1. Introduction
In modern medicine, mesenchymal stem cells (MSCs) are widely used as a therapeutic remedy: in fact, these multipotent cells are able to self-regenerate and differentiate towards cells of the mesodermal lineage, i.e. bone, cartilage, and fat cells [
1]. MSCs, alone or integrated with scaffolds, can be used in the treatment of various pathologies. The efficacy of these cells has been evaluated in wound therapy [
2], cartilage and bone dehiscence [
3], graft rejection disease (GVHD) [
4], cardiovascular [
5], and neural disorders [
6]. The most studied MSCs are those residents in the bone marrow. However, bone marrow harvesting implies physical pain and is psychologically demanding for the donor, so other sources of MSCs, such as dental tissues, were investigated to overcome these complications. MSCs can derive from both mature and immature dental tissues [
7]. Dental pulp stem cells (DPSCs) are usually extracted from the pulp of the wisdom tooth, while dental bud stem cells (DBSCs) come from the immature and therefore not yet calcified form of the tooth, i.e. the Dental Bud (DB) [
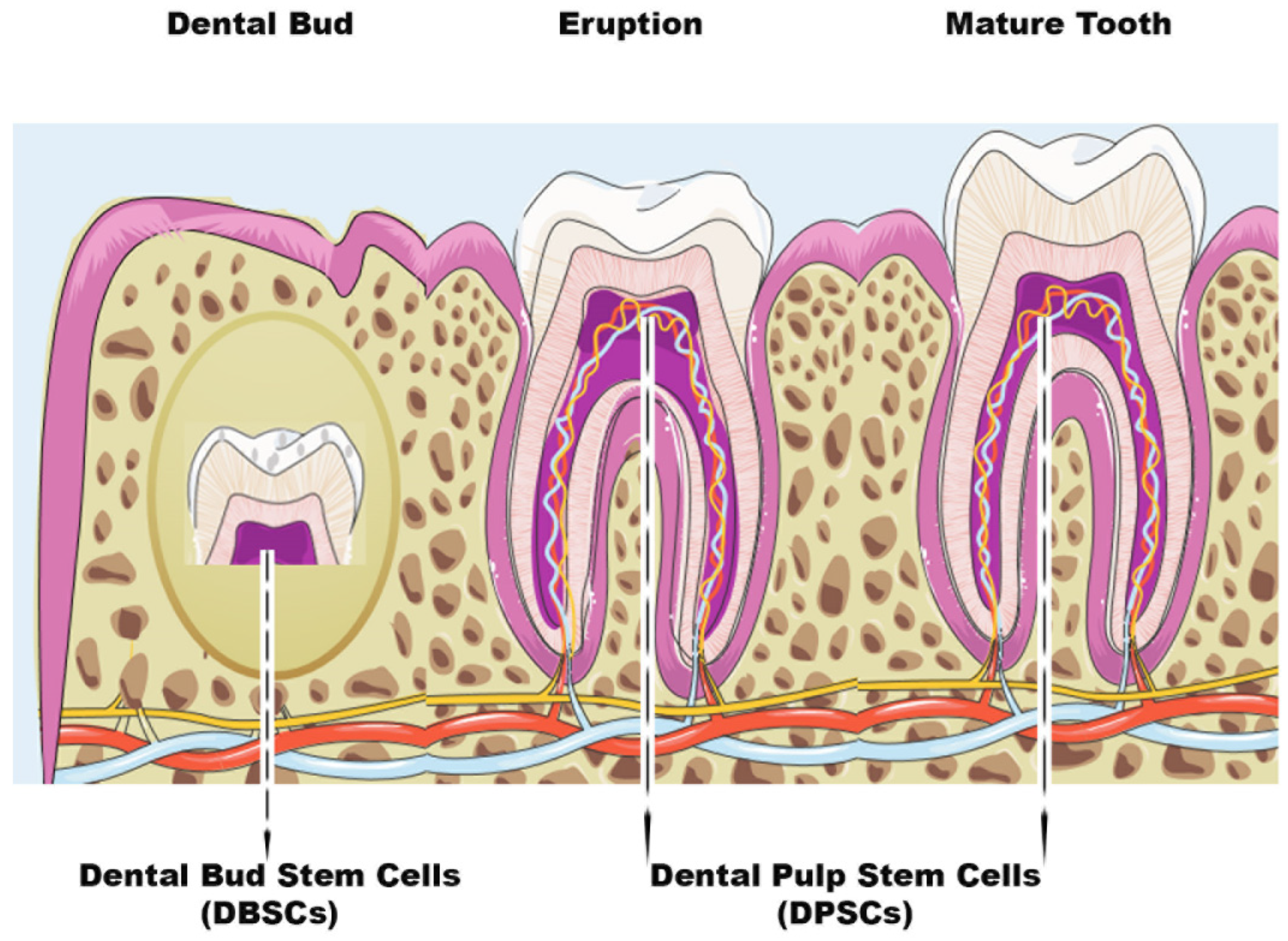
8] (
Figure 1). DBSCs and DPSCs express Nanog, OCT4, Sox2, c-Myc, and Klf4, transcription factors responsible for the pluripotency of stem cells, as they regulate multiple processes such as self-renewal, the expression of specific genes responsible for the differentiation in diverse tissues and the transformation of stem cells into mature cells [
8]. These stem cells have been seen as an attractive way to repair and regenerate damaged tissue, among not only dentists and orthopedists but also in the regenerative medicine community; they are a valid alternative solution to be used for bone regeneration thanks to their biological characteristics. Research has definitively demonstrated the differentiation of both bone and cartilage from MSCs. However, the reconstruction of these hard tissues is still a challenge for regenerative medicine because, to date, treatments are not yet fully effective and not always feasible. In this review, we present the study of molecules, of natural and non-natural compounds, and their effects on the differentiation of cells from dental bud and dental pulp: it may provide the right input to continue the search for new molecules to be used for the bioengineering of bone.
Figure 1.
Scheme of Dental Bud Stem Cells (DBSCs) and Dental Pulp Stem Cells (DPSCs). The figure summarizes the stages of tooth growth. As discussed in the review, DBSCs can be obtained from the Dental Bud and DPSCs can be isolated both at the Eruption or Mature stage of the tooth.
Figure 1.
Scheme of Dental Bud Stem Cells (DBSCs) and Dental Pulp Stem Cells (DPSCs). The figure summarizes the stages of tooth growth. As discussed in the review, DBSCs can be obtained from the Dental Bud and DPSCs can be isolated both at the Eruption or Mature stage of the tooth.
2. DPSCs
The dental pulp (DP) is a connective tissue located in the center of the tooth and highly vascularized. It is encircled by dentin, a mineralized hard tissue, and includes several cell types, such as undifferentiated progenitor cells and odontoblasts [9-15]. The DP derives from multipotent mesenchymal cells of the cranial neural crest, which migrate, during early embryonic development, towards the first and second branchial arch [
12]. This dental tissue contains numerous stem cells in the perivascular niche, just like in the embryonic mesenchyme [
16]. Postnatal stem cells possess immense potential for tissue regeneration and they constantly work to maintain homeostasis. The identification of these stem cells, in the orofacial system, occurred after the 2000s, when they were first discovered in the DP and called dental pulp stem cells (DPSCs). Gronthos
et al. were the first to identify and isolate DPSCs [
14]. DPSCs come from the DP of permanent teeth and are usually extracted from wisdom teeth because third molars result in an extra mostly of little use for chewing and often there is not enough space for their eruption; thus they can be extracted without critical problems and their DP tissue can be easily isolated. Grottkau
et al. demonstrated the potential of these newly isolated cells that can self-renew, proliferate and differentiate into cell lines such as osteoblasts, chondrocytes, adipocytes, neurocytes, and smooth muscle cells [
15]. Recent studies show that DPSCs have the capacity,
in vitro, to differentiate into bone cells and,
in vivo studies, to form mineralized tissue [
17]. These data suggest that DPSCs may have great value for dental and periodontal tissue engineering and efficacy on bone regeneration. Many scientists have considered these cells, thanks to their ability to differentiate into many cell types, a useful source of pluripotent stem cells to deepen the knowledge of multilineage differentiation
in vitro and to adopt them for therapeutic applications.
3. Differentiative role of natural molecules in DPSCs
Different molecules, such as vitamins, natural compounds, or drugs positively influence DPSC differentiation. The discovery of the activity of these molecules has helped researchers to know and better understand the osteogenic characteristics of these stem cells. Below we describe some molecules of natural and non-natural compounds, studied in recent years, associated with the DPSC osteogenic differentiation and activity.
3.1. Resveratrol
Several studies have been conducted on natural antioxidant molecules, such as resveratrol (RSV), present in grape skin, cranberries, peanuts, and root extracts of Polygonum cuspidatum [
18]. A variety of biological functions of RSV have already been studied. It often acts as an important and functional activator of Sirtuin 1 (Sirt1) [
19] and studies have been carried out on it in the field of traditional medicine
[20,21].
For the first time Feng et al. [
22], studied the effects of Sirt1 in DPSCs using RSV to favor the function of Sirt1 and siRNA to silence Sirt1 gene expression. The silencing, regardless of the presence of RSV, led to a reduced expression of Sirt1. Furthermore, RSV was able to up-regulate the expression of Runx-2, BMP2, and Collagen I (Col I), while this process would have been weakened with the siRNA transfection of Sirt1. Interestingly the Alkaline Phosphatase (ALP) staining result confirmed RSV capacity in stimulating DPSC osteogenic differentiation. This study validates the function of RSV in promoting osteogenic differentiation of DPSCs via Sirt1 [
22].
It is well known that RSV shows antioxidant properties [
20]; in this regard, Zhang et al. investigated its capacity to induce osteogenic differentiation of DPSCs in the presence of oxidative stress [
23]. Their data confirmed RSV ability to potentiate osteogenesis and to be an antioxidant for DPSCs. The study showed an upregulation of Sirt1/Nrf2 expression, which appeared to be reduced due to oxidative stress, and osteogenic markers such as Runx-2 and Osteocalcin (OCN) were rescued by the treatment with RSV. A similar pattern for Sirt1 expression was observed in in vivo experiments where a potentiation of the bone matrix and collagen was noted in mice that had defects in the cranial structure. These results confirm the role of RSV as a promoter of bone formation and enhancer of DPSC proliferation; moreover, they provide useful information for regenerative medicine and for the development of bone therapies based on RSV [
23].
Although RSV represents a molecule particularly suitable for bone regeneration therapies, its characteristics make it difficult to study and deepen its effects in vivo through oral administration [
24]. As a matter of fact, this molecule has little solubility in water, deficient pharmacokinetics, and rapid metabolism. Therefore, to overcome these limitations, alternative administration methods became necessary to favour a direct RSV action on the site to be treated.
A new strategy currently in use is represented by electrospinning membranes, with biodegradable characteristics, which can be combined with the molecule and released in a controlled manner on the affected area that presents a bone defect. There are still few works that have employed RSV with this type of membrane [
25,
26].
Riccitiello
et al. were the first to use and study some polymers for electrospinning membranes, to be used as ideal substrates to administer RSV, evaluating its effect on osteoblasts and osteoclasts [
27]. They designed two materials: poly (ε-caprolactone) PCL and poly (lactic) acid (PLA). These materials have already been studied in the past both on bone marrow MSCs, where they led to an accretion of them [
28], and on DPSCs that differentiated into osteoblast-like cells, overexpressing the most common markers of bone formation [
29]. The authors loaded RSV on these substrates and showed that the release of the molecule on the affected area in a late manner favored the differentiation of DPSCs into osteoblast-like cells and blocked osteoclastogenesis. This study gives an interesting starting point for regenerative medicine as these materials, associated with RSV, can provide useful support to repair bone defects, and therefore help bone formation, counteracting resorption [
27].
3.2. Flavonoids
Among the various studies on natural molecules, it has emerged that some flavonoids also promote osteoblast differentiation [30-32].
Taxifolin (dihydroquercetin) is the most common flavonoid and its presence has been demonstrated in a wide range of foods [
33,
34]. This compound shows many beneficial biological and pharmacological properties: it displays anti-inflammatory, antioxidant, anti-apoptotic, and anti-cancer effects [35-38]. These healthful features prompted the researchers to find out whether taxifolin could protect DPSCs from apoptosis and be involved in osteogenic differentiation process. Fu
et al. revealed the surprising effects of the flavonoid on DPSCs highlighting its synergistic action with carbonic anhydrase IX (CA9), an enzyme which is often related to the aggressiveness and prognosis of many cancers. The study pointed out the combined effect of the two molecules in inhibiting DPSC apoptosis under inflammation and hypoxia conditions. Silencing CA9 nullifies the effect of Taxifolin on DPSCs [
33]. These data lay the bases for the possible use of anti-apoptotic and hypoxia-fighting molecules to prevent the reduction of osteogenic differentiation in inflammatory conditions. The natural molecule taxifolin could be used for bone regeneration starting from a MSCs source, and could protect DPSC osteogenic differentiation in inflammatory conditions.
3.2.1. Chrysin
Chrysin (5,7-dihydroxyflavone) is a natural flavonoid which can be extracted from the seeds of
Oroxylum indicum and has a wide pharmacological activity [
39]. Several studies have shown that Chrysin possesses anti-inflammatory and anti-carcinogenic properties [40-46]. It is one of the polyphenolic compounds abundant in propolis, which inhibits osteoclast maturation and reduces bone resorption [
47]. Studies on this flavonoid have been conducted in the past demonstrating its effect in enhancing the proliferation and osteogenic differentiation of MSCs [45, 48]. An
in vitro study demonstrated that Chrysin induces osteogenic differentiation and mineralization by activation of Erk1/2 signaling in the MC3T3-E1 cell line [
40], but its role on DPSCs was unclear, this aspect was then investigated by Huo
et al. [
39]. They experimented
in vivo grafting of scaffolds with DPSCs integrated on β-tricalcium phosphate (β – TCP), treated or not with Chrysin, in nude mice and repeated the same experiment on a rat model presenting a cranial defect. It emerged that Chrysin stimulated DPSCs leading to the abundant bone formation on both models tested. Furthermore, Huo and colleagues confirmed that Chrysin upregulates the expression of OCN, Runx-2 and Col I proteins in DPSCs, thus demonstrating that the flavonoid enhances the osteogenic differentiation process [
39]. This study could be useful in bioengineering techniques and regenerative medicine in fact, it has indicated new patterns and new natural molecules to counteract bone defects.
3.2.2. Prenylflavonoids
Prenylflavonoids are a class of compounds found in a variety of plants and known for their anti-inflammatory, antioxidant, and osteogenic effects [
49]. Several types of prenylflavonoids have been identified in the fruit of
Macaranga tanarius and, among them, nymphaeol B, isonymphaeol B (INB) and 3'-geranyl-naringenin have no cytotoxic effect on DPSCs [
50]. Nam
et al. identified in INB the most efficient compound in stimulating DPSC differentiation and in inducing mineralization in a dose-dependent manner. The same
in vitro study proved that INB directly affected DPSC differentiation: the treatment for one week with this prenylflavonoid increased the expression of odontogenesis marker genes such as BSP, DMP1 and DSPP. Likewise, after two weeks, there was also an increase of Runx-2 expression levels. INB effect on mineralization was confirmed by
in vivo experiments; the tooth bud INB treatment of post-natal day five (PN5) ICR mice significantly induced tooth root elongation when transplanted into sub-renal capsules of six-week-old mice, compared with untreated tooth buds. These data confirmed that INB effectively promotes dentin formation by odontoblasts [
50]. These findings suggest that prenylflavonoids may have potential applications in the field of bone bioengineering.
3.2.3. Hesperetin
Citrus fruits are known to have beneficial effects on health. In particular, oranges, lemons, and grapefruits have been demonstrated to contain a flavanone called hesperetin (HS) which belongs to a subclass of flavonoids and presents antioxidant, anti-inflammatory, antimicrobial, and antiviral properties [
51]. This bioflavonoid has been clinically tested showing the capacity to promote hard tissues repair: it can be used to treat bone fractures and as a long-term preventive treatment of arthritis; HS also helps to maintain bone density and reduce the severity of joint pain [
52]. Furthermore, HS has been shown to suppress the expression of the inflammation marker p65, suggesting that HS may be effective in the treatment against inflammation in preclinical and clinical models [
53]. One strategy that is currently applied to make the best use of natural molecules is represented by nanotechnology. In fact, pharmacologically active molecules often have dimensions and characteristics that are not always suitable for the administration, therefore it is possible to exploit nanotechnology to improve their bioavailability. Research has recently focused on nanomedicine, which uses nanoparticles as vectors for drug delivery, allowing treatment to be more effective than conventional methods [
54]. Alipour
et al. have taken advantage of this kind of approach evaluating the osteoinductive ability of HS on DPSCs through nano-HS particles. They created nanoparticles of HS, obtained using a spray dryer, from a solution of HS and acetone, and subsequently in sodium dodecyl sulfonate. Then they treated DPSCs with HS administered as soluble molecule or nano-HS and observed that, in both treatments, DPSCs proliferated without undergoing any cytotoxic effect. In addition, when low concentrations of nano-HS (0.5 and 1 μM) were used, DPSCs showed a remarkable proliferation. Furthermore, HS nanoparticles enhanced the expression of key osteogenic markers (Col I, BSP, Runx-2, OCN and ALP), as well as mineral matrix deposition, compared to HS-treated and untreated groups [
55]. As previously mentioned, similarly to other flavonoids, HS plays an anti-inflammatory role [
51] and Alipour and colleagues found that HS nanoparticles reduced inflammation in DPSCs compared to the treatment with HS [
55]. These studies confirm that flavonoids have proliferative effects on MSCs at low concentrations and, furthermore, their osteogenic activity could be improved by the application of approaches at the nanoscale level.
3.3. Curcumin
Curcumin is a polyphenol found in turmeric, a well-known yellow spice widely used in Oriental cooking and traditional Chinese and Ayurvedic medicine [
56] for its healing benefits [
57]. It is known for its powerful anti-inflammatory, antioxidant, and anti-cancer properties [
58]. Additionally, curcumin may be helpful in preventing bone loss due to osteoporosis [
59]. It has been shown that curcumin stimulates osteoblastic proliferation and differentiation [
60]; moreover, Samiei
et al. showed that the use of curcumin and calcitriol enhanced DPSC differentiation and mineralization [
61]. This study demonstrated that low doses of curcumin (0.5-1 μM) have no-toxic effects and promote DPSC growth.
DPSCs respond to growth factors that promote osteogenic differentiation and mineral matrix production; in particular, curcumin stimulation increases ALP expression at both mRNA and protein levels in DPSCs [
61].
Interestingly, these results indicate that curcumin could be used as a biological agent to promote osteogenic differentiation of DPSCs and could be an alternative to the drugs commonly used in clinical practice for bone regeneration. Crucial to the use of curcumin in these studies is its bioavailability, as curcumin is absorbed to a very small extent compared to the amount ingested. This means that high quantities are required to achieve the intended effects and these in turn may increase the risk of adverse effects [
62]. A strategy to overcome this problem could be represented by nanotechnology. Nanoparticles complexed with curcumin could improve its bioavailability and optimize its therapeutic effects [
63]. Samiei
et al., in another study, used this approach and evaluated the cytotoxic effects of nanocurcumin at different concentrations on DPSCs [
64]. They showed that nanocurcumin, with concentrations ranging from 0.5 to 10 μM, had no toxic effects and rather promoted DPSC proliferation. On the contrary, when used at higher concentrations (25 μM) and for extended exposure times, only the lowest doses were non-cytotoxic; furthermore, the higher concentrations inhibited DPSC growth. Nanocurcumin, therefore, could present a dose- and time-dependent effect on DPSCs and, in conclusion, it could be a feasible alternative to promote osteo/odontogenic differentiation of dental stem cells. Moreover, curcumin administered in nanoparticles exhibited high bioavailability, low toxicity, and strong biological activity, thus making it an excellent resource for hard tissue repair.
3.4. Irisin
Irisin is a hormone produced especially in skeletal muscle during physical exercise. It has been studied for its potential health benefits, including its ability to increase energy expenditure, reduce fat mass, and improve metabolic health [
65]. Son
et al. investigated and demonstrated that myokine Irisin promoted odontogenic differentiation of DPSCs by inducing mineralized nodule formation, enhancing ALP activity, and upregulating odontogenic markers [
66]. Moreover, the researchers evaluated a significant increase in DPSC migration through scratch wound tests. The results show that the healing of the scratch was significantly increased, due to the Irisin administration for 24 hours, in which period cell proliferation was significantly enhanced both in the control group and in the group with Irisin. It is interesting to highlight that the study by Son
et al. is the first of its kind, which examines Irisin as an odontogenic potential of DPSCs, indicating that this myokine can enhance odontoblastic differentiation and mineralization in DPSCs.
3.5. Vitamins
The formation and preservation of bone health are processes that require an adequate supply of essential nutrients, including vitamins. Only by a diet rich in these nutrients, it would be possible to limit the onset of bone diseases such as osteoporosis. Among vitamins related to bone health Vitamins C and D are known to help the formation of new bone tissue. Vitamin C stimulates the production and correct structural formation of collagen, a major component of bone tissue extracellular matrix (ECM), while Vitamin D increases the absorption of calcium, which is also fundamental for bone formation [67-69]. In addition, Vitamin K and other vitamins such as Vitamin B6, B12, and folic acid have also been shown to have an efficacious effect on bone [70-72].
Several studies on MSCs revealed that the treatment with different vitamins can positively affect the osteoblastic differentiation of these stem cells [
68,
69,
71,
73]. Studies show that Vitamin D (Vit D) and Vitamin E (Vit E) play an important role in promoting DPSC differentiation [74-76]. An interesting
in vitro study evaluated the effect of both Vit D and Vit E, individually or in a combined way, on DPSCs during their osteogenic differentiation [
75]. Vit D treatment appeared to enhance the formation of calcified nodules, but this increase was minimal when the two vitamins were used simultaneously. This result showed that the use of the two vitamins together did not strengthen the prompting effect on osteoblastic differentiation in DPSCs. Furthermore, Vit D and Vit E, individually, stimulated an increase in the expression of cell differentiation genes such as Runx-2 and Osterix (OSX). While in a combined manner, in addition to a decrease in cell proliferation compared to single treatments, there were changes in the morphology of the cells following exposure to the two vitamins [
75].
There are also studies on Vitamin K demonstrating positive effects on MSC osteogenic differentiation, in fact, Vitamin K2 (MK-4) has been shown to be associated with increased bone mineralization and greater resistance to fractures [70-72]. An
in vitro study analyzed the effects of this vitamin on DPSCs used at different concentrations [
77]. At 14 days, the positive effects of MK-4 were evident in the differentiation of DPSCs into osteoblasts with extracellular calcium deposition [
77].
Interestingly, the effects of these vitamins are aimed at models for tissue engineering. The study by Khanna-Jain
et al. highlighted the ability of DPSCs to proliferate and differentiate on a poly (l-lactic acid/caprolactone) scaffold [
29], thanks to Vitamin D3 and dexamethasone, a powerful anti-inflammatory known as an inducer of osteogenic differentiation in DPSCs [
78]. These two enhancers have been shown to have a potentiating role in DPSC osteogenesis, improving their adhesion to scaffolds and upregulating the osteogenic marker OCN. Therefore, vitamins may also have an interesting role in regenerative medicine.
4. Aspirin: a non-natural compound that promotes bone regeneration
Acetylsalicylic acid (ASA), commonly known as Aspirin, is a pain reliever and fever reducer that belongs to a class of drugs called nonsteroidal anti-inflammatory drugs (NSAIDs) [
79]. Aspirin has been shown to have a positive effect on bone metabolism, including the capacity of increasing bone mineral density and bone formation and of improving the renewal of bone marrow MSCs [
80,
81]. Aspirin can be also used in dentistry: it can accelerate the repair of damaged periodontal ligaments since it improves cellular function favoring the differentiation of stem cells and thus modulating the healing process of deep periodontal wounds [
82,
83]. However, the efficacy of Aspirin on osteogenic differentiation of DPSCs needed further investigation
in vivo and Yuan
et al. studied its impact on bone repair in a rat skull defect model, using bovine Bio-Oss where DPSCs were seeded [
84]. Bio-Oss is a natural alternative bone compound widely used in regenerative dentistry during surgical treatments for bone regeneration. It can become an integral part of the newly formed bone structure [
83,
85]. Yuan and colleagues demonstrated that, after seeding DPSCs on Bio-Oss, Aspirin enhanced cell proliferation and DPSCs were able to cover the Bio-Oss scaffolds [
83]. Aspirin effect on DPSC proliferation was recently corroborated also by Khampatee
et al., which found an increased proliferation rate as well as an enhanced odontogenesis of DPSCs when a low dose of Aspirin (25–50 μg/mL) was used
in vitro [
86]. These works represent a promising study for new therapies on DPSC-based bone regeneration.
5. DBSCs
The DB is the immature part of the teeth. It has been observed that it contains MSCs that can differentiate into osteoblast-like cells [
87]. DBSCs can give rise to all the tissues that we find in the mature tooth such as enamel, pulp, dentin, periodontal ligament, and cementum. The DB, being an immature organ, consists of more undifferentiated cells than the DP [
8]. The dimension represents another advantage since the DB is considerably larger than the pulp. Such properties induce to consider the DB as a convenient source of MSCs, but obtaining these postnatal stem cells requires the extraction of a tooth before it erupts. It is necessary to proceed with an early removal (age 7-12) of the third molar DB, using preferably a reliable technique called piezosurgery, which is a more conservative surgical approach. Furthermore, the patients selected for extraction will not lose any functional teeth, since they are often subjects who, due to the birth of a wisdom tooth, could face dental overcrowding [
88].
DBSCs exhibited similarities to bone marrow-derived MSCs. This was verified by studying the expression pattern of adhesion molecules during DBSC differentiation [
89]. Several studies have confirmed that cadherins promote the osteogenesis of bone precursor cells [
90,
91]. Indeed, undifferentiated DBSCs express adhesion receptors such as N-cadherin and cadherin-11, which change their localization during osteogenic differentiation, thus confirming their mesenchymal derivation and osteogenic capacity [
89]. The same studies indicate that DBSCs can be used for bone formation: to fill bone gaps and to replace damaged parts of the bone. Further exploitation can be to regenerate or strengthen bone in case of fractures or bone diseases such as osteoporosis. These cells may also be employed in stomatognathic systems, such as the repair or replacement of damaged or lost teeth making them an important resource for tissue engineering. The optimal reconstruction of bone tissue requires that stem cells have to be grown and differentiated on biomaterial scaffolds under osteogenic conditions and then implanted
in vivo. To do that properly, cells need to interact not only with each other but also with their ECM to acquire and maintain adequate tissue architecture. It was found that by culturing DBSCs with ECM proteins such as osteopontin (OPN), fibronectin (FN), and vitronectin (VTN) there was an enhancement of the differentiation into osteoblast-like cells [
89]. This interesting study suggests the application of DBSCs in the reconstruction of bone tissue, which could be remarkably improved by adding some ECM proteins to the biomaterial used.
6. Differentiative role of natural molecules in DBSCs
6.1. Polydatin
Non-pharmacological therapies based on natural compounds have recently become of great interest to researchers. They are extensively studied for their antioxidant and anti-inflammatory effects [
92,
93]. Studies conducted on bone tissue have shown that the antioxidant properties of phytochemicals, present in fruits and vegetables, have positive effects on bone health [
94]. Polydatin (Pol), a natural glycosylated precursor of the well-known compound RSV, is a powerful stilbenoid polyphenol, which is present in nature and particularly abundant in
Polygonum Cuspidatum. This glucoside, presenting numerous therapeutic potentials, has recently been the object of several studies due to its possible applications as a curative agent [
95]. Regarding the effects of Pol on the skeletal system, studies have demonstrated that the molecule can have an anti-osteoporotic effect and, in particular, can induce osteogenic differentiation and migration of MSCs from bone marrow [
96,
97].
Of note, only the study carried out by Di Benedetto
et al. focused on the involvement of the oligostilbene Pol in DBSC osteogenic differentiation [
98]. The research in question investigated and compared how both, RSV and Pol, affect DBSC differentiation and therefore could aid bone formation. They approached the study by verifying whether DBSCs were responsive to RSV and Pol treatment and found an enhanced Sirt1 expression when the molecules were used. Subsequently, they demonstrated that a lower dose of Pol (0.1 μM) stimulated osteogenic differentiation of DBSCs, upregulating the expression of activating transcription factor 4 (ATF-4), Osteopontin (OPN) and ALP, typical osteoblast markers, and increased the deposition of mineral matrix. On the contrary, a higher dose of Pol (1.0 μM) appeared to be ineffective on the osteogenic differentiation process. Interestingly, low doses of Pol showed an effect comparable to that of RSV used at higher concentrations [
98]. These results propose an effective role of Pol in enhancing the osteogenic differentiation of DBSCs by sharing similar properties with RSV and, surprisingly, showing a real effect at considerably lower concentrations. These findings suggest that Pol may be an alternative to RSV, even more effective, strengthening its positive aspects as an antioxidant or anti-inflammatory and its strong bioactivity [
99,
100]. Thus, Pol could represent a valid alternative to RSV for medical or industrial applications.
6.2. Vitamin D
We have previously illustrated how extensively the role of vitamins in DPSC osteogenic differentiation has been investigated. On the contrary, there are few data in literature related to Vitamins and their possible influence on DBSCs. In 2016, Posa
et al. focused, for the first time, on the potential effect of Vit D on DBSCs differentiating into osteoblasts [
101]. The experiments demonstrated that cultures of DBSCs, with a suitable osteogenic medium, showed an improvement in the differentiation into osteoblastic cells, when treated with the vitamin. In fact, Vit D increased the expression of Runx-2 and Col I, typical early markers of osteoblastogenesis, and favored the formation of mineralized matrix nodules. Later, the authors investigated Vit D influence on the expression of α
Vβ
3 integrin [
102], which is well known for its involvement in the commitment of MSCs towards the osteogenic lineage. The observed findings clarified that Vit D acted on the integrin expression favoring its disposition at the focal adhesion sites level. This action would drive the cells toward their differentiation and would also be supported by the presence of FN, an adhesive glycoprotein of the ECM [
102].
These findings highlight that Vit D enhances the osteoblastic characteristics of DBSCs and that the supplementation of this vitamin could potentially be used to improve DBSC osteogenic capacity and therefore lead to bone regeneration applications.
6.3. Irisin
Irisin, a myokine recently identified, stimulates migration, growth, osteogenic differentiation of periodontal ligament cells (PDLCs), and odontogenic differentiation of DPSCs [
103,
66]. An important role of Irisin has been demonstrated in bone remodeling and in fracture healing [104-108]. In a recent study, it has been observed that Irisin, bound to an αv integrin receptor, acts on osteocytes [
109]. An
in vitro study, conducted on the MLO-Y4 osteocyte cell line, showed that the integrin receptor, when bound by its ligand, activates one of the main signaling pathways: the phosphorylation of Erk1/2 [
110]. Recently the effect of Irisin has been studied in a model of osteoblastogenesis, represented by DBSCs, focusing on the expression of the most important markers of osteoblast differentiation [
111]. In this work by Posa
et al., the responsiveness of DBSCs to Irisin was evaluated for the first time and a cellular response emerged through Erk1/2 phosphorylation. Moreover, the cells maintained the response to the myokine and were induced to osteoblastic differentiation even when cultured under osteogenic conditions. Furthermore, there was an increase in the mineral matrix deposited by DBSCs, again under osteogenic conditions and treatment with Irisin. DBSCs, by means of the myokine effect, also presented an important expression of OCN, a late marker of osteoblastic differentiation. These effects indicate that Irisin plays a relevant role in the osteogenic differentiation process of DBSCs [
111].
7. T-LysYal: a non-natural compound that promotes osteogenic differentiation
Other studies have investigated novel ways to enhance osteogenic differentiation and promote mineral matrix deposition [
87,
112,
113]. Glycosaminoglycans (GAGs) are polysaccharides that play a vital role in the function of many tissues, such as skin, mucous membranes, ligaments, and tendons. They are involved in the regulation of cell permeability, cell adhesion, and tissue regeneration. GAGs are also involved in the synthesis of chemical signaling molecules, an example is hyaluronic acid (HA). HA is a polysaccharide acid abundantly present in the ECM of many tissues. Recently, a new derivative of HA has been developed, called T-LysYal (T-Lys) formed by a combination of HA, lysine hyaluronate, sodium chloride, and thymine. Research has proven encouraging results of T-Lys in tissue regeneration, particularly in bone and cartilage [
88]. T-Lys has been shown to promote wound healing in the nasal mucosa [
114] and the cornea [
115], provide anti-inflammatory effects, and reduce the formation of scar tissue, demonstrating to be effective in the treatment of ulcers and chronic wounds [
116]. In 2020, di Benedetto
et al. conducted a work on T-Lys to evaluate its effect on MSCs from DB and study the regenerative potential of this molecule on bone [
88]. They found that DBSCs responded to treatment with T-Lys, which considerably promotes the expression of Runx-2 and Col I, both at the protein and mRNA level, demonstrating its ability in enhancing the osteogenic differentiation potential of these stem cells. Furthermore, the expression of α
Vβ
3 integrin in DBSCs by administering T-Lys was examined. The literature shows that cell adhesions are crucial for differentiation processes [
117,
118] and that α
Vβ
3 integrin is essential for MSC commitment into the osteoblast line [
87]. The research of Di Benedetto
et al. revealed that T-Lys treatment increased α
Vβ
3 integrin expression directing its localization mainly at focal adhesion sites [
88]. These data suggest that T-Lys influences integrin disposition by affecting DBSC differentiation.
8. Perspectives and Limitations
The current need in the field of bone regeneration is to develop new models to treat bone defects. The studies mentioned in this review indicate DPSCs and DBSCs as innovative and alternative postnatal stem cells sharing similar properties to bone marrow MSCs and easily accessible in sites and organs that are not essential. In fact, it has been demonstrated that these DSCs have a remarkable ability to proliferate, even if they are not inexhaustible like immortalized cell lines. A limitation is that of paying attention and avoiding too many passages during experimentation to prevent consequences that could lead to cell aging. In the field of bone regeneration and therefore to reconstruct bone tissue optimally, in addition to having ideal cell lines as MSCs, it is essential to develop adequate strategies. In recent years, researchers have developed biomaterial scaffolds with specific chemical and physical properties to support the growth and differentiation of stem cells, in osteogenic conditions, to implant in vivo. There are cases where problems have been identified with these strategies. Complications have usually emerged from the non-recruitment and non-adherence of stem cells to the scaffold. In this case, one of the major problems is the inability to obtain a sufficient cell population to ensure quality tissue regeneration. If the cells do not integrate with the scaffold, they will not be able to proliferate and differentiate effectively, limiting regeneration. Furthermore, if the cells do not remain adherent to the scaffold, they can release toxic substances that can damage surrounding stem cells.
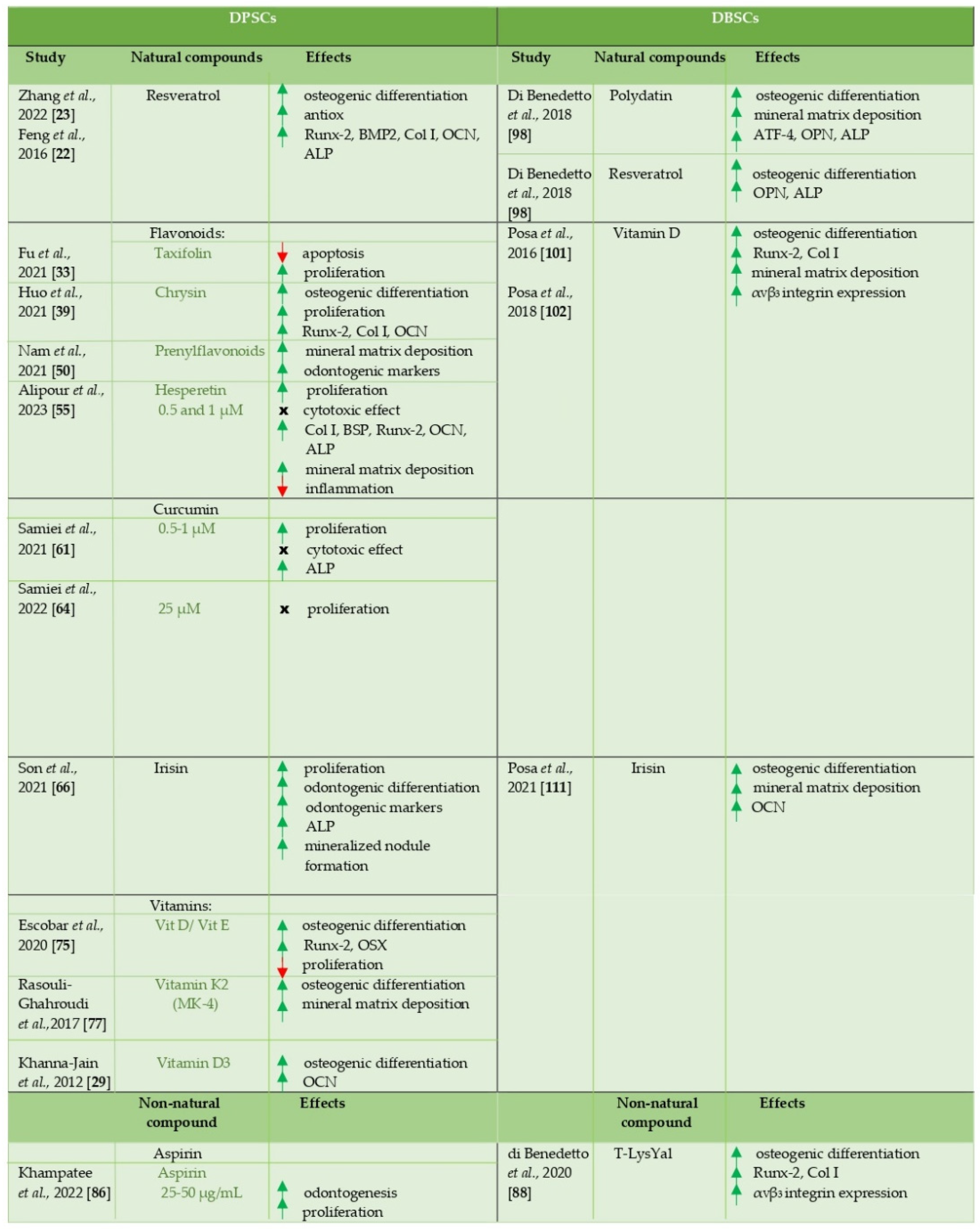
The works here analyzed confirm the potential of DPSCs and DBSCs to differentiate into osteoblast-like cells and, above all, highlight the role that natural and non-natural molecules could have in favoring this process (Table 1), thus laying the foundations for the development of future studies aimed at therapeutic applications, such as bone regeneration, based on the combined use of these cells and compounds.
We would like to encourage further research on these promising molecules, which could clarify the mechanisms underlying their action and their effects on bone health, favoring in vivo applications.
Table 1.
A schematic overview about effects of natural and non-natural compounds on DPSCs and DBSCs.
Table 1.
A schematic overview about effects of natural and non-natural compounds on DPSCs and DBSCs.
Author Contributions
Conceptualization, A.A., F.P. and G.M.; resources, A.A., F.P. and G.M.; writing—original draft preparation, A.A.; writing—review and editing, A.A., F.P., G.S. and G.M.; visualization, F.P., G.S.; supervision, G.M.; project administration, G.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Uccelli, A.; Moretta, L.; Pistoia, V. Mesenchymal Stem Cells in Health and Disease. Nat Rev Immunol 2008, 8, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, K.; Jadhav, S.S. The Application of Mesenchymal Stem Cells to Treat Thermal and Radiation Burns. Adv Drug Deliv Rev 2018, 123, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Rodeo, S.A. Cell Therapy in Orthopaedics: Where Are We in 2019? Bone Joint J 2019, 101-B, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Kebriaei, P.; Isola, L.; Bahceci, E.; Holland, K.; Rowley, S.; McGuirk, J.; Devetten, M.; Jansen, J.; Herzig, R.; Schuster, M.; et al. Adult Human Mesenchymal Stem Cells Added to Corticosteroid Therapy for the Treatment of Acute Graft-versus-Host Disease. Biol Blood Marrow Transplant 2009, 15, 804–811. [Google Scholar] [CrossRef]
- Madonna, R.; Geng, Y.J.; de Caterina, R. Adipose Tissue-Derived Stem Cells: Characterization and Potential for Cardiovascular Repair. Arterioscler Thromb Vasc Biol 2009, 29, 1723–1729. [Google Scholar] [CrossRef]
- Řehořová, M.; Vargová, I.; Forostyak, S.; Vacková, I.; Turnovcová, K.; Kupcová Skalníková, H.; Vodička, P.; Kubinová, Š.; Syková, E.; Jendelová, P. A Combination of Intrathecal and Intramuscular Application of Human Mesenchymal Stem Cells Partly Reduces the Activation of Necroptosis in the Spinal Cord of SOD1G93A Rats. Stem Cells Transl Med 2019, 8, 535–547. [Google Scholar] [CrossRef]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal Human Dental Pulp Stem Cells (DPSCs) in Vitro and in Vivo. Proc Natl Acad Sci U S A 2000, 97, 13625–13630. [Google Scholar] [CrossRef]
- Rodas-Junco, B.A.; Villicaña, C. Dental Pulp Stem Cells: Current Advances in Isolation, Expansion and Preservation. Tissue Eng Regen Med 2017, 14, 333–347. [Google Scholar] [CrossRef]
- Ballini, A.; Mastrangelo, F.; Gastaldi, G.; Tettamanti, L.; Bukvic, N.; Cantore, S.; Cocco, T.; Saini, R.; Desiate, A.; Gherlone, E.; et al. Osteogenic Differentiation and Gene Expression of Dental Pulp Stem Cells under Low-Level Laser Irradiation: A Good Promise for Tissue Engineering. J Biol Regul Homeost Agents 2021, 29, 813–822. [Google Scholar]
- Kawashima, N. Characterisation of Dental Pulp Stem Cells: A New Horizon for Tissue Regeneration? Arch Oral Biol 2012, 57, 1439–1458. [Google Scholar] [CrossRef]
- Liu, H.; Gronthos, S.; Shi, S. Dental Pulp Stem Cells. Methods Enzymol 2006, 419, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, L.; Xiao, L.; Zhang, D. Recycle the Dental Fairy’s Package: Overview of Dental Pulp Stem Cells. Stem Cell Res Ther 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; van den Dolder, J.; Walboomers, X.F.; Zhang, W.; Bian, Z.; Fan, M.; Jansen, J.A. The Odontogenic Potential of STRO-1 Sorted Rat Dental Pulp Stem Cells in Vitro. J Tissue Eng Regen Med 2007, 1, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Brahim, J.; Li, W.; Fisher, L.W.; Cherman, N.; Boyde, A.; DenBesten, P.; Robey, P.G.; Shi, S. Stem Cell Properties of Human Dental Pulp Stem Cells. 2016, 81, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Grottkau, B. E.; Prasad Purudappa, P.; Yun-feng, L. Multilineage Differentiation of Dental Pulp Stem Cells from Green Fluorescent Protein Transgenic Mice. Int J Oral Sci. 2010, 2, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Gronthos, S. Perivascular Niche of Postnatal Mesenchymal Stem Cells in Human Bone Marrow and Dental Pulp. Journal of Bone and Mineral Research 2003, 18, 696–704. [Google Scholar] [CrossRef]
- Sloan, A.J.; Smith, A.J. Stem Cells and the Dental Pulp: Potential Roles in Dentine Regeneration and Repair. Oral Dis 2007, 13, 151–157. [Google Scholar] [CrossRef]
- Baolin, L.; Inami, Y.; Tanaka, H.; Inagaki, N.; Iinuma, M.; Nagai, H. Resveratrol Inhibits the Release of Mediators from Bone Marrow-Derived Mouse Mast Cells in Vitro. Planta Med 2004, 70, 305–309. [Google Scholar] [CrossRef]
- Knutson, M.D.; Leeuwenburgh, C. Resveratrol and Novel Potent Activators of SIRT1: Effects on Aging and Age-Related Diseases. Nutr Rev 2008, 66, 591–596. [Google Scholar] [CrossRef]
- Biswas, P.; Dellanoce, C.; Vezzoli, A.; Mrakic-Sposta, S.; Malnati, M.; Beretta, A.; Accinni, R. Antioxidant Activity with Increased Endogenous Levels of Vitamin C, E and A Following Dietary Supplementation with a Combination of Glutathione and Resveratrol Precursors. Nutrients 2020, 12, 1–14. [Google Scholar] [CrossRef]
- Wu, L.; Li, F.; Zhao, C.; Ming, Y.; Zheng, C.; Li, Y.; Lei, S.; Chen, C. Effects and Mechanisms of Traditional Chinese Herbal Medicine in the Treatment of Ischemic Cardiomyopathy. Pharmacol Res 2020, 151. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Zheng, K.; Song, D.; Xu, K.; Huang, D.; Zhang, Y.; Cao, P.; Shen, S.; Zhang, J.; Feng, X.; et al. SIRT1 Was Involved in TNF-α-Promoted Osteogenic Differentiation of Human DPSCs through Wnt/β-Catenin Signal. In Vitro Cell Dev Biol Anim 2016, 52, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, R.; Man, K.; Yang, X.B. Enhancing Osteogenic Potential of HDPSCs by Resveratrol through Reducing Oxidative Stress via the Sirt1/Nrf2 Pathway. Pharm Biol 2022, 60, 501. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, M.M.; Ornstrup, M.J.; Harsløf, T.; Jessen, N.; Langdahl, B.L.; Richelsen, B.; Jørgensen, J.O.L.; Pedersen, S.B. Short-Term Resveratrol Supplementation Stimulates Serum Levels of Bone-Specific Alkaline Phosphatase in Obese Non-Diabetic Men. J Funct Foods 2014, 6, 305–310. [Google Scholar] [CrossRef]
- Poornima, B.; Korrapati, P.S. Fabrication of Chitosan-Polycaprolactone Composite Nanofibrous Scaffold for Simultaneous Delivery of Ferulic Acid and Resveratrol. Carbohydr Polym 2017, 157, 1741–1749. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wu, Y.; Wang, J.; Zhang, C.; Yan, H.; Zhu, M.; Wang, K.; Li, C.; Xu, Q.; Kong, D. Effect of Resveratrol on Modulation of Endothelial Cells and Macrophages for Rapid Vascular Regeneration from Electrospun Poly(ϵ-Caprolactone) Scaffolds. ACS Appl Mater Interfaces 2017, 9, 19541–19551. [Google Scholar] [CrossRef] [PubMed]
- Riccitiello, F.; de Luise, A.; Conte, R.; D’Aniello, S.; Vittoria, V.; di Salle, A.; Calarco, A.; Peluso, G. Effect of Resveratrol Release Kinetic from Electrospun Nanofibers on Osteoblast and Osteoclast Differentiation. Eur Polym J 2018, 99, 289–297. [Google Scholar] [CrossRef]
- Chen, J.P.; Chang, Y.S. Preparation and Characterization of Composite Nanofibers of Polycaprolactone and Nanohydroxyapatite for Osteogenic Differentiation of Mesenchymal Stem Cells. Colloids Surf B Biointerfaces 2011, 86, 169–175. [Google Scholar] [CrossRef]
- Khanna-Jain, R.; Mannerström, B.; Vuorinen, A.; Sándor, G.K.B.; Suuronen, R.; Miettinen, S. Osteogenic Differentiation of Human Dental Pulp Stem Cells on β-Tricalcium Phosphate/Poly (l-Lactic Acid/Caprolactone) Three-Dimensional Scaffolds. J Tissue Eng 2012, 3, 1–11. [Google Scholar] [CrossRef]
- Swarnkar, G.; Sharan, K.; Siddiqui, J.A.; Mishra, J.S.; Khan, K.; Khan, M.P.; Gupta, V.; Rawat, P.; Maurya, R.; Dwivedi, A.K.; et al. A Naturally Occurring Naringenin Derivative Exerts Potent Bone Anabolic Effects by Mimicking Oestrogen Action on Osteoblasts. Br J Pharmacol 2012, 165, 1526–1542. [Google Scholar] [CrossRef]
- Sak, K. Characteristic Features of Cytotoxic Activity of Flavonoids on Human Cervical Cancer Cells. Asian Pacific Journal of Cancer Prevention 2014, 15, 8007–8018. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.P.; Sheu, S.Y.; Sun, J.S.; Chen, M.H. Icariin Inhibits Osteoclast Differentiation and Bone Resorption by Suppression of MAPKs/NF-ΚB Regulated HIF-1α and PGE2 Synthesis. Phytomedicine 2011, 18, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Feng, Y.; Shao, B.; Zhang, Y. Taxifolin Protects Dental Pulp Stem Cells under Hypoxia and Inflammation Conditions. Cell Transplant. 2021. [Google Scholar] [CrossRef] [PubMed]
- Sunil, C.; Xu, B. An Insight into the Health-Promoting Effects of Taxifolin (Dihydroquercetin). Phytochemistry 2019, 166. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Gu, N.; Xue, C.; Li, B.R. Plant Flavonoid Taxifolin Inhibits the Growth, Migration and Invasion of Human Osteosarcoma Cells. Mol Med Rep 2018, 17, 3239–3245. [Google Scholar] [CrossRef] [PubMed]
- Anthony, K.P.; Saleh, M.A. Free Radical Scavenging and Antioxidant Activities of Silymarin Components. Antioxidants 2013, 2, 398. [Google Scholar] [CrossRef] [PubMed]
- Topal, F.; Nar, M.; Gocer, H.; Kalin, P.; Kocyigit, U.M.; Gülçin, I.; Alwasel, S.H. Antioxidant Activity of Taxifolin: An Activity-Structure Relationship. J Enzyme Inhib Med Chem 2016, 31, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ma, Y.; Luo, L.; Zong, D.; Li, H.; Zeng, Z.; Cui, Y.; Meng, W.; Chen, Y. Dihydroquercetin Suppresses Cigarette Smoke Induced Ferroptosis in the Pathogenesis of Chronic Obstructive Pulmonary Disease by Activating Nrf2-Mediated Pathway. Phytomedicine 2022, 96. [Google Scholar] [CrossRef]
- Huo, J.F.; Zhang, M.L.; Wang, X.X.; Zou, D.H. Chrysin Induces Osteogenic Differentiation of Human Dental Pulp Stem Cells. Exp Cell Res 2021, 400, 112466. [Google Scholar] [CrossRef]
- Zeng, W.; Yan, Y.; Zhang, F.; Zhang, C.; Liang, W. Chrysin Promotes Osteogenic Differentiation via ERK/MAPK Activation. Protein Cell 2013, 4, 539–547. [Google Scholar] [CrossRef]
- Woo, K.J.; Jeong, Y.J.; Park, J.W.; Kwon, T.K. Chrysin-Induced Apoptosis Is Mediated through Caspase Activation and Akt Inactivation in U937 Leukemia Cells. Biochem Biophys Res Commun 2004, 325, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Weng, M.S.; Ho, Y.S.; Lin, J.K. Chrysin Induces G1 Phase Cell Cycle Arrest in C6 Glioma Cells through Inducing P21Waf1/Cip1 Expression: Involvement of P38 Mitogen-Activated Protein Kinase. Biochem Pharmacol 2005, 69, 1815–1827. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.K.; Kwon, H.S.; Kim, Y.H.; Shin, H.K.; Kim, J.K. Chrysin, a Natural Flavone, Improves Murine Inflammatory Bowel Diseases. Biochem Biophys Res Commun 2009, 381, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.K.; Moon, E.; Kim, S.Y. Chrysin Suppresses LPS-Stimulated Proinflammatory Responses by Blocking NF-ΚB and JNK Activations in Microglia Cells. Neurosci Lett 2010, 485, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X. <p>Chrysin Attenuates High Glucose-Induced BMSC Dysfunction via the Activation of the PI3K/AKT/Nrf2 Signaling Pathway</P>. Drug Des Devel Ther 2022, 16, 165–182. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Qin, H.; Shi, Q. Chrysin attenuates inflammation by regulating M1/M2 status via activating PPARgamma, Biochem. Pharmacol. 2014, 89, 503–514. [Google Scholar] [CrossRef]
- Pileggi, R.; Antony, K.; Johnson, K.; Zuo, J.; Shannon Holliday, L. Propolis Inhibits Osteoclast Maturation. Dent Traumatol 2009, 25, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Z.; Luo, Y.; Li, X.; Huang, G.; Chen, H.; Li, A.; Qin, S. The Role of Flavonoids in the Osteogenic Differentiation of Mesenchymal Stem Cells. Front Pharmacol 2022, 13. [Google Scholar] [CrossRef]
- Santos, C.M.M.; Silva, A.M.S. The Antioxidant Activity of Prenylflavonoids. Molecules 2020, 25. [Google Scholar] [CrossRef]
- Nam, S.H.; Yamano, A.; Kim, J.A.; Lim, J.; Baek, S.H.; Kim, J.E.; Kwon, T.; Saito, Y.; Teruya, T.; Choi, S.Y.; et al. Prenylflavonoids Isolated from Macaranga Tanarius Stimulate Odontoblast Differentiation of Human Dental Pulp Stem Cells and Tooth Root Formation via the Mitogen-Activated Protein Kinase and Protein Kinase B Pathways. Int Endod J 2021, 54, 1142–1154. [Google Scholar] [CrossRef]
- Galati, E.M.; Montforte, M.T.; Kirjavainen, S.; Forestieri, A.M.; Trovato, A.; Tripodo, M.M. Biological Effects of Hesperidin, a Citrus Flavonoid. (Note I): Antiinflammatory and Analgesic Activity. Farmaco 1994, 40, 709–712. [Google Scholar] [PubMed]
- Xue, D.; Chen, E.; Zhang, W.; Gao, X.; Wang, S.; Zheng, Q.; Pan, Z.; Li, H.; Liu, L. The Role of Hesperetin on Osteogenesis of Human Mesenchymal Stem Cells and Its Function in Bone Regeneration. Oncotarget 2017, 8, 21031. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Liu, W.; Bi, J.; Liu, S.; Zhao, H.; Gong, N.; Xing, D.; Gao, H.; Gong, M. Anti-Inflammatory Effect of Hesperidin Enhances Chondrogenesis of Human Mesenchymal Stem Cells for Cartilage Tissue Repair. J Inflamm (Lond) 2018, 15. [Google Scholar] [CrossRef] [PubMed]
- Farokhzad, O.C.; Langer, R. Impact of nanotechnology on drug delivery. ACS Nano 2009, 3, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Alipour, M.; Sharifi, S.; Samiei, M.; Shahi, S.; Aghazadeh, M.; Dizaj, S.M. Synthesis, Characterization, and Evaluation of Hesperetin Nanocrystals for Regenerative Dentistry. Scientific Reports 2023 13:1 2023, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak-Drozd, K.; Oniszczuk, T.; Gancarz, M.; Kondracka, A.; Rusinek, R.; Oniszczuk, A. Curcumin and Weight Loss: Does It Work? Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef]
- Hatcher, H.; Planalp, R.; Cho, J.; Torti, F.M.; Torti, S. V Curcumin: From Ancient Medicine to Current Clinical Trials. Cell Mol Life Sci 2008, 65, 1631–52. [Google Scholar] [CrossRef]
- Sharifi, S.; Zununi Vahed, S.; Ahmadian, E.; Maleki Dizaj, S.; Abedi, A.; Hosseiniyan Khatibi, S.M.; Samiei, M. Stem Cell Therapy: Curcumin Does the Trick. Phytotherapy Research 2019, 33, 2927–2937. [Google Scholar] [CrossRef]
- Khanizadeh, F.; Rahmani, A.; Asadollahi, K.; Ahmadi, M.R.H. Combination Therapy of Curcumin and Alendronate Modulates Bone Turnover Markers and Enhances Bone Mineral Density in Postmenopausal Women with Osteoporosis. Arch Endocrinol Metab 2018, 62, 438–445. [Google Scholar] [CrossRef]
- Son, H.E.; Kim, E.J.; Jang, W.G. Curcumin Induces Osteoblast Differentiation through Mild-Endoplasmic Reticulum Stress-Mediated Such as BMP2 on Osteoblast Cells. Life Sci 2018, 193, 34–39. [Google Scholar] [CrossRef]
- Samiei, M.; Abedi, A.; Sharifi, S.; Dizaj, S.M.; Maleki, S.; Rodr Guez Lozano, F.J. Early Osteogenic Differentiation Stimulation of Dental Pulp Stem Cells by Calcitriol and Curcumin. Stem Cells Int 2021, 23. [Google Scholar] [CrossRef] [PubMed]
- Tahmasebi Birgani, M.; Erfani-Moghadam, V.; Babaei, E.; Najafi, F.; Zamani, M.; Shariati, M.; Nazem, S.; Farhangi, B.; Motahari, P.; Sadeghizadeh, M. Dendrosomal Nano-Curcumin; The Novel Formulation to Improve the Anticancer Properties of Curcumin. Prog Biol Sci 2015, 5, 143–158. [Google Scholar] [CrossRef]
- Rajalakshmi, N.; Dhivya, S. A Review on the Preparation Methods of Curcumin Nanoparticles. PharmaTutor 2018, 6, 6–10. [Google Scholar] [CrossRef]
- Samiei, M.; Arablouye Moghaddam, F.; Dalir Abdolahinia, E.; Ahmadian, E.; Sharifi, S.; Maleki Dizaj, S. Influence of Curcumin Nanocrystals on the Early Osteogenic Differentiation and Proliferation of Dental Pulp Stem Cells. J Nanomater 2022, 2022. [Google Scholar] [CrossRef]
- Colaianni, G.; Cinti, S.; Colucci, S.; Grano, M. Irisin and Musculoskeletal Health. Ann N Y Acad Sci 2017, 1402, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Son, J.W.; Choi, S.H.; Jang, J.H.; Koh, J.T.; Oh, W.M.; Hwang, Y.C.; Lee, B.N. Irisin Promotes Odontogenic Differentiation and Angiogenic Potential in Human Dental Pulp Cells. Int Endod J 2021, 54, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Oakes, B.; Bolia, I.K.; Weber, A.E.; Petrigliano, F.A. Vitamin C in Orthopedic Practices: Current Concepts, Novel Ideas, and Future Perspectives. Journal of Orthopaedic Research 2021, 39, 698–706. [Google Scholar] [CrossRef] [PubMed]
- De Kok, I.J.; Hicok, K.C.; Padilla, R.J.; Young, R.G.; Cooper, L.F. Effect of Vitamin D Pretreatment of Human Mesenchymal Stem Cells on Ectopic Bone Formation. Journal of Oral Implantology 2006, 32, 103–109. [Google Scholar] [CrossRef]
- Curtis, K.M.; Aenlle, K.K.; Roos, B.A.; Howard, G.A. 24R,25-Dihydroxyvitamin D3 Promotes the Osteoblastic Differentiation of Human Mesenchymal Stem Cells. Molecular Endocrinology 2014, 28, 644–658. [Google Scholar] [CrossRef]
- Urayama, S.; Kawakami, A.; Nakashima, T.; Tsuboi, M.; Yamasaki, S.; Hida, A.; Ichinose, Y.; Nakamura, H.; Ejima, E.; Aoyagi, T.; et al. Effect of Vitamin K2 on Osteoblast Apoptosis: Vitamin K2 Inhibits Apoptotic Cell Death of Human Osteoblasts Induced by Fas, Proteasome Inhibitor, Etoposide, and Staurosporine. Journal of Laboratory and Clinical Medicine 2000, 136, 181–193. [Google Scholar] [CrossRef]
- Koshihara, Y.; Hoshi, K.; Okawara, R.; Ishibashi, H.; Yamamoto, S. Vitamin K Stimulates Osteoblastogenesis and Inhibits Osteoclastogenesis in Human Bone Marrow Cell Culture. J Endocrinol 2003, 176, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Atkins, G.J.; Welldon, K.J.; Wijenayaka, A.R.; Bonewald, L.F.; Findlay, D.M. Vitamin K promotes mineralization, osteoblast-to-osteocyte transition, and an anticatabolic phenotype by γ-carboxylation-dependent and-independent mechanisms. American Journal of Physiology-Cell Physiology 2009, 297, C1358–C67. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.H.; Jung, H.K.; Jung, S.E.; Yi, K.W.; Park, H.T.; Shin, J.H.; Kim, Y.T.; Hur, Y.; Kim, S.H.; Kim, T. Microarray Analysis of Gene Expression During Differentiation of Human Mesenchymal Stem Cells Treated with Vitamin E in Vitro into Osteoblasts. Korean Journal of Bone Metabolism 2011, 18, 23–32. [Google Scholar]
- Mojarad, F.; Amiri, I.; Rafatjou, R.; Janeshin, A.; Farhadian, M.; Author, C. The Effect of 1α,25(OH)2D3 on Osteogenic Differentiation of Stem Cells from Dental Pulp of Exfoliated Deciduous Teeth. J Dent Shiraz Univ Med Sci 2016, 17, 348–353. [Google Scholar]
- Escobar, L.M.; Bendahan, Z.; Bayona, A.; Castellanos, J.E.; González, M.C. Effect of Vitamins D and E on the Proliferation, Viability, and Differentiation of Human Dental Pulp Stem Cells: An in Vitro Study. Int J Dent 2020, 2020. [Google Scholar] [CrossRef]
- Khanna-Jain, R.; Vuorinen, A.; Sándor, G.K.B.; Suuronen, R.; Miettinen, S. Vitamin D3 Metabolites Induce Osteogenic Differentiation in Human Dental Pulp and Human Dental Follicle Cells. J Steroid Biochem Mol Biol 2010, 122, 133–141. [Google Scholar] [CrossRef]
- Rasouli-Ghahroudi, A.A.; Akbari, S.; Najafi-Alishah, M.; Bohloli, M. The Effect of Vitamin K2 on Osteogenic Differentiation of Dental Pulp Stem Cells: An In Vitro Study. Journal of “Regeneration, Reconstruction & Restoration” (Triple R) 2017, 2, 26–29. [Google Scholar] [CrossRef]
- Rickard, D.J.; Sullivan, T.A.; Shenker, B.J.; Leboy, P.S.; Kazhdan, I. Induction of Rapid Osteoblast Differentiation in Rat Bone Marrow Stromal Cell Cultures by Dexamethasone and BMP-2. Dev Biol 1994, 161, 218–228. [Google Scholar] [CrossRef]
- Smith, J.B.; Willis, A.L. Aspirin Selectively Inhibits Prostaglandin Production in Human Platelets. Nat New Biol 1971, 231, 235–237. [Google Scholar] [CrossRef]
- Yamaza, T.; Miura, Y.; Bi, Y.; Liu, Y.; Akiyama, K.; Sonoyama, W.; Patel, V.; Gutkind, S.; Young, M.; Gronthos, S.; et al. Pharmacologic Stem Cell Based Intervention as a New Approach to Osteoporosis Treatment in Rodents. PLoS One 2008, 3. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, C.; Liu, S.; Liu, D.; Xu, X.; Chen, X.; Shi, S. Acetylsalicylic Acid Treatment Improves Differentiation and Immunomodulation of SHED. J Dent Res 2015, 94, 209–218. [Google Scholar] [CrossRef]
- Rahman, F.A.; Ali, J.M.; Abdullah, M.; Kasim, N.H.A.; Musa, S. Aspirin Enhances Osteogenic Potential of Periodontal Ligament Stem Cells (PDLSCs) and Modulates the Expression Profile of Growth Factor–Associated Genes in PDLSCs. J Periodontol 2016, 87, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Sculean, A.; Chiantella, G.C.; Windisch, P.; Gera, I.; Reich, E. Clinical evaluation of an enamel matrix protein derivative (Emdogain) combined with a bovine-derived xenograft (Bio-Oss) for the treatment of intrabony periodontal defects in humans. Int J Periodontics Restorative Dent. 2002, 22, 259–267. [Google Scholar] [PubMed]
- Yuan, M.; Zhan, Y.; Hu, W.; Li, Y.; Xie, X.; Miao, N.; Jin, H.; Zhang, B. Aspirin Promotes Osteogenic Differentiation of Human Dental Pulp Stem Cells. Int J Mol Med 2018, 42, 1967–1976. [Google Scholar] [CrossRef] [PubMed]
- Klinge, B.; Alberius, P.; Isaksson, S.; Jönsson, J. Osseous reponse to implant natural bone mineral and synthetic hydroxylapatite ceramic in the repair of experimental skull bone defects. J Oral Maxillofac Surg. 1992, 50, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Khampatee, V.; Zhang, C.; Chou, L. Effects of Aspirin on Odontogenesis of Human Dental Pulp Cells and TGF- β 1 Liberation from Dentin In Vitro. Int J Dent 2022, 2022. [Google Scholar] [CrossRef] [PubMed]
- Di Benedetto, A.; Brunetti, G.; Posa, F.; Ballini, A.; Grassi, F.R.; Colaianni, G.; Colucci, S.; Rossi, E.; Cavalcanti-Adam, E.A.; Lo Muzio, L.; et al. Osteogenic Differentiation of Mesenchymal Stem Cells from Dental Bud: Role of Integrins and Cadherins. Stem Cell Res 2015, 15, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Di Benedetto, A.; Posa, F.; Marazzi, M.; Kalemaj, Z.; Grassi, R.; lo Muzio, L.; Comite, M. di; Cavalcanti-Adam, E.A.; Grassi, F.R.; Mori, G. Osteogenic and Chondrogenic Potential of the Supramolecular Aggregate T-LysYal®. Front Endocrinol (Lausanne) 2020, 11, 285. [Google Scholar] [CrossRef]
- Di Benedetto, A.; Brunetti, G.; Posa, F.; Ballini, A.; Grassi, F.R.; Colaianni, G.; Colucci, S.; Rossi, E.; Cavalcanti-Adam, E.A.; lo Muzio, L.; et al. Osteogenic Differentiation of Mesenchymal Stem Cells from Dental Bud: Role of Integrins and Cadherins. Stem Cell Res 2015, 15, 618–628. [Google Scholar] [CrossRef]
- Mbalaviele, G.; Donsante, C.; Watkins, M.; Radice, G.L.; Civitelli, R. Accentuated Ovariectomy-Induced Bone Loss and Altered Osteogenesis in Heterozygous N-Cadherin Null Mice. Journal of Bone and Mineral Research 2006, 21, 1897–1906. [Google Scholar] [CrossRef]
- Greenbaum, A.M.; Revollo, L.D.; Woloszynek, J.R.; Civitelli, R.; Link, D.C. N-Cadherin in Osteolineage Cells Is Not Required for Maintenance of Hematopoietic Stem Cells. Blood 2012, 120, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Levis, S.; Theodore, G. Summary of AHRQ’s Comparative Effectiveness Review of Treatment to Prevent Fractures in Men and Women with Low Bone Density or Osteoporosis: Update of the 2007 Report. J Manag Care Pharm 2012, 18. [Google Scholar] [CrossRef]
- Conti, V.; Izzo, V.; Corbi, G.; Russomanno, G.; Manzo, V.; de Lise, F.; di Donato, A.; Filippelli, A. Antioxidant Supplementation in the Treatment of Aging-Associated Diseases. Front Pharmacol 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Gunn, C.A.; Weber, J.L.; McGill, A.T.; Kruger, M.C. Increased Intake of Selected Vegetables, Herbs and Fruit May Reduce Bone Turnover in Post-Menopausal Women. Nutrients 2015, 7, 2499–2517. [Google Scholar] [CrossRef] [PubMed]
- Şöhretoğlu, D.; Baran, M.Y.; Arroo, R.; Kuruüzüm-Uz, A. Recent Advances in Chemistry, Therapeutic Properties and Sources of Polydatin. Phytochemistry Reviews 2018 17:5 2018, 17, 973–1005. [Google Scholar] [CrossRef]
- Shen, Y.-S.; Chen, X.-J.; Wuri, S.-N.; Yang, F.; Pang, F.-X.; Xu, L.-L.; He, W.; Wei, Q.-S. Polydatin Improves Osteogenic Differentiation of Human Bone Mesenchymal Stem Cells by Stimulating TAZ Expression via BMP2-Wnt/β-Catenin Signaling Pathway. Stem Cell Res Ther 2020, 11, 204. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Q.; Wei, Q.S.; Hong, G.J.; Chen, D.; Liang, J.; He, W.; Chen, M.H. Polydatin Induces Bone Marrow Stromal Cells Migration by Activation of ERK1/2. Biomed Pharmacother 2016, 82, 49–53. [Google Scholar] [CrossRef] [PubMed]
- di Benedetto, A.; Posa, F.; de Maria, S.; Ravagnan, G.; Ballini, A.; Porro, C.; Trotta, T.; Grano, M.; Muzio, L. lo; Mori, G. Polydatin, Natural Precursor of Resveratrol, Promotes Osteogenic Differentiation of Mesenchymal Stem Cells. Int J Med Sci 2018, 15, 944–952. [Google Scholar] [CrossRef]
- Wang, H.L.; Gao, J.P.; Han, Y.L.; Xu, X.; Wu, R.; Gao, Y.; Cui, X.H. Comparative Studies of Polydatin and Resveratrol on Mutual Transformation and Antioxidative Effect in Vivo. Phytomedicine 2015, 22, 553–559. [Google Scholar] [CrossRef]
- Lanzilli, G.; Cottarelli, A.; Nicotera, G.; Guida, S.; Ravagnan, G.; Fuggetta, M.P. Anti-Inflammatory Effect of Resveratrol and Polydatin by In Vitro IL-17 Modulation. Inflammation 2012. [Google Scholar] [CrossRef]
- Posa, F.; di Benedetto, A.; Colaianni, G.; Cavalcanti-Adam, E.A.; Brunetti, G.; Porro, C.; Trotta, T.; Grano, M.; Mori, G. Vitamin D Effects on Osteoblastic Differentiation of Mesenchymal Stem Cells from Dental Tissues. Stem Cells Int 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Posa, F.; Di Benedetto, A.; Cavalcanti-Adam, E.A.; Colaianni, G.; Porro, C.; Trotta, T.; Brunetti, G.; Lo Muzio, L.; Grano, M.; Mori, G. Vitamin D Promotes MSC Osteogenic Differentiation Stimulating Cell Adhesion and αVβ3 Expression. Stem Cells Int 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Pullisaar, H.; Colaianni, G.; Lian, A.M.; Vandevska-Radunovic, V.; Grano, M.; Reseland, J.E. Irisin Promotes Growth, Migration and Matrix Formation in Human Periodontal Ligament Cells. Arch Oral Biol 2020, 111. [Google Scholar] [CrossRef]
- Colaianni, G.; Cuscito, C.; Mongelli, T.; Pignataro, P.; Buccoliero, C.; Liu, P.; Lu, P.; Sartini, L.; Comite, M.D.; Mori, G.; et al. The Myokine Irisin Increases Cortical Bone Mass. Proc Natl Acad Sci U S A 2015, 112, 12157–12162. [Google Scholar] [CrossRef]
- Luo, Y.; Ma, Y.; Qiao, X.; Zeng, R.; Cheng, R.; Nie, Y.; Li, S.; A, R.; Shen, X.; Yang, M.; et al. Irisin Ameliorates Bone Loss in Ovariectomized Mice. Climacteric 2020, 23, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Qiao, X.; Ma, Y.; Deng, H.; Xu, C.C.; Xu, L. Disordered Metabolism in Mice Lacking Irisin. Sci Rep 2020, 10. [Google Scholar] [CrossRef]
- Colucci, S.C.; Buccoliero, C.; Sanesi, L.; Errede, M.; Colaianni, G.; Annese, T.; Khan, M.P.; Zerlotin, R.; Dicarlo, M.; Schipani, E.; et al. Systemic Administration of Recombinant Irisin Accelerates Fracture Healing in Mice. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- Oranger, A.; Zerlotin, R.; Buccoliero, C.; Sanesi, L.; Storlino, G.; Schipani, E.; Kozloff, K.M.; Mori, G.; Colaianni, G.; Colucci, S.; et al. Irisin Modulates Inflammatory, Angiogenic, and Osteogenic Factors during Fracture Healing. Int J Mol Sci 2023, 24, 1809. [Google Scholar] [CrossRef]
- Kim, H.; Wrann, C.D.; Jedrychowski, M.; Vidoni, S.; Kitase, Y.; Nagano, K.; Zhou, C.; Chou, J.; Parkman, V.J.A.; Novick, S.J.; et al. Irisin Mediates Effects on Bone and Fat via AV Integrin Receptors. Cell 2018, 175, 1756–1768.e17. [Google Scholar] [CrossRef]
- Storlino, G.; Colaianni, G.; Sanesi, L.; Lippo, L.; Brunetti, G.; Errede, M.; Colucci, S.; Passeri, G.; Grano, M. Irisin Prevents Disuse-Induced Osteocyte Apoptosis. J Bone Miner Res 2020, 35, 766–775. [Google Scholar] [CrossRef]
- Posa, F.; Colaianni, G.; di Cosola, M.; Dicarlo, M.; Gaccione, F.; Colucci, S.; Grano, M.; Mori, G. The Myokine Irisin Promotes Osteogenic Differentiation of Dental Bud-Derived MSCs. Biology (Basel) 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, D.T. “Ins” and “Outs” of Mesenchymal Stem Cell Osteogenesis in Regenerative Medicine. World J Stem Cells 2014, 6, 94–110. [Google Scholar] [CrossRef] [PubMed]
- Barthes, J.; Özçelik, H.; Hindié, M.; Ndreu-Halili, A.; Hasan, A.; Engin Vrana, N. Cell Microenvironment Engineering and Monitoring for Tissue Engineering and Regenerative Medicine: The Recent Advances. Biomed Res Int. 2014. [Google Scholar] [CrossRef] [PubMed]
- Cantone, E.; Castagna, G.; Sicignano, S.; Ferranti, I.; Rega, F.; di Rubbo, V.; Iengo, M. Impact of Intranasal Sodium Hyaluronate on the Short-Term Quality of Life of Patients Undergoing Functional Endoscopic Sinus Surgery for Chronic Rhinosinusitis. Int Forum Allergy Rhinol 2014, 4, 484–487. [Google Scholar] [CrossRef] [PubMed]
- Barabino, S.; de Servi, B.; Aragona, S.; Manenti, D.; Meloni, M. Efficacy of a New Ocular Surface Modulator in Restoring Epithelial Changes in an In Vitro Model of Dry Eye Syndrome. Curr Eye Res 2017, 42, 358–363. [Google Scholar] [CrossRef]
- Felzani, G.; Spoletini, I.; Convento, A.; di Lorenzo, B.; Rossi, P.; Miceli, M.; Rosano, G. Effect of Lysine Hyaluronate on the Healing of Decubitus Ulcers in Rehabilitation Patients. Adv Ther 2011, 28, 439–445. [Google Scholar] [CrossRef]
- Docheva, D.; Popov, C.; Mutschler, W.; Schieker, M. Human Mesenchymal Stem Cells in Contact with Their Environment: Surface Characteristics and the Integrin System. J Cell Mol Med 2007, 11, 21–38. [Google Scholar] [CrossRef]
- Wang, Y.K.; Chen, C.S. Cell Adhesion and Mechanical Stimulation in the Regulation of Mesenchymal Stem Cell Differentiation. J Cell Mol Med 2013, 17, 823. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).