1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has brought unprecedented health, economic and social crises around the world. Although the COVID-19 infection and death rate per capita has been relatively low in sub-Saharan Africa, South Africa has been significantly affected by the pandemic, with a highly contagious viral variant (501Y.V2) identified in late 2020 (1,2). As of 11 April, 2023, South Africa has recorded over 4 million cases and 102 595 COVID-19 related deaths. Nevertheless, it appears that SARS-CoV-2 is here for the long haul and could likely follow the path of most respiratory viruses such as influenza and other human coronaviruses, which have a relatively predictable seasonal pattern (3,4). Therefore, implementing effective control measures will require an understanding of the extent of natural immunity after infection. However, many questions remain about the durability of the immune response to SARS-CoV-2 infection. Also, the effect of established risk factors on the longevity of the humoral immune response remains a subject of intense investigation.
Ageing and chronic comorbidities have been identified as major risk factors for SARS-CoV-2 infection (5). Furthermore, diabetes mellitus (DM), hypertension, obesity and cardiovascular diseases have been recognised as the most prevalent comorbidities in individuals infected with SARS-CoV-2 (5,6). It has been shown that these comorbidities are risk factors for poor outcome and mortality among infected patients (6). The mechanism in which these comorbidities worsen COVID-19 outcomes may involve immune and inflammatory responses, modulated by hyperglycaemia, and the Renin-Angiotensin System (7–9).
Antibodies play a crucial role in neutralising viruses and preventing reinfection. Upon infection, immunoglobulin M (IgM) antibodies are produced and start to decrease by the third week, while immunoglobulin G (IgG) antibodies are expected to remain detectable after infection (10). For the human coronaviruses which cause the common cold, IgG antibodies last for at least a year after infection. In Middle East Respiratory Syndrome (MERS-CoV) and severe acute respiratory syndrome coronavirus-1 (SARS-CoV-1), IgG antibodies can be detected for years after infection (11). Bichara et al (12) reported a high frequency of loss of SARS-CoV-2 IgG antibodies within three months after COVID-19 diagnosis, while other studies have shown a short to medium term protection against reinfection with similar levels of prevention of symptomatic infection as currently available vaccines (13,14).
Genetic variability in the immune system, more specifically in the human leukocyte antigen (HLA), may influence the strength of immune responses to SARS-CoV-2 (15,16). The variations in the HLA system are linked to ethnicity and geographical location primarily due to pathogen-driven selection and past interbreeding with archaic human lineages (17,18). The duration of COVID-19 immunity after a natural infection may also vary with age, comorbidities, and severity of infection (19). A similar study from USA also revealed that female sex, age, symptom duration, and disease severity were associated with the persistence and loss of serum IgG levels in individuals who have recovered from SARS-CoV-2 infection (20).
Current understanding of SARS-CoV-2 antibody durability is limited by small sample sizes and the lack of African-specific data. This study aimed to investigate the durability of the humoral immune response (SARS-CoV-2 anti-N IgG antibody) to primary COVID-19 infection, and its association with age, sex, ethnicity, and co-morbidities in a cohort of HCWs in South Africa. The findings from this study might improve our understanding of the humoral immune response to SARS-CoV-2 among the African population.
2. Materials and Methods
Study Design and Settings
This prospective cohort study was nested within the larger study, the Eastern Cape Healthcare Workers Acquisition of the SARS-CoV-2 (ECHAS) study, which aimed at determining the cumulative incidence of SARS-CoV-2 infection among HCWs in the Eastern Cape, South Africa (21). The full methodology of the study had been published previously (22). Briefly, this study was conducted in two academic hospitals in the central region of the Eastern Cape (South Africa): Frere and Cecilia Makiwane Hospitals. These two hospitals serve a combined population of about three million people residing in four district municipalities: Buffalo City Metropolitan, Amathole, Joe Gqabi and Chris Hani districts.
Participants and Procedure
Participants in this nested cohort study were recruited in two phases prior to the vaccine roll out in South Africa. In Phase 1, all the HCWs workers in the ECHAS study who self-reported a diagnosis of COVID-19 and were confirmed to have a positive SARS-CoV-2 reverse transcription-polymerase chain reaction (RT-PCR) result (N = 390), were selected. Between November and December 2020, a trained research nurse collected 5mL of venous blood from each participant for SARS-CoV-2 anti-N IgG antibody testing using aseptic technique. Participants who tested positive for SARS-CoV-2 anti-N IgG antibodies were considered for follow-up after two months.
In the Phase 2, participants with persistent SARS-CoV-2 anti-N IgG antibody at the end of Phase 1 were invited telephonically for the follow-up study, and dates and time were agreed with the research team. A trained research nurse collected another 5mL of venous blood for SARS-CoV-2 anti-N IgG antibody testing using aseptic technique. In the event of a difficult venipuncture, one of the doctors belonging to the research team collected the blood sample. Participants also answered questions on whether they had been diagnosed with COVID-19 within the previous two months.
Laboratory Testing
All venous blood samples were tested by the National Health Laboratory Service (NHLS) for IgG antibodies against the SARS-CoV-2 nucleocapsid protein in accordance with standard protocols. Serum samples were analysed on an Abbott ARCHITECT i1000SR instrument using the Abbott SARS-CoV-2 IgG assay in accordance with the manufacturer’s instructions. This is a chemiluminescent microparticle immunoassay for the qualitative detection of IgG against the SARS-CoV-2 nucleocapsid protein. The strength of response in relative light units reflects the quantity of IgG present which is compared with a calibrator to determine the calculated index (specimen/calibrator) for a sample (with positive at 1.4 or greater). This assay has a sensitivity of 100% at 17 days after symptom onset and 13 days after PCR positivity, and a specificity of 99.9% from 1020 pre-COVID-19 serum specimens (23).
All results were captured on the REDCap online database of the South African Medical Research Council server. This database housed the baseline dataset from the ECHAS study, by assigning unique identifying numbers to encode the participants’ personal information (names and dates of births) but also allowing for linkage of the follow-up data. This process ensures confidentiality and privacy of medical information.
Main Outcome Measures
The main outcome measure was the persistence of SARS-CoV-2 anti-N IgG antibodies at the end of Phase 1 (categorised as; 1-3 months, 4-5 months and 6-7 months) and Phase 2 (7-8 months) following natural infection. In addition, factors associated with persistence of IgG antibodies were examined by extracting participants’ demographic and clinical characteristics from the ECHAS database.
The following explanatory variables were extracted from the database: age, gender, ethnicity, place of residence, highest level of education, smoking status and certain comorbidities – human immunodeficiency virus (HIV), hypertension, chronic kidney disease, DM, obesity, and other cardiovascular diseases (CVDs). These comorbid conditions have been shown to be associated with more severe disease or mortality (24–28). In addition, the severity of COVID-19 disease was coded as severe if HCWs received oxygen or were admitted to the hospital.
Data Analysis
Data were exported from the REDCap online database into the IBM SPSS Version 27.0 software (IBM SPSS, Chicago, Illinois, USA) and cross-checked for accuracy. Demographic characteristics of the study participants were summarised using descriptive statistics: counts and proportions for categorical data and means for continuous data. Given the variable intervals between RT-PCR confirmed infections among the 390 participants and the first assay of SARS-CoV-2 anti-N IgG antibodies (Phase 1), the durability of these antibodies was assessed and categorised as: 1–3 months, 4–5 months and 6–7 months. The mean durability of SARS-CoV-2 anti-N IgG was also reported. Similarly, the extended durability of the SARS-CoV-2 anti-N IgG was tested at 7–8 months among 202 participants (Phase 2) who had persistent antibodies at the end of Phase 1.
We performed Chi-square test to assess the association between the baseline characteristics and the persistence of SARS-CoV-2 anti-N IgG antibodies at 4-5 months and 6-7 months in Phase 1 and extended duration of 7-8 months in Phase 2. We excluded the 1-3 months from further analysis due to the small number of participants in this category (n=31). Subsequently, we performed multivariate logistic regression model analysis fitted with a 95% confidence interval (95% CI) after controlling for other covariates to assess the independent association of participants’ characteristics and persistence of SARS-CoV-2 IgG antibodies at 4-5 months, 6-7 months and extended duration of 7-8 months. A two-tailed p value of less than 0.05 was considered statistically significant.
3. Results
Characteristics of the Participants (Phase 1)
A total of 390 HCWs who had tested RT-PCR positive for COVID-19 during the first wave of infection were enrolled in this study. The cohort comprises 332 female (85.12%) and 325 black participants (83.33%). Most of the study participants (72.82%) were classified as obese (BMI ≥30 kg/m
2), had tertiary education (73.33%), and never smoked cigarette (94.87%) (
Table 1).
SARS-CoV-2 anti-N IgG Antibody Durability
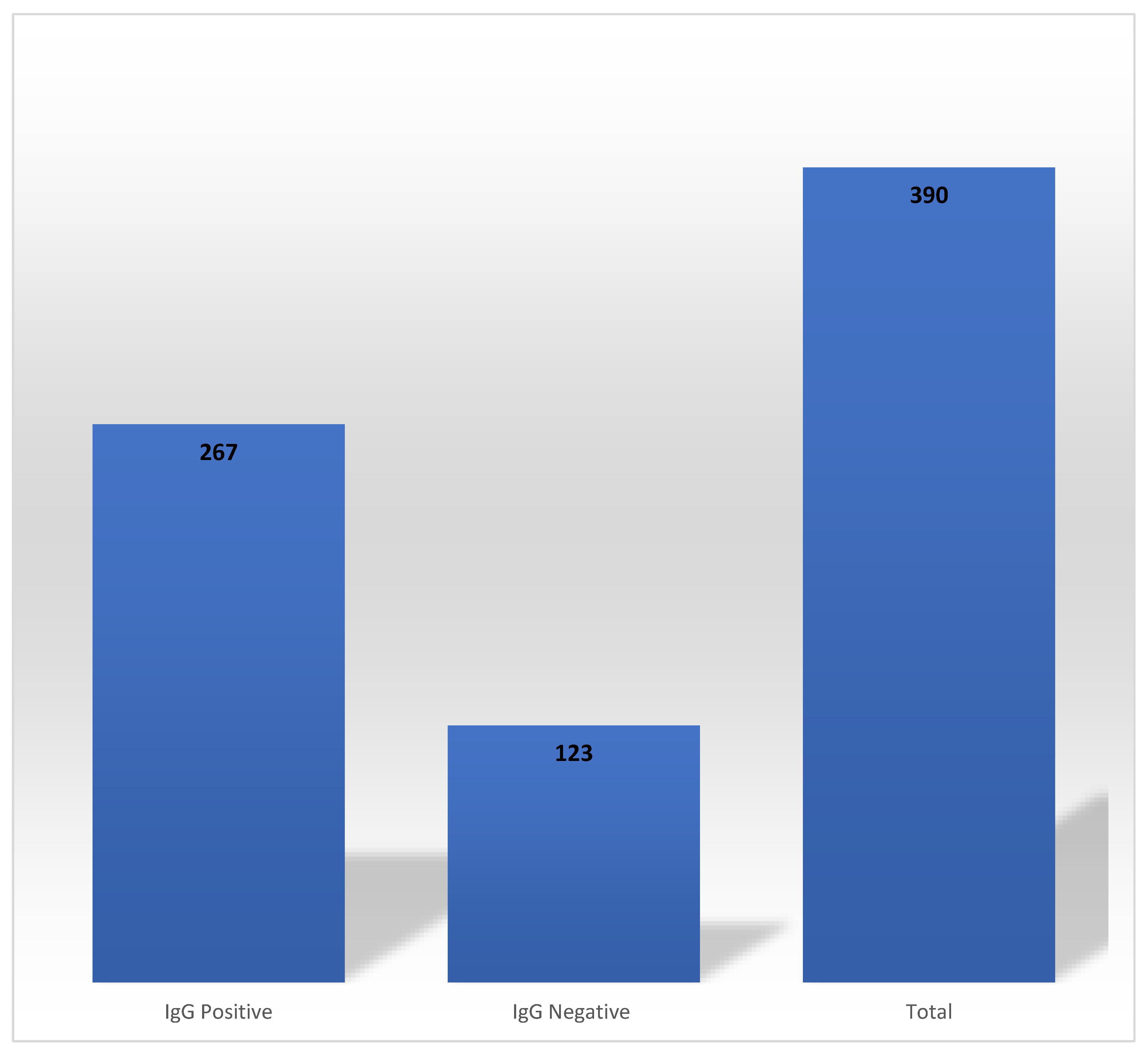
Out of 390 HCWs who had tested RT-PCR positive for COVID-19 at baseline, 68.46% (n = 267) had detectable SARS-CoV-2 anti-N IgG antibodies at the end of Phase 1 (
Figure 1). These antibodies persisted for 1-3, 4-5 and 6-7 months in 7.49% (n = 20), 76.40% (n = 204) and 16.10% (n = 43), respectively. The mean durability of SARS-CoV-2 anti-N IgG antibodies was 143 days (4.67 months).
Factors Associated with Antibody Durability
In the Chi-square analysis, age (
p <0.001), hypertension (
p = 0.013), and obesity (
p = 0.027) showed a significant association with SARS-CoV-2 anti-N IgG antibody durability for 4-5 months. Besides obesity (
p <0.001), there were no associations established between the selected variables and SARS-CoV-2 anti-N IgG antibody durability at 6-7 months (
Table 2).
In the multivariate logistic regression model analysis, black participants were more likely to sustain SARS-CoV-2 IgG antibodies for 4-5 months (adjusted odds ratio [AOR], 7.85; 95% confidence interval [CI] 1.49 - 41.14; p=0.02). Participants who were human immunodeficiency virus (HIV) positive were less likely to sustain SARS-CoV-2 anti-N IgG antibodies for 4-5 months (AOR=0.16; 95% CI 0.03-0.76; p=0.02). In addition, individuals who were <45 years of age were more likely to sustain SARS-CoV-2 IgG antibodies for 6-7 months (AOR, 3.38; 95% CI, 1.02-11.22;
p = 0.046). Other selected variables were not associated with SARS-CoV-2 anti-N IgG antibody persistence (
Table 3).
Phase 2: Extended Antibody Durability (7-8 Months)
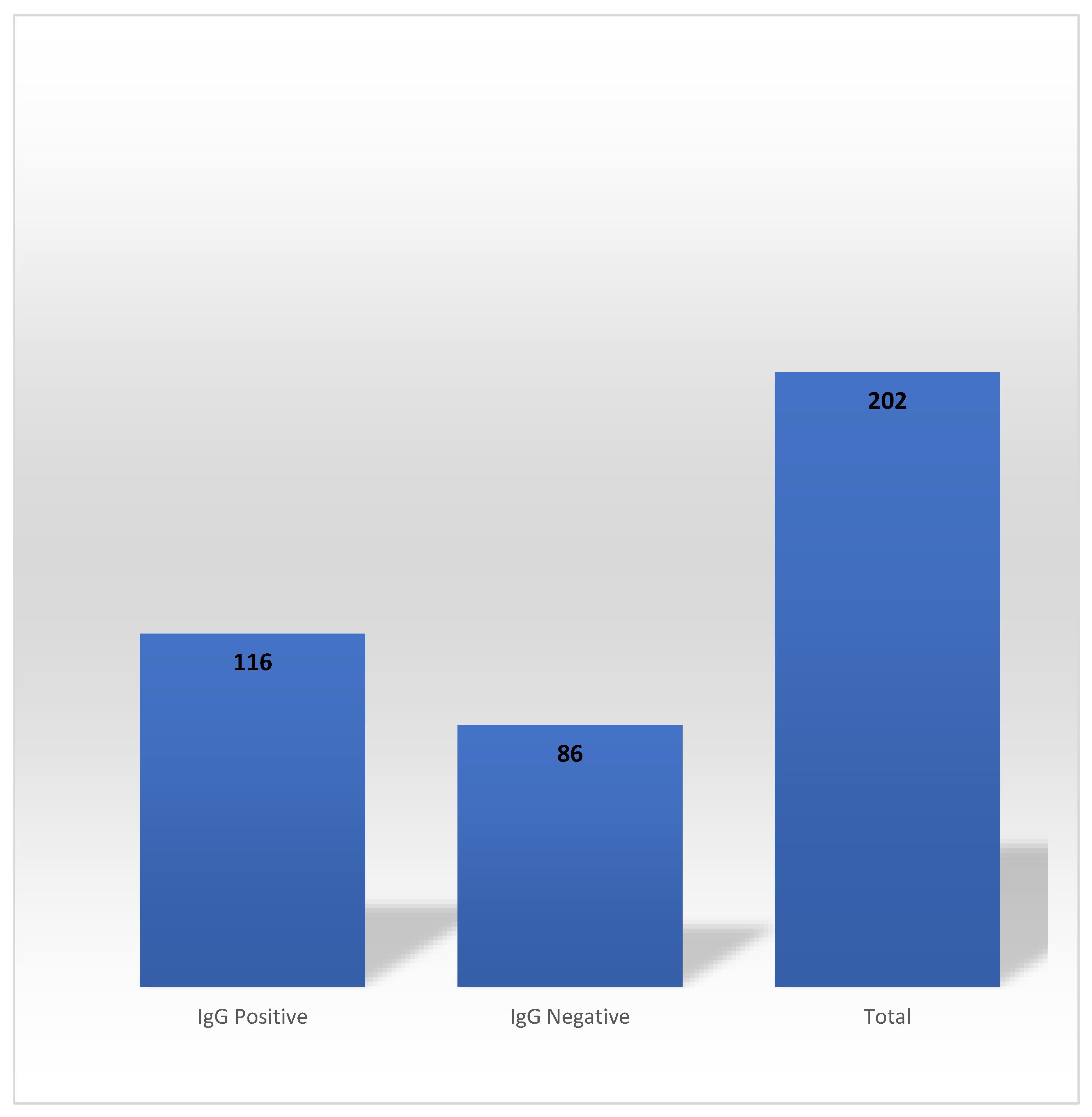
In the Phase 2, 202 HCWs out of 267 who exhibited SARS-CoV-2 anti-N IgG antibody seropositivity during the Phase 1 participated. Persistence of the SARS-CoV-2 anti-N IgG antibody occurred in 57.43% of the participants (n=116) (
Figure 2).
The mean extended durability of SARS-CoV-2 anti-N IgG antibodies was 223 days (7.46 months). Both the Chi-square (
Table 4) and multivariate logistic regression model analysis (
Table 5) showed that the selected variables had no significant associations with the extended durability of SARS-CoV-2 anti-N IgG antibodies.
4. Discussion
One of the key questions in predicting the trajectory of the COVID-19 pandemic and future interventions is how well and how long protective immunity might last after infection or vaccination. However, the exact duration of SARS-CoV-2 immunity after a natural infection is not fully understood. In this study, we investigated the durability of the humoral immune response (SARS-CoV-2 anti-N IgG antibodies) to primary infection, and its association with age, sex, ethnicity, and co-morbidities among South African HCWs.
In our study, the mean durability of SARS-CoV-2 anti-N IgG antibodies was approximately five months. In some individuals, the antibody response persisted for over seven months. Like other studies, we identified a proportion of individuals with RT-PCR-confirmed SARS-CoV-2 who did not have detectable antibodies (29–32). The reasons why some patients with confirmed COVID-19 did not develop antibodies against the virus are unclear but may be related to asymptomatic infection or milder disease (31,32). Nonetheless, our findings add to the growing evidence that SARS-CoV-2 anti-N IgG antibodies can persist for at least three months, and possibly longer, after infection. To the best of our knowledge, the current study is the first to explore the durability of SARS -CoV-2 anti-N IgG antibodies after natural infection in the South African setting.
People with HIV, especially those with advanced disease, have an increased risk of severe complications resulting from SARS-CoV-2 infection independent of sex and age (33). However, limited data exist on natural immunity after SARS-CoV-2 infection among this subgroup. In the current study, HIV positive participants were more likely to be seronegative at 4-5 months. A study conducted by Spinelli et al (34) demonstrated that individuals with HIV had lower neutralising antibody titres and SARS-CoV-2 IgG concentrations after natural infection. These findings suggest that immunosuppressive conditions may influence immune response to SARS-CoV-2 and increase the risk of reinfection.
Demonstrating the ability to mount and sustain appropriate immune response in the presence of DM is important for preventing SARS-CoV-2 re-infection and mortality. In this study, pre-existing DM was associated with a sustained anti-N IgG antibody response. The reason for this observation is unclear, although a previous study reported that DM was associated with higher IgG levels (35), while others reported that individuals with good glycaemic control had less severe COVID-19 pneumonia and lower mortality risk in comparison to those with poor glycaemic control (36,37). Evidence has shown that hyperglycaemia does not impair antibody response against SARS-CoV-2 (37). While more studies are needed to examine the SARS-CoV-2 IgG antibody responses in this subpopulation, our findings create optimism with regard to the durability of SARS-CoV-2 vaccines responses among this subgroup.
The effects of ethnicity/race, age, and disease severity on SARS-CoV-2 IgG antibody longevity remain largely unknown. Unlike similar studies (20,31,38), we did not identify age or disease severity to be associated with a longer lasting immune response. However, black participants were more likely to have detectable antibodies at 4-5 months. We found no record of the longevity of SARS-CoV-2 IgG antibody among black individuals in any study. Even so, other studies reported that non-white races, including African-Americans, may be associated with higher prevalence of antibodies following infection (31,38). Smith et al(39)., reported a significant difference in the breadth and strength of the humoral immune response in relation to ethnicity, which may be attributed to genetic and lifestyle factors. It should be noted that the majority of the participants in the current study were black. Therefore, these findings should be interpreted with caution. Future studies from South Africa should target other racial groups to gain a better understanding of the effect of race on humoral immune response against SARS-CoV-2 infection.
The humoral immune response to SARS-CoV-2 includes antibodies against specific viral antigens such as the nucleocapsid protein and spike (S) protein. The S protein harbours the receptor binding domains (RBD) that allows the virus bind and enter susceptible host cells. Neutralizing antibodies block the RBD. Though, most of the participants in our study developed detectable levels of SARS-CoV-2 anti-N IgG antibodies, it is unclear the extent and duration of protection these antibodies offer against SARS-CoV-2 reinfection. The development of antibodies may result in some level of protection against SARS-CoV-2 reinfection (40). We could not assess the functional ability of the detected antibodies by a neutralisation test due to lack of required resources in our setting. Nevertheless, several studies have shown that individuals with SARS-CoV-2 IgG antibodies are less likely to be reinfected in the short-term than those who lack the antibodies (13,41,42). The emergence of new SARS-CoV-2 variants with potential immune escape calls for further studies to assess the breadth of neutralising antibodies and protection from subsequent infection.
Study Limitations
Our study is geographically specific and includes a relatively small number of HCWs, potentially limiting generalisability to other settings or populations. It is notable, however, that the results of our study are consistent with other studies on the durability of SARS-CoV-2 anti-N IgG antibodies. In addition, we could not assess how SARS-CoV-2 anti-N IgG antibodies correlate with functionality and protection against reinfection.
5. Conclusion
Our study showed sustained humoral immunity in a cohort of African HCWs who recovered from COVID-19. The sustained antibody response is not affected by age, race/ethnicity, comorbidities, or disease severity. Our findings support the longevity of vaccine responses against SARS CoV-2 in black Africans. However, periodic vaccinations with updated vaccines or boosters may be required, as they are for seasonal influenza.
Author Contributions
OVA conceptualised, designed and implemented the study protocol. CM analysed the data. OVA, CM and OCD jointly drafted the paper, critical revised and approved the final draft for submission for publication.
Funding
The research project was supported by the South African Medical Research Council (SAMRC) Grant (Reference; 0000062597106824), and the Walter Sisulu University Faculty of Health Sciences Personal Research Publication Funds (OVA).
Institutional Review Board Statement
This study received approvals from the Walter Sisulu University Faculty of Health Sciences Ethics Committee (Reference: 087/2020) and Cecilia Makiwane and Frere Hospitals Research Ethics Committee (FCMHREC/A068/2020). In addition, the clinical governance committees of the two hospitals granted permission for the implementation of the study protocols.
Informed Consent Statement
All participants gave written informed consent for this nested cohort study in addition to the main study. Privacy and confidentiality of medical information were maintained during and after the study. The study was implemented in accordance with the Helsinki Declaration and Good Clinical Practice governing human research.
Data Availability Statement
The dataset analysed in this study are available with the corresponding author upon reasonable written request.
Acknowledgments
The authors are grateful to the management and staff of the two hospitals for the warm embrace of our project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Coronavirus disease (COVID-19) – World Health Organization [Internet]. [cited 2021 Sep 14]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
- Tegally H, Wilkinson E, Giovanetti M, Iranzadeh A, Fonseca V, Giandhari J, Doolabh D, Pillay S, San EJ, Msomi N, Mlisana K. Emergence and rapid spread of a new severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) lineage with multiple spike mutations in South Africa. Nature. 2021;592(7854):438-443.
- Li Q, Wu J, Nie J, Zhang L, Hao H, Liu S, et al. The Impact of Mutations in SARS-CoV-2 Spike on Viral Infectivity and Antigenicity. Cell. 2020 Sep 3;182(5):1284-1294.e9.
- Scudellari, M. How the pandemic might play out in 2021 and beyond. Nature. 2020 Aug 5;584(7819):22–5. [CrossRef]
- Sanyaolu A, Okorie C, Marinkovic A, Patidar R, Younis K, Desai P, et al. Comorbidity and its Impact on Patients with COVID-19. Sn Compr Clin Med. 2020 Jun 25;1–8. [CrossRef]
- Varghese E, Samuel SM, Liskova A, Kubatka P, Büsselberg D. Diabetes and coronavirus (SARS-CoV-2): Molecular mechanism of Metformin intervention and the scientific basis of drug repurposing. PLOS Pathog. 2021 Jun 22;17(6):e1009634.
- Ni W, Yang X, Yang D, Bao J, Li R, Xiao Y, et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit Care. 2020 Jul 13;24(1):422. [CrossRef]
- Cooper SL, Boyle E, Jefferson SR, Heslop CRA, Mohan P, Mohanraj GGJ, et al. Role of the Renin–Angiotensin–Aldosterone and Kinin–Kallikrein Systems in the Cardiovascular Complications of COVID-19 and Long COVID. Int J Mol Sci. 2021 Jan;22(15):8255.
- Lim S, Bae JH, Kwon HS, Nauck MA. COVID-19 and diabetes mellitus: from pathophysiology to clinical management. Nat Rev Endocrinol. 2021 Jan;17(1):11–30. [CrossRef]
- Jacofsky D, Jacofsky EM, Jacofsky M. Understanding Antibody Testing for COVID-19. J Arthroplasty. 2020 Jul;35(7):S74–81. [CrossRef]
- Huang AT, Garcia-Carreras B, Hitchings MDT, Yang B, Katzelnick LC, Rattigan SM, et al. A systematic review of antibody mediated immunity to coronaviruses: kinetics, correlates of protection, and association with severity. Nat Commun. 2020 Sep 17;11(1):4704. [CrossRef]
- Bichara CDA, da Silva Graça Amoras E, Vaz GL, da Silva Torres MK, Queiroz MAF, do Amaral IPC, et al. Dynamics of anti-SARS-CoV-2 IgG antibodies post-COVID-19 in a Brazilian Amazon population. BMC Infect Dis. 2021 Dec;21(1):443.
- Hall VJ, Foulkes S, Charlett A, Atti A, Monk EJM, Simmons R, et al. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: a large, multicentre, prospective cohort study (SIREN). Lancet Lond Engl. 2021 Apr 17;397(10283):1459–69.
- Abu-Raddad LJ, Chemaitelly H, Coyle P, Malek JA, Ahmed AA, Mohamoud YA, Younuskunju S, Ayoub HH, Al Kanaani Z, Al Kuwari E, Butt AA. SARS-CoV-2 antibody-positivity protects against reinfection for at least seven months with 95% efficacy. EClinicalMedicine. 2021 May 1 ;35:100861.
- Barquera R, Collen E, Di D, Buhler S, Teixeira J, Llamas B, et al. Binding affinities of 438 HLA proteins to complete proteomes of seven pandemic viruses and distributions of strongest and weakest HLA peptide binders in populations worldwide. HLA. 2020 Sep;96(3):277–98. [CrossRef]
- Nguyen A, David JK, Maden SK, Wood MA, Weeder BR, Nellore A, et al. Human Leukocyte Antigen Susceptibility Map for Severe Acute Respiratory Syndrome Coronavirus 2. J Virol. 2020 Jun 16;94(13):e00510-20. [CrossRef]
- Benton ML, Abraham A, LaBella AL, Abbot P, Rokas A, Capra JA. The influence of evolutionary history on human health and disease. Nat Rev Genet. 2021 May;22(5):269–83. [CrossRef]
- Saad-Roy CM, Wagner CE, Baker RE, Morris SE, Farrar J, Graham AL, et al. Immune life history, vaccination, and the dynamics of SARS-CoV-2 over the next 5 years. Science. 2020 Nov 13;370(6518):811–8.
- Kist NC, Lambert B, Campbell S, Katzourakis A, Lunn D, Lemey P, et al. HIV-1 p24Gag adaptation to modern and archaic HLA-allele frequency differences in ethnic groups contributes to viral subtype diversification. Virus Evol. 2020 Jul;6(2):veaa085. [CrossRef]
- Ibarrondo FJ, Fulcher JA, Goodman-Meza D, Elliott J, Hofmann C, Hausner MA, et al. Rapid Decay of Anti–SARS-CoV-2 Antibodies in Persons with Mild Covid-19. N Engl J Med. 2020 Sep 10;383(11):1085–7.
- Stead D, Adeniyi OV, Singata-Madliki M, Abrahams S, Batting J, Jelliman E, et al. Cumulative incidence of SARS-CoV-2 and associated risk factors among healthcare workers: a cross-sectional study in the Eastern Cape, South Africa. BMJ Open. 2022 Mar 18;12(3):e058761.
- Adeniyi OV, Stead D, Singata-Madliki M, Batting J, Hyera L, Jelliman E, et al. Eastern Cape Healthcare Workers Acquisition of SARS-CoV-2 (ECHAS): Cross-Sectional (Nested Cohort) Study Protocol. Int J Environ Res Public Health. 2021 Jan 5;18(1):E323.
- Bryan A, Pepper G, Wener MH, Fink SL, Morishima C, Chaudhary A, et al. Performance Characteristics of the Abbott Architect SARS-CoV-2 IgG Assay and Seroprevalence in Boise, Idaho. J Clin Microbiol. 2020 Jul 23;58(8):e00941-20.
- Nguyen LH, Drew DA, Graham MS, Joshi AD, Guo CG, Ma W, et al. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Health. 2020 Sep;5(9):e475–83. [CrossRef]
- Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020 Mar 26;382(13):1199–207.
- Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020 Apr 30;382(18):1708–20. [CrossRef]
- Iversen K, Bundgaard H, Hasselbalch RB, Kristensen JH, Nielsen PB, Pries-Heje M, et al. Risk of COVID-19 in health-care workers in Denmark: an observational cohort study. Lancet Infect Dis. 2020 Dec;20(12):1401–8. [CrossRef]
- Pillay-van Wyk V, Bradshaw D, Groenewald P, Seocharan I, Manda S, Roomaney RA, et al. COVID deaths in South Africa: 99 days since South Africa’s first death. South Afr Med J Suid-Afr Tydskr Vir Geneeskd. 2020 Oct 1;110(11):1093–9. [CrossRef]
- Wajnberg A, Amanat F, Firpo A, Altman DR, Bailey MJ, Mansour M, McMahon M, Meade P, Mendu DR, Muellers K, Stadlbauer D. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020 Dec 4;370(6521):1227-30.
- Isho B, Abe KT, Zuo M, Jamal AJ, Rathod B, Wang JH, et al. Persistence of serum and saliva antibody responses to SARS-CoV-2 spike antigens in COVID-19 patients. Sci Immunol. 2020 Oct 8;5(52):eabe5511.
- Petersen LR, Sami S, Vuong N, Pathela P, Weiss D, Morgenthau BM, et al. Lack of antibodies to SARS-CoV-2 in a large cohort of previously infected persons. Clin Infect Dis Off Publ Infect Dis Soc Am. 2020 Nov 4;ciaa1685.
- Liu X, Wang J, Xu X, Liao G, Chen Y, Hu CH. Patterns of IgG and IgM antibody response in COVID-19 patients. Emerg Microbes Infect. 2020 Dec;9(1):1269–74. [CrossRef]
- Dauby N, Martin C. SARS-CoV-2 immunity and HIV infection: total recall? Lancet HIV. 2021 Jun 1;8(6):e312–3.
- Spinelli MA, Lynch KL, Yun C, Glidden DV, Peluso MJ, Henrich TJ, et al. SARS-CoV-2 seroprevalence, and IgG concentration and pseudovirus neutralising antibody titres after infection, compared by HIV status: a matched case-control observational study. Lancet HIV. 2021 Jun 1;8(6):e334–41. [CrossRef]
- Huang M, Lu QB, Zhao H, Zhang Y, Sui Z, Fang L, et al. Temporal antibody responses to SARS-CoV-2 in patients of coronavirus disease 2019. Cell Discov. 2020 Sep 15;6(1):1–4.
- Shauly-Aharonov M, Shafrir A, Paltiel O, Calderon-Margalit R, Safadi R, Bicher R, et al. Both high and low pre-infection glucose levels associated with increased risk for severe COVID-19: New insights from a population-based study. PLOS ONE. 2021 Jul 22;16(7):e0254847. [CrossRef]
- Lampasona V, Secchi M, Scavini M, Bazzigaluppi E, Brigatti C, Marzinotto I, et al. Antibody response to multiple antigens of SARS-CoV-2 in patients with diabetes: an observational cohort study. Diabetologia. 2020 Dec 1;63(12):2548–58.
- Staines HM, Kirwan DE, Clark DJ, Adams ER, Augustin Y, Byrne RL, Cocozza M, Cubas-Atienzar AI, Cuevas LE, Cusinato M, Davies BM. IgG seroconversion and pathophysiology in severe acute respiratory syndrome coronavirus 2 infection. Emerging infectious diseases. 2021 Jan;27(1):85. [CrossRef]
- Smith M, Abdesselem HB, Mullins M, Tan TM, Nel AJM, Al-Nesf MAY, et al. Age, Disease Severity and Ethnicity Influence Humoral Responses in a Multi-Ethnic COVID-19 Cohort. Viruses. 2021 Apr 28;13(5):786. [CrossRef]
- Kopel J, Perisetti A, Roghani A, Aziz M, Gajendran M, Goyal H. Racial and Gender-Based Differences in COVID-19. Front Public Health. 2020;8:418. [CrossRef]
- Lumley SF, O’Donnell D, Stoesser NE, Matthews PC, Howarth A, Hatch SB, et al. Antibody Status and Incidence of SARS-CoV-2 Infection in Health Care Workers. N Engl J Med. 2021 Feb 11;384(6):533–40. [CrossRef]
- Jeffery-Smith A, Iyanger N, Williams SV, Chow JY, Aiano F, Hoschler K, et al. Antibodies to SARS-CoV-2 protect against re-infection during outbreaks in care homes, September and October 2020. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull. 2021 Feb;26(5).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).