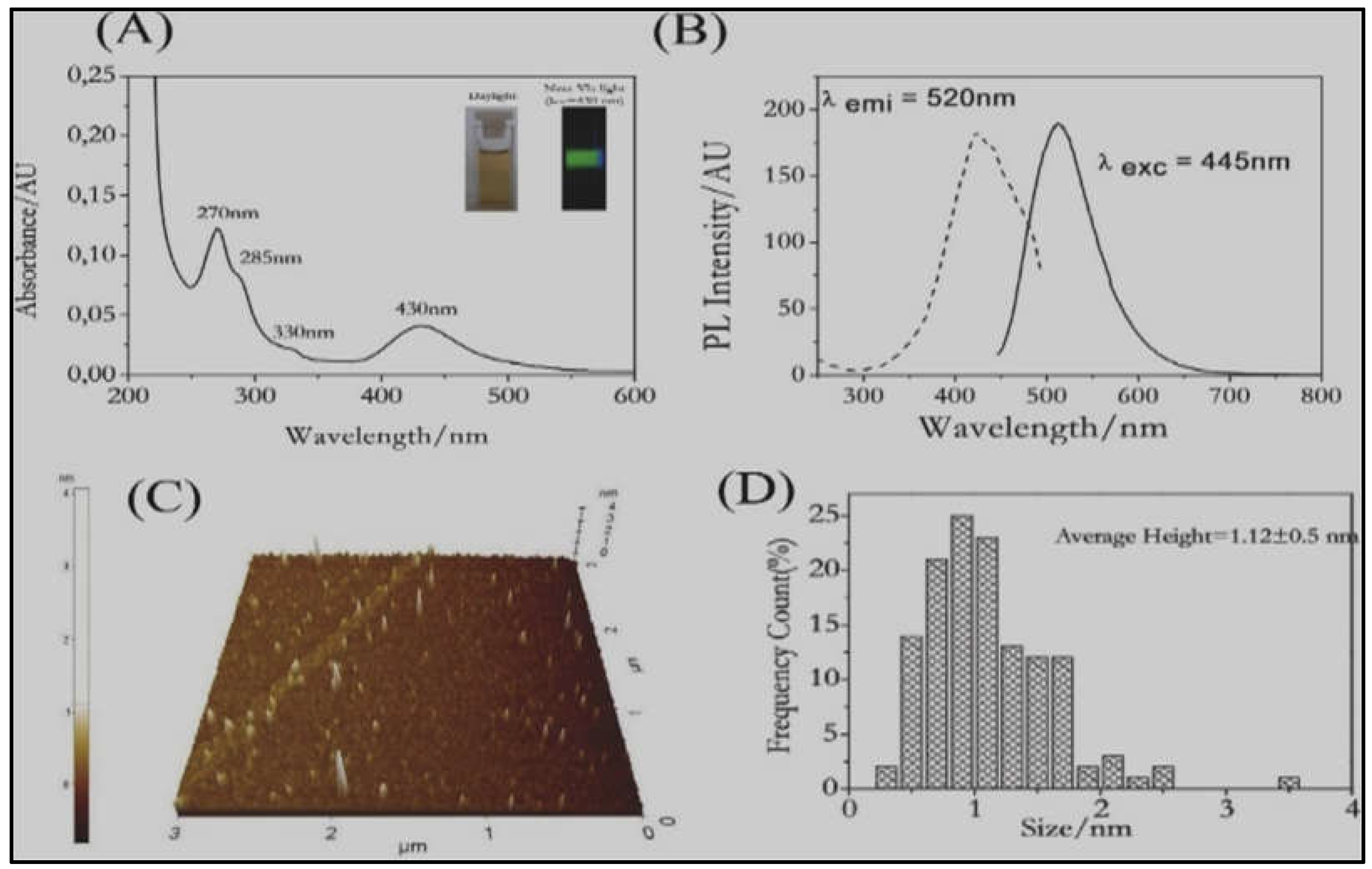
- Chen, Y.-S.; Kamat, P.V. Glutathione-Capped Gold Nanoclusters as Photosensitizers. Visible Light-Induced Hydrogen Generation in Neutral Water. J. Am. Chem. Soc. 2014, 136, 6075–6082. [CrossRef]
- Zhang, L.; Can, M.; Ragsdale, S.W.; Armstrong, F.A. Fast and Selective Photoreduction of CO 2 to CO Catalyzed by a Complex of Carbon Monoxide Dehydrogenase, TiO 2 , and Ag Nanoclusters. ACS Catal. 2018, 8, 2789–2795. [CrossRef]
- El-Roz, M.; Telegeiev, I.; Mordvinova, N.E.; Lebedev, O.I.; Barrier, N.; Behilil, A.; Zaarour, M.; Lakiss, L.; Valtchev, V. Uniform Generation of Sub-Nanometer Silver Clusters in Zeolite Cages Exhibiting High Photocatalytic Activity under Visible Light. ACS Appl. Mater. Interfaces 2018, 10, 28702–28708. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





