Submitted:
05 May 2023
Posted:
06 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Collection of shells and removal of outer prismatic layer
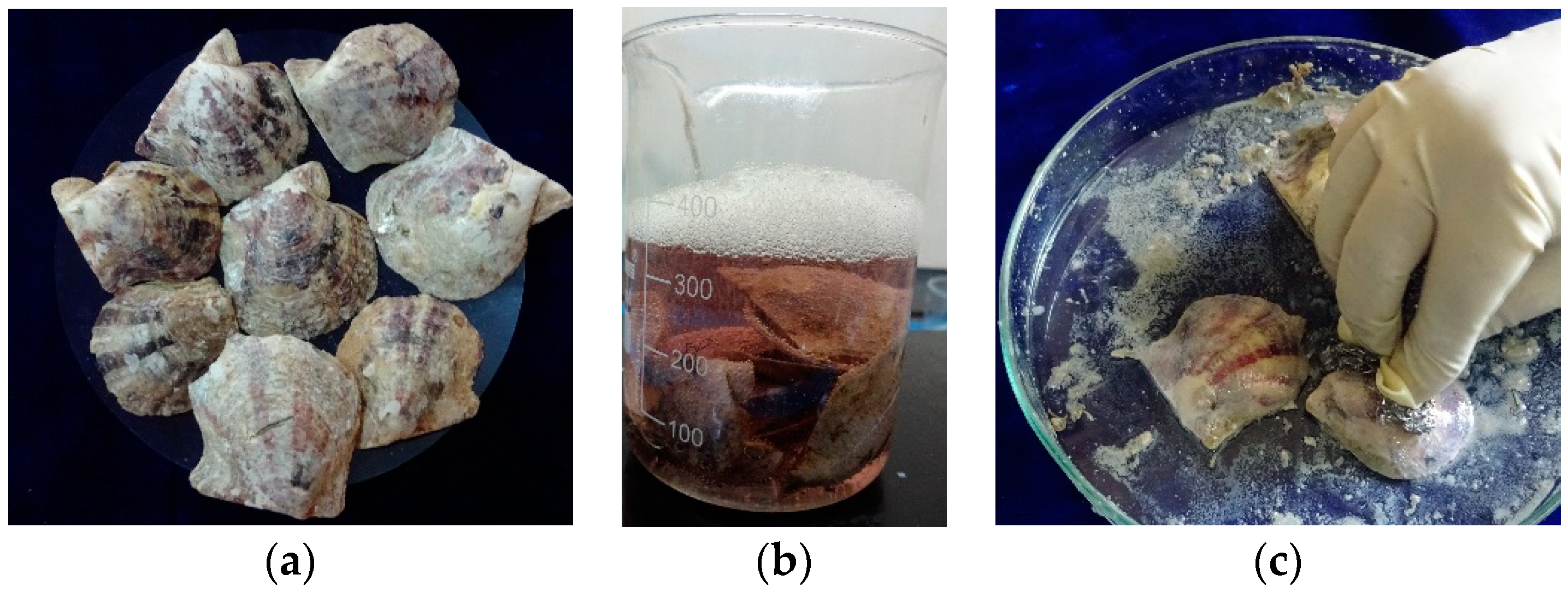
2.1.1. Processing method for shell nacre powder
2.1.2. Characterization of shell nacre powder
2.2. Synthesis of SNLSM 1 and SNLSM 2
2.3. Characterization of SNLSM resins
2.4. Formulation of shell nacre cements
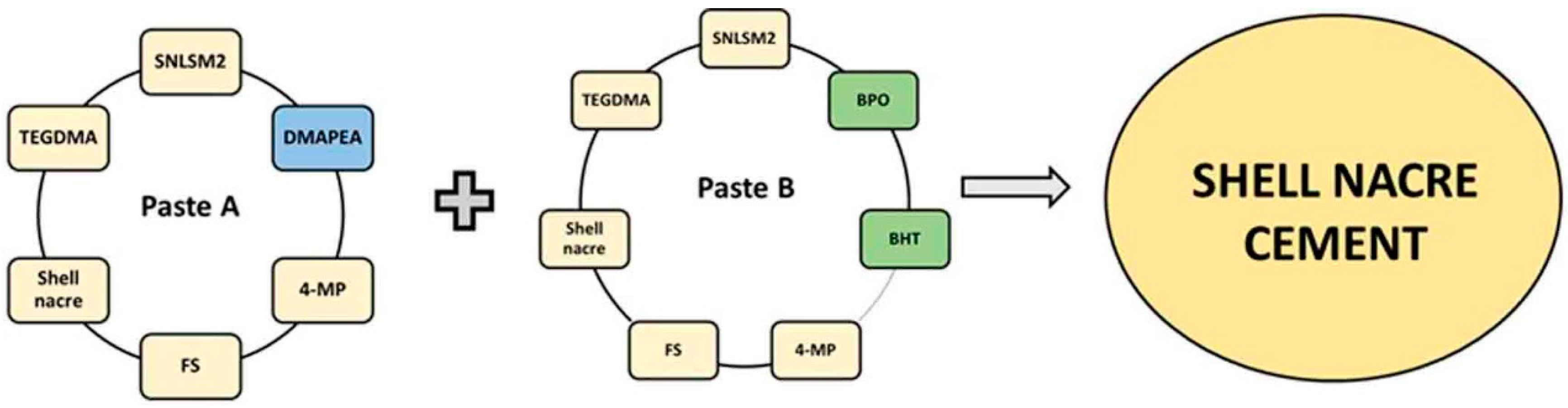
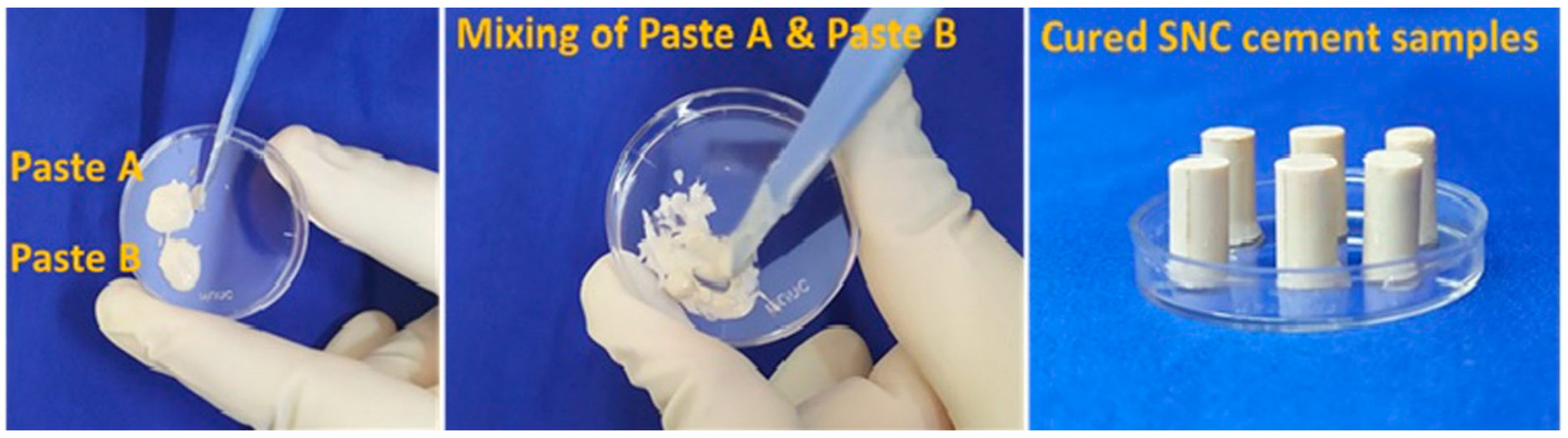
2.4.1. Preparation of Paste A and Paste B (SNC 24/48/72)
2.4.2. Preparation of shell nacre cement samples
2.5. Characterization of shell nacre cement
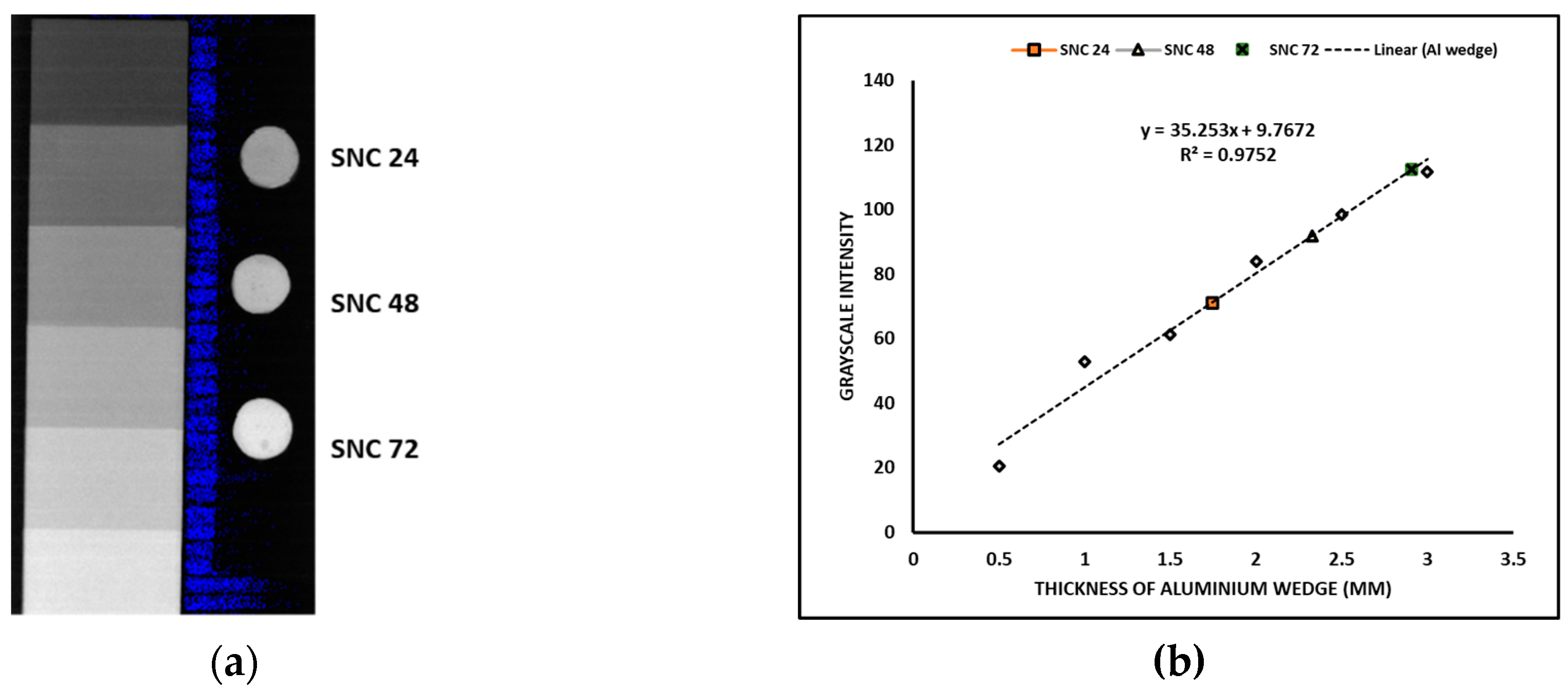
2.5.1. Evaluation of radiopacity
2.5.2. Evaluation of linear polymerization shrinkage (LPS)
2.5.3. Evaluation of mechanical properties
2.5.4. Investigation of exotherm generated
2.5.5. Cytotoxicity evaluation
2.6. Statistical analysis
3. Results
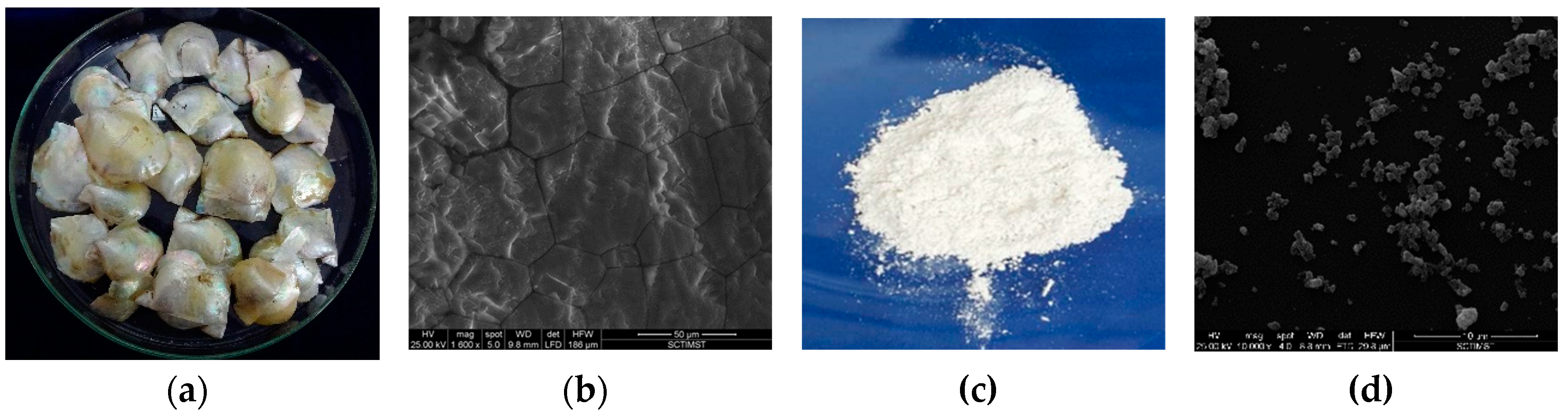
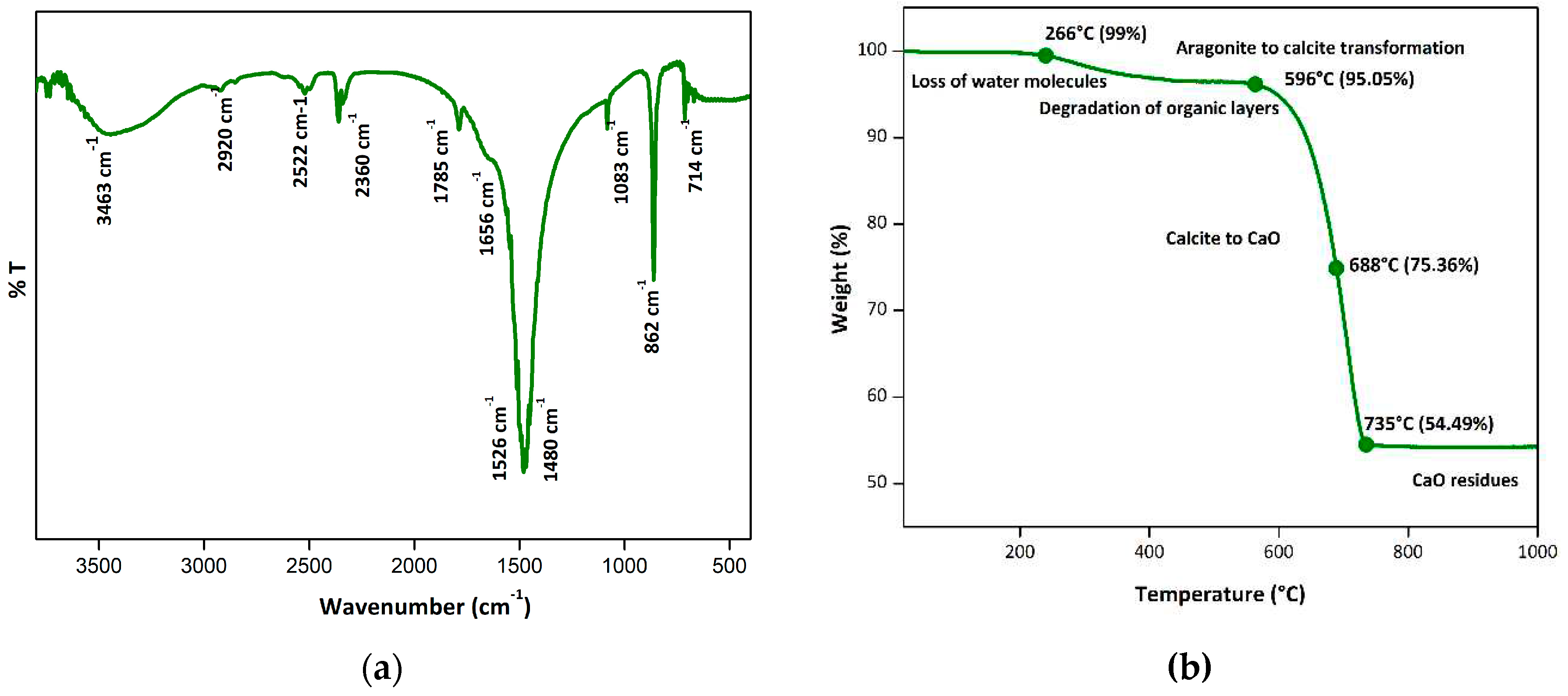
3.1. Shell nacre powder comprised both organic and inorganic constituents
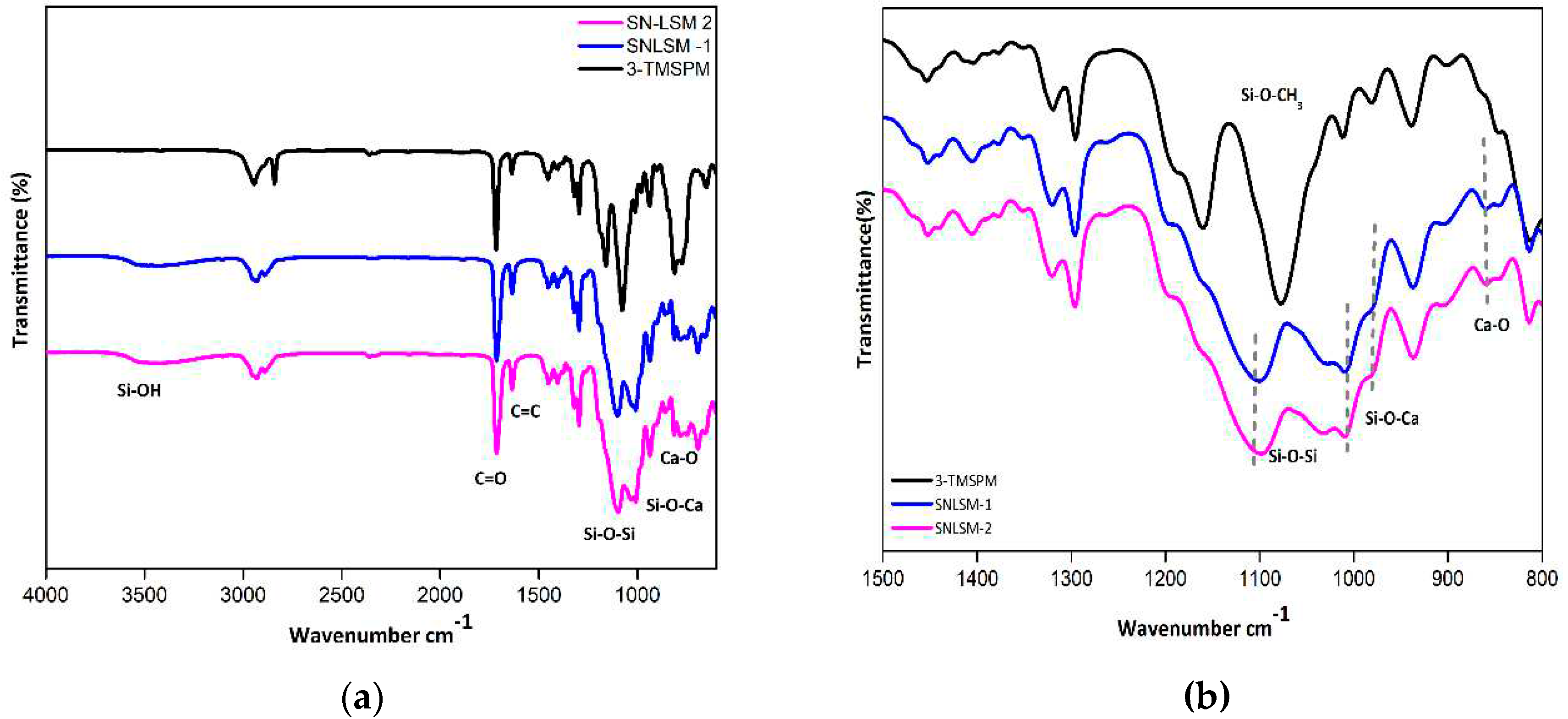
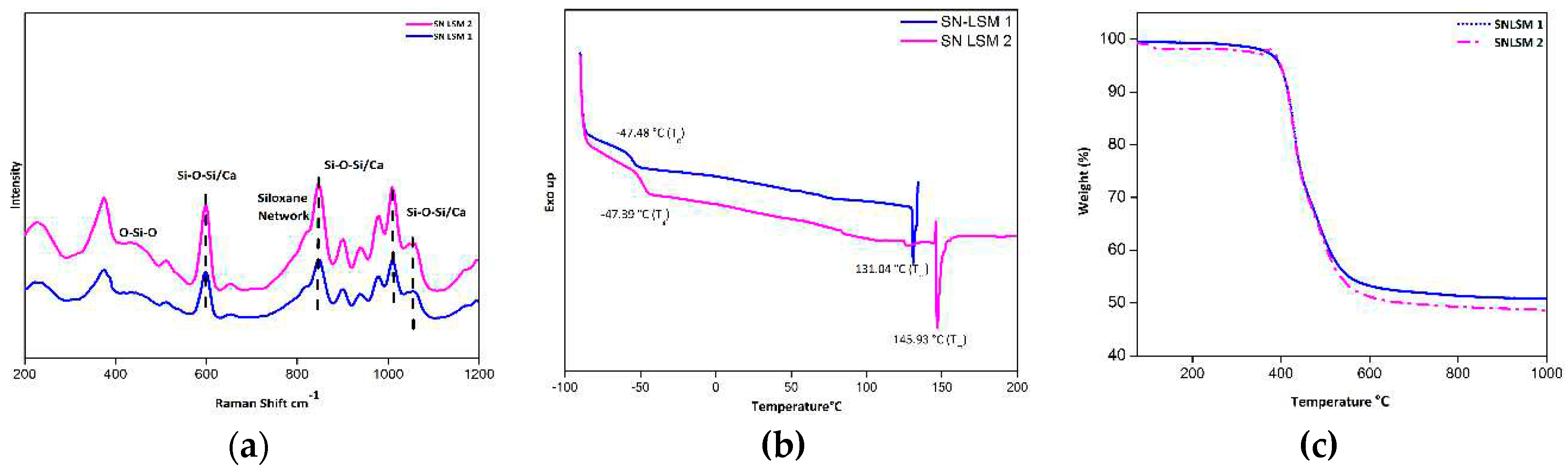
3.2. Characterization of SN-LSM 1 and SN LSM 2
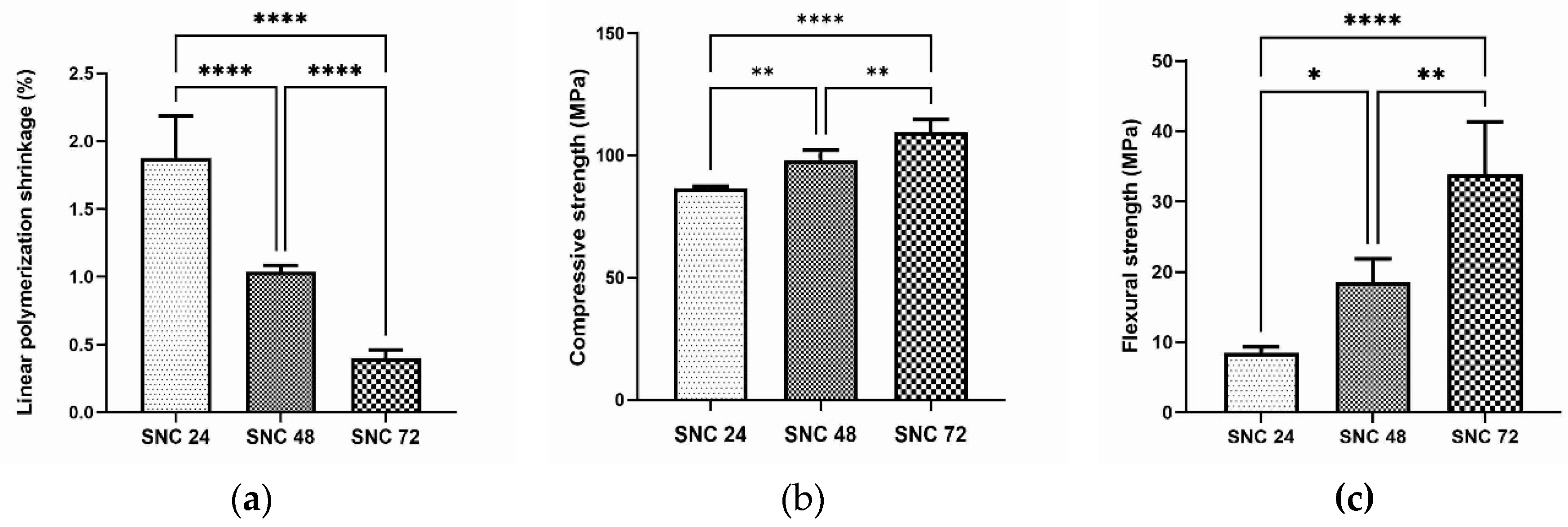
3.3. Radiopaque cement with low linear polymerization shrinkage and better mechanical properties
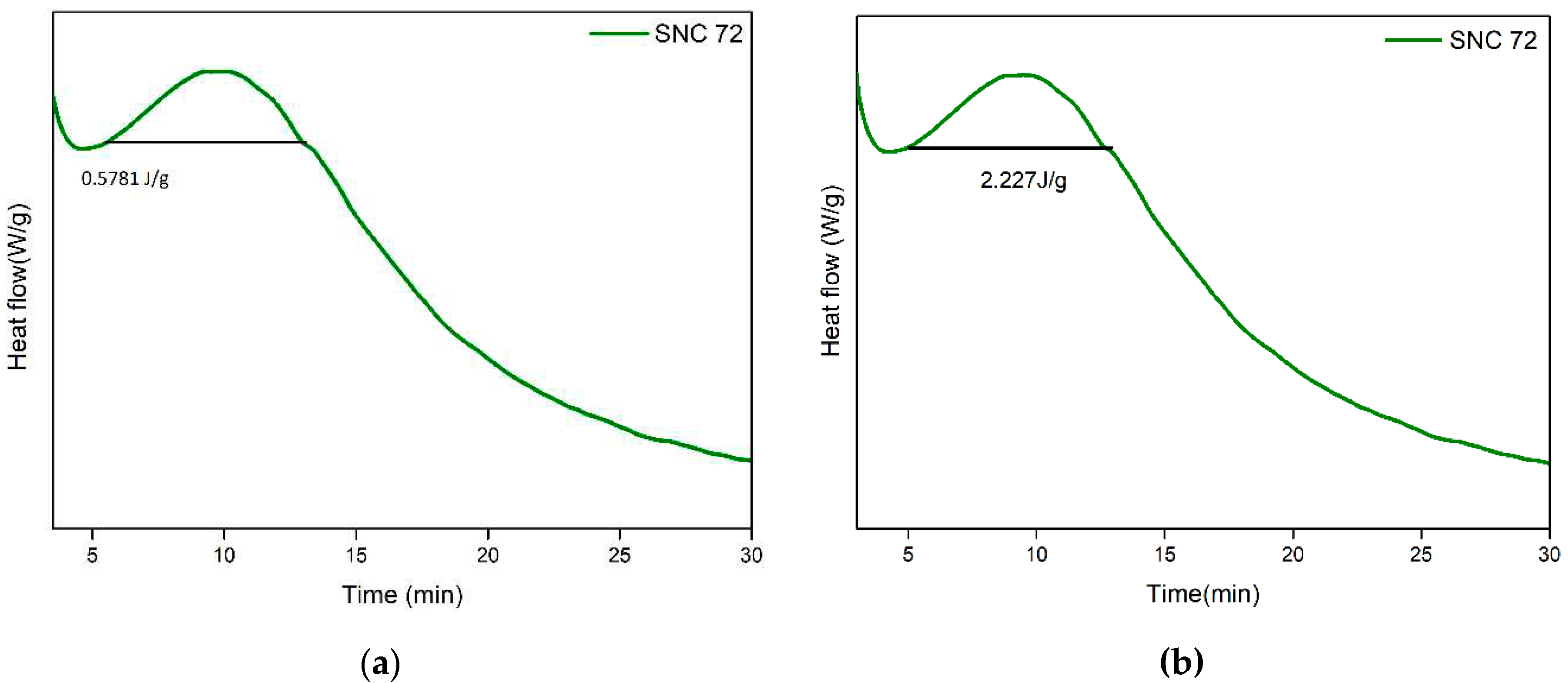
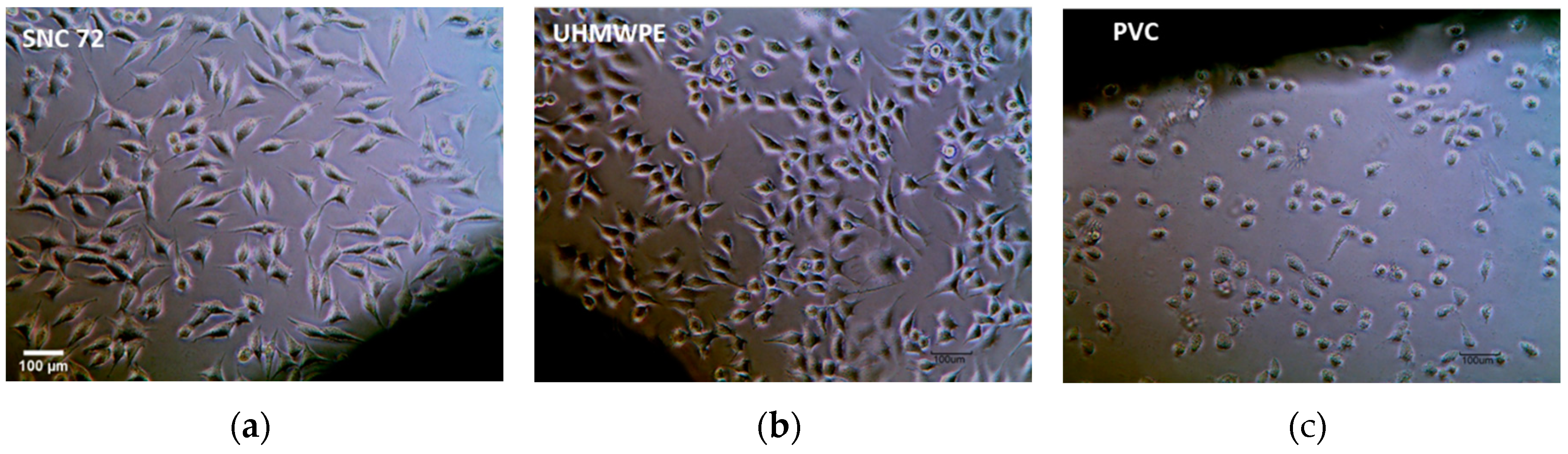
3.4. Minimal exotherm generation and lack of cytotoxicity
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Mancuso, F.; Beltrame, A.; Colombo, E.; Miani, E.; Bassini, F. Management of Metaphyseal Bone Loss in Revision Knee Arthroplasty. Acta Biomed 2017, 88, 98–111. https://doi.org/10.23750/abm.v88i2-S.6520. [CrossRef]
- Hasandoost, L.; Rodriguez, O.; Alhalawani, A.; Zalzal, P.; Schemitsch, E.H.; Waldman, S.D.; Papini, M.; Towler, M.R. The Role of Poly(Methyl Methacrylate) in Management of Bone Loss and Infection in Revision Total Knee Arthroplasty: A Review. JFB 2020, 11, 25. https://doi.org/10.3390/jfb11020025. [CrossRef]
- Blokhuis, T.J. Management of Traumatic Bone Defects: Metaphyseal versus Diaphyseal Defects. Injury 2017, 48, S91–S93. https://doi.org/10.1016/j.injury.2017.04.021. [CrossRef]
- Piccirilli, E.; Cariati, I.; Primavera, M.; Triolo, R.; Gasbarra, E.; Tarantino, U. Augmentation in Fragility Fractures, Bone of Contention: A Systematic Review. BMC Musculoskelet Disord 2022, 23, 1046. https://doi.org/10.1186/s12891-022-06022-0. [CrossRef]
- He, Z.; Zhai, Q.; Hu, M.; Cao, C.; Wang, J.; Yang, H.; Li, B. Bone Cements for Percutaneous Vertebroplasty and Balloon Kyphoplasty: Current Status and Future Developments. Journal of Orthopaedic Translation 2015, 3, 1–11. https://doi.org/10.1016/j.jot.2014.11.002. [CrossRef]
- Reito, A.; Ylitalo, A. Polymethyl Methacrylate Cement Fill as a Definitive Treatment for Massive Bone Defect After Infected Internal Fixation in Bicondylar Tibial Fracture: A Case Report. JBJS Case Connector 2020, 10, e19.00286. https://doi.org/10.2106/JBJS.CC.19.00286. [CrossRef]
- Wu, M.; Yao, S.; Xie, Y.; Yan, F.; Deng, Z.; Lei, J.; Cai, L. A Novel Subchondral Bone-Grafting Procedure for the Treatment of Giant-Cell Tumor around the Knee: A Retrospective Study of 27 Cases. Medicine (Baltimore) 2018, 97, e13154. https://doi.org/10.1097/MD.0000000000013154. [CrossRef]
- Vaishya, R.; Chauhan, M.; Vaish, A. Bone Cement. Journal of Clinical Orthopaedics and Trauma 2013, 4, 157–163. https://doi.org/10.1016/j.jcot.2013.11.005. [CrossRef]
- O’dowd-Booth, C.J.; White, J.; Smitham, P.; Khan, W.; Marsh, D.R. Bone Cement: Perioperative Issues, Orthopaedic Applications and Future Developments. Journal of Perioperative Practice 2011, 21, 304–308. https://doi.org/10.1177/175045891102100902. [CrossRef]
- Soleymani Eil Bakhtiari, S.; Bakhsheshi-Rad, H.R.; Karbasi, S.; Tavakoli, M.; Razzaghi, M.; Ismail, A.F.; RamaKrishna, S.; Berto, F. Polymethyl Methacrylate-Based Bone Cements Containing Carbon Nanotubes and Graphene Oxide: An Overview of Physical, Mechanical, and Biological Properties. Polymers (Basel) 2020, 12, E1469. https://doi.org/10.3390/polym12071469. [CrossRef]
- Kawanabe, K.; Tamura, J.; Yamamuro, T.; Nakamura, T.; Kokubo, T.; Yoshihara, S. A New Bioactive Bone Cement Consisting of BIS-GMA Resin and Bioactive Glass Powder. Journal of Applied Biomaterials 1993, 4, 135–141. https://doi.org/10.1002/jab.770040204. [CrossRef]
- Mousa, W.F.; Kobayashi, M.; Shinzato, S.; Kamimura, M.; Neo, M.; Yoshihara, S.; Nakamura, T. Biological and Mechanical Properties of PMMA-Based Bioactive Bone Cements. Biomaterials 2000, 21, 2137–2146. https://doi.org/10.1016/S0142-9612(00)00097-1. [CrossRef]
- Goto, K.; Tamura, J.; Shinzato, S.; Fujibayashi, S.; Hashimoto, M.; Kawashita, M.; Kokubo, T.; Nakamura, T. Bioactive Bone Cements Containing Nano-Sized Titania Particles for Use as Bone Substitutes. Biomaterials 2005, 26, 6496–6505. https://doi.org/10.1016/j.biomaterials.2005.04.044. [CrossRef]
- Deb, S.; Aiyathurai, L.; Roether, J.A.; Luklinska, Z.B. Development of High-Viscosity, Two-Paste Bioactive Bone Cements. Biomaterials 2005, 26, 3713–3718. https://doi.org/10.1016/j.biomaterials.2004.09.065. [CrossRef]
- Ni, G.X.; Chiu, K.Y.; Lu, W.W.; Wang, Y.; Zhang, Y.G.; Hao, L.B.; Li, Z.Y.; Lam, W.M.; Lu, S.B.; Luk, K.D.K. Strontium-Containing Hydroxyapatite Bioactive Bone Cement in Revision Hip Arthroplasty. Biomaterials 2006, 27, 4348–4355. https://doi.org/10.1016/j.biomaterials.2006.03.048. [CrossRef]
- Liu, Z.; Tang, Y.; Kang, T.; Rao, M.; Li, K.; Wang, Q.; Quan, C.; Zhang, C.; Jiang, Q.; Shen, H. Synergistic Effect of HA and BMP-2 Mimicking Peptide on the Bioactivity of HA/PMMA Bone Cement. Colloids Surf B Biointerfaces 2015, 131, 39–46. https://doi.org/10.1016/j.colsurfb.2015.04.032. [CrossRef]
- Erbe, E.M.; Clineff, T.D.; Gualtieri, G. Comparison of a New Bisphenol-a-Glycidyl Dimethacrylate-Based Cortical Bone Void Filler with Polymethyl Methacrylate. Eur Spine J 2001, 10, S147–S152. https://doi.org/10.1007/s005860100288. [CrossRef]
- Zhang, H.; Cui, Y.; Zhuo, X.; Kim, J.; Li, H.; Li, S.; Yang, H.; Su, K.; Liu, C.; Tian, P.; et al. Biological Fixation of Bioactive Bone Cement in Vertebroplasty: The First Clinical Investigation of Borosilicate Glass (BSG) Reinforced PMMA Bone Cement. ACS Appl. Mater. Interfaces 2022, 14, 51711–51727. https://doi.org/10.1021/acsami.2c15250. [CrossRef]
- Tan, Q.-C.; Jiang, X.-S.; Chen, L.; Huang, J.-F.; Zhou, Q.-X.; Wang, J.; Zhao, Y.; Zhang, B.; Sun, Y.-N.; Wei, M.; et al. Bioactive Graphene Oxide-Functionalized Self-Expandable Hydrophilic and Osteogenic Nanocomposite for Orthopaedic Applications. Materials Today Bio 2023, 18, 100500. https://doi.org/10.1016/j.mtbio.2022.100500. [CrossRef]
- Han, Z.; Wang, B.; Ren, B.; Liu, Y.; Zhang, N.; Wang, Z.; Liu, J.; Mao, K. Characterization and Biomechanical Study of a Novel Magnesium Potassium Phosphate Cement. Life (Basel) 2022, 12, 997. https://doi.org/10.3390/life12070997. [CrossRef]
- Moore, W.R.; Graves, S.E.; Bain, G.I. Synthetic Bone Graft Substitutes. ANZ Journal of Surgery 2001, 71, 354–361. https://doi.org/10.1046/j.1440-1622.2001.02128.x. [CrossRef]
- Nandi, S.K.; Roy, S.; Mukherjee, P.; Kundu, B.; De, D.K.; Basu, D. Orthopaedic Applications of Bone Graft & Graft Substitutes: A Review. Indian Journal of Medical Research 2010, 132, 15.
- Wang, W.; Yeung, K.W.K. Bone Grafts and Biomaterials Substitutes for Bone Defect Repair: A Review. Bioactive Materials 2017, 2, 224–247. https://doi.org/10.1016/j.bioactmat.2017.05.007. [CrossRef]
- Marongiu, G.; Verona, M.; Cardoni, G.; Capone, A. Synthetic Bone Substitutes and Mechanical Devices for the Augmentation of Osteoporotic Proximal Humeral Fractures: A Systematic Review of Clinical Studies. Journal of Functional Biomaterials 2020, 11, 29. https://doi.org/10.3390/jfb11020029. [CrossRef]
- Fillingham, Y.; Jacobs, J. Bone Grafts and Their Substitutes. The Bone & Joint Journal 2016, 98-B, 6–9. https://doi.org/10.1302/0301-620X.98B.36350. [CrossRef]
- Gu, X.; Li, Y.; Qi, C.; Cai, K. Biodegradable Magnesium Phosphates in Biomedical Applications. J. Mater. Chem. B 2022, 10, 2097–2112. https://doi.org/10.1039/D1TB02836G. [CrossRef]
- Liu, Z.; He, X.; Chen, S.; Yu, H. Advances in the Use of Calcium Silicate-Based Materials in Bone Tissue Engineering. Ceramics International 2023. https://doi.org/10.1016/j.ceramint.2023.03.063. [CrossRef]
- Atlan, G.; Delattre, O.; Berland, S.; LeFaou, A.; Nabias, G.; Cot, D.; Lopez, E. Interface between Bone and Nacre Implants in Sheep. Biomaterials 1999, 20, 1017–1022. https://doi.org/10.1016/S0142-9612(98)90212-5. [CrossRef]
- Camprasse, S.; Camprasse, G.; Pouzol, M.; Lopez, E. Artificial Dental Root Made of Natural Calcium Carbonate (Bioracine). Clinical Materials 1990, 5, 235–250. https://doi.org/10.1016/0267-6605(90)90022-N. [CrossRef]
- Lamghari, M.; Berland, S.; Laurent, A.; Huet, H.; Lopez, E. Bone Reactions to Nacre Injected Percutaneously into the Vertebrae of Sheep. Biomaterials 2001, 22, 555–562. https://doi.org/10.1016/S0142-9612(00)00213-1. [CrossRef]
- Berland, S.; Delattre, O.; Borzeix, S.; Catonné, Y.; Lopez, E. Nacre/Bone Interface Changes in Durable Nacre Endosseous Implants in Sheep. Biomaterials 2005, 26, 2767–2773. https://doi.org/10.1016/j.biomaterials.2004.07.019. [CrossRef]
- Kim, H.; Lee, K.; Ko, C.-Y.; Kim, H.-S.; Shin, H.-I.; Kim, T.; Lee, S.H.; Jeong, D. The Role of Nacreous Factors in Preventing Osteoporotic Bone Loss through Both Osteoblast Activation and Osteoclast Inactivation. Biomaterials 2012, 33, 7489–7496. https://doi.org/10.1016/j.biomaterials.2012.06.098. [CrossRef]
- Lee, K.; Kim, H.; Kim, J.M.; Chung, Y.H.; Lee, T.Y.; Lim, H.-S.; Lim, J.-H.; Kim, T.; Bae, J.S.; Woo, C.-H.; et al. Nacre-Driven Water-Soluble Factors Promote Wound Healing of the Deep Burn Porcine Skin by Recovering Angiogenesis and Fibroblast Function. Mol Biol Rep 2012, 39, 3211–3218. https://doi.org/10.1007/s11033-011-1088-4. [CrossRef]
- Gerhard, E.M.; Wang, W.; Li, C.; Guo, J.; Ozbolat, I.T.; Rahn, K.M.; Armstrong, A.D.; Xia, J.; Qian, G.; Yang, J. Design Strategies and Applications of Nacre-Based Biomaterials. Acta Biomaterialia 2017, 54, 21–34. https://doi.org/10.1016/j.actbio.2017.03.003. [CrossRef]
- Zhang, G.; Brion, A.; Willemin, A.-S.; Piet, M.-H.; Moby, V.; Bianchi, A.; Mainard, D.; Galois, L.; Gillet, P.; Rousseau, M. Nacre, a Natural, Multi-Use, and Timely Biomaterial for Bone Graft Substitution. Journal of Biomedical Materials Research Part A 2017, 105, 662–671. https://doi.org/10.1002/jbm.a.35939. [CrossRef]
- Sol–Gel Based Materials for Biomedical Applications - ScienceDirect Available online: https://www.sciencedirect.com/science/article/pii/S0079642516000025 (accessed on 9 April 2020).
- Lizymol, P.P. Studies on Shrinkage, Depth of Cure, and Cytotoxic Behavior of Novel Organically Modified Ceramic Based Dental Restorative Resins. Journal of Applied Polymer Science 2010, 116, 2645–2650. https://doi.org/10.1002/app.31762. [CrossRef]
- Lizymol, P.P. Effects of Diluent’s Concentration upon the Properties of Organically Modified Ceramics Based Composites for Application in Dentistry. Journal of Applied Polymer Science 2004, 94, 469–473. https://doi.org/10.1002/app.20891. [CrossRef]
- Vibha, C.; Lizymol, P.P. Development of Hydroxyapatite-Reinforced Biocomposites Based on Polymerizable Multifunctional Strontium Containing Inorganic-Organic Hybrid Resins for Biomedical Applications. Materials Letters 2017, 197, 63–66. https://doi.org/10.1016/j.matlet.2017.03.098. [CrossRef]
- Vibha, C.; Lizymol, P.P. Synthesis and Characterization of a Novel Radiopaque Dimethacrylate Zirconium Containing Pre-Polymer for Biomedical Applications. Materials Letters 2019, 237, 294–297. https://doi.org/10.1016/j.matlet.2018.11.098. [CrossRef]
- Wolter, H.; Glaubitt, W.; Rose, K. Multifunctional (Meth)Acrylate Alkoxysilanes a New Type of Reactive Compounds. MRS Online Proceedings Library 1992, 271, 719–724. https://doi.org/10.1557/PROC-271-719. [CrossRef]
- Dirè, S.; Borovin, E.; Ribot, F. Architecture of Silsesquioxanes. In Handbook of Sol-Gel Science and Technology: Processing, Characterization and Applications; Klein, L., Aparicio, M., Jitianu, A., Eds.; Springer International Publishing: Cham, 2018; pp. 3119–3151 ISBN 978-3-319-32101-1.
- Kim, Y.H.; Choi, G.-M.; Bae, J.G.; Kim, Y.H.; Bae, B.-S. High-Performance and Simply-Synthesized Ladder-Like Structured Methacrylate Siloxane Hybrid Material for Flexible Hard Coating. Polymers 2018, 10, 449. [CrossRef]
- Haas, K.-H. Hybrid Inorganic–Organic Polymers Based on Organically Modified Si-Alkoxides. Advanced Engineering Materials 2000, 2, 571–582. https://doi.org/10.1002/1527-2648(200009)2:9<571::AID-ADEM571>3.0.CO;2-M. [CrossRef]
- Bridget Jeyatha, W.; Paul, W.; Mani, S.; Lizymol, P.P. Synthesis and Characterization of Ladder Structured Ormocer Resin of Siloxane Backbone and Methacrylate Side Chain. Materials Letters 2022, 310, 131192. https://doi.org/10.1016/j.matlet.2021.131192. [CrossRef]
- INTERNATIONAL STANDARD ISO 10993-5.Biological Evaluation of Medical Devices —Part 5: Tests for in Vitro Cytotoxicity.
- Standard Test Methods for Determining Radiopacity for Medical Use Available online: https://www.astm.org/f0640-20.html (accessed on 27 February 2023).
- Orthopaedics | Bone Repair. MEGA BIOPHARMA.
- Shen, Y.; Yang, S.; Liu, J.; Xu, H.; Shi, Z.; Lin, Z.; Ying, X.; Guo, P.; Lin, T.; Yan, S.; et al. Engineering Scaffolds Integrated with Calcium Sulfate and Oyster Shell for Enhanced Bone Tissue Regeneration. ACS Appl. Mater. Interfaces 2014, 6, 12177–12188. https://doi.org/10.1021/am501448t. [CrossRef]
- Ruan, R.; Zheng, M.; Gao, J.; Landao-Bassonga, E.; Chen, L.; Chen, P.; Wang, T.; Zhao, X. Improved Biological Properties of Calcium Phosphate Cement by Nacre Incorporation: An In Vitro Study. Journal of Biomaterials and Tissue Engineering 2018, 8, 67–79. https://doi.org/10.1166/jbt.2018.1720. [CrossRef]
- Simu, M.-R.; Pall, E.; Radu, T.; Miclaus, M.; Culic, B.; Mesaros, A.-S.; Muntean, A.; Filip, G.A. Development of a Novel Biomaterial with an Important Osteoinductive Capacity for Hard Tissue Engineering. Tissue and Cell 2018, 52, 101–107. https://doi.org/10.1016/j.tice.2018.04.004. [CrossRef]
- Du, M.; Li, Q.; Chen, J.; Liu, K.; Song, C. Design and Characterization of Injectable Abalone Shell/Calcium Sulfate Bone Cement Scaffold for Bone Defect Repair. Chemical Engineering Journal 2021, 420, 129866. https://doi.org/10.1016/j.cej.2021.129866. [CrossRef]
- Pei, J.; Wang, Y.; Zou, X.; Ruan, H.; Tang, C.; Liao, J.; Si, G.; Sun, P. Extraction, Purification, Bioactivities and Application of Matrix Proteins From Pearl Powder and Nacre Powder: A Review. Frontiers in Bioengineering and Biotechnology 2021, 9.
- Sun, J.; Bhushan, B. Hierarchical Structure and Mechanical Properties of Nacre: A Review. RSC Adv. 2012, 2, 7617–7632. https://doi.org/10.1039/C2RA20218B. [CrossRef]
- Iandolo, D.; Laroche, N.; Nguyen, D.K.; Normand, M.; Met, C.; Zhang, G.; Vico, L.; Mainard, D.; Rousseau, M. Preclinical Safety Study of Nacre Powder in an Intraosseous Sheep Model. BMJ Open Science 2022, 6. https://doi.org/10.1136/bmjos-2021-100231. [CrossRef]
- Atlan, G.; Balmain, N.; Berland, S.; Vidal, B.; Lopez, É. Reconstruction of Human Maxillary Defects with Nacre Powder: Histological Evidence for Bone Regeneration. Comptes Rendus de l’Académie des Sciences - Series III - Sciences de la Vie 1997, 320, 253–258. https://doi.org/10.1016/S0764-4469(97)86933-8. [CrossRef]
- Balmain, J.; Hannoyer, B.; Lopez, E. Fourier Transform Infrared Spectroscopy (FTIR) and X-Ray Diffraction Analyses of Mineral and Organic Matrix during Heating of Mother of Pearl (Nacre) from the Shell of the Mollusc Pinctada Maxima. Journal of Biomedical Materials Research 1999, 48, 749–754. https://doi.org/10.1002/(SICI)1097-4636(1999)48:5<749::AID-JBM22>3.0.CO;2-P. [CrossRef]
- Bellaaj-Zouari, A.; Chérif, K.; Elloumi-Hannachi, I.; Slimane, N.; Jaafoura, M.H. Characterization of Mineral and Organic Phases in Nacre of the Invasive Pearl Oyster Pinctada Radiata (Leach, 1814).
- In Vivo Characterization of Bivalve Larval Shells: A Confocal Raman Microscopy Study | Journal of The Royal Society Interface Available online: https://royalsocietypublishing.org/doi/full/10.1098/rsif.2017.0723 (accessed on 28 February 2023).
- Standard Guide for Characterization and Testing of Chitosan Salts as Starting Materials Intended for Use in Biomedical and Tissue-Engineered Medical Product Applications Available online: https://www.astm.org/f2103-18.html (accessed on 25 February 2023).
- Standard Specification for Calcium Phosphate Coatings for Implantable Materials Available online: https://www.astm.org/f1609-08r14.html (accessed on 28 February 2023).
- Baatti, A.; Erchiqui, F.; Bébin, P.; Godard, F.; Bussières, D. A Two-Step Sol-Gel Method to Synthesize a Ladder Polymethylsilsesquioxane Nanoparticles. Advanced Powder Technology 2017, 28, 1038–1046. https://doi.org/10.1016/j.apt.2017.01.009. [CrossRef]
- Mark, J.E. Some Interesting Things about Polysiloxanes. Acc. Chem. Res. 2004, 37, 946–953. https://doi.org/10.1021/ar030279z. [CrossRef]
- Lewis, G. Alternative Acrylic Bone Cement Formulations for Cemented Arthroplasties: Present Status, Key Issues, and Future Prospects. J Biomed Mater Res B Appl Biomater 2008, 84, 301–319. https://doi.org/10.1002/jbm.b.30873. [CrossRef]
- Jacobs, E.; Saralidze, K.; Roth, A.K.; de Jong, J.J.A.; van den Bergh, J.P.W.; Lataster, A.; Brans, B.T.; Knetsch, M.L.W.; Djordjevic, I.; Willems, P.C.; et al. Synthesis and Characterization of a New Vertebroplasty Cement Based on Gold-Containing PMMA Microspheres. Biomaterials 2016, 82, 60–70. https://doi.org/10.1016/j.biomaterials.2015.12.024. [CrossRef]
- Gilbert, J.L.; Hasenwinkel, J.M.; Wixson, R.L.; Lautenschlager, E.P. A Theoretical and Experimental Analysis of Polymerization Shrinkage of Bone Cement: A Potential Major Source of Porosity. Journal of Biomedical Materials Research 2000, 52, 210–218. https://doi.org/10.1002/1097-4636(200010)52:1<210::AID-JBM27>3.0.CO;2-R. [CrossRef]
- Wu, T.; Gao, S.; Cui, Y.; Qiao, Y.; Zhou, F.; Qiu, D. Amphiphilic Bioactive Filler for Acrylic Bone Cement to Enhance Its Cell Adhesion. Journal of Biomedical Nanotechnology 2018, 14, 795–801. https://doi.org/10.1166/jbn.2018.2543. [CrossRef]
- Khandaker, M.; Vaughan, M.B.; Morris, T.L.; White, J.J.; Meng, Z. Effect of Additive Particles on Mechanical, Thermal, and Cell Functioning Properties of Poly(Methyl Methacrylate) Cement. Int J Nanomedicine 2014, 9, 2699–2712. https://doi.org/10.2147/IJN.S61964. [CrossRef]
- Yang, Z.; Chen, L.; Hao, Y.; Zang, Y.; Zhao, X.; Shi, L.; Zhang, Y.; Feng, Y.; Xu, C.; Wang, F.; et al. Synthesis and Characterization of an Injectable and Hydrophilous Expandable Bone Cement Based on Poly(Methyl Methacrylate). ACS Appl. Mater. Interfaces 2017, 9, 40846–40856. https://doi.org/10.1021/acsami.7b12983. [CrossRef]
- Wolf-Brandstetter, C.; Roessler, S.; Storch, S.; Hempel, U.; Gbureck, U.; Nies, B.; Bierbaum, S.; Scharnweber, D. Physicochemical and Cell Biological Characterization of PMMA Bone Cements Modified with Additives to Increase Bioactivity. Journal of Biomedical Materials Research Part B: Applied Biomaterials 2013, 101B, 599–609. https://doi.org/10.1002/jbm.b.32862. [CrossRef]
- Paz, E.; Forriol, F.; Del Real, J.C.; Dunne, N. Graphene Oxide versus Graphene for Optimisation of PMMA Bone Cement for Orthopaedic Applications. Mater Sci Eng C Mater Biol Appl 2017, 77, 1003–1011. https://doi.org/10.1016/j.msec.2017.03.269. [CrossRef]
- Chiang, C.-C.; Hsieh, M.-K.; Wang, C.-Y.; Tuan, W.-H.; Lai, P.-L. Cytotoxicity and Cell Response of Preosteoblast in Calcium Sulfate-Augmented PMMA Bone Cement. Biomed Mater 2021, 16. https://doi.org/10.1088/1748-605X/ac1ab5. [CrossRef]

| Elements analyzed | Total amount (PPM) |
|---|---|
| Cu | BDL1 |
| Fe | 5.889 |
| Mg | 102.87 |
| Mn | 0.772 |
| Zn | 0.4827 |
| Cd | BDL1 |
| Pb | 0.772 |
| Hg | 1.4 |
| Se | BDL1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




